Bacterial toxin-antitoxin (TA) modules confer multidrug tolerance (persistence) that may contribute to the recalcitrance of chronic and recurrent infections. The first high-persister gene identified was hipA of Escherichia coli strain K-12, which encodes a kinase that inhibits glutamyl-tRNA synthetase. The hipA gene encodes the toxin of the hipBA TA module, while hipB encodes an antitoxin that counteracts HipA. Here, we describe a novel, widespread TA gene family, hipBST, that encodes HipT, which exhibits sequence similarity with the C terminus of HipA. HipT is a kinase that phosphorylates tryptophanyl-tRNA synthetase and thereby inhibits translation and induces the stringent response. Thus, this new TA gene family may contribute to the survival and spread of bacterial pathogens.
KEYWORDS: persistence, ppGpp, toxin/antitoxin systems, translation, tRNA synthetase
ABSTRACT
Type II toxin-antitoxin (TA) modules encode a stable toxin that inhibits cell growth and an unstable protein antitoxin that neutralizes the toxin by direct protein-protein contact. hipBA of Escherichia coli strain K-12 codes for HipA, a serine-threonine kinase that phosphorylates and inhibits glutamyl-tRNA synthetase. Induction of hipA inhibits charging of glutamyl-tRNA that, in turn, inhibits translation and induces RelA-dependent (p)ppGpp synthesis and multidrug tolerance. Here, we describe the discovery of a three-component TA gene family that encodes toxin HipT, which exhibits sequence similarity with the C-terminal part of HipA. A genetic screening revealed that trpS in high copy numbers suppresses HipT-mediated growth inhibition. We show that HipT of E. coli O127 is a kinase that phosphorylates tryptophanyl-tRNA synthetase in vitro at a conserved serine residue. Consistently, induction of hipT inhibits cell growth and stimulates production of (p)ppGpp. The gene immediately upstream from hipT, called hipS, encodes a small protein that exhibits sequence similarity with the N terminus of HipA. HipT kinase was neutralized by cognate HipS in vivo, whereas the third component, HipB, encoded by the first gene of the operon, did not counteract HipT kinase activity. However, HipB augmented the ability of HipS to neutralize HipT. Analysis of two additional hipBST-homologous modules showed that, indeed, HipS functions as an antitoxin in these cases also. Thus, hipBST constitutes a novel family of tricomponent TA modules where hipA has been split into two genes, hipS and hipT, that function as a novel type of TA pair.
INTRODUCTION
Prokaryotic toxin-antitoxin (TA) modules are usually composed of two elements, a toxin that can inhibit cell growth and an antitoxin that counteracts the inhibitory effect of the toxin (1, 2). Based on the molecular modes of antitoxin activity, TA modules have been divided into different types (3). The abundant type II modules are characterized by protein antitoxins that bind directly to and inhibit their cognate toxins by tight molecular interaction. Type II antitoxins usually contain a DNA-binding motif used to regulate TA operon transcription via binding to operators in the promoter region and a separate domain that interacts with and neutralizes the cognate toxin. Moreover, antitoxins are degraded by cellular proteases, such as Lon and/or Clp, and the cellular activity and amount synthesized of a given toxin are thus determined by the concentration of cognate antitoxin (4).
Type II modules are highly abundant; that is, most prokaryotic chromosomes encode at least one and some chromosomes encode cohorts of them. For example, Mycobacterium tuberculosis has 88 known, well-conserved type II TAs, while the insect pathogen Photorhabdus luminescens has a similarly large cohort (5). Toxin gene similarities were used to divide type II modules into superfamilies (6, 7). Thus, in general, toxins that exhibit sequence similarity inhibit cell growth by identical or related molecular mechanisms and can be grouped into the same family. Type II toxins belonging to the RelE, MazF, VapC, HipA, and TacT families curtail cell growth by inhibiting translation, CcdB and ParE inhibit DNA replication, Zeta toxins inhibit cell wall synthesis, and RES toxins inhibit cell growth by depleting NAD+ (8–18).
The biological functions of TAs have been debated. For type II modules, many studies now point to a function in survival during stress, including tolerance of multiple antibiotics (1). Stochastic or stress-induced activation of TA modules can protect bacteria from unfavorable environmental conditions by inducing persister formation (19, 20), a transient, slow-growing state in which the bacteria are tolerant of antibiotics and various other forms of stress (21). The stochastic formation of persisters is due to phenotypic heterogeneity in clonal populations of cells and can be viewed as a bet-hedging strategy that increases the survival rate in rapidly changing environments (22). Moreover, sublethal concentrations of antibiotics and other stresses have been found to stimulate the formation of persisters (23, 24).
The first gene associated with persistence was hipA (high persister gene A) of Escherichia coli strain K-12, identified as a gain-of-function allele, hipA7 (25). This allele, found also in clinical isolates of uropathogenic E. coli (26), showed a 100- to 1,000-fold increase in persistence due to two amino acid changes in HipA (changes of G to S at position 22 [G22S] and D to A at positions 291 [D291A]) (27). The hipA toxin gene and the upstream hipB antitoxin gene together constitute a type II TA module (28). Modest ectopic expression of wild-type HipA causes severe growth inhibition that can be countered by the HipB antitoxin, which interacts directly with HipA (28). HipA and HipB form a complex that represses hipBA transcription via binding to operators in the promoter region (26). HipA is a Hanks serine-threonine kinase (29, 30) and was found to specifically phosphorylate and inhibit glutamyl-tRNA synthetase (GltX or GltRS), causing strong inhibition of translation and induction of guanosine tetra- and pentaphosphate [(p)ppGpp] synthesis and persistence (11, 31, 32). HipA-mediated phosphorylation of the conserved residue Ser239 inhibits the activity of GltX (11), thereby preventing charging of tRNAGlu. As a consequence, the ratio of charged to uncharged tRNAGlu decreases, which in turn stimulates binding of RelA-tRNA complexes to the ribosome, leading to activation of RelA (33). The resulting increase in the cellular (p)ppGpp level triggers the stringent response (11, 27, 32).
Here, we describe a novel family of three-component TA modules encoding toxins exhibiting sequence similarity to HipA. We discovered that HipT of the enteropathogenic E. coli O127:H6 strain E2348/69 (HipTO127) is a toxin that can be counteracted by overproduction of tryptophanyl-tRNA synthetase (TrpS or TrpRS). Consistently, our in vitro data show that HipTO127 is a serine-threonine kinase that inhibits translation by phosphorylating TrpS. HipTO127 aligns colinearly with HipA but lacks ∼100 amino acids (aa) at its N terminus (Fig. 1A). Interestingly, hipTO127 is preceded by hipSO127, encoding HipSO127 (103 aa), which exhibits sequence similarity with the N-terminal part of HipA that is missing from HipTO127 (Fig. 1A). Finally, hipSO127 is preceded by a gene encoding a HipB homolog containing a helix-turn-helix (HTH) DNA-binding motif. HipB, HipS, and HipT form a complex in vivo and in vitro, and HipSO127 alone counteracts HipTO127 activity in vivo. The HipB homolog (called HipBO127) does not counteract HipTO127 but instead augments the ability of HipSO127 to counteract HipTO127. Analysis of the hipBST modules of Haemophilus influenzae and Tolumonas auensis revealed that the HipT proteins of these organisms also are counteracted by overproduction of TrpS. Moreover, cognate HipS neutralizes HipT in both these cases. In summary, we describe here a family of novel three-component TA modules that potentially can increase the stress resilience and spread of bacterial pathogens.
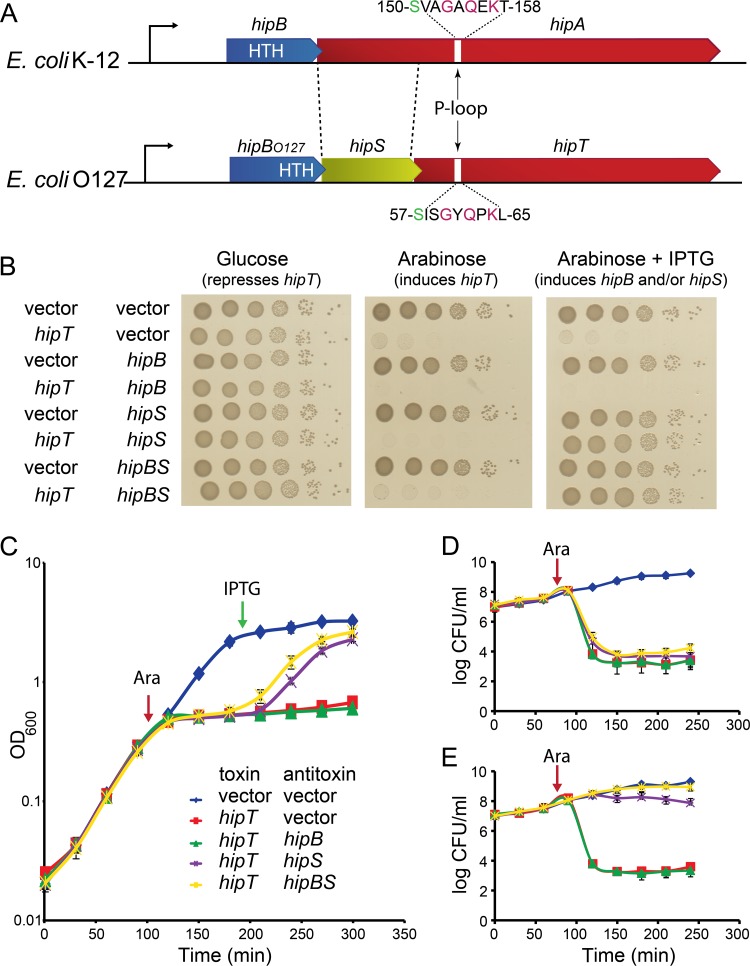
FIG 1.
hipBST of E. coli O127 encodes a three-component toxin-antitoxin module. (A) Schematic showing a comparison of the hipBA and hipBST operons of E. coli K-12 and O127, respectively. Bent arrows pointing right indicate promoters. The hipBA operon contains two genes, hipB and hipA, while hipBSTO127 contains three genes, hipBO127, hipSO127, and hipTO127. The region of hipA between the dashed lines exhibits sequence similarity to hipSO127. The 8 amino acid residues of the P loop in HipA (150-VAGAQEKT-158) that binds phosphates of ATP are shown; the autophosphorylated S150 residue is shown in green (35). The homologous P loop and autophosphorylated serine in HipTO127 were inferred by sequence similarity. (B) Overnight cultures of E. coli MG1655 harboring pSVN1 (pBAD33::hipTO127) or the empty pBAD33 vector combined with pSVN111 (pNDM220::hipBO127), pSVN109 (pNDM220::hipSO127), pSVN110 (pNDM220::hipBSO127), or the empty low-copy-number pNDM220 vector, as indicated, were diluted to obtain the same values of OD600, centrifuged at 5,000 rpm for 5 min, washed in phosphate-buffered saline (PBS), and serially diluted before being spotted onto LB nutrient agar plates containing 0.2% glucose (to repress hipTO127), 0.2% arabinose (to induce hipTO127), or 0.2% arabinose plus 200 μM IPTG (to induce hipBO127, hipSO127, or hipBSO127). (C) The strains used in the experiment whose results are shown in panel B were grown in LB medium plus appropriate antibiotics. Overnight cultures were diluted, cells were grown exponentially for at least 3 h until the doubling time appeared constant, and at an OD600 of ≈0.3, arabinose (0.2%) was added to induce hipTO127 (red arrow). After a further 1.5 h, IPTG (200 μM) was added to induce hipSO127, hipBO127, or hipBSO127 (green arrow). (D and E) Viable counts of strains from the experiment whose results are shown in panels B and C before and after the addition of arabinose (0.2%) at an OD600 of ≈0.3 (red arrow). At each time point, cell samples (0.5 ml) were washed in PBS before a 10-times dilution series was spotted on agar plates with glucose (0.2%) to repress hipTO127 expression (D) or with glucose (0.2%) to repress hipTO127 expression and IPTG (200 μM) to induce hipBO127, hipSO127, or hipBSO127 (E). Plates were incubated for 16 h at 37°C before counting. Data points in panels C, D, and E represent mean values of results from at least three independent experiments, and error bars indicate standard deviations.
RESULTS
Homologs of HipA are encoded by three-gene operons.
