Abstract
In Japan, a research center network consisting of Kyoto University to provide clinical-grade induced Pluripotent Stem Cells (iPSC) and several major research centers to develop iPSC-based regenerative therapies was formed for the clinical application of iPSCs. This network is under the supervision of a newly formed funding agency, the Japan Agency for Medical Research and Development. In parallel, regulatory authorities of Japan, including the Ministry of Health, Labour and Welfare, and Pharmaceuticals and Medical Devices Agency, are trying to accelerate the development process of regenerative medicine products (RMPs) by several initiatives: 1) introduction of a conditional and time-limited approval scheme only applicable to RMPs under the revised Pharmaceuticals and Medical Devices Act, 2) expansion of a consultation program at the early stage of development, 3) establishment of guidelines to support efficient development and review and 4) enhancement of post-market safety measures such as introduction of patient registries and setting user requirements with cooperation from relevant academic societies and experts. Ultimately, the establishment of a global network among iPSC banks that derives clinical-grade iPSCs from human leukocyte antigens homozygous donors has been proposed. In order to share clinical-grade iPSCs globally and to facilitate global development of iPSC-based RMPs, it will be necessary to promote regulatory harmonization and to establish common standards related to iPSCs and differentiated cells based on scientific evidence.
Keywords: Regenerative medicine, Policy, Regulation, iPS cells, Haplobank, Japan
Abbreviations: iPSC, induced pluripotent stem cell; ESC, embryonic stem cell; HLA, human leukocyte antigen; RMP, regenerative medicine product; R&D, research and development; AMED, Japan Agency for Medical Research and Development; MEXT, Ministry of Education, Culture, Sports, Science and Technology; MHLW, Ministry of Health, Labour and Welfare; METI, Ministry of Economy, Trade and Industry; JST, Japan Science and Technology Agency; NIBIO, National Institute of Biomedical Innovation; NEDO, New Energy and Industrial Technology Development Organization; FY, fiscal year; CiRA, Center for iPS Cell Research and Application; IRB, Institutional Review Board; RM Act, the Act on the Safety of Regenerative Medicine; Riken CDB, Riken Center for Developmental Biology; IBRI, Institution of Biomedical Research and Innovation; PMDA, Pharmaceuticals and Medical Devices Agency; NIHS, National Institute of Health Science; PAL, Pharmaceutical Affairs Law; PMD Act, Pharmaceuticals and Medical Devices Act; GMP, good manufacturing practice; GCTP, Good Gene, Cell, Cellular and Tissue-based Products Manufacturing Practice; DMF, Drug Master File; LVAD, left ventricular assist device; J-MACS, Japanese Registry for Mechanically Assisted Circulatory Support; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; U.S., United States; GAiT, Global Alliance for iPS Cell Therapies; WHO, World Health Organization; CFR, Code of Federal Regulations; FDA, Food and Drug Administration; BLA, Biological License Approval; IND, Investigational New Drug; ICH, The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; PIC/S, The Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme
Highlights
-
•
Overview of collaborative research center network program related to iPSC-based regenerative therapies in Japan.
-
•
Recent initiatives that promote R&D and review of regenerative medicine products by regulatory authorities of Japan.
-
•
Challenges related to global cooperation among iPSC banks overseas under current regulations.
1. Introduction
Induced pluripotent stem cells (iPSCs) were firstly made from mice in 2006 [1] and from humans in 2007 [2], [3]. iPSCs share many characteristics with embryonic stem cells (ESCs), including the ability to self-renew and differentiate into all cell types of the adult body. However, because iPSCs are made not from a fertilized egg but from somatic cells, their creation avoids the destruction of fertilized eggs and allows them to be acquired from donors whose genetic characteristics and other health records are already well documented. Like ESCs, iPSC-based products are used as research tools for drug toxicity testing, such as drug-induced QT prolongation [4], [5], [6]. A unique application of iPSCs is that they can be derived from diseased patients, which gives them a distinct advantage over ESCs for disease modeling and drug discovery [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21].
Regarding cell therapy applications, it is expected that iPSC-based products from healthy donors can be used for allogeneic therapies. By carefully selecting donors that have homozygous human leukocyte antigens (HLA), it will be possible to reduce immune rejection and thus also the dose of immunosuppressive agents [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. In addition, iPSCs have the potential for autologous therapies, further reducing the risk of immune rejection [36], [37].
In Japan, the discovery of iPSCs has stimulated strong government support for the development and commercialization of iPSC-based technology, including generous research funding. This support was amplified after the Nobel Prize in Physiology or Medicine was awarded in 2012. In addition, regulatory authorities have reevaluated policy in order to facilitate effective and efficient regulatory clearance for marketing approval of this technology [38], [39], [40], [41], [42], [43], [44], [45]. In this manuscript, we will review relevant policies and collaborative efforts among academia, industry and government agencies. We also discuss potential regulatory and ethical challenges related to future international cooperation for HLA homozygous iPSC banks, which are being used as sources for iPSC-based regenerative medicine products (RMPs).
2. Role of public funding to facilitate R&D on iPSC-based regenerative therapies
2.1. Establishment of Japan Agency for Medical Research and Development
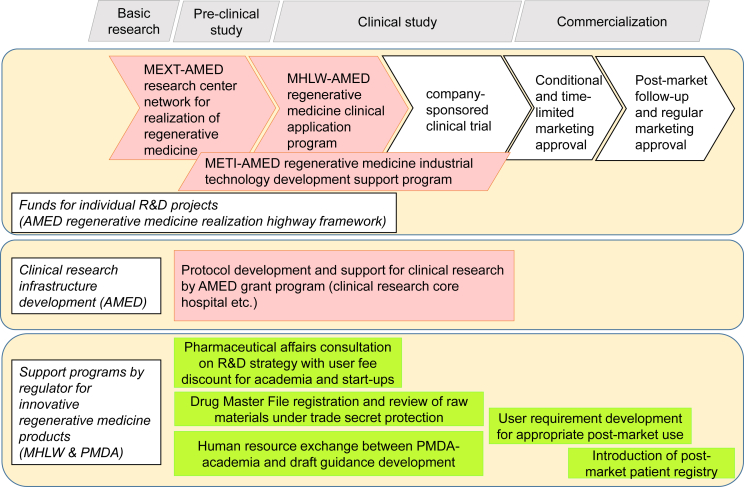
In order to promote medical research and development (R&D), including regenerative medicine, the Japan Agency for Medical Research and Development (AMED) was established as a new National Research and Development Agency on April 2015 [40], [43], [46]. AMED is organized to consolidate national medical R&D funding, which was previously operated directly by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Ministry of Health, Labour and Welfare (MHLW) and Ministry of Economy, Trade and Industry (METI) or through the Japan Science and Technology Agency (JST), National Institute of Biomedical Innovation (NIBIO) and New Energy and Industrial Technology Development Organization (NEDO) [47]. AMED also consolidates the operation of the research center network for realization of regenerative medicine (fiscal year (FY) 2015 budget: 8.99 billion yen) from MEXT, regenerative medicine clinical application program (FY2015 budget: 2.78 billion yen) from MHLW and regenerative medicine industrial technology development support program (FY2015 budget: 2.5 billion yen) from METI (Fig. 1) [48], [49].
Fig. 1.
Overview of support programs for the R&D of regenerative medicine products. AMED, Japan Agency for Medical Research and Development; MEXT, Ministry of Education, Culture, Sports, Science and Technology; MHLW, Ministry of Health, Labour and Welfare; METI, Ministry of Economy, Trade and Industry.
2.2. Collaboration for regenerative medicine related to the iPSC stock project
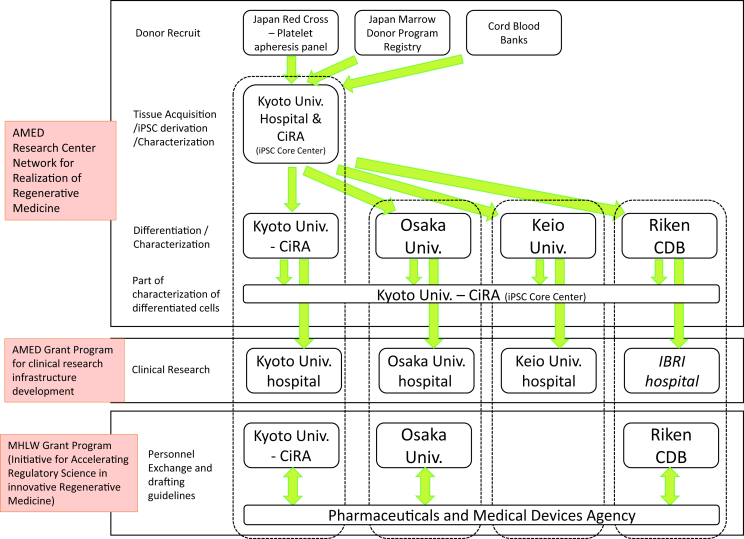
For R&D toward iPSC-based regenerative therapies, close collaboration between research centers that generate and provide (or distribute) iPSCs and those that differentiate iPSCs into final products are important. Therefore, MEXT and JST initiated a new program, “Research center network for realization of regenerative medicine” in FY 2013 (Fig. 2) [50]. Under this program, the Center for iPS Cell Research and Application (CiRA), Kyoto University, was selected as a core center for iPSC research to conduct “iPSC stock development projects for regenerative medicine”. Four research centers (Keio University, CiRA, Riken and Osaka University) were selected as “Centers for Clinical Application Research on Specific Disease/Organ (Type A Centers/Institutions)”, which aim at the clinical application of iPSC-based regenerative therapies by FY 2017, making them national leaders in iPSC-based regenerative therapy R&D. Overall, the program consists of many other iPSC-based regenerative therapy R&D projects. Table 1 shows examples of disease/organ research projects related to iPSC-based regenerative therapy being done by at least one of the four institutions under this program. Many of these projects involve collaborations using the iPSC stocks provided by CiRA.
