Abstract
For the past few decades, spinal cord injury (SCI) has been believed to be an incurable traumatic condition, but with recent developments in stem cell biology, the field of regenerative medicine has gained hopeful momentum in the development of a treatment for this challenging pathology. Among the treatment candidates, transplantation of neural precursor cells has gained remarkable attention as a reasonable therapeutic intervention to replace the damaged central nervous system cells and promote functional recovery. Here, we highlight transplantation therapy techniques using induced pluripotent stem cells to treat SCI and review the recent research giving consideration to future clinical applications.
Keywords: Spinal cord injury, Neural precursor cells, Induced pluripotent stem cells, Clinical application
Abbreviations: SCI, spinal cord injury; NPCs, neural precursor cells; iPSCs, induced pluripotent stem cells; ESCs, embryonic stem cells; NHPs, nonhuman primates; CST, corticospinal tract; HMGB1, high mobility group box-1; SLA, swine leukocyte antigen; MLR, mixed lymphocyte reaction; HLA, human leukocyte antigen; PBMCs, peripheral blood mononuclear cells; drNPCs, directly reprogrammed neural precursor cells; C-ABC, chondroitinase ABC; GSI, γ-secretase inhibitor; HSVtk, herpes simplex virus type I thymidine kinase; GCV, ganciclovir; OPCs, oligodendrocyte progenitor cells; CiRA, the Center for iPS Cell Research and Application; ASIA, American Spinal Injury Association; CSPGs, chondroitin sulfate proteoglycans
Highlights
-
•
Transplantation of iPSC-derived neural precursor cells (NPCs) shows beneficial effects for spinal cord injury (SCI).
-
•
Because unsafe iPSC-NPC lines can form tumors after grafting, provisions to attenuate this risk are substantially important.
-
•
Clinical application for SCI patients using iPSCs will be conducted in the near future.
1. Introduction
Spinal cord injury (SCI) is a devastating event with sudden onset of motor and sensory dysfunction. Damage to autonomic neurons at and below the level of injury leads to bowel, bladder, and sexual functional loss. This trauma was formerly found primarily in young patients due to high-energy accidents and contact sports, but cervical canal stenosis in elderly patients is increasing with the progress of our aging society, and elderly patients can easily injure their spinal cord from a fall. Currently, surgical intervention and subsequent rehabilitation are the only options for SCI treatment. Although methylprednisolone is administered at the acute stage of injury, a consensus of its use has not yet been reached from the aspects of both safety and effectiveness [1], [2].
Recent developments in stem cell research have indicated that researchers are close to a breakthrough in this challenging field. Numerous preclinical studies have demonstrated the effectiveness of neural precursor cell (NPC) transplantation in animal models of SCI. Ethical concerns remain about the use of NPCs harvested from fetal or embryonic stem cells (ESCs). However, Yamanaka and his colleagues developed induced pluripotent stem cells (iPSCs), which opened a new avenue toward the clinical application of stem cells for regenerative medicine [3], [4]. iPSCs exhibit characteristics similar to those of ESCs and can generate all three germ layers. Therefore, iPSCs can enrich ectodermal neural-lineage cells with appropriate culture induction. Because iPSCs can be derived from human somatic cells, they have the potential to overcome ethical problems. Although the potential use of iPSCs is very attractive, artificial induction methods raise alternative problems, such as genetic and epigenetic abnormalities and subsequent tumorigenicity. Thus, solving these problems to promote the clinical application of stem cells is essential [5].
The purpose of this review article is to present the current status of cell therapy using NPCs, especially derived from iPSCs. In addition, we report several approaches to overcome the posttransplant tumorigenic problems and introduce interesting studies. We are currently preparing for the first human clinical trial of iPSC-NPC therapy for subacute SCI, and we are planning to start this research soon.