Using similarity searching with HipA (440 amino acids [aa]) of E. coli K-12 as the query sequence, we identified a number of genes encoding HipA homologs that aligned colinearly with the C terminus of HipA but were shortened by ∼100 aa at their N termini (Fig. 1A, and see Fig. S1A in the supplemental material) (34). The HipA homologs contain P-loop motifs matching the experimentally validated P loop of HipA, as well as conserved catalytic domains and Mg2+ binding motifs, suggesting that, like HipA, HipT proteins are kinases (Fig. S1A) (35). A phylogenetic analysis showed that HipA and HipT group monophyletically in a cladogram based on 8 HipA and 40 HipT sequences (Fig. S1D) (36). The majority of the hipT genes were from gammaproteobacteria, but two HipT homologs deeply embedded in the phylogenetic tree were from a deltaproteobacterium and a firmicute (Streptococcus pneumoniae) (Fig. S1D). The HipT homolog from S. pneumoniae is identical to the HipT homolog of H. influenzae strain 10810 (Fig. S1D). These two organisms separated more than a billion years ago, and both are highly competent for DNA uptake and live in the same biological habitat (the upper respiratory tract). These observations raise the possibility that hipBST loci undergo lateral gene transfer between distantly related organisms.
Alignments of protein sequences encoded by hipBST loci from five different gammaproteobacteria and a phylogenetic analysis of HipT. (A) Alignment of HipT homologs with the C-terminal part of HipA of E. coli K-12. The 8-aa conserved P loop (35) in HipA indicated below the sequences was used as a fixed point to generate the alignment. The autophosphorylated Ser150 in HipA is fully conserved in the HipT homologs, and the HipT proteins contain conserved catalytic domains and Mg2+ binding motifs as indicated (34, 35, 38). The HipT sequences were from E. coli O127:H6 strain E2348/69 (GenBank accession number CAS11333.1), Haemophilus influenzae Rd KW20 (NCBI accession number NP_438824), Tolumonas auensis DSM 9187 (NCBI accession number WP_015879003.1), Vibrio cholerae O1 strain NHCM-017 (WP_050916634.1), and Vibrio halioticoli (GenBank accession number GAD88429.1). (B) Alignment of five HipS homologs encoded by genes just upstream of the genes encoding the HipT homologs shown in panel A with the N-terminal part of HipA. The HipS sequences were from Escherichia coli O127:H6 strain E2348/69 (GenBank accession number CAS11334.1), H. influenzae Rd KW20 (NCBI accession number NP_438825.1), Tolumonas auensis (NCBI accession number WP_015879002.1), V. cholerae O1 strain NHCM-017 (accession number WP_044125702.1), and Vibrio halioticoli (GenBank accession number GAD88428.1). (C) Alignment of five HipB homologs encoded by genes just upstream of the genes encoding the HipS homologs shown in panel B. The HipB sequences were from E. coli O127:H6 strain E2348/69 (GenBank accession number CAS11335.1), H. influenzae Rd KW20 (NCBI accession number NP_438826.1), Tolumonas auensis (NCBI accession number WP_083757795.1), V. cholerae O1 strain NHCM-017 (WP_050916568.1), and V. halioticoli (GenBank accession number AD88427.1). Color codes of the alignments are as follows: black on white, nonsimilar residues; blue on cyan, consensus residue derived from a block of similar residues at a given position; black on green, consensus residue derived from the occurrence of greater than 50% of a single, distinct residue; red on yellow, consensus residue derived from a completely conserved residue; and green on white, residue weakly similar to consensus residue. The alignments in panels A, B, and C were generated by using the commercial version of Vector NTI (Invitrogen). (D) Phylogenetic tree of 8 HipA and 40 HipT homologs. A sequence alignment of the proteins was accomplished using an online version of MUSCLE (36) provided by European Bioinformatics Institute at EMBL (https://www.ebi.ac.uk/Tools/msa/muscle/) and used to derive the phylogenetic tree presented as a cladogram. Sampling of the HipA and HipT sequences was accomplished using BLASTP at NCBI and was not exhaustive. Accession numbers of the proteins included in the cladogram were as follows: Photorhabdus luminescens TT01, CAE17272.1; Vibrio parahaemolyticus, WP_020904255.1; Vibrio cyclitrophicus, WP_102385597.1; Pantoea vagans, WP_095707190.1; Enterobacter ludwigii, WP_081112566.1; Pantoea GM01, EJL89497.1; E. coli K-12, P23874.2; Shigella dysenteriae, WP_119170656.1; Paraglaciecola psychrophila 170, AGH46782.1; Desulfobacula toluolica Tol2, CCK78946.1; Marinobacter LQ44, AMQ90498.1; Alteromonas mediterranea U7, AGP89851.1; V. halioticoli NBRC 102217, GAD88429.1; Tatumella ptyseos NCTC11468, SQK77238.1; Serratia plymuthica PRI-2C, ANS44223.1; Yersinia frederiksenii FDAARGOS 417, ATM86408.1; Pantoea agglomerans C410P1, AOE38443.1; Pantoea agglomerans L15, AZI51552.1; Pantoea agglomerans TH81, AYP22744.1; T. auensis DSM 9187, ACQ93535.1; E. coli MVAST0167, AML11457.1; E. coli O127:H6 E2348/69, CAS11333.1; Buttiauxella 3AFRM03, AYN26082.1; Pectobacterium atrosepticum 36A, ATY88941.1; Pectobacterium carotovorum 3-2, AVT56767.1; P. carotovorum 14A, AZK60881.1; Paraglaciecola polaris NIBIO1006, ASY75229.1; P. polaris NIBIO1392, ASY81571.1; P. carotovorum PCC21, AFR01461.1; P. carotovorum subsp. brasiliense, ARA78327.1; Actinobacillus porcitonsillarum 9953L55, AWI50429.1; H. influenzae NML-Hia-1, AOZ67195.1; H. influenzae R2866, ADO81720.1; H. influenzae F3031, CBY81777.1; H. influenzae 723, AJO91476.1; H. influenzae 10810, CBW28981.1; Streptococcus pneumoniae 2842STDY5881852, CVQ15586.1; H. influenzae 67P38H1, AVJ03036.1; H. influenzae FDAARGOS199, ARB90233.1; H. influenzae Rd KW20, NP_438824; Haemophilus aegyptius NCTC8502, SQH35811.1; H. influenzae F3047, CBY86290.1; H. influenzae NCTC8455 SQK92775.1; H. influenzae P650-8603, AXP41025.1; H. influenzae 86-028NP, AAX87695.1; H. influenzae C486, AJO89092.1; H. influenzae 84P36H1, AWP55634.1; and H. influenzae 5P28H1, AVI99364.1. Download FIG S1, PDF file, 2.2 MB (2.2MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In all the hipT-containing organisms examined, we discovered short open reading frames adjacent to and upstream from hipT, which are herein called hipS, encoding proteins of ∼100 aa that exhibit sequence similarity to the missing N-terminal part of HipA (Fig. S1B). In all these cases, open reading frames upstream from hipS encode putative proteins of ∼100 aa containing HTH DNA-binding motifs (Fig. S1C). These putative HipB homologs may thus autoregulate the hipBST operons. We chose the hipBST module of E. coli O127:H6 strain E2348/69 as our primary model system for functional analysis (Fig. 1A). The hipBSTO127 module encodes HipBO127 (107 aa), HipSO127 (103 aa), and HipTO127 (335 aa). Gene pair hipBO127 and hipSO127 overlaps by 16 nucleotides (nt), and gene pair hipSO127 and hipTO127 overlaps by 1 nt, suggesting that the genes may be translationally coupled.
HipTO127 inhibits cell growth and is counteracted by HipSO127.
We validated the components encoded by hipBSTO127 experimentally by inserting hipTO127 into plasmid vector pBAD33 (carrying the arabinose-inducible pBAD promoter) and hipSO127, hipBO127, and hipBSO127 into the low-copy-number R1 vector pNDM220 (carrying the synthetic, isopropyl-β-d-thiogalactopyranoside [IPTG]-inducible pA1/O4/O3 promoter) and subjected the standard E. coli K-12 strain MG1655 carrying combinations of these plasmids to growth assays and viable-count measurements. Induction of hipTO127 resulted in strong inhibition of cell growth, both on plates and in liquid medium, supporting the hypothesis that HipTO127 can function as a toxin (Fig. 1B and C). Growth was rescued by induction of hipSO127 alone but not by hipBO127 alone, suggesting that HipSO127 functions as the antitoxin (Fig. 1B and C). Coinduction of hipBO127 and hipSO127 provided a consistent, yet mild growth rescue advantage compared to the results for hipSO127 alone, suggesting that HipBO127 augments the antitoxin activity of HipSO127 (Fig. 1C). Thus, HipBO127 does not function as a classical antitoxin.
Upon induction of hipTO127, CFU decreased dramatically (Fig. 1D). However, later induction of hipSO127 or hipSO127 plus hipBO127 (by adding IPTG and glucose to the agar plates to induce PA1/O4/O3::hipBSO127 and repress pBAD::hipTO127, respectively) fully rescued cell viability (Fig. 1E). This result showed that ectopic production of HipTO127 induces a bacteriostatic condition from which the cells can be resuscitated. In support of this conclusion, strains in which hipTO127 was induced recovered viability after prolonged incubation times (∼40 h), even in the absence of hipSO127 or hipBSO127 (Fig. S2).
Long-term incubation shows HipT of E. coli O127 induces bacteriostasis. Viable counts (CFU/ml) before and after hipTO127 induction in the presence or absence of induction of hipBO127 or hipSO127 and hipBSO127. Cultures of E. coli MG1655 harboring pSVN1 (pBAD33::hipTO127) or the empty pBAD33 vector combined with pSVN111 (pND::hipBO127), pSVN109 (pND::hipSO127), pSVN110 (pND::hipBSO127), or the empty low-copy-number pND vector were growing exponentially in LB medium at 37°C. The pBAD promoter was induced by the addition of arabinose (0.2%) at an OD600 of ≈0.3 (red arrow). At each time point, 0.5-ml cell samples were washed in PBS before 10-times dilution series were spotted on agar plates with glucose only (0.2%), to repress hipTO127 expression (A), or glucose plus IPTG (200 μM) to also induce hipBO127 or hipSO127 and hipBSO127 (B) on the plates. Plates were incubated for 40 hours at 37°C before counting. Data points represent mean values from at least three independent experiments, and error bars indicate standard deviations. As seen by the results, the prolonged incubation period (16 h versus 40 h) allowed for the full recovery of viability, even in the absence of hipS or hipBS (compare Fig. S2 with Fig. 1D and E). Download FIG S2, PDF file, 0.4 MB (393.9KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
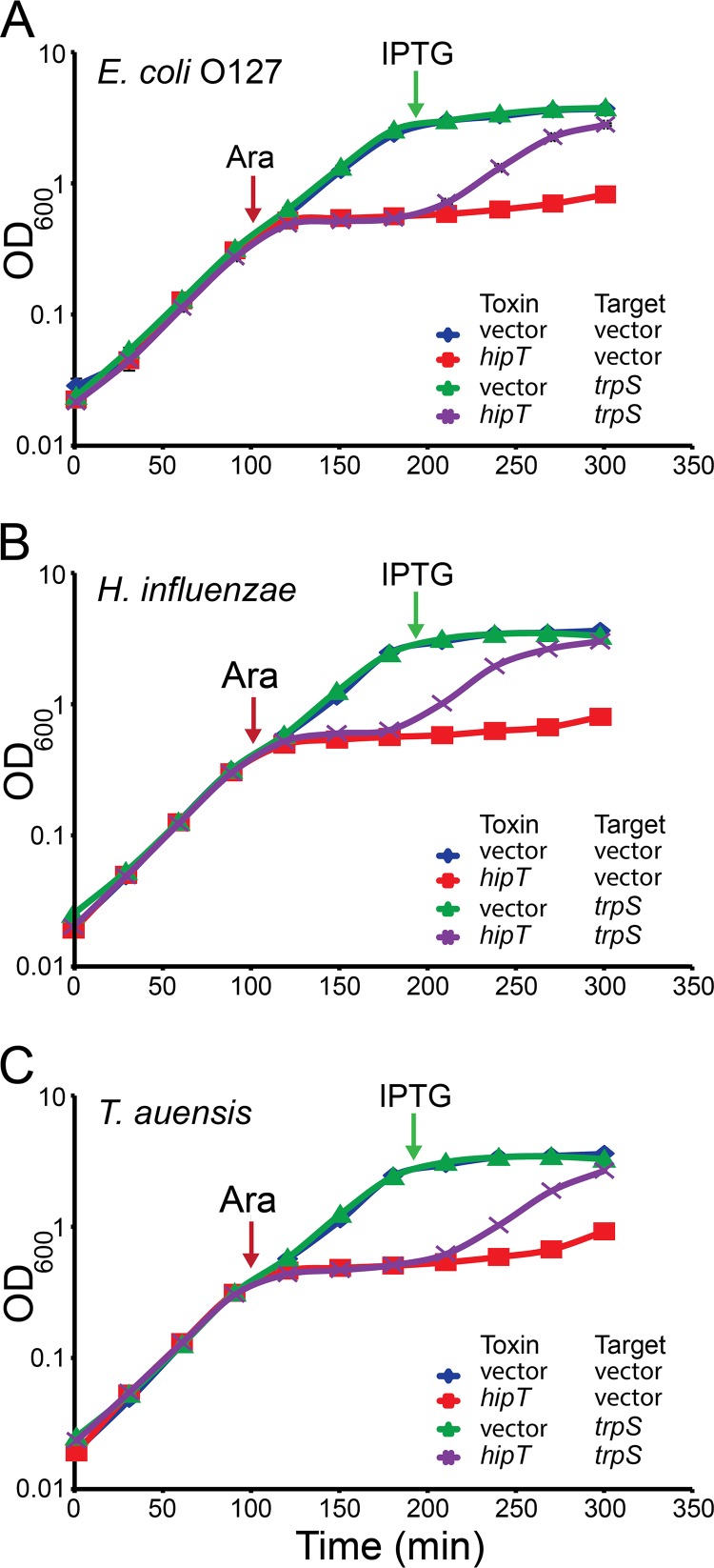
We were puzzled by the observation that HipSO127 but not HipBO127 exhibited antitoxin activity and therefore decided to analyze the hipBST modules of two additional gammaproteobacteria, Haemophilus influenzae Rd KW20 (hipBSTHi) and Tolumonas auensis DSM 9187 (hipBSTTa) (Fig. S1). Induction of hipTHi or hipTTa inhibited cell growth of E. coli MG1655 in liquid medium in both cases, and induction of cognate hipS genes was sufficient to neutralize the two HipT toxins (Fig. S3A and B). Like HipBO127, HipBHi augmented the ability of HipSHi to neutralize HipTHi, as the presence of the HipBSHi-encoding plasmid almost entirely prevented growth inhibition after induction of hipTHi (Fig. S3A). HipBTa did not detectably augment the antitoxin effect of HipSTa in this experimental setup (Fig. S3B). We also tested hipT genes from strains of Vibrio cholerae and Vibrio halioticoli, but their induction was, for unknown reasons, not toxic in E. coli K-12 and the corresponding hipBST modules were not analyzed further.
