Abstract
Introduction
Autologous, allogeneic, and artificial bones are clinically applied as graft materials for bone reconstruction, with each having their own advantages and disadvantages. Although artificial bones with various shapes are currently available, a product with a morphology that may be freely modified by operators has not yet been developed. In the present study, we developed a full custom-made artificial bone, and applied it to form the maxillofacial region. We herein report treatment outcomes.
Methods
An artificial bone was prepared on a 3-dimensional solid model, and data of its shape was collected on CT. A full custom-made artificial bone was prepared by laminating α-tricalcium phosphate powder using an aqueous polysaccharide curing solution and the ink-jet powder-laminating device, Z406 3D Printer (DICO, USA). Subjects comprised patients who underwent maxillofacial plasty using this artificial bone between March 2006 and September 2009.
Results
Maxillofacial plasty using the full custom-made artificial bone was applied to 23 regions in 20 patients (14 females and 6 males). The recipient region was the maxilla in 3, mandibular ramus in 13, mental region in 7, and frontal bone in 1. Postoperative courses were favorable in 18 out of the 23 regions; however, the fit was insufficient in 2 regions and the recipient regions were exposed within 1 year after surgery. Three regions were exposed 1 year or more after surgery.
Conclusion
We developed a novel reconstruction method using a full custom-made artificial bone. Its fit with the recipient bone was considered to be important, since an ill fit between the recipient and artificial bones potentially resulting in the artificial bone being detached. Therefore, fixation is important in order to prevent the detachment, and careful course observations are required when an ill fit is concerned during the follow-up period.
Keywords: Full custom-made, Artificial bones, Oral-maxillofacial region, Complication
1. Introduction
Autologous [1], allogeneic [2], and artificial bones have been used to reconstruct the craniomaxillofacial bone [3], [4]. Autologous bone is the clinical gold standard and is superior to the other bone types; however, it is invasive because the collection of bone from a healthy region is necessary, and the amount collectable is limited, which is disadvantageous [5]. Allogeneic bone may be used as an alternative, but has not been widely applied in Japan because of cultural and religious differences, the risk of unknown infections and concerns onethical issues, such as corpse trade in other countries [2]. In contrast, artificial bones are not only capable of avoiding the collection of healthy bone, but are also superior to autologous bone grafting in biocompatibility without the risk of unknown viral infection. One of the challenges associated with the application of graft materials is the preparation of an appropriate morphology for reconstruction [5]. Many artificial bones that have been developed to date cannot be freely handed, and thus the difficulties are associated with forming an appropriate shape. Moreover, a sintered ceramic artificial bone cannot be absorbed or replaced by bone because of its high crystallinity, in addition to the disadvantage of shrinking [6]. In order to overcome these issues, we therefore developed a novel full custom-made artificial bone with a shape in cooperation with NEXT21 K.K. and started its clinical application in 2006 [7]. We herein report the treatment long-term outcomes of clinical cases.
2. Materials and methods
2.1. Materials
Subjects comprised patients who underwent maxillofacial plasty with custom-made artificial bone in an investigator-initiated clinical study (the approval number by the Ethics Committee of the University of Tokyo: 1310) and clinical study (the approval number by the Ethics Committee of the University of Tokyo: 3DB-01/CT-1) between March 2006 and September 2009.
2.2. Preparation of the artificial bone
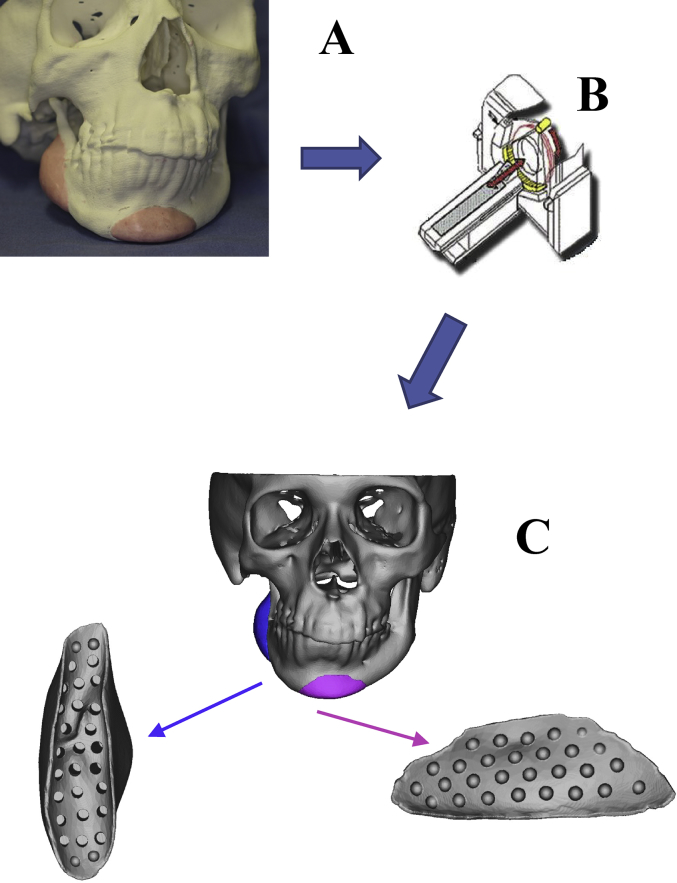
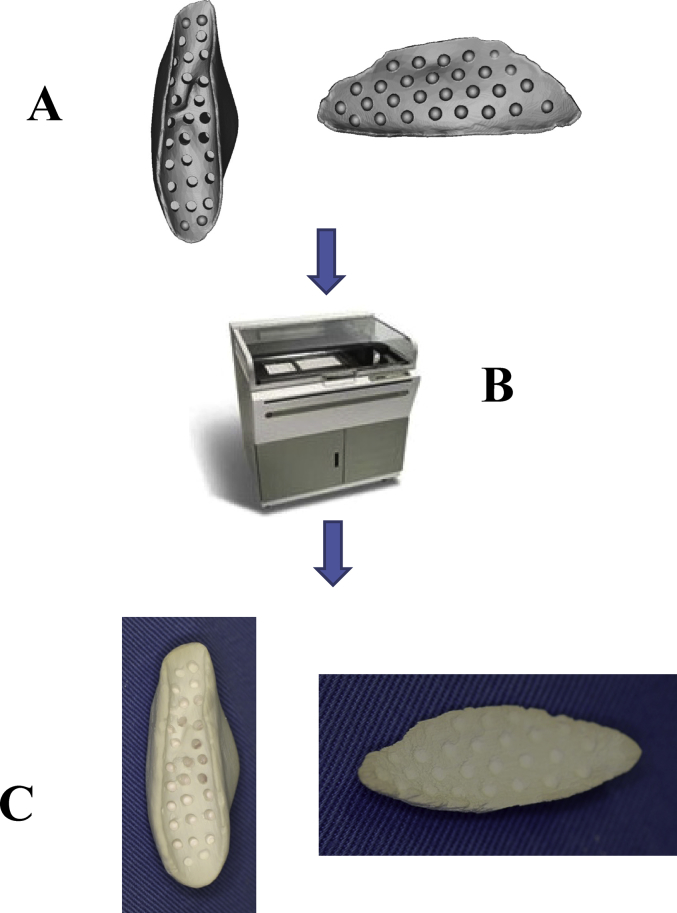
An artificial bone model was prepared by building up contrast-enhancing wax in the bone defect or filled region on a 3-dimensional solid model [8]. This solid model was scanned by CT, and the DICOM data was output. Then, the region of designed artificial bone was extracted from CAD data (Fig. 1). A detailed design was prepared on CAD based on the extracted data of the artificial bone. In the bone-contact region, an inner surface structure advantageous for vascularization was formed with small holes, while holes for fixation were also prepared based on the data. α-tricalcium phosphate (TCP) was used as the base material, and the bone was prepared using the ink-jet powder laminating device, Z406 3D Printer (DICO, USA) [9]. α-TCP has been used because it cures upon addition of water, and is more rapidly replaced by bone tissue than β-TCP (Fig. 2).