Fig. 2.
iPSC stock related R&D collaboration involving major research centers and regulatory agencies. AMED, Japan Agency for Medical Research and Development; MHLW, Ministry of Health, Labour and Welfare.
Table 1.
iPSC-based regenerative therapy R&D projects under the AMED regenerative medicine network program.
| Kyoto University | Riken | Osaka University | Keio University |
|---|---|---|---|
| Dopamine-producing neurons | Retinal pigment epithelium cells | Corneal epithelium cells | Neural progenitor cells |
| Platelet | Photoreceptor cells | Corneal endothelium cells | Corneal endothelium cells |
| Cartilage | Natural killer T cells | Cardiomyocytes | Cardiomyocytes |
| Cardiomyocytes | Teeth | Hepatocytes | |
| Kidney | Hair | ||
| Pancreas | Secretory glands | ||
| Skeletal muscle |
Note: Other institutions participating but not shown include Osaka National Hospital (neural progenitor cells), Yokohama City University (liver), the University of Tokyo (liver and pancreas), Kumamoto University (liver), Chiba University (liver) and National Center of Neurology and Psychiatry (skeletal muscle).
Reference: summarized from the JST regenerative medicine network program website (http://www.jst.go.jp/saisei-nw/).
One of the primary roles of CiRA is to provide seed stocks of iPSCs for the development of therapeutic products [51], [52], [53]. The stock generated from HLA-homozygous donors is expected to reduce the risk of immune rejection upon the transplantation of differentiated cells to recipients having the same HLA haplotype [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Therefore, CiRA is developing a series of clinical grade iPSCs from HLA-homozygous donors in collaboration with the Japan Red Cross, the Japan Marrow Donor Program and cord blood banks.
In general, user research centers will expand and establish their own cell banks of iPSCs or of downstream stem/progenitor cells for pre-clinical and clinical studies. In the case that the research center derives iPSCs from a source other than the CiRA iPSC stock for therapeutic purposes, CiRA will provide technical assistance when necessary. For example, the two institutes will characterize and analyze the iPSC-based differentiated cells together. For the development of iPSC-based regenerative therapy, in addition to general pre-clinical toxicity testing, additional consideration of tumorigenicity related to residual undifferentiated cells, contaminant cells, cells genetically transformed to malignancy during culture, or residual vectors is essential [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]. The relationship between genomic abnormalities of iPSCs or final products and tumorigenicity is not yet elucidated. To appropriately analyze genomic abnormalities of the final products, it is necessary to compare genome information from the original donor cells, iPSCs and differentiated cells. CiRA will be responsible for the genome analysis, and user research centers will be responsible for tumorigenicity studies. The results of these works will be used to optimize quality standards for both iPSCs as raw material and differentiated cells as final products.
2.3. Expansion of clinical research infrastructure
To facilitate academia-based clinical research of iPSC-based regenerative therapies, collaboration with hospitals that have experts who can prepare clinical research protocols and manage clinical research, is important. Therefore, MHLW and MEXT started several grants for clinical research infrastructure [40]. These grants facilitate early-phase and exploratory clinical trials using iPSC-based regenerative therapies. Osaka University and Keio University Hospitals have been awarded the early/exploratory clinical trial center grant since 2011 [64], and Kyoto University Hospital the clinical research core center grant since 2012 [65]. MHLW certified Institutional Review Boards (IRBs) at these three institutions in April 2015 based on a new certification program [66]. Osaka, Keio and Kyoto Universities also established specially certified regenerative medicine committees to review clinical research protocols for iPSC-based therapies under the new Act on the Safety of Regenerative Medicine (RM Act) enacted in November 2014 [39], [43], [67], [68]. Although the Center for Developmental Biology (CDB), Riken, does not have its own hospital, it has already begun iPSC-based retinal pigment epithelium (RPE) clinical research by coordinating with Institution of Biomedical Research and Innovation (IBRI) Hospital, which has been awarded the Japan initiated global trial center grant since 2012 [69]. These grants were transferred from MHLW to AMED as part of the “AMED innovative medical technology generation center program” [48], [49].
2.4. Collaboration between PMDA and research institutions to develop guidelines for iPSC-based regenerative medicine products
A unique scheme that encourages close interactions between regulatory authorities and research institutions was introduced recently in Japan. For innovative products under development by academia, such as iPSC-based RMPs, regulatory requirements have sometimes been unclear. However, effective regulatory guidelines cannot be made by the regulatory authorities without input from active scientists. Therefore, MHLW initiated a new grant program, “Initiative for Accelerating Regulatory Science in Innovative Drugs, Medical Devices and Regenerative Medicine”, in 2012 [70], [71]. R&D programs related to iPSC-based RMPs at CiRA, Osaka University and Riken CDB have been awarded these grants. In this program, reviewers at the Pharmaceuticals and Medical Devices Agency (PMDA) or scientific experts at National Institute of Health Science (NIHS) visit research institutions periodically to give scientific advise and remain updated with on-going development of innovative technologies. In exchange, researchers visit PMDA as part-time or full-time employees and provide technical advice to support PMDA's review and consultation. The intent of these bi-directional exchanges is to have all parties better understand their partners' motivations and goals. An expected outcome of this five-year program (FY2012–FY2016) is to have researchers and PMDA's or NIHS's visiting scientists together develop a draft of guidelines. After appropriate public consultation, MHLW will finalize and publish these documents as official guidelines that clarify regulatory requirements to facilitate the commercialization of innovative iPSC derived RMPs.
In addition to this scheme, there are other mechanisms for developing such guidelines. One is MHLW's regulatory science research grants, which have led to general guidelines for iPSC-based RMPs that were published in 2012 [72], [73], [74], [75]. Another is a specific program that began in 2006 and drafts evaluation guidance for innovative products. Under this program, MHLW, NIHS, and PMDA work together with academic experts and have published guidelines for iPSC-based RPE cells in 2013 (autologous) and 2014 (allogeneic) [76], [77]. Currently, new guidelines for iPSC-based cartilage products are under preparation [78].
3. Revised regulatory policy to promote R&D of RMPs
Along with the actions described above, there are several regulator-led initiatives that are designed to promote the R&D of RMPs (Fig. 1). In this section, we review current regulatory policy related to RMPs relevant to future iPSC-based RMPs.
3.1. Introduction of conditional, time-limited approval for RMPs
The Pharmaceutical Affairs Law (PAL) was revised and renamed to the Pharmaceuticals and Medical Devices (PMD) Act, which became effective in November 2014 [38], [39], [40], [41], [42], [43], [44], [45], [79], [80]. In this revision, a new product category, RMPs, which is separate from drugs and medical devices, was introduced, and its specific regulation was established. Because RMPs are extremely difficult to evaluate for effectiveness due to the heterogeneity of cells, a new approval system in which MHLW can give conditional and time-limited approval after confirmation of probable benefit and safety was introduced. Upon this probationary approval, approval holders must apply for regular approval within a specified period (normally maximum 7 years) or the approval will expire. In addition, Good Manufacturing Practice (GMP) that considers the unique characteristics of RMPs was introduced as Good Gene, Cell, Cellular and Tissue-based products Manufacturing Practice (GCTP) [81].
Under the new PMD Act, two products were approved as new RMPs on September 18 2015. One product, Temcell® HS Inj. by JCR Pharmaceuticals Co., Ltd for Acute Graft-versus-Host Disease following hematopoietic stem cell transplant, was awarded regular approval [82], [83]. Another product, HeartSheet® Autologous Skeletal Myoblast Sheets by Terumo Corporation for severe heart failure caused by chronic ischemic heart disease, was awarded conditional and time-limited approval for 5 years [84], [85].
3.2. Introduction of new consultation program for early stage development and simplification of clinical trial plan review
Under the PMD Act, article 80-2, intensive review by PMDA and MHLW is required for the first clinical trial protocol of any new drug, medical device or RMP within 30 days of application submission. However, this time period is demanding when considering the quality and safety of RMPs. Therefore, pre-clinical review by the Pharmaceutical and Medical Device Evaluation Center of NIHS (consolidated to the PMDA in 2004) and the MHLW Pharmaceutical and Food Sanitation Council before submission of the first clinical trial protocol had been required since 1999 [86], [87], [88].
Prior to the recent revision of the PMD Act, MHLW held “the committee of regenerative medicine regulatory framework”, which consisted of regenerative medicine experts, from 2009 to 2011. This committee discussed improvements to the PMD Act. Accordingly, in July 2011, MHLW and PMDA introduced an early stage consultation program that mainly targets academia and start-up companies and replace the official additional pre-clinical review of quality and safety of RMPs [89], [90]. In this new consultation program, named pharmaceutical affairs strategy consultation, PMDA gives advice related to R&D strategy [91]. The review of quality and safety data under the pharmaceutical affairs strategy consultation is required before submission of the first clinical trial protocol for any RMP. Pharmaceutical affairs strategy consultation usually requires fees for each official meeting, but for the mandatory consultation related to quality and safety, a one-time consultation fee can be paid. In addition, by subsidy from MHLW, discounts of up to 90% can be procured for academia and start-up companies. This consultation has considerably simplified pre-clinical reviews and reduced uncertainty of what quality and safety data are required in the pre-clinical phases because of constant communication between the sponsors and PMDA. CiRA has used this pharmaceutical affairs strategy consultation to confirm the quality and safety of its iPSC stocks and other iPSC-based RMPs [92].
More recently, MHLW announced the introduction of fast-track consultation and review program designed specifically for drugs, medical devices and RMPs that act against diseases in urgent need of innovative therapy and are initially developed in Japan or will include Japan in a multi-national clinical trial [93], [94].
