Abstract
The development of biologically relevant three-dimensional (3D) tissue constructs is essential for the alternative methods of organ transplantation in regenerative medicine, as well as the development of improved drug discovery assays. Recent technological advances in hydrogel microfabrication, such as micromolding, 3D bioprinting, photolithography, and stereolithography, have led to the production of 3D tissue constructs that exhibit biological functions with precise 3D microstructures. Furthermore, microfluidics technology has enabled the development of the perfusion culture of 3D tissue constructs with vascular networks. In this review, we present these hydrogel microfabrication technologies for the in vitro reconstruction and cultivation of 3D tissues. Additionally, we discuss current challenges and future perspectives of 3D tissue engineering.
Keywords: Hydrogels, Microfabrication, Tissue engineering, Microfluidics, Perfusion
Highlights
-
•
The overview of recent technological advances in hydrogel microfabrication.
-
•
Hydrogel microfabrication technologies in 3D tissue engineering.
-
•
The usability of microfluidics combined with hydrogel microfabrication.
1. Introduction
A multicellular three-dimensional (3D) cell culture model in a collagen hydrogel prior to implantation was constructed in the 1990s [1], with the aim of repairing vascular tissues using hydrogels with encapsulated cells. Over the past several decades, in vitro tissue model reconstruction in tissue engineering relied on hydrogels to mimic native tissue, owing to the biocompatibility of hydrogels, their ability to encapsulate bioactive molecules and cells, and the efficient mass transfer by diffusion [2]. The hydrogels composed of natural materials, including collagen, alginate, gelatin, hyaluronic acid, chitosan, and fibrin, are useful for the investigations of cell–cell and cell–extracellular matrix (ECM) interactions as well [3], [4]. Although these hydrogels provide a microenvironment that chemically mimics cell–cell and cell–ECM interactions, they may lack an appropriate mechanical strength. In order to improve the mechanical properties of hydrogels, synthetic polymers, including poly(vinyl alcohol) (PVA), poly(ethylene glycol) (PEG), and poly(lactic-co-glycolic acid) (PLGA), have been widely used [2], [3]. Numerous strategies have been developed in order to alter the biochemical and mechanical properties of the hydrogels. For example, ECM proteins (e.g., collagen, fibronectin, and laminin) and/or their functional peptide sequences, may be chemically incorporated into hydrogels to prompt the cells to adhere to the surface of a hydrogel [5]. The mechanical strength of hydrogels is often adjusted by controlling the cross-linking density.
A key requirement for the replication of functional organs and tissues is a comprehensive knowledge of the organization and composition of their components, based on the in vivo model, and the desirable 3D microstructure in the reconstructed tissue. Recent advances in the field of tissue engineering have been based on the precise 3D microfabrication technologies, such as micromolding, 3D bioprinting, photolithography, and stereolithography [6]. These technologies allow the fabrication of precise 3D architectures at the micron scale. Additionally, microfluidics technology has been used for the fabrication of building blocks for 3D tissue engineering, while the medical imaging technologies are attractive systems for the design of 3D tissue constructs, and they include X-ray computed tomography (CT), and magnetic resonance imaging (MRI). The architectural parameters can be designed by the application of computer-aided design (CAD), using the captured 3D image of the normal tissue. Furthermore, microfluidics technologies [7] offer an attractive platform for the enhancement of the biological functions of 3D tissues. The combination of the existing biomaterial [8], microfabrication, and microfluidics approaches has an excellent potential for the reconstruction of large organ models in the future. Here, we provide an overview of these microfabrication and microfluidics technologies using hydrogels, in 3D tissue model engineering.
2. Hydrogel microfabrication in tissue engineering
Hydrogel microfabrication technologies in tissue engineering have been extensively reviewed [9]. These technologies include micromolding, 3D bioprinting [10], [11], photolithography [12], and microfluidics [13], [14]. Here, we focus on hydrogel microfabrication, and highlight the abundance of recent studies in the field of tissue engineering. These approaches provide different advantages or disadvantages in the selection of material, complexity of the 3D architecture, resolution, damage to the cells, and fabrication speed, and we have taken into consideration these properties and compared them.
2.1. Micromolding
Various micromolding approaches for the fabrication of 3D tissue constructs have been reported. Most of the other microfabrication technologies are limited by the selection of suitable materials for each fabrication process, and this suitability depends on their physicochemical properties. The micromolding approach allows this limitation to be overcome, while offering the advantages of short processing time and easy-to-use procedures. In this technique, elastomers, such as polydimethylsiloxane (PDMS) and poly(methyl methacrylate) (PMMA), have been employed as templates for the creation of tissue constructs. Although alginate and poly l-lactic acid based polymers are often used as sacrificial hydrogels for the fabrication of complex structures [15], there are no technical limitations for the use of other materials as templates, and numerous materials, including sugar [16] and gelatin [17], have also been used to create microvascular networks in hydrogels.
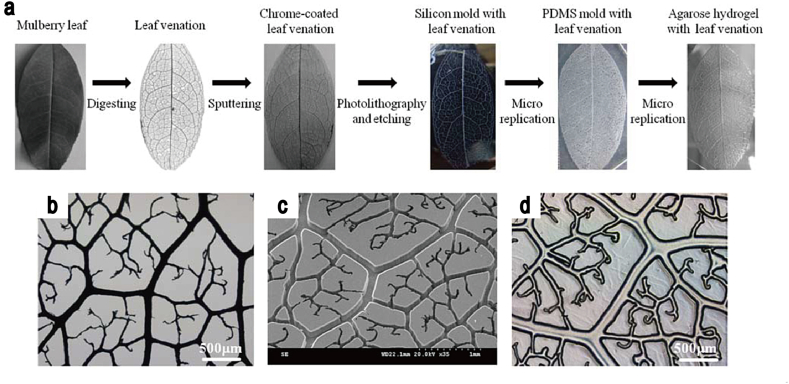
In a recent report [18], He et al. demonstrated the use of a sputtered natural leaf as a replica mold for the fabrication of a microvascular network in agarose hydrogels (Fig. 1). The layer-by-layer process allowed the fabrication of complex 3D structures. Minimizing the processing time for the fabrication of the desired structures presents a key challenge in the micromolding approach. A rapid micromolding, powered by an electrochemical cellular detachment, was performed by Seto et al. [19], who demonstrated that a microvascular 3D capillary-like structure can be created using this procedure, while providing the homogeneous cell adhesion inside the capillary structure [20]. Providing a perfusable platform for cell culturing inside the capillary structures, in order to enhance their biological functions present in normal tissues represents an additional challenge. In order to provide this platform, PEG diacrylate-based hydrogels with capillary structures were fabricated in a PDMS device by Cuchiara et al. [21], [22]. These techniques allowed for the fabrication of perfusable hydrogel networks independent of overall scaffold geometry. Additional examples of a microfluidic approach for the development of a perfusion culture are outlined in the subsequent section on microfluidics.
Fig. 1.
The production of nature-inspired perfusable microfluidic network in the hydrogels, using micromolding technique. (a–c) Fabrication of agarose gel micromold using leaves. (d) The fabricated 3D perfusable structure in the hydrogel.
Source: He et al. [18], copyright (2013) with permission from John Wiley & Sons, Inc.
2.2. 3D bioprinting
3D bioprinting technologies have been applied for the fabrication of 3D tissue constructs, using different biomaterials, and they have a high potential of precise deposition of materials to a desired location, enabling the production of a well-defined 3D architecture. These techniques allow the rapid prototyping of complex 3D tissue constructs containing cells. Additionally, they provide the possibility of using the direct copies of patients' architectural parameters, obtained by different scanning systems, such as X-ray CT [23], [24], and MRI, and reproducing a precise biomimetic 3D-engineered tissue. In the cases of injury and disease, when a direct copy of structural parameters cannot be obtained from the tissues of the patients, the application of CAD [25], [26] can be useful for the reproduction of 3D tissues and organs [10].
The conventional 3D bioprinting approaches in tissue engineering are classified into three major groups: (i) inkjet, (ii) microextrusion, and (iii) laser-assisted bioprinting (LAB). In most cases, the most important factor is the selection of a suitable material for each approach [27]. The printability depends not only on the physicochemical properties of pregel solutions (e.g., viscosity) but on the gelation process as well. Recently, several reviews have provided an overview of these 3D bioprinting approaches [28], [29], and here, we discuss recent advancements in 3D bioprinting hydrogel microfabrication.
2.2.1. Inkjet bioprinting
Inkjet bioprinters have recently been customized to print biocompatible materials with increased resolution and speed. The two approaches most commonly used to eject bioink onto a substrate are the thermal- and piezoelectric-nozzle approaches. Even though the advantages of inkjet bioprinting are high printing speed and low cost, the printing process usually requires: (i) a quick crosslinking reaction for gelation, (ii) the removal of nozzle clogging, (iii) the removal of cavitation bubbles. Alginate is a material commonly used in inkjet bioprinting, owing to its quick crosslinking through an ionic reaction. A PEG based polymer is used as well, as it has high biocompatibility and can be tailored to specific needs by adjusting its physical and chemical properties [30]. Although PEG based hydrogels can provide higher mechanical strength compared with the natural hydrogels, the cellular response to the PEG-based hydrogels (e.g., adhesion) is very limited, and, in order to address this issue, gelatin [31], [32], [33] and hyaluronan [32], [34] are used for the generation of 3D tissue constructs. Furthermore, an advantage of inkjet bioprinting is the possibility of printing multiple cell types and materials. Recently, Xu et al. demonstrated a novel method, fabricating complex and heterogeneous 3D constructs, while using multiple cell types, including stem cells, muscle cells, and endothelial cells [35].
