Dephosphorylation of the lipid A 1-phosphate by LpxE in Gram-negative bacteria plays important roles in antibiotic resistance, bacterial virulence, and modulation of the host immune system. Our results demonstrate that in addition to removing the 1-phosphate from lipid A, LpxEs also dephosphorylate undecaprenyl pyrophosphate, an important metabolite for the synthesis of the essential envelope components, peptidoglycan and O-antigen. Therefore, LpxEs participate in multiple layers of biogenesis of the Gram-negative bacterial envelope and increase antibiotic resistance. This discovery marks an important step toward understanding the regulation and biogenesis of the Gram-negative bacterial envelope.
KEYWORDS: bacterial cell envelope biogenesis, lipid A 1-phosphate phosphatase, phosphatidylglycerol phosphate phosphatase, type 2 phosphatidic acid phosphatase (PAP2) superfamily, undecaprenyl pyrophosphate phosphatase
ABSTRACT
Although distinct lipid phosphatases are thought to be required for processing lipid A (component of the outer leaflet of the outer membrane), glycerophospholipid (component of the inner membrane and the inner leaflet of the outer membrane), and undecaprenyl pyrophosphate (C55-PP; precursors of peptidoglycan and O antigens of lipopolysaccharide) in Gram-negative bacteria, we report that the lipid A 1-phosphatases, LpxEs, functionally connect multiple layers of cell envelope biogenesis in Gram-negative bacteria. We found that Aquifex aeolicus LpxE structurally resembles YodM in Bacillus subtilis, a phosphatase for phosphatidylglycerol phosphate (PGP) with a weak in vitro activity on C55-PP, and rescues Escherichia coli deficient in PGP and C55-PP phosphatase activities; deletion of lpxE in Francisella novicida reduces the MIC value of bacitracin, indicating a significant contribution of LpxE to the native bacterial C55-PP phosphatase activity. Suppression of plasmid-borne lpxE in F. novicida deficient in chromosomally encoded C55-PP phosphatase activities results in cell enlargement, loss of O-antigen repeats of lipopolysaccharide, and ultimately cell death. These discoveries implicate LpxE as the first example of a multifunctional regulatory enzyme that orchestrates lipid A modification, O-antigen production, and peptidoglycan biogenesis to remodel multiple layers of the Gram-negative bacterial envelope.
INTRODUCTION
The Gram-negative bacterial cell envelope consists of three essential molecular architectures—the inner membrane, the peptidoglycan layer, and the outer membrane—that together protect bacteria against mechanical stress, maintain cell shape, and shield these microorganisms from the damage of detergents and antibiotics. These architectures are formed by distinct molecules, with phospholipids constituting the inner membrane and inner leaflet of the outer membrane, peptide-conjugated carbohydrates constituting the peptidoglycan layer, and lipopolysaccharides (LPS) anchoring at the outer leaflet of the outer membrane through the hydrophobic lipid A moiety. As peptidoglycan, phospholipids, and LPS are synthesized through distinct pathways, how Gram-negative bacteria orchestrate the biogenesis and remodeling across three layers of the cell envelope for optimal bacterial growth and virulence remains incompletely understood.
As the major lipid species coating the outer surface of Gram-negative bacteria, lipid A is the predominant signaling molecule that is detected by the mammalian Toll-like receptor 4 (TLR4)/myeloid differentiation factor 2 (MD-2) innate immune receptor (1) and caspase-4/-5/-11 (2) to trigger the host innate immune response to bacterial infection. With few exceptions, Gram-negative bacteria constitutively synthesize the 1,4′-bisphosphorylated tetra-acyl-lipid A intermediate, 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo)-linked lipid IVA (Kdo2-lipid IVA), via the action of seven conserved enzymes in the Raetz pathway (3) (see Fig. S1A in the supplemental material), which are essential to nearly all Gram-negative bacteria and are attractive targets for novel antibiotics (4–6). Gram-negative bacteria additionally harbor modification enzymes that further process the Kdo2-lipid IVA intermediate to generate unique lipid A molecules in each bacterial species to adapt to environmental changes and evade the host immune response (7). For example, the lipid A 1-phosphate is a key determinant for lipid A recognition by the mammalian TLR4/MD-2 innate immune receptor (8). Removal of the lipid A 1-phosphate by the membrane-embedded phosphatase LpxE strongly protects bacteria against host cationic peptides and the last-resort antibiotic colistin (9), significantly dampens the host innate immune response, and dramatically increases colonization and survival of Helicobacter pylori in the gastric mucosa (10).
Lipid A biosynthesis and modification. (A) Schematic illustration of Kdo2-lipid A biosynthesis and modification. The lipid A 1-phosphatase LpxE is in pink. (B) Lipid A structure of A. pyrophilus. Download FIG S1, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In order to gain molecular insights into the structure and function of the lipid A 1-phosphatase LpxE, we identified the previously uncharacterized gene aq_1706 from Aquifex aeolicus as the gene for the thermophilic LpxE enzyme (LpxEAA). Our structural analysis of LpxEAA shows distinct features between LpxEAA and Escherichia coli PgpB (PgpBEC) enzymes but reveals a surprising structural similarity to YodM, a phosphatase of phosphatidylglycerol phosphate (PGP) in the Gram-positive bacterium Bacillus subtilis with a weak in vitro activity on undecaprenyl pyrophosphate (C55-PP). Consistent with our structural analysis, we found that LpxEAA possesses substantial in vitro activities toward Kdo2-lipid A/lipid IVA, C55-PP, and PGP and complements E. coli strains deficient in C55-PP phosphatase and PGP phosphatase activities. In addition to the LpxE enzyme from A. aeolicus, distant LpxE orthologs from Francisella, Helicobacter, and Rhizobium also complement E. coli strains deficient in the C55-PP phosphatase activity, supporting the notion that the multifunctional lipid phosphatase activity is a general feature of LpxE enzymes. Significantly, deletion of the native lpxE gene sensitizes Francisella novicida to bacitracin, an antibiotic that sequesters C55-PP to disrupt peptidoglycan synthesis; furthermore, suppression of plasmid-encoded lpxE in the F. novicida strain deficient in the endogenous C55-PP phosphatase activity results in noticeable changes in cell morphology, profound reduction of O-antigen repeats in LPS, and loss of cell viability. Taken together, these observations reveal a previously unappreciated contribution of LpxE to peptidoglycan biogenesis and LPS O-antigen modification beyond its well-recognized role as the lipid A 1-phosphatase to orchestrate the remodeling of multiple layers of the Gram-negative bacterial envelope to respond to environmental changes, evade host immune surveillance, and promote bacterial viability and virulence.
RESULTS
A distant ortholog of LpxEFN in A. aeolicus.
LpxE is a member of the lipid phosphatase/phosphotransferase (LPT) family, a well-distributed family of lipid-processing enzymes also known as the integral transmembrane branch of the type II phosphatidic acid phosphatase (PAP2) superfamily (11, 12). This family is characterized by a conserved tripartite active site motif of KX6RP---PSGH---SRX5HX3D and activity independent of Mg2+ or other cations (13). The LPT family includes enzymes responsible for processing several types of lipids in Gram-negative bacteria, including the membrane-embedded PgpB, which dephosphorylates PGP and C55-PP (14) (Fig. S2). Even though PgpB and LpxE are both members of the LPT family, they have been reported to have distinct substrate specificities: PgpB is unable to utilize lipid A as a substrate (15), whereas purified LpxE from Rhizobium leguminosarum (LpxERL) utilizes PGP ∼1,000 times less efficiently than lipid A species as a substrate in vitro (16).
Lipid phosphatases. Reactions of PGP and C55-PP phosphatases are shown in panels A and B, respectively. Download FIG S2, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In order to gain a molecular understanding of the LpxE structure and function, we searched for a thermophilic LpxE enzyme from Aquificae to facilitate structural analysis. The lipid A of Aquifex pyrophilus LPS contains d-galacturonic acid in place of phosphates at the 1- and 4′-positions (17) (Fig. S1B). As the 1,4′-bisphosphorylated lipid IVA is a common lipid A intermediate before further modification (18, 19) and as Aquificae has the conserved biosynthetic enzymes to make 1,4′-bisphosphorylated lipid IVA, incorporation of the d-galacturonic acid moiety requires the removal of 1-phosphate from lipid A, indicating the presence of the lipid A 1-phosphatase activity in Aquificae. Such a rationale led us to search for the gene responsible for the lipid A 1-phosphatase activity in A. aeolicus VF5, as no lipid A 1-phosphatase has been reported in any Aquifex species.