3.3. Expansion of Drug Master File registration systems for raw materials of RMPs
A Drug Master File (DMF) registration system for the raw materials of drugs or medical devices, such as active pharmaceutical ingredients, was introduced in 2005 [95]. This system allows PMDA to review information directly submitted by raw material manufacturers who are not applying for clinical trials or seeking regulatory approval, thus allowing these manufacturers to protect trade secrets. In December 2012, subjects of DMF registration were expanded to include raw materials related to RMPs, such as cells (iPSCs and ESCs), media, medium additives (sera, growth factors and cytokines) and other materials [96], [97]. Raw material manufacturers can directly register manufacturing and quality control information, specifications related to human or animal derived materials, information related to the donors of the cells, specifications of the cells and non-clinical test data [98], [99]. These data can be reviewed directly by PMDA not only during the approval review, but also before clinical trials, including during the pharmaceutical affairs strategy consultation, to confirm the quality and safety of the raw materials and final products.
3.4. Introduction of post-market patient registry for RMPs
Unlike most drugs, which disappear within a few days by metabolism or elimination after the end of administration, many RMPs stay in the patient body long after transplantation. Therefore, long-term patient follow-up is essential for reliable evaluation. From this perspective, RMPs share similar characteristics with implantable medical devices, such as left ventricular assist devices or hip replacement implants, which are often included in a patient registry for recognition of post-market safety issues [100], [101]. PMDA has experience in developing and operating J-MACS (Japanese registry for Mechanically Assisted Circulatory Support), which is a patient registry for mechanical circulatory support implantation devices and built on a similar data structure as North America's INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) [102], [103], [104], [105], [106]. Leveraging this experience and in response to the introduction of the new conditional, time-limited approval system, a new patient cohort registry that records post-market safety and efficacy data of RMPs is currently under development by MHLW and PMDA [41], [44].
A MHLW-sponsored committee to discuss the design of a patient registry system for RMPs was formed in January 2013. Based on their conclusions, PMDA commenced the development of a registry, aiming for launch by the end of FY2015 [107], [108]. It is expected that this new patient cohort registry of RMPs will generate useful post-market safety and efficacy data, which can be used for regular approval review after conditional and time-limited approval.
3.5. User requirements for appropriate use of RMPs
Another safety measure to consider for conditional, time-limited approval is the requirements of users, such as medical institutions and medical doctors, for RMPs that require novel or complex surgical techniques. In 2011, in response to increasing complexity, MHLW introduced a new program to facilitate the development of post-market user requirements for facilities and physicians [109]. In this program, MHLW, PMDA and related academic societies worked together to set post-market user requirements during the approval review of innovative medical devices and RMPs. These requirements have been adopted as conditions for National Health Insurance reimbursement coverage. These standards are already being applied to an autologous cultured cartilage product, JACC®, that is manufactured by Japan Tissue Engineering Co., Ltd. and was approved in July 2012 [110], [111], [112], [113], [114]. The Japanese Orthopaedic Association proposed the user requirements of JACC®, including the necessary facilities, human resources, experience, training, expertise, etc. [115]. These standards are also used to determine the conditions for reimbursement [116], [117]. Currently, user requirements for autologous skeletal muscle derived cell sheets for heart failure are under preparation [118]. It is expected that future iPSC-based RMPs will also fall under this scheme and appropriate human and facility standards in order to secure the product efficacy and safety set by MHLW, PMDA and relevant professional societies.
3.6. Alternative scheme to provide regenerative therapies under early stage, small-scale human clinical research
Apart from clinical trials under the PMD Act, which aims for the commercialization of RMPs, there is an alternative scheme to use iPSC-based regenerative therapies under physician discretion. This scheme is recognized as “clinical research” and is not intended for commercialization [40], [43], [86], [87]. It is unique to Japan, but shares some common characteristics with the “Hospital Exemption” scheme in EU member states [119].
Until recently, clinical research using human stem cells was regulated under the independent, non-legally binding guideline, “Guidelines for Clinical Research Using Human Stem Cells” [120]. The RM Act [41], [43], [68], enacted since November 2014, replaced this guideline. The RM Act stipulates stricter standards to provide regenerative medicine for medical institutions, and manufacturing and quality control standards for cell processing centers. However, the RM Act also removed a ban on the outsourcing of cell processing to companies outside medical institutions. Previously, such outsourcing was considered an infringement of the PMD Act, which prohibits the provision of unapproved products except for those used in clinical trials.
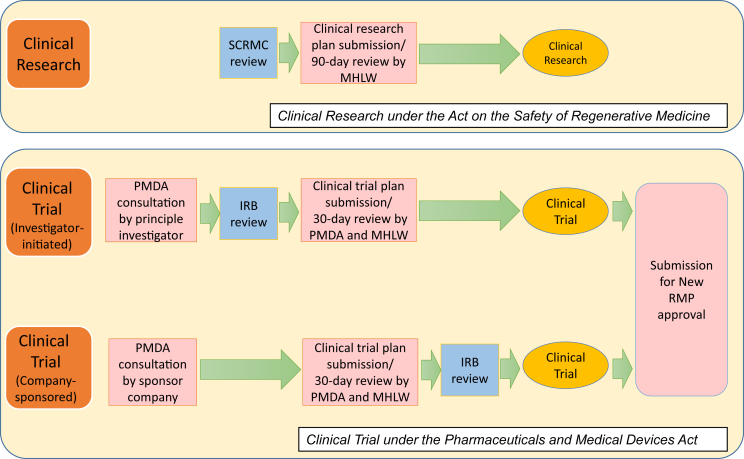
There are several differences in procedures and requirements between clinical research under the RM Act and clinical trial under the PMD Act. Fig. 3 summarizes the differences. As described in the Section 3.2, PMDA consultations are required before the initiation of clinical trial under the PMD Act. This process is not trivial, but provides clarity for regulatory clearance when submitting a clinical trial plan and new RMP approval application later. On the other hand, there is no official consultation mechanism before submitting a clinical research plan to the MHLW under the RM Act. Therefore, the review period of the clinical research plan is longer in the RM Act (90 days) than is the clinical trial plan in the PMD Act (30 days). Furthermore, additional data might be requested after submitting a clinical research plan. The technical requirement of IRBs (named “specially certified regenerative medicine committees” under the RM Act for high risk regenerative therapies such as iPSC-based therapies) is also stricter in the RM Act than in the PMD Act.
Fig. 3.
Procedures to initiate Clinical Research under the Act on the Safety or Regenerative Medicine (RM Act) and Clinical Trial under the Pharmaceuticals and Medical Devices Act (PMD Act) for iPSC-based regenerative therapies. SCRMC, Specially Certified Regenerative Medicine Committee; IRB, Institutional Review Board.
Clinical research under the RM Act is not subject to Good Clinical Practice (GCP), which means clinical research data are not accepted in the new RMP approval application under the PMD Act. However, in Japan, without first-in-human clinical research experience, companies tend to hesitate to invest money and effort on clinical trials. Therefore, it is generally considered that clinical research is more suitable for small-scale clinical study, such as first-in-human study by academia using limited government research funding, since the costs of clinical research is cheaper than that of clinical trial. Upon confirmation of feasibility, the technology is transferred to a company. For example, regarding recently approved “HeartSheet®”, after initial clinical research conducted by a university, a company conducted additional clinical trials for regulatory submission according to the PMD Act [84], [85].
However, it is also argued that considering the commercialization of regenerative medicine products in the future, it might be better to switch from early stage clinical research to clinical trial as soon as possible or even begin at clinical trial [87]. Since regulatory requirements for iPSC-based regenerative therapy under the RM Act are strict, whether the clinical research scheme will be used the same way as previous guidelines is unclear.
There is another argument that because the RM Act regulates “clinical practice” outside of “clinical research”, it might be possible to proceed from clinical research to clinical practices under the RM Act without commercialization as RMPs. However, this alternative route may not be suitable for iPSC-based regenerative therapies, because (1) it is unclear when the iPSC-based regenerative therapies are considered not “clinical research” but rather “clinical practice”, (2) the RM Act requires a burdensome procedure such as IRB and MHLW review even for “clinical practice”, and (3) National Health Insurance coverage scheme for “clinical practice” under the RM Act is unclear.
4. Challenges for international collaboration using clinical grade iPSCs
In addition to the iPSC stock project at CiRA [51], [52], several other projects, such as those at Cellular Dynamics International Inc. in the United States (U.S.) and Cell Therapy Catapult in the United Kingdom, are generating iPSCs prepared from HLA homozygous donors [121], [122], [123]. Other countries are in planning phases for the same [33]. Assuming these nations meet minimal standards, it is expected that they can provide iPSCs of a specific HLA haplotype to other countries where the same HLA haplotype is relatively rare in donors [28], [29], [30], [31], [32], [33], [34]. Thus, a global cooperation network among these iPSC banks (haplobanks), or the Global Alliance of iPSC Therapies (GAiT), has been proposed.