The limiting factors in inkjet bioprinting are ink viscosity, due to excessive force required to eject droplets [36], [37], and the potential of cell damage during the printing process [38]. During the thermal nozzle printing, the heat generated to eject droplets causes cell damage, whereas during piezoelectric-nozzle printing, even though there is no heating, the high pressure required to eject bioink droplets from the nozzle may cause some damage to the cells.
2.2.2. Microextrusion bioprinting
As an alternative approach to inkjet bioprinting, microextrusion bioprinting is often used to fabricate biomimetic 3D tissue constructs. The three typical techniques for the dispersion of the biomaterials onto a substrate, widely used in microextrusion bioprinting, are: (i) pneumatic-, (ii) piston-, and (iii) screw-dispensers.
Piston-dispenser is used most commonly, as it is suitable for the deposition of highly viscous materials onto the substrate. Most of the existing studies using this dispenser reported that 3D tissue constructs were printed using alginate [24], [33], [39] and agarose [40], [41], and the gelation processes were ionic and thermal cross-linking. One of the reasons for the popularity of the microextrusion bioprinting is the compatibility of the dispensing system with the various cross-linking mechanisms. A robust hydrogel is required to maintain high resolution of 3D structures after printing, and the classical approach has been to increase the polymer concentrations and cross-linking density. Additionally, materials can be dispensed through small diameter nozzles under high pressure [42]. However, the dispensing pressure can affect cell viability during the printing process. Therefore, Cohen et al. [43] evaluated a relationship between the resolution of printed constructs and the mixing process of alginate and cross-linkers, and found that an increased mixing procedure before printing affects the resolution of printed materials without causing cell damage.
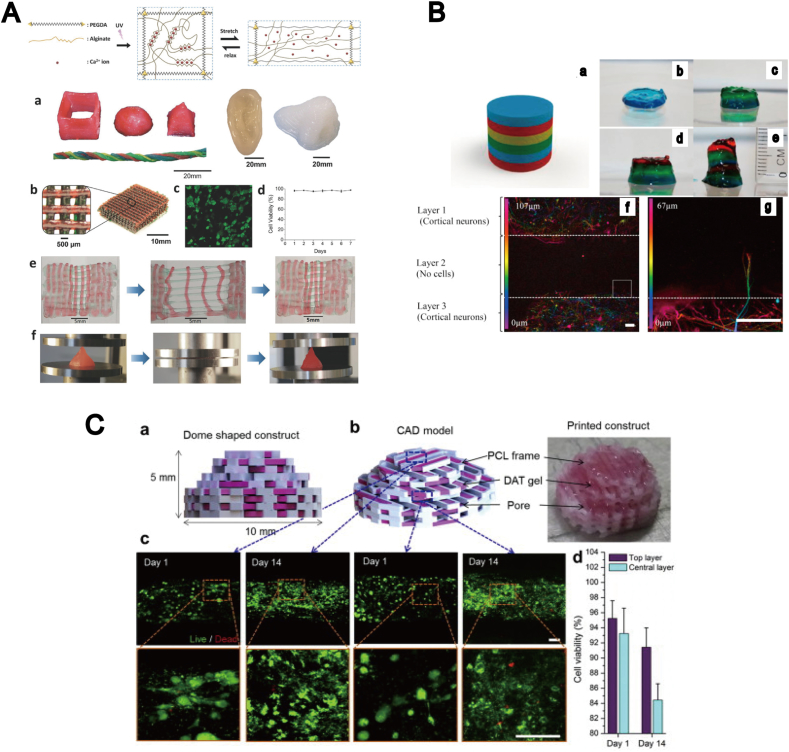
Generally, highly viscous materials are preferred for 3D bioprinting than less viscous ones, because the 3D shape needs to be maintained during the cross-linking reactions following the printing process. The main advantage of microextrusion bioprinting is that it enables the use of highly viscous materials, such as gelatin- and collagen-based materials. This type of bioprinting has been applied to combined gelation processes, including ionic/thermal [33], ionic/chemical [44] and thermal/chemical [45] processes, providing a biomimetic microenvironment with a high resolution for cell growth after the printing. Recent microextrusion techniques tend to be performed with photo-crosslinking reactions using photosensitive polymers, such as PEG diacrylate [40] and gelatin methacrylate [46], [47]. In a recent study, Hong et al. [48] generated highly stretchable and tough hydrogels containing mesenchymal stem cells (MSCs) in the alginate and PEG-based hybrid hydrogels, combining ionic- and photo-crosslinking reactions (Fig. 2A). Pescosolido et al. adopted the semi-interpenetrating network in photo-polymerization in order to optimize the rheological properties of hydrogels [49], and produced 3D printed constructs with hyaluronic acid and hydroxymethacrylate. In the most recent example, production of an in vitro 3D brain model was demonstrated by Lozano et al. [50], where layered 3D tissue constructs were fabricated, and biological function of these models were enhanced with peptide-modified gellan gum, in order for this model to resemble a cortical network with primary neural and glial cells (Fig. 2B). The axon elongation into adjacent hydrogel layers was observed without significant cell damage during the printing and gelation process. Another recent example, together with the application of CAD format, was reported by Pati et al. [51], who fabricated adipose 3D tissue constructs using human matrix bioink, encapsulating human adipose tissue-derived mesenchymal stem cells (Fig. 2C). Although these approaches can be used for the production of simple 3D architecture and porous 3D structures with cells, they may have some limitations in the fabrication of complex 3D architectures. Additionally, the clogging of bioink in the nozzle may present a potential problem related with this approach.
Fig. 2.
3D tissue constructs fabricated by microextrusion bioprinting. (A) Microextrusion bioprinting of tough and highly stretchable hydrogels composed of PEG-alginate-nanoclay polymer through ionic/photo crosslinking. (a) 3D structure printed using the hydrogel. (b) A 3D-printed mesh geometry with the hydrogels. (c–d) Cell viability test in a hydrogel, following the printing. (e) A printed bilayer mesh structure. (f) Compression test. Source: Hong et al. [48], copyright (2015) with permission from John Wiley & Sons, Inc. (B) Bioengineered layered 3D brain-like structures, produced using peptide-modified gellan gum substrates. (a–e) Printed brain-like layered 3D structure. (f–g) Axon elongation into adjacent hydrogels layer. Source: Lozano et al. [50], copyright (2015) with permission from Elsevier Ltd. (C) Microextrusion printing of biomimetic 3D tissue from CAD format. (a–b) CAD format model. (c) Confocal images of cell viability (green: live; red: dead) in printed tissue construct after 2 weeks, in top and central layers. (d) Cell viability in top and central layers. Source: Pati et al. [51], copyright (2015) with permission from Elsevier Ltd.
2.2.3. Laser-assisted bioprinting (LAB)
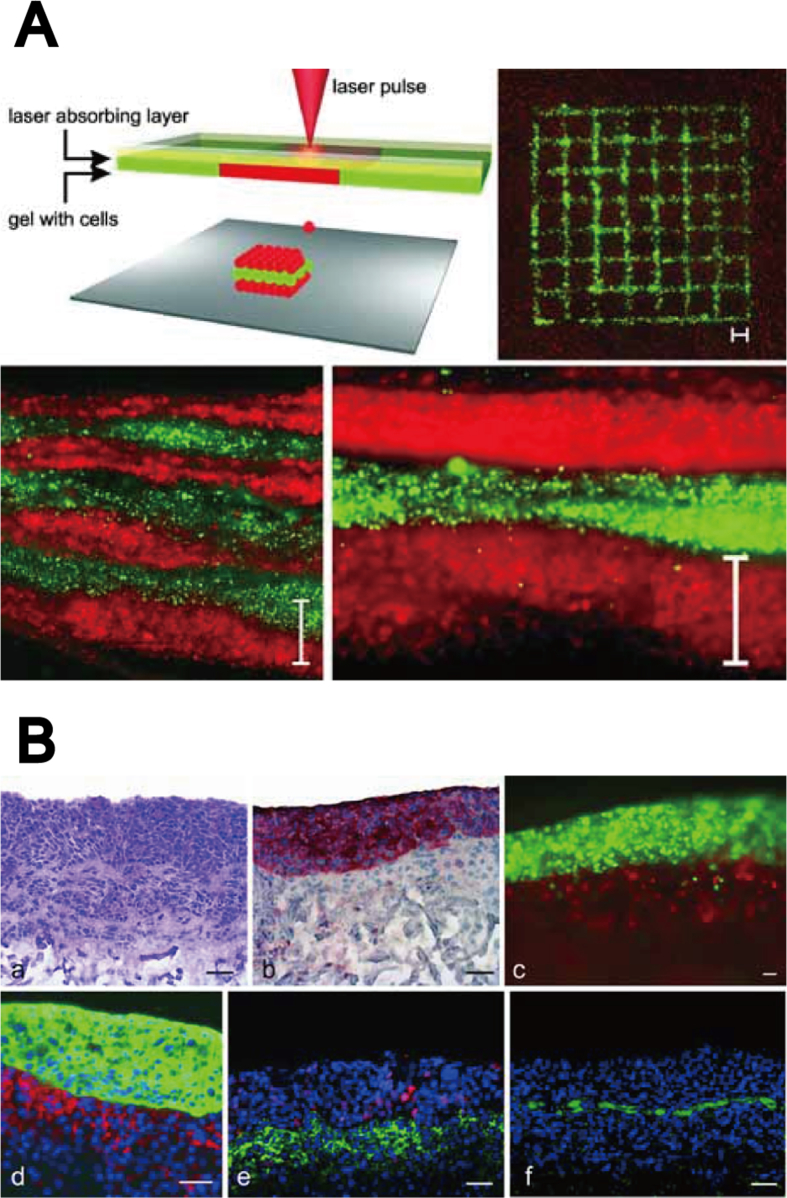
LAB has been used to avoid the previously mentioned issues in the microextrusion bioprinting, since the laser-assisted approach is nozzle-free and can avoid clogging. The laser-induced forward transfer technique, which allows the printing with both inorganic and organic ink at micrometer resolution, was developed by Bohandy et al. [52]. The LAB designed for bioink printing was also reported in 2004 [53]. A typical LAB is composed of three components: (i) a pulsed laser beam, (ii) a ribbon that prints the scaffold, (iii) a substrate that collects the printed materials [54]. Concentrated laser beam pulses on the absorbing layer of the ribbon cause bioink to be propelled by a high-pressure gas towards the collector side. Therefore, LAB enables the generation of the desired geometry at a high resolution without cell damage [55]. Another advantage of LAB is that it allows the cell deposition on the substrate at high densities. Guillemot et al. developed a high-throughput laser printer for tissue engineering [56], and demonstrated the fabrication of microscale cell patterning using alginate hydrogels at high cell density [54]. An alginate solution is commonly used as a bioink to print the engineered tissue, owing to the rapid gelation during the printing process. Although the typical concentration range is 1–2%, a higher concentration of alginate is required to fabricate 3D engineered tissue at millimeter scale. Yan et al. succeeded in the fabrication of long tubes and annular structures using 2–8% alginate [57]. More recent LAB approaches tend to focus on the use of natural hydrogels that provide improved biomimetic microenvironment, for better cell growth. In a recent study by Koch et al. [58], a skin tissue model was constructed, demonstrating the fabrication of 3D thick-tissue constructs with collagen hydrogels, including fibroblasts and keratinocytes, based on a layer-by-layer approach (Fig. 3A and B). These 3D tissue constructs exhibited cell–cell interactions, such as gap junction.