HipT of H. influenzae and T. auensis inhibit cell growth and can be neutralized by cognate HipS. Cells were grown as described in the legend to Fig. 1C. As seen by the results, cognate HipBHi augments HipSHi in the neutralization of HipTHi. Strains used in the experiment whose results are shown in panel A were E. coli MG1655 harboring pSVN135 (pBAD33::hipTHi) or the empty pBAD33 vector combined with pSVN122 (pNDM220::hipBHi), pSVN123 (pNDM220::hipSHi), or pSVN139 (pNDM220::hipBSHi) or the empty low-copy-number pNDM220 as indicated, and strains in the experiment whose results are shown in panel B were E. coli MG1655 harboring pSVN129 (pBAD33::hipTTa) or the empty pBAD33 vector combined with pSVN126 (pNDM220::hipBTa), pSVN127 (pNDM220::hipSTa), or pSVN138 (pNDM220::hipBSTa) or the empty low-copy-number pNDM220 as indicated. Data points represent mean values from at least two independent experiments, and error bars indicate standard deviations. Download FIG S3, PDF file, 0.3 MB (343KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HipB, HipS, and HipT form a ternary complex in vivo.
The above-described observations suggest that HipBO127, HipSO127, and HipTO127 might form a protein complex in vivo, as seen for other type II TA modules. To test this, we constructed a plasmid (pSVN94) encoding N-terminally His6-tobacco etch virus (TEV)-tagged HipBO127, HipSO127, and the enzymatically inactive HipTO127D233Q mutant protein in which all three genes had optimized translation signals (Shine-Dalgarno [SD] sequences and ATG start codons) to increase translation rates. His6-TEV-HipBO127 was purified under native conditions and analyzed by denaturing polyacrylamide gel electrophoresis (SDS-PAGE). Indeed, three proteins of the expected molecular weights (MWs) copurified (Fig. S4A), indicating that the HipBSTO127 proteins form a complex in vivo. Further separation of the protein complex using a heparin column allowed isolation of three samples containing HipTO127, HipBTO127, and HipBSTO127 (Fig. S4B, top). Gel filtration chromatography further confirmed that HipT and HipBST are monodispersed in solution, suggesting that HipBSTO127 is a heterotrimer (Fig. S4B, bottom).
HipBO127, HipSO127, and HipTO127 form a complex. (A) Purification of His6-TEV-HipBO127, HipSO127, and HipTD233QO127 from E. coli strain BL21 containing pSVN94 analyzed by SDS-PAGE. E. coli BL21 containing pSVN94 was grown in LB medium at 37°C and His6-TEV-HipBO127 purified according to standard procedure. As seen by the results, the N-terminally His-tagged HipBO127 pulled down HipSO127 and HipTO127. Lanes 1 to 5 contained samples from washes with buffer B (50 mM NaH2PO4 [pH 8], 0.3 M NaCl, 35 mM imidazole, and 1 mM β-mercaptoethanol). Lanes 6 to 10 show samples from washes with buffer C (100 mM NaH2PO4 [pH 8], 10 mM Tris-HCl [pH 8], and 1 mM β-mercaptoethanol) with different concentrations of urea, as follows: 0 M, 2.45 M, 4.9 M, 7.35 M, and 9.8 M urea, respectively. Lane 11 shows a sample from flowthrough after overnight wash with buffer C containing 9.8 M urea. Lanes 12 and 13 show samples from additional washes with buffer C containing 9.8 M urea after overnight incubation. Lane 14 contained a sample from elution with buffer D (100 mM NaH2PO4 [pH 8], 10 mM Tris-HCl [pH 8], 9.8 M urea, 0.5 M imidazole, and 1 mM β-mercaptoethanol). (B) Top, purification of HipBSTO127 subcomplexes using a heparin column and elution using increasing concentrations of salt. Bottom, purification of HipBSTO127 and isolated HipTO127 toxin on a gel filtration column. Elution positions of standard proteins with known mass (indicated in kDa) are shown with vertical arrows. Download FIG S4, PDF file, 2.9 MB (2.9MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Multicopy suppression of HipT by trpS.
Previously, we showed that overproduction of GltX suppresses HipA-mediated growth inhibition and that HipA phosphorylates GltX in vitro (11). Unexpectedly, overproduction of GltX did not suppress HipT-mediated growth inhibition (Fig. S5A). Therefore, we performed a second multicopy gene library screening in an attempt to identify genes that in high copy numbers could suppress the effect of HipTO127 (see Materials and Methods). Using a pBR322-based Sau3A-derived gene library of E. coli MG1655ΔydeA, ∼8,300 colonies with an average insert size of ∼3,300 bp were screened, resulting in a coverage of roughly 5.8 times. In this screening, 105 hits were obtained, of which 19 were retransformed. Six of these plasmids exhibited a stable phenotype and were sent for sequencing. Thereby, we identified a DNA fragment containing rpe, gph, and trpS that suppressed HipTO127. Of these genes, only conditional induction of trpS, which encodes tryptophanyl-tRNA synthetase (TrpS), suppressed HipTO127-mediated growth inhibition, both on solid medium (Fig. S5B) and in liquid culture (Fig. 2A). TrpS also suppressed HipTHi and HipTTa (Fig. 2B and C and Fig. S5B), whereas GltX had no such effect (Fig. S5A).
FIG 2.
Overproduction of TrpS counteracts HipTO127. (A to C) Growth curves of strains of E. coli MG1655 harboring pSVN1 (pBAD33::hipTO127) (A), pSVN135 (pBAD33::hipTHi) (B), and pSVN129 (pBAD33::hipTTa) (C) or the empty pBAD33 vector combined with pSVN37 (pEG25::trpS) or the empty high-copy-number pEG25 vector, as indicated. Cells were grown in LB medium supplemented with the appropriate antibiotics. Overnight cultures were diluted and grown exponentially for at least 3 h until the doubling time appeared constant. The pBAD promoter of the pBAD33 derivatives was induced by arabinose (0.2%) at an OD600 of ≈0.3 (red arrow). The PA1/O4/O3 promoter of the pEG25-derived plasmids was induced by the addition of IPTG (200 μM; green arrow) 1.5 h later. Data points represent mean values from at least two independent experiments, and error bars indicate standard deviations.
Overproduction of TrpS suppresses HipTO127, HipTHi, and HipTTa-mediated growth inhibition, whereas GltX has no such effect. (A) Overnight cultures of E. coli MG1655 harboring (left) pSVN1 (pBAD33::hipTO127), (middle) pSVN135 (pBAD33::hipTHi), or (right) pSVN129 (pBAD33::hipTTa) or the empty pBAD33 vector combined with pEG::gltX (pMG25::gltX) or the empty high-copy-number vector pMG25, as indicated, were diluted to similar OD600 values and washed in PBS before 10-times dilution series were spotted on LB agar plates containing the appropriate antibiotics and glucose (0.2%), arabinose (0.2%), or arabinose (0.2%) plus IPTG (200 μM), respectively. (B) Overnight cultures of E. coli MG1655 harboring (left) pSVN1 (pBAD33::hipTO127), (middle) pSVN135 (pBAD33::hipTHi), or (right) pSVN129 (pBAD33::hipTTa) or the empty pBAD33 vector combined with pSVN103 (pNDM220::trpS) or the empty low-copy-number vector pNDM220, as indicated, were diluted to similar OD600 values and washed in PBS before 10-times dilution series were spotted on LB agar plates containing the appropriate antibiotics and glucose (0.2%), arabinose (0.2%), or arabinose (0.2%) plus IPTG (200 μM), respectively. Download FIG S5, PDF file, 0.2 MB (210KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HipT phosphorylates TrpS at a conserved sequence motif.
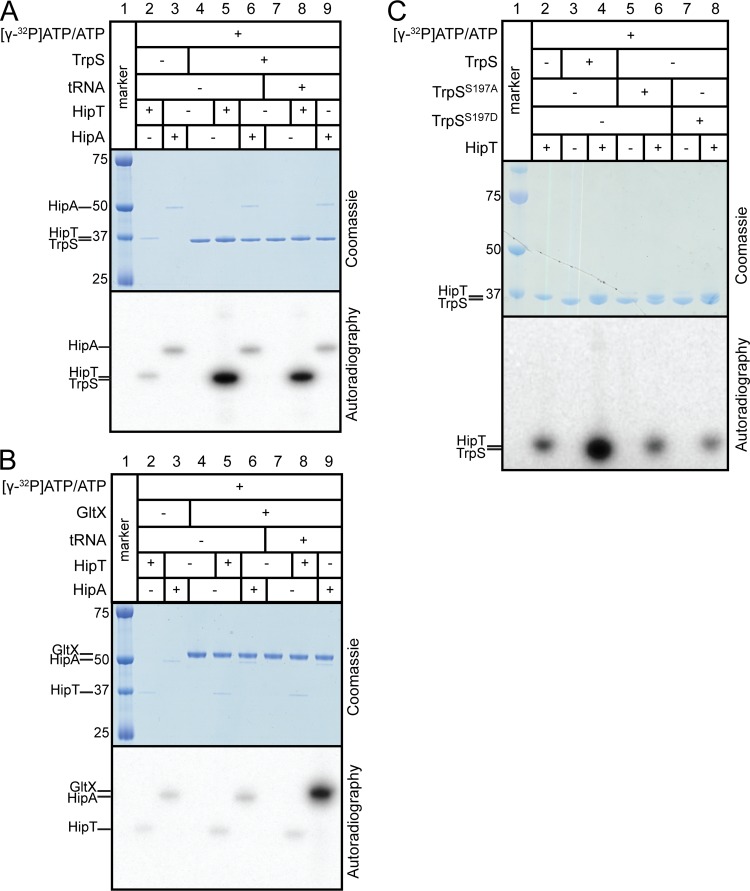
The above-described results suggested that HipT phosphorylates TrpS. To analyze HipT kinase activity directly, we purified HipTO127 and its presumed target, TrpS. For comparison, we included HipA and its known target GltX in the analysis. Indeed, HipTO127 phosphorylated TrpS in vitro (Fig. 3A, lanes 5 and 8) in a reaction that did not require tRNA (Fig. S6). We showed previously that HipA phosphorylates GltX in vitro in a reaction that requires the addition of tRNAGlu (11). Here, we were able to reproduce the results showing that HipA phosphorylates GltX in the presence but not in the absence of tRNA (Fig. 3B, lanes 6 and 9). Thus, HipT and HipA kinases differ not only with respect to their specific target but also by whether there is a requirement for the presence of tRNA in the in vitro reaction mixtures (see Discussion).