Fig. 1.
(A) An artificial bone with a planned shape was simulated by applying contrast medium-containing wax to the 3-dimensional solid plaster model. (B) The simulated model was imaged using helical CT. (C) CAD data was extracted from the DICOM data of CT.
Fig. 2.
(A) CAD data of an artificial bone. (B) CAD data of an artificial bone was output by a 3-dimensional printer. (C) A fabricated artificial bone.
2.3. Surgical procedures
Surgery was performed under general anesthesia through an intra- or extraoral approach. A periosteal flap was prepared by subperiosteal dissection, and the recipient bone region was clearly exposed. The prepared custom-made artificial bone was inserted to confirm its fit with the recipient bone and accompanying changes in soft tissue. When fixation of the artificial bone to the recipient bone was necessary, holes for fixation were made using a surgical drill to fix the bones at several sites with polyglycolic acid absorbable sutures (2-0 Vicryl®, Johnson & Johnson, USA). The surgical wound was tightly closely by concomitantly applying periosteal sutures in order to prevent postoperative deviation of the artificial bone. When the graft was inserted through the oral cavity, fixation was not always applicable due to a limited surgical field. In such cases, the wound was closed after confirming the absence of mobility of the graft. After surgery, CT was periodically performed in order to evaluate the fit between the artificial and recipient bones.
3. Results
Maxillofacial plasty with custom-made artificial bones was applied to 23 regions in 20 patients during the survey period. There were 14 females and 6 males, and the recipient regions were the maxilla in 3, mandibular ramus in 13, mental region in 7, and frontal bone in 1. Artificial bones were fixed or not to recipient bones in 16 and 7 patients, respectively (Table 1).
Table 1.
| Case | Age (years) | Sex | Diagnosis | Implantation site | Follow-up period (months) | Timing of adverse events (months) | Fixation |
|---|---|---|---|---|---|---|---|
| 1 | 26 | F | Right maxillary and mandibular hypoplasia | Right maxilla | 115 | − | |
| Right mandibule | 14 | − | |||||
| 2 | 55 | F | Left mandibular deformity after reconstruction | Left mandibule | 108 | + | |
| 3 | 41 | F | Micrognathia | Chin | 13 | 1 | + |
| 4 | 23 | M | Right hemifacial microsomia | Right mandibule | 14 | − | |
| Chin | 108 | − | |||||
| 5 | 23 | F | Left hemifacial microsomia | Left mandibule | 13 | − | |
| 6 | 30 | F | Right mandibular deformity after reconstruction | Right mandibule | 102 | + | |
| 7 | 53 | F | Left mandibular deformity after reconstruction | Left mandibule | 101 | + | |
| 8 | 18 | F | Micrognathia | Chin | 103 | + | |
| 9 | 38 | F | Left mandibular hypoplasia | Left mandibule | 25 | − | |
| 10 | 43 | F | Mandibular deformity after trauma | Chin | 74 | + | |
| 11 | 44 | M | Mandibular deformity after reconstruction | Left and Right mandibule | 1 | + | |
| 12 | 26 | M | Micrognathia | Chin | 12 | + | |
| 13 | 32 | F | Mandibular deformity after reconstruction | Right mandibule | 71 | + | |
| Left mandibule | 71 | + | |||||
| 14 | 26 | M | Right mandibular hypoplasia | Right mandibule | 24 | + | |
| 15 | 30 | F | Right frontal bone deformity after reconstruction | Right frontal bone | 15 | − | |
| 16 | 24 | M | Right hemifacial microsomia | Right mandibule | 12 | + | |
| 17 | 20 | F | Right maxillary deformity after trauma | Right maxilla | 48 | + | |
| 18 | 20 | F | Treacher Collins' syndrome | Chin | 68 | + | |
| 19 | 39 | F | Left maxillary deformity after reconstruction | Left maxilla | 30 | + | |
| 20 | 23 | M | Micrognathia | Chin | 13 | + |
Adverse events occurred within 1 year of surgery in 2 patients, and fixation with the recipient bone had been performed in both. In one patient, an umbrella shaft blown by a strong wind hit the graft-recipient mental region and thus the artificial bone was broken. Artificial bone was grafted again, and the recovery was uneventful thereafter. The other patient was an MRSA carrier, and wound infection occurred early after surgery. It rapidly disseminated to the artificial bone, which was ultimately removed. Adverse events occurred after more than one year in 3 patients, with fixation with the recipient bone not being performed applied in 2. On the other patient, the bone moved after more than 5 years and the recipient bone was exposed. In all cases, an ill fit with the recipient bone was noted in images early after surgery. Redness and swelling of the recipient region were observed as early symptoms. The graft was removed, and granulation was noted between the artificial and recipient bones.