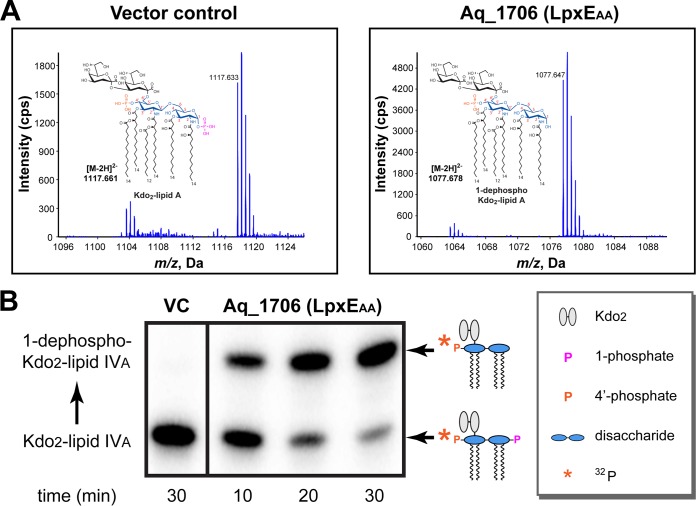
A Position-Specific Iterated Basic Local Alignment Search Tool (PSI-BLAST) (20) search revealed a distant ortholog of F. novicida LpxE (LpxEFN) (15), Aq_1706 (E value, 0.81; sequence identity, 13.84%), in the genome of A. aeolicus VF5. Aq_1706 shares little sequence identity with other LpxE enzymes (sequence identities of Aq_1706 with LpxE of Helicobacter and Rhizobium are 16.58% and 14.57%, respectively), except for the well-conserved tripartite active-site motif of KX6RP---PSGH---SRX5HX3D (Fig. S3). In order to determine if aq_1706 encodes the lipid A 1-phosphatase activity in vivo, we overexpressed Aq_1706 in the heptose transferase-deficient E. coli strain WBB06, which produces Kdo2-lipid A instead of full-length LPS, to facilitate mass spectrometry analysis of lipid A modifications (21). Since E. coli does not encode LpxE activity, mass spectrometry analysis of the extracted lipids showed normal lipid A containing 1-phosphate with an m/z of 1,117.633 for the [M-2H]2− ion species (calculated m/z, 1,117.661 for the exact mass of 2,237.336 of Kdo2-lipid A) from E. coli cells expressing a control vector; in contrast, overexpression of Aq_1706 in E. coli led to the disappearance of the intact lipid A species and significant accumulation of lipid A molecules lacking the 1-phosphate group, with an m/z of 1,077.647 for the [M-2H]2− ion species (calculated m/z, 1,077.678 for the exact mass of 2,157.370 of 1-dephospho Kdo2-lipid A), consistent with the anticipated lipid A 1-phosphatase activity (Fig. 1A). In order to verify that the loss of phosphate occurred at the 1-position, but not at the 4′-position, we further tested the ability of LpxE to dephosphorylate 4′-32P-labeled Kdo2-lipid IVA, which was previously shown to be an efficient substrate for LpxE enzymes with specific activity comparable to that for the substrate Kdo2-lipid A (16). We found that treatment of Kdo2-[4′-32P] lipid IVA with membrane extracts from E. coli overexpressing Aq_1706, but not those carrying a control vector, resulted in time-dependent reduction of the Kdo2-lipid IVA band and accumulation of an upper-shifted band on the thin-layer chromatography (TLC) plate (Fig. 1B), reflecting the removal of 1-phosphate but retention of the 32P-labeled 4′-phosphate group. Taken together, these observations verify aq_1706 in A. aeolicus as the gene that encodes the thermophilic lipid A 1-phosphatase LpxE (LpxEAA).
FIG 1.
Characterization of Aq_1706 from A. aeolicus as the lipid A 1-phosphatase LpxEAA. (A) Mass spectrometry analysis of lipid A species in the heptose-deficient E. coli strain WBB06 (left) and the WBB06 strain overexpressing LpxEAA (right). (B) 32P-autoradiographic TLC-based Kdo2-lipid IVA 1-dephosphorylation assay of the membrane extract of the C41(DE3) strain overexpressing LpxEAA.
Sequence alignment of LpxE orthologs. Alignment was achieved by Clustal Omega (F. Sievers, A. Wilm, D. Dineen, T. J. Gibson, et al., Mol Syst Biol 7:539, 2011) with manual adjustment. Conserved residues in the signature motifs of the PAP2 family of enzymes are labeled. Download FIG S3, TIF file, 2.9 MB (3MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Structural analysis of LpxEAA reveals a striking similarity to YodM in B. subtilis.
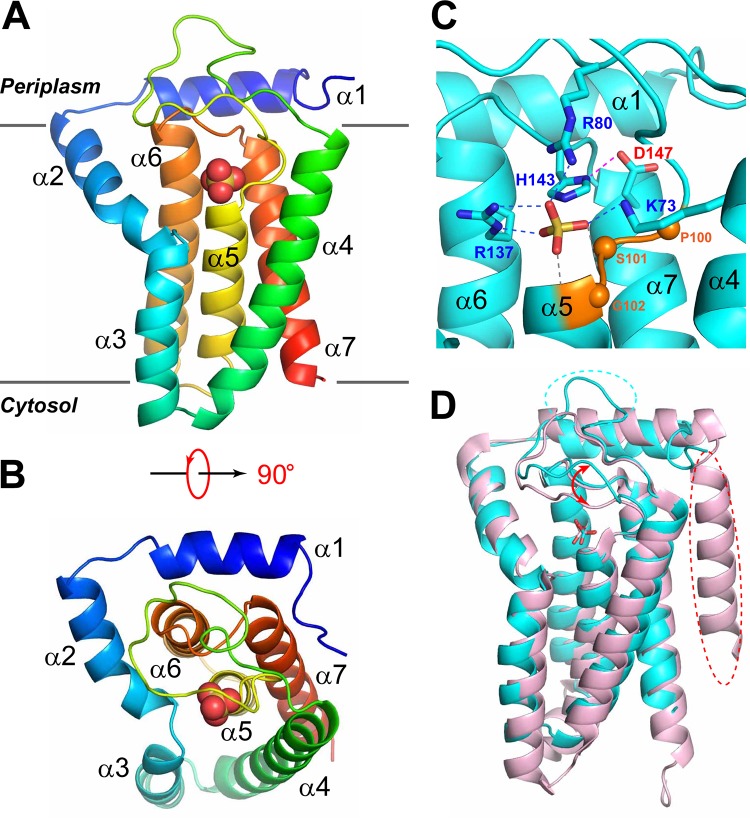
After verifying the lipid A 1-phosphatase activity of LpxEAA, we cloned and purified LpxEAA. Consistent with the TMHMM analysis (http://www.cbs.dtu.dk/services/TMHMM/), high-yield expression of LpxEAA was achieved in a maltose-binding protein (MBP) fusion construct containing an N-terminal PelB secretion signal (22), suggesting that the N terminus of LpxEAA is located at the periplasmic side of the inner membrane. The crystal structure of LpxEAA containing an I63M mutation was determined at 2.38 Å (Fig. 2A; statistics shown in Table S1). The selenomethionine substitution of the nonconserved I63 residue (I63M) was designed to enhance the selenium single anomalous dispersion (Se-SAD) signal for de novo phasing. The overall structure of LpxEAA contains seven α-helices, including an N-terminal amphiphilic helix lying at the periplasmic surface of the inner membrane and five tightly packed transmembrane helices (α3 to α7). Apart from α2, which originates from the periplasmic surface and penetrates halfway across the inner membrane at an ∼45° angle and immediately connects to transmembrane helix α3, the remaining helices are oriented largely in parallel or antiparallel with each other and perpendicularly to the membrane plane. Looking from the periplasmic surface, helix 5 (α5) is located at the center, which is surrounded by α2, α3, α4, α7, and α6 in a counterclockwise fashion (Fig. 2B).