2. iPSC-derived NPCs for the treatment of spinal cord injury
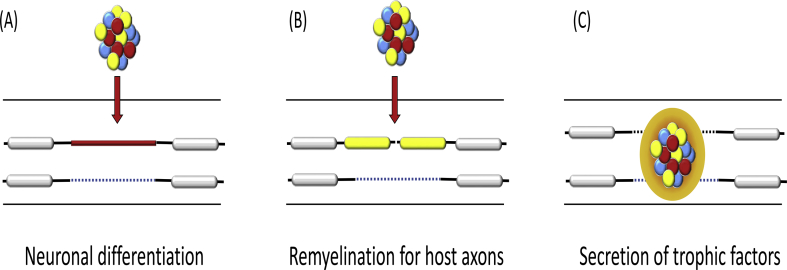
Previous studies have demonstrated the efficacy of NPC transplantation for animal SCI models, and several mechanisms of functional recovery have been noted (Fig. 1) [6]. 1) Grafted NPCs secrete neurotrophic factors to attenuate secondary damage, which is supported by the evidence of early functional improvement before complete maturation of the transplanted cells [7], [8]. 2) Transplanted NPCs differentiate into neurons that make synaptic connections with host axons [9], [10]. 3) The transplants generate oligodendrocytes and myelinate host axons to improve the saltatory conduction along the neuronal axons [11], [12].
Fig. 1.
Roles of transplanted neural precursor cells in spinal cord injury. There are three mechanisms for tissue recovery after cell transplantation. (A) The grafted cells differentiate into neurons and form synapses with host neuronal cells. (B) The transplanted cells differentiate into oligodendrocytes and myelinated host axons. (C) The grafts secrete neurotrophic factors and prevent secondary damage.
Our group previously established an induction protocol to make NPCs from several mouse iPSC lines by mimicking ESC technology [13], [14]. However, the cellular characteristics varied depending on the iPSC lines, and some of the iPSC-derived NPCs formed tumors when transplanted into CNS tissues. The diversity of properties among iPSC lines may be attributed to the expression profiles of the other remaining genes in the original somatic cells. Based on the results, we selected nontumorigenic, safe iPSCs and transplanted these iPSC-NPCs into an SCI mouse model [15]. As expected, the grafted NPCs differentiated into three neuronal lineages without tumor formation. In particular, the transplanted cells enhanced remyelination of host neurons and induced neurite regrowth around the lesion area (Fig. 1). These mechanisms contributed to locomotor functional recovery, which was quite similar to the regenerative potential in NPCs derived from the fetal CNS or from ESCs [16], [17], [18]. In contrast to the good and safe iPSC clones, unstable tumorigenic iPSC-NPCs formed tumors and resulted in neurological deterioration after transplantation in an SCI model. Therefore, although iPSC-NPCs have great potential to restore the histologically and functionally damaged spinal cord, the cellular characteristics such as genome stability and tumorigenicity must be evaluated before transplantation to select safe clones.
The effectiveness of human iPSCs is directly connected to the realization of their clinical application for SCI treatment. In 2011, we transplanted human iPSC-NPCs into a rodent SCI model and determined that the transplanted cells mainly differentiated into neurons that formed synaptic connections with host axons [19]. The human iPSC-NPCs enhanced axonal regrowth, angiogenesis, and preservation of the whole spinal cord. These beneficial effects contributed to neurological and electrophysiological recovery. Importantly, the NPCs did not create tumors for up to three months after transplantation. Although a longer observational period is essential to validate the nontumorigenic properties, this was the first study to demonstrate the efficacy and safety of human iPSCs for therapeutic strategies in SCI. To investigate the efficacy of human iPSCs in nonhuman primates (NHPs), we next induced NHP SCI models using common marmosets and transplanted human iPSC-NPCs at the subacute stage [20]. Again, the transplanted cells predominantly differentiated into neurons around the lesion site without tumorigenicity and promoted axonal regrowth and angiogenesis. These positive mechanisms resulted in significant functional recovery in the NPC recipients compared to that in the vehicle control group.
The efficacy of human iPSC-NPCs has also been demonstrated in laboratories worldwide. Tuszynski's group initially transplanted human NPCs harvested from fetal CNS tissues, and in contrast to the NPCs with brain characteristics, the cells with spinal cord properties promoted regeneration of the host corticospinal tract (CST) with motor functional improvement [21], [22], [23]. Accordingly, this group induced human ESCs and iPSCs into NPCs with spinal cord characteristics and demonstrated CST regeneration and neurological recovery after transplantation [21], [24]. These studies indicate that NPCs exert their beneficial effects on an injured spinal cord if they are cultured for caudalization along the neural axis and imitate the characteristics of the host tissue. In fact, transplantation of iPSC-NPCs that were not induced to exhibit spinal cord properties was performed in a previous study, and the animals failed to achieve motor recovery [25].
