Abstract
Cell surface engineering using single-stranded DNA–poly(ethylene glycol)-conjugated phospholipid (ssDNA–PEG-lipid) is useful for inducing cell–cell attachment two and three dimensionally. In this review, we summarize our recent techniques for cell surface engineering and their applications to islet transplantation. Because any DNA sequence can be immobilized onto the cell surface by hydrophobic interactions between ssDNA–PEG-lipid and the cellular membrane without impairing cell function, a cell–cell hybrid can be formed through the DNA hybridization. With this technique, it would be possible to create three-dimensional hybrid structures of pancreatic islets coated with various accessory cells, such as patients’ own cells, mesenchymal and adipose-derived stem cells, endothelial progenitor cells, neural crest stem cells or regulatory T cells, which might significantly improve the outcome of islet transplantation in diabetic patients.
Keywords: Cell surface modification, PEG-conjugated phospholipid (PEG-lipid), Pancreatic islet, Instant blood-mediated inflammatory reaction (IBMIR), Diabetes
Abbreviations: PEG-lipid, poly(ethylene glycol)-conjugated phospholipid; IBMIR, instant blood-mediated inflammatory reaction; islets, islets of Langerhans; PMPC, poly(2-methacryloyloxyethyl phosphorylcholine)
Highlights
-
•
DNA can be immobilized onto cell by DNA–PEG-lipid to induce cell–cell attachment.
-
•
Cell surface engineering with DNA–PEG-lipid can develop 3D cell hybrid.
-
•
3D hybrid of pancreatic islet and cells can be potential for type 1 diabetes cure.
1. Introduction
Diabetes is characterized by hyperglycemia due to an absolute or relative lack of insulin to cover the metabolic needs of the body. The disease is commonly divided in type 1 and type 2 diabetes but its etiology and pathogenesis is quite heterogeneous. A common denominator is, however, the loss of functional insulin producing cell (beta-cell) mass. This is caused in by immunological mechanisms in type 1 diabetes and is probably inherent when exposed to external stress in type 2 diabetes. Exogenous insulin therapy cannot approximate normal physiological pulsatile insulin secretory patterns with complete integrity and rarely attains normal blood glucose levels without the risk of major hypoglycemic episodes and devastating complications including retinopathy, nephropathy, and neuropathy; therefore, more effective therapy needs to be established.
Presently, diabetes can neither be prevented nor cured by other means that cell replacement including pancreas and pancreatic islet transplantation. Islets are aggregates of 1000–2000 endocrine cells (including beta-cells) that form cell clusters of up to 300 μm within the pancreas. For clinical islet transplantation, these cells are isolated from the pancreases of a few brain-dead donors and infused into the liver via the portal vein of diabetic recipients or their body. Because the procedure is less invasive to patients, this treatment is very promising, and various related clinical reports have been published since the beginning of the 1970s [1], [2], [3]. However, recipients must take immune-suppressive drugs to protect grafts from immune rejection.
In addition, the first days after transplantation are characterized by dynamic changes resulting in substantial early cell death and dysfunction due to multiple factors including insufficient graft revascularization [4], and re-innervation [5], alloimmune rejection and recurrence or persistence of autoimmunity [6], toxicity of immunosuppressive regimens [7], liver ischemia with subsequent cytotoxicity [8] and inflammatory reactions. Exposure of the islet surface to recipient blood activates blood coagulation and a complement response, which subsequently induces inflammation after infusion into the liver [9], [10], [11]. This series of reactions, is recognized as instant blood-mediated inflammatory reaction (IBMIR), leads to immediate islet destruction immediately after intraportal transplantation [12]. Despite intense scientific efforts, this issue still remains unresolved in clinical islet transplantation. Several studies have been conducted to examine ways to protect islets from IBMIR using systemic administration of anticoagulants, anti-thrombin inhibitors, melagatran [13], low-molecular weight dextran sulfate [14], and some complement inhibitors [9], [15]. However, systemic administration is always associated with a bleeding risk. Alternatively, our group has examined immobilization of bioactive substances and living functional cells onto the islet surface, which could provide local regulation of unfavorable reactions [16], [17], [18], [19], [20]. By using this technique the risk of bleeding, associated with systemic modulation of coagulation and complement after intraportal islet transplantation would be avoided. In preclinical studies, co-transplantation of islets of Langerhans with accessory non-islet cells, such as mesenchymal and adipose-derived stem cells [21], [22], [23], [24], endothelial progenitor cells [25], [26], [27], neural crest stem cells [28], [29] or regulatory T cells [30], has been show to improve the outcome of islet transplantation. Thanks to their pleiotropic effects, including angiogenic, anti-apoptotic and immunomodulatory effects, these cells might prove to be superior compared to drug-based approaches that often target single components of islet graft failure. In particular, our group has already shown that coating of the islet surface with endothelial cells has the potential to significantly inhibit IBMIR completely because endothelial cells express regulators for coagulation and complement systems and the exposed surface can mimic the endothelium of the recipient. In fact, our group has already published some promising results [31].
