Abstract
Recently, regenerative medicine has become a highlighted field because it has great potential to induce a paradigm shift of supportive conventional therapy into definitive treatment. The cornea is the avascular, transparent, dome-shaped outermost layer of the eyeball, and it consists of three layers: epithelium, stroma, and endothelium. Conventional corneal transplantation, known as keratoplasty, has two main problems, a donor shortage and immunological rejection. Therefore, regenerative medicine has been applied to overcome these challenges. Regenerative medicine involving the corneal epithelium has been clinically applied, along with an understanding of corneal epithelial stem cell biology, earlier than that of the corneal stroma or endothelium. Thus, the effectiveness and safety of cultivated corneal or oral mucosal epithelial cell sheet transplantation have been reported by many researchers. Clinical studies on regenerative medicine for corneal stroma or endothelium have begun after basic and nonclinical study. Translational research has been performed to make corneal regenerative medicine a universal therapy. This article reviews corneal regenerative medicine.
Keywords: Regenerative medicine, Cornea, Translational research
Abbreviations: LEC, limbal epithelial crypts; LSCD, limbal stem-cell deficiency; COMET, cultivated oral mucosal epithelial cell sheet transplantation; iPS, induced pluripotent stem; GAG, glycosaminoglycan; PMD Act, Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics (PMD Act)
Highlights
-
•
Cultivated corneal or oral mucosal epithelial cell sheet has been used for patients with limbal stem-cell deficiency.
-
•
Clinical studies on regenerative medicine for corneal stroma or endothelium have begun after basic and nonclinical study.
-
•
Translational research has been performed to make corneal regenerative medicine a universal therapy.
1. Structure of the cornea
The cornea is the avascular, transparent, dome-shaped outermost layer of the eyeball. The main optical function of the cornea, together with the lens, is the refraction of light to focus on the retina. Thus, the cornea and lens in the eye function similarly to the lens in a camera, while the retina functions analogously to film in a camera (Fig. 1). The cornea consists of three layers: corneal epithelium, stroma, and endothelium (Fig. 2). All of the layers play important roles to maintain corneal homeostasis. The corneal epithelium blocks the passage of foreign materials, including bacteria, fungi, and dust into the eye and provides a smooth surface that absorbs oxygen from the tear film. The corneal stroma is the middle connective tissue, and it consists of three main groups of proteins, collagens, proteoglycans, glycoproteins, and a small amount of stromal cells known as keratocytes. Regular alignment and the consistent diameter of collagen fibers are crucial for the maintenance of transparency and the strength of the cornea. The corneal endothelium is a single cell layer that forms a boundary between the cornea and the anterior chamber. Proteoglycans are associated with stromal collagens that bind to water and produce a pressure gradient. A major function of the corneal endothelium is to maintain corneal transparency using Na/K ATPase pump function, which regulates corneal hydration [1]. In addition, the endothelium forms leaky tight junctions by permitting a paracellular pathway of aqueous humor into the cornea.
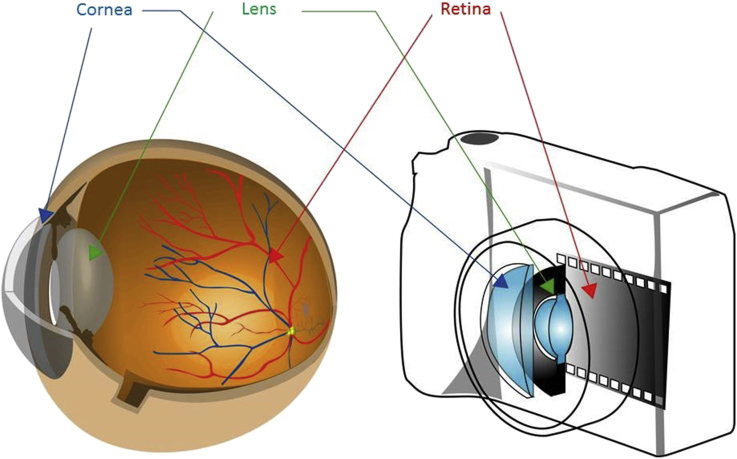
Fig. 1.
Structure of the eyeball. The structure of the eyeball is similar to that of a camera. The cornea and lens in the eyeball function similarly to the lens in a camera. The retina in the eyeball functions similarly to film in a camera.