FIG 3.
HipTO127 phosphorylates TrpS at S197 in vitro, and HipTO127 is autophosphorylated in vitro. (A) Phosphorylation of TrpS and autophosphorylation of HipTO127 and HipA in vitro. HipTO127 or HipA (0.2 μM), [γ-32P]ATP (0.1 μM), and ATP (66 μM) were incubated with (+) or without (−) TrpS [purified from strain C41(DE3)/pSVN46], as well as with (+) or without (−) a mixture of all E. coli tRNAs (0.5 μg) per microliter of reaction mixture as indicated. (B) Phosphorylation of GltX and autophosphorylation of HipTO127 and HipA in vitro. HipTO127 or HipA (0.2 μM), [γ-32P]ATP (0.1 μM), and ATP (66 μM) were incubated with (+) or without (−) GltX (purified from strain JW2395), as well as with (+) or without (−) a mixture of E. coli tRNAs (0.5 μg) per microliter of reaction mixture as indicated. (C) Phosphorylation of TrpS at S197 and autophosphorylation of HipTO127 in vitro. HipTO127 (1.5 μM) [purified from strain C41(DE3)/pSVN42] was added to the reaction mixtures as indicated, in addition to [γ-32P]ATP (0.1 μM) and ATP (66 μM) with (+) or without (−)TrpS (1.5 μM), TrpSS197A (1.5 μM), or TrpSS197D (1.5 μM) as indicated.
tRNA is not required for in vitro phosphorylation of TrpS by HipTO127. Purified TrpS (1 μM; purified from E. coli BL21/pSVN46), HipTO127 (0.5 μM) (purified from E. coli BL21/pSVN42), and RNase A (0.1 mg/ml) were mixed with 0.1 μM [γ-32P]ATP and 66 μM ATP as indicated. Samples were treated as described in Materials and Methods. Download FIG S6, PDF file, 1.1 MB (1.2MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The best-conserved stretch of amino acids between GltX and TrpS are the highly conserved KLS239KR/KMS197KS flexible-loop motifs (Fig. S7). Lys237 and Lys195 participate in the catalytic reaction by stabilizing the transition state of ATP, and intact loop motifs are required for catalysis (37). The observation that HipA phosphorylates GltX at S239 (11) raised the possibility that HipT phosphorylates TrpS at the homologous S197. To test this, we introduced two amino acid changes, S197A and S197D, into TrpS, the latter to mimic a phosphorylated serine. Both changes abolished phosphorylation of HipTO127, consistent with the proposal that HipT phosphorylates TrpS at S197 (Fig. 3C). Finally, mass-spectrometric analysis revealed that, indeed, HipTO127 phosphorylates TrpS at S197 in vitro (Table 1). We also note that HipA did not phosphorylate TrpS (Fig. 3A, lanes 6 and 9), while HipTO127 did not phosphorylate GltX (Fig. 3B, lanes 5 and 8). This lack of cross-reactivity in the in vitro reactions is consistent with the specificity of the multicopy suppression data.
TABLE 1.
Phosphorylation sites identified by LC-MS/MS analysis of products of in vitro phosphorylation reaction between HipTO127 and TrpS
| Protein | Amino acid |
Andromeda score |
Localization probability |
Mass error (ppm) |
Phosphopeptide sequence of the best localized MS/MS spectrum |
|---|---|---|---|---|---|
| HipT | S57 | 104.24 | 0.999992 | 0.93417 | GMS(1)ISGYQPK |
| HipT | S59 | 139.32 | 0.999224 | 0.14379 | GMS(0.001)IS(0.999)GYQPK |
| TrpS | S197 | 304.44 | 0.999903 | −0.17001 | KMS(1)KSDDNRNNVIGLLEDPK |
| TrpS | S199 | 309.72 | 0.864936 | −0.094932 | SGARVMSLLEPTKKMS(0.135)KS(0.865)DDNRNNVIGLLEDPK |
Sequence alignment of GltX and TrpS. The alignment shows that S239 in GltX aligns to S197 in TrpS in the conserved sequence motifs KKLSKR and KKMSKS in GltX and TrpS, respectively. Download FIG S7, PDF file, 0.4 MB (461.9KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HipA is known to inactivate itself by trans autophosphorylation at Ser150 (35, 38). In the reaction mixture containing only HipTO127, a faint radioactive band corresponding to the MW of HipTO127 was observed (Fig. 3A and B, lane 2). Since HipTO127 was the only protein in the reaction mixture, we infer that HipTO127 phosphorylates itself. Consistently, the weak HipTO127 band also appeared when HipTO127 was mixed with the noncognate target GltX (Fig. 3B, lanes 5 and 8). Accordingly, the analysis of the products of the in vitro reaction between HipTO127 and TrpS by mass spectrometry showed that HipTO127 autophosphorylates either on S57 or S59 (Table 1).
HipTO127 stimulates production of (p)ppGpp.
We and others showed previously that HipA activates RelA to synthesize (p)ppGpp (11, 31, 32). Here, we measured whether induction of hipTO127 induces (p)ppGpp synthesis and compared it to the effect of induction of hipA or relE of E. coli K-12, the latter of which inhibits translation by ribosome-dependent mRNA cleavage (8). Indeed, induction of both hipTO127 and hipA resulted in increased levels of (p)ppGpp, albeit at a somewhat lower level in the case of hipTO127 (Fig. 4A and B). The latter observation is consistent with the fact that tryptophan is encoded by one codon only, compared to two in the case of glutamate, and the fraction of tryptophanyl-tRNA is less than 2% of total tRNA, whereas that of glutamyl-tRNA is more than 7% (39). Thus, deficiency of charged tRNAGlu leads to a higher level of hungry ribosomal A sites and, therefore, a higher number of activated RelA molecules and a higher level of (p)ppGpp. Consistent with previous results (40), induction of relE did not stimulate (p)ppGpp synthesis, showing that inhibition of translation per se is not sufficient to stimulate (p)ppGpp accumulation (Fig. 4A and B).
FIG 4.
HipTO127 induces (p)ppGpp accumulation in vivo. Levels of (p)ppGpp of E. coli MG1655 containing pNDM220 (vector), pAH1 (pNDM220::hipA), pSVN116 (pNDM220::hipTO127), or pAH2 (pNDM220::relE). The toxin-encoding genes were located downstream from the IPTG-inducible PA1/O3/O4 promoter (56, 57). (A) Cells were grown exponentially at 37°C in low-phosphate MOPS (morpholinepropanesulfonic acid) minimal medium containing radiolabeled H332PO4 (see Materials and Methods). Samples were withdrawn before and 10, 30, and 60 min after the addition of IPTG (1 mM) and analyzed by thin-layer chromatography (TLC) and phosphor imaging. (B) Quantification of the results of experiment shown in panel A and of repetitions of the experiment shown in Fig. S8. Materials and Methods gives additional experimental details. Error bars indicate standard deviations of three independent experiments.
(p)ppGpp accumulation following overproduction of HipA, HipTO127, and RelE. The figure shows a repetition of the experiment whose results are shown in Fig. 4A. Cells of E. coli MG1655 carrying pAH1 (pNDM220::hipA), pSVN116 (pNDM220::hipTO127), or pAH2 (pNDM220::relE) were grown exponentially in low-phosphate MOPS minimal medium containing H32PO4. Samples were withdrawn before and 10, 30, and 60 minutes after induction of the toxin genes by the addition of IPTG (1 mM) and analyzed by TLC and phosphor imaging. The data contributed to the quantification shown in Fig. 4B. Materials and Methods gives additional experimental details. Download FIG S8, PDF file, 2.7 MB (2.7MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
In this paper, we describe the discovery of a novel family of bacterial serine/threonine kinases, HipT kinases, that exhibit sequence similarity with HipA of E. coli K-12. HipA inhibits GltX (glutamyl-tRNA synthetase) by phosphorylation and thereby triggers RelA-dependent synthesis of (p)ppGpp (11, 31, 32). We found that HipT of E. coli O127 phosphorylates and inhibits TrpS (tryptophanyl-tRNA synthetase) and thereby, similarly to HipA, stimulates synthesis of (p)ppGpp (Fig. 4). Even though TrpS and GltX belong to the same class of tRNA synthetases (41), HipTO127 and HipA do not exhibit cross-phosphorylation of TrpS and GltX in vitro, implying that the two kinases exhibit substrate specificity (Fig. 3A and B). We showed previously that HipA phosphorylates S239 of the conserved KLS239KR motif in GltX (11). A variant of this motif (KMS197KS) is present in TrpS. Even though there are two amino acid differences between the two motifs, they represent the overall highest degree of sequence similarity between the two synthetases, suggesting that HipT phosphorylates S197 of TrpS. Indeed, this proposal was confirmed by our mass spectrometric and mutational analysis of TrpS (Table 1 and Fig. 3C).
We showed previously that phosphorylation of the conserved S239 of GltX by HipA requires the presence of tRNAGlu in the in vitro reaction mixture (11). We proposed that the binding of tRNAGlu to GltX would induce a conformational change of the motif KLS239KR that would make S239 accessible to phosphorylation (11). In contrast, even though GltX and TrpS belong to the same class of tRNA synthetases and the structural organization of their active sites is similar (42), phosphorylation of TrpS by HipTO127 does not require the addition of tRNA (Fig. S6 in the supplemental material). We believe that this difference is consistent with the requirement of GltX for the presence of cognate tRNA to activate glutamate to glutamyl-adenylate (41), a property shared with only two other type I tRNA synthetases (GlnRS and ArgRS). Thus, TrpS does not require the presence of tRNATrp to activate tryptophan to tryptophanyl-adenylate and does not require tRNATrp to be phosphorylated by HipT (Fig. S6).
HipA inactivates itself by autophosphorylation at the fully conserved, essential S150 located adjacent to the P loop of the kinase (35). Structural analysis revealed that autophosphorylation stabilizes a conformation of HipA that disrupts the ATP-binding pocket. It was proposed that autophosphorylation of HipA functions in the resuscitation of cells inhibited by HipA by preventing further activity of available toxins. This explanation is plausible, because cells inhibited by HipA somehow must revert the inhibition of GltX before the cells can resuscitate. We observed that HipTO127 is autophosphorylated in vitro (Fig. 3A and B) at the fully conserved S57 adjacent to the P-loop motif in HipT and, to a minor extent, at S59 in the P-loop motif, both of which are likely to inactivate the enzyme (Table 1).
The hipT gene is the third gene of the hipBST operon, and HipS and HipT exhibit sequence similarity with either end of HipA. The most parsimonious explanation as to how this arrangement appeared seems to be that hipA was duplicated during evolution and split into hipS and hipT, shifting the kinase specificity during this evolutionary trajectory. Analysis of the structure of HipBA reveals that HipS likely corresponds to the N-terminal subdomain of HipA, which was found to be involved in dimerization during DNA binding, as well as to harbor several mutations associated with high-persister phenotypes (Fig. S9A and B, blue) (26). A more detailed look at the N-terminal subdomain of HipA shows that residues involved in forming the hydrophobic core of the domain are well conserved in HipS, suggesting that HipS and the N-terminal subdomain of HipA share structure, while residues that are involved in HipA-HipA dimerization appear to differ in HipS while being conserved between HipS orthologs. This could suggest that the higher-order structure of HipBST differs from that of HipBA. We also note that several of the known high-persister mutations found in the N-terminal subdomain of HipA (including one of the mutations responsible for the hipA7 genotype) are naturally present in HipS, which raises the possibility that HipS is HipA7-like (Fig. S9C). Finally, the structural analysis also reveals that HipB (of HipBST) closely matches the corresponding antitoxin HipB in HipBA and likely harbors a DNA-binding domain (Fig. S9A and B). Of the three proteins, the function of HipS as the “third TA component” is clearly the most intriguing. We found that all three HipS orthologs investigated are able to counteract cognate HipT toxins on their own, while the HipB proteins do not have such an effect (Fig. 1). However, in two cases, we observed that the HipB proteins augmented HipS-mediated neutralization of HipT, suggesting that HipB somehow increases the activity of HipS, for example, by increasing HipS metabolic stability or by stabilizing the HipS-HipT interaction. The latter proposal is consistent with the observation that HipBST form a stable complex in vivo (Fig. S4). A summary of our findings is presented in Fig. 5 and described further in the legend to the figure.