Co-transplantation of islets and other cells thus can be an alternative to the surface-modification approach. However, the hybrid of islets and other cells is not easy to achieve because cell–cell attachment cannot be induced without general cadherin–cadherin interactions. Although interaction with collagen on the islet surface can be available for attaching endothelial cells, an engineering approach should be established to expand this idea to the use of various functional cells. To address this issue, we have used a cell surface-modification technique with single-stranded DNA–PEG-conjugated phospholipid (ssDNA–PEG-lipid), which enabled us to induce cell–cell attachment two dimensionally (2D) and three dimensionally (3D). In this review, we summarize our recent techniques for cell surface engineering and their applications to islets transplantation.
2. Immobilization of ssDNA on the cell surface by hydrophobic interactions
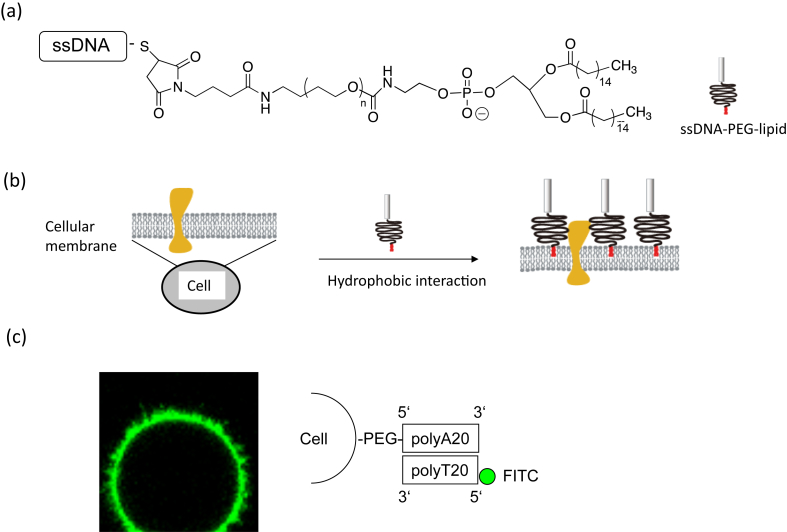
ssDNA can be immobilized on the living cell surface without influencing cell viability by using an amphiphilic polymer, PEG-conjugated phospholipid (PEG-lipid), which consists of both a hydrophilic domain (PEG) and a hydrophobic domain (lipid) (Fig. 1A) [19], [32], [33]. For this purpose, ssDNA–PEG-lipid is used where any sequence of DNA is available for the conjugation [34]. When the lipid domain of ssDNA–PEG-lipids is spontaneously incorporated into the lipid bilayer membrane by hydrophobic interactions, the hydrophilic ssDNA–PEG domain is displayed on the cell surface (Fig. 1B). Here the molecular weight of PEG of ssDNA–PEG-lipids was 5 kD. The role of the PEG is a spacer for anchoring ssDNA on cell surface. Thus, it is possible to immobilize ssDNA on the cell surface. Although ssDNA–PEG-lipids are incorporated into the cellular membrane, there was no cytotoxicity for primary cells, cell lines, and islets after the cell surface modification. In addition, this surface modification of islets with ssDNA–PEG-lipids does not impair insulin secretion ability from the insulin release assay [20]. These results indicated that our approach with ssDNA–PEG-lipids did not influence cellular function.
Fig. 1.
Amphiphilic polymers employed for cell surface modifications. (a) Chemical structures of amphiphilic polymers: polyethylene glycol-conjugated phospholipid (PEG-lipid). (b) Cell surface modification by hydrophobic interaction. Cell surfaces can be modified with PEG-lipid that interacts with the membrane through hydrophobic interactions. (c) Confocal laser scanning microscopy image of a CCRF-CEM cell that was modified with polyA20–PEG-lipids and then reacted with FITC-polyT20.