FIG 2.
Crystal structure of LpxEAA. (A) Ribbon representation of LpxEAA, with blue to red colors corresponding to the N to C termini. The sulfate molecule is shown in the space-filling model. Individual helices and membrane locations are labeled. (B) Top view of LpxEAA. (C) The active site of LpxEAA. The sulfate molecule is shown in the stick model. Side chains of H143 and D147 from the RX5HX3D motif and conserved residues coordinating the sulfate molecule, including K73 and R80 from the KX6RP motif and R137 of the RX5HX3D motif, are shown in the stick model. Hydrogen bonds are shown by dashed lines. The sulfate group is additionally stabilized by the interaction with the electrical dipole of helix α5 (indicated by gray hydrogen bonds). The conserved PSG motif is colored in coral, with Cα atoms shown in spheres. (D) Superimposition of LpxEAA (cyan) with YodMBS (PDB code 5JKI; pink), revealing striking structural similarities. The major differences between the two structures are highlighted, with dashed circles indicating missing structural features (helix or loop) and arrows indicating conformational discrepancy.
Data collection and refinement statistics of LpxEAA. Download Table S1, DOCX file, 0.01 MB (14.3KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The active site of LpxE is located at the periplasmic surface of the inner membrane and is defined by conserved motifs specific to the PAP2 enzymes (K73X6 R80P---R137X5 H143X3 D147) located at the C-terminal end of α4, the α4-α5 loop, α6, the α6-α7 loop, and the N terminus of α7 (Fig. 2C). Fortuitously, a sulfate molecule is found in the active site, which is a structural analog of the 1-phosphate group of lipid A. The sulfate group is extensively recognized by K73 and R80 of the K73X6R80P motif and R137 of the R137X5H143X3D147 motif. The catalytically important H143 is located 3.3 Å away from the sulfur atom of the sulfate group, ready to carry out inline attack to remove the phosphate group of the lipid substrate. D147, the last residue of the R137X5H143X3D147 motif, forms a hydrogen bond with H143. Although the corresponding aspartate residue is found in most LpxE enzymes (Fig. S3), it is absent in the LpxE ortholog from H. pylori (LpxEHP), suggesting that it is not absolutely required for catalysis. The first three residues of the PSGH motif are conserved in LpxEAA, with the central serine residue (S101) serving as a helix cap to stabilize helix α5, but the histidine residue is replaced with an aspartate residue in LpxEAA (Fig. 2C).
The LpxEAA structure shows noticeable conformational discrepancy with the previously reported structures of PgpBEC (PDB codes 4PX7 and 5JWY) (23, 24), another PAP2 family enzyme, with overall backbone root mean square deviations (RMSDs) of ∼4.5 Å (Fig. S4); surprisingly, LpxEAA is structurally similar to the recently reported YodM in B. subtilis (PDB code 5JKI) (25), a PGP phosphatase with a weak in vitro activity on C55-PP, with an overall backbone RMSD of 1.2 Å (Fig. 2D). The major differences of these two enzymes are the absence of an N-terminal transmembrane helix in LpxEAA in comparison with YodM, a longer α4-α5 loop in LpxEAA, and a significant conformational variation of the α4-α5 loop surrounding the active site.
Superimposition of LpxEAA with PgpBEC structures. RMSDs of ∼4.5 Å were observed between the coordinates of LpxEAA and PgpBEC (PDB codes 4PX7 [J. Fan, D. Jiang, Y. Zhao, J. Liu, et al., Proc Natl Acad Sci U S A 111:7636–7640, 2014] and 5JWY [S. L. Tong, Y. B. Lin, S. Lu, M. T. Wang, et al., J Biol Chem 291:18342–18352, 2016]). LpxEAA and PgpBEC are shown in Cα traces, with LpxEAA colored in cyan and PgpBEC in gray. Download FIG S4, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
LpxEAA is a trifunctional lipid phosphatase in vitro and functionally complements E. coli mutants deficient in C55-PP or PGP phosphatase activities.
Surprised by the structural similarity between LpxEAA and YodMBS, we asked whether LpxEAA could function as a C55-PP and PGP phosphatase. To address this question, we compared the specific activities of purified LpxEAA toward Kdo2-lipid A, PGP, and C55-PP using the malachite green assay to detect the release of inorganic phosphate. As expected, LpxEAA efficiently catalyzed the hydrolysis of 1-phosphate from Kdo2-lipid A, with a specific activity of 2.04 ± 0.46 μmol/mg/min. Moreover, LpxEAA catalyzed C55-PP more efficiently than it catalyzed Kdo2-lipid A, with a specific activity of 3.58 ± 0.47 μmol/mg/min—a value that is ∼1.8-fold higher than that toward Kdo2-lipid A. Finally, LpxEAA also displayed significant activity toward PGP, with a specific activity of 0.75 ± 0.11 μmol/mg/min, ∼40% of its activity toward Kdo2-lipid A (Table 1). Taken together, our biochemical assays validate LpxEAA as a trifunctional LPT enzyme that efficiently dephosphorylates chemically diverse Kdo2-lipid A (glycolipids), PGP (phosphoglycerol lipid), and C55-PP (isoprenyl lipid) in vitro.
TABLE 1.
Specific activities of enzymes in this study
| Enzyme | Specific activity (μmol/mg/min) |
||
|---|---|---|---|
| Kdo2-lipid A | C55-PP | PGP | |
| LpxEAA | 2.04 ± 0.46 | 3.58 ± 0.47 | 0.75 ± 0.11 |
| LpxEFN | 3.25 ± 0.21 | 2.99 ± 0.45 | 0.038 ± 0.009 |
| UppPFN | 0.010 ± 0.005 | 22.71 ± 2.62 | 0.031 ± 0.007 |
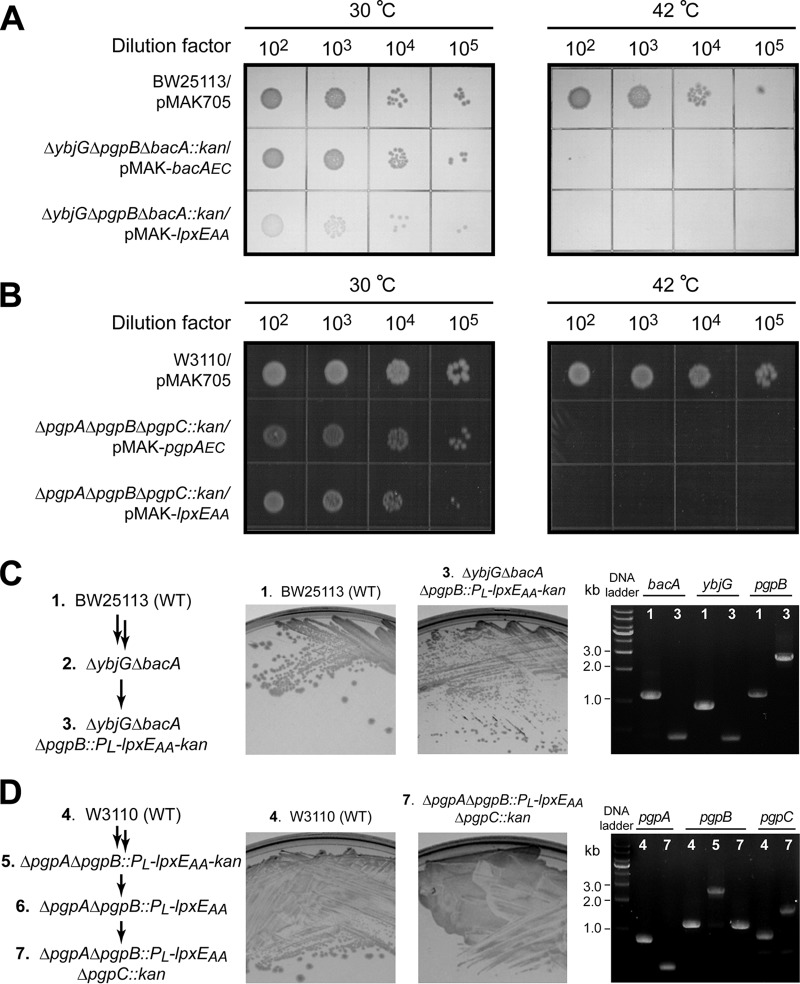
In order to obtain further evidence of the trifunctional role of LpxEAA in cells, we examined whether LpxEAA could functionally rescue lethal E. coli mutants lacking C55-PP phosphatase or PGP phosphatase activities. E. coli contains four C55-PP phosphatases, BacA, PgpB, YbjG, and LpxT. A deletion mutant, ΔybjG ΔbacA ΔpgpB::kan, in E. coli is lethal unless rescued by a plasmid expressing BacA, PgpB, or YbjG (26). To examine if LpxEAA could function as a C55-PP phosphatase in cells, we set up complementation of the lethal ΔybjG ΔpgpB ΔbacA::kan E. coli mutant carrying lpxEAA on a low-copy-number, temperature-sensitive pMAK705 vector (pMAK-lpxEAA). The E. coli bacA gene, encoding the C55-PP phosphatase, was used as the positive control (pMAK-bacAEC). We found that overexpression of LpxEAA and BacAEC from pMAK705-derived plasmids complemented the lethal phenotype of the ΔybjG ΔpgpB ΔbacA::kan triple knockout in E. coli on an LB agar plate at 30°C; such a complementation effect was lost when cells were grown at 42°C, consistent with the loss of the temperature-sensitive pMAK705 plasmid encoding LpxEAA or BacAEC and confirming that LpxEAA functionally complements the loss of C55-PP phosphatase activity in E. coli (Fig. 3A).