Fig. 3.
3D tissue constructs fabricated by LAB. (A) Skin tissue generation using LAB. (B) 3D skin tissue model printed using the layer-by-layer approach: Murine fibroblasts (10–20 layers) and human keratinocytes (10–20 layers) encapsulated in collagen hydrogel.
Source: Koch et al. [58], copyright (2012) with permission from John Wiley and Sons, Inc.
2.3. Photolithography
Conventional types of photolithography used for tissue engineering are generally photomask-based photolithography and maskless photolithography, which can be either digital light projection stereolithography or laser-based stereolithography [59]. These approaches have different characteristics: cost issues, cell damage levels, resolution, and fabrication speed. Here, we compare these approaches taking into consideration their features.
2.3.1. Photomask-based photolithography
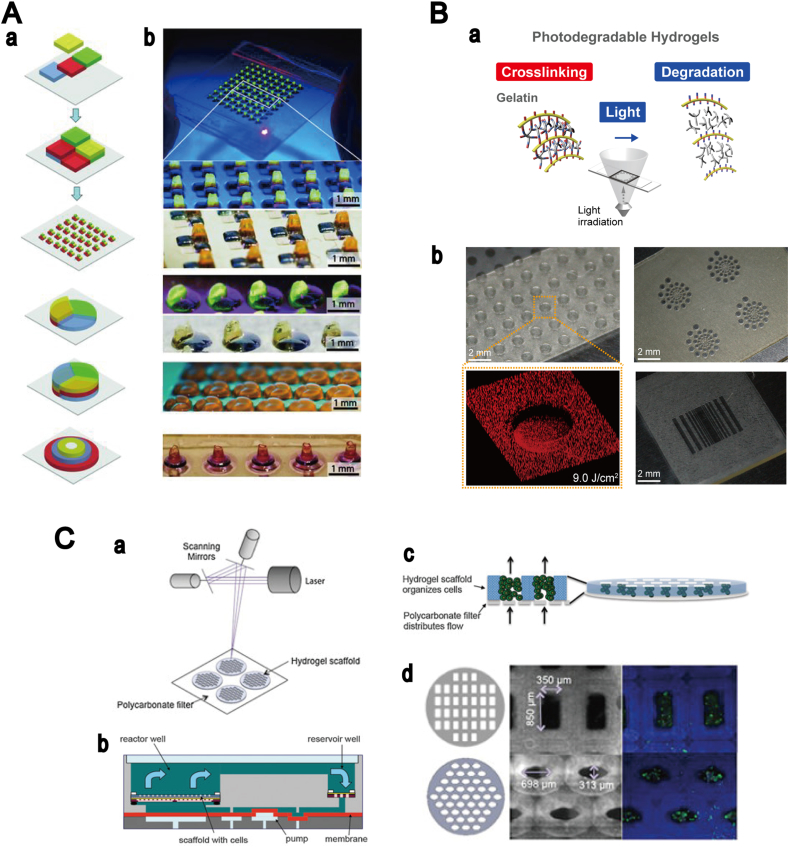
Several studies on 2D hydrogel micropatterning by photolithography have reported the regeneration of biomimetic tissue constructs. The early investigations in tissue engineering relied on photomask-based photolithography because of the simple and low-cost preparation process. A key engineering challenge in photomask-based photolithography is building thick 3D constructs [60]. In recent studies, three groups reported the production of 3D hydrogel constructs with a millimeter-scale thickness, by photomask-based photolithography. Gurkan et al. employed a multilayer photolithography system, used in semiconductor technology development, to fabricate 3D digitally specified hydrogels with multiple cell types (Fig. 4A) [61]. A different approach was used by Hammoudi et al. [62], who succeeded in culturing cells in thick hydrogels, in order to understand stem cell interactions with injured tissue. An alternative approach was reported by Occhetta et al. [63], demonstrating the production of 3D cell-laden microgels through photo-mold patterning. Although photomask-based photolithography is a very simple and low-cost process for the fabrication of 2D patterned hydrogels, this approach requires a substantial amount of photomasks for the generation of 3D architectures.
Fig. 4.
3D tissue constructs created using photolithography. (A) Multilayer digitally specified hydrogels with spatially heterogeneous 3D structures. (a) The process of fabrication of 3D structured hydrogels using multilayer photolithography. (b) Microfabricated array of multilayer digitally specified 3D tissue prototypes. Source: Gurkan et al. [61], copyright (2013) with permission from John Wiley and Sons, Inc. (B) 3D hydrogels patterning using gelatin based photodegradable hydrogels. (a) Micropatterning procedure. (b) Micropatterned photodegradable hydrogels. Source: Yanagawa et al. [75], copyright (2014) with permission from John Wiley and Sons, Inc. (C) Photopatterning of hydrogel scaffolds for perfusion culture. (a) Hydrogel scaffold fabrication. (b–c) Design of perfused bioreactor. (d) Cell viability in fabricated 3D scaffold. Green: live; red: dead cells. Source: Neiman et al. [80], copyright (2015) with permission from John Wiley and Sons, Inc.
2.3.2. Maskless photolithography (stereolithography)
Stereolithography has been used for the production of many well-defined scaffolds for implantation [64]. These techniques offer a lot of potential for the fabrication of 3D structures using non-biological materials, such as resin. Two different methods, digital light projection and laser-based stereolithography, combined with CAD format [65], enable the generation of 3D tissue or organs based on the geometrical information obtained from patient databases, with feature sizes ranging from micro- to millimeters. An important challenge in stereolithography is finding a suitable photosensitive materials for the fabrication of 3D tissue containing cells. Cell encapsulation in hydrogels offers several advantages compared with the seeding of cells on hydrogels.
2.3.2.1. Digital light projection stereolithography
The cell encapsulation in biocompatible hydrogels was first shown by Dhariwala et al. in 2004 [66], who succeeded in the fabrication of engineered hydrogels with polyethylene oxide and PEG dimethacrylate. Early studies in tissue engineering using the digital light projection relied on PEG based hydrogels [67] to fabricate a 3D tissue with fibroblasts, hepatocytes, and MSCs [68], due to the strong mechanical properties of this material and high resolution. However, PEG based hydrogels are inert to cell adhesion, and therefore, their application is limited [69]. In order to improve the characteristics of the materials used, Arg–Gly–Asp (RGD) peptide has been used additionally in hydrogels, to provide cell adhesion on PEG based hydrogels. Another approach uses natural polymers, such as gelatin and polysaccharide, for these purposes. These natural polymers require chemical modification for the introduction of photosensitive moiety, because the materials are not photo-reactive. Recently, Gauvin et al. reported the production of well-designed 3D cell-laden hydrogels with gelatin methacrylate, using the digital light projection approach and layer-by-layer process [70]. A similar approach was employed by Zhang et al., using the CAD format [71], who succeeded in fabricating well-defined 3D architecture with human umbilical vein endothelial cells (HUVECs) and mouse embryonic fibroblast cells, using the layer-by-layer process.
