Abstract
The first issue of Nature Medicine published 20 years ago featured an article that reported Japan's critical situation regarding clinical trials, calling for major reform. Twenty years later, Japan has enacted three laws to promote the use of regenerative medicine as a national policy. The first law to be enacted was the Regenerative Medicine Promotion Act, which represents the country's determination to work toward the promotion of regenerative medicine. Subsequently, the Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act (PMD Act) and the Act on the Safety of Regenerative Medicine (RM Act) came into effect. The PMD Act created a new category for regenerative medicine products, and established the process for obtaining approval for cell therapy and other regenerative therapies through the implementation of clinical trials. The RM Act specified the regulations that doctors, review committees, and cell culture/processing facilities must adhere to when providing regenerative medicine in medical care, not only in clinical research but also in private practice.
Previously, researchers in regenerative medicine only had a set of guidelines to follow for conducting clinical research. Now, with the enactment of the RM Act, all areas for improvement that had been enumerated 20 years ago—such as the lack of appropriate review committees and governmental control—have been addressed by law, creating a system that gives the highest priority to patient safety. In this paper, we present the particularly noteworthy points of the RM Act, along with the actual current conditions of regenerative medicine in Japanese medical care.
Keywords: Regenerative medicine, Act on Safety for Regenerative Medicine
1. Perspective
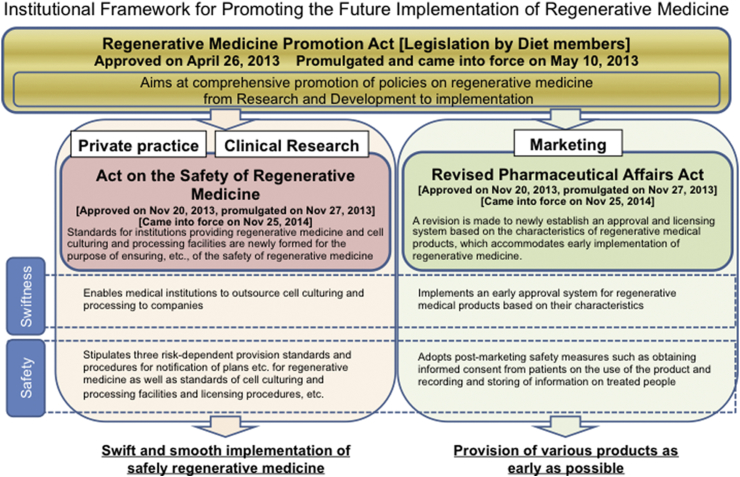
In the first issue of Nature Medicine published in 1995, Fukushima [1] emphasized inadequacy in the implementation of clinical trials in Japan. In the report, the author pointed out the lack of infrastructure for informed consent, as well as institutional review board (IRB), and governmental control, indicating the need for an overhaul of the entire field of translational medicine in Japan. In 2014, approximately 20 years after this initial report, Japan enacted the Regenerative Medicine Promotion Act, followed by two other associated Acts concerning translational regenerative medicine [2], [3] (Fig. 1). The first is the Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act (PMD Act, renamed from the Revised Pharmaceutical Affairs Act), which newly created a category for regenerative medical products in addition to the existing categories pharmaceutical products, medical device products, quasi-drugs and cosmetics. Furthermore, by adopting a system with conditional and time-limited approval for regenerative medicine products, the PMD Act established the process for obtaining approval for cell therapy and other regenerative therapies through the implementation of clinical trials [4]. The PMD Act regulates the production and marketing of regenerative and cellular therapeutic products by firms.
Fig. 1.
Japan has enacted three laws to promote the use of regenerative medicine as a national policy. The first law to be enacted was the Regenerative Medicine Promotion Act, which represents the country's determination to work toward the promotion of regenerative medicine, following which the Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act (PMD Act) and the Act on the Safety of Regenerative Medicine (RM Act) came into effect.
The second law is the Act on the Safety of Regenerative Medicine (RM Act), which established a framework for regenerative medicine provided both in clinical research (not including clinical trials complying with international guidelines, including the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use [ICH]-Good Clinical Practice [GCP]) and private practice (not covered by health insurance) and clarified the measures necessary for ensuring patient safety. Under the Medical Care Act and the Medical Practitioner's Act, the RM Act regulates regenerative medical technologies using processed cells for ensuring the safety and adequacy.