FIG 3.
LpxEAA complements E. coli strains deficient in C55-PP phosphatase or PGP phosphatase activities. (A) Complementation of the C55-PP phosphatase-deficient E. coli strain (BW25113 ΔybjG ΔpgpB ΔbacA::kan) by the temperature-sensitive pMAK705 plasmid harboring bacAEC (positive control, pMAK-bacAEC) or lpxEAA (pMAK-lpxEAA). WT E. coli cells carrying pMAK705 or C55-PP phosphatase-deficient E. coli cells carrying pMAK-bacAEC or pMAK-lpxEAA were grown at 30°C or 42°C. From left to right are spots of 10-fold serial dilutions from 102 to 105. (B) Complementation of the PGP phosphatase-deficient E. coli strain (W3110 ΔpgpA ΔpgpB ΔpgpC::kan) by the temperature-sensitive pMAK705 plasmid harboring pgpAEC (positive control, pMAK-pgpAEC) or lpxEAA (pMAK-lpxEAA). WT E. coli cells carrying pMAK705 or PGP phosphatase-deficient E. coli cells carrying pMAK-pgpAEC or pMAK-lpxEAA were grown at 30°C or 42°C. From left to right are spots of 10-fold serial dilutions from 102 to 105. (C) Chromosomal complementation of C55-PP phosphatase activity-deficient E. coli with lpxEAA. The left, middle, and right images show the construction of different E. coli C55-PP phosphatase gene deletion mutants, the growth of WT E. coli cells and C55-PP phosphatase-deficient cells complemented by a chromosomal copy of lpxEAA, and the PCR verification of ybjG, bacA, and pgpB knockouts of the target mutant strain, respectively. (D) Chromosomal complementation of PGP phosphatase activity-deficient E. coli with lpxEAA. The left, middle, and right images show the construction of different E. coli PGP phosphatase gene deletion mutants, the growth of WT E. coli cells and PGP phosphatase-deficient cells complemented by a chromosomal copy of lpxEAA, and PCR verification of pgpA, pgpC, and pgpB knockouts of the target mutant strain, respectively. Since the expected sizes of pgpB (1,106 bp) and pgpB::PL-lpxEAA (1,093 bp) are similar using primers flanking pgpB in the final strain, the knockout of pgpB was established by also verifying the PCR result of the mother strain (strain 5: W3110 ΔpgpA ΔpgpB::PL-lpxEAA-frt-kan-frt).
We similarly tested whether LpxEAA functionally complements the loss of PGP phosphatase activity in E. coli. E. coli has three PGP phosphatases, PgpA, PgpB, and PgpC (27). A ΔpgpA ΔpgpB ΔpgpC::kan triple-knockout mutant is lethal unless it is rescued by a plasmid harboring an active PGP phosphatase (27). Overexpression of LpxEAA or the positive control PgpAEC from the temperature-sensitive pMAK705 plasmid supported the growth of the ΔpgpA ΔpgpB ΔpgpC::kan triple-knockout mutant strains at 30°C but not at 42°C. In contrast, the control strain (W3110/pMAK705) grew well at both temperatures (Fig. 3B). These observations confirm that LpxEAA is a functional PGP phosphatase in E. coli.
While the pMAK705 vector-encoded LpxEAA complemented E. coli triple knockouts lacking C55-PP phosphatase or PGP phosphatase activities, pMAK705 has a higher copy number (pSC101 origin, ∼5 copies/cell) than that of the chromosome in E. coli (single copy/cell). In order to mitigate the concern that the observed genetic complementation was caused by multiple copies of the lpxEAA gene, we replaced the pgpB gene in the chromosome of E. coli (BW25113) ΔybjG ΔbacA with a gene cassette (PL-lpxEAA-FRT-kan-FRT) containing lpxEAA and a kanamycin resistance gene under the control of the PL promoter (28). The resulting E. coli strain (E. coli BW25113 ΔybjG ΔbacA ΔpgpB::PL-lpxEAA-FRT-kan-FRT) grew on an LB agar plate, and the proper knockouts of bacA, ybjG, and pgpB were verified by PCR (Fig. 3C), confirming that the chromosomal copy of lpxEAA complemented the loss of C55-PP phosphatase activity. Using a similar approach, we also replaced the pgpB gene of E. coli (W3110) ΔpgpA with PL-lpxEAA-FRT-kan-FRT, removed the kanamycin resistance cassette (29), and then knocked out pgpC. The resulting strain (E. coli W3110 ΔpgpA ΔpgpB::PL-lpxEAA ΔpgpC::kan) also grew on an LB agar plate, and knockouts of pgpA, pgpB, and pgpC were verified by PCR (Fig. 3D), confirming that the chromosomal copy of lpxEAA similarly complemented the loss of PGP phosphatase activity.
Altogether, the substantial phosphatase activities of LpxEAA toward Kdo2-lipid A, C55-PP, and PGP in vitro and its ability to complement the loss of C55-PP and PGP phosphatase activities in E. coli—both via the plasmid-borne gene and via chromosomal knock-in—strongly support the multifunctionality of LpxEAA in Gram-negative bacterial envelope biogenesis.
LpxEFN is a bifunctional lipid phosphatase in vitro and functionally complements an E. coli mutant deficient in the C55-PP phosphatase activity.
Despite the intriguing observation of the multifunctionality of LpxEAA, it is challenging to establish the biological consequence in its native host due to the difficulty of culturing and genetic manipulation of A. aeolicus. Therefore, we asked if other LpxE enzymes from genetically trackable bacteria similarly display multifunctional lipid phosphatase activities. In order to answer this question, we chose LpxEFN, a distant ortholog of LpxEAA, for further characterization. The ability of LpxEFN to dephosphorylate lipid A at the 1-position was previously reported (15), but its activity toward other lipid substrates has not been thoroughly investigated. We first conducted similar complementation experiments using E. coli strains deficient in either the C55-PP phosphatase activity or PGP phosphatase activity carrying the temperature-sensitive pMAK-lpxEFN. We found that LpxEFN complemented the loss of C55-PP phosphatase activity of E. coli (ΔybjG ΔpgpB ΔbacA::kan) at 30°C but not at 42°C, indicating that LpxEFN is a functional C55-PP phosphatase in E. coli (Fig. 4A). However, we were unable to complement E. coli deficient in the PGP activity (ΔpgpA ΔpgpB ΔpgpC::kan) with a plasmid encoding LpxEFN (pMAK-lpxEFN). Consistently, we found that purified LpxEFN displayed significant phosphatase activity toward both Kdo2-lipid A and C55-PP and processed these two substrates with similar efficiencies (specific activities of 3.25 ± 0.21 μmol/mg/min for Kdo2-lipid A and 2.99 ± 0.45 μmol/mg/min for C55-PP), but its activity toward PGP was ∼100-fold lower (specific activity of 0.038 ± 0.009 μmol/mg/min) (Table 1), confirming that LpxEFN is a bifunctional lipid phosphatase.