Recent investigations have been devoted to the studies of the regulation of the growth of different types of cells, including fibroblasts, endothelial, smooth muscle, and MSCs cells, by the mechanical properties of hydrogels. Additionally, the studies of stereolithography moved to the use of photodegradable hydrogels based on PEG [72], [73] and gelatin [74], [75]. These hydrogels were used in the studies of cell behaviors, on and in the hydrogels, in contrast to the fabrication of tissue constructs. Providing a platform for the control of the mechanical properties of hydrogels is crucial for the formation of an appropriate microenvironment, aimed at obtaining the desired cellular functions. The dynamic modification of the elasticity of the hydrogels has been a focus of many studies recently. Photodegradable hydrogels offer several advantages as compared to photopolymerized hydrogels, such as the spatiotemporally tunable physicochemical properties, controlled by light. 3D hydrogel patterning on photodegradable hydrogels was first reported by the Anseth group [72], where the fabrication of microstructures in photodegradable hydrogels, and cell adhesion, migration, and spreading in the photodegradable hydrogels were demonstrated and investigated [76]. Yanagawa et al. reported the use of photodegradable hydrogel [75], [77] in the combination with the digital light projection, and they were able to produce micropatterned structure with HUVECs, using gelatin based photodegradable hydrogels (Fig. 4B), and showed the elasticity pattering of hydrogels by light irradiation [78].
2.3.2.2. Laser-based stereolithography
Conventional laser-based stereolithography uses an ultraviolet laser and photosensitive materials, and this approach was used in 3D hydrogel production by Zorlutuna et al. [79], producing multifunctional polymer hydrogels that recapitulate cell–cell interactions between skeletal muscle myoblast cells and primary hippocampus neuron cells. The production of perfusable structure in these hydrogels is required for the long-term culturing of 3D structured tissue, even though the research of stereolithography in tissue engineering has a tendency of focusing on static cultures. Neiman et al. [80] recently produced an open channel structure in 3D hydrogels for the perfusion culture (Fig. 4C).
An alternative laser-based stereolithography uses two- or multi-photon laser system, in order to fabricate 3D hydrogels using photosensitive materials [81]. The advantage of these approaches is that they are able to produce 3D tissue constructs with micro- or nanometer-scale precisions [82], [83], [84]. Compared with short-wavelength lasers, such as an ultraviolet laser, both two- and multi-photon laser systems provide a mild processing environment, which does not cause photochemical damage to the cells. Although a few researchers reported the use of two- and multi-photon laser systems for the production of well-defined 3D hydrogels with cells, most investigations demonstrated a significant cell damage, caused by the photoinitiator during the gelation process [85]. In response to this, a photoinitiator-free multi-photon method was recently developed by Applegate et al. [86], who showed 3D multiscale micropatterning on the silk hydrogels by a multi-photon laser without significant cell damage. Although two- and multi-photon laser-based stereolithographic approaches are attractive options for the production of 3D tissue constructs, many additional issues need to be investigated and resolved, including the toxicity of the initiator and the possibility of high speed fabrication of large tissue constructs [85].
3. Microfluidics
Many researchers use microfluidics technologies for the development of new drug testing platforms [7], [13], and they offer a new opportunity as attractive platforms for the fabrication of functional 3D tissue constructs on a micrometer scale. The main microfluidics technologies are building-block and microfiber approaches, and both are predominately affected by the viscosity of materials used, as well as the various factors described above in Section 2.2. An advantage of microfluidics technologies is the ability to control the flow of fluids, which allows the control of the size and shape of fabricated structures. Additionally, these technologies provide a potential platform for culture medium perfusion through the vascular network in the 3D tissue constructs. The different properties of microfluidics technologies, enabling the fabrication of engineered 3D tissue constructs and the development of perfusion cultures in the following sections.
3.1. Microfluidics for microfabrication
3.1.1. Building-block microfabrication
Microfluidics technologies are often used for the fabrication of tissue building blocks containing cells, known as cell-laden microgels, in order to construct complex 3D tissue structures. Various factors, such as the viscosity of the materials, fluid flow rate, and substrate wettability, must be considered during the creation of these building blocks [87]. An emulsion-based approach is commonly used to fabricate microsphere-shaped microgels as building blocks, using PEG based polymers [88], [89], agarose [90], and alginate [91], [92], [93], [94]. This approach can be divided into methods based on flow-focusing and T-junction microchannels. Comprehensive reviews of microfluidics technologies for the fabrication of microsphere-shaped microgels exist [95], while we focus on the research in the field of 3D tissue engineering.
Manipulating the fabricated cell-laden microgels to construct thick 3D tissue structures represents a major challenge in microfluidics technologies for tissue engineering. Matsunaga et al. [96] reported a novel method for rapid assembly of well-designed tissue on millimeter scale. The cell-laden collagen microspheres produced using the microfluidics technologies were stacked, in order to construct thick tissue structures. Although the emulsion-based approach enables precise control of microsphere size, the possible fabrication shapes are limited. To address this issue, Doyle et al. developed flow lithography, combining photolithography with microfluidics technologies [97], [98]. Flow lithography requires the use of photosensitive materials and patterned light projection, as described previously, in Section 2.3. The application of flow lithography in the tissue engineering was reported by Panda et al. [99], who demonstrated the fabrication of well-designed architectural microgels containing cells. In order to construct 3D tissue structures from the microgels, Chung et al. developed a railed track microfluidics channel that uses flow to guide and assemble the cell-laden microgels inside the microfluidic device [100]. While this approach allows high-throughput fabrication, the ability to create microgels with cells is restricted, due to the higher concentration of monomer and the presence of photoinitiator.
3.1.2. Microfiber-based microfabrication
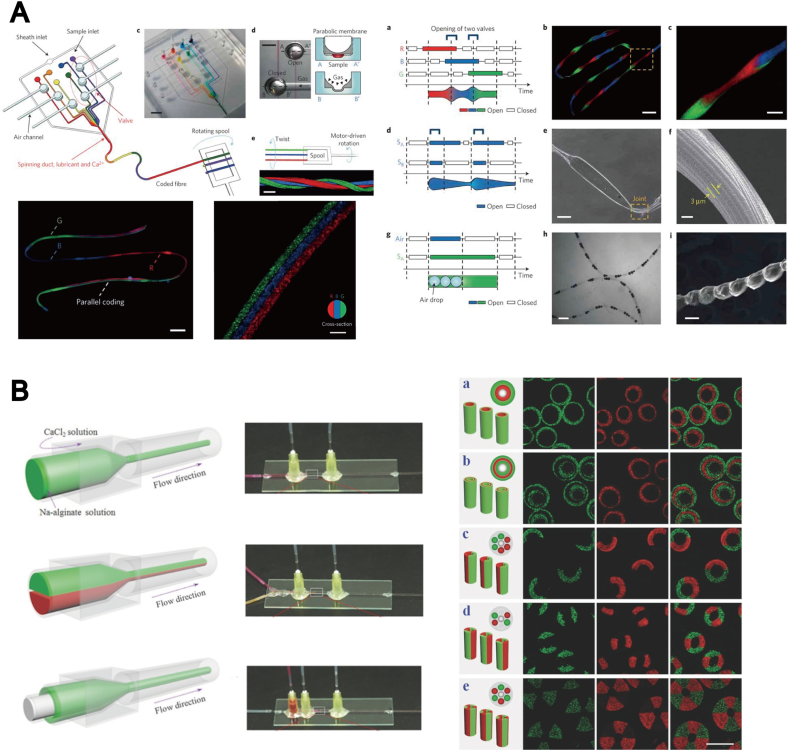
Microfibers in tissue engineering have been widely used for the fabrication of 3D complex fiber geometry [101]. Several approaches have been described, such as electrospinning [102], [103], [104], wet spinning [105], [106], and melt spinning [107]. Single fibers with a simple, homogeneous chemical structure have been produced using these methods. Additionally, microfiber encapsulated cells were produced with a microfluidics-based approach known as microfluidics fiber spinning [108]. A main advantage of this process is the reduced damage to cells during the process of cell encapsulation within microfibers. This approach allows the precise control of the diameter, which is predominately controlled by fluid flow rate in addition to the building block microfabrication described previously. Microfluidics based microfibers have been developed with various hydrogels [109], including PLGA [110], alginate [111], [112] mixed with PLL [113], chitosan [114], and collagen [115]. Most of the existing approaches use alginate [116], due to the rapid gelation based on the ionic crosslinking.
Recently, the advancements in microfluidics fiber spinning allowed a precise design of 3D fiber geometry with multiple layers. Kang et al. [117] developed microfibers with tunable morphological and chemical properties, using digital and programmable flow control system (Fig. 5A). A similar method was developed by Yamada et al. [118], who fabricated an anisotropic microfiber structure with primary rat hepatocyte and feeder cells, mimicking a hepatic micro-organoid. Cheng et al. [119] reported a multiple-laminar-flow microfluidics method for the fabrication of multicomponent 3D microfibers using alginate (Fig. 5B). This approach permits the precise control of the morphology of cells encapsulated in the fibers.
Fig. 5.
Microfluidic procedure. (A) Microfluidic devices for the fabrication of spatially tunable microfibers. Source: Kang et al. [117], copyright (2011) with permission from Nature Publishing Group. (B) Microfluidic injection channels, creating different hollow microfibers. Source: Cheng et al. [119], copyright (2014) with permission from John Wiley and Sons, Inc.
3.2. Microfluidic scaffold for 3D perfusion culture
The microfluidics technologies have been used to generate 3D tissue constructs with perfusable microvascular networks [120], [121], because these networks play a vital role in the distribution of oxygen and nutrients within the engineered thick tissue [122]. The introduction of a flow medium into hydrogels is a necessary process, in order to maintain the viability of fabricated tissue constructs. Therefore, combining microfluidic platforms and 3D microfabrication systems, such as micromolding [123], bioprinting [124] and photolithography [80], is an important task during the generation of microfluidic perfusion cultures.