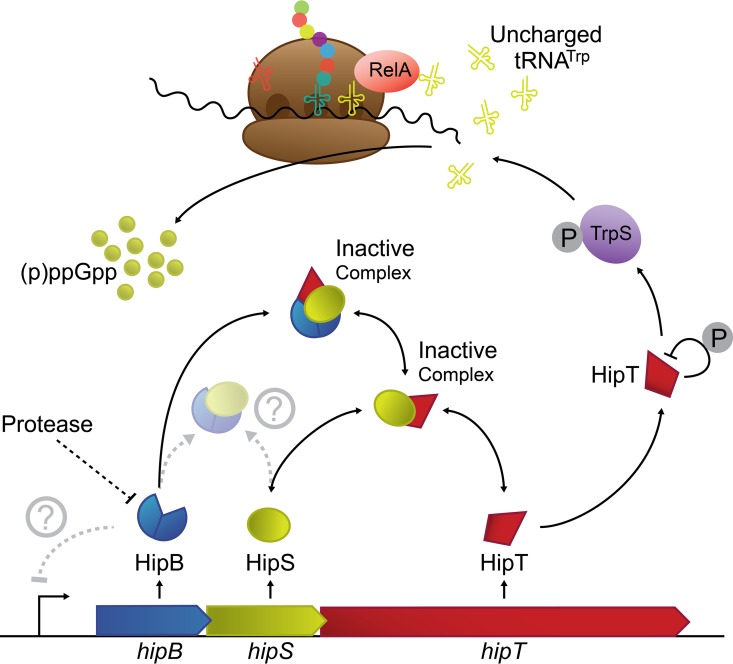
FIG 5.
Schematic overview of the components encoded by hipBST and their interaction. Our evidence supports the idea that HipT inactivates TrpS by phosphorylation and that HipT phosphorylates itself. Inactivation of TrpS, in turn, increases the level of uncharged tRNATrp that, in complex with RelA, loads at hungry ribosomal A sites loaded with tryptophan codons. Loading of the binary RelA-tRNATrp complex at an A site activates RelA to synthesize (p)ppGpp (33). The function of HipT autophosphorylation is unknown, but it may play a role in the resuscitation of HipT-induced persister cells. Furthermore, our data show that the HipBST proteins form one or more complexes and that HipT is inactivated by HipS. HipB contains an HTH DNA-binding motif and probably autoregulates the hipBST operon. Speculative interactions are indicated by stippled lines.
Modelling of the HipBSTO127 complex structure. (A). Top, the crystal structure of E. coli HipBA (PDB ID 4YG7) colored according to a sequence alignment with HipBSTO127, with the part corresponding to HipBO127 in purple, HipSO127 in blue, and HipTO127 in yellow. Bottom, schematic overview of the sequence alignment with colors as described above. HipA of E. coli K-12 is shown in red. (B) The structure of HipBA bound to DNA shows that the part corresponding to HipSO127 (the HipA N-terminal subdomain) mediates complex dimerization in the DNA-bound form. (C) The N-terminal subdomain of HipA contains a hydrophobic core conserved in HipSO127 (green) but a divergent dimerization interface (red). Two high-persister mutations in the N-subdomain-1 of HipA conserved among HipS orthologs are shown in yellow. Download FIG S9, PDF file, 0.2 MB (243.1KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Although two-component TA modules are by far the most common, a number of other three-component TA modules have been identified (1). In many of these cases, two adjacent genes exhibit sequence similarity with known type II TA modules, while the function of the third component often remains unclear. However, in a few cases, the function of the third component is known. For example, M. tuberculosis contains a three-gene TA module that encodes a RelE-homologous HigB toxin and the HigA antitoxin. The third gene encodes a SecB-like chaperone that controls the stability of HigA such that the antitoxin becomes metabolically unstable under environmental stress, thereby leading to activation of HigB and inhibition of translation by mRNA cleavage (43). Thus, in this TA module, the third component provides a link between cellular physiology and activation of the TA module. ω-ε-ζ of Streptococcus pyogenes is a three-component TA module in which ω is a DNA-binding autorepressor of the operon and ε is an antitoxin that neutralizes the ζ toxin by direct protein-protein contact (44). In the paaR-paaA-parE modules of E. coli O157:H7, the first gene encodes a transcriptional regulator of the module operon and the second a type II antitoxin that counteracts the activity of the ParE toxin (45). Thus, our work presented here reveals a novel type of three-component TA modules with unknown regulator properties that will be important and exciting to study. We hope that future biochemical and structural studies will be helpful in revealing the mechanisms of HipBST activation and regulation, as well as why this locus is configured as a three-component TA module.
MATERIALS AND METHODS
Strains, plasmids, media, and growth conditions.
Strains and plasmids are listed in Table 2, and DNA oligonucleotides in Table 3. Cultures were grown at 37°C with shaking at 160 rpm in Luria-Bertani (LB) medium. When required, the medium was supplemented with 25 μg/ml chloramphenicol, 30 μg/ml or 100 μg/ml ampicillin, and 25 μg/ml kanamycin. Gene expression from plasmids carrying the pBAD promoter was induced by 0.2% arabinose and repressed by 0.2% glucose. Gene expression from plasmids carrying the synthetic PA1/O4/O3 promoter was induced by 200 μM isopropyl β-d-1 thiogalactopyranoside (IPTG). The solid medium used to grow cells was Luria-Bertani agar (LB agar) medium supplemented with the appropriate antibiotics and incubated at 37°C for approximately 16 h unless otherwise stated.
TABLE 2.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| E. coli strains | ||
| MG1655 | Wild-type K-12 | 58 |
| MG1655ΔydeA | K-12 MG1655ΔydeA::FRT | This work |
| BL21 | F− ompT hsdSB (rB− mB−) gal dcm | 59 |
| C41 (DE3) | Derived from BL21(DE3): F− ompT hsdSB (rB− mB−) gal dcm (DE3) | 60 |
| EG235 | C41 (DE3) ΔhipBA::kan, pMG25::gltX (optimized SD), pBAD33::His6::hipA (SD8 and start codon ATG) | Laboratory collection |
| JW2395 | AG1 [recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1] carrying pCA24N::gltX, GltX purification plasmid encoding N-terminally His6-tagged gltX, from ASKA collection | 55 |
| Plasmids | ||
| pBAD33 | p15 araC PBAD, Cmr | 61 |
| pNDM220 | Mini-R1 lacIq PA1/04/03, Ampr | 56 |
| pCP20 | pSC101 rep(Ts), Ampr Cmr | 47 |
| pBR322 | pMB1 rop, Ampr Tetr | 62 |
| pMG25 | pUC lacIq PA1/O4/O3, Ampr | Laboratory collection |
| pEG25 | pMG25 derivative that has reduced leakiness of the IPTG-inducible PA1/O4/O3 promoter | Laboratory collection |
| pEG::gltX | pMG25::gltX, optimized SD | Laboratory collection |
| pEG::His6hipA | pBAD33::His6hipA, HipA purification plasmid harboring N-terminally His6-tagged hipA | Laboratory collection |
| pET-15b | pBR322 lacI PT7, Ampr | Novagen |
| pKG127 | pUC57::hipBSTO127 | Genscript |
| pSVN1 | pBAD33::hipTO127, start codon GTG | This work |
| pSVN42 | pEG25::hipTO127::His6, optimized SD, HipTO127 purification plasmid C-terminally His6-tagged hipTO127 | This work |
| pSVN46 | pEG25::trpSHis6, optimized SD, TrpS purification plasmid harboring C-terminally His6-tagged trpS | This work |
| pSVN49 | pEG25::trpSS197DHis6, optimized SD, TrpSS197D purification plasmid harboring C-terminally His6-tagged trpSS197D |
This work |
| pSVN52 | pEG25::trpSS197AHis6, optimized SD, TrpSS197A purification plasmid harboring C-terminally His6-tagged trpSS197A |
This work |
| pSVN60 | pUC57::His6-TEVhipB::hipS::hipTO127, optimized SDs for all genes | Genscript |
| pSVN61 | pUC57::hipB::hipS::hipTO127::His6, optimized SDs for all genes used for TA complex purification via C-terminally His6-tagged hipTO127 |
Genscript |
| pSVN37 | pEG25::trpS, optimized SD | This work |
| pSVN44 | pBAD33::hipBSO127, optimized SD for hipBO127 | This work |
| pSVN94 | pET-15b::His6-TEVhipB::hipS::hipTD233QO127, optimized SDs for all genes | This work |
| pSVN103 | pNDM220::trpS, optimized SD | This work |
| pSVN109 | pNDM220::hipSO127, optimized SD | This work |
| pSVN110 | pNDM220::hipBSO127, optimized SDs | This work |
| pSVN111 | pNDM220::hipBO127, optimized SD | This work |
| pSVN113 | pUC57::hipBSTHi | Genscript |
| pSVN114 | pUC57::hipBSTTa | Genscript |
| pSVN116 | pNDM220::hipTO127, start codon GTG | This work |
| pSVN122 | pNDM220::hipBHi, optimized SD | This work |
| pSVN123 | pNDM220::hipSHi, optimized SD | This work |
| pSVN124 | pNDM220::hipBSHi, optimized SD for hipBHi, overlapping hipBHi and hipSHi | This work |
| pSVN126 | pNDM220::hipBTa, optimized SD | This work |
| pSVN127 | pNDM220::hipSTa, optimized SD | This work |
| pSVN128 | pNDM220::hipBSTa, optimized SD for hipBTa, overlapping hipBTa and hipSTa | This work |
| pSVN129 | pBAD33::hipTTa, optimized SD, start codon GTG | This work |
| pSVN135 | pBAD33::hipTHi, optimized SD | This work |
| pSVN138 | pNDM220::hipBSTa, optimized SDs | This work |
| pSVN139 | pNDM220::hipBSHi, optimized SDs | This work |
| pAH1 | pNDM220::hipA | A. Harms |
| pAH2 | pNDM220::relE | A. Harms |
SD, Shine-Dalgarno sequence.
TABLE 3.
Oligonucleotides
| Oligonucleotide | Sequence |
|---|---|
| FP1(GTG) | CCCCCGTCGACGGATCCAAGGAGTTTTATAAGTGGCGAATTGTCGTATTCTG |
| RP1 | CCCCCGCATGCGAATTCGCTCACAGCAGCCCCAGACG |
| FP25 | CCCCCTCGAGGGATCCAAAATAAGGAGGAAAAAAAAATGATCTGCTCAGGACCAC |
| RP15 | GGGGGAATTCAAGCTTTCACTCGCCGATGCATAG |
| FP22 | CCCCCTCGAGGGATCCAAAATAAGGAGGAAAAAAAAATGCATCGGCGAGTGAAAG |
| RP14 | GGGGGAATTCAAGCTTTTATTCCTCCCAAGGTAAAATC |
| FP39 | CCCCGGGGGATCCAAAATAAGGAGGAAAAAAAAATGAATTTTTGTCGTATTTTATTAAAG |
| RP21 | GGGGGTACCCTGCAGTTATAGTTCAGGTTCATTTAATAG |
| FP29 | CCCCCGGGGGATCCAAAATAAGGAGGAAAAAAAAATGGACAATCTTAGTGCAC |
| RP19 | GGGGGTACCCTGCAGCTAAATCGCGCATAGTGAAAC |
| FP30 | CCCCCGGGGGATCCAAAATAAGGAGGAAAAAAAAATGCGCGATTTAGTCCGC |
| RP20 | GGGGGTACCCTGCAGTCATTGTTTTTCTTCCTG |
| FP42 | AAAATAAGGAGGAAAAAAAAATGCGCGATTTAGTCCG |
| RP30 | TTTTTTTTCCTCCTTATTTTCTAAATCGCGCATAGTGAAAC |
| FP34 | CCCCCGGGGGATCCAAAATAAGGAGGAAAAAAAAGTGGACCGTTGTCTGATCAC |
| RP24 | GGGGGTACCCTGCAGTTACCGGTCGAGATCGACAAC |
| FP32 | CCCCCGGGGGATCCAAAATAAGGAGGAAAAAAAAATGAGCCATAGAAATCTACTCG |
| RP22 | GGGGGTACCCTGCAGTTACTTTGCGGCCCATAACTTG |
| FP33 | CCCCCGGGGGATCCAAAATAAGGAGGAAAAAAAAATGGGCCGCAAAGTAATTG |
| RP23 | GGGGGTACCCTGCAGTTAATCATTAACCTCAAG |
| FP41 | AAAATAAGGAGGAAAAAAAAATGGGCCGCAAAGTAATT |
| RP29 | TTTTTTTTCCTCCTTATTTTCTATTTGGCGGCCCATAACTTGATAC |
| trpS Fw | CCCCCGGATCCAAAATAAGGAGGAAAAAAAAATGACTAAGCCCATCG |
| trpS RP4 | GGGGGAATTCTTACGGCTTCGCCACAAAACC |
| trpS Rv | CCCCCAAGCTTTTACGGCTTCGCCACAAAAC |
| FP13 | CCCCGGATCCAAAATAAGGAGGAAAAAAAAATGGCGAATTGTCGTATTC |
| RP5 | GGGGAAGCTTTCAGTGATGGTGATGGTGATGCAGCAGCCCCAGACGATG |
| trpS RP3 | GGGGGAAGCTTTTAGTGATGGTGATGGTGATGCGGCTTCGCCACAAAACC |
| trpS S197A Fw | AGAAGATGGCCAAGTCTGACGATAATCGC |
| trpS S197A Rv | AGACTTGGCCATCTTCTTGGTCGGCTC |
| trpS S197D Fw | AGAAGATGGACAAGTCTGACGATAATCGCA |
| trpS S197D Rv | CAGACTTGTCCATCTTCTTGGTCGGCTC |
| FP5 | CCCCCGTCGACGGATCCAAGGAAAAAAAAAGTGGCGAATTGTCGTATTCTG |
| FP15 | CCCCGTCGACAAAATAAGGAGGAAAAAAAAATGATCTGCTCAGGACCA |
| RP6 | GGGGGCATGCTTATTCCTCCCAAGGTAAAA |
| hipX D233Q Fw | CGGTGTATCAGTTTGTTTCTGTCGCTCCC |
| hipX D233Q Rv | GAAACAAACTGATACACCGGCGCTAACG |
| FP16 | CCCCGAATTCAAAATAAGGAGGAAAAAAAAATGCATCACCATCACCATCACGAAAACCTGTACTTCCAAGGGATCTGCTCAGGACCACAAAATC |
| RP7 | GGGGAAGCTTTCACTCGCCGATGCATAGTTTC |
| RP13 | GGGGGAATTCAAGCTTTTAGTGATGGTGATGGTGATGTTCCTCCCAAGGTAAAATC |
Gene knockout by P1 transduction.