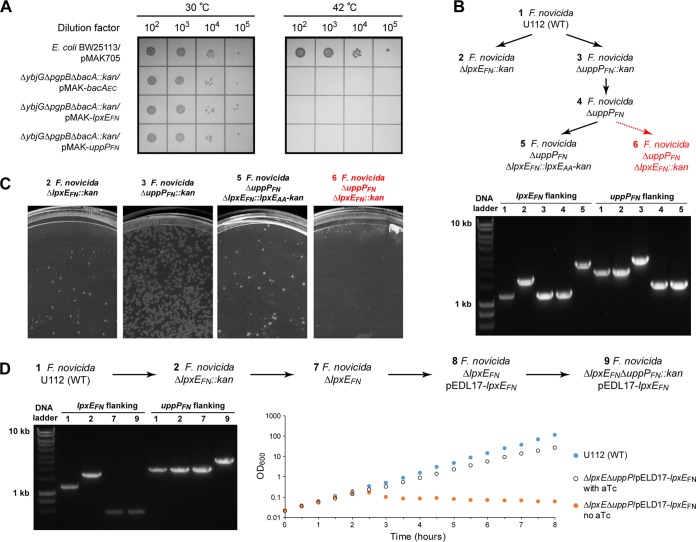
FIG 4.
F. novicida harbors two C55-PP phosphatases, LpxEFN and UppPFN. (A) Complementation of the C55-PP phosphatase-deficient E. coli strain (BW25113 ΔybjG ΔbacA ΔpgpB::kan) by the temperature-sensitive pMAK705 plasmid harboring bacAEC (positive control, pMAK-bacAEC), lpxEFN (pMAK-lpxEFN), or uppPFN (ftn_1552, pMAK-uppPFN). WT E. coli cells carrying pMAK705 or C55-PP phosphatase-deficient E. coli cells carrying pMAK-bacAEC, pMAK-lpxEFN, or pMAK-uppPFN were grown at 30°C or 42°C. From left to right are spots of 10-fold serial dilutions from 102 to 105. (B) Schematic illustration of the construction of different F. novicida gene deletion strains. Viable and lethal strains are in black and red, respectively. The presence of the proper gene deletion was verified by PCR using primers at ∼0.25-kb or 0.6-kb positions flanking lpxE or uppP, respectively. (C) Viability of F. novicida mutants. While F. novicida mutants containing ΔlpxEFN::kan, ΔuppPFN::kan, or ΔuppPFN ΔlpxEFN::lpxEAA were viable, no colonies could be isolated for F. novicida mutants containing ΔuppPFN ΔlpxEFN::kan. (D) Construction of the conditional lethal F. novicida strain containing ΔlpxEFN ΔuppPFN complemented by aTc-inducible pEDL17-lpxEFN. The sequence of strain construction is shown at the top. The presence of the desired gene deletion was verified by PCR (left). Prolonged withdrawal of aTc from the growth medium resulted in a slow bactericidal phenotype (right).
F. novicida harbors two C55-PP phosphatases: LpxEFN and FTN_1552.
It is important to note that the lipid A 1-phosphatase activity is not essential in bacteria but the C55-PP phosphatase activity is. Prior to this study, no enzyme encoding the C55-PP phosphatase activity had been identified in F. novicida. As the transposon mutant of lpxEFN is not lethal in F. novicida (30), we reasoned that there must exist another enzyme encoding the C55-PP phosphatase activity in F. novicida. By searching for F. novicida proteins homologous to E. coli enzymes containing C55-PP phosphatase activity (i.e., BacAEC, YbjGEC, PgpBEC, and LpxTEC) using PSI-BLAST (20), we have identified a PAP2 family protein of unknown function, FTN_1552, as a potential candidate of the C55-PP phosphatase (PSI-BLAST of PgpBEC: E value of 0.003 and sequence identity of 16.47%). We found that the temperature-sensitive pMAK705 vector harboring ftn_1552 complemented the E. coli strain deficient in C55-PP phosphatase activity (ΔybjG ΔpgpB ΔbacA::kan), confirming ftn_1552 as the gene encoding the C55-PP phosphatase activity (Fig. 4A). FTN_1552 was subsequently renamed UppPFN. Purified UppPFN appears to be a specific enzyme for C55-PP, with a specific activity of 22.71 ± 2.62 μmol/mg/min, and displays little activity toward Kdo2-lipid A and PGP (specific activities of 0.010 ± 0.005 μmol/mg/min and 0.031 ± 0.007 μmol/mg/min, respectively [Table 1]). Importantly, while F. novicida strains containing a chromosomal deletion of either lpxEFN or uppPFN were viable, we were unable to generate F. novicida strains containing both deletions (ΔlpxEFN ΔuppPFN) in the chromosome (Fig. 4B and C). However, F. novicida cells were viable in the ΔuppPFN background when lpxEFN was replaced with lpxEAA (Fig. 4B and C). Furthermore, when F. novicida was first transformed with a plasmid (pEDL17) bearing lpxEFN under the control of an anhydrotetracycline (aTc) promoter, we were also able to obtain viable F. novicida colonies containing chromosomal deletions of both lpxEFN and uppPFN (U112 ΔlpxEFN ΔuppPFN::kan/pEDL17-lpxEFN). The presence of proper chromosomal deletions of the lpxEFN and uppPFN genes was verified by PCR (Fig. 4D). As expected, the viability of such a strain depends on the expression of plasmid-encoded LpxEFN: prolonged withdrawal of aTc suppressed the bacterial growth and slowly resulted in cell lysis in culture (Fig. 4D), reinforcing the notion that UppPFN and LpxEFN share redundant C55-PP phosphatase activities in Francisella.
LpxEFN functionally connects multiple layers of envelope biogenesis in F. novicida.
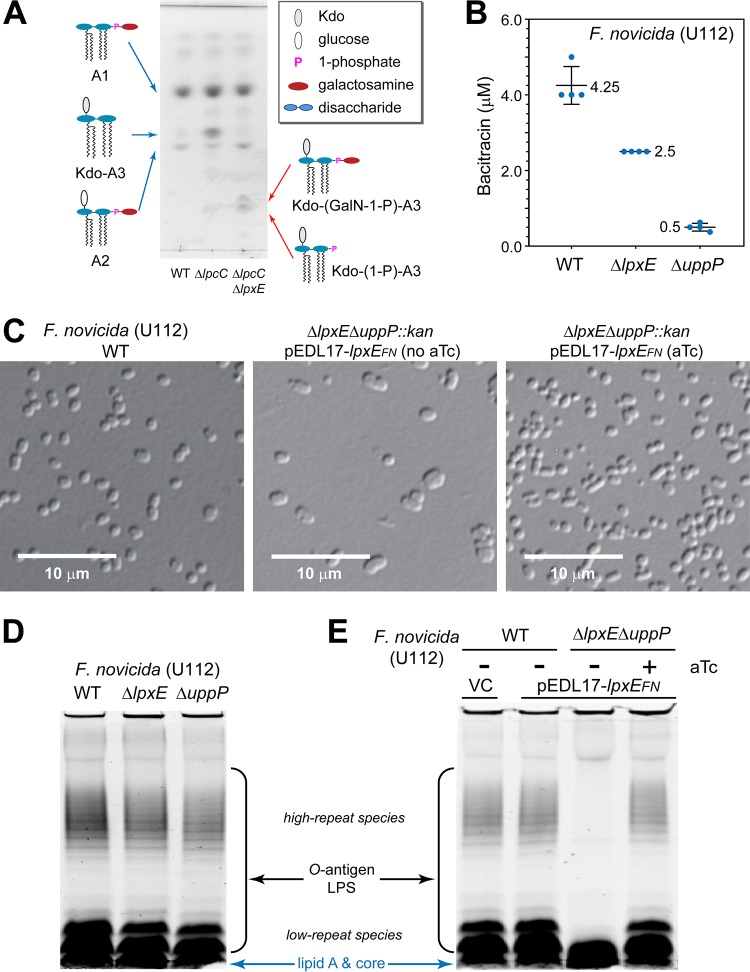
After establishing that LpxEFN shares C55-PP phosphatase activity with UppPFN, we further examined the biological implication of the multifunctional enzymatic activity of LpxEFN in its native host, F. novicida. We first verified the role of LpxEFN as a lipid A 1-phosphatase. Wild-type (WT) F. novicida cells contain both LPS (i.e., core oligosaccharide and O-antigen-modified Kdo-lipid A3 without 1- and 4′-phosphates) and free lipid A species A1 and A2, which do not contain core oligosaccharides/Kdo or O-antigen (lipid A2 differs from lipid A1 in that it has an additional α-linked glucose moiety attached to its 6′-position; also see the schematic lipid A structures of WT F. novicida in Fig. 5A) (31). Both lipid A1 and lipid A2 are further modified by FlmK, which transfers galactosamine from C55-P-galactosamine to the 1-phosphate of lipid A (32, 33). As the core oligosaccharide and O-antigen-modified lipid A are inefficiently extracted by the Bligh-Dyer method for mass spectrometry analysis, we examined the effect of ΔlpxEFN in the F. novicida strain deficient in the glycosyltransferase activity (ΔlpcC), which produces Kdo-lipid A3 (instead of LPS), in addition to lipids A1 and A2 found in the wild-type cells (Fig. 5A). Accumulations of Kdo-(1-phospho)-lipid A3 and Kdo-(galactosamine-1-phospho)-lipid A3, as well as the disappearance of Kdo-lipid A3, were observed in F. novicida when lpxEFN was deleted (ΔlpxEFN), confirming the lipid A 1-phosphatase activity of LpxEFN in cells (Fig. 5A and Fig. S5).