Nakashima's group reported the efficacy of medication against neural inflammation associated with iPSC-NPC transplantation [26]. This group focused on high mobility group box-1 (HMGB1), which plays an important role in triggering the inflammatory response in CNS injury, and developed an anti-HMGB1 antibody. When administrating this antibody to a mouse SCI model, disruption of the blood-spinal cord barrier was reduced, and subsequent edema formation and cell apoptosis were alleviated. This medication also enhanced the survival and preservation of host neurons. After iPSC-NPCs were transplanted into this modified environment of an injured spinal cord, the cells formed abundant synaptic connections with host neurons, which promoted functional locomotor recovery.
In the actual clinical setting, autologous cell transplantation is ideal for preventing immune rejection, but it requires a considerable number of trials and a high cost for the quality assessment of iPSC-NPCs before transplantation. Therefore, allogenic grafts using iPSC stocks would be more realistic [27], [28]. To achieve successful allogenic transplantation, however, immunosuppressant drugs should be considered. Indeed, Romanyuk et al. transplanted human iPSC-NPCs into a rodent SCI model with the use of immunosuppressant medications [29]. Their results demonstrated that the grafted cells survived with sufficient neuronal maturation, preserved the damaged spinal cord, and secreted neurotrophic factors. A recent study reported the establishment of swine-derived iPSC-NPCs transplanted into porcine SCI models. If the swine leukocyte antigen (SLA)-matched NPCs were used for syngeneic grafting, the transplanted cells survived without any immunosuppressant treatment for three months. Even if SLA-mismatched NPCs were transplanted, administration of an immunosuppressant for only one month resulted in good graft survival with motor improvement. Our group performed a mixed lymphocyte reaction (MLR) assay to evaluate the significance of human leukocyte antigen (HLA)-matching in human iPSC-NPC transplantation [30]. When HLA-mismatched iPSC-NPCs were cultured with peripheral blood mononuclear cells (PBMCs), the immune response was surprisingly low, and the reaction levels were comparable between HLA-matched and HLA-mismatched NPCs. Moreover, iPSC-NPCs suppressed the proliferative reaction of allogenic HLA-mismatched PBMCs in a dose-dependent manner. Because the iPSC-NPCs exhibited low antigen-presenting function even under stimulation by inflammatory cytokines [30], those studies including ours indicate that the cells possess immunosuppressive effects even under an allogenic leukocyte antigen-mismatching condition. Therefore, the dose and duration of immunosuppressant should be verified cautiously. However, further study is necessary to validate and reproduce this low-immunogenic phenomenon in in vivo NHP models for clinical application.
Recently, Fehlings's group reported unique studies using directly reprogrammed human NPCs (drNPCs), which were produced from bone marrow somatic cells using transient transfection of the three factors musashi-1, neurogenin-2, and methyl-CpG binding domain protein 2. This induction method does not have the cells passing through pluripotent status as in iPSCs and may shorten the duration of NPC production while enhancing the efficiency of the reprogramming process [31], [32]. These authors established a protocol to generate cells with more of an oligodendrogenic fate from drNPCs and transplanted these cells into a rat SCI model [31]. The oligogenic NPCs, which were transplanted at the subacute phase, survived at the lesion site at a rate of approximately 30%, myelinated host axons and spared white matter area. These beneficial effects contributed to locomotor functional recovery without tumorigenicity. Transplanting the cells at the chronic phase, approximately seven weeks post injury, to enhance the survival rate of the graft was also attempted. Chondroitinase ABC (C-ABC)-included hydrogel was added one week before transplantation to digest glial scar tissue [32]. Similar to the results observed in the subacute study, the cells mainly differentiated into oligodendrocytes, created nodes of Ranvier for saltatory conduction, and formed synaptic connections with host neurons. These mechanisms accompanied by histological recovery led to functional restoration even at the chronic stage of transplantation.