To construct strain E. coli MG1655ΔydeA, gene knockout was obtained by phage P1 transduction using a strain of the Keio collection as donor according to standard procedure (46, 47).
Multicopy suppression screening.
Genomic DNA (gDNA) of E. coli MG1655ΔydeA was purified according to the manufacturer’s instructions (EdgeBio). The gDNA was then partially digested with Sau3AI (Bsp143I) and fragments inserted into pBR322, which had been digested with BamHI and dephosphorylated. The gene library was transformed into a strain harboring the pBAD33::hipTO127 plasmid and plated on agar plates containing arabinose.
Site-directed mutagenesis.
Amino acid changes TrpSS197A, TrpSS197D, and HipTO127D233Q were constructed by PCR mutagenesis (Table 3). The PCR products were digested with DpnI, and the resulting plasmids were transformed into E. coli strain DH5α.
Protein purification.
HipTO127 (produced by pSVN42) was purified from E. coli strain BL21 that also produced HipBO127 and HipSO127 (pSVN44). Overnight cultures were diluted 100-fold into 350 ml fresh LB medium. At an optical density at 600 nm (OD600) of ≈0.3, the toxin gene was induced by the addition of 1 mM IPTG for 4 h, and cells were harvested by centrifugation. Pellets were resuspended in 25 ml cold buffer A (50 mM NaH2PO4 [pH 8], 0.3 M NaCl, 10 mM imidazole, 5 mM β-mercaptoethanol [BME]) with the addition of half a protease inhibitor cocktail each. Cells were carefully sonicated for 5 min at 60% amplification (2 s on and 2 s off) while still kept cold. The cell lysate was spun at 16,000 rpm for 30 min at 4°C, and the cleared lysate was incubated at 4°C for 1 h with 1 ml Ni beads that had been freshly equilibrated in the same buffer for 1 h. Protein-bound beads were then applied to gravity flow columns and washed with 50 ml of buffer B (50 mM NaH2PO4 [pH 8], 0.3 M NaCl, 35 mM imidazole, 1 mM BME). As described previously, the toxin and antitoxins were separated with urea washes to leave the His-tagged protein on the affinity column (48). His-tagged proteins were purified according to the manufacturer’s protocol, further purified using an Äkta Pure (GE Healthcare) fast protein liquid chromatography (FPLC) instrument, and stored in 200 mM NaCl, 50 mM Tris-HCl, and 5% glycerol. All proteins purified with His tags were tested and compared to wild-type proteins in vivo prior to purification in order to assess their functionality.
Phosphorylation in vitro.
Phosphorylation reactions were performed in the presence of 0.05 μl [γ-32P]ATP (6,000 Ci/mmol; Perkin Elmer) per microliter of reaction mixture, 66.6 μM ATP (nonradioactive), and aminoacylation buffer (1 mM dithiothreitol [DTT], 10 mM KCl, 16 μM ZnSO4, and 20 mM MgCl2) for 45 min as described previously (11). Each reaction was stopped by the addition of 1 volume Laemmli loading buffer, the reaction mixture was incubated for 10 min at 95°C, and proteins were resolved by SDS-PAGE and exposed using phosphorimaging (GE Healthcare) overnight.
Phosphorylation in vitro measured by LC-MS.
The phosphorylation reaction was performed with 13.5 μM TrpS and 6.7 μM HipTO127 in the presence of 5 mM ATP and aminoacylation buffer for 45 min at 37°C. The reaction was stopped by the addition of 4 volumes of denaturation buffer (6 M urea, 2 M thiourea, 1 mM DTT, and 10 mM Tris-HCl, pH 8.0), and the reaction mixture incubated for 30 min at room temperature, followed by incubation with 5.5 mM iodoacetamide for 45 min at room temperature. Denatured proteins were digested overnight either with endoproteinase Lys-C (1:100 [wt/wt]; Wako) in 20 mM bicarbonate, pH 8.0, or with endoproteinase Arg-C (1:100 [wt/wt]; Roche) in 90 mM Tris-HCl, pH 7.6, 8.5 mM CaCl2, 5 mM DTT, 0.5 mM EDTA. Digested peptides were purified via Pierce C18 Spin Tips, and 0.5 μg of each sample was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously (49). Briefly, peptides were separated by an Easy-nL 1200 ultra-high-performance liquid chromatography (UHPLC) instrument (Thermo Fisher Scientific) and transferred into an online coupled Q Exactive HF mass spectrometer (Thermo Fisher Scientific) by nanoelectrospray ionization. Peptides were eluted from a 20-cm-long analytical column packed with 1.9-μm reverse-phase particles using a 33-min segmented gradient of 5% to 50% solvent B (80% [vol/vol] acetonitrile, 0.1% [vol/vol] formic acid) at a constant flow rate of 300 nl/min. Full-scan MS spectra were acquired in a mass range from 300 to 1,650 m/z with a maximum injection time of 45 ms and a resolution of 60,000. Higher-energy collisional dissociation MS/MS scans of the 7 (Top7 data-dependent method) most abundant peaks were recorded with a maximum injection time of 220 ms at a resolution of 60,000. Acquired raw data were processed with MaxQuant software (version 1.5.2.8) (50) using default settings if not stated otherwise. The derived peak list was searched against a reference E. coli K-12 proteome (Taxon identifier 83333) containing 4,324 entries (UniProt, release 2017/12), the protein sequence of HipT, HipS, and HipB from E. coli O127:H6, and a file containing 245 common laboratory contaminants using a built-in Andromeda search engine (51). Methionine oxidation, protein N-terminal acetylation, and Ser/Thr/Tyr phosphorylation were defined as variable modifications, and carbamidomethylation of cysteines was set as a fixed modification. The maximum number of missed cleavages allowed was set to 3 for the endoproteinase Lys-C and to 2 for Arg-C. Only phosphopeptides with an Andromeda score of >70 and a localization probability of >0.75 were considered, and their MS/MS spectra were inspected manually (Fig. S10).
Representative mass spectra after in vitro kinase assay revealing HipTO127-mediated phosphorylation sites on TrpS (A, B) and autophosphorylation sites of HipTO127 (C, D). Download FIG S10, PDF file, 0.2 MB (177KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Measurement of cellular (p)ppGpp levels.
Measurement of cellular (p)ppGpp levels was performed as described previously (52, 53).
Mass spectrometry.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (54) partner repository with the data set identifier PXD012023.
Construction of plasmids.
Construction of plasmids is summarized below.
pKG127.
The region of the E. coli O127 E2348/69 genome (accession number NC_011601.1) containing the hipBST locus (+3,948,403 to +3,950,320) was synthesized (GeneScript) and inserted into the SalI restriction site of pUC57.
pSVN1.
hipTO127 with start codon GTG was amplified from pKG127 using primers FP1(GTG) and RP1. The resulting PCR product was digested with SalI and SphI and ligated with pBAD33.
pSVN37.
trpS was amplified from pCA24N::trpS from the ASKA collection (55) using primers trpS Fw and trpS Rv. The resulting PCR product was digested with BamHI and HindIII and ligated into pEG25.
pSVN42.
hipTO127::His6 was amplified from pKG127 using primers FP13 and RP5. The resulting PCR product was digested with BamHI and HindIII and ligated into pEG25.
pSVN44.
hipBSO127 was amplified from pKG127 using primers FP15 and RP6. The resulting PCR product was digested with SalI and SphI and ligated into pBAD33.
pSVN46.
trpSHis6 was amplified from pSVN37using primers trpS Fw and trpS RP3. The resulting PCR product was digested with BamHI and HindIII and ligated into pEG25.
pSVN49.
The mutation in trpSS197DHis6 was created using pSVN46 and primers trpS S197D Fw and trpS S197D Rv in a site-directed plasmid mutagenesis PCR. The fragment was digested with DpnI before being transformed into E. coli DH5α.
pSVN52.
The mutation in trpSS197AHis6 was created using pSVN46 and primers trpS S197A Fw and trpS S197A Rv in a site-directed plasmid mutagenesis PCR. The fragment was digested with DpnI before being transformed into E. coli DH5α.
pSVN94.
His6-tevhipBO127::hipSO127::hipTO127 with optimized SDs for all three genes was subcloned from pSVN60 by digesting with XbaI and XhoI, purifying the DNA fragment from a 1% agarose gel, and ligating into pET-15b. The mutation in His6-tevhipBO127::hipS::hipTD233Q was created using primers hipX D233Q Fw and hipX D233Q Rv in a site-directed plasmid mutagenesis PCR. The fragment was digested with DpnI before transformation.
pSVN103.
trpS was amplified from pCA24N::trpS from the ASKA collection (55) using primers trpS Fw and trpS RP4. The resulting PCR product was digested with BamHI and EcoRI and ligated into pNDM220.
pSVN109.
hipSO127 was amplified from pKG127 using primers FP22 and RP14. The resulting PCR product was digested with XhoI and EcoRI and ligated into pNDM220.
pSVN110.
hipBSO127 was amplified from pSVN61 using primers FP25 and RP14. The resulting PCR product was digested with XhoI and EcoRI and ligated into pNDM220.
pSVN111.
hipBO127 was amplified from pSVN61 using primers FP25 and RP15. The resulting PCR product was digested with XhoI and EcoRI and ligated into pNDM220.
pSVN113.
The region of the H. influenzae Rd KW20 genome (NC_000907.1) containing the hipBST locus (+710,585 to +712,589) was synthesized and inserted into the SalI site of pUC57 (GeneScript).
pSVN114.
The region of the Tolumonas auensis DSM 9187 genome (NC_012691.1) containing the hipBST locus (+2,117,168 to +2,119,170) was synthesized and inserted into the SalI site of pUC57 (GeneScript).
pSVN116.
hipTO127 was amplified using primers FP5 and RP1 from pSVN1. The fragment was then cloned into cut pNDM220 using BamHI and EcoRI, resulting in pSVN116 (pNDM220::hipTO127).
pSVN122.
hipBHi was amplified from pSVN113 using primers FP29 and RP19. The resulting PCR product was digested with BamHI and KpnI and ligated into pNDM220.
pSVN123.
hipSHi was amplified from pSVN113 using primers FP30 and RP20. The resulting PCR product was digested with BamHI and KpnI and ligated into pNDM220.
pSVN124.
hipBSHi was amplified from pSVN113 using primers FP29 and RP20. The resulting PCR product was digested with BamHI and KpnI and ligated into pNDM220.
pSVN126.
hipBTa was amplified from pSVN114 using primers FP32 and RP22. The resulting PCR product was digested with BamHI and KpnI and ligated into pNDM220.
pSVN127.
hipSTa was amplified from pSVN114 using primers FP33 and RP23. The resulting PCR product was digested with BamHI and KpnI and ligated into pNDM220.
pSVN128.
hipBSTa was amplified from pSVN114 using primers FP32 and RP23. The resulting PCR product was digested with BamHI and KpnI and ligated into pNDM220.
pSVN129.
hipTTa was amplified from pSVN114 using primers FP34 and RP24. The resulting PCR product was digested with XmaI and PstI and ligated into pBAD33.
pSVN135.
hipTHi was amplified from pSVN113 using primers FP39 and RP21. The resulting PCR product was digested with XmaI and PstI and ligated into pBAD33.
pSVN138.