FIG 5.
LpxEFN functionally contributes to multiple layers of the F. novicida bacterial envelope biogenesis. (A) Deletion of lpxEFN results in accumulation of 1-phosphorylated Kdo-lipid A3 species. The profiles of total lipid extracts from F. novicida U112 WT, ΔlpcC, and ΔlpcC ΔlpxE strains were analyzed by TLC. Lipid A species are labeled. Abbreviations: GalN, galactosamine; A1, lipid A1; A2, lipid A2; Kdo-A3, Kdo-lipid A3; Kdo-(GalN-1-P)-A3, Kdo-(galactosamine-1-phospho)-lipid A3; Kdo-(1-P)-A3, Kdo-(1-phospho)-lipid A3. (B) Deletion of lpxEFN sensitizes F. novicida to bacitracin as reflected by reduced MIC. Error bars represent standard deviations from quadruplet measurements. (C) Suppression of the plasmid-encoded LpxEFN expression in the ΔlpxEFN ΔuppPFN::kan mutant of F. novicida causes cell deformation. Images of wild-type cells and ΔlpxEFN ΔuppPFN::kan F. novicida mutant cells without and with LpxEFN expression are shown in the left, middle, and right images, respectively. (D) Lack of LPS phenotypes in F. novicida cells containing the single deletion of lpxEFN or uppPFN. (E) Suppression of the plasmid-encoded LpxEFN expression in the ΔlpxEFN ΔuppPFN::kan mutant of F. novicida causes the loss of O-antigen repeats in LPS. LPS profiles from wild-type F. novicida cells carrying the pDEL17 vector (VC) or the C55-PP phosphatase-deficient (ΔlpxEFN ΔuppPFN::kan) cells carrying the pDEL17-lpxEFN vector without or with aTc were analyzed on SDS-PAGE gels using the Pro-Q Emerald LPS staining kit. O-antigen-containing LPS, including both high- and low-repeat species, and free lipid A/core species are labeled.
Mass spectrometry analysis of purified lipid A species of F. novicida mutants from preparative TLC. Accumulated new lipid A species from F. novicida mutants (ΔlpcC::tet and ΔlpxE::kan ΔlpcC::tet) isolated from TLC are identified using mass spectrometry as Kdo-lipid A3 (A), Kdo-(galactosamine-1-phospho)-lipid A3 (B), and Kdo-(1-phospho)-lipid A3 (C). Download FIG S5, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
As the C55-PP phosphatase activity of LpxEFN (2.99 ± 0.45 μmol/mg/min) is only ∼7-fold smaller than that of UppPFN (22.71 ± 2.62 μmol/mg/min), we asked whether LpxEFN could functionally contribute to the bacterial envelope biogenesis beyond lipid A modification at the 1-phosphate position. We first compared the sensitivities of the wild-type F. novicida strain (U112) and mutant strains containing either the lpxE or uppP deletion to bacitracin, an antibiotic sequestering C55-PP. We found that while the loss of uppP in F. novicida generated an 8.5-fold drop of MIC in comparison with that of the WT strain (0.5 μM versus 4.25 μM), as expected, the loss of lpxE also resulted in ∼1.7-fold drop of the MIC of bacitracin (2.5 μM for F. novicida ΔlpxE) (Fig. 5B), implicating a functional role of LpxEFN in the recycling of C55-PP.
In order to isolate the biological effect of LpxEFN, we utilized the F. novicida strain containing chromosomal deletions of both uppPFN and lpxEFN, which is complemented by a plasmid carrying lpxEFN under the control of an aTc promoter. We found that the loss of plasmid-mediated expression of LpxEFN due to withdrawal of aTc in the growth medium resulted in cell enlargement, reflecting defective peptidoglycan biosynthesis (Fig. 5C). Strikingly, while no change of O-antigen repeats was observed in F. novicida cells containing the chromosomal deletion of either lpxE or uppP in comparison with WT cells (Fig. 5D), transient suppression of LpxE led to a dramatic reduction of the LPS O-antigen repeats, including both high- and low-repeat species (Fig. 5E) (34), suggesting a contribution of LpxE to the O-antigen biogenesis. These observations are consistent with the notion that the biosynthesis and transport of peptidoglycan and O-antigen depend on C55-P, the product of LpxEFN (and UppPFN) activity, and reveal a previously unappreciated function of LpxE in the biogenesis and remodeling of multiple components across the bacterial envelope: peptidoglycan, free lipid A, and the O-antigen repeat of LPS.
DISCUSSION
LpxE enzymes are important virulence factors that promote bacterial survival, fitness, and pathogenicity. In H. pylori and Rhizobium etli CE3, the chromosomal knockout of lpxE resulted in increased susceptibility to positively charged antimicrobial peptides such as polymyxin B and colistin (9, 35), presumably due to the retention of 1-phosphate of lipid A. Previous studies showed that Rhizobium LpxE displays over a 1,000-fold preference of Kdo2-lipid A/lipid IVA over PGP; therefore, LpxE has been regarded as a highly specific monofunctional enzyme whose sole activity is to remove the 1-phosphate from lipid A. In this study, based on the striking structural similarity between LpxEAA and YodMBS, a PGP phosphatase with a weak in vitro activity on C55-PP phosphatase, we discovered that LpxE is a multifunctional lipid phosphatase. The LpxE enzyme from A. aeolicus displays significant activities toward Kdo2-lipid A/lipid IVA, C55-PP and PGP and functionally complements E. coli strains deficient in C55-PP or PGP phosphatase activities. Likewise, the LpxE enzyme from F. novicida is a dual-function enzyme that processes Kdo2-lipid A and C55-PP with similar efficiencies. Strikingly, deletion of lpxEFN in its native host F. novicida resulted in accumulation of phosphorylated lipid A species and increased sensitivity to bacitracin; in the C55-PP phosphatase-deficient (ΔuppP and ΔlpxE double-knockout mutant) F. novicida, suppression of LpxEFN expression from the plasmid resulted in cell deformation due to defective peptidoglycan biosynthesis and the loss of O-antigen repeats in LPS associated with reduced O-antigen transport, both of which are critically dependent on the recycling of C55-PP to C55-P. Taken together, these results show that LpxE enzymes from A. aeolicus and F. novicida functionally connect multiple layers of bacterial envelope biogenesis and remodeling. Such multiple functional roles are not unique to LpxE enzymes from A. aeolicus and F. novicida: we found that LpxE enzymes from H. pylori and R. leguminosarum also complemented E. coli deficient in C55-PP phosphatase activities (Fig. S6), suggesting that these LpxE enzymes can similarly process Kdo2-lipid A and C55-PP to synchronize lipid A modification with peptidoglycan biosynthesis and O-antigen modification of LPS.
