Abstract
Much attention has been paid to three-dimensional cell culture systems in the field of regenerative medicine, since three-dimensional cellular aggregates, or spheroids, are thought to better mimic the in vivo microenvironments compared to conventional monolayer cultured cells. Synthetic calcium phosphate (CaP) materials are widely used as bone substitute materials in orthopedic and dental surgeries. Here we have developed a technique for constructing a hybrid spheroid consisting of mesenchymal stem cells (MSCs) and synthetic CaP materials using a spheroid culture device. We found that the device is able to generate uniform-sized CaP/cell hybrid spheroids rapidly and easily. The results showed that the extent of osteoblastic differentiation from MSCs was different when cells were grown on octacalcium phosphate (OCP), hydroxyapatite (HA), or β-tricalcium phosphate (β-TCP). OCP showed the greatest ability to increase the alkaline phosphatase activity of the spheroid cells. The results suggest that the spheroids with incorporated OCP may be an effective implantable hybrid consisting of scaffold material and cells for bone regeneration. It is also possible that this CaP–cell spheroid system may be used as an in vitro method for assessing the osteogenic induction ability of CaP materials.
Keywords: Spheroids, Calcium phosphate, Octacalcium phosphate, Oxygen-permeable chip, Osteoblastic differentiation
Highlights
-
•
Constructing a hybrid spheroid consisting of MSCs and calcium phosphates was examined.
-
•
A culture device can generate uniform-sized hybrid spheroids rapidly and easily.
-
•
A spheroid with incorporated octacalcium phosphate was an effective implantable hybrid.
1. Introduction
The development of three dimensional (3-D) cell culture models has attracted a great deal of attention in the field of tissue engineering, since 3-D cell cultures appear to better mimic the microenvironment around cells within the body as well as stimulate physiological responses compared to conventional two dimensional (2-D; monolayer) cultures. The creation of functional 3-D tissue with or without scaffold materials could be useful not only for tissue engineering, but also for helping to better understand basic mechanisms of cell–cell and/or cell–matrix interactions and tissue development.
One of the biggest problems of using 3-D cell aggregates in regenerative medicine is the development of hypoxia and subsequent cell death due to a lack of oxygen supply in the center of cell aggregates. To overcome this problem, we have developed an oxygen-permeable spheroid culture device [1]. As an alternative approach, other groups have reported the use of microspheres in cell aggregates in order to prevent a lack of oxygen and nutrients in the center of spheroids. In those studies, it was shown that the incorporation of microbeads made of gelatin [2] and alginate [3] into cell aggregates promoted cell activities.
Here we present a methodology to promote osteoblastic differentiation of mesenchymal stem cells by incorporating microparticles consisting of calcium phosphate (CaP) materials. We hypothesized that the presence of the CaP minerals may favor osteoblastic differentiation of cells in the cell aggregates. In this paper, we compare the formation of aggregates and the osteoblastic differentiation of mesenchymal stem cell strain D1 with or without three types of CaP materials: hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), or octacalcium phosphate (OCP). HA and β-TCP are commonly used bone substitutes in clinical use. Our previous study showed that OCP promotes new bone formation compared to HA and β-TCP in bone defects of rat tibia [4]. In this study we present a novel 3-D cell culture system that could be an effective strategy for promoting osteoblastic differentiation of MSCs in vitro.
2. Materials and methods
2.1. Fabrication of spheroid culture chips
We prepared a spheroid culture chip as previously described [1]. Briefly, a polydimethylsiloxiane (PDMS) negative mold was replicated from a prototype culture device utilizing the thin PDMS membrane deformation by applying negative pressure [5]. A PDMS (Silpot 184, Dow Corning Toray, Co. Ltd., Tokyo, Japan) prepolymer was prepared by mixing the base and curing agent at a ratio of 10:1. The negative mold was immersed in 4% Pluronic F-127 (Sigma–Aldrich, St. Louis, MO, USA) solution for 24 h to facilitate wetting of the surface of the mold and to prevent PDMS-to-PDMS adhesion [1]. PDMS prepolymer was poured into the PDMS negative mold and cured at 70 °C for 1 h. The PDMS replica was peeled off from the mold and used in the cell culture in the present study (Oxy chip). The Oxy chip was designed to consist of multicavities (512 wells, 1.00 mm in diameter, 1.05 mm pitch, 1.06 mm in depth) in a triangular arrangement on a 25 × 25 mm section of the cell culture area.