3.1. Case
3.1.1. Case 7
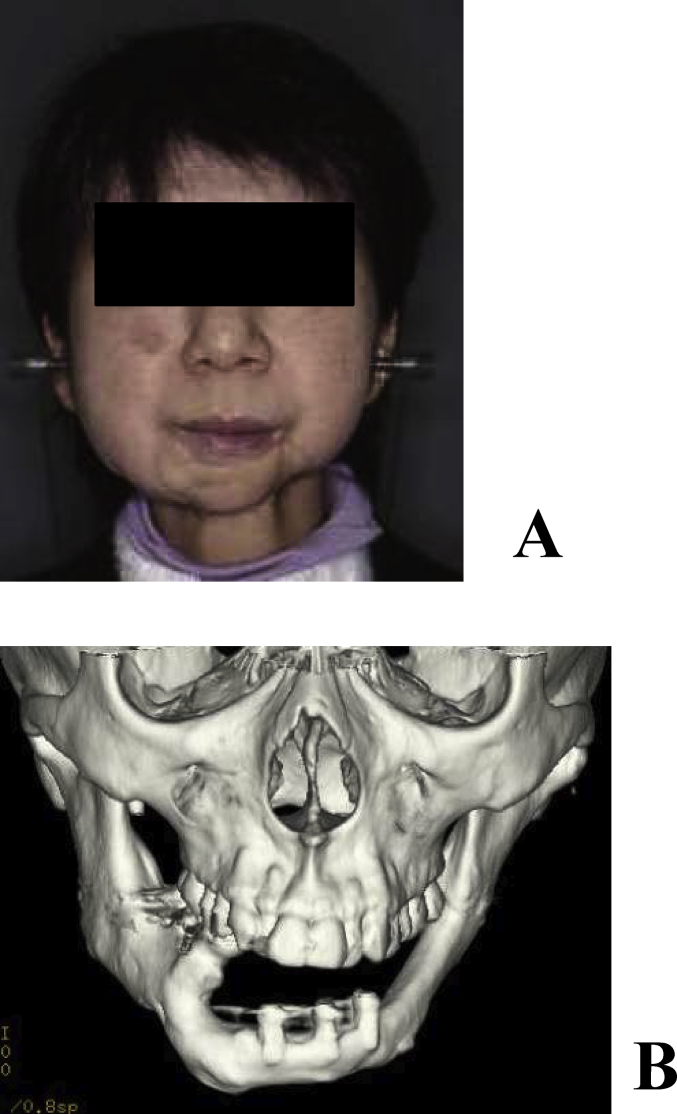
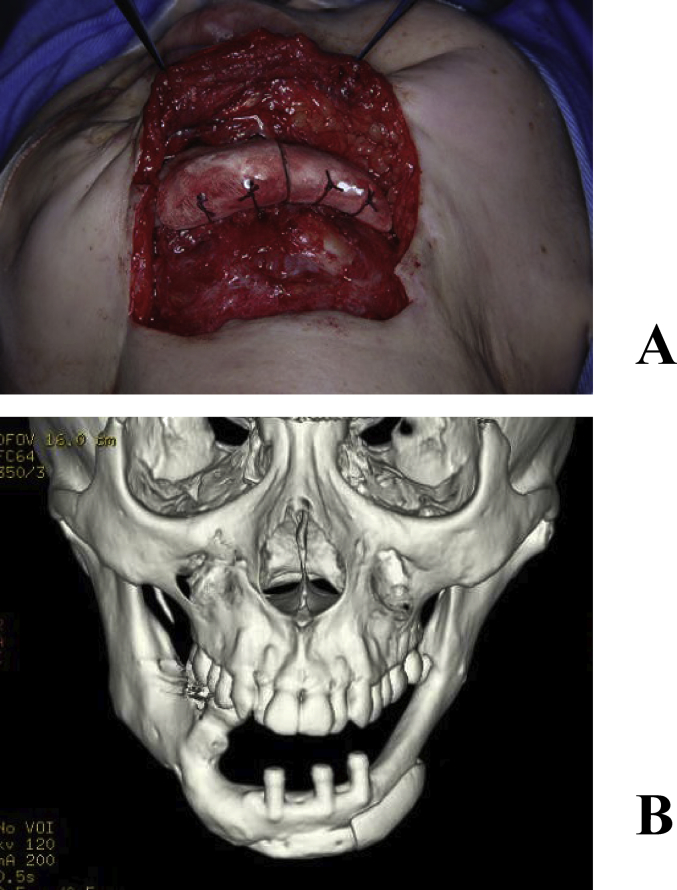
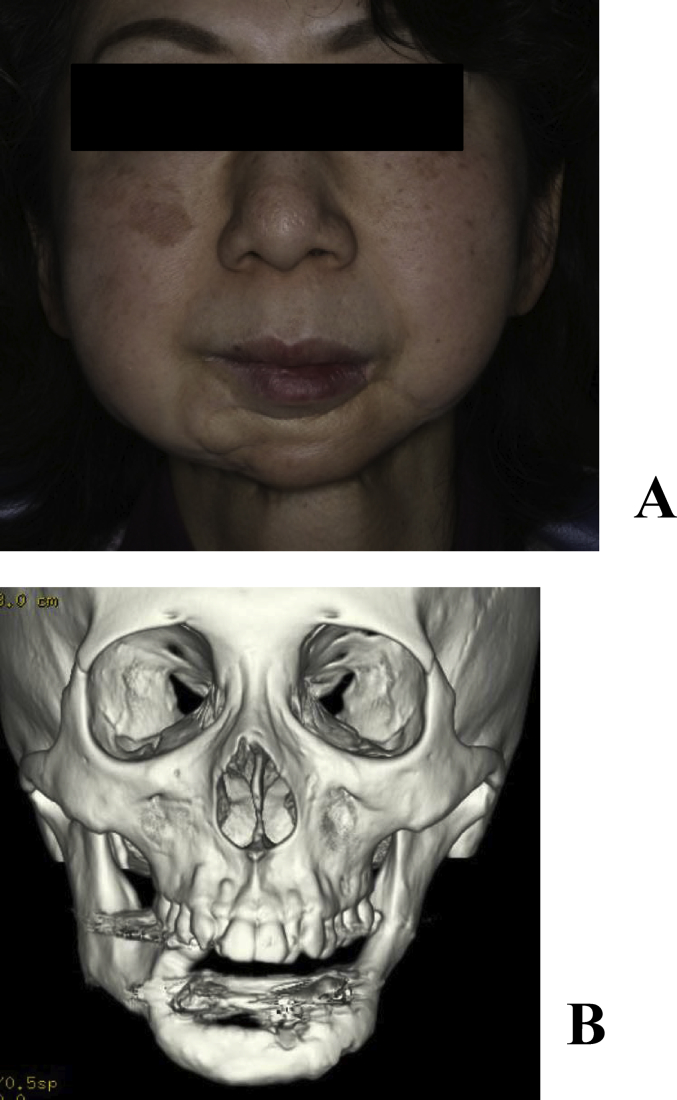
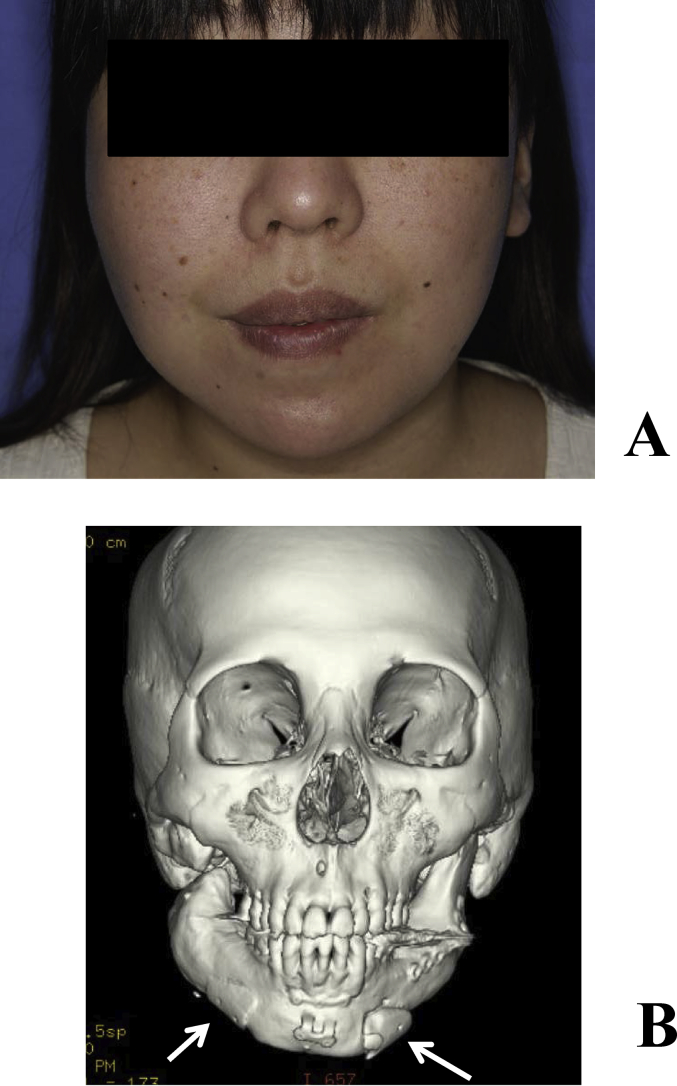
A 53-year-old female underwent vascular pedicle fibular bone grafting for reconstruction after surgery for mandibular sarcoma 7 years earlier; however, a facial deformity remained. Although autologous bone grafting and cicatrization were subsequently performed, her outcome was not satisfactory. Our department therefore performed mandibular plasty with a full custom-made artificial bone for the left mandibular concavity (Fig. 3A, B). A simulation was performed on a 3-dimensional plaster model prepared before surgery, in which deformation of the mandibular ramus was marked and the curvature was complex. In order to improve the fit and disperse stress, a 2-piece custom-made artificial bone was prepared. In surgery, after the recipient region was revealed through an extraoral approach, the artificial bone was inserted, which was then fixed to the recipient bone with absorbable sutures (Fig. 4A, B). Facial morphology improved after surgery, and the patient was satisfied. New bone formation was observed between the recipient and artificial bones including the hole prepared on the inner surface of the artificial bone on CT (Fig. 5A, B). As of 8 years and 5 months after surgery, her postoperative course has been favorable.
Fig. 3.
(A) Preoperative frontal view. A concave deformity was noted in the left mandibular body. (B) Preoperative 3-dimensional CT.
Fig. 4.
(A) Intraoperative findings. The artificial bone was fixed to the recipient bone with absorbable sutures. (B) Postoperative 3-dimensional CT.
Fig. 5.
Outcomes 2 years after surgery. (A) Facial photograph. (B) 3-dimensional CT.