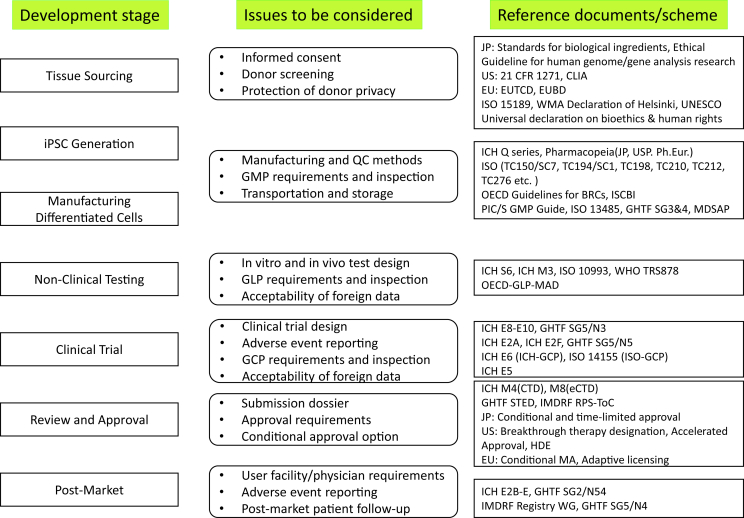
In relation to the commercialization of iPSC therapies, each country or region has its own regulations and ethical standards. Although some international guidelines or voluntary standards for other products can be applied (Fig. 4), because RMPs such as iPSCs are relatively new, most R&D processes are not in global agreement. Therefore, to share clinical-grade iPSCs internationally, there are several potential regulatory challenges that must be overcome. Discussion about these regulatory challenges has been initiated by several international expert groups such as International Stem Cell Banking Initiative (ISCBI), International Alliance for Biological Standardization (IABS) and GAiT [33], [124], [125], [126].
Fig. 4.
Overview of development stage, issues and major reference documents/schemes related to iPSC-based regenerative medicine products. CLIA, Clinical Laboratory Improvement Amendments; EUTCD, European Union Tissues and Cells Directives (2004/23); EUBD, European Union Blood Directive (2002/98); WMA, World Medical Association; UNESCO, United Nations Educational, Scientific and Cultural Organization; ICH, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; JP, Japanese Pharmacopoeia; USP, United States Pharmacopeia; Ph.Eur, European Pharmacopoeia; ISO, International Organisation for Standardization; TC, Technical Committee; SC, Subcommittee OECD, Organisation for Economic Co-operation and Development; GL, Guideline; BRC, Biological Resource Centres; ISCBI, International Stem Cell Banking Initiative; PIC/S, Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme; GMP, Good Manufacturing Practices; GHTF, Global Harmonization Task Force; SG, Study Group; MDSAP, Medical Device Single Audit Program; WHO, World Health Organization; TRS, Technical Report Series; GLP, Good Laboratory Practice; MAD, Mutual Acceptance of Data; GCP, Good Clinical Practice; STED, Summary Technical Documentation; IMDRF, International Medical Device Regulators Forum; RPS-ToC, Regulated Product Submission- Table of Contents; HDE, Humanitarian Device Exemption; MA, Marketing Authorization.
4.1. Cell sourcing and donor eligibility before iPSC derivation
4.1.1. Informed consent and donor protection
When cells or tissues are sourced from HLA homozygous donors, it is necessary to meet regulation and ethical standards, such as informed consent, protection of donor privacy, and (non-)remuneration [127], [128], [129], [130], [131], [132]. As recommended for blood donations by the World Health Organization (WHO) [133], voluntary and non-remunerated donations of source cells for RMPs are required in several countries including Japan [130]. As a result, iPSCs derived from remunerated donors may not be suitable for international exchange when used as RMPs.
Considering the protection of privacy, how to access potential donors from cooperating blood centers, cord blood banks or bone marrow registries are under discussion [29], [30], [32], [126], [127], [129]. Donor privacy related to genome analysis, including issues around incidental findings, are regulated under diverse ethical standards among countries and also under wider discussion within medical and ethical communities [9], [30], [33], [127], [134], [135], [136], [137], [138], [139], [140], [141], [142].
4.1.2. Donor screening and testing for infectious agents
The diversity of donor screening regulations might hinder the acceptance of iPSCs from foreign haplobanks [127], [130], [131], [143], [144], [145], [146]. For example, the Code of Federal Regulations (CFR) of the U.S. Food and Drug Administration (FDA) requires the use of “appropriate FDA-licensed, approved, or cleared donor screening tests” (21 CFR1271.80(c)) and also requires testing be “performed by a laboratory that either is certified to perform such testing on human specimens under the Clinical Laboratory Improvement Amendments of 1988 (42 U.S.C. 263a) and 42 CFR part 493 or has met equivalent requirements, as determined by the Centers for Medicare and Medicaid Services” (21 CFR1271.80(c)) [144]. The exchange of iPSCs from foreign haplobanks might be hampered by difficulties in conforming specific requirements across borders, such as specifically approved kits and certified laboratory. A flexible policy regarding donor screening results under appropriate foreign regulations is desirable.
The acceptance of iPSCs derived from previously collected cord blood in foreign countries is also not clear, as cord blood regulations are not internationally harmonized. For example, in the U.S., cord blood is subject to biological license approval (BLA) or investigational new drug (IND) requirements under the Federal Food, Drug, and Cosmetic Act [123], [147], but in Japan cord blood is subject to different regulations under the Act for Appropriate Provision of Hematopoietic Stem Cells to be Used in Transplantations [131], [148], [149]. Like above, flexible policy for iPSCs derived from cord blood is desirable.
4.2. Manufacturing and quality control of iPSC and differentiated cells
4.2.1. Manufacturing methods and raw material information
There are a variety of methods for generating clinical grade iPSCs using non-integrated vectors such as episomal plasmid, Sendai virus and mRNA [150], [151], [152], [153], [154], [155], [156], [157], [158], [159]. It is expected that several manufacturing methods will be acceptable from a regulatory perspective [33]. The use of xeno-free, feeder-free raw materials for manufacturing clinical grade RMPs is generally desirable and rapidly increasing [154]. It should be carefully checked, however, whether animal-derived materials are used as or in the manufacturing process of raw materials and whether the safety of these materials, such as traceability and virus clearance, if applicable, meet standards. In order to use RMPs based on iPSCs provided from foreign haplobanks for clinical trials, it is also important to gain the cooperation of the raw material supplier who provides necessary information to the manufacturer of the RMPs or to the foreign regulatory authorities through DMF registration, as described in the Section 3.3, or similar mechanisms.
4.2.2. Quality standards
Quality standards for iPSCs of research-grade and clinical-grade have been already discussed in several academic societies or consortiums, such as the International Stem Cell Banking Initiative [33], [124], [155], [157], [160], [161]. Other existing guidelines for biotechnology products, such as those of the International Conference on Harmonisation1 of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) might be applicable for the generation of a master cell bank [162], [163]. Clear regulatory guidelines for the evaluation of iPSCs and RMPs may be helpful to promote efficient R&D. However, to further refine the standards necessary for iPSCs, it might be necessary to gather more data of both iPSCs and iPSC-based RMPs under a collaborative mechanism between haplobanks and user research centers developing RMPs, as exemplified in the Section 2.2. For the time being, it is expected that so long final products are safe and effective, they will receive approval by regulatory authorities that consider appropriate specification of iPSCs as a raw material and final product, even without international consensus on standards or regulatory guidelines.
Because different clones of iPSCs from the same donor may have different propensities of differentiation [164], it is probably best to defer to user research centers for the selection of clones until better predictive methods for differentiation propensity are established [165]. In addition, because iPSC-based RMPs vary in characteristics and number of cells, quality standards for one product may not be universal. For example, there is less need to concern platelets derived from iPSCs [166] with tumorigenicity resulting from genomic abnormalities compared with other RMPs, because of the absence of a genome in platelets. Therefore, universal standards risk inefficiencies. Another issue relates to the donor background. Depending on the disease targeted by the RMP, additional requirements of the donor's health and genetic background or specific virus screening might be necessary [167]. For example, allogeneic clinical trials of retinal pigment epithelium cells need screenings for retinal diseases [77], [168]. Considering the above issues, it will be desirable to have regulator-led international forums, such as ICH, include academic experts in order to develop flexible but sufficient criteria for multi-purpose iPSC haplobanks that leverage empirical evidence generated from scientific studies.
4.2.3. GMP standard and inspection
GMP for RMPs or GCTP in many countries is generally consistent with the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (jointly referred to as PIC/S) GMP Guide, which was drafted in consultation with pharmaceutical inspection authorities from 46 countries [169], [170], [171]. However, it is not yet clear to what extent GMP controls are required for the generation of iPSCs, which are at a relatively early stage of the manufacturing process and well before the stage of a master cell bank for specific RMPs [32], [143], [172]. In addition global cooperation for haplobanks may require each haplobank receives inspection from several national regulatory authorities. A higher level of regulatory cooperation in order to mutually rely on the GMP inspection reports from other regulatory authorities is desirable [173].
4.2.4. Addition of iPSCs to existing RMPs
Another unresolved issue unique to iPSC-based RMPs, especially regarding haplobanks, is the extent of additional tests for the inclusion of new iPSC lines [33], [143]. The same product from different cell lines may not have universal properties. Therefore, a number of causes for the variability must be considered, including
-
(1)
changes from clones of the same donor,
-
(2)
different donors from the same manufacturing site and manufacturing method,
-
(3)
changes in manufacturing methods (including changes in media or reagents) from the same manufacturing site,
-
(4)
different manufacturing sites from the same manufacturing method, and
-
(5)
different manufacturing methods that meet the same specifications and criteria.
Changes in cell lines and master cell banks are allowed in previously approved allogeneic RMPs, such as Apligraf®, or Gintuit™, both from Organogenesis Inc, in the U.S. based on extensive characterization and through premarket approval supplement for manufacture process change [172], [174]. A similar approach for new iPSC lines would allow approval holders of RMPs derived from HLA-homozygous iPSCs to add new HLA-type iPSC lines to existing RMPs. However, there is neither agreement for required comparability data nor scientific evidence to justify that changes in the cell bank do not affect product safety or efficacy. If the new iPSC line requires excessive testing, cost may prohibit the inclusion of this line for RMP development, potentially resulting in the RMP only serving patients with frequent HLA phenotypes, thus creating an ethical conflict. Regulatory judgment that considers maximization of public health in accordance with the concept of regulatory science should be applied [175].