2.2. Cell culture
Mouse bone marrow-derived mesenchymal stem cells (D1 ORL UVA [D1]) were obtained from ATCC (Rockville, MD, USA). The cells were maintained in minimum Dulbecco's Modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS; Sigma–Aldrich, St. Louis, MO) and 1% penicillin/streptomycin (PS, Invitrogen-Gibco, Carlsbad, CA) at 37 °C in a 5% carbon dioxide environment. The PDMS chips were sterilized in an oven (160 °C, 2 h). Before use, the PDMS chips were incubated with 2 ml of 4% Pluronic F-127 solution overnight. The polymer is adsorbed on the surface of the PDMS and prevents cell attachment [1], [6]. The chips were then rinsed three times with DMEM to remove excess Pluronic F-127.
OCP was prepared by mixing calcium and phosphate solutions as previously described [7]. Commercially available sintered β-TCP (OSferion: OLIMPUS TERUMO BIOMATERIALS, Tokyo, Japan) and HA (APACERAM: PENTAX, Tokyo, Japan) were purchased. OCP, β-TCP, and HA granules were obtained by passing them through a standard testing sieve (270-mesh sieve and 53 μm). The sieved granules were sterilized by heating at 120 °C for 12 h. The average particle size of CaPs was measured using a SHIMAZDU SALD-2000J laser diffraction particle size analyzer. The analysis revealed that the average size of OCP, HA, and β-TCP were 30.5, 18.8, 41.9 μm, respectively.
D1 cells (1.0 × 106 cells) were mixed with OCP granules (1.0 mg), β-TCP granules (2.0 mg), or HA granules (5.0 mg) in 3 ml of osteogenic differentiation medium (DMEM supplemented with 10% FBS, 1% PS, 50 μg/ml ascorbate 2-phosphate, 10 mM β-glycero phosphate, and 100 nM dexamethasone). Cells (1.0 × 106 cells) without calcium phosphate granules were inoculated in the Oxy chip as a control group. All cells were cultured at 37 °C, 5% CO2, and 95% air in humidified incubators. The culture medium was changed every two days.
2.3. Spheroid diameter measurement
To evaluate changes in spheroid diameter, spheroids were photographed with a photomicroscope (Leica DFC300 FX, Leica Microsystems Japan, Tokyo, Japan). Spheroid diameters were analyzed using an image analysis program for Windows (Image-Pro Plus 7.0, Media Cybernetics Inc., Bethesda, MD, USA). A minimum of 30 spheroids on each chip were photographed and diameters were measured. Spheroid diameter was defined as the average length of diameters measured at two-degree intervals joining two outline points and passing through the centroid.
2.4. Measurement of DNA content and alkaline phosphatase (ALP) of D1 cells
Cells on culture chips were rinsed three times with phosphate buffered saline (PBS). Spheroids were then retrieved from culture chips by washing them out with PBS using a plastic pipette. The collected spheroids were suspended in 0.5 ml of 0.2% Triton X-100 solution and sonicated in an ice bath. DNA concentration in cell lysate was measured using a Quant-iT™ PicoGreen® dsDNA kit (Invitrogen). ALP activity was measured using a commercially available kit (Wako Pure Chemical Industries, Ltd.). The ALP activity was normalized using DNA amounts as determined with the Pico Green kit.
2.5. Analysis by histochemistry
Cells with or without calcium phosphate materials were incubated in the culture chip for 7 days as described above. At day 7, cell culture chips were rinsed three times with PBS. The spheroids collected from the culture chips were fixed in 10% formalin for 24 h. Spheroids were then rinsed with PBS and embedded in 2.5% fibrin gel. Fibrin clot containing spheroids were fixed in 10% formalin for 24 h at 4 °C. Serial sections (3.5 μm) were mounted onto silane-coated slides and stained with hematoxylin-eosin (HE). Photographs were taken with a photomicroscope (Leica DFC300 FX, Leica Microsystems Japan, Tokyo, Japan).