3. Provisions for safety issues in human iPSC-NPC transplantation
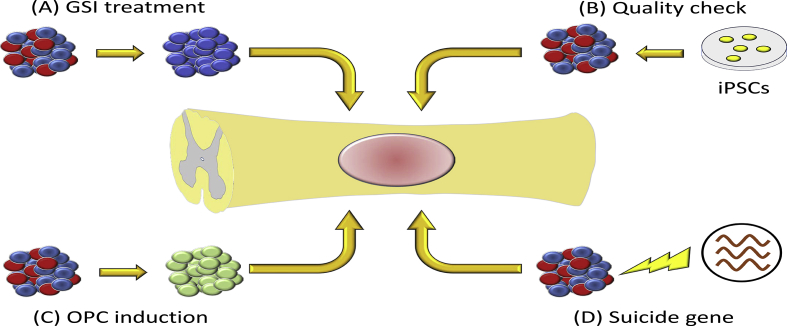
As we described above, histological and functional recovery were achieved after NPC transplantation if we chose the safe iPSC cell line [19], [20]. However, unsafe and unstable human iPSC lines have shown tumorigenic potential after grafting [33]. Our group has approached this critical issue and achieved significant results (Fig. 2).
Fig. 2.
Strategy to prevent tumorigenicity after transplantation. (A) A γ-secretase inhibitor (GSI) promotes maturation and neuronal differentiation of neural precursor cells (NPCs). As a result, tumor-initiating cells are removed before transplantation. (B) Thorough investigation is important for the iPSCs and the derivative NPCs prior to transplantation. Only safe and effective cells after a proper quality check should be transplanted. (C) The NPCs are pushed to become more mature oligodendrocyte progenitor cells (OPCs) before transplantation. (D) Suicide genes are integrated into NPCs beforehand. If transplanted cells show tumorigenicity, an apoptosis inducer should be injected systematically.
First, the best method for examining which cell lines have the risk for tumor formation remains elusive. Therefore, identifying the factors that regulate the tumorigenicity of iPSC-NPCs prior to transplantation would be ideal. We focused on genetic and epigenetic mechanisms underlying the tumor pathogenesis by comprehensive DNA methylation analyses [34]. In this study, we clarified that distinct differences in the DNA methylation pattern were detected between safe and tumorigenic iPSC-NPCs. In particular, large differences in the DNA methylation status of several tumor suppressor genes were involved. Intriguingly, the methylation patterns were affected by differences not only in cell lines but also in passage number. Thus, the methylation profiles could be included in the criteria to choose safe iPSC-NPCs in an actual clinical setting.
Even if the NPCs are judged as safe cell lines after a quality check, the risk for tumorigenicity cannot be excluded due to the contamination of undifferentiated cells prior to transplantation. To solve this issue, one approach is to eliminate these cells using a γ-secretase inhibitor (GSI), which inhibits Notch signaling [35]. The status of undifferentiated NPCs is controlled by Notch signaling, and inhibition of this signaling promotes the additional maturation and neuronal differentiation of NPCs. In our study, tumorigenic human iPSC-NPCs were treated with GSI for only one day in vitro, and they exhibited neuronal differentiation, reduction in cell proliferation, and suppression of tumor-related gene expression. When transplanted into the SCI model of NOD/SCID mice, the iPSC-NPCs mainly generated mature neurons around the injury site and did not form tumors as late as 89 days after transplantation. On the other hand, non-GSI-treated NPCs formed tumors and resulted in declining motor function. Thus, pretreatment with GSI can eliminate tumor-initiating cells in human iPSC-NPCs and promote the potential to overcome the safety issues related to tumorigenicity after cell transplantation.