3.1.2. Case 8
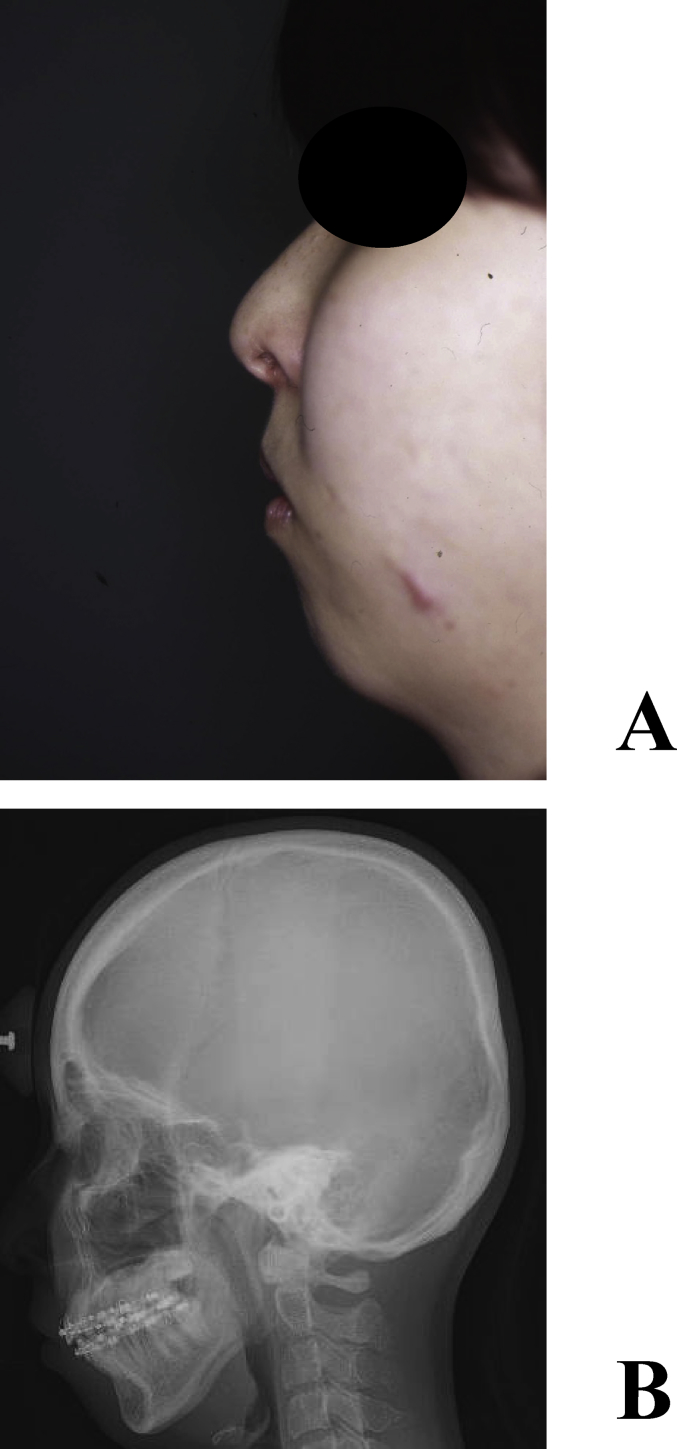
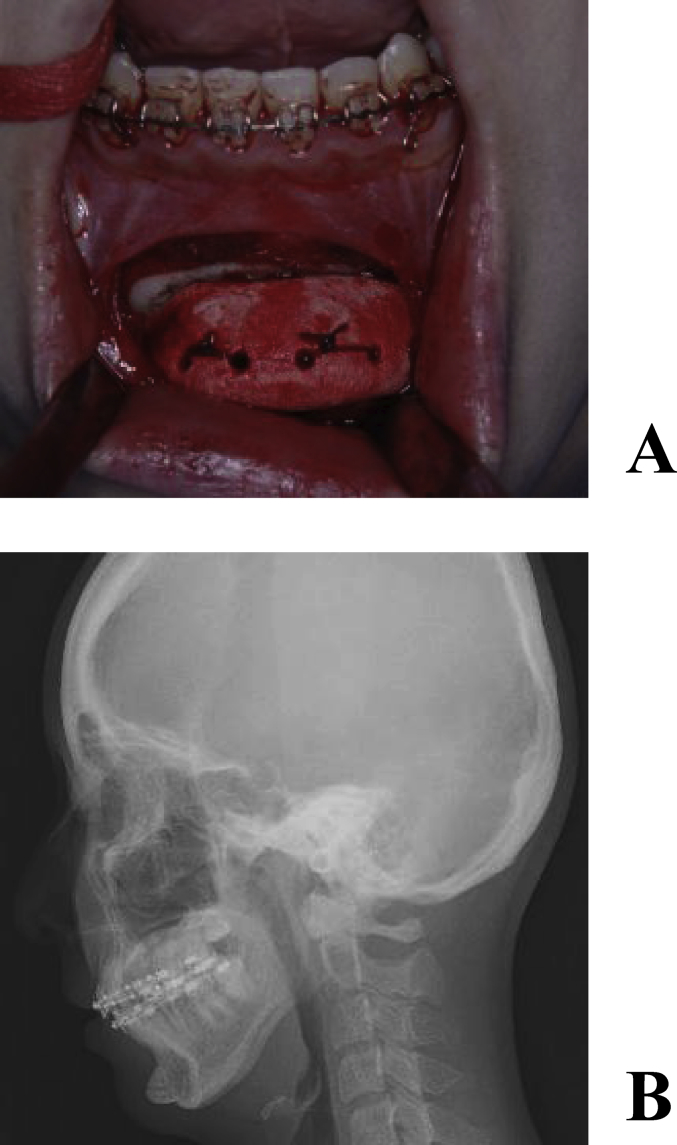
A 23-year-old female underwent osteotomy of the upper and lower jaw bones for a jaw deformity. Although the occlusal relationship was improved, the patient sensed retrogression of the mental region and requested its improvement, for which reconstruction with a full custom-made artificial bone was performed (Fig. 6A, B). A custom-made artificial bone was fabricated, the recipient bone was exposed through an intraoral incision, and the fit of the artificial bone and morphological changes in soft tissue were confirmed. Holes were made in the recipient bone using a surgical bar, and the artificial bone was fixed to the recipient bone using 2-0 Vicryl® (Johnson & Johnson K.K, Tokyo) in order to stabilize it (Fig. 7A, B). The CT images at 8 years after surgery showed that the artificial bone had completely fused with the recipient bone, and the mental morphology was also stabilized (Fig. 8A–B).
Fig. 6.
Mental plasty case. (A) Retrogression of the mental region was observed in a preoperative photograph of the lateral face. (B) Cephalometric radiography (lateral view).
Fig. 7.
Intraoperative photograph. (A) The artificial bone was fixed to the recipient bone with absorbable sutures. (B) Postoperative cephalometric radiogram.
Fig. 8.
(A) Facial outcomes at 8 years after surgery. (B) 3-dimensional CT.