5. Conclusion
In this article, we reviewed recent developments of collaborative R&D schemes between clinical grade iPSC manufacturers and user research centers, as well as government policies to facilitate the establishment of R&D and regulatory infrastructures in Japan. In addition, we reviewed potential challenges for further collaboration with research center overseas. We expect some of these challenges will be resolved with growing scientific knowledge gained from pre-clinical studies and the clinical development of RMPs. The resolution of other issues for the wide deployment of iPSC therapies should come from further collaboration between regulators and researchers.
Conflict of interest
Shinya Yamanaka is a scientific advisor of iPS Academia Japan without salary.
Acknowledgment
We express our sincere gratitude to all our coworkers and collaborators. We thank Peter Karagiannis for critical reading of the manuscript; Shinji Asonuma, Jun Takahashi, Koji Eto, Noriyuki Tsumaki, Megumu Saito, Shin Kaneko, Masato Nakagawa, Keisuke Okita, Kazutoshi Takahashi, Shin Shimizu, Naoko Takasu, Iori Kuranaga, Masafumi Umekage, Hiromi Dohi and Ayumi Matsunaga for scientific discussion; Fumitaka Kokubo, Hisae Takenakajima, Kana Matsumoto and Yoko Miyake for coordination and administrative assistance. We apologize to the researchers whose contributions to iPSC-based regenerative therapies could not be cited because of space limitations.
This work was supported in part by the Core Center for iPS Cell Research grant from Japan Agency for Medical Research and Development (AMED), Research Center Network for Realization of Regenerative Medicine.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Renamed to the International Council for Harmonisation on October 2015 (http://www.ich.org/about/organisational-changes.html).
References
- 1.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Chi K.R. Revolution drawing in cardiotoxicity testing. Nat Rev Drug Discov. 2013;12:565–567. doi: 10.1038/nrd4083. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y., Matsuo J., Miyamoto N., Ojima A., Ando K., Kanda Y. Assessment of testing methods for drug-induced repolarization delay and arrhythmias in an iPS cell-derived cardiomyocyte sheet: multi-site validation study. J Pharmacol Sci. 2014;124(4):494–501. doi: 10.1254/jphs.13248fp. [DOI] [PubMed] [Google Scholar]
- 6.Asakura K., Hayashi S., Ojima A., Taniguchi T., Miyamoto N., Nakamori C. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. J Pharmacol Toxicol Methods. 2015;75:17–26. doi: 10.1016/j.vascn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 7.McKernan R., Watt F.M. What is the point of large - scale collections of human induced pluripotent stem cells? Nat Biotechnol. 2013;31(10):875–877. doi: 10.1038/nbt.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares F.A., Sheldon M., Rao M., Mummery C., Vallier L. International coordination of large-scale human induced pluripotent stem cell initiatives: Wellcome Trust and ISSCR workshops white paper. Stem Cell Rep. 2014;3(6):931–939. doi: 10.1016/j.stemcr.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtz A., Stacey G., Kidane L., Seriola A., Stachelscheid H., Veiga A. Regulatory insight into the European human pluripotent stem cell registry. Stem Cells Dev. 2014;23(S1):51–55. doi: 10.1089/scd.2014.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engle S.J., Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell. 2013;12(6):669–677. doi: 10.1016/j.stem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Kolaja K. Stem cells and stem cell-derived tissues and their use in safety assessment. J Biol Chem. 2014;289(8):4555. doi: 10.1074/jbc.R113.481028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engle S.J., Vincent F. Small molecule screening in human induced pluripotent stem cell-derived terminal cell types. J Biol Chem. 2014;289(8):4562–4570. doi: 10.1074/jbc.R113.529156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue H., Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther. 2011;89(5):655–661. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H., Nagata N., Kurokawa H., Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33(5):409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida M., Kitaoka S., Egawa N., Yamane M., Ikeda R., Tsukita K. Modeling the early phenotype at the neuromuscular junction of spinal muscular atrophy using patient-derived iPSCs. Stem Cell Rep. 2015;4(4):561–568. doi: 10.1016/j.stemcr.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N.M., Niwa A., Yabe M., Hira A., Okada C., Amano N. Pluripotent cell models of fanconi anemia identify the early pathological defect in human hemoangiogenic progenitors. Stem Cells Transl Med. 2015;4:333–338. doi: 10.5966/sctm.2013-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto Y., Ikeya M., Hino K., Horigome K., Fukuta M., Watanabe M. New protocol to optimize iPS cells for genome analysis of fibrodysplasia ossificans progressiva. Stem Cells. 2015;33(6):1730–1742. doi: 10.1002/stem.1981. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama K., Ikeya M., Umeda K., Oda H., Nodomi S., Nasu A. Enhanced chondrogenesis of induced pluripotent stem cells from patients with neonatal-onset multisystem inflammatory disease occurs via the caspase 1-independent cAMP/protein kinase A/CREB pathway. Arthritis Rheumatol. 2015;67(1):302–314. doi: 10.1002/art.38912. [DOI] [PubMed] [Google Scholar]
- 19.Shoji E., Sakurai H., Nishino T., Nakahata T., Heike T., Awaya T. Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci Rep. 2015;5:12831. doi: 10.1038/srep12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita A., Morioka M., Kishi H., Kimura T., Yahara Y., Okada M. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014;513(7519):507–511. doi: 10.1038/nature13775. [DOI] [PubMed] [Google Scholar]
- 21.Karagiannis P., Tsumaki N. Cell reprogramming for skeletal dysplasia drug repositioning. Cell Cycle. 2014;13(24):3791–3792. doi: 10.4161/15384101.2014.989944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor C.J., Bolton E.M., Pocock S., Sharples L.D., Pedersen R.A., Bradley J.A. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366(9502):2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima F., Tokunaga K., Nakatsuji N. Human leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapy. Stem Cells. 2007;25(4):983–985. doi: 10.1634/stemcells.2006-0566. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuji N., Nakajima F., Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol. 2008;26(7):739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- 25.Lin G., Xie Y., Ouyang Q., Qian X., Xie P., Zhou X. HLA-matching potential of an established human embryonic stem cell bank in China. Cell Stem Cell. 2009;5(5):461–465. doi: 10.1016/j.stem.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Taylor C.J., Bolton E.M., Bradley J.A. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366(1575):2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 28.Gourraud P.A., Gilson L., Girard M., Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30(2):180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- 29.Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11(2):147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Turner M., Leslie S., Martin N.G., Peschanski M., Rao M., Taylor C.J. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13(4):382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Solomon S., Pitossi F., Rao M.S. Banking on iPSC – is it doable and is it worthwhile. Stem Cell Rev Rep. 2015;11(1):1–10. doi: 10.1007/s12015-014-9574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter M.K., Rao M.S. Concise review: making and using clinically compliant pluripotent stem cell lines. Stem Cells Transl Med. 2015;4(4):381–388. doi: 10.5966/sctm.2014-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barry J., Hyllner J., Stacey G., Taylor C.J., Turner M. Setting up a haplobank: issues and solutions. Curr Stem Cell Rep. 2015;1(2):110–117. doi: 10.1007/s40778-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilmut I., Leslie S., Martin N.G., Peschanski M., Rao M., Trounson A. Development of a global network of induced pluripotent stem cell haplobanks. Regen Med. 2015;10(3):235–238. doi: 10.2217/rme.15.1. [DOI] [PubMed] [Google Scholar]
- 35.Pappas D.J., Gourraud P.A., Le Gall C., Laurent J., Trounson A., DeWitt N. Proceedings: human leukocyte antigen haplo-homozygous induced pluripotent stem cell haplobank modeled after the California population: evaluating matching in a multiethnic and admixed population. Stem Cells Transl Med. 2015;4(5):413–418. doi: 10.5966/sctm.2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Rep. 2013;1(4):283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamao H., Mandai M., Okamoto S., Sakai N., Suga A., Sugita S. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2(2):205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cyranoski D. Japan to offer fast-track approval path for stem cell therapies. Nat Med. 2013;19(5):510. doi: 10.1038/nm0513-510. [DOI] [PubMed] [Google Scholar]
- 39.Hara A., Sato D., Sahara Y. New governmental regulatory system for stem cell-based therapies in Japan. Ther Innov Regul Sci. 2014;48(6):681–688. doi: 10.1177/2168479014526877. [DOI] [PubMed] [Google Scholar]
- 40.Okada K. A new national framework for clinical trials and evaluation of innovative medical care technologies using living cell transplantation in Japan. J Transplant Technol Res. 2014;4(2):137. [Google Scholar]
- 41.Konomi K., Tobita M., Kimura K., Sato D. New Japanese initiatives on stem cell therapies. Cell Stem Cell. 2015;16(4):350–352. doi: 10.1016/j.stem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Sipp D. Conditional approval: Japan lowers the bar for regenerative medicine products. Cell Stem Cell. 2015;16(4):353–356. doi: 10.1016/j.stem.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Azuma K. Regulatory landscape of regenerative medicine in Japan. Curr Stem Cell Rep. 2015;1(2):118–128. [Google Scholar]
- 44.Okada K., Koike K., Sawa Y. Consideration of and expectations for the pharmaceuticals, medical devices and other therapeutic products act in Japan. Regen Ther. 2015;1:80–83. doi: 10.1016/j.reth.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kusakabe T. Regulatory perspectives of Japan. Biologicals. 2015;43:422–424. doi: 10.1016/j.biologicals.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Government of Japan . Agency for Medical Research and Development; 2014. The act on the National Research and Development Agency of Japan. Act No. 49. [in Japanese] [Google Scholar]
- 47.Office of Healthcare Policy, Cabinet Secretariat. The healthcare policy and the new system of medical R&D. http://www.kantei.go.jp/jp/singi/kenkouiryou/en/pdf/doc1.pdf [accessed 30.10.15].