Previously, oligodendrocyte progenitor cells (OPCs) have been demonstrated to be a cellular source for transplantation therapy in SCI. Keirstead and his colleagues transplanted ESC-derived human OPCs and observed robust remyelination of host neuronal axons without tumor formation [36]. Following the beneficial results, the Geron Corporation begun a clinical trial using ESC-OPCs in 2010, which was taken over by Asterias Biotherapeutics Inc. and validated the safety and efficacy of the cells for transplantation [37], [38]. This phase I/IIa trial targets cervical SCI patients at the subacute phase, and enrollment of 25 participants has already been completed (ClinicalTrials.gov Identifier: NCT02302157). Patients were evaluated for their neurological outcomes one year after transplantation, and the results are expected to be published soon. With respect to the oligogenic culture system, we also succeeded in establishing a protocol to enrich OPCs from human iPSC-NPCs [39] and grafted the cells into a rodent SCI model [12]. Around the lesion area at the spinal cord, the frequency of graft differentiation was 40% in oligodendrocytes, which was a dramatic enhancement compared to the 3% of oligo-lineage differentiation from the original NPCs [19]. The grafts promoted remyelination and axonal growth with synapse formation, resulting in functional and electrophysiological recovery. Similar results were observed in another study, which established a culture protocol to generate OPCs from human iPSCs [40]. The OPCs were transplanted into the rat spinal cord 24 h after injury, and more than 70% of the grafts differentiated into mature oligodendrocytes around the lesion site without tumorigenicity. The cells protected host axons by remyelination and reduced the cavity and glial scar area, leading to functional recovery three months after SCI. Together with the results from several studies, these observations indicate that biasing NPCs toward oligodendrogenic cells could reduce the potential for tumorigenicity by pushing immature cells to be more committed premature cells. Moreover, transplantation of oligodendrogenic NPCs themselves may be a beneficial strategy for regenerative therapy to remyelinate damaged host axons and recover saltatory conduction, lending potential for neurological restoration.
Even if the NPCs were pretreated with GSI or pushed toward oligogenic cells, concern still remains about posttransplantation tumor formation depending on iPSC lines. Therefore, considering the provisions for tumor ablation after transplantation is critically important. We performed unique studies using a suicide gene system [41], [42]. One of the suicide genes, inducible caspase-9, was transduced into iPSC lines with potential tumorigenicity, and after NPC differentiation, the cells were transplanted into the injured spinal cord of NOD/SCID mice. When the apoptosis inducer was systematically injected into the animals, the transplanted cells disappeared histologically, and further functional loss did not occur [41]. However, this genomic system killed all types of transplanted cells, and ideally, only proliferating tumor cells would be eliminated, while differentiated, mature neural cells would be spared. We therefore transduced the herpes simplex virus type I thymidine kinase (HSVtk) gene into tumorigenic human iPSC-NPCs [42]. HSVtk phosphorylates its prodrug ganciclovir (GCV), which leads to the production of cytotoxic GCV phosphate and kills immature and/or proliferating tumor cells while sparing postmitotic mature neural cells. Upon transplantation of iPSC-NPCs transduced with HSVtk into a rodent SCI model, only the tumorigenic cells were ablated after GCV injection, and mature neuronal cells were preserved, contributing to the maintenance of the recovered locomotor function. Because the HSVtk/GCV system has already been applied in some clinical trials without any safety problems [43], [44], this technique can also be used in the clinical setting of our iPSC project for SCI patients.
4. Clinical trial for treating SCI using iPSC-derived NPCs
Based on the efficacy and safety results of cell transplantation therapy, we have prepared a clinical study using human iPSC-NPCs for SCI patients [28]. For the clinical application, we have collaborated with the Center for iPS Cell Research and Application (CiRA) at Kyoto University, which establishes clinical-grade, integration-free human iPSC lines. We have already succeeded in generating NPCs from the provided iPSCs and thoroughly evaluated their characteristics and quality by various endpoints, such as characteristic marker expression and functional, genomic, and safety analyses in vitro [45], [46]. We performed an in vivo study by transplanting the cells into animal models of SCI to assess functional recovery. To examine safety issues, we grafted the cells into the rodent CNS and observed the animals over three months after transplantation to evaluate tumor formation. After completing all subitems, our plan for this trial was eventually approved by the Keio University Certified Special Committee for Regenerative Medicine on November 2018. At present, SCI patients with ASIA impairment scale A are the target subjects for our clinical study, and 2 × 106 iPSC-NPCs will be transplanted at 14–28 days after injury. The patients will be followed-up for one year, undergoing neurological and imaging evaluations and rehabilitation [28]. In the future, validation of iPSC-NPC transplantation at the subacute phase will expand toward graft indication for chronic SCI.