3.1.3. Case 13
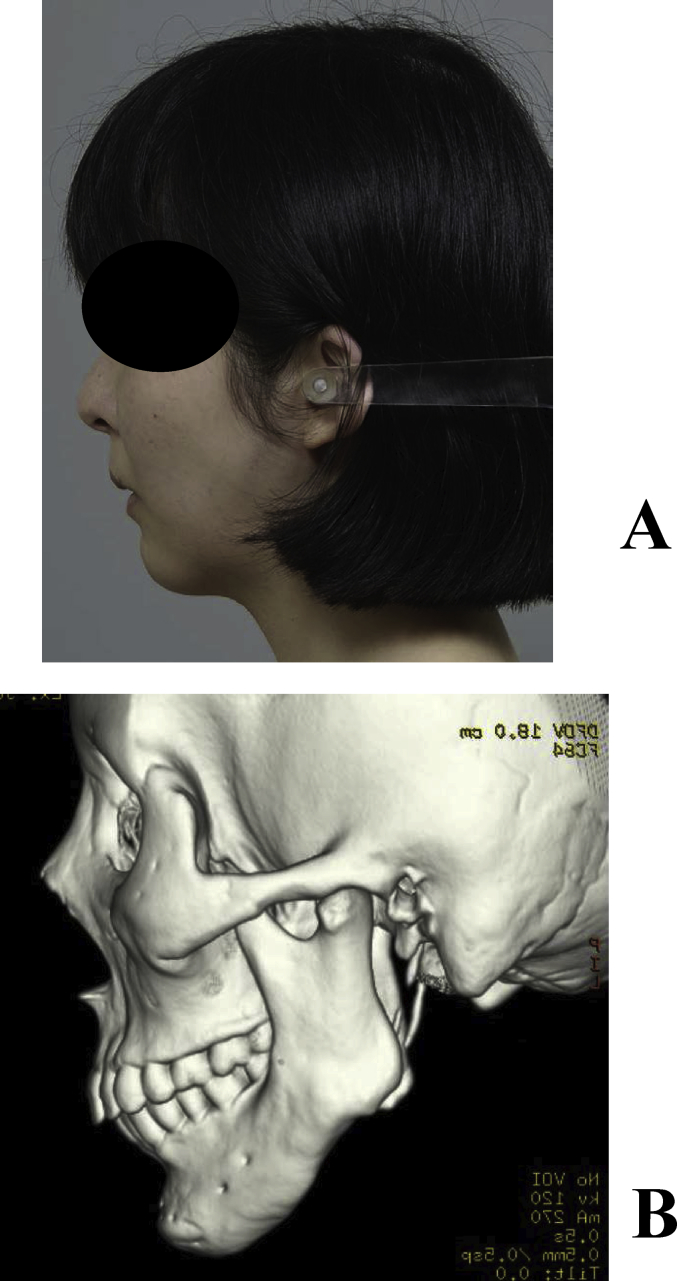
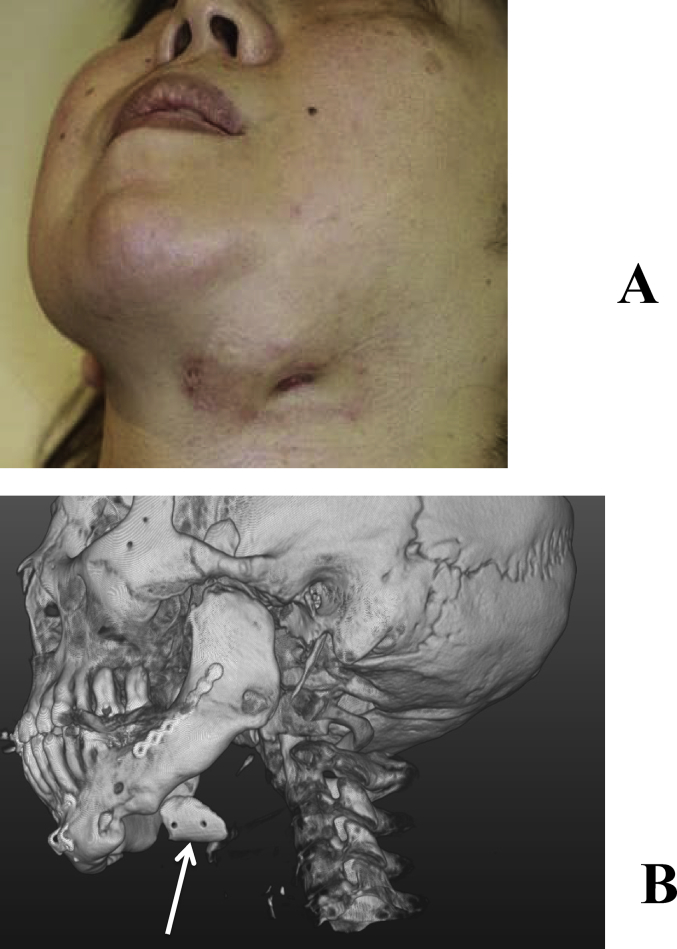
A 32-year-old female underwent temporomandibular joint mobilization at 6 and 18 years old in order to treat bilateral temporomandibular joint ankyloses, and bilateral mandibular distraction at 22 years old. Mental plasty was performed when the patient was 30 years old; however, she noted concaved bilateral mandibular bodies, and visited our department for its improvement. A close examination before surgery showed bone concavities in the distracted regions, for which mandibular plasty with full custom-made artificial bones was performed. Artificial bones were fabricated for the bilateral sides on a surgical simulation. Surgery was performed under general anesthesia through an extraoral incision. After inserting and confirming the fit, the artificial bones were fixed by sutures with absorbable 2-0 Vicryl®. Her postoperative course was favorable, and the balance of facial morphology was improved (Fig. 9A, B). However, acute apical periodontitis of the left molar developed 3 months after surgery, and a fistula formed in the gingiva. The fistula was resected, granulation tissue was curetted, the root canal was simultaneously treated, and the fistula was resolved. On imaging, the fit with the recipient bone was slightly unfavorable on the left side. The periodontal tissue condition became stabilized, and no issues with facial morphology were noted. CT images of 5 years after surgery showed the favorable fusion of the graft with the recipient bone on the right side; however, the fit on the left side was still slightly unfavorable. Since there were no subjective or objective symptoms at that time, course observations were selected (Fig. 9A–B). However, redness and swelling became apparent in the left mandibular body region approximately 6 years after surgery. As CT indicated that the artificial bone had been detached, it was removed (Fig. 10A–B).
Fig. 9.
(A) Facial outcomes at 5 years after surgery. (B) CT. The arrow indicates grafted artificial bones. The fit of the left artificial bone was not as favorable as that on the right side; however, no abnormal finding was noted in the skin or oral cavity.
Fig. 10.
(A) A fistula appeared in the skin at 5 years and 11 months after surgery. (B) CT showed that the artificial bone had completely detached from the recipient bone (arrow).