- 48.Government of Japan . Committee for Promotion of Healthcare and Medical Strategy; Jan 21 2015. Summary of medical research and development budget for FY2015.http://www.kantei.go.jp/jp/singi/kenkouiryou/suisinkaigi/dai9/siryou2.pdf [in Japanese, 30.10.15] [Google Scholar]
- 49.The Japan Agency for Medical Research and Development (AMED). http://www.amed.go.jp/en/program/ [accessed 30.10.15].
- 50.The Japan Science and Technology Agency. Research center network for realization of regenerative medicine. http://www.jst.go.jp/saisei-nw/ [in Japanese] [accessed 30.10.15].
- 51.Cyranoski D. Stem-cell pioneer banks on future therapies. Nature. 2012;488(7410):139. doi: 10.1038/488139a. [DOI] [PubMed] [Google Scholar]
- 52.Saito M.K., Matsunaga A., Takasu N., Yamanaka S. Stem cell banking. Springer; New York: 2014. Donor recruitment and eligibility criteria for HLA-homozygous iPS cell bank in Japan; pp. 67–76. [Google Scholar]
- 53.Okano H., Yamanaka S. iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain. 2014;7:22. doi: 10.1186/1756-6606-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuroda T., Yasuda S., Kusakawa S., Hirata N., Kanda Y., Suzuki K. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One. 2012;7(5):e37342. doi: 10.1371/journal.pone.0037342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanemura H., Go M.J., Shikamura M., Nishishita N., Sakai N., Kamao H. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamata S., Kanemura H., Sakai N., Takahashi M., Go M.J. Design of a tumorigenicity test for induced pluripotent stem cell (iPSC)-derived cell products. J Clin Med. 2015;4(1):159–171. doi: 10.3390/jcm4010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujita M., Hatta T., Sawai T., Takahashi J. Risk of tumorigenesis and patient hope. AJOB Neurosci. 2015;6(1):69–70. [Google Scholar]
- 58.Yasuda S., Sato Y. Tumorigenicity assessment of human cell-processed therapeutic products. Biologicals. 2015;43:416–421. doi: 10.1016/j.biologicals.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Kuroda T., Yasuda S., Matsuyama S., Tano K., Kusakawa S., Sawa Y. Highly sensitive droplet digital PCR method for detection of residual undifferentiated cells in cardiomyocytes derived from human pluripotent stem cells. Regen Ther. 2015;2:17–23. doi: 10.1016/j.reth.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garber K. Inducing translation. Nat Biotechnol. 2013;31(6):483–486. doi: 10.1038/nbt.2602. [DOI] [PubMed] [Google Scholar]
- 61.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson S.E., Loring J.F. Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem. 2014;289(8):4578–4584. doi: 10.1074/jbc.R113.516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harding J., Mirochnitchenko O. Preclinical studies for induced pluripotent stem cell-based therapeutics. J Biol Chem. 2014;289(8):4585–4593. doi: 10.1074/jbc.R113.463737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The Ministry of Health, Labour and Welfare . July 22 2011. The selection result for the early-phase/exploratory clinical trial centers.http://www.mhlw.go.jp/stf/houdou/2r9852000001jym4.html [in Japanese] [accessed 30.10.15] [Google Scholar]
- 65.The Ministry of Health, Labour and Welfare . May 25 2012. The selection result for clinical research core hospitals in FY2012.http://www.mhlw.go.jp/stf/shingi/2r9852000002bfqp.html [in Japanese] [accessed 30.10.15] [Google Scholar]
- 66.The Ministry of Health, Labour and Welfare . April 1 2015. Institutional review boards certified by the Institutional Review Board Certification Program in FY2014.http://www.mhlw.go.jp/topics/bukyoku/isei/chiken/ [in Japanese] [accessed 30.10.15] [Google Scholar]
- 67.The Ministry of Health, Labour and Welfare. List of specially certified regenerative medicine committees certified by the pursuant to the Act on the Safety of Regenerative Medicine article 26 paragraph 4 as of October 16 2015. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryou/saisei_iryou/index.html [in Japanese] [accessed 30.10.15].
- 68.Government of Japan . 2014. The Act on the Safety of Regenerative Medicine. Act No. 85. [in Japanese] [Google Scholar]
- 69.The Ministry of Health, Labour and Welfare . June 12 2012. The selection result for research support centers for Japan initiated global clinical trials in FY2012.http://www.mhlw.go.jp/topics/bukyoku/isei/chiken/dl/120615-01.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 70.The Ministry of Health, Labour and Welfare. Initiative for accelerating regulatory science in innovative drugs, medical devices and regenerative medicine website. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iyakuhin/kakushin/index.html [in Japanese] [accessed 30.10.15].
- 71.The Pharmaceuticals and Medical Devices Agency (PMDA). Initiative for accelerating regulatory science in innovative drugs, medical devices and regenerative medicine website. http://www.pmda.go.jp/rs-std-jp/facilitate-developments/0001.html [in Japanese] [accessed 30.10.15].
- 72.The Ministry of Health, Labour and Welfare . September 7 2012. Guideline on ensuring the quality and safety of products derived from processed autologous human induced pluripotent stem(-like) cells. Pharmaceutical and Food Safety Bureau (PFSB) Director Notice 0907 No.4. [in Japanese] [Google Scholar]
- 73.The Ministry of Health, Labour and Welfare . September 7 2012. Guideline on ensuring the quality and safety of products derived from processed allogeneic human induced pluripotent stem(-like) cells. PFSB Director Notice 0907 No.5. [in Japanese] [Google Scholar]
- 74.Hayakawa T., Aoi T., Umezawa A., Ozawa K., Sato Y., Sawa Y. A study on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of autologous human induced pluripotent stem(-like) cells. Regen Ther. 2015;2:81–94. doi: 10.1016/j.reth.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayakawa T., Aoi T., Umezawa A., Ozawa K., Sato Y., Sawa Y. A study on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of allogeneic human induced pluripotent stem(-Like) cells. Regen Ther. 2015;2:95–108. doi: 10.1016/j.reth.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.The Ministry of Health, Labour and Welfare . May 29 2013. Points to considers for the evaluation of specific products: autologous iPS cells-derived retinal pigment epithelial cells. Office of Medical Device Evaluation Director notice 0529 No.1. [in Japanese] [Google Scholar]
- 77.The Ministry of Health, Labour and Welfare . September 12 2014. Points to considers for the evaluation of specific products: allogeneic iPS cells-derived retinal pigment epithelial cells. Medical Device and Regenerative Medicine Product Evaluation Division (MRED) Director Notice 0912 No.2. [in Japanese] [Google Scholar]
- 78.National Institute of Health Science. Working group to develop evaluation guidance for innovative medical devices and regenerative medicine products. http://dmd.nihs.go.jp/jisedai/ [in Japanese] [accessed 30.10.15].
- 79.Government of Japan . 1960. The Pharmaceuticals and Medical Devices Act (The Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, etc.) Act No. 145. [in Japanese] [Google Scholar]
- 80.Government of Japan . 2013. The Act on the Partial Revision of the Pharmaceutical Affairs Law. Act No.84. [in Japanese] [Google Scholar]
- 81.The Ministry of Health, Labour and Welfare . August 6 2014. Good gene, cellular, and tissue-based products manufacturing practice. 2014 MHLW Ministerial Ordinance No.93. [in Japanese] [Google Scholar]
- 82.The Ministry of Health, Labour and Welfare . September 2 2015. Review result report of TEMCELL HS Inj.http://www.pmda.go.jp/regenerative_medicines/2015/R20151009001/530210000_22700FZX00001_A100_1.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 83.JCR Pharmaceuticals Co., Ltd. September 18 2015. JCR receives approval for TEMCELL® HS Inj., the first allogeneic regenerative medicine in Japan.http://www.jcrpharm.co.jp/en/site/en/ir/pdf/ir_news_20150918.pdf [accessed 30.10.15] [Google Scholar]
- 84.The Ministry of Health, Labour and Welfare . September 2 2015. Review result report of HeartSheet.http://www.pmda.go.jp/regenerative_medicines/2015/R20151008001/470034000_22700FZX00002_A100_2.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 85.Terumo receives approval for the manufacturer and sale of its HeartSheet Autologous Skeletal Myoblast Sheets in Japan. September 18 2015. http://www.terumo.com/about/pressrelease/2015/20150918.html [accessed 30.10.15] [Google Scholar]
- 86.Yanagi K., Fukuda E., Jotatsu Y., Shikano M., Miyake S. Regulatory frameworks for cell therapy products in Japan. J Artif Organs. 2012;15:325–330. doi: 10.1007/s10047-012-0653-5. [DOI] [PubMed] [Google Scholar]
- 87.Tsubouchi M., Matsui S., Banno Y., Kurokawa K., Kawakami K. Overview of the clinical application of regenerative medicine products in Japan. Health Policy. 2008;88(1):62–72. doi: 10.1016/j.healthpol.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 88.The Ministry of Health, Labour and Welfare . July 30 1999. Notice for quality and safety of cellular and tissue-based medical devices and pharmaceuticals. Pharmaceutical Safety Bureau Director Notice No.906. [in Japanese] [Google Scholar]
- 89.The Ministry of Health, Labour and Welfare . April 28 2011. Regulatory framework to make it possible to seamless transition from clinical research to commercialization of regenerative and cell therapies. Health Policy Bureau Director Notice 0428 No.7 and PFSB 0428 No.1. [in Japanese] [Google Scholar]
- 90.The Ministry of Health, Labour and Welfare. Revision for regulatory procedure of cellular and tissue-based pharmaceuticals and medical devices by the introduction of pharmaceutical affairs strategy consultation. PFSB Director Notice No. 0630002 [in Japanese].