5. Regeneration during the chronic phase of SCI
Most SCI studies focus on the acute to subacute phases because of the plasticity and reactivity of therapeutic interventions. Demographically, however, a great majority of SCI patients suffer from their impairment and disability at the chronic phase. At this stage, substantial loss of neural tissue has led to the formation of a cavity surrounded by glial scar tissue, and suppression of axonal elongation occurs due to neurite inhibitory molecules [45], [47]. In this unfavorable environment for regeneration, transplanted NPCs do not exert their beneficial potential, with low survival rates and failure to achieve histological and neurological restoration.
Despite this complicated pathology, several studies have reported on the efficacy of cell transplantation therapy for chronic SCI. Recently, our group reported modest but significant functional recovery using GSI for transplanting iPSC-NPCs [48]. We transplanted GSI-treated iPSC-NPCs into the spinal cord of NOD/SCID mice 6 weeks after injury and found that the grafted cells predominantly differentiated into mature neuronal cells, while the number of undifferentiated, immature NPCs was reduced 12 weeks after transplantation. The differentiated neuronal axons formed inhibitory synaptic connections with host tissues, indicating that suppression of spasticity by the grafts contributed to improved motor coordination. Interestingly, inhibition of Notch signaling by GSI resulted in enhanced phosphorylation of p38 MAPK, which plays an important role in axonal regeneration. Thus, GSI treatment for NPCs may have favorable outcomes for functional recovery even in the chronic phase. However, combination therapy with rehabilitation should be considered for spinal cord regeneration to enhance the restoration of locomotor function.
Although mouse CNS-derived NPCs were used, we previously evaluated the effectiveness of NPC transplantation for chronic SCI combined with rehabilitation therapy [49]. The cells were transplanted 49 days after SCI, and their recipients continued to conduct treadmill training for 8 weeks. This combination therapy promoted neuronal differentiation of the grafted cells, increased serotonergic neurons and axonal regeneration, and enhanced coordination gait. Moreover, these interventions ameliorated sensory abnormalities, such as thermal allodynia and tactile hyperalgesia [50]. Therefore, treadmill exercise with NPC transplantation promoted neuronal differentiation, regeneration and maturation of neural circuits and enhanced the recovery of motor and sensory functions.
Chondroitin sulfate proteoglycans (CSPGs), which are produced from reactive astrocytes and distribute to glial scar tissue at the chronic phase of SCI, are inhibitory molecules of neuronal regeneration [51]. C-ABC is a bacterial enzyme that detaches the GAG from CSPG proteins, and this degradation allows neural axons to grow [51], [52]. Our group evaluated the efficacy of C-ABC treatment at the chronic phase of SCI combined with treadmill exercise and demonstrated functional motor recovery with a significant increase in regenerating neurite axons [53]. Another group performed NPC transplantation with C-ABC infusion and rehabilitation and revealed enhanced cell survival rates and increased motor-related neurons with functional improvement [32], [54], [55]. Together with the studies above, these observations suggest that the regenerative capacity can still be preserved even in the chronic phase of SCI, and appropriate therapeutic intervention could provide beneficial effects to enhance this potential.
6. Conclusions
Although iPSC-NPCs are at risk for tumorigenicity, we can achieve safe transplantation therapy by comprehensively evaluating the cell quality before grafting and taking precautions against tumor formation. Our many years of efforts have almost reached the realization of clinical trials using iPSCs, but continuous basic and clinical research are indispensable to enhancing the regenerative capacity of the spinal cord, especially during the chronic phase of injury.
Conflicts of interest
H.O. is a founding scientist of SanBio Co. Ltd. and K Pharma Inc. M.N. is a founding scientist of K Pharma Inc. N.N. and O.T. declare no competing interests.