2.6. Statistical analysis
Results were expressed as the mean ± standard deviation (SD). All experiments were performed at least three times and showed reliable reproducibility. Statistical differences among specimens were evaluated by Tukey–Kramer multiple comparison analysis. A value of p < 0.05 was regarded as statistically significant.
3. Results
3.1. Formation of D1 cell spheroids and CaP/cell spheroids
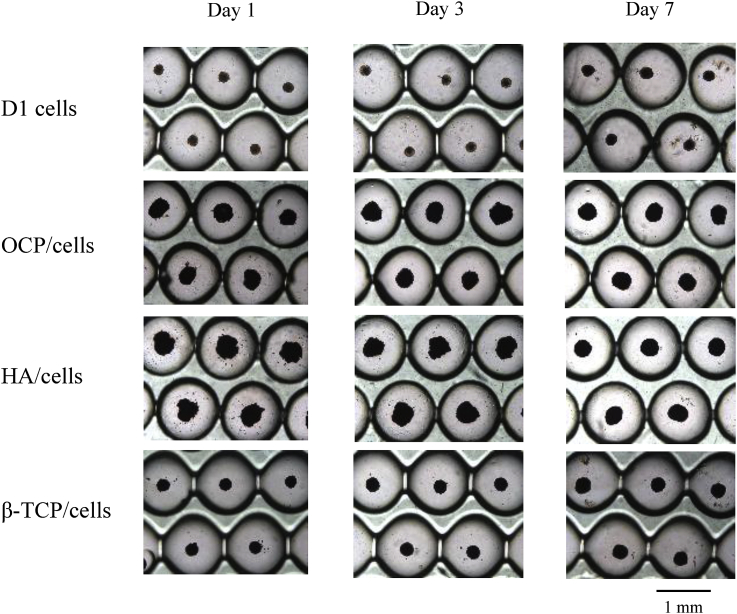
Fig. 1 shows light microscopic images of spheroid formation of D1 cells and formation of CaP/cell spheroids on the culture chips. Spheroids of only D1 cells formed on the chip within one day. The size of CaP/cell spheroids was always larger than that of spheroids consisting of only D1 cells during the culture period. An assembly of cells with OCP or HA was slower than that with β-TCP. However, all spheroids of CaP/cells became well-assembled at day 7. This result indicates that the efficiency of incorporation of CaP granules was very high with the Oxy chip without rotation or shaking regardless of the type of CaP material used.
Fig. 1.
Light microscopic images of spheroid formation on the Oxy chips. Bar = 1 mm.
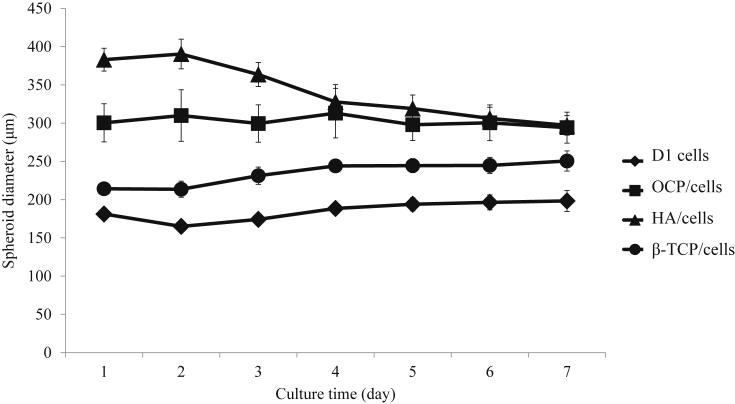
Changes in spheroid diameter of each cell aggregate on the culture chips were measured and shown in Fig. 2. The initial mean diameter of OCP/cell, HA/cell, and β-TCP/cell spheroids was 300 μm, 383 μm, and 214 μm, respectively, and larger than D1 cells alone (181 μm). However, the size of the spheroids of HA/cells at day 7 became gradually smaller and approached the diameter of the OCP/cell spheroids. In contrast, the size of OCP/cells, β-TCP/cells, and D1 cells alone remained a constant size during the culture period.