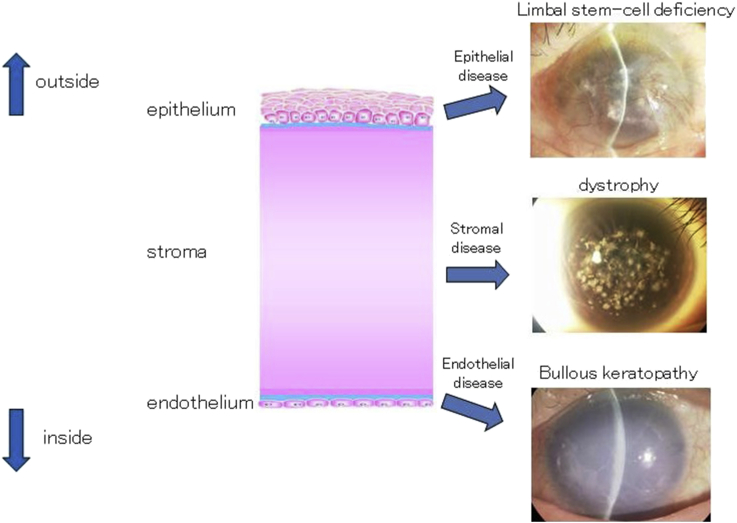
Fig. 2.
Structure of the cornea and corneal disease. The cornea consists of three layers: epithelium, stroma, and endothelium. Vision can deteriorate due to disease in any layer: epithelial disease (e.g., limbal stem-cell deficiency), stromal disease (e.g., dystrophy), and endothelial disease (e.g., bullous keratopathy).
2. Regenerative medicine for corneal epithelium
2.1. Corneal epithelium and limbal stem cells
The corneal epithelium is stratified squamous nonkeratinized epithelium. The thickness of the corneal epithelium has been reported to be 48–53 μm [2], [3], [4], [5], [6], [7]. This layer is five to seven cells thick, and consists of small basal cells, flattened middle cells (wing cells), and polygonal flattened superficial cells.
It has been proposed that corneal epithelial stem cells are located in the basal layer of the limbus, the transitional zone between the cornea and sclera, due to much evidence: label-retaining cells in the limbus [8], the higher proliferative potential of limbal basal cells than that of other parts of the cornea [9], the lower expression of the differentiation marker by limbal basal cells [10], the successful reconstruction of the corneal epithelium by limbal transplantation in patients with limbal stem-cell deficiency [11], and the centripetal movement of pigmented cells in the cornea [12]. Several markers, including p63 [13], ATP-binding cassette subfamily G member 2 (ABCG2) [14], N-cadherin [15], NGF receptors (TrkA) [16], integrin a6 [17], and ABCB5 [18] have been reported as candidate markers of limbal stem cells. However, a specific marker has not yet been identified.
Clinically, the limbal characteristic observation “palisades of Vogt” suggests normal corneal epithelial stem cell function. However, Dua HS et al. reported that the role of limbal epithelial stem cells is limited in the physiological homeostasis of the corneal epithelium, and corneal central basal cells are independently capable of regenerating and maintaining the corneal epithelial layer [19]. Recently, a new phenomenon, “limbal epithelial crypts (LEC)”, has been reported as a putative limbal stem cell niche [20], [21]. Cells within the LEC have the phenotype of CK3-/CK19+/CD34-/Vimentin+/p63+/Connexin43+/MlB(Ki67)-.
2.2. Limbal stem-cell deficiency
If limbal stem cells are depleted, then corneal neovascularization occurs, and stem-cell deficiency (LSCD). The causative diseases of LSCD are classified in Table 1 [22], [23].
Table 1.
Causative diseases of limbal stem-cell deficiency.
| Category | Disease |
|---|---|
| Congenital | Aniridia, sclerocornea |
| External | Thermal, alkali, acid burns, pseudopemphigoid |
| Internal | Stevens-Johnson syndrome, ocular pemphigoid |
| Idiopathic | Unknown |
In patients with LSCD, functional stem cells must be transplanted to sustainably reconstruct the ocular surface. Although limbal autograft transplantation and keratolimbal autograft have been performed, long-term outcomes have been reported to be unsatisfactory due to bacterial keratitis or immunological rejection [11], [24], [25]. Patients with LSCD often have coexisting ocular abnormalities, including severe dry eye due to the destruction of lachrymal glands, trichiasis, eyelid defect, and chronic inflammation. Furthermore, these abnormalities will negatively affect stem cell survival after transplantation. Thus, the management of these ocular surface abnormalities and stem cell transplantation is very important for the treatment of LSCD.