Acknowledgments
This work was supported by the Research Center Network for the Realization of Regenerative Medicine and the Japan Agency for Medical Research and Development (AMED) (18bk0104050h0003) (to H.O.).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Fehlings M.G., Wilson J.R., Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(Suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 2.Hurlbert R.J. Methylprednisolone for the treatment of acute spinal cord injury: point. Neurosurgery. 2014;61(Suppl 1):32–35. doi: 10.1227/NEU.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Okano H., Nakamura M., Yoshida K., Okada Y., Tsuji O., Nori S. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 6.Barnabe-Heider F., Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Kumamaru H., Ohkawa Y., Saiwai H., Yamada H., Kubota K., Kobayakawa K. Direct isolation and RNA-seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat Commun. 2012;3:1140. doi: 10.1038/ncomms2132. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura S., Yasuda A., Iwai H., Takano M., Kobayashi Y., Nori S. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abematsu M., Tsujimura K., Yamano M., Saito M., Kohno K., Kohyama J. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Investig. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings B.J., Uchida N., Tamaki S.J., Salazar D.L., Hooshmand M., Summers R. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi-Abdolrezaee S., Eftekharpour E., Wang J., Morshead C.M., Fehlings M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci :Off J Soc Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawabata S., Takano M., Numasawa-Kuroiwa Y., Itakura G., Kobayashi Y., Nishiyama Y. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura K., Okada Y., Aoi T., Okada A., Takahashi K., Okita K. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 14.Okada Y., Matsumoto A., Shimazaki T., Enoki R., Koizumi A., Ishii S. Spatiotemporal recapitulation of central nervous system development by murine embryonic stem cell-derived neural stem/progenitor cells. Stem Cells. 2008;26:3086–3098. doi: 10.1634/stemcells.2008-0293. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji O., Miura K., Okada Y., Fujiyoshi K., Mukaino M., Nagoshi N. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai G., Okada Y., Yamane J., Nagoshi N., Kitamura K., Mukaino M. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald J.W., Liu X.Z., Qu Y., Liu S., Mickey S.K., Turetsky D. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y., Sawamoto K., Miyata T., Miyao S., Watanabe M., Nakamura M. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 19.Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi Y., Okada Y., Itakura G., Iwai H., Nishimura S., Yasuda A. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadoya K., Lu P., Nguyen K., Lee-Kubli C., Kumamaru H., Yao L. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–487. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenzweig E.S., Brock J.H., Lu P., Kumamaru H., Salegio E.A., Kadoya K. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24:484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumamaru H., Kadoya K., Adler A.F., Takashima Y., Graham L., Coppola G. Generation and post-injury integration of human spinal cord neural stem cells. Nat Methods. 2018;15:723–731. doi: 10.1038/s41592-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 25.Lu P., Woodruff G., Wang Y., Graham L., Hunt M., Wu D. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uezono N., Zhu Y., Fujimoto Y., Yasui T., Matsuda T., Nakajo M. Prior treatment with anti-high mobility group box-1 antibody boosts human neural stem cell transplantation-mediated functional recovery after spinal cord injury. Stem Cells. 2018;36:737–750. doi: 10.1002/stem.2802. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama Y., Iwanami A., Kohyama J., Itakura G., Kawabata S., Sugai K. Safe and efficient method for cryopreservation of human induced pluripotent stem cell-derived neural stem and progenitor cells by a programmed freezer with a magnetic field. Neurosci Res. 2016 doi: 10.1016/j.neures.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji O., Sugai K., Yamaguchi R., Tashiro S., Nagoshi N., Kohyama J. Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells. 2018 doi: 10.1002/stem.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romanyuk N., Amemori T., Turnovcova K., Prochazka P., Onteniente B., Sykova E. Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant. 2015;24:1781–1797. doi: 10.3727/096368914X684042. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki M., Iwanami A., Nagoshi N., Kohyama J., Itakura G., Iwai H. Evaluation of the immunogenicity of human iPS cell-derived neural stem/progenitor cells in vitro. Stem Cell Res. 2017;19:128–138. doi: 10.1016/j.scr.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Nagoshi N., Khazaei M., Ahlfors J.E., Ahuja C.S., Nori S., Wang J. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cell Trans Med. 2018;7:806–818. doi: 10.1002/sctm.17-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nori S., Khazaei M., Ahuja C.S., Yokota K., Ahlfors J.E., Liu Y. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Rep. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nori S., Okada Y., Nishimura S., Sasaki T., Itakura G., Kobayashi Y. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015;4:360–373. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iida T., Iwanami A., Sanosaka T., Kohyama J., Miyoshi H., Nagoshi N. Whole-genome DNA methylation analyses revealed epigenetic instability in tumorigenic human iPS cell-derived neural stem/progenitor cells. Stem Cells. 2017;35:1316–1327. doi: 10.1002/stem.2581. [DOI] [PubMed] [Google Scholar]