4. Discussion
Reconstruction with hard tissue is useful for treating bone defects in the craniomaxillofacial region [10]. Invasiveness cannot be avoided in reconstructions with autologous bones because they require the excision and collection of healthy bone [11]. A vascular pedicle bone flap is needed for a large bone defect, which requires not only microvascular anastomosis, but also modifications to the morphology of the graft bone in order for it to fit the recipient region [12], [13], thereby necessitating a prolonged operative time and skilled techniques. In reconstructions with allogeneic bones, in addition to the risk of unknown viral infections and associated ethical issues, modifications to the bone morphology are needed in order for it to fit to the recipient region. The advantages of artificial bones are as follows: they do not require the excision and collection of a bone graft, they have good biocompatibility, and it involves a simple and straightforward surgical procedure [14], [15]. Artificial bones currently applied worldwide are roughly divided into hydroxyapatite (HA) [16], α-TCP and β-TCP [17]. Each has its own unique characteristics, and is clinically applied based on the bone defect region [18], [19]. HA artificial bones induce new bone formation in pores by introducing collagen tissue for bone formation, and are characterized by strong affinity to biological tissues and a direct union with bone tissue [20], [21]. The mechanical strength of HA artificial bones is also comparable to that of human bones, and they are widely applied clinically to fill defects. Products in porous block, granular, and paste forms have been commercialized [22]. However, HA is unlikely to be absorbed and remains in a stable state because of its high crystallinity, i.e., HA processed by heating at a high temperature to high crystallinity is a biologically non-absorbable material [23]. TCP is absorbed and replaced by bone in the process of bone repair, while it is also superior in tissue affinity, exhibiting osseous conductivity [24]. Regarding the mechanism of new bone formation, TCP is gradually converted to hydroxyapatite in the body, its replacement with new bone by osteoblasts progresses simultaneously with artificial bone resorption [25]. Both types of TCP (α and β) have superior osseous conductivity and biocompatibility and are ultimately replaced by bone [26], [27].
The main ingredient of the artificial bone in the present study was α-TCP powder, which reacted with the curing solution to form unsintered cured TCP. Therefore, it is not only as biocompatible as HA, but is also replaced and absorbed in the body [28], [29], suggesting that grafted artificial bones can be replaced by bone in a long term.
Although the strength is inferior to sintered artificial bone, it is higher than 20 Mp, considered to be sufficient for facial bones. However, our artificial bone may not tolerate high weight-bearing sites such as the spine and crus.
In the 19 regions with stable postoperative courses, new bone formation was noted at the boundary between the grafted and recipient bones on CT images at 3–6 months after surgery. In patients who were followed-up for a longer period of time, new bone was formed near the grafted artificial bone and eventually surrounded the artificial bone, showing a characteristic imaging finding. This artificial bone may be characterized by a bone union pattern that differs from that of hydroxyapatite, which has been clinically applied, in addition to high biocompatibility. In patients in whom the artificial bone was detached or removed, a space was present between the recipient and artificial bones immediately after surgery, suggesting an ill fit as the cause of unfavorable outcomes. Even if conformity is good, from the result of this study, the fixation has the need to perform by all means in art. The fit of the artificial bone with the recipient bone in the early phase may be important for successful grafting, even for full custom-made artificial bones. In the patient whose graft was removed after 6 years, odontogenic infection was present around the grafted artificial bone, which may have disseminated to the artificial bone. Therefore, a preoperative close examination of periodontal tissue is necessary in such cases. The patient who developed an infection early after surgery was an MRSA carrier. Since MRSA was detected in the wound, it might have disseminated to the artificial bone, making it difficult to controlling the infection. Reconstructions with artificial bones are considered to be difficult in MRSA carriers, and thus they should be excluded from the indication.
We established a minimally-invasive method of reconstruction with full custom-made artificial bones that did not require modifications. These grafts were stable for a prolonged period of time and no serious adverse events occurred, thereby confirming their safety. However, even though they were custom-made, their fit to recipient bones was important. In the investigation of the long-time follow up, the need to fix an artificial bone and a recipient bone was suggested. When the fit between recipient and artificial bones is unfavorable, strict course observations may be necessary. The preparation of more precise custom-made artificial bones and development of bioactive custom-made artificial bones supplemented with growth factors are expected in order to achieve earlier and more reliable fusion with recipient bones.
5. Conclusions
We herein reconstructed facial bones with full-custom-made artificial bones made of α-TCP employing a minimally-invasive method that does not require the excision of healthy bone, and obtained favorable long-term outcomes.
Acknowledgments
This study was performed in cooperation with NEXT21 K.K. after approval by the University of Tokyo Faculty of Medicine Ethics Committee as an investigator-initiated clinical study (approval number: 1310) and clinical study (approval number: 3DB-01/CT-1).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Hideto Saijo, Email: saijyoh-ora@h.u-tokyo.ac.jp.
Yuko Fujihara, Email: fujiharay-ora@h.u-tokyo.ac.jp.
Yuki Kanno, Email: kannoy-eme@h.u-tokyo.ac.jp.
Kazuto Hoshi, Email: pochi-tky@umin.net.
Atsuhiko Hikita, Email: ahikita-tky@umin.ac.jp.
Ung-il Chung, Email: tei@bioeng.t.u-tokyo.ac.jp.
Tsuyoshi Takato, Email: takato-ora@h.u-tokyo.ac.jp.