One approach is the use of polyA20–PEG-lipid and polyT20–PEG-lipid for attaching different cells through DNA hybridization. Because the hybridization between polyA20 and polyT20 is a rapid and specific reaction, it is easy to design 2D and 3D cell organization. Addition of ssDNA–PEG-lipid solution to the cell suspension and incubation at room temperature for 30 min leads to modification of the cell surface with ssDNA–PEG-lipid. To examine the existence of ssDNA (i.e., polyA20) on the cell surface, FITC-labeled complementary ssDNA′ (FITC-polyT20) is used (Fig. 1C). Clear fluorescence from FITC-polyT20 is observed only on the cell surface, which is treated with polyA20–PEG-lipid, indicating the immobilization of polyA20–PEG-lipid. Of importance, the whole cell surface is uniformly covered with polyA20–PEG-lipid. Actually, there are lots of membrane proteins existing on cellular membranes. Since ssDNA–PEG-lipids are hydrophobically interactive with lipid bilayer membrane domains, they are separately located on the cell membrane and available for DNA hybridization. Therefore, using ssDNA–PEG-lipid makes it possible to immobilize any DNA sequence on the cell surface for further reactions that lead to 2D and 3D cell organization. Multiple membrane proteins and glycocalyx components such as glycoproteins and glycolipids cover the cell membrane. The combined thickness of this layer is assumed to be up to several hundred nanometers. When the cell membrane is modified with ssDNA–PEG-lipids with 5 kD of PEG, they are surrounded with membrane proteins and glycocalyx, where ssDNAs are presumably located at the lower position than various membrane proteins. However, ssDNA molecules on the cell membrane can be accessible to the complementary DNAs when they are added. Flexible PEG chain might be useful for the reaction.
3. 2D cell alignment by ssDNA–PEG-lipid
To align cells on the substrate in a patterned way, various approaches have been reported. Usually, extracellular matrix such as fibronectin, vitronectin, or RGD peptide is immobilized onto the specific area, which allows cells to adhere selectively. Although the initial cell attachment is controlled by this approach, cells gradually migrate freely on the substrate because of the non-specific binding of extracellular matrix onto the substrate from serum in culture medium. Therefore, the surface treatment with PEG or poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) [35] is usually necessary to repel the non-specific protein adsorption and cell attachment. For the patterning of extracellular matrix on the substrate surface, a micro contact-printing method and photolithography have been used [36], [37], [38], [39]. Poly(dimethyl siloxane) is often used in the micro contact-printing method to produce a stamp with a pattern, in which extracellular matrix dipped onto the stamp can be printed to the substrate. For photolithography, the cell adhesion area and non-cell adhesion area can be patterned by UV irradiation through a photomask with micro-patterning.
With these approaches, adherent cells can be aligned in 2D via integrins, i.e. an interactive extracellular matrix, while floating cells cannot. In addition, one single cell type can be successfully aligned with these methods but more than two types of cells cannot be patterned separately as all adherent cells use the same integrins to bind extracellular matrix. Another approach is the use of antibodies against membrane proteins that are specific for each cell. Although available antibodies are usually limited, this approach might be effective to align several kinds of cells on the substrate surface.