- 35.Okubo T., Iwanami A., Kohyama J., Itakura G., Kawabata S., Nishiyama Y. Pretreatment with a gamma-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injury. Stem Cell Rep. 2016;7:649–663. doi: 10.1016/j.stemcr.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keirstead H.S., Nistor G., Bernal G., Totoiu M., Cloutier F., Sharp K. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci : Off J Soc Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manley N.C., Priest C.A., Denham J., Wirth E.D., 3rd, Lebkowski J.S. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cell Trans Med. 2017;6:1917–1929. doi: 10.1002/sctm.17-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priest C.A., Manley N.C., Denham J., Wirth E.D., 3rd, Lebkowski J.S. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. 2015;10:939–958. doi: 10.2217/rme.15.57. [DOI] [PubMed] [Google Scholar]
- 39.Numasawa-Kuroiwa Y., Okada Y., Shibata S., Kishi N., Akamatsu W., Shoji M. Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes. Stem Cell Rep. 2014;2:648–661. doi: 10.1016/j.stemcr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.All A.H., Gharibani P., Gupta S., Bazley F.A., Pashai N., Chou B.K. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itakura G., Kawabata S., Ando M., Nishiyama Y., Sugai K., Ozaki M. Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Rep. 2017;8:673–684. doi: 10.1016/j.stemcr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kojima K., Miyoshi H., Nagoshi N., Kohyama J., Itakura G., Kawabata S. Selective ablation of tumorigenic cells following human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in spinal cord injury. Stem Cell Trans Med. 2018 doi: 10.1002/sctm.18-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillat C., Carrio M., Cascante A., Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 44.Sangro B., Mazzolini G., Ruiz M., Ruiz J., Quiroga J., Herrero I. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837–843. doi: 10.1038/cgt.2010.40. [DOI] [PubMed] [Google Scholar]
- 45.Nagoshi N., Okano H. iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol Life Sci : CMLS. 2018;75:989–1000. doi: 10.1007/s00018-017-2676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isoda M., Kohyama J., Iwanami A., Sanosaka T., Sugai K., Yamaguchi R. Robust production of human neural cells by establishing neuroepithelial-like stem cells from peripheral blood mononuclear cell-derived feeder-free iPSCs under xeno-free conditions. Neurosci Res. 2016;110:18–28. doi: 10.1016/j.neures.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 48.Okubo T., Nagoshi N., Kohyama J., Tsuji O., Shinozaki M., Shibata S. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC- derived transplants for chronic spinal cord injury. Stem Cell Rep. 2018;11:1416–1432. doi: 10.1016/j.stemcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tashiro S., Nishimura S., Iwai H., Sugai K., Zhang L., Shinozaki M. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in mice with chronic spinal cord injury. Sci Rep. 2016;6:30898. doi: 10.1038/srep30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tashiro S., Nishimura S., Shinozaki M., Takano M., Konomi T., Tsuji O. The amelioration of pain-related behavior in mice with chronic spinal cord injury treated with neural stem/progenitor cell transplantation combined with treadmill training. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5537. [DOI] [PubMed] [Google Scholar]
- 51.Bradbury E.J., Carter L.M. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84:306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Zhao R.R., Fawcett J.W. Combination treatment with chondroitinase ABC in spinal cord injury--breaking the barrier. Neurosci Bull. 2013;29:477–483. doi: 10.1007/s12264-013-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinozaki M., Iwanami A., Fujiyoshi K., Tashiro S., Kitamura K., Shibata S. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res. 2016 doi: 10.1016/j.neures.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Karimi-Abdolrezaee S., Eftekharpour E., Wang J., Schut D., Fehlings M.G. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci : Off J Soc Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki H., Ahuja C.S., Salewski R.P., Li L., Satkunendrarajah K., Nagoshi N. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]