Fig. 2.
Changes in the diameter of D1 cell spheroids and CaP/cell spheroids. N = 30 spheroids at each point.
3.2. Effect of CaP granule incorporation into the spheroids on osteoblastic differentiation of D1 cells
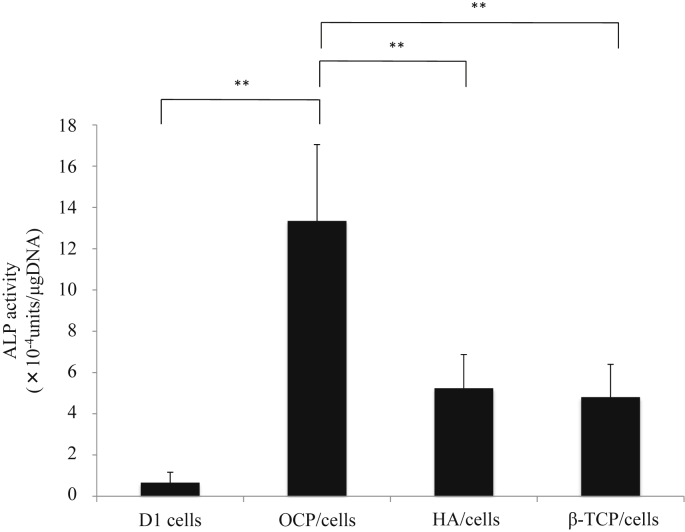
To evaluate the effect of CaP material incorporation on osteoblastic differentiation, the ALP activity of D1 cells in each spheroid was measured at day 7 (Fig. 3). Incorporation of CaP materials in the spheroids promoted ALP activity compared to spheroids consisting of only cells. In particular, the incorporation of OCP in the spheroids dramatically improved the ALP activity of D1 cells, which was approximately 20 times higher than that of D1 cells alone.
Fig. 3.
The ALP activity of D1 cell spheroids and CaP/cell spheroids after 7 days in culture.
3.3. Histological analysis of D1 cell spheroids and CaP/cell spheroids
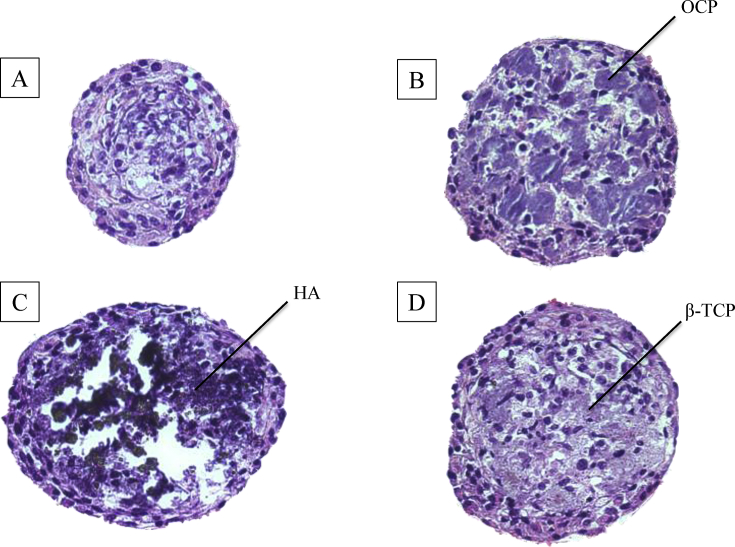
Fig. 4 shows the photographs of H-E staining of D1 cell spheroids and CaP/cell spheroids. No necrotic regions were found in the spheroid cores for all experimental conditions based on cross-sections of H-E staining. In the CaP/cell spheroids, CaP granules were incorporated in the spheroids and surrounded by cells.
Fig. 4.