Complementation of the C55-PP phosphatase-deficient E. coli strain by LpxE enzymes from R. leguminosarum (LpxERL) and H. pylori (LpxEHP). Wild-type E. coli cells carrying an empty vector (BW25113/pMAK705) or C55-PP phosphatase-deficient E. coli cells (BW25113 ΔybjG ΔpgpB ΔbacA::kan) carrying pMAK-lpxERL or lpxEHP were grown at 30°C or 42°C, respectively. From left to right are spots of 10-fold serial dilutions from 102 to 105. Download FIG S6, TIF file, 2.2 MB (2.3MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
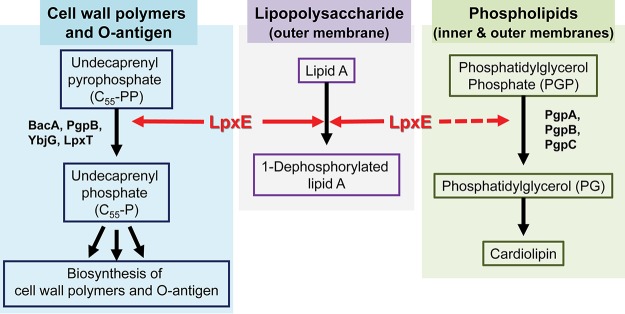
It is appropriate to ask why LpxE has evolved into a multifunctional enzyme. There are several potential explanations. First, it is conceivable that the peptidoglycan biosynthesis is such an essential process that multiple enzymes, including LpxE, are employed as the backup enzymes for the C55-PP phosphatase-mediated recycling reaction for peptidoglycan charging and biosynthesis. Second, it is possible that LpxE from Aquifex species represents an ancestral lipid phosphatase, which, although primitive, is sufficient to conduct all lipid phosphatase activities to support the bacterial envelope biogenesis and remodeling, while other LPT family of lipid phosphatases, such as the PGP phosphatase, evolved later as specialized, highly efficient enzymes. Third, it is also likely that LpxE evolved as a multifunctional enzyme to coordinate lipid A modification and the biogenesis of other layers of bacterial envelope. As 1,4′-bisphosphorylated lipid A chelates metal ions to form a fortified layer for bacterial protection, removal of the 1-phosphate could weaken the lipid A layer and increase membrane permeability. It is conceivable that the weakened lipid A layer is compensated by the elevated peptidoglycan biosynthesis and enhanced O-antigen decoration of LPS. Thus, bestowing LpxE with the multifunctionality toward Kdo2-lipid A and C55-PP (and, in the case of A. aeolicus, PGP) enables LpxE to orchestrate lipid A modification with bacterial envelope remodeling at multiple layers (Fig. 6) in order to promote the optimal bacterial growth and enhance bacterial survival in nature and the human host.
FIG 6.
Multiple roles of LpxE in the biogenesis of the Gram-negative bacterial envelope. Shown is a schematic illustration of the multifunctional roles of LpxE in the biogenesis of the cell wall polymer (peptidoglycan), outer membrane (LPS), and inner membrane (PGP). Shared phosphatase activities of LpxEs from diverse bacteria, including Aquifex (Aquificales), Francisella (Gammaproteobacteria), Rhizobium (Alphaproteobacteria), and Helicobacter (Epsilonproteobacteria), toward lipid A and C55-PP are indicated by solid arrows, whereas the unique PGP phosphatase activity of LpxEAA is indicated by a dashed red line.
The Gram-negative bacterial envelope contains three layers. How Gram-negative bacteria coordinate the biogenesis and remodeling of different layers of the bacterial envelope has remained an area of active investigation. Our study has revealed the first biological evidence of a multifunctional enzyme, LpxE in F. novicida, that natively couples lipid A 1-dephosphorylation with C55-PP recycling to enhance peptidoglycan biogenesis and O-antigen decoration of LPS, promote cell viability against antimicrobial peptides, evade host immune surveillance, and ultimately support bacterial pathogenesis. We suggest that such a multifunctional role represents a common but previously unappreciated mechanism for Gram-negative bacteria to coordinate bacterial envelop biogenesis across different layers.
MATERIALS AND METHODS
Data collection and refinement statistics of LpxEAA are listed in Table S1. All strains and plasmids used in this work are listed in Tables S2 and S3, respectively.
Plasmids used in this work. Download Table S2, DOCX file, 0.02 MB (20.5KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains used in this work. Download Table S3, DOCX file, 0.02 MB (25.6KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Plasmid and strain constructions and growth conditions are described in the supplemental material in detail.
Extraction of lipid A species, TLC and mass spectrometry analyses of lipid A species, and assay conditions are described in the Supplementary Methods section of the supplemental material.
Characterizations of F. novicida U112 mutants are described in the Supplementary Methods section of the supplemental material.
Supplemental methods. Download Text S1, DOCX file, 0.1 MB (78.3KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We thank the late professor Christian R. H. Raetz for building the foundation of this work and his mentorship.
This work was supported by the Intramural Research Program of KIST, by the Pioneer Research Center Program (2014M3C1A3054141) through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning, National Research Foundation of Korea (NRF) grant founded by the Korea government (2018R1A2B2008995), and National Institutes of Health (NIH) grants (GM51310 to Christian R. H. Raetz and P.Z. and GM115355 to P.Z.). X-ray diffraction data were collected at the Northeastern Collaborative Access Team beam line 24-ID-C, which is funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Pilatus 6M detector on 24-ID-C beam line is funded by a NIH-ORIP HEI grant (S10 RR029205). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Zhao J, An J, Hwang D, Wu Q, Wang S, Gillespie RA, Yang EG, Guan Z, Zhou P, Chung HS. 2019. The lipid A 1-phosphatase, LpxE, functionally connects multiple layers of bacterial envelope biogenesis. mBio 10:e00886-19. https://doi.org/10.1128/mBio.00886-19.
Contributor Information
M. Stephen Trent, University of Georgia.
Nina R. Salama, Fred Hutchinson Cancer Research Center.