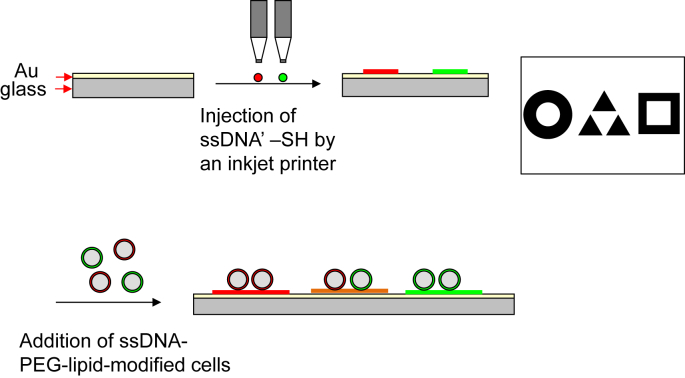
The use of ssDNA immobilized on the cell surface is also available for patterning of cells. The advantage of using ssDNA is the possibility of many combinations. In addition, any kind of cells—adherent cells and floating cells—can be applied due to the fact that DNA hybridization is specific. Here we show some results of 2D cell patterning with ssDNA–PEG-lipid. First, we prepared the complementary sequence of DNA with thiolation (ssDNA′-SH) and printed onto a gold-coated glass surface (Fig. 2). For printing ssDNA′-SH, a micro contact-printing method and photolithography are available as described above. It is possible to use an inkjet printer as well. Because the gold–thiol reaction is rapid and proceeds under mild conditions, DNA is printed easily and stably with the desired patterns. In addition, immobilized DNA is not denatured under dry conditions, so it is convenient to use. Second, the complementary sequence ssDNA is conjugated to maleimide–PEG-lipid to prepare ssDNA–PEG-lipid, and cells are then treated with ssDNA–PEG-lipid. Finally, those treated cells are seeded to the ssDNA′-patterned surface for immobilization of cells through DNA hybridization [34]. As multiple DNA hybridization reactions take place simultaneously, cell immobilization reactions on the substrate are rapid. Once the treated cells contact the substrate, cell immobilization is immediately completed. Although attached adherent cells move to non-DNA-immobilized area eventually without blocking treatment, the initial attachment of cells could be easily controlled by this approach. When cells are cultured on patterned areas, the surface treatment with PEG or PMPC could be useful to repel the non-specific protein adsorption and cell attachment.
Fig. 2.
Schematic illustration of a method for cell immobilization on a pattern printed in DNA. First, immobilization of DNA with a specific sequence on the cell surface was done with DNA–PEG-lipids. Second, printing a pattern with DNA′-SH was performed by an inkjet printer. The cells modified by DNA–PEG-lipid were applied to the substrate and immobilized on the pattern.
An example of a cell-printed pattern using an inkjet printer has been described [40]. In this case, a solution of polyT20-SH was injected through the nozzle of an inkjet printer to draw the logo of a university. Then, cells treated with polyA20–PEG-lipid were added to the substrate (Fig. 2). There is also an example of patterning with two kinds of cells using two DNA sequences. The area where SeqA′-SH or SeqB′-SH is injected exhibits selective binding of SeqA-cells or SeqB-cells from the cell mixture. In addition, both SeqA-cells and SeqB-cells are immobilized on the area with both SeqA′-SH and SeqB′-SH injection.
4. 3D alignment of cells by ssDNA–PEG-lipid
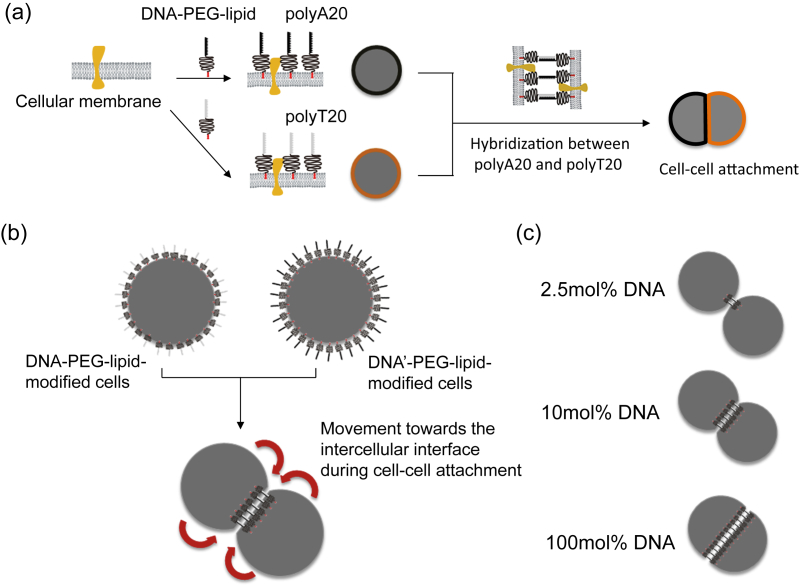
Above, we gave some examples of 2D cell alignment using ssDNA–PEG-lipid. With this DNA hybridization technique, it is also possible to elicit 3D cell organization [34] (Fig. 3). Here, one cell is treated with polyA20–PEG-lipids, and the other is treated with polyT20–PEG-lipids. These two modified cells then can be attached through DNA hybridization. During the cell–cell attachment process, polyA20–PEG-lipids and polyT20–PEG-lipids on the cell surface move towards the intercellular interface between two cells by lateral diffusion and form the DNA hybridization. The DNA hybridization proceeds at the intercellular interface with time [41]. Therefore, the total amount of ssDNA–PEG-lipids on the cell surface is the limiting factor for cell–cell attachment. When a low number of ssDNA–PEG-lipids is present, the cell–cell attachment is weak because the intercellular interface is limited. On the other hand, when cells are treated with a higher number of ssDNA–PEG-lipids, the cell–cell attachment is strong, and most of the ssDNA–PEG-lipids contribute to the DNA hybridization at the interface to strengthen the attachment. Thus, it is possible to elicit 3D cell organization with this technique.