2.3. Cultivated epithelial cell sheet transplantation
Pellegrini G et al. reported that the ocular surface in patients with LSCD caused by ocular burns can be restored using expanded limbal stem cells for as long as two years [26]. Following this report, many researchers have reported the effectiveness and safety of this method for ocular surface reconstruction. The same group reported that permanent restoration of a transparent corneal epithelium was attained in 76.6% of eyes using autologous limbal stem cells cultivated on fibrin in patients with ocular burns [27]. The European Medicines Agency has recommended Holoclar, cultivated limbal epithelial stem cells, for approval in the European Union in 2014. Holoclar is the first regenerative and cellular therapeutic product in the cornea field worldwide. It is believed that this technique should be more widely used as a universal treatment because its effectiveness and safety have been confirmed by many clinical studies and trials.
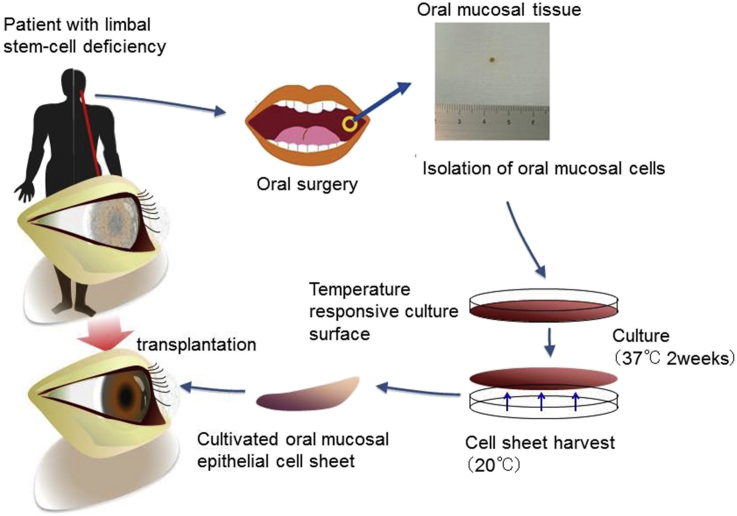
Although this treatment has been successfully applied for patients with LSCD, it can only be used for unilateral patients because limbal tissue from the healthy eye is used as the cell source. Thus, the patients' own oral mucosal epithelial cells are used as a cell source for bilateral patients, and cultivated oral mucosal epithelial cell sheet transplantation (COMET) has been reported (Fig. 3) [28]. Although corneal neovascularization or subepithelial scarring can occur following COMET, long-term outcomes are favorable (Fig. 4) [29], [30].
Fig. 3.
Ocular surface reconstruction by autologous transplantation of tissue-engineered cell sheets fabricated from oral mucosal epithelial cells. Oral mucosal tissue containing whole epithelial cell layers was excised from the oral cavity of a patient. The cells were then seeded onto a temperature-responsive culture dish. The cultured cells were harvested as a cell sheet by reducing the culture temperature. The cells were then transplanted onto the corneal surface of the patient.
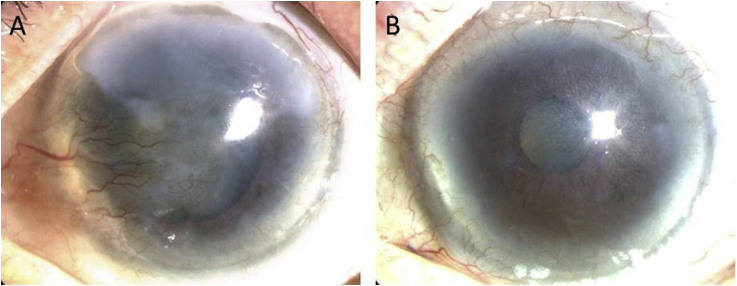
Fig. 4.
Slit lamp photographs of patients before and after cultivated oral mucosal epithelial cell transplantation. (A) The left eye has a total limbal stem-cell deficiency. The visual acuity (VA) was 20/2000. (B) One year postoperatively, the corneal epithelial clarity was well maintained, and the VA was 20/33.