REFERENCES
- 1.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 3.Raetz CRH, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Zhao J. 2017. Structure, inhibition, and regulation of essential lipid A enzymes. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1424–1438. doi: 10.1016/j.bbalip.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CJ, Liang X, Wu Q, Najeeb J, Zhao J, Gopalaswamy R, Titecat M, Sebbane F, Lemaitre N, Toone EJ, Zhou P. 2016. Drug design from the cryptic inhibitor envelope. Nat Commun 7:10638. doi: 10.1038/ncomms10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemaitre N, Liang X, Najeeb J, Lee CJ, Titecat M, Leteurtre E, Simonet M, Toone EJ, Zhou P, Sebbane F. 2017. Curative treatment of severe Gram-negative bacterial infections by a new class of antibiotics targeting LpxC. mBio 8:e00674-17. doi: 10.1128/mBio.00674-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem 76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scior T, Alexander C, Zaehringer U. 2013. Reviewing and identifying amino acids of human, murine, canine and equine TLR4/MD-2 receptor complexes conferring endotoxic innate immunity activation by LPS/lipid A, or antagonistic effects by Eritoran, in contrast to species-dependent modulation by lipid IVa. Comput Struct Biotechnol J 5:e201302012. doi: 10.5936/csbj.201302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran AX, Whittimore JD, Wyrick PB, McGrath SC, Cotter RJ, Trent MS. 2006. The lipid A 1-phosphatase of Helicobacter pylori is required for resistance to the antimicrobial peptide polymyxin. J Bacteriol 188:4531–4541. doi: 10.1128/JB.00146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. 2011. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog 7:e1002454. doi: 10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carman GM. 1997. Phosphatidate phosphatases and diacylglycerol pyrophosphate phosphatases in Saccharomyces cerevisiae and Escherichia coli. Biochim Biophys Acta 1348:45–55. doi: 10.1016/S0005-2760(97)00095-7. [DOI] [PubMed] [Google Scholar]
- 12.Sigal YJ, McDermott MI, Morris AJ. 2005. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem J 387:281–293. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stukey J, Carman GM. 1997. Identification of a novel phosphatase sequence motif. Protein Sci 6:469–472. doi: 10.1002/pro.5560060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillon DA, Wu WI, Riedel B, Wissing JB, Dowhan W, Carman GM. 1996. The Escherichia coli pgpB gene encodes for a diacylglycerol pyrophosphate phosphatase activity. J Biol Chem 271:30548–30553. doi: 10.1074/jbc.271.48.30548. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CR. 2004. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of Francisella novicida LpxE expressed in Escherichia coli. J Biol Chem 279:49470–49478. doi: 10.1074/jbc.M409078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karbarz MJ, Six DA, Raetz CR. 2009. Purification and characterization of the lipid A 1-phosphatase LpxE of Rhizobium leguminosarum. J Biol Chem 284:414–425. doi: 10.1074/jbc.M808390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotz BM, Lindner B, Stetter KO, Holst O. 2000. Characterization of a novel lipid A containing D-galacturonic acid that replaces phosphate residues. The structure of the lipid A of the lipopolysaccharide from the hyperthermophilic bacterium Aquifex pyrophilus. J Biol Chem 275:11222–11228. doi: 10.1074/jbc.275.15.11222. [DOI] [PubMed] [Google Scholar]
- 18.Metzger LE IV, Raetz CR. 2010. An alternative route for UDP-diacylglucosamine hydrolysis in bacterial lipid A biosynthesis. Biochemistry 49:6715–6726. doi: 10.1021/bi1008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzger LE, Lee JK, Finer-Moore JS, Raetz CRH, Stroud RM. 2012. LpxI structures reveal how a lipid A precursor is synthesized. Nat Struct Mol Biol 19:1132–1140. doi: 10.1038/nsmb.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brabetz W, Muller-Loennies S, Holst O, Brade H. 1997. Deletion of the heptosyltransferase genes rfaC and rfaF in Escherichia coli K-12 results in an Re-type lipopolysaccharide with a high degree of 2-aminoethanol phosphate substitution. Eur J Biochem 247:716–724. doi: 10.1111/j.1432-1033.1997.00716.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SH, Kim SK, Kim JF. 2010. Secretory production of recombinant proteins in Escherichia coli. Recent Pat Biotechnol 4:23–29. doi: 10.2174/187220810790069550. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Jiang D, Zhao Y, Liu J, Zhang XC. 2014. Crystal structure of lipid phosphatase Escherichia coli phosphatidylglycerophosphate phosphatase B. Proc Natl Acad Sci U S A 111:7636–7640. doi: 10.1073/pnas.1403097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong SL, Lin YB, Lu S, Wang MT, Bogdanov M, Zheng L. 2016. Structural insight into substrate selection and catalysis of lipid phosphate phosphatase PgpB in the cell membrane. J Biol Chem 291:18342–18352. doi: 10.1074/jbc.M116.737874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghachi ME, Howe N, Auger R, Lambion A, Guiseppi A, Delbrassine F, Manat G, Roure S, Peslier S, Sauvage E, Vogeley L, Rengifo-Gonzalez J-C, Charlier P, Mengin-Lecreulx D, Foglino M, Touzé T, Caffrey M, Kerff F. 2017. Crystal structure and biochemical characterization of the transmembrane PAP2 type phosphatidylglycerol phosphate phosphatase from Bacillus subtilis. Cell Mol Life Sci 74:2319–2332. doi: 10.1007/s00018-017-2464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Ghachi M, Derbise A, Bouhss A, Mengin-Lecreulx D. 2005. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli. J Biol Chem 280:18689–18695. doi: 10.1074/jbc.M412277200. [DOI] [PubMed] [Google Scholar]
- 27.Lu YH, Guan Z, Zhao J, Raetz CR. 2011. Three phosphatidylglycerol-phosphate phosphatases in the inner membrane of Escherichia coli. J Biol Chem 286:5506–5518. doi: 10.1074/jbc.M110.199265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menart V, Jevsevar S, Vilar M, Trobis A, Pavko A. 2003. Constitutive versus thermoinducible expression of heterologous proteins in Escherichia coli based on strong PR, PL promoters from phage lambda. Biotechnol Bioeng 83:181–190. doi: 10.1002/bit.10660. [DOI] [PubMed] [Google Scholar]
- 29.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher LA, Ramage E, Jacobs MA, Kaul R, Brittnacher M, Manoil C. 2007. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proc Natl Acad Sci U S A 104:1009–1014. doi: 10.1073/pnas.0606713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ribeiro AA, Guan Z, McGrath SC, Cotter RJ, Raetz CR. 2006. Structure and biosynthesis of free lipid A molecules that replace lipopolysaccharide in Francisella tularensis subsp. novicida. Biochemistry 45:14427–14440. doi: 10.1021/bi061767s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanistanon D, Hajjar AM, Pelletier MR, Gallagher LA, Kalhorn T, Shaffer SA, Goodlett DR, Rohmer L, Brittnacher MJ, Skerrett SJ, Ernst RK. 2008. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog 4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song F, Guan Z, Raetz CR. 2009. Biosynthesis of undecaprenyl phosphate-galactosamine and undecaprenyl phosphate-glucose in Francisella novicida. Biochemistry 48:1173–1182. doi: 10.1021/bi802212t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai XH, Shirley RL, Crosa L, Kanistanon D, Tempel R, Ernst RK, Gallagher LA, Manoil C, Heffron F. 2010. Mutations of Francisella novicida that alter the mechanism of its phagocytosis by murine macrophages. PLoS One 5:e11857. doi: 10.1371/journal.pone.0011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram BO, Sohlenkamp C, Geiger O, Raetz CR. 2010. Altered lipid A structures and polymyxin hypersensitivity of Rhizobium etli mutants lacking the LpxE and LpxF phosphatases. Biochim Biophys Acta 1801:593–604. doi: 10.1016/j.bbalip.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lipid A biosynthesis and modification. (A) Schematic illustration of Kdo2-lipid A biosynthesis and modification. The lipid A 1-phosphatase LpxE is in pink. (B) Lipid A structure of A. pyrophilus. Download FIG S1, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Lipid phosphatases. Reactions of PGP and C55-PP phosphatases are shown in panels A and B, respectively. Download FIG S2, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sequence alignment of LpxE orthologs. Alignment was achieved by Clustal Omega (F. Sievers, A. Wilm, D. Dineen, T. J. Gibson, et al., Mol Syst Biol 7:539, 2011) with manual adjustment. Conserved residues in the signature motifs of the PAP2 family of enzymes are labeled. Download FIG S3, TIF file, 2.9 MB (3MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data collection and refinement statistics of LpxEAA. Download Table S1, DOCX file, 0.01 MB (14.3KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Superimposition of LpxEAA with PgpBEC structures. RMSDs of ∼4.5 Å were observed between the coordinates of LpxEAA and PgpBEC (PDB codes 4PX7 [J. Fan, D. Jiang, Y. Zhao, J. Liu, et al., Proc Natl Acad Sci U S A 111:7636–7640, 2014] and 5JWY [S. L. Tong, Y. B. Lin, S. Lu, M. T. Wang, et al., J Biol Chem 291:18342–18352, 2016]). LpxEAA and PgpBEC are shown in Cα traces, with LpxEAA colored in cyan and PgpBEC in gray. Download FIG S4, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mass spectrometry analysis of purified lipid A species of F. novicida mutants from preparative TLC. Accumulated new lipid A species from F. novicida mutants (ΔlpcC::tet and ΔlpxE::kan ΔlpcC::tet) isolated from TLC are identified using mass spectrometry as Kdo-lipid A3 (A), Kdo-(galactosamine-1-phospho)-lipid A3 (B), and Kdo-(1-phospho)-lipid A3 (C). Download FIG S5, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complementation of the C55-PP phosphatase-deficient E. coli strain by LpxE enzymes from R. leguminosarum (LpxERL) and H. pylori (LpxEHP). Wild-type E. coli cells carrying an empty vector (BW25113/pMAK705) or C55-PP phosphatase-deficient E. coli cells (BW25113 ΔybjG ΔpgpB ΔbacA::kan) carrying pMAK-lpxERL or lpxEHP were grown at 30°C or 42°C, respectively. From left to right are spots of 10-fold serial dilutions from 102 to 105. Download FIG S6, TIF file, 2.2 MB (2.3MB, tif) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Plasmids used in this work. Download Table S2, DOCX file, 0.02 MB (20.5KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains used in this work. Download Table S3, DOCX file, 0.02 MB (25.6KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental methods. Download Text S1, DOCX file, 0.1 MB (78.3KB, docx) .
Copyright © 2019 Zhao et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.