H-E staining of (A) D1 cell spheroids, (B) OCP/cell spheroids, (C) HA/cell spheroids, and (D) β-TCP/cell spheroids after 7 days in culture. Mean diameter of OCP/cell, HA/cell, and β-TCP/cell spheroids in the medium was 294 μm, 297 μm, and 250 μm, respectively.
4. Discussion
The present study describes methods for preparing spheroids consisting of D1 cells and CaP materials by using a spheroid culture device, Oxy chip. Our findings suggest that these spheroids have potential utility in bone regeneration. Osteoblastic differentiation of D1 cells tended to occur in the CaP/cell spheroids, but was dramatically induced in OCP/cell spheroids. These results suggest that OCP provides an appropriate scaffold for supporting the osteoblastogenesis of MSCs.
Previous studies have reported that a 3-D culture system is useful for promoting cell differentiation because it can better mimic in vivo conditions compared to 2-D cultures. For example, Garreta et al. showed that mouse embryonic fibroblasts (MEFs) cultured in a 3-D environment show enhanced osteoblastic differentiation compared to those in a 2-D environment [8]. Other groups have shown that proliferation and osteoblastic differentiation of human MSCs is promoted when cells are cultured in a 3-D culture with a radial flow bioreactor [9]. We preliminarily confirmed that D1 cell spheroids prepared with the Oxy chip exhibited significantly increased osteoblastic differentiation compared to D1 cells cultured in a conventional (gas non-permeable) chip or in 2-D cultures. In the present study, the formation of spheroids with CaP materials tended to promote osteoblastic differentiation compared to D1 cells cultured alone, indicating that CaP adjacent to D1 cells represents an effective material for inducing osteoblastogenesis in the 3-D spheroid cultures. The light microscopic images and histochemical analysis demonstrated that the surface structure and topology of the Oxy chip provide appropriate environments for the incorporation of CaP materials into the spheroids. Moreover, histological analysis revealed that CaP granules were incorporated into the core of the spheroid and covered by cells. One of the challenges with a spheroid structure is the prevention of oxygen and nutrient exchange within the center of the spheroid due to its tightly packed and highly dense structures. It is likely that oxygenation by PDMS as well as the reduction in cell density through CaP granule incorporation within the scaffold promotes osteoblastic differentiation of MSCs in the hybrid spheroids.
Among CaP materials, it is noteworthy that OCP induced the greatest ALP activity of D1 cells in a 3-D spheroid system (2.5 times higher than HA, 2.8 times higher than β-TCP). OCP possesses unique characteristics, including the gradual conversion to HA accompanied with the incorporation of calcium ions and release of phosphate ions under physiological conditions [10], [11]. We previously showed that OCP can induce osteoblastic differentiation of a mouse stromal cell line in vitro [12], [13] and rat MSCs in vivo [14].
The physicochemical properties of CaP also influenced the formation of the spheroids. We postulate that the different behaviors of spheroid formation are due to the characteristics of CaP crystals, such as solubility, crystal surface topography, hydrophilicity, and electrical potential when CaP is incorporated in the spheroids of D1 cells. Solubility decreases in the order of OCP, β-TCP, and HA at physiologic pH 7.4 and 25 °C [11], [15], [16]. The difference in solubility among CaP materials and the process of converting OCP to HA would affect cell activity [17]. A previous study revealed that the size and microstructure of CaP dramatically altered initial cell adhesion, which affected the bone regeneration of a mouse calvarial defect [17]. Consistent with our previous studies, the physicochemical and biological characteristics of OCP affected the activity of D1 cells and enhanced osteoblastic differentiation, even in 3-D spheroids. The present technique is currently being applied to primary MSCs and in vivo bone regeneration experiments.
5. Conclusions
In the present study, we have developed a method to successfully incorporate CaPs into D1 cell aggregates using the Oxy chip. Incorporation of CaP microparticles promoted ALP activity of D1 cells. However, the factors responsible for regulating osteoblastic differentiation of MSCs in the spheroids remain unknown. Further studies are needed to elucidate the mechanism facilitating osteoblastic differentiation over a longer time period and to identify the appropriate conditions of CaP/cells for application in bone regeneration. Moreover, it is possible that this CaP/cell spheroid system may be useful as an in vitro assessment method for determining the osteogenic induction capacity of CaP materials.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgment
This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas “Bio Assembler” (23106010) and (25670829, 26282133, 26670846 and 15K15720) from the Ministry of Education, Science, Sports and Culture of Japan (MEXT).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Takahisa Anada, Email: anada@m.tohoku.ac.jp.