The induction of pluripotent stem cells was reported from adult somatic cells by introducing factors, Oct3/4, Sox2, c-Myc, and Klf4, under embryonic stem (ES) cell culture conditions [31], [32], [33]. Induced pluripotent stem (iPS) cells can be applied to regenerative medicine, drug development, and analysis of disease pathogenesis. Co-ordinate ocular development from human iPS cells and the recovery of corneal function have recently been reported [34]. The first-in human clinical trial of anterior eye transplantation to restore visual function using this method will be performed in the near future.
3. Regenerative medicine for the corneal stroma
3.1. Structure of the corneal stroma
The corneal stroma consists of collagens and associated proteoglycans, other glycoproteins, and corneal stromal cells (keratocytes). In total, 71% of the dry weight of the cornea is collagen [35]. Collagen and proteoglycans form the scaffolding of many tissues, including the cornea, cartilage, skin, and tendon. These two types of proteins make up the majority of the extracellular matrix between cells. The cellular component (keratocytes) is thought to represent only 2–3% of the matrix [36].
Collagens can be divided into several subfamilies based on their structure and organization [37]. Fibrillar collagens, including types I to III, V, and XI, have the same general structure and play an important role in the formation of cross-banded fibrils. Other types of collagens can be categorized as nonfibrillar collagens. For example, basement membrane (type IV) collagen forms networks. Type VI collagen forms beaded filaments. Type VII collagen forms anchoring fibrils and is involved in anchorage of the basement membrane. Corneal collagens mostly consist of type I with smaller amounts of types III, V, and VI [38], [39]. Both the mean diameter of collagen fibers and the mean distance between these fibers are homogeneous and 400–700 μm. These anatomical characteristics are thought to be crucial for the maintenance of corneal transparency because the incident ray of light by each collagen fiber is canceled by interference from other scattered rays, which allows light to pass through the cornea.
The second major group of proteins in the corneal stroma is proteoglycans. They consist of a core protein containing one or more glycosaminoglycan (GAG) side chains, which are also known as mucopolysaccharides. Approximately 65% of the corneal GAG is keratin sulfate, and the remaining amount is chondroitin sulfate and dermatan sulfate. The core proteins of the corneal stroma include lumican, keratocan, and mimecan as keratin sulfate proteoglycans, as well as decorin and biglycan as chondroitin sulfate or dermatan sulfate. GAG has the ability to absorb and retain large amounts of water, and it generates stromal swelling pressure. The precise regulation of the diameter and orientation of fibers, as well as the interfibrillar spaces, is partially attributed to the interactions between glycosaminoglycans and collagens [37].
Keratocytes predominantly comprise cells of the corneal stroma. It is believed that they turn over approximately every 2–3 years. They are connected with extended long processes by gap junctions. Keratocytes are normally quiescent but can change into myofibroblasts, which are positive for a-smooth muscle actin in response to various types of insults to the stroma [40].
3.2. Treatment for corneal stromal disease
Penetrating keratoplasty has been the only choice for corneal stromal disease for more than half a century, but the adoption by specialist surgeons of newer forms of lamellar transplantation surgery, which selectively replaces only diseased layers of the cornea, has been a fundamental change in recent years [41]. Deep anterior lamellar keratoplasty, one procedure of anterior lamellar transplantation, has an advantage over penetrating graft in eliminating the risk of endothelial rejection. Once endothelial rejection occurs, it can directly cause graft failure due to endothelial dysfunction. However, donor shortage is a very large problem in many countries that perform keratoplasty.
3.3. Regenerative medicine for corneal stromal disease
Many researchers have reported basic or clinical studies of regenerative medicine for corneal stromal diseases. Griffith M et al. reported functional human corneal equivalents constructed from cell lines [42] and have also applied corneal implants made from interpenetrating networks of cross-linked recombinant human collagen type III and 2-methacryloyloxyethil phosphorylcholine in three patients [43]. The potential use of human limbal biopsy-derived stromal, dental pulp stem cells, and embryonic stem cell-derived keratocytes for corneal disorders has been reported [44], [45], [46]. These types of cells can differentiate into keratocytes that are positive for keratocan [47]. In addition, mesenchymal stem cells have a great capacity for differentiation and immunosuppression. It has been reported that they have an ability to differentiate into corneal epithelial cells, keratocytes, and endothelial cells [48]. There have been several clinical trials using mesenchymal stem cells in patients with corneal disease, such as sterile corneal epithelial defect and chronic graft-versus-host disease [49], [50].