Fig. 3.
(a) Schematic illustration of cell surface modification with ssDNA–PEG-lipid by hydrophobic interaction and of cell–cell attachment by DNA hybridization. Cells were modified with polyA20–PEG-lipid and polyT20–PEG-lipid and then mixed to induce attachment. (b) During the cell–cell attachment, ssDNA–PEG-lipid on the cell surface moved towards the interface between the two cells and elicited DNA hybridization, which induced the cell–cell attachment. (c) Influence of the shape of cells attached by DNA hybridization with different ssDNA ratios to PEG-lipid without DNA: 2.5, 10, and 100 mol%. With an increased ssDNA ratio on the cell surface, the cell–cell attachment strengthened with the larger contact area.
5. 3D hybrid of pancreatic islets with living cells
Here we introduce an example of a 3D hybrid of pancreatic islets with living cells produced by DNA hybridization using ssDNA–PEG-lipids. Such an approach could significantly improve islet transplantation outcomes by modulation of multiple processes important for islet graft survival, including revascularization, local immunomodulation and apoptosis.
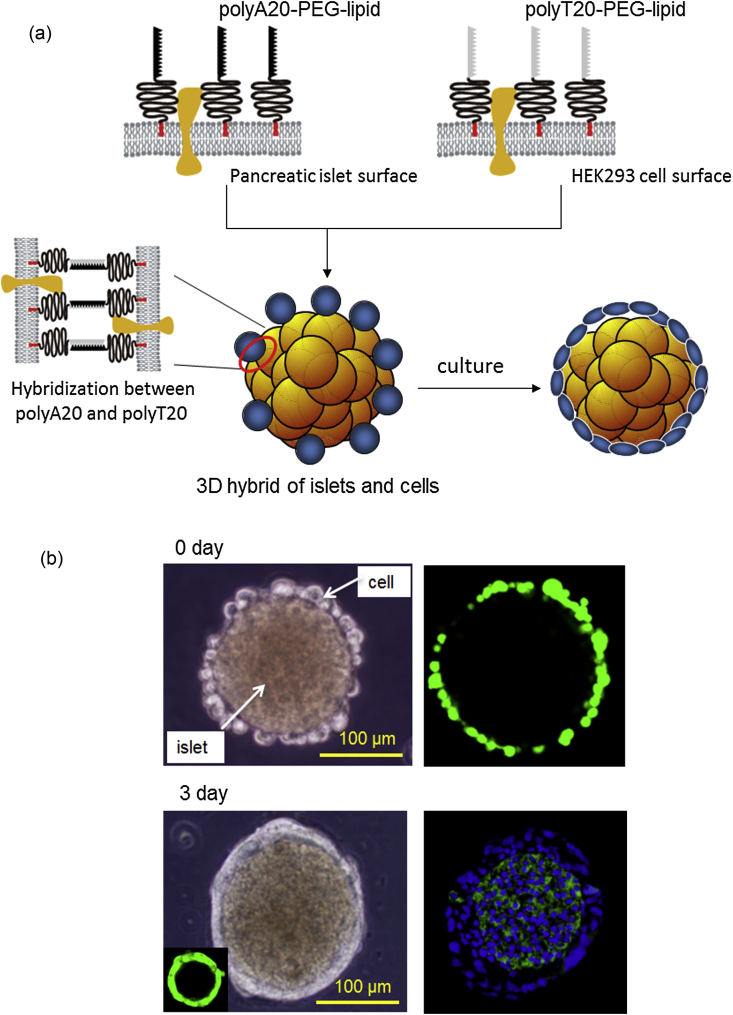
In this case, a complementary pair of polyA20–PEG-lipid and polyT20–PEG-lipid can be used to form the 3D hybrid of islet and non-islet derived cells [34], [42] (Fig. 4). The surfaces of accessory cells are treated with polyT20–PEG-lipid, and the surfaces of islets are modified with polyA20–PEG-lipids for the attachment. When the islets modified with polyA20–PEG-lipid are mixed with cells treated with polyT20–PEG-lipid, accessory cells are attached to the islet surface through the hybridization of the polyA20 and polyT20. We used the human endoderm kidney cell line HEK293 for immobilization on mouse islets with polyT20–PEG-lipid and polyA20–PEG-lipid. Of interest, when the 3D hybrid of islet and living cells was cultured, the attached cells proliferated on the islet surface without detaching from it. The attached cells initially spread at 1 day and proliferated eventually onto islets surface. During the suspension culture for 2–3 days, a layer of HEK293 cells was formed on the islet surface where the whole islet surface was fully covered with cell layer, with no central necrosis inside the islet surrounded by living cells, based on immune staining. The thickness of the cell layer is approximately 10 μm [42].