Osamu Suzuki, Email: suzuki-o@m.tohoku.ac.jp.
References
- 1.Anada T., Fukuda J., Sai Y., Suzuki O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials. 2012;33:8430–8441. doi: 10.1016/j.biomaterials.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi K., Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7:2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Kojima N., Takeuchi S., Sakai Y. Fabrication of microchannel networks in multicellular spheroids. Sensors Actuators B Chem. 2014;198:249–254. [Google Scholar]
- 4.Miyatake N., Kishimoto K.N., Anada T., Imaizumi H., Itoi E., Suzuki O. Effect of partial hydrolysis of octacalcium phosphate on its osteoconductive characteristics. Biomaterials. 2009;30:1005–1014. doi: 10.1016/j.biomaterials.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Anada T., Masuda T., Honda Y., Fukuda J., Arai F., Fukuda T. Three-dimensional cell culture device utilizing thin membrane deformation by decompression. Sensors Actuators B Chem. 2010;147:376–379. [Google Scholar]
- 6.Neff J.A., Caldwell K.D., Tresco P.A. A novel method for surface modification to promote cell attachment to hydrophobic substrates. J Biomed Mater Res. 1998;40:511–519. doi: 10.1002/(sici)1097-4636(19980615)40:4<511::aid-jbm1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki O., Nakamura M., Miyasaka Y., Kagayama M., Sakurai M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J Exp Med. 1991;164:37–50. doi: 10.1620/tjem.164.37. [DOI] [PubMed] [Google Scholar]
- 8.Garreta E., Genove E., Borros S., Semino C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215–2227. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura I., Hisanaga R., Sato T., Arano T., Nomoto S., Ikada Y. Effect of osteogenic differentiation medium on proliferation and differentiation of human mesenchymal stem cells in three-dimensional culture with radial flow bioreactor. Regen Ther. 2015;2:24–31. doi: 10.1016/j.reth.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki O., Kamakura S., Katagiri T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J Biomed Mater Res B Appl Biomater. 2006;77:201–212. doi: 10.1002/jbm.b.30407. [DOI] [PubMed] [Google Scholar]
- 11.Sakai S., Anada T., Tsuchiya K., Yamazaki H., Margolis H., Suzuki O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent Mater J. 2016 doi: 10.4012/dmj.2015-255. [in press] [DOI] [PubMed] [Google Scholar]
- 12.Anada T., Kumagai T., Honda Y., Masuda T., Kamijo R., Kamakura S. Dose-dependent osteogenic effect of octacalcium phosphate on mouse bone marrow stromal cells. Tissue Eng Part A. 2008;14:965–978. doi: 10.1089/ten.tea.2007.0339. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki O., Kamakura S., Katagiri T., Nakamura M., Zhao B., Honda Y. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials. 2006;27:2671–2681. doi: 10.1016/j.biomaterials.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T., Anada T., Masuda T., Honda Y., Sakai Y., Kato Y. The effect of synthetic octacalcium phosphate in a collagen scaffold on the osteogenicity of mesenchymal stem cells. Eur Cell Mater. 2011;22:124–136. doi: 10.22203/ecm.v022a10. [DOI] [PubMed] [Google Scholar]
- 15.Lc C. Next generation calcium phosphate-based biomaterials. Dent Mater J. 2009;28:1–10. doi: 10.4012/dmj.28.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown W.E., Mathew M., Tung M.S. Crystal chemistry of octacalcium phosphate. Prog Cryst Growth Charact. 1981;4:59–87. [Google Scholar]
- 17.Suzuki O. Octacalcium phosphate (OCP)-based bone substitute materials. Jpn Dent Sci Rev. 2013;49:58–71. [Google Scholar]