4. Regenerative medicine for corneal endothelium
Corneal endothelial dysfunction causes bullous keratopathy, which is clinically characterized by corneal epithelial and endothelial edema, Descemet's membrane fold. Cataract surgery and Fuchs' endothelial corneal dystrophy are is a main causes of bullous keratopathy. In Japan, bullous keratopathy secondary to laser iridotomy is the second most common cause [51]. Although penetrating keratoplasty has been the only treatment for bullous keratopathy, endothelial keratoplasty for a long time, endothelial keratoplasty including Descemet-stripping automated endothelial keratoplasty or Descemet membrane endothelial keratoplasty, has recently been applied in an increasing number of patients [52], [53], [54], [55], [56]. Cultivated corneal endothelial transplantation using the ROCK inhibitor has also been reported [57], [58], [59], [60], [61], [62]. This novel treatment has a great possibility of overcoming the large problem of donor shortage. Another potential solution is corneal endothelium derived from corneal stroma stem cells [63], [64]. It was reported that the corneal endothelium was induced by retinoic acid and Wnt/β-catenin signaling. Autologous or allogenic therapy using this technology can be applied in the near future.
5. Translational research towards a universal therapy
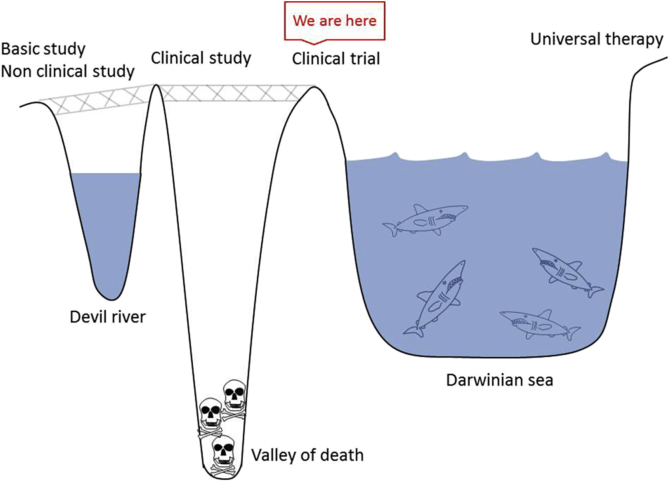
Universal therapy means that patients can access treatment regardless of their location. To achieve this goal, translational research must be performed to develop new treatment methods based on basic research findings in the health care field (Fig. 5). Translational research steps include basic research, nonclinical research, clinical research, and universal therapy. However, there is a gap between basic research and clinical research known as “Devil River”. For example, even if a novel induction method of pluripotent or multipotent stem cells to a specific type of cell is established, the safety of animal-derived materials used during the induction process must be confirmed prior to the first clinical application. A patent that sufficiently covers the developed method must be also acquired. An interval between clinical research and clinical trials can be called the “Valley of death.” Here, regulatory issues must be addressed before the clinical trial, and the cost for the clinical trial and the approval process must be covered. A gap between clinical trials and universal therapy can be called a “Darwinian sea,” which indicates severe natural selection in the market. In the field of regenerative medicine, the cost of maintaining and running the good manufacturing practice facility where the cells are manipulated is high. In the market, the cost-benefit performance should be evaluated. Thus, manufacturing cost should be cut in some way (see Fig. 5).
Fig. 5.
Translational research. The steps from basic research to universal therapy are referred to as “translational research”.
Multidisciplinary groups, including not only basic scientists and clinicians but also bioinformaticists, statisticians, engineers and industry experts, are required for translational research. Scientists will be evaluated using business techniques, such as milestones and the ability to work in multidisciplinary groups, rather than by their publications alone [65].
Our group has been working on translational research using cultivated oral mucosal epithelial cell sheets [66]. We developed a new culture method without xenogenic feeder layer nor bovine fetal serum, sheet transportation technique [67], [68]. We are performing a multicenter clinical study and trial based on these results. Unfortunately, COMET has been performed in a limited number of institutes for a small number of patients thus far. Approval as a regenerative and cellular therapeutic product is essential for universal treatment. One goal of our translational research is that many patients access this treatment and benefit from the approved products as a universal therapy.