Fig. 4.
A 3D hybrid of pancreatic islets and living cells created via DNA hybridization. (a) Schematic illustration of the coating of an islet within living cells. Both the cell and islet surfaces are modified with polyDNA. polyT20–PEG-lipid is immobilized on living HEK293 cells, and polyA20–PEG-lipid is immobilized on the islet surface. During mixing of the modified cells and islets, DNA hybridization causes the attachment of HEK293 cells onto the islet surfaces. After several days of culture, HEK293 proliferation encloses the islet within a cellular capsule. (b) Phase contrast microscopy and fluorescence microscopy of islets with attached HEK293 cells. At 0 day, GFP-HEK293 cells immobilized to islets were observed with confocal laser-scanning microscopy, and after 3 day, frozen sections of islets with attached GFP-HEK293 cells were stained with Alexa488-labeled anti-insulin antibody (green) and Hoechst 33342 dye (blue) for nuclear staining (Partially modified from Ref. [42]).
Moreover, HEK293-coated islets where positive for insulin and able to respond by increasing insulin secretion following glucose challenge. However, insulin secretion was reduced compared to that observed in control non-coated islets. Although HEK293 cells are a cell line, and therefore with no direct relevance for a clinical perspective, these results demonstrate that the 3D-hybridization approach proposed here can lead to a complete cell coating of islets with non-islet cells without significantly inhibiting function. This method may lead to a clinical procedure for encapsulating isolated pancreatic islets with accessory cells able to significantly enhance graft survival in patients with type 1 diabetes.
6. Conclusions and outlook
The shortage of human donors is a major problem in transplantation therapy but some day may not be a serious one because functional cells, tissues, and organs could be available from embryonic stem cells or induced pluripotent stem cells in the near future [43]. Functional cells with simple roles can be transplanted directly into patients; however, complicated functions such as those of liver, heart, and kidney cells are not as straightforward to replicate because both cell function and 3D structure are important. Thus, the reorganization of various cells by engineering approaches is critical. Cell surface engineering with ssDNA–PEG-lipids makes it possible to achieve 2D and 3D alignment of cells for further functionalization. The hybrid cellular complex may be promising for producing complicated 3D structures in regenerative medicine using stem cells. Furthermore, with our surface modification technique, solid organ can also be functionalized with living cells as seen in 3D hybrid of pancreatic islets and cells. For instance, damaged tissues or endothelial cells in solid organ can be replaced or repaired by new cells, which are modified with PEG-lipid derivatives. Thus, cell surface modification can be available in various transplantation therapies.