The optimized SD inserted between hipBTa and hipSTa was created using pSVN128 and primers FP41 and RP29 in a site-directed plasmid mutagenesis PCR. Eight reactions were carried out at different temperatures with a gradient PCR. The samples were pooled and digested with DpnI to digest the parental plasmid before being transformed into E. coli DH5α.
pSVN139.
The optimized SD inserted between hipBHi and hipSHi was created using pSVN124 and primers FP42 and RP30 in a site-directed plasmid mutagenesis PCR. The fragment was digested with DpnI before transformation.
ACKNOWLEDGMENTS
We thank Alexander Harmes, Biozentrum, Center for Molecular Life Sciences, University of Basel, Switzerland, and Elsa Germain, Laboratoire de Chimie Bactérienne for donation of plasmids, CNRS-Aix Marseille University, Institut de Microbiologie de la Méditerranée, Marseille, France, for the donation of plasmids.
This work was funded by a Novo Nordisk Foundation laureate research grant to K.G. and by the Danish Natural Research Foundation’s Centre of Excellence for Bacterial Stress Response and Persistence (grant number DNRF120 to K.G.).
Footnotes
Citation Vang Nielsen S, Turnbull KJ, Roghanian M, Bærentsen R, Semanjski M, Brodersen DE, Macek B, Gerdes K. 2019. Serine-threonine kinases encoded by split hipA homologs inhibit tryptophanyl-tRNA synthetase. mBio 10:e01138-19. https://doi.org/10.1128/mBio.01138-19.
Contributor Information
Michael T. Laub, Massachusetts Institute of Technology.
Sophie Helaine, Imperial College London.
Cecilia Arraiano, Instituto de Tecnologia Quimica e Biologica-Universidade Nova de Lisboa.
REFERENCES
- 1.Harms A, Brodersen DE, Mitarai N, Gerdes K. 2018. Toxins, targets, and triggers: an overview of toxin-antitoxin biology. Mol Cell 70:768–784. doi: 10.1016/j.molcel.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Page R, Peti W. 2016. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol 12:208. doi: 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- 3.Hayes F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301:1496–1499. doi: 10.1126/science.1088157. [DOI] [PubMed] [Google Scholar]
- 4.Chan WT, Espinosa M, Yeo CC. 2016. Keeping the wolves at bay: antitoxins of prokaryotic type II toxin-antitoxin systems. Front Mol Biosci 3:9. doi: 10.3389/fmolb.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen MG, Pandey DP, Jaskolska M, Gerdes K. 2009. HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J Bacteriol 191:1191–1199. doi: 10.1128/JB.01013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerdes K. 2000. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress. J Bacteriol 182:561–572. doi: 10.1128/jb.182.3.561-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen K, Zavialov AV, Pavlov MY, Elf J, Gerdes K, Ehrenberg M. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:131–140. doi: 10.1016/S0092-8674(02)01248-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell 12:913–923. doi: 10.1016/S1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 10.Winther KS, Gerdes K. 2011. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc Natl Acad Sci U S A 108:7403–7407. doi: 10.1073/pnas.1019587108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain E, Castro-Roa D, Zenkin N, Gerdes K. 2013. Molecular mechanism of bacterial persistence by HipA. Mol Cell 52:248–254. doi: 10.1016/j.molcel.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Cheverton AM, Gollan B, Przydacz M, Wong CT, Mylona A, Hare SA, Helaine S. 2016. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol Cell 63:86–96. doi: 10.1016/j.molcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard P, Couturier M. 1992. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J Mol Biol 226:735–745. doi: 10.1016/0022-2836(92)90629-X. [DOI] [PubMed] [Google Scholar]
- 14.Jurėnas D, Chatterjee S, Konijnenberg A, Sobott F, Droogmans L, Garcia-Pino A, Van Melderen L. 2017. AtaT blocks translation initiation by N-acetylation of the initiator tRNA(fMet). Nat Chem Biol 13:640–646. doi: 10.1038/nchembio.2346. [DOI] [PubMed] [Google Scholar]
- 15.Miki T, Park JA, Nagao K, Murayama N, Horiuchi T. 1992. Control of segregation of chromosomal DNA by sex factor F in Escherichia coli. Mutants of DNA gyrase subunit A suppress letD (ccdB) product growth inhibition. J Mol Biol 225:39–52. doi: 10.1016/0022-2836(92)91024-J. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Pogliano J, Helinski DR, Konieczny I. 2002. ParE toxin encoded by the broad-host-range plasmid RK2 is an inhibitor of Escherichia coli gyrase. Mol Microbiol 44:971–979. doi: 10.1046/j.1365-2958.2002.02921.x. [DOI] [PubMed] [Google Scholar]
- 17.Freire DM, Gutierrez C, Garza-Garcia A, Grabowska AD, Sala AJ, Ariyachaokun K, Panikova T, Beckham KSH, Colom A, Pogenberg V, Cianci M, Tuukkanen A, Boudehen YM, Peixoto A, Botella L, Svergun DI, Schnappinger D, Schneider TR, Genevaux P, de Carvalho LPS, Wilmanns M, Parret AHA, Neyrolles O. 2019. An NAD(+) phosphorylase toxin triggers Mycobacterium tuberculosis cell death. Mol Cell 73:1282–1291.e8. doi: 10.1016/j.molcel.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skjerning RB, Senissar M, Winther KS, Gerdes K, Brodersen DE. 2019. The RES domain toxins of RES-Xre toxin-antitoxin modules induce cell stasis by degrading NAD. Mol Microbiol 111:221–236. doi: 10.1111/mmi.14150. [DOI] [PubMed] [Google Scholar]
- 19.Arnoldini M, Mostowy R, Bonhoeffer S, Ackermann M. 2012. Evolution of stress response in the face of unreliable environmental signals. PLoS Comput Biol 8:e1002627. doi: 10.1371/journal.pcbi.1002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radzikowski JL, Vedelaar S, Siegel D, Ortega AD, Schmidt A, Heinemann M. 2016. Bacterial persistence is an active sigmaS stress response to metabolic flux limitation. Mol Syst Biol 12:882. doi: 10.15252/msb.20166998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms A, Maisonneuve E, Gerdes K. 2016. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354:aaf4268. doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- 22.Veening JW, Smits WK, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 23.Dorr T, Vulic M, Lewis K. 2010. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol 8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goneau LW, Yeoh NS, MacDonald KW, Cadieux PA, Burton JP, Razvi H, Reid G. 2014. Selective target inactivation rather than global metabolic dormancy causes antibiotic tolerance in uropathogens. Antimicrob Agents Chemother 58:2089–2097. doi: 10.1128/AAC.02552-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyed HS, Bertrand KP. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol 155:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher MA, Balani P, Min J, Chinnam NB, Hansen S, Vulić M, Lewis K, Brennan RG. 2015. HipBA–promoter structures reveal the basis of heritable multidrug tolerance. Nature 524:59. doi: 10.1038/nature14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korch SB, Henderson TA, Hill TM. 2003. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol 50:1199–1213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- 28.Korch SB, Hill TM. 2006. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J Bacteriol 188:3826–3836. doi: 10.1128/JB.01740-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stancik IA, Šestak MS, Ji B, Axelson-Fisk M, Franjevic D, Jers C, Domazet-Lošo T, Mijakovic I. 2018. Serine/threonine protein kinases from Bacteria, Archaea and Eukarya share a common evolutionary origin deeply rooted in the tree of life. J Mol Biol 430:27–32. doi: 10.1016/j.jmb.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Hanks SK, Quinn AM, Hunter T. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 31.Kaspy I, Rotem E, Weiss N, Ronin I, Balaban NQ, Glaser G. 2013. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat Commun 4:3001. doi: 10.1038/ncomms4001. [DOI] [PubMed] [Google Scholar]
- 32.Bokinsky G, Baidoo EE, Akella S, Burd H, Weaver D, Alonso-Gutierrez J, García-Martín H, Lee TS, Keasling JD. 2013. HipA-triggered growth arrest and β-lactam tolerance in Escherichia coli are mediated by RelA-dependent ppGpp synthesis. J Bacteriol 195:3173–3182. doi: 10.1128/JB.02210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winther KS, Roghanian M, Gerdes K. 2018. Activation of the stringent response by loading of RelA-tRNA complexes at the ribosomal A-site. Mol Cell 70:95–105.e4. doi: 10.1016/j.molcel.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher MA, Piro KM, Xu W, Hansen S, Lewis K, Brennan RG. 2009. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 323:396–401. doi: 10.1126/science.1163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher MA, Min J, Link TM, Guan Z, Xu W, Ahn Y-H, Soderblom EJ, Kurie JM, Evdokimov A, Moseley MA, Lewis K, Brennan RG. 2012. Role of unusual P-loop ejection and autophosphorylation in HipA-mediated persistence and multidrug tolerance. Cell Rep 2:518–525. doi: 10.1016/j.celrep.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekine S, Nureki O, Dubois DY, Bernier S, Chenevert R, Lapointe J, Vassylyev DG, Yokoyama S. 2003. ATP binding by glutamyl-tRNA synthetase is switched to the productive mode by tRNA binding. EMBO J 22:676–688. doi: 10.1093/emboj/cdg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correia FF, D’Onofrio A, Rejtar T, Li L, Karger BL, Makarova K, Koonin EV, Lewis K. 2006. Kinase activity of overexpressed HipA is required for growth arrest and multidrug tolerance in Escherichia coli. J Bacteriology 188:8360–8367. doi: 10.1128/JB.01237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Nilsson L, Kurland CG. 1996. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J Mol Biol 260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- 40.Germain E, Roghanian M, Gerdes K, Maisonneuve E. 2015. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc Natl Acad Sci U S A 112:5171–5176. doi: 10.1073/pnas.1423536112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Giege R, Springer M. 23 May 2016, posting date. Aminoacyl-tRNA synthetases in the bacterial world. EcoSal Plus 2016 . doi: 10.1128/ecosalplus.ESP-0002-2016. [DOI] [PubMed] [Google Scholar]
- 42.Ribas de Pouplana L, Schimmel P. 2001. Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell 104:191–193. doi: 10.1016/S0092-8674(01)00204-5. [DOI] [PubMed] [Google Scholar]
- 43.Bordes P, Cirinesi A-M, Ummels R, Sala A, Sakr S, Bitter W, Genevaux P. 2011. SecB-like chaperone controls a toxin-antitoxin stress-responsive system in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 108:8438–8443. doi: 10.1073/pnas.1101189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volante A, Soberon NE, Ayora S, Alonso JC. 2014. The interplay between different stability systems contributes to faithful segregation: Streptococcus pyogenes pSM19035 as a model. Microbiol Spectr 2(4):PLAS-0007-2013. doi: 10.1128/microbiolspec.PLAS-0007-2013. [DOI] [PubMed] [Google Scholar]
- 45.Hallez R, Geeraerts D, Sterckx Y, Mine N, Loris R, Van Melderen L. 2010. New toxins homologous to ParE belonging to three-component toxin-antitoxin systems in Escherichia coli O157:H7. Mol Microbiol 76:719–732. doi: 10.1111/j.1365-2958.2010.07129.x. [DOI] [PubMed] [Google Scholar]
- 46.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherny I, Overgaard M, Borch J, Bram Y, Gerdes K, Gazit E. 2007. Structural and thermodynamic characterization of the Escherichia coli RelBE toxin-antitoxin system: indication for a functional role of differential stability. Biochemistry 46:12152–12163. doi: 10.1021/bi701037e. [DOI] [PubMed] [Google Scholar]
- 49.Semanjski M, Germain E, Bratl K, Kiessling A, Gerdes K, Macek B. 2018. The kinases HipA and HipA7 phosphorylate different substrate pools in Escherichia coli to promote multidrug tolerance. Sci Signal 11:eaat5750. doi: 10.1126/scisignal.aat5750. [DOI] [PubMed] [Google Scholar]