The Regenerative Medicine Promotion Law and the Act on the Safety of Regenerative Medicine was recently established in Japan. The Pharmaceutical Affairs Law was revised as the Pharmaceuticals, Medical Devices and Other Therapeutic Products Act (PMD Act), which introduced early approval for regenerative and cellular therapeutic products. Before this revision, confirmation of a product's effectiveness and safety was required for its approval. However, the confirmation of safety and the suggestion of effectiveness are now required for conditional approval. These changes will expedite the approval of regenerative medicine products and will promote the standardization of regenerative medicine.
There are two ways for a new treatment to become widely available as a general medical treatment: coverage by health insurance or approval as a medical device under the PMD Act. A treatment that receives approval as a regenerative and cellular therapeutic product under the PMD Act will be more accessible to patients because all hospitals can purchase commercially available products. We believe that it is time to make regenerative medicine a universal therapy.
Conflicts of interest and source of funding
Kohji Nishida has a patent. Yoshinori Oie has no conflicts of interest to declare. This study was supported by the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and development, AMED.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Maurice D.M. The location of the fluid pump in the cornea. J Physiol. 1972;221:43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Tan O., Brass R., Weiss J.L., Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119:2425–2433. doi: 10.1016/j.ophtha.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan E.H., Chen L., Yu F., Deng S.X. Epithelial thinning in limbal stem cell deficiency. Am J Ophthalmol. 2015;160 doi: 10.1016/j.ajo.2015.06.029. 669–677. [DOI] [PubMed] [Google Scholar]
- 4.Rocha K.M., Krueger R.R. Spectral-domain optical coherence tomography epithelial and flap thickness mapping in femtosecond laser-assisted in situ keratomileusis. Am J Ophthalmol. 2014;158 doi: 10.1016/j.ajo.2014.04.012. 293–301. [DOI] [PubMed] [Google Scholar]
- 5.Reinstein D.Z., Silverman R.H., Rondeau M.J., Coleman D.J. Epithelial and corneal thickness measurements by high-frequency ultrasound digital signal processing. Ophthalmology. 1994;101:140–146. doi: 10.1016/s0161-6420(94)31373-x. [DOI] [PubMed] [Google Scholar]
- 6.Haque S., Simpson T., Jones L. Corneal and epithelial thickness in keratoconus: a comparison of ultrasonic pachymetry, Orbscan II, and optical coherence tomography. J Refract Surg. 2006;22:486–493. doi: 10.3928/1081-597X-20060501-11. [DOI] [PubMed] [Google Scholar]
- 7.Li H.F., Petroll W.M., Moller-Pedersen T., Maurer J.K., Cavanagh H.D., Jester J.V. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF) Curr Eye Res. 1997;16:214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- 8.Cotsarelis G., Cheng S.Z., Dong G., Sun T.T., Lavker R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini G., Golisano O., Paterna P., Lambiase A., Bonini S., Rama P. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schermer A., Galvin S., Sun T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon K.R., Tseng S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. discussion 722–703. [DOI] [PubMed] [Google Scholar]
- 12.Davanger M., Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini G., Dellambra E., Golisano O., Martinelli E., Fantozzi I., Bondanza S. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe K., Nishida K., Yamato M., Umemoto T., Sumide T., Yamamoto K. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi R., Yamato M., Sugiyama H., Sumide T., Yang J., Okano T. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25:289–296. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T., Endo K., Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells. 2007;25:628–638. doi: 10.1634/stemcells.2006-0494. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi R., Yamato M., Saito T., Oshima T., Okano T., Tano Y. Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin alpha6 and CD71. Biochem Biophys Res Commun. 2008;367:256–263. doi: 10.1016/j.bbrc.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 18.Ksander B.R., Kolovou P.E., Wilson B.J., Saab K.R., Guo Q., Ma J. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dua H.S., Miri A., Alomar T., Yeung A.M., Said D.G. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–863. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Dua H.S., Shanmuganathan V.A., Powell-Richards A.O., Tighe P.J., Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanmuganathan V.A., Foster T., Kulkarni B.B., Hopkinson A., Gray T., Powe D.G. Morphological characteristics of the limbal epithelial crypt. Br J Ophthalmol. 2007;91:514–519. doi: 10.1136/bjo.2006.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oie Y., Nishida K. Regenerative medicine for the cornea. BioMed Res Int. 2013;2013:428247. doi: 10.1155/2013/428247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida K. Tissue engineering of the cornea. Cornea. 2003;22:S28–S34. doi: 10.1097/00003226-200310001-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gomes J.A., Santos M.S., Ventura A.S., Donato W.B., Cunha M.C., Hofling-Lima A.L. Amniotic membrane with living related corneal limbal/conjunctival allograft for ocular surface reconstruction in Stevens-Johnson syndrome. Arch Ophthalmol. 2003;121:1369–1374. doi: 10.1001/archopht.121.10.1369. [DOI] [PubMed] [Google Scholar]
- 25.Ilari L., Daya S.M. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1278–1284. doi: 10.1016/s0161-6420(02)01081-3. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 27.Rama P., Matuska S., Paganoni G., Spinelli A., De Luca M., Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 28.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T., Takeda K., Inatomi T., Sotozono C., Kinoshita S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–946. doi: 10.1136/bjo.2010.188714. [DOI] [PubMed] [Google Scholar]
- 30.Satake Y., Higa K., Tsubota K., Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118:1524–1530. doi: 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi R., Ishikawa Y., Sasamoto Y., Katori R., Nomura N., Ichikawa T. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531:376–380. doi: 10.1038/nature17000. [DOI] [PubMed] [Google Scholar]
- 35.Miller E.J., Gay S. Collagen: an overview. Methods Enzymol. 1982;82(Pt A):3–32. doi: 10.1016/0076-6879(82)82058-2. [DOI] [PubMed] [Google Scholar]
- 36.Otori T. Electrolyte content of the rabbit corneal stroma. Exp Eye Res. 1967;6:356–367. doi: 10.1016/s0014-4835(67)80010-1. [DOI] [PubMed] [Google Scholar]
- 37.Robert L., Legeais J.M., Robert A.M., Renard G. Corneal collagens. Pathol Biol (Paris) 2001;49:353–363. doi: 10.1016/s0369-8114(01)00144-4. [DOI] [PubMed] [Google Scholar]
- 38.Fitch J.M., Gross J., Mayne R., Johnson-Wint B., Linsenmayer T.F. Organization of collagen types I and V in the embryonic chicken cornea: monoclonal antibody studies. Proc Natl Acad Sci U S A. 1984;81:2791–2795. doi: 10.1073/pnas.81.9.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue B.Y., Sugar J., Schrode K. Collagen staining in corneal tissues. Curr Eye Res. 1986;5:559–564. doi: 10.3109/02713688609015119. [DOI] [PubMed] [Google Scholar]
- 40.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 41.Tan D.T., Dart J.K., Holland E.J., Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 42.Griffith M., Osborne R., Munger R., Xiong X., Doillon C.J., Laycock N.L. Functional human corneal equivalents constructed from cell lines. Science. 1999;286:2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- 43.Buznyk O., Pasyechnikova N., Islam M.M., Iakymenko S., Fagerholm P., Griffith M. Bioengineered corneas grafted as alternatives to human donor corneas in three high-risk patients. Clin Transl Sci. 2015;8:558–562. doi: 10.1111/cts.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syed-Picard F.N., Du Y., Lathrop K.L., Mann M.M., Funderburgh M.L., Funderburgh J.L. Dental pulp stem cells: a new cellular resource for corneal stromal regeneration. Stem Cells Transl Med. 2015;4:276–285. doi: 10.5966/sctm.2014-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu S., Hertsenberg A.J., Funderburgh M.L., Burrow M.K., Mann M.M., Du Y. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hertsenberg A.J., Funderburgh J.L. Generation of corneal keratocytes from human embryonic stem cells. Methods Mol Biol. 2016;1341:285–294. doi: 10.1007/7651_2015_231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funderburgh J.L., Funderburgh M.L., Du Y. Stem cells in the limbal stroma. Ocul Surf. 2016;14:113–120. doi: 10.1016/j.jtos.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Coulson-Thomas V.J., Ferreira T.G., Kao W.W. Mesenchymal stem cells for treating ocular surface diseases. BMC Ophthalmol. 2015;15(Suppl. 1):155. doi: 10.1186/s12886-015-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agorogiannis G.I., Alexaki V.I., Castana O., Kymionis G.D. Topical application of autologous adipose-derived mesenchymal stem cells (MSCs) for persistent sterile corneal epithelial defect. Graefes Arch Clin Exp Ophthalmol. 2012;250:455–457. doi: 10.1007/s00417-011-1841-3. [DOI] [PubMed] [Google Scholar]