Regenerative medical technologies and Regenerative medical products are defined as processed live human/animal cells that are intended to be used for either (1) the reconstruction, repair, or formation of structures or functions of the human body, or (2) the treatment or prevention of human diseases. Regenerative medical products also include gene therapy products.
November 2015 marked the end of the one-year period of transitional measures for the RM Act, and we are now able to witness the actual conditions of the clinical research activities and therapies in regenerative medicine currently conducted in Japan under this Act. In this paper, we provide an overview of how translational regenerative medicine in Japan has changed in the past 20 years, along with the actual conditions of regenerative medicine provided under the RM Act.
Previously, researchers in regenerative medicine had only one major guideline to follow (“Guidelines on Clinical Research Using Human Stem Cells”) when conducting clinical research. Starting in November 2014, researchers have been required to comply with the RM Act in the provision of regenerative medicine. Furthermore, the RM Act applies not only to clinical research but also to private practice. The intention behind this Act is to have a clear grasp of the actual conditions of regenerative medicine used as therapies to ensure patient safety. In this paper, we present some of the regulatory requirements specified in the RM Act. Regarding informed consent, which was one of the aspects that Fukushima [1] strongly pointed out as in need of reform, the RM Act presents the requirements for not only recipients of regenerative therapies but also for the donors. Furthermore, the Act includes requirements for the protection of vulnerable populations involved in research and appropriate compensation/treatment for subjects who may be harmed as a result of participating in research, both of which were included in the revised Declaration of Helsinki in 2013 [5].
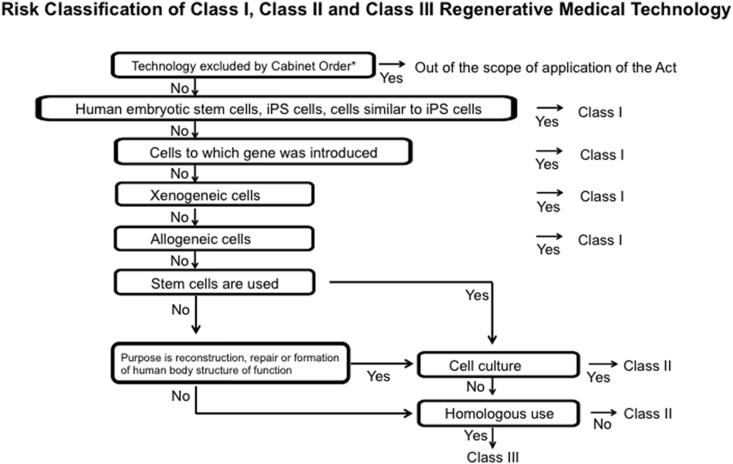
Another area for improvement identified by Fukushima involved the empowerment of IRBs. In the RM Act, regenerative medical techniques are classified into high risk (Class I), intermediate risk (Class II), and low risk (Class III) (Fig. 2). To review these classified techniques properly, the Act specifies the requirements for two types of certified committees: certified committees for regenerative medicine to review Class III techniques, and certified special committees for regenerative medicine to review Class I and II techniques. The latter committee requires higher review capabilities and objectivity.
Fig. 2.
Risk Classification of Class I, Class II, and Class III Regenerative Medical Technology. *: Medical technologies such as blood transfusion (excluding those that use gene-transferred cells), hematopoietic stem cell transplantation (excluding those that use gene-transferred cells), and assisted reproductive technology (excluding those that use embryotic stem cells established from human sperm or unfertilized eggs) are excluded by the cabinet order.
These certified committees for regenerative medicine can be formed by universities, academic societies of medical science, and general incorporated associations; moreover, there must be no conflict of interest within the committee. Certified committees for regenerative medicine are authorized to issue opinions regarding the provision of regenerative medicine in both clinical research and private practice, adverse event reports, and annual reports. Additionally, information on reviews conducted by the committees is to be shared with the public.
Another notable characteristic of the RM Act is that, when providing regenerative medicine in research or private practice, it is now possible to outsource the cell culture/processing to private contractors. The use of companies that specialize in cell cultivation can secure the safety of cell culture and ensures a steady supply of cells. This allows doctors to focus specifically on treatment and research, potentially further promoting the use of regenerative medicine.