Conformal coating or thin polymer membrane coating of islets based on surface modification is promising in clinical islet transplantation because there is no volume increase after coating and any transplantation site in human body is available as non-coated islets, which is advantage over microencapsulated and macroencapsulated islets using hydrogels and devices. On the other hand, the membrane stability is not enough to suppress immune rejection reactions for the long time. The more stable polymer membrane will be necessary before the clinical use.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Young Scientists (A) (No. 26702017) and a Grant-in-Aid for Scientific Research on Innovative Areas “Bio Assembler” (No. 26106709) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan and by the Bilateral Joint Research Projects (Japan–Sweden) of the Japan Society for the Promotion of Science (JSPS) and the Swedish Foundation for International Cooperation in Research and Higher Education (STINT).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Ballinger W.F., Lacy P.E. Transplantation of intact pancreatic-islets in rats. Surgery. 1972;72:175–186. [PubMed] [Google Scholar]
- 2.Ryan E.A., Lakey J.R., Rajotte R.V., Korbutt G.S., Kin T., Imes S. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson P.O., Palm F., Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87:5418–5423. doi: 10.1210/jc.2002-020728. [DOI] [PubMed] [Google Scholar]
- 5.Korsgren O., Jansson L., Andersson A., Sundler F. Reinnervation of transplanted pancreatic islets. A comparison among islets implanted into the kidney, spleen, and liver. Transplantation. 1993;56:138–143. [PubMed] [Google Scholar]
- 6.Roep B.O., Stobbe I., Duinkerken G., van Rood J.J., Lernmark A., Keymeulen B. Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes. 1999;48:484–490. doi: 10.2337/diabetes.48.3.484. [DOI] [PubMed] [Google Scholar]
- 7.Desai N.M., Goss J.A., Deng S., Wolf B.A., Markmann E., Palanjian M. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76:1623–1625. doi: 10.1097/01.TP.0000081043.23751.81. [DOI] [PubMed] [Google Scholar]
- 8.Yin D., Ding J.W., Shen J., Ma L., Hara M., Chong A.S. Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6:60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 9.Bennet W., Sundberg B., Groth C.G., Brendel M.D., Brandhorst D., Brandhorst H. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 10.Bennet W., Sundberg B., Lundgren T., Tibell A., Groth C.G., Richards A. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69:711–719. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Moberg L., Johansson H., Lukinius A., Berne C., Foss A., Kallen R. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 12.Naziruddin B., Iwahashi S., Kanak M.A., Takita M., Itoh T., Levy M.F. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transpl. 2014;14:428–437. doi: 10.1111/ajt.12558. [DOI] [PubMed] [Google Scholar]
- 13.Ozmen L., Ekdahl K.N., Elgue G., Larsson R., Korsgren O., Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 14.Goto M., Johansson H., Maeda A., Elgue G., Korsgren O., Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77:741–747. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 15.Goto M., Tjernberg J., Dufrane D., Elgue G., Brandhorst D., Ekdahl K.N. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15:225–234. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H., Teramura Y., Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)-conjugated phospholipid. J Control Release. 2011;150:229–234. doi: 10.1016/j.jconrel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Luan N.M., Teramura Y., Iwata H. Immobilization of soluble complement receptor 1 on islets. Biomaterials. 2011;32:4539–4545. doi: 10.1016/j.biomaterials.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Takemoto N., Teramura Y., Iwata H. Islet surface modification with urokinase through DNA hybridization. Bioconjug Chem. 2011;22:673–678. doi: 10.1021/bc100453r. [DOI] [PubMed] [Google Scholar]
- 19.Teramura Y., Iwata H. Islets surface modification prevents blood-mediated inflammatory responses. Bioconjug Chem. 2008;19:1389–1395. doi: 10.1021/bc800064t. [DOI] [PubMed] [Google Scholar]
- 20.Teramura Y., Iwata H. Improvement of graft survival by surface modification with poly(ethylene glycol)-lipid and urokinase in intraportal islet transplantation. Transplantation. 2011;91:271–278. doi: 10.1097/tp.0b013e3182034fa4. [DOI] [PubMed] [Google Scholar]
- 21.Berman D.M., Willman M.A., Han D., Kleiner G., Kenyon N.M., Cabrera O. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558–2568. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg D.J., Weigelt M., Wilhelm C., Gerlach M., Bickle M., Speier S. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia. 2014;57:522–531. doi: 10.1007/s00125-013-3109-4. [DOI] [PubMed] [Google Scholar]