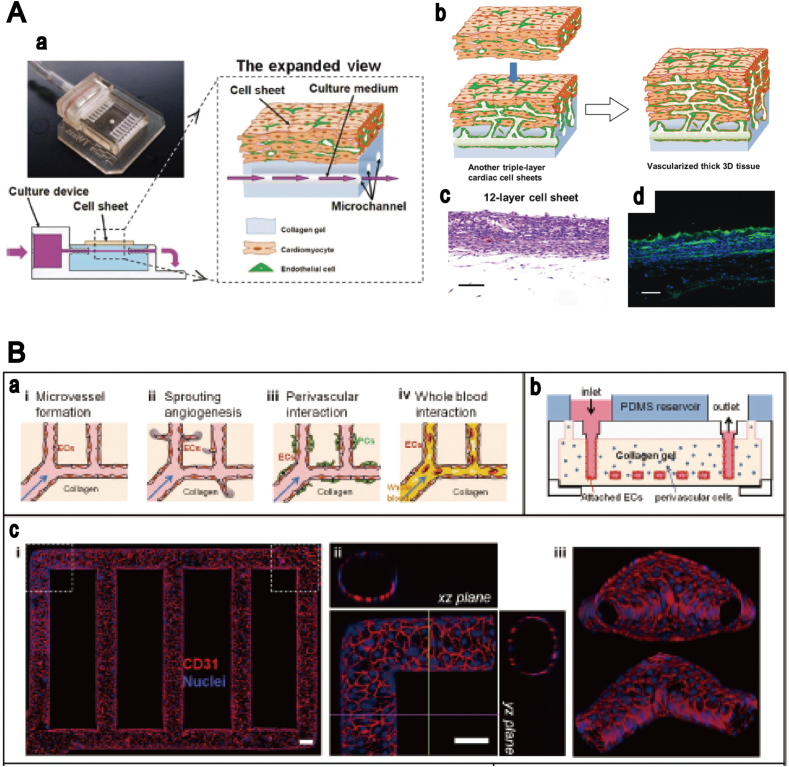
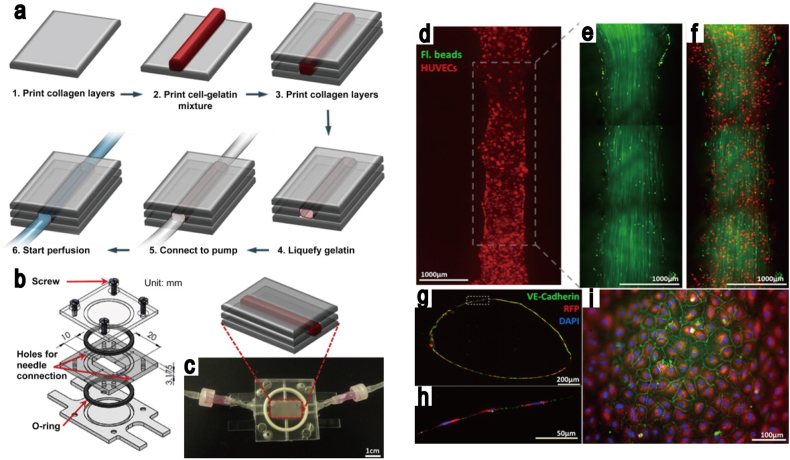
The typical materials that represent a suitable microenvironment for endothelial cell growth in microvascular networks are based on naturally derived polymers, such as (i) collagen [125], [126], [127], (ii) fibrin [128], [129], [130], [131] and (iii) gelatin [132]. Cell culturing in collagen hydrogels with fluid flow was reported by the Tien group [133], who were successful in obtaining cultured HUVECs in a cylindrical channel. They demonstrated that the lifespan of microvascular cells engineered in collagen gel depends on the flow rate of the medium used. Recently, perfusable hydrogels were combined with microfluidics technologies, and a multi-layered bioreactor was developed by Sakaguchi et al. [134], who produced thick tissue with a microchannel in a collagen hydrogel (Fig. 6A). Although a removable substrate, for example, needles and steel, is often used for the fabrication of 3D tubular structures in hydrogels, the technique is limited to the generation of simple structures, such as single and straight channels. In order to improve the applicability of this model, alginate hydrogels have been used for complex channel production inside hydrogels. Golden and Tien used sacrificial hydrogels to create a perfusable microchannel in the collagen hydrogels [17], while a similar method was developed recently by Baker et al. [135], where the effect of angiogenetic growth factors on angiogenesis were investigated in patterned collagen hydrogels. An alternative approach has been reported by Zheng et al. [123], where the perfusion culture was obtained in 3D patterned hydrogels that were fabricated using the micromolding technique combined with a microfluidics system. The endothelial cells were cultured for up to two weeks in the microvascular networks developed in the collagen hydrogels, in order to study the angiogenesis and thrombosis (Fig. 6B). The combination of micromolding and 3D bioprinting systems was also developed by Lee et al. [136], who used the alginate layer as a sacrificial layer, creating a single channel in the collagen hydrogels, and obtained the HUVEC culture in collagen hydrogels with fluid flow (Fig. 7).
Fig. 6.
Microfluidic devices for the culturing of cells in perfusable hydrogels. (A)In vitro development of vascularized tissue using perfusable collagen hydrogel. (a) Culturing device and cell system with medium perfusion. (b) The process of fabrication of vascularized 3D tissue based on a layer-by-layer technique. (c) Twelve-layer cell sheet on collagen hydrogel. (d) Six-layer cell sheet of cardiac muscle. Source: Sakaguchi et al. [134], copyright (2013), with permission from Nature Publishing Group. (B) Microvascular network in a collagen hydrogel, fabricated by micromolding. (a) The processes of fabrication and microvascularization. (b) Design of microfluidic device. (c) Confocal images of an established microvascular structure. Source: Zheng et al. [123], copyright (2012), with permission from the National Academy of Sciences.
Fig. 7.
The fabrication of perfused functional vascular channels, using 3D bioprinting technology. (a) The schematics of the vascular channel construction procedure using cell gelatin mixture. (b–c) Custom-designed flow chamber. (d) Fluorescent images of printed vascular channel with perfusion, after five days of culture. (e–f) The visualization of fluorescent bead motion with flow. (g–i) Vascular channel images, following five days of cell culture, with flow. Blue: DAPI nuclei staining; Red: RFP-transfected HUVECs; Green: VE-cadherin.
Source: Lee et al. [136], copyright (2014), with permission from Elsevier Ltd.
The inability of this methodology to generate well-defined 3D vascular structures with enhanced biological functions, for example a barrier function, similar to what occurs in in vivo models, is their prominent feature. More relevant tissue models, developed with stem cells [137] and with the aim of performing drug testing and investigation of disease mechanisms using them, require not only key structural functionality but also biofunctional features, in order to investigate molecular responses to receptors and transporters. These areas require further research in the future in order to appreciate their full potential.
4. Limitations and future challenges
The goal of 3D tissue engineering is not only the fabrication of whole-organ structures, but also the generation of functional engineered organs and tissues, in order to restore the sites of injury [13], [138]. Although the hydrogels are useful for fabricating and maintaining 3D structure, the engineered tissue for transplantation therapy should synchronize with the tissues of the recipient following the transplantation. Therefore the ideal transplantation scaffolds should be hydrolytically or enzymatically degradable. A number of studies have focused on the development of biodegradable hydrogels [139], [140], designed to degrade by hydrolysis [141], reduction [142], enzymatic reaction [143], [144], or a combination. Since the hydrogel degradation can, in practice, be tuned by chemical moieties of the hydrogels, the degradation rate and profile are controllable as well.
The future challenge for 3D tissue engineering in the field of regenerative therapy is the development of 3D tissue constructs without hydrogels. One of the recently developed hydrogel-free approaches is the use of a decellularized extracellular matrix (dECM) as a native scaffold [145]. This approach was first reported by Cho et al. [146], who fabricated the complex channel structure with dECM, using 3D printing technology. Despite this success, numerous issues remain, with the most significant being the potential removal of the various types of molecules in the ECM during the decellularization process. Therefore, the mechanical properties of fabricated tissues remain less strong as compared to those of the native tissues. Another example of hydrogel-free approaches is the use of cellular aggregates, such as spheroids. Hydrogel-free tubular tissues have been created by the 3D printing of spheroids into needle array [147]. These approaches may overcome the limitations of the hydrogels, but their potential is still being investigated. The understanding and methodology developed in the hydrogel microfabrication can contribute to the advancements in hydrogel-free technologies.