- 91.The Pharmaceuticals and Medical Devices Agency. Pharmaceutical affairs strategy consultation website. http://www.pmda.go.jp/review-services/f2f-pre/strategies/0003.html [in Japanese] [accessed 30.10.15].
- 92.Center for iPS Cell Research and Application, Kyoto University . October 18 2011. Initiation of pharmaceutical affairs strategy consultation towards development of iPS cell bank for regenerative medicine.http://www.cira.kyoto-u.ac.jp/j/pressrelease/news/111018-151957.html [in Japanese] [accessed 30.10.15] [Google Scholar]
- 93.The Ministry of Health, Labour and Welfare. Strategy of SAKIGAKE website. http://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/140729-01.html [accessed 30.10.15].
- 94.The Ministry of Health, Labour and Welfare . July 1 2015. SAKIGAKE priority review designation pilot program for medical devices, in vitro diagnostics, and regenerative medicine products. MRED Director Notice 0701 No.1. [in Japanese] [Google Scholar]
- 95.Government of Japan . 2002. The Act on the Partial Revision of the Pharmaceutical Affairs Law and the Blood Collection and Donation Service Control Act. Act No. 96. [in Japanese] [Google Scholar]
- 96.The Ministry of Health, Labour and Welfare . December 28 2012. Partial revision of “Guidance on the use of Drug Master File”. Evaluation and Licensing Division (ELD) Director Notice 1228 No.27. [in Japanese] [Google Scholar]
- 97.The Ministry of Health, Labour and Welfare . December 28 2012. Q&A on Drug Master File (Part 3). ELD Administrative Notice. [in Japanese] [Google Scholar]
- 98.The Ministry of Health, Labour and Welfare . November 17 2014. Guidance on the use of Drug Master File. ELD Director Notice 1117 No.3 and MRED Director Notice No.1. [in Japanese] [Google Scholar]
- 99.The Ministry of Health, Labour and Welfare . March 8 2015. Guidance on the application dossier for the registration of the Drug Master File related to the manufacture of cell and tissue-based products. ELD Administrative Notice. [in Japanese] [Google Scholar]
- 100.Köckerling F. The need for registries in the early scientific evaluation of surgical innovations. Front Surg. 2014;1:12. doi: 10.3389/fsurg.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Therapeutic Goods Administration, Department of Health, Australian Government. Metal-on-metal hip replacement implants website. http://www.tga.gov.au/metal-metal-hip-replacement-implants [accessed 30.10.15].
- 102.The Pharmaceuticals and Medical Devices Agency. J-MACS: Japanese Registry for Mechanically Assisted Circulatory Support website. http://www.pmda.go.jp/safety/surveillance-analysis/0009.html [in Japanese] [accessed 30.10.15].
- 103.Uchida T., Ikeno F., Ikeda K., Suzuki Y., Todaka K., Yokoi H. Global cardiovascular device innovation: Japan–USA synergies. Circ J. 2013;77(7):1714–1718. doi: 10.1253/circj.cj-12-1431. [DOI] [PubMed] [Google Scholar]
- 104.Tamura A., Kutsumi H. Multiregional medical device development: regulatory perspective. Clin J Gastroenterol. 2014;7(2):108–116. doi: 10.1007/s12328-014-0478-2. [DOI] [PubMed] [Google Scholar]
- 105.University of Alabama at Birmingham. INTERMACS website. http://www.uab.edu/medicine/intermacs/ [accessed 30.10.15].
- 106.Holman W.L. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) what have we learned and what will we learn? Circulation. 2012;126(11):1401–1406. doi: 10.1161/CIRCULATIONAHA.112.097816. [DOI] [PubMed] [Google Scholar]
- 107.The Ministry of Health, Labour and Welfare . July 4 2014. Report of the committee on patient registry system of regenerative medicine products.http://www.mhlw.go.jp/stf/shingi/0000050197.html [in Japanese] [accessed 30.10.15] [Google Scholar]
- 108.The Pharmaceuticals and Medical Devices Agency . April 1 2015. FY2015 PMDA annual plan.http://www.pmda.go.jp/files/000204288.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 109.The Ministry of Health, Labour and Welfare . June 24 2014. Medical device evaluation system reinforcement programs.http://www.mhlw.go.jp/jigyo_shiwake/dl/h26_gaiyou02a_day2.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 110.The Ministry of Health, Labour and Welfare . June 19 2012. Public procurement notice: new medical device user requirement development program (autologous cultured cartilage)http://www.mhlw.go.jp/sinsei/chotatu/chotatu/kikaku/2012/06/kk0619-01.html [in Japanese] [accessed 30.10.15] [Google Scholar]
- 111.The Ministry of Health, Labour and Welfare . 22 Jun 2012. Review result report of JACC.http://www.pmda.go.jp/files/000203221.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 112.Yano K., Watanabe N., Tsuyuki K., Ikawa T., Kasanuki H., Yamato M. Regulatory approval for autologous human cells and tissue products in the United States, the European Union, and Japan. Regen Ther. 2015;1:45–56. doi: 10.1016/j.reth.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yano K., Watanabe N., Tsuyuki K., Ikawa T., Kasanuki H., Yamato M. Diverse approval systems for autologous human cells and tissue products. Chemother Open Access. 2015;4:157. doi: 10.1016/j.reth.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sengoku S., Sakurai M., Yashiro Y. Japan's regulatory framework: seeking to provide impetus to the commercialization of regenerative medicine products. Cell Gene Ther Insights. 2015;1(1):83–92. [Google Scholar]
- 115.The Japanese Orthopaedic Association . May 22 2013. User requirements for autologous cultured cartilage (JACC)http://www.joa.or.jp/jp/media/institution/files/jack_reference.pdf [in Japanese] [accessed 30.10.15] [Google Scholar]
- 116.Japan Tissue Engineering Co., Ltd. List of medical institutions in conformity with conditions for insurance reimbursement specified by MHLW. http://www.jpte.co.jp/JACC_institutions.html [in Japanese] [accessed 30.10.15].
- 117.Japan Tissue Engineering Co., Ltd. Conditions for insurance reimbursement. http://www.jpte.co.jp/ins.html [in Japanese] [accessed 30.10.15].
- 118.The Ministry of Health, Labour and Welfare . June 22 2015. Public procurement notice: new regenerative medicine product user requirement development program (human autologous skeletal muscle derived cell sheet)http://www.mhlw.go.jp/sinsei/chotatu/chotatu/kikaku/2015/06/kk0622-01.html [in Japanese] [accessed 30.10.15] [Google Scholar]
- 119.Cuende N., Boniface C., Bravery C., Forte M., Giordano R., Hildebrandt M. The puzzling situation of hospital exemption for advanced therapy medicinal products in Europe and stakeholders' concerns. Cytotherapy. 2014;16(12):1597–1600. doi: 10.1016/j.jcyt.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 120.The Ministry of Health, Labour and Welfare . 2006. Guidelines for clinical research using human stem cells. MHLW Ministerial Notification No.425, 2010 MHLW Ministerial Notification No.380, 2013 MHLW Ministerial Notification No.317. [in Japanese] [Google Scholar]
- 121.Cellular Dynamics International, Inc. Feb 9 2015. Cellular dynamics manufactures cGMP HLA “Superdonor” stem cell lines to enable cell therapy with genetic matching.http://files.shareholder.com/downloads/AMDA-1ZQS9K/2959357463x0x807953/C9093013-5DA7-4F32-BFA4-466532611116/ICEL_News_2015_2_9_General_Releases.pdf [accessed 30.10.15] [Google Scholar]
- 122.The Cell Therapy Catapult and Roslin Cells Ltd . September 11 2013. Cell Therapy Catapult & Roslin Cells to create clinical grade stem cells to accelerate research into new treatments.https://ct.catapult.org.uk/-/cell-therapy-catapult-roslin-cells-to-create-clinical-grade-stem-cells-to-accelerate-research-into-new-treatments [accessed 30.10.15] [Google Scholar]
- 123.Zhou H., Rao M.S. Can cord blood banks transform into induced pluripotent stem cell banks? Cytotherapy. 2015;17(6):756–764. doi: 10.1016/j.jcyt.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 124.Andrews P., Baker D., Benvinisty N., Miranda B., Bruce K., Brustle O. Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI) Regen Med. 2015;10(2s):1–44. doi: 10.2217/rme.14.93. [DOI] [PubMed] [Google Scholar]
- 125.Hayakawa T., Aoi T., Bravery C., Hoogendoorn K., Knezevic I., Koga J. Report of the international conference on regulatory endeavors towards the sound development of human cell therapy products. Biologicals. 2015;43(5):283–297. doi: 10.1016/j.biologicals.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 126.Andrews P.W., Cavagnaro J., Deans R., Feigal E., Horowitz E., Keating A. Harmonizing standards for producing clinical-grade therapies from pluripotent stem cells. Nat Biotechnol. 2014;32(8):724–726. doi: 10.1038/nbt.2973. [DOI] [PubMed] [Google Scholar]
- 127.Jonlin E.C. Consent for pluripotent cell use for therapy. Curr Stem Cell Rep. 2015;1(2):92–101. [Google Scholar]
- 128.Lowenthal J., Lipnick S., Rao M,Hull S.C. Specimen collection for induced pluripotent stem cell research: harmonizing the approach to informed consent. Stem Cells Transl Med. 2012;1(5):409–421. doi: 10.5966/sctm.2012-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lomax G.P., Hull S.C., Isasi R.M. The DISCUSS project: revised points to consider for the derivation of induced pluripotent stem cell lines from previously collected research specimens. Stem Cells Transl Med. 2015;4(2):123–129. doi: 10.5966/sctm.2014-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.The Ministry of Health, Labour and Welfare . May 20 2003. Standards for biological ingredients. 2003 MHLW Minister's Notification No. 210. [in Japanese] [Google Scholar]
- 131.The Ministry of Health, Labour and Welfare . October 2 2014. Administration of standards for biological ingredients. ELD Director Notice 1002 No.1 & MRED Director Notice 1002 No.5. [in Japanese] [Google Scholar]