Regarding the current implementation status of regenerative medicine research and therapies under the RM Act as of the end of November 2015, the numbers of regenerative medicine provision plans submitted are 11 for Class I (9 research studies and 2 private practice applications), 48 for Class II (24 research and 24 private practice), and 1831 for Class III (37 research and 1794 private practice). There are 26 certified special committees to review Class I and Class II regenerative medicine provision plans, and there are 88 certified committees to review Class III regenerative medicine provision plans. With respect to the operating status of cell culture/processing facilities, there are 2194 facilities established within medical institutions and 41 facilities that are outside hospitals or are run by corporations. Currently, no overseas facilities manufacture the processed cells for use in Japan (Table 1). Since the RM Act also applies to the use of platelet-rich plasma (PRP), operating rooms that perform minimal manipulations such as collection of PRP are also required to file an application to be registered as cell culture/processing facilities. These operating rooms are included in the number of cell culture/processing facilities within medical institutions.
Table 1.
Current implementation status of regenerative medicine research and therapies under the RM Acta (the end of November 2015).
| Number of the regenerative medicine provision plans | |||
|---|---|---|---|
| Type of classes | Number of provision plans (Total) | Number of provision plans (Clinical research and private practice) | Others (The target diseases or type of regenerative medical technologies) |
| Class I | 11 | Clinical research: 9 | The processed cells of regenerative medical technologies are mainly shown as follows.
|
| Private practice: 2 | Under deliberation in the Health Science Council.b | ||
| Class II | 48 | Clinical research: 24 | The processed cells of regenerative medical technologies are mainly shown as follows.
|
| Private practice: 24 | The processed cells of regenerative medical technologies are mainly shown as follows.
|
||
| Class III | 1831 | Clinical research: 37 | The processed cells of regenerative medical technologies are mainly shown as follows.
|
| Private practice: 1794 | The processed cells of regenerative medical technologies are mainly shown as follows.
|
||
The act on safety of regenerative medicine.
In class I regenerative medicine, a certain period of restricted implementation period will be imposed, and the Ministry of Health, Labour and Welfare will confirm the safety, etc., by hearing opinions of the Health Science Council within the period.
We have presented the current status of clinical research and private practice in regenerative medicine in Japan after the one-year transitional period has ended. Regarding the lack of governmental control, which Fukushima regarded as the most pressing issue in 1995, Japan has now adopted a new system to protect patients' lives, in which the government is authorized to issue requests for reports, improvement orders, and emergency interim orders to regenerative medicine institutes, certified committees for regenerative medicine, and cell culture/processing facilities. Although the RM Act was finally enacted after 20 years of anticipation among many researchers and patients, many issues still need to be resolved for the promotion of regenerative medicine. Considerations are underway on how to best evaluate the safety of cell transplantation using induced pluripotent stem cells and other cells, and the establishment of support systems to implement high-quality clinical research. There is also a plan to help citizens understand the regenerative medicine currently applied in both research and therapeutic contexts, by disclosing the implementation status to the public. Moreover, Japan has been preparing laws concerning the implementation of other translational research activities outside of regenerative medicine, thereby promoting the reform of translational medicine as a whole.
As a law that regulates clinical research and therapies in regenerative medicine, the RM Act is globally unprecedented. Although it will take some time before we can determine its effects on the promotion of regenerative medicine, at this point, we can at least say that many Japanese people support the law. Finally, we would like to express our deep appreciation to everyone involved in the efforts to enact the RM Act. We sincerely hope that many Japanese people will benefit from regenerative medicine.
Competing financial interests
The author declares no competing financial interests.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Fukushima M. Clinical trials in Japan. Nat Med. 1995;1:12–13. doi: 10.1038/nm0195-12. [DOI] [PubMed] [Google Scholar]
- 2.Konomi K., Tobita M., Kimura K., Sato D. New Japanese initiatives on stem cell therapies. Cell Stem Cell. 2015;16:350–352. doi: 10.1016/j.stem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Hara A., Sato D., Sahara Y. New governmental regulatory system for stem cell-based therapies in Japan. Ther Innov Regul Sci. 2014;48:681–688. doi: 10.1177/2168479014526877. [DOI] [PubMed] [Google Scholar]
- 4.Cyranoski D. Japan to offer fast-track approval path for stem cell therapies. Nat Med. 2013;19:510. doi: 10.1038/nm0513-510. [DOI] [PubMed] [Google Scholar]
- 5.World Medical Association World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]