5. Concluding remarks
With recent advancements in 3D tissue engineering, hydrogel microfabrication technologies, such as micromolding, 3D bioprinting, photolithography, and stereolithography, are providing a more realistic approach to drug discovery and the development of alternative methods of organ transplantation. The production of well-defined architectures that mimic natural tissues and organs, without causing a significant cell damage, is one of the key challenges during the fabrication process. Additionally, combining the hydrogel microfabrication technology with cell culture platform, such as microfluidics devices, to provide nutrients and oxygen to the cells within the hydrogels, has not been investigated thoroughly to date. The progress in the fabrication of hydrogels and the development of methodology for cell cultivation will offer long-term improvement of the biological functions in 3D tissue constructs.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgments
F.Y. and S.S. wrote this review. F.Y. would like to acknowledge the support of KAKENHI (grant number 26870904), while S.S. would like to acknowledge KAKENHI (grant number 26106726) for funding this work. F.Y., S.S., and T.K. revised the manuscript. All authors would like to thank Ms. Kristen J. Lowndes for helpful technical comments during the editing of this manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Ishibashi K., Matsuda T. Reconstruction of a hybrid vascular graft hierarchically layered with three cell types. ASAIO J. 1994;40:M284–M290. doi: 10.1097/00002480-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Peppas N.A., Hilt J.Z., Khademhosseini A., Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 3.Annabi N., Nichol J.W., Zhong X., Ji C., Koshy S., Khademhosseini A. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 2010;16:371–383. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Rodrigues J., Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012;41:2193–2221. doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 5.Khetani S.R., Bhatia S.N. Engineering tissues for in vitro applications. Curr Opin Biotechnol. 2006;17:524–531. doi: 10.1016/j.copbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Whitesides G.M. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 8.Thiele J., Ma Y., Bruekers S.M., Ma S., Huck W.T. 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv Mater. 2014;26:125–147. doi: 10.1002/adma.201302958. [DOI] [PubMed] [Google Scholar]
- 9.Zorlutuna P., Annabi N., Camci-Unal G., Nikkhah M., Cha J.M., Nichol J.W. Microfabricated biomaterials for engineering 3D tissues. Adv Mater. 2012;24:1782–1804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 11.Ho C.M., Ng S.H., Li K.H., Yoon Y.J. 3D printed microfluidics for biological applications. Lab Chip. 2015;15:3627–3637. doi: 10.1039/c5lc00685f. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj P., Schweller R.M., Khademhosseini A., West J.L., Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 14.Barata D., Blitterswijk C.V., Habibovic P. High-throughput screening approaches and combinatorial development of biomaterials using microfluidics. Acta Biomater. 2015 doi: 10.1016/j.actbio.2015.09.009. in press. [DOI] [PubMed] [Google Scholar]
- 15.Weinandy S., Laffar S., Unger R.E., Flanagan T.C., Loesel R., Kirkpatrick C.J. Biofunctionalized microfiber-assisted formation of intrinsic three-dimensional capillary-like structures. Tissue Eng Part A. 2014;20:1858–1869. doi: 10.1089/ten.tea.2013.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.H.T., Cohen D.M. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden A.P., Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 18.He J.K., Mao M., Liu Y.X., Shao J.Y., Jin Z.M., Li D.C. Fabrication of nature-inspired microfluidic network for perfusable tissue constructs. Adv Healthc Mater. 2013;2:1108–1113. doi: 10.1002/adhm.201200404. [DOI] [PubMed] [Google Scholar]
- 19.Seto Y., Inaba R., Okuyama T., Sassa F., Suzuki H., Fukuda J. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials. 2010;31:2209–2215. doi: 10.1016/j.biomaterials.2009.11.104. [DOI] [PubMed] [Google Scholar]
- 20.Sadr N., Zhu M., Osaki T., Kakegawa T., Yang Y., Moretti M. SAM-based cell transfer to photopatterned hydrogels for microengineering vascular-like structures. Biomaterials. 2011;32:7479–7490. doi: 10.1016/j.biomaterials.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuchiara M.P., Allen A.C., Chen T.M., Miller J.S., West J.L. Multilayer microfluidic PEGDA hydrogels. Biomaterials. 2010;31:5491–5497. doi: 10.1016/j.biomaterials.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Cuchiara M.P., Gould D.J., McHale M.K., Dickinson M.E., West J.L. Integration of self-assembled microvascular networks with microfabricated PEG-based hydrogels. Adv Funct Mater. 2012;22:4511–4518. doi: 10.1002/adfm.201200976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inzana J.A., Olvera D., Fuller S.M., Kelly J.P., Graeve O.A., Schwarz E.M. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hockaday L.A., Kang K.H., Colangelo N.W., Cheung P.Y., Duan B., Malone E. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4:035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutmacher D.W., Sittinger M., Risbud M.V. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Cohen D.L., Malone E., Lipson H., Bonassar L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12:1325–1335. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 27.Malda J., Visser J., Melchels F.P., Jungst T., Hennink W.E., Dhert W.J.A. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 28.Stanton M.M., Samitier J., Sanchez S. Bioprinting of 3D hydrogels. Lab Chip. 2015;15:3111–3115. doi: 10.1039/c5lc90069g. [DOI] [PubMed] [Google Scholar]
- 29.Wu G.H., Hsu S.H. Review: polymeric-based 3D printing for tissue engineering. J Med Biol Eng. 2015;35:285–292. doi: 10.1007/s40846-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skardal A., Zhang J.X., Prestwich G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–6181. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 31.Billiet T., Gevaert E., De Schryver T., Cornelissen M., Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35:49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 32.Skardal A., Zhang J.X., McCoard L., Xu X.Y., Oottamasathien S., Prestwich G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A. 2010;16:2675–2685. doi: 10.1089/ten.tea.2009.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan B., Hockaday L.A., Kang K.H., Butcher J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101:1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skardal A., Zhang J.X., McCoard L., Oottamasathien S., Prestwich G.D. Dynamically crosslinked gold nanoparticle–hyaluronan hydrogels. Adv Mater. 2010;22:4736–4740. doi: 10.1002/adma.201001436. [DOI] [PubMed] [Google Scholar]
- 35.Xu T., Zhao W.X., Zhu J.M., Albanna M.Z., Yoo J.J., Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34:130–139. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 36.Murphy S.V., Skardal A., Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013;101:272–284. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 37.Kim J.D., Choi J.S., Kim B.S., Choi Y.C., Cho Y.W. Piezoelectric inkjet printing of polymers: stem cell patterning on polymer substrates. Polymer. 2010;51:2147–2154. [Google Scholar]
- 38.Cui X.F., Dean D., Ruggeri Z.M., Boland T. Cell damage evaluation of thermal inkjet printed Chinese Hamster ovary cells. Biotechnol Bioeng. 2010;106:963–969. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Yu Y., Chen H., Ozbolat I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication. 2013;5:025004. doi: 10.1088/1758-5082/5/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maher P.S., Keatch R.P., Donnelly K., Mackay R.E., Paxton J.Z. Construction of 3D biological matrices using rapid prototyping technology. Rapid Prototype J. 2009;15:204–210. [Google Scholar]
- 41.Duarte Campos D.F., Blaeser A., Weber M., Jakel J., Neuss S., Jahnen-Dechent W. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication. 2013;5:015003. doi: 10.1088/1758-5082/5/1/015003. [DOI] [PubMed] [Google Scholar]
- 42.Nair K., Gandhi M., Khalil S., Yan K.C., Marcolongo M., Barbee K. Characterization of cell viability during bioprinting processes. Biotechnol J. 2009;4:1168–1177. doi: 10.1002/biot.200900004. [DOI] [PubMed] [Google Scholar]
- 43.Cohen D.L., Lo W., Tsavaris A., Peng D., Lipson H., Bonassar L.J. Increased mixing improves hydrogel homogeneity and quality of three-dimensional printed constructs. Tissue Eng Part C Methods. 2011;17:239–248. doi: 10.1089/ten.TEC.2010.0093. [DOI] [PubMed] [Google Scholar]
- 44.Yan Y., Wang X., Pan Y., Liu H., Cheng J., Xiong Z. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005;26:5864–5871. doi: 10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Yan Y., Pan Y., Xiong Z., Liu H., Cheng J. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006;12:83–90. doi: 10.1089/ten.2006.12.83. [DOI] [PubMed] [Google Scholar]
- 46.Schuurman W., Levett P.A., Pot M.W., van Weeren P.R., Dhert W.J.A., Hutmacher D.W. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013;13:551–561. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 47.Levato R., Visser J., Planell J.A., Engel E., Malda J., Mateos-Timoneda M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6:035020. doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 48.Hong S.M., Sycks D., Chan H.F., Lin S.T., Lopez G.P., Guilak F. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv Mater. 2015;27:4035–4040. doi: 10.1002/adma.201501099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pescosolido L., Schuurman W., Malda J., Matricardi P., Alhaique F., Coviello T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules. 2011;12:1831–1838. doi: 10.1021/bm200178w. [DOI] [PubMed] [Google Scholar]