References
- 1.Tessier P., Kawamoto H., Matthews D., Posnick J., Raulo Y., Tulasne J.F. Autogenous bone grafts and bone substitutes – tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116:6S–24S. doi: 10.1097/01.prs.0000173862.20563.12. [DOI] [PubMed] [Google Scholar]
- 2.Eppley B.L., Pietrzak W.S., Blanton M.W. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981–989. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 3.Fischer-Brandies E., Dielert E. Clinical use of tricalciumphosphate and hydroxyapatite in maxillofacial surgery. J Oral Implantol. 1985;12:40–44. [PubMed] [Google Scholar]
- 4.Nishiyama T., Nakajima T., Yoshimura Y., Nakanishi Y. Utilizing solid model for preoperative shaping of HAP-TCP ceramic bone substitute: application for craniomaxillofacial surgery. Eur J Plast Surg. 1994;17:173–177. [Google Scholar]
- 5.Eppley B.L. Craniofacial reconstruction with computer-generated HTR patient-matched implants use in primary bone tumor excision. J Craniofac Surg. 2002;13:650–657. doi: 10.1097/00001665-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Kay D.B., Njus G., Parrish W., Theken R. Basilar crescentic osteotomy. A three-dimensional computer simulation. Orthop Clin N Am. 1989;20:571–582. [PubMed] [Google Scholar]
- 7.Saijo H., Chung U.I., Igawa K., Mori Y., Chikazu D., Iino M. Clinical application of artificial bone in the maxillofacial region. J Artif Organs. 2008;11:171–176. doi: 10.1007/s10047-008-0425-4. [DOI] [PubMed] [Google Scholar]
- 8.Saijo H., Kanno Y., Mori Y., Suzuki S., Ohkubo K., Chikazu D. A novel method for designing and fabricating custom-made artificial bones. Int J Oral Maxillofac Surg. 2011;40:955–960. doi: 10.1016/j.ijom.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Takato T., Mori Y., Fujihara Y., Asawa Y., Nishizawa S., Kanazawa S. Preclinical and clinical research on bone and cartilage regenerative medicine in oral and maxillofacial region. Oral Sci Int. 2014;11:45–51. [Google Scholar]
- 10.Tada H., Hatoko M., Tanaka A., Kuwahara M., Mashiba Kumi, Yurugi S. Preshaped hydroxyapatite tricalcium-phosphate implant using three-dimensional computed tomography in the reconstruction of bone deformities of craniomaxillofacial region. J Craniofac Surg. 2002;13:287–292. doi: 10.1097/00001665-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Eppley B.L. Donor site morbidity of rib graft harvesting in primary alveolar cleft bone grafting. J Craniofac Surg. 2005;16:335–338. doi: 10.1097/00001665-200503000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Yonehara Y., Takato T., Susami T., Nakatsuka T. Large myxofibroma of the mandible treated with segmental mandibular resection and vascularized fibular graft. Ann Plast Surg. 2000;44:440–443. doi: 10.1097/00000637-200044040-00016. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsuka T., Harii K., Yamada A., Ueda K., Ebihara S., Takato T. Surgical treatment of mandibular osteoradionecrosis: versatility of the scapular osteocutaneous flap. Scand J Plast Reconstr Surg Hand Surg. 1996;30:291–298. doi: 10.3109/02844319609056407. [DOI] [PubMed] [Google Scholar]
- 14.Takato T., Fujihara Y., Hoshi K. Bone and cartilage regenerative medicine in maxillofacial region. Jpn J Oral Maxillofac Surg. 2015;61:262–269. [Google Scholar]
- 15.Ono I., Tateshita T., Satou M., Sasaki T., Matsumoto M., Kodama N. Treatment of large complex cranial bone defects by using hydroxyapatite ceramic implants. Plast Reconstr Surg. 1999;104:339–349. doi: 10.1097/00006534-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Eppley B.L. Hydroxyapatite cranioplasty: I. Experimental results from a new quick-setting material. J Craniofac Surg. 2003;14:85–88. doi: 10.1097/00001665-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa A., Matsuya S., Takeuchi A., Ishikawa K. Comparison of the effects of added alpha- and beta- tricalcium phosphate on the basic properties of apatite cement. Dent Mater J. 2007;26:342–347. doi: 10.4012/dmj.26.342. [DOI] [PubMed] [Google Scholar]
- 18.Merten H.A., Wiltfang J., Grohmann U., Hoenig J.F. Intraindividual comparative animal study of alpha- and beta-tricalcium phosphate degradation in conjunction with simultaneous insertion of dental implants. J Craniofac Surg. 2001;12:59–68. doi: 10.1097/00001665-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Hallman M., Sennerby L., Lundgren S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int J Oral Maxillofac Implant. 2002;17:635–643. [PubMed] [Google Scholar]
- 20.Hallman M., Lundgren S., Sennerby L. Histologic analysis of clinical biopsies taken 6 months and 3 years after maxillary sinus floor augmentation with 80% bovine hydroxyapatite and 20% autogenous bone mixed with fibrin glue. Clin Implant Dent Relat Res. 2001;3:87–96. doi: 10.1111/j.1708-8208.2001.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 21.Pretorius J.A., Melsen B., Nel J.C., Germishuys P.J. A histomorphometric evaluation of factors influencing the healing of bony defects surrounding implants. Int J Oral Maxillofac Implant. 2005;20:387–398. [PubMed] [Google Scholar]
- 22.Song Y., Feng Z., Wang T. In situ study on the curing process of calcium phosphate bone cement. J Mater Sci Mater Med. 2007;18:1185–1193. doi: 10.1007/s10856-007-0138-x. [DOI] [PubMed] [Google Scholar]
- 23.Eppley B.L. Craniofacial reconstruction with computer-generated HTR patient-matched implants: use in primary bony tumor excision. J Craniofac Surg. 2002;13:650–657. doi: 10.1097/00001665-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Camiré C.L., Nevsten P., Lidgren L., McCarthy I. The effect of crystallinity on strength development of alpha-TCP bone substitutes. J Biomed Mater Res B Appl Biomater. 2006;79:159–165. doi: 10.1002/jbm.b.30526. [DOI] [PubMed] [Google Scholar]
- 25.Yamada M., Shiota M., Yamashita Y., Kasugai S. Histological and histomorphometrical comparative study of the degradation and osteoconductive characteristics of alpha- and beta-tricalcium phosphate in block grafts. J Biomed Mater Res B Appl Biomater. 2007;82:139–148. doi: 10.1002/jbm.b.30715. [DOI] [PubMed] [Google Scholar]
- 26.Kihara H., Shiota M., Yamashita Y., Kasugai S. Biodegradation process of alpha-TCP particles and new bone formation in a rabbit cranial defect model. J Biomed Mater Res B Appl Biomater. 2006;79:284–291. doi: 10.1002/jbm.b.30540. [DOI] [PubMed] [Google Scholar]
- 27.Yuan H., De Bruijn J.D., Li Y., Feng J., Yang Z., Groot D. Bone formation induced by calcium phosphate ceramics in soft tissue of dogs: a comparative study between porous alpha-TCP and beta-TCP. J Mater Sci Mater Med. 2001;12:7–13. doi: 10.1023/a:1026792615665. [DOI] [PubMed] [Google Scholar]
- 28.Igawa K., Mochizuki M., Sugimori O., Shimizu K., Yamazawa K., Kawaguchi H. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J Artif Organs. 2006;9:234–240. doi: 10.1007/s10047-006-0347-y. [DOI] [PubMed] [Google Scholar]
- 29.Takato T., Fujihara Y., Hoshi K., Ogasawara T., Saijo H., Abe T. Basic and clinical research on bone and cartilage regenerative medicine in the oral and maxillofacial region. J Jpn Stomatol Soc. 2014;63:207–215. [Google Scholar]