- 132.Ministry of Education, Culture, Sports, Science and Technology (MEXT), Ministry of Health, Labour and Welfare (MHLW) and Ministry of Economy, Trade and Industry (METI) February 8 2013. Ethical guideline for human genome/gene analysis research.http://www.meti.go.jp/english/press/2013/0208_02.html 2013 MEXT, MHLW and METI Ministers' Notification No. 1. [Google Scholar]
- 133.The World Health Organization . May 29 1975. World Health Assembly Resolution 28.72.http://www.who.int/bloodsafety/BTS_ResolutionsAdopted.pdf [accessed 02.07.15] [Google Scholar]
- 134.Caulfield T., McGuire A.L., Cho M., Buchanan J.A., Burgess M.M., Danilczyk U. Research ethics recommendations for whole-genome research: consensus statement. PLoS Biol. 2008;6(3):e73. doi: 10.1371/journal.pbio.0060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rehm H.L., Bale S.J., Bayrak-Toydemir P., Berg J.S., Brown K.K., Deignan J.L. ACMG clinical laboratory standards for next-generation sequencing. Genet Med. 2013;15(9):733–747. doi: 10.1038/gim.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Green R.C., Berg J.S., Grody W.W., Kalia S.S., Korf B.R., Martin C.L. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aziz N., Zhao Q., Bry L., Driscoll D.K., Funke B., Gibson J.S. College of American Pathologists' laboratory standards for next-generation sequencing clinical tests. Arch Pathol Lab Med. 2015;139(4):481–493. doi: 10.5858/arpa.2014-0250-CP. [DOI] [PubMed] [Google Scholar]
- 138.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Middleton A., Morley K.I., Bragin E., Firth H.V., Hurles M.E., Wright C.F. Attitudes of nearly 7000 health professionals, genomic researchers and publics toward the return of incidental results from sequencing research. Eur J Hum Genet. 2016;24(1):21–29. doi: 10.1038/ejhg.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.The International Society for Stem Cell Research (ISSCR) June 26 2015. Draft guidelines for stem cell science and clinical translation.http://www.isscr.org/home/publications/2015-guidelines-draft [accessed 30.10.15] [Google Scholar]
- 141.Government of the United Kingdom . Advisory Committee on the Safety of Blood, Tissues and Organs; June 2014. Donation of starting material for cell-based advanced therapies: a SaBTO review.https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/326823/Cellular_Therapy.pdf [accessed 30.10.15] [Google Scholar]
- 142.The United States Food and Drug Administration (USFDA) February 20 2015. Public workshop – optimizing FDA's regulatory oversight of next generation sequencing diagnostic tests public workshop.http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm427296.htm [accessed 30.10.15] [Google Scholar]
- 143.Harris I.R. Extent and content of data for regulatory submissions: first-in-human and marketing authorization – viewpoint of US industry. Biologicals. 2015;43(5):402–405. doi: 10.1016/j.biologicals.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 144.Federal government of the United States. 21CFR1271 subpart C (donor eligibility) Sec. 1271.80. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1271.80 [accessed 30.10.15].
- 145.The European Union Tissues and Cells Directive Directive. 2004/23/EC. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004L0023&from=EN [accessed 30.10.15].
- 146.The European Union Blood Directive. 2002/98/EC. http://ec.europa.eu/health/files/eudralex/vol-1/dir_2002_98/dir_2002_98_en.pdf [accessed 30.10.15].
- 147.Boo M., Ballen K., Maiers M. Cord blood unit access and selection: 2010 and beyond: best practices and emerging trends in cord blood unit selection. Biol Blood Marrow Transplant. 2010;17(1):S46–S51. doi: 10.1016/j.bbmt.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 148.Nishiwaki S. Rules of providing cord blood for induced pluripotent stem cells for research. Cytotherapy. 2015;17(7):1008. doi: 10.1016/j.jcyt.2015.03.606. [DOI] [PubMed] [Google Scholar]
- 149.Government of Japan . 2012. The act for appropriate provision of hematopoietic stem cells to be used in transplantations.http://www.jshct.com/english/pdf/act_en.pdf Act No.90. [accessed 30.10.15] [Google Scholar]
- 150.Okita K., Yamakawa T., Matsumura Y., Sato Y., Amano N., Watanabe A. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31(3):458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 151.Nakagawa M., Taniguchi Y., Senda S., Takizawa N., Ichisaka T., Asano K. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rao M.S., Malik N. Assessing iPSC reprogramming methods for their suitability in translational medicine. J Cell Biochem. 2012;113(10):3061–3068. doi: 10.1002/jcb.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsumaki N., Okada M., Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2015;70:48–54. doi: 10.1016/j.bone.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 154.Seki T., Fukuda K. Methods of induced pluripotent stem cells for clinical application. World J Stem Cells. 2015;7(1):116–125. doi: 10.4252/wjsc.v7.i1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Silva M., Daheron L., Hurley H., Bure K., Barker R., Carr A.J. Generating iPSCs: translating cell reprogramming science into scalable and robust biomanufacturing strategies. Cell Stem Cell. 2015;16(1):13–17. doi: 10.1016/j.stem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 156.Revilla A., González C., Iriondo A., Fernández B., Prieto C., Marín C. Current advances in the generation of human iPS cells: implications in cell-based regenerative medicine. J Tissue Eng Regen Med. Mar 11 2015 doi: 10.1002/term.2021. [DOI] [PubMed] [Google Scholar]
- 157.Baghbaderani B.A., Tian X., Neo B.H., Burkall A., Dimezzo T., Sierra G. Cgmp-manufactured human induced pluripotent stem cells are available for pre-clinical and clinical applications. Stem Cell Rep. 2015;5(4):647–659. doi: 10.1016/j.stemcr.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rao M., Ahrlund-Richter L., Kaufman D.S. Concise review: cord blood banking, transplantation and induced pluripotent stem cell: success and opportunities. Stem Cells. 2012;30(1):55–60. doi: 10.1002/stem.770. [DOI] [PubMed] [Google Scholar]
- 159.Karagiannis P., Yamanaka S. The fate of cell reprogramming. Nat Methods. 2014;11(10):1006–1008. doi: 10.1038/nmeth.3109. [DOI] [PubMed] [Google Scholar]
- 160.Crook J.M., Hei D., Stacey G. The international stem cell banking initiative (ISCBI): raising standards to bank on. In Vitro Cell Dev Biol Anim. 2010;46(3–4):169–172. doi: 10.1007/s11626-010-9301-7. [DOI] [PubMed] [Google Scholar]
- 161.Stacey G.N., Crook J.M., Hei D., Ludwig T. Banking human induced pluripotent stem cells: lessons learned from embryonic stem cells? Cell Stem Cell. 2013;13(4):385–388. doi: 10.1016/j.stem.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 162.The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . September 23 1999. Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin Q5A(R1)http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5A_R1/Step4/Q5A_R1__Guideline.pdf [accessed 30.10.15] [Google Scholar]
- 163.The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . July 16 1997. Derivation and characterisation of cell substrates used for production of biotechnological/biological products.http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5D/Step4/Q5D_Guideline.pdf [accessed 30.10.15] [Google Scholar]
- 164.Koyanagi-Aoi M., Ohnuki M., Takahashi K., Okita K., Noma H., Sawamura Y. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110(51):20569–20574. doi: 10.1073/pnas.1319061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10(6):678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 166.Nakamura S., Takayama N., Hirata S., Seo H., Endo H., Ochi K. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14:535–548. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 167.Heslop J.A., Hammond T.G., Santeramo I., Piella A.T., Hopp I., Zhou J. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med. 2015;4(4):389–400. doi: 10.5966/sctm.2014-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 169.XXX . March 1 2014. The pharmaceutical inspection convention and pharmaceutical inspection co-operation scheme (PIC/S) GMP guide (PE009-11)http://www.picscheme.org/publication.php [accessed 30.10.15] [Google Scholar]
- 170.European Commission . September 9 2013. EU guidelines for good manufacturing practice for medicinal products for human and veterinary use: annex 2 on manufacture of biological active substances and medicinal products for human use. [Google Scholar]
- 171.The Ministry of Health, Labour and Welfare . July 8 2015. Partial Revision of “Guidance on the application of PIC/S GMP Guide”. Compliance and Narcotics Division Administrative Notice. [in Japanese] [Google Scholar]
- 172.Feigal E.G., Tsokas K., Viswanathan S., Zhang J., Priest C., Pearce J. Proceedings: international regulatory considerations on development pathways for cell therapies. Stem Cells Transl Med. 2014;3(8):879–887. doi: 10.5966/sctm.2014-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tominaga T. The ICH, the GHTF, and the future of harmonization initiatives. Ther Innov Regul Sci. 2013;47(5):572–580. doi: 10.1177/2168479013494393. [DOI] [PubMed] [Google Scholar]
- 174.Yano K., Tsuyuki K., Watanabe N., Kasanuki H., Yamato M. The regulation of allogeneic human cells and tissue products as biomaterials. Biomaterials. 2013;34(13):3165–3173. doi: 10.1016/j.biomaterials.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 175.Tominaga T., Asahina Y., Uyama Y., Kondo T. Regulatory science as a bridge between science and society. Clin Pharmacol Ther. 2011;90(1):29–31. doi: 10.1038/clpt.2011.89. [DOI] [PubMed] [Google Scholar]