- 50.Lozano R., Stevens L., Thompson B.C., Gilmore K.J., Gorkin R., III, Stewart E.M. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials. 2015;67:264–273. doi: 10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Pati F., Ha D.H., Jang J., Han H.H., Rhie J.W., Cho D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 52.Bohandy J., Kim B.F., Adrian F.J. Metal deposition from a supported metal film using an excimer laser. J Appl Phys. 1986;60:1538. [Google Scholar]
- 53.Barron J.A., Wu P., Ladouceur H.D., Ringeisen B.R. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices. 2004;6:139–147. doi: 10.1023/b:bmmd.0000031751.67267.9f. [DOI] [PubMed] [Google Scholar]
- 54.Guillotin B., Souquet A., Catros S., Duocastella M., Pippenger B., Bellance S. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31:7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 55.Catros S., Guillemot F., Nandakumar A., Ziane S., Moroni L., Habibovic P. Layer-by-layer tissue microfabrication supports cell proliferation in vitro and in vivo. Tissue Eng Part C Methods. 2012;18:62–70. doi: 10.1089/ten.TEC.2011.0382. [DOI] [PubMed] [Google Scholar]
- 56.Guillemot F., Souquet A., Catros S., Guillotin B., Lopez J., Faucon M. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6:2494–2500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 57.Yan J.Y., Huang Y., Chrisey D.B. Laser-assisted printing of alginate long tubes and annular constructs. Biofabrication. 2014;6:015002. doi: 10.1088/1758-5082/5/1/015002. [DOI] [PubMed] [Google Scholar]
- 58.Koch L., Deiwick A., Schlie S., Michael S., Gruene M., Coger V. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109:1855–1863. doi: 10.1002/bit.24455. [DOI] [PubMed] [Google Scholar]
- 59.Skoog S.A., Goering P.L., Narayan R.J. Stereolithography in tissue engineering. J Mater Sci Mater Med. 2014;25:845–856. doi: 10.1007/s10856-013-5107-y. [DOI] [PubMed] [Google Scholar]
- 60.Yanagawa F., Kaji H., Jang Y.H., Bae H., Yanan D., Fukuda J. Directed assembly of cell-laden microgels for building porous three-dimensional tissue constructs. J Biomed Mater Res A. 2011;97:93–102. doi: 10.1002/jbm.a.33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurkan U.A., Fan Y.T., Xu F., Erkmen B., Urkac E.S., Parlakgul G. Simple precision creation of digitally specified, spatially heterogeneous, engineered tissue architectures. Adv Mater. 2013;25:1192–1198. doi: 10.1002/adma.201203261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammoudi T.M., Lu H., Temenoff J.S. Long-term spatially defined coculture within three-dimensional photopatterned hydrogels. Tissue Eng Part C Methods. 2010;16:1621–1628. doi: 10.1089/ten.tec.2010.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Occhetta P., Sadr N., Piraino F., Redaelli A., Moretti M., Rasponi M. Fabrication of 3D cell-laden hydrogel microstructures through photo-mold patterning. Biofabrication. 2013;5:035002. doi: 10.1088/1758-5082/5/3/035002. [DOI] [PubMed] [Google Scholar]
- 64.Melchels F.P.W., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 65.Ovsianikov A., Schlie S., Ngezahayo A., Haverich A., Chichkov B.N. Two-photon polymerization technique for microfabrication of CAD-designed 3D scaffolds from commercially available photosensitive materials. J Tissue Eng Regen Med. 2007;1:443–449. doi: 10.1002/term.57. [DOI] [PubMed] [Google Scholar]
- 66.Dhariwala B., Hunt E., Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10:1316–1322. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 67.Arcaute K., Mann B.K., Wicker R.B. Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann Biomed Eng. 2006;34:1429–1441. doi: 10.1007/s10439-006-9156-y. [DOI] [PubMed] [Google Scholar]
- 68.Leigh S.J., Gilbert H.T.J., Barker I.A., Becker J.M., Richardson S.M., Hoyland J.A. Fabrication of 3-dimensional cellular constructs via microstereolithography using a simple, three-component, poly(ethylene glycol) acrylate-based system. Biomacromolecules. 2013;14:186–192. doi: 10.1021/bm3015736. [DOI] [PubMed] [Google Scholar]
- 69.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gauvin R., Chen Y.C., Lee J.W., Soman P., Zorlutuna P., Nichol J.W. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33:3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang A.P., Qu X., Soman P., Hribar K.C., Lee J.W., Chen S.C. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv Mater. 2012;24:4266–4270. doi: 10.1002/adma.201202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kloxin A.M., Kasko A.M., Salinas C.N., Anseth K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeForest C.A., Anseth K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamura M., Yanagawa F., Sugiura S., Takagi T., Sumaru K., Matsui H. Optical cell separation from three-dimensional environment in photodegradable hydrogels for pure culture techniques. Sci Rep. 2014;4:4793. doi: 10.1038/srep04793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanagawa F., Sugiura S., Takagi T., Sumaru K., Camci-Unal G., Patel A. Activated-ester-type photocleavable crosslinker for preparation of photodegradable hydrogels using a two-component mixing reaction. Adv Healthc Mater. 2014;4:246–254. doi: 10.1002/adhm.201400180. [DOI] [PubMed] [Google Scholar]
- 76.DeForest C.A., Anseth K.S. Cytocompatible click-based hydrogels with dynamically-tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2012;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tamura M., Yanagawa F., Sugiura S., Takagi T., Sumaru K., Kanamori T. Click-crosslinkable and photodegradable gelatin hydrogels for cytocompatible optical cell manipulation in natural environment. Sci Rep. 2015;5:15060. doi: 10.1038/srep15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yanagawa F., Mizutani T., Sugiura S., Takagi T., Sumaru K., Kanamori T. Partially photodegradable hybrid hydrogels with elasticity tunable by light irradiation. Colloids Surf B, Biointerf. 2015;126:575–579. doi: 10.1016/j.colsurfb.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Zorlutuna P., Jeong J.H., Kong H., Bashir R. Stereolithography-based hydrogel microenvironments to examine cellular interactions. Adv Funct Mater. 2011;21:3642–3651. [Google Scholar]
- 80.Neiman J.A.S., Raman R., Chan V., Rhoads M.G., Raredon M.S.B., Velazquez J.J. Photopatterning of hydrogel scaffolds coupled to filter materials using stereolithography for perfused 3D culture of hepatocytes. Biotechnol Bioeng. 2015;112:777–787. doi: 10.1002/bit.25494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hahn M.S., Miller J.S., West J.L. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv Mater. 2006;18:2679–2684. [Google Scholar]
- 82.Farsari M., Chichkov B.N. Two-photon fabrication. Nat Photonics. 2009;3:450–452. [Google Scholar]
- 83.Ciuciu A.I., Cywinski P.J. Two-photon polymerization of hydrogels – versatile solutions to fabricate well-defined 3D structures. RSC Adv. 2014;4:45504–45516. [Google Scholar]
- 84.Xing J.F., Zheng M.L., Duan X.M. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem Soc Rev. 2015;44:5031–5039. doi: 10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 85.Torgersen J., Qin X.H., Li Z.Q., Ovsianikov A., Liska R., Stampfl J. Hydrogels for two-photon polymerization: a toolbox for mimicking the extracellular matrix. Adv Funct Mater. 2013;23:4542–4554. [Google Scholar]
- 86.Applegate M.B., Coburn J., Partlow B.P., Moreau J.E., Mondia J.P., Marelli B. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc Natl Acad Sci U S A. 2015;112:12052–12057. doi: 10.1073/pnas.1509405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wan J.D. Microfluidic-based synthesis of hydrogel particles for cell microencapsulation and cell-based drug delivery. Polymers. 2012;4:1084–1108. [Google Scholar]
- 88.Allazetta S., Hausherr T.C., Lutolf M.P. Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules. 2013;14:1122–1131. doi: 10.1021/bm4000162. [DOI] [PubMed] [Google Scholar]
- 89.Koster S., Angile F.E., Duan H., Agresti J.J., Wintner A., Schmitz C. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip. 2008;8:1110–1115. doi: 10.1039/b802941e. [DOI] [PubMed] [Google Scholar]
- 90.Capretto L., Mazzitelli S., Luca G., Nastruzzi C. Preparation and characterization of polysaccharidic microbeads by a microfluidic technique: application to the encapsulation of sertoli cells. Acta Biomater. 2010;6:429–435. doi: 10.1016/j.actbio.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 91.Kim C., Lee K.S., Kim Y.E., Lee K.J., Lee S.H., Kim T.S. Rapid exchange of oil-phase in microencapsulation chip to enhance cell viability. Lab Chip. 2009;9:1294–1297. doi: 10.1039/b819044e. [DOI] [PubMed] [Google Scholar]
- 92.Kim C., Chung S., Kim Y.E., Lee K.S., Lee S.H., Oh K.W. Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid. Lab Chip. 2011;11:246–252. doi: 10.1039/c0lc00036a. [DOI] [PubMed] [Google Scholar]
- 93.Sugiura S., Oda T., Izumida Y., Aoyagi Y., Satake M., Ochiai A. Size control of calcium alginate beads containing living cells using micro-nozzle array. Biomaterials. 2005;26:3327–3331. doi: 10.1016/j.biomaterials.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 94.Utech S., Prodanovic R., Mao A.S., Ostafe R., Mooney D.J., Weitz D.A. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv Healthc Mater. 2015;4:1628–1633. doi: 10.1002/adhm.201500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang A., Park J., Ju J., Jeong G.S., Lee S.H. Cell encapsulation via microtechnologies. Biomaterials. 2014;35:2651–2663. doi: 10.1016/j.biomaterials.2013.12.073. [DOI] [PubMed] [Google Scholar]
- 96.Matsunaga Y.T., Morimoto Y., Takeuchi S. Molding cell beads for rapid construction of macroscopic 3D tissue architecture. Adv Mater. 2011;23:H90–H94. doi: 10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- 97.Dendukuri D., Gu S.S., Pregibon D.C., Hatton T.A., Doyle P.S. Stop-flow lithography in a microfluidic device. Lab Chip. 2007;7:818–828. doi: 10.1039/b703457a. [DOI] [PubMed] [Google Scholar]