- 23.Ohmura Y., Tanemura M., Kawaguchi N., Machida T., Tanida T., Deguchi T. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation. 2010;90:1366–1373. doi: 10.1097/TP.0b013e3181ffba31. [DOI] [PubMed] [Google Scholar]
- 24.Rackham C.L., Chagastelles P.C., Nardi N.B., Hauge-Evans A.C., Jones P.M., King A.J. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011;54:1127–1135. doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 25.Kang S., Park H.S., Jo A., Hong S.H., Lee H.N., Lee Y.Y. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes. 2012;61:866–876. doi: 10.2337/db10-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.I., Yu J.E., Lee S.Y., Sul A.Y., Jang M.S., Rashid M.A. The effect of composite pig islet–human endothelial cell grafts on the instant blood-mediated inflammatory reaction. Cell Transplant. 2009;18:31–37. doi: 10.3727/096368909788237113. [DOI] [PubMed] [Google Scholar]
- 27.Penko D., Rojas-Canales D., Mohanasundaram D., Peiris H.S., Sun W.Y., Drogemuller C.J. Endothelial progenitor cells enhance islet engraftment, influence beta-cell function, and modulate islet connexin 36 expression. Cell Transplant. 2015;24:37–48. doi: 10.3727/096368913X673423. [DOI] [PubMed] [Google Scholar]
- 28.Grapensparr L., Vasylovska S., Li Z., Olerud J., Jansson L., Kozlova E. Co-transplantation of human pancreatic islets with post-migratory neural crest stem cells increases beta-cell proliferation and vascular and neural regrowth. J Clin Endocrinol Metab. 2015;100:E583–E590. doi: 10.1210/jc.2014-4070. [DOI] [PubMed] [Google Scholar]
- 29.Olerud J., Kanaykina N., Vasylovska S., King D., Sandberg M., Jansson L. Neural crest stem cells increase beta cell proliferation and improve islet function in co-transplanted murine pancreatic islets. Diabetologia. 2009;52:2594–2601. doi: 10.1007/s00125-009-1544-z. [DOI] [PubMed] [Google Scholar]
- 30.Takemoto N., Konagaya S., Kuwabara R., Iwata H. Coaggregates of regulatory T cells and islet cells allow long-term graft survival in liver without immunosuppression. Transplantation. 2015;99:942–947. doi: 10.1097/TP.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 31.Johansson U., Elgue G., Nilsson B., Korsgren O. Composite islet–endothelial cell grafts: a novel approach to counteract innate immunity in islet transplantation. Am J Transpl. 2005;5:2632–2639. doi: 10.1111/j.1600-6143.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 32.Miura S., Teramura Y., Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)–phospholipids anchored to cell membrane. Biomaterials. 2006;27:5828–5835. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 33.Teramura Y., Iwata H. Cell surface modification with polymers for biomedical studies. Soft Matter. 2010;6:1081–1091. [Google Scholar]
- 34.Teramura Y., Chen H., Kawamoto T., Iwata H. Control of cell attachment through polyDNA hybridization. Biomaterials. 2010;31:2229–2235. doi: 10.1016/j.biomaterials.2009.11.098. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara K., Aragaki R., Ueda T., Watenabe A., Nakabayashi N. Reduced thrombogenicity of polymers having phospholipid polar groups. J Biomed Mater Res. 1990;24:1069–1077. doi: 10.1002/jbm.820240809. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi F., Okada M., Kato K., Jakt L.M., Iwata H. Array-based functional screening for genes that regulate vascular endothelial differentiation of Flk1-positive progenitors derived from embryonic stem cells. Biochim Biophys Acta. 2007;1770:1085–1097. doi: 10.1016/j.bbagen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Hu J., Shi J., Zhang F., Lei L., Li X., Wang L. High resolution and hybrid patterning for single cell attachment. Microelectron Eng. 2010;87:726–729. [Google Scholar]
- 38.Ingham C., Bomer J., Sprenkels A., van den Berg A., de Vos W., van Hylckama Vlieg J. High-resolution microcontact printing and transfer of massive arrays of microorganisms on planar and compartmentalized nanoporous aluminium oxide. Lab Chip. 2010;10:1410–1416. doi: 10.1039/b925796a. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki H., Maki T., Kato K., Iwata H. Surface-displayed antibodies as a tool for simultaneously controlling the arrangement and morphology of multiple cell types with microscale precision. ACS Appl Mater Interf. 2009;1:53–55. doi: 10.1021/am800147x. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai K., Teramura Y., Iwata H. Cells immobilized on patterns printed in DNA by an inkjet printer. Biomaterials. 2011;32:3596–3602. doi: 10.1016/j.biomaterials.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 41.Teramura Y. Cell surface modification with ssDNA–PEG-lipid for analysing intercellular interactions between different cells. Biomaterials. 2015;48:119–128. doi: 10.1016/j.biomaterials.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 42.Teramura Y., Minh L.N., Kawamoto T., Iwata H. Microencapsulation of islets with living cells using polyDNA–PEG-lipid conjugate. Bioconjug Chem. 2010;21:792–796. doi: 10.1021/bc900494x. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]