- 50.Weng J., He C., Lai P., Luo C., Guo R., Wu S. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther J Am Soc Gene Ther. 2012;20:2347–2354. doi: 10.1038/mt.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimazaki J., Amano S., Uno T., Maeda N., Yokoi N., Japan Bullous Keratopathy Study G National survey on bullous keratopathy in Japan. Cornea. 2007;26:274–278. doi: 10.1097/ICO.0b013e31802c9e19. [DOI] [PubMed] [Google Scholar]
- 52.Gorovoy M.S. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25:886–889. doi: 10.1097/01.ico.0000214224.90743.01. [DOI] [PubMed] [Google Scholar]
- 53.Ham L., Dapena I., Moutsouris K., Balachandran C., Frank L.E., van Dijk K. Refractive change and stability after Descemet membrane endothelial keratoplasty. Effect of corneal dehydration-induced hyperopic shift on intraocular lens power calculation. J Cataract Refract Surg. 2011;37:1455–1464. doi: 10.1016/j.jcrs.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Price M.O., Giebel A.W., Fairchild K.M., Price F.W., Jr. Descemet's membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Price M.O., Gorovoy M., Benetz B.A., Price F.W., Jr., Menegay H.J., Debanne S.M. Descemet's stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117:438–444. doi: 10.1016/j.ophtha.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melles G.R., Ong T.S., Ververs B., van der Wees J. Descemet membrane endothelial keratoplasty (DMEK) Cornea. 2006;25:987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 57.Koizumi N., Okumura N., Ueno M., Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea. 2014;33(Suppl. 11):S25–S31. doi: 10.1097/ICO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 58.Koizumi N., Sakamoto Y., Okumura N., Okahara N., Tsuchiya H., Torii R. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48:4519–4526. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]
- 59.Koizumi N., Sakamoto Y., Okumura N., Tsuchiya H., Torii R., Cooper L.J. Cultivated corneal endothelial transplantation in a primate: possible future clinical application in corneal endothelial regenerative medicine. Cornea. 2008;27(Suppl. 1):S48–S55. doi: 10.1097/ICO.0b013e31817f2298. [DOI] [PubMed] [Google Scholar]
- 60.Okumura N., Kinoshita S., Koizumi N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea. 2014;33(Suppl. 11):S37–S41. doi: 10.1097/ICO.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 61.Okumura N., Koizumi N., Ueno M., Sakamoto Y., Takahashi H., Tsuchiya H. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181:268–277. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 62.Okumura N., Ueno M., Koizumi N., Sakamoto Y., Hirata K., Hamuro J. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 63.Hatou S., Yoshida S., Higa K., Miyashita H., Inagaki E., Okano H. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem Cells Dev. 2013;22:828–839. doi: 10.1089/scd.2012.0286. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida S., Shimmura S., Nagoshi N., Fukuda K., Matsuzaki Y., Okano H. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 65.Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- 66.Oie Y., Nishida K. Translational research on ocular surface reconstruction using oral mucosal epithelial cell sheets. Cornea. 2014;33(Suppl. 11):S47–S52. doi: 10.1097/ICO.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 67.Oie Y., Hayashi R., Takagi R., Yamato M., Takayanagi H., Tano Y. A novel method of culturing human oral mucosal epithelial cell sheet using post-mitotic human dermal fibroblast feeder cells and modified keratinocyte culture medium for ocular surface reconstruction. Br J Ophthalmol. 2010;94:1244–1250. doi: 10.1136/bjo.2009.175042. [DOI] [PubMed] [Google Scholar]
- 68.Oie Y., Nozaki T., Takayanagi H., Hara S., Hayashi R., Takeda S. Development of a cell sheet transportation technique for regenerative medicine. Tissue Eng Part C Methods. 2014;20:373–382. doi: 10.1089/ten.tec.2013.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]