- 98.Bong K.W., Lee J., Doyle P.S. Stop flow lithography in perfluoropolyether (PFPE) microfluidic channels. Lab Chip. 2014;14:4680–4687. doi: 10.1039/c4lc00877d. [DOI] [PubMed] [Google Scholar]
- 99.Panda P., Ali S., Lo E., Chung B.G., Hatton T.A., Khademhosseini A. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8:1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chung S.E., Park W., Shin S., Lee S.A., Kwon S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat Mater. 2008;7:581–587. doi: 10.1038/nmat2208. [DOI] [PubMed] [Google Scholar]
- 101.Daniele M.A., Boyd D.A., Adams A.A., Ligler F.S. Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications. Adv Healthc Mater. 2015;4:11–28. doi: 10.1002/adhm.201400144. [DOI] [PubMed] [Google Scholar]
- 102.Lee K.J., Yoon J., Rahmani S., Hwang S., Bhaskar S., Mitragotri S. Spontaneous shape reconfigurations in multicompartmental microcylinders. Proc Natl Acad Sci U S A. 2012;109:16057–16062. doi: 10.1073/pnas.1213669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhaskar S., Hitt J., Chang S.W., Lahann J. Multicompartmental microcylinders. Angew Chem. 2009;48:4589–4593. doi: 10.1002/anie.200806241. [DOI] [PubMed] [Google Scholar]
- 104.Bhaskar S., Lahann J. Microstructured materials based on multicompartmental fibers. J Am Chem Soc. 2009;131:6650–6651. doi: 10.1021/ja900354b. [DOI] [PubMed] [Google Scholar]
- 105.Jalili R., Aboutalebi S.H., Esrafilzadeh D., Shepherd R.L., Chen J., Aminorroaya-Yamini S. Scalable one-step wet-spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: towards multifunctional textiles. Adv Funct Mater. 2013;23:5345–5354. [Google Scholar]
- 106.Tuzlakoglu K., Pashkuleva I., Rodrigues M.T., Gomes M.E., van Lenthe G.H., Muller R. A new route to produce starch-based fiber mesh scaffolds by wet spinning and subsequent surface modification as a way to improve cell attachment and proliferation. J Biomed Mater Res A. 2010;92A:369–377. doi: 10.1002/jbm.a.32358. [DOI] [PubMed] [Google Scholar]
- 107.Chung S.W., Ingle N.P., Montero G.A., Kim S.H., King M.W. Bioresorbable elastomeric vascular tissue engineering scaffolds via melt spinning and electrospinning. Acta Biomater. 2010;6:1958–1967. doi: 10.1016/j.actbio.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Chung B.G., Lee K.H., Khademhosseini A., Lee S.H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip. 2012;12:45–59. doi: 10.1039/c1lc20859d. [DOI] [PubMed] [Google Scholar]
- 109.Onoe H., Takeuchi S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov Today. 2015;20:236–246. doi: 10.1016/j.drudis.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 110.Hwang C.M., Khademhosseini A., Park Y., Sun K., Lee S.H. Microfluidic chip-based fabrication of PLGA microfiber scaffolds for tissue engineering. Langmuir. 2008;24:6845–6851. doi: 10.1021/la800253b. [DOI] [PubMed] [Google Scholar]
- 111.Yu Y., Wen H., Ma J., Lykkemark S., Xu H., Qin J. Flexible fabrication of biomimetic bamboo-like hybrid microfibers. Adv Mater. 2014;26:2494–2499. doi: 10.1002/adma.201304974. [DOI] [PubMed] [Google Scholar]
- 112.Park D.Y., Mun C.H., Kang E., No D.Y., Ju J., Lee S.H. One-stop microfiber spinning and fabrication of a fibrous cell-encapsulated scaffold on a single microfluidic platform. Biofabrication. 2014;6:024108. doi: 10.1088/1758-5082/6/2/024108. [DOI] [PubMed] [Google Scholar]
- 113.Sugiura S., Oda T., Aoyagi Y., Satake M., Ohkohchi N., Nakajima M. Tubular gel fabrication and cell encapsulation in laminar flow stream formed by microfabricated nozzle array. Lab Chip. 2008;8:1255–1257. doi: 10.1039/b803850c. [DOI] [PubMed] [Google Scholar]
- 114.Lee B.R., Lee K.H., Kang E., Kim D.S., Lee S.H. Microfluidic wet spinning of chitosan-alginate microfibers and encapsulation of HepG2 cells in fibers. Biomicrofluidics. 2011;5:22208. doi: 10.1063/1.3576903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaubaroux C., Perrin-Schmitt F., Senger B., Vidal L., Voegel J.C., Schaaf P. Cell alignment driven by mechanically induced collagen fiber alignment in collagen/alginate coatings. Tissue Eng Part C Methods. 2015;21:881–888. doi: 10.1089/ten.tec.2014.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kang E., Choi Y.Y., Chae S.K., Moon J.H., Chang J.Y., Lee S.H. Microfluidic spinning of flat alginate fibers with grooves for cell-aligning scaffolds. Adv Mater. 2012;24:4271–4277. doi: 10.1002/adma.201201232. [DOI] [PubMed] [Google Scholar]
- 117.Kang E., Jeong G.S., Choi Y.Y., Lee K.H., Khademhosseini A., Lee S.H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat Mater. 2011;10:877–883. doi: 10.1038/nmat3108. [DOI] [PubMed] [Google Scholar]
- 118.Yamada M., Sugaya S., Naganuma Y., Seki M. Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter. 2012;8:3122–3130. [Google Scholar]
- 119.Cheng Y., Zheng F.Y., Lu J., Shang L.R., Xie Z.Y., Zhao Y.J. Bioinspired multicompartmental microfibers from microfluidics. Adv Mater. 2014;26:5184–5190. doi: 10.1002/adma.201400798. [DOI] [PubMed] [Google Scholar]
- 120.van Duinen V., Trietsch S.J., Joore J., Vulto P., Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Wong K.H., Chan J.M., Kamm R.D., Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng. 2012;14:205–230. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- 122.Sakai Y., Hattori K., Yanagawa F., Sugiura S., Kanamori T., Nakazawa K. Detachably assembled microfluidic device for perfusion culture and post-culture analysis of a spheroid array. Biotechnol J. 2014;9:971–979. doi: 10.1002/biot.201300559. [DOI] [PubMed] [Google Scholar]
- 123.Zheng Y., Chen J., Craven M., Choi N.W., Totorica S., Diaz-Santana A. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gelber M.K., Bhargava R. Monolithic multilayer microfluidics via sacrificial molding of 3D-printed isomalt. Lab Chip. 2015;15:1736–1741. doi: 10.1039/c4lc01392a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jeon J.S., Zervantonakis I.K., Chung S., Kamm R.D., Charest J.L. In vitro model of tumor cell extravasation. PLoS ONE. 2013;8:e56910. doi: 10.1371/journal.pone.0056910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S., Lee H., Chung M., Jeon N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 127.Shin Y., Yang K., Han S., Park H.J., Seok Heo Y., Cho S.W. Reconstituting vascular microenvironment of neural stem cell niche in three-dimensional extracellular matrix. Adv Healthc Mater. 2014;3:1457–1464. doi: 10.1002/adhm.201300569. [DOI] [PubMed] [Google Scholar]
- 128.Jeon J.S., Bersini S., Whisler J.A., Chen M.B., Dubini G., Charest J.L. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol UK. 2014;6:555–563. doi: 10.1039/c3ib40267c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jeona Jessie S., Bersini Simone, Gilardi Mara, Dubini Gabriele, Charest Joseph L., Moretti Matteo. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee H., Kim S., Chung M., Kim J.H., Jeon N.L. A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res. 2014;91:90–98. doi: 10.1016/j.mvr.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 131.Ehsan S.M., Welch-Reardon K.M., Waterman M.L., Hughes C.C.W., George S.C. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol UK. 2014;6:603–610. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen M.B., Srigunapalan S., Wheeler A.R., Simmons C.A. A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell–cell interactions. Lab Chip. 2013;13:2591–2598. doi: 10.1039/c3lc00051f. [DOI] [PubMed] [Google Scholar]
- 133.Chrobak K.M., Potter D.R., Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 134.Sakaguchi K., Shimizu T., Horaguchi S., Sekine H., Yamato M., Umezu M. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baker B.M., Trappmann B., Stapleton S.C., Toro E., Chen C.S. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip. 2013;13:3246–3252. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee V.K., Kim D.Y., Ngo H., Lee Y., Seo L., Yoo S.S. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35:8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cosson S., Lutolf M.P. Hydrogel microfluidics for the patterning of pluripotent stem cells. Sci Rep. 2014;4:4462. doi: 10.1038/srep04462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mao A.S., Mooney D.J. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112:14452–14459. doi: 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee S.H., Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 140.Nicodemus G.D., Bryant S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peister A., Deutsch E.R., Kolambkar Y., Hutmacher D.W., Guldberg R.E. Amniotic fluid stem cells produce robust mineral deposits on biodegradable scaffolds. Tissue Eng Part A. 2009;15:3129–3138. doi: 10.1089/ten.tea.2008.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chien H.W., Tsai W.B., Jiang S.Y. Direct cell encapsulation in biodegradable and functionalizable carboxybetaine hydrogels. Biomaterials. 2012;33:5706–5712. doi: 10.1016/j.biomaterials.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y., Rossi F., Papa S., Violatto M.B., Bigini P., Sorbona M. Non-invasive in vitro and in vivo monitoring of degradation of fluorescently labeled hyaluronan hydrogels for tissue engineering applications. Acta Biomater. 2016;30:188–198. doi: 10.1016/j.actbio.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 144.Daemi H., Rajabi-Zeleti S., Sardon H., Barikani M., Khademhosseini A., Baharvand H. A robust super-tough biodegradable elastomer engineered by supramolecular ionic interactions. Biomaterials. 2016;84:54–63. doi: 10.1016/j.biomaterials.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 145.Badylak S.F., Taylor D., Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pati F., Jang J., Ha D.H., Kim S.W., Rhie J.W., Shim J.H. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Itoh M., Nakayama K., Noguchi R., Kamohara K., Furukawa K., Uchihashi K. Scaffold-free tubular tissues created by a Bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One. 2015;10:e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]