- 50.Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 51.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. 2011. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 52.Cashel M. 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants. Methods Mol Genet 3:341–356. [Google Scholar]
- 53.Tian C, Roghanian M, Jorgensen MG, Sneppen K, Sorensen MA, Gerdes K, Mitarai N. 2016. Rapid curtailing of the stringent response by toxin-antitoxin module-encoded mRNases. J Bacteriol 198:1918–1926. doi: 10.1128/JB.00062-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vizcaíno JA, Csordas A, del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu Q-W, Wang R, Hermjakob H. 2016. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 44:D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 56.Gotfredsen M, Gerdes K. 1998. The Escherichia coli relBE genes belong to a new toxin–antitoxin gene family. Mol Microbiol 29:1065–1076. doi: 10.1046/j.1365-2958.1998.00993.x. [DOI] [PubMed] [Google Scholar]
- 57.Lanzer M, Bujard H. 1988. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A 85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blattner F, Plunkett GI, Bloch C, Perna N, Burland V, Riley M, Collado-Vides J, Glasner J, Rode C, Mayhew G, Gregor J, Davis N, Kirkpatrick H, Goeden M, Rose D, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 59.Studier FW, Moffatt BA. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 60.Miroux B, Walker JE. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 61.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose P(BAD) promoter. J Bacteriol 177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. 1977. Construction and characterization of new cloning vehicle. II. A multipurpose cloning system. Gene 2:95–113. doi: 10.1016/0378-1119(77)90000-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignments of protein sequences encoded by hipBST loci from five different gammaproteobacteria and a phylogenetic analysis of HipT. (A) Alignment of HipT homologs with the C-terminal part of HipA of E. coli K-12. The 8-aa conserved P loop (35) in HipA indicated below the sequences was used as a fixed point to generate the alignment. The autophosphorylated Ser150 in HipA is fully conserved in the HipT homologs, and the HipT proteins contain conserved catalytic domains and Mg2+ binding motifs as indicated (34, 35, 38). The HipT sequences were from E. coli O127:H6 strain E2348/69 (GenBank accession number CAS11333.1), Haemophilus influenzae Rd KW20 (NCBI accession number NP_438824), Tolumonas auensis DSM 9187 (NCBI accession number WP_015879003.1), Vibrio cholerae O1 strain NHCM-017 (WP_050916634.1), and Vibrio halioticoli (GenBank accession number GAD88429.1). (B) Alignment of five HipS homologs encoded by genes just upstream of the genes encoding the HipT homologs shown in panel A with the N-terminal part of HipA. The HipS sequences were from Escherichia coli O127:H6 strain E2348/69 (GenBank accession number CAS11334.1), H. influenzae Rd KW20 (NCBI accession number NP_438825.1), Tolumonas auensis (NCBI accession number WP_015879002.1), V. cholerae O1 strain NHCM-017 (accession number WP_044125702.1), and Vibrio halioticoli (GenBank accession number GAD88428.1). (C) Alignment of five HipB homologs encoded by genes just upstream of the genes encoding the HipS homologs shown in panel B. The HipB sequences were from E. coli O127:H6 strain E2348/69 (GenBank accession number CAS11335.1), H. influenzae Rd KW20 (NCBI accession number NP_438826.1), Tolumonas auensis (NCBI accession number WP_083757795.1), V. cholerae O1 strain NHCM-017 (WP_050916568.1), and V. halioticoli (GenBank accession number AD88427.1). Color codes of the alignments are as follows: black on white, nonsimilar residues; blue on cyan, consensus residue derived from a block of similar residues at a given position; black on green, consensus residue derived from the occurrence of greater than 50% of a single, distinct residue; red on yellow, consensus residue derived from a completely conserved residue; and green on white, residue weakly similar to consensus residue. The alignments in panels A, B, and C were generated by using the commercial version of Vector NTI (Invitrogen). (D) Phylogenetic tree of 8 HipA and 40 HipT homologs. A sequence alignment of the proteins was accomplished using an online version of MUSCLE (36) provided by European Bioinformatics Institute at EMBL (https://www.ebi.ac.uk/Tools/msa/muscle/) and used to derive the phylogenetic tree presented as a cladogram. Sampling of the HipA and HipT sequences was accomplished using BLASTP at NCBI and was not exhaustive. Accession numbers of the proteins included in the cladogram were as follows: Photorhabdus luminescens TT01, CAE17272.1; Vibrio parahaemolyticus, WP_020904255.1; Vibrio cyclitrophicus, WP_102385597.1; Pantoea vagans, WP_095707190.1; Enterobacter ludwigii, WP_081112566.1; Pantoea GM01, EJL89497.1; E. coli K-12, P23874.2; Shigella dysenteriae, WP_119170656.1; Paraglaciecola psychrophila 170, AGH46782.1; Desulfobacula toluolica Tol2, CCK78946.1; Marinobacter LQ44, AMQ90498.1; Alteromonas mediterranea U7, AGP89851.1; V. halioticoli NBRC 102217, GAD88429.1; Tatumella ptyseos NCTC11468, SQK77238.1; Serratia plymuthica PRI-2C, ANS44223.1; Yersinia frederiksenii FDAARGOS 417, ATM86408.1; Pantoea agglomerans C410P1, AOE38443.1; Pantoea agglomerans L15, AZI51552.1; Pantoea agglomerans TH81, AYP22744.1; T. auensis DSM 9187, ACQ93535.1; E. coli MVAST0167, AML11457.1; E. coli O127:H6 E2348/69, CAS11333.1; Buttiauxella 3AFRM03, AYN26082.1; Pectobacterium atrosepticum 36A, ATY88941.1; Pectobacterium carotovorum 3-2, AVT56767.1; P. carotovorum 14A, AZK60881.1; Paraglaciecola polaris NIBIO1006, ASY75229.1; P. polaris NIBIO1392, ASY81571.1; P. carotovorum PCC21, AFR01461.1; P. carotovorum subsp. brasiliense, ARA78327.1; Actinobacillus porcitonsillarum 9953L55, AWI50429.1; H. influenzae NML-Hia-1, AOZ67195.1; H. influenzae R2866, ADO81720.1; H. influenzae F3031, CBY81777.1; H. influenzae 723, AJO91476.1; H. influenzae 10810, CBW28981.1; Streptococcus pneumoniae 2842STDY5881852, CVQ15586.1; H. influenzae 67P38H1, AVJ03036.1; H. influenzae FDAARGOS199, ARB90233.1; H. influenzae Rd KW20, NP_438824; Haemophilus aegyptius NCTC8502, SQH35811.1; H. influenzae F3047, CBY86290.1; H. influenzae NCTC8455 SQK92775.1; H. influenzae P650-8603, AXP41025.1; H. influenzae 86-028NP, AAX87695.1; H. influenzae C486, AJO89092.1; H. influenzae 84P36H1, AWP55634.1; and H. influenzae 5P28H1, AVI99364.1. Download FIG S1, PDF file, 2.2 MB (2.2MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Long-term incubation shows HipT of E. coli O127 induces bacteriostasis. Viable counts (CFU/ml) before and after hipTO127 induction in the presence or absence of induction of hipBO127 or hipSO127 and hipBSO127. Cultures of E. coli MG1655 harboring pSVN1 (pBAD33::hipTO127) or the empty pBAD33 vector combined with pSVN111 (pND::hipBO127), pSVN109 (pND::hipSO127), pSVN110 (pND::hipBSO127), or the empty low-copy-number pND vector were growing exponentially in LB medium at 37°C. The pBAD promoter was induced by the addition of arabinose (0.2%) at an OD600 of ≈0.3 (red arrow). At each time point, 0.5-ml cell samples were washed in PBS before 10-times dilution series were spotted on agar plates with glucose only (0.2%), to repress hipTO127 expression (A), or glucose plus IPTG (200 μM) to also induce hipBO127 or hipSO127 and hipBSO127 (B) on the plates. Plates were incubated for 40 hours at 37°C before counting. Data points represent mean values from at least three independent experiments, and error bars indicate standard deviations. As seen by the results, the prolonged incubation period (16 h versus 40 h) allowed for the full recovery of viability, even in the absence of hipS or hipBS (compare Fig. S2 with Fig. 1D and E). Download FIG S2, PDF file, 0.4 MB (393.9KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HipT of H. influenzae and T. auensis inhibit cell growth and can be neutralized by cognate HipS. Cells were grown as described in the legend to Fig. 1C. As seen by the results, cognate HipBHi augments HipSHi in the neutralization of HipTHi. Strains used in the experiment whose results are shown in panel A were E. coli MG1655 harboring pSVN135 (pBAD33::hipTHi) or the empty pBAD33 vector combined with pSVN122 (pNDM220::hipBHi), pSVN123 (pNDM220::hipSHi), or pSVN139 (pNDM220::hipBSHi) or the empty low-copy-number pNDM220 as indicated, and strains in the experiment whose results are shown in panel B were E. coli MG1655 harboring pSVN129 (pBAD33::hipTTa) or the empty pBAD33 vector combined with pSVN126 (pNDM220::hipBTa), pSVN127 (pNDM220::hipSTa), or pSVN138 (pNDM220::hipBSTa) or the empty low-copy-number pNDM220 as indicated. Data points represent mean values from at least two independent experiments, and error bars indicate standard deviations. Download FIG S3, PDF file, 0.3 MB (343KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HipBO127, HipSO127, and HipTO127 form a complex. (A) Purification of His6-TEV-HipBO127, HipSO127, and HipTD233QO127 from E. coli strain BL21 containing pSVN94 analyzed by SDS-PAGE. E. coli BL21 containing pSVN94 was grown in LB medium at 37°C and His6-TEV-HipBO127 purified according to standard procedure. As seen by the results, the N-terminally His-tagged HipBO127 pulled down HipSO127 and HipTO127. Lanes 1 to 5 contained samples from washes with buffer B (50 mM NaH2PO4 [pH 8], 0.3 M NaCl, 35 mM imidazole, and 1 mM β-mercaptoethanol). Lanes 6 to 10 show samples from washes with buffer C (100 mM NaH2PO4 [pH 8], 10 mM Tris-HCl [pH 8], and 1 mM β-mercaptoethanol) with different concentrations of urea, as follows: 0 M, 2.45 M, 4.9 M, 7.35 M, and 9.8 M urea, respectively. Lane 11 shows a sample from flowthrough after overnight wash with buffer C containing 9.8 M urea. Lanes 12 and 13 show samples from additional washes with buffer C containing 9.8 M urea after overnight incubation. Lane 14 contained a sample from elution with buffer D (100 mM NaH2PO4 [pH 8], 10 mM Tris-HCl [pH 8], 9.8 M urea, 0.5 M imidazole, and 1 mM β-mercaptoethanol). (B) Top, purification of HipBSTO127 subcomplexes using a heparin column and elution using increasing concentrations of salt. Bottom, purification of HipBSTO127 and isolated HipTO127 toxin on a gel filtration column. Elution positions of standard proteins with known mass (indicated in kDa) are shown with vertical arrows. Download FIG S4, PDF file, 2.9 MB (2.9MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overproduction of TrpS suppresses HipTO127, HipTHi, and HipTTa-mediated growth inhibition, whereas GltX has no such effect. (A) Overnight cultures of E. coli MG1655 harboring (left) pSVN1 (pBAD33::hipTO127), (middle) pSVN135 (pBAD33::hipTHi), or (right) pSVN129 (pBAD33::hipTTa) or the empty pBAD33 vector combined with pEG::gltX (pMG25::gltX) or the empty high-copy-number vector pMG25, as indicated, were diluted to similar OD600 values and washed in PBS before 10-times dilution series were spotted on LB agar plates containing the appropriate antibiotics and glucose (0.2%), arabinose (0.2%), or arabinose (0.2%) plus IPTG (200 μM), respectively. (B) Overnight cultures of E. coli MG1655 harboring (left) pSVN1 (pBAD33::hipTO127), (middle) pSVN135 (pBAD33::hipTHi), or (right) pSVN129 (pBAD33::hipTTa) or the empty pBAD33 vector combined with pSVN103 (pNDM220::trpS) or the empty low-copy-number vector pNDM220, as indicated, were diluted to similar OD600 values and washed in PBS before 10-times dilution series were spotted on LB agar plates containing the appropriate antibiotics and glucose (0.2%), arabinose (0.2%), or arabinose (0.2%) plus IPTG (200 μM), respectively. Download FIG S5, PDF file, 0.2 MB (210KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
tRNA is not required for in vitro phosphorylation of TrpS by HipTO127. Purified TrpS (1 μM; purified from E. coli BL21/pSVN46), HipTO127 (0.5 μM) (purified from E. coli BL21/pSVN42), and RNase A (0.1 mg/ml) were mixed with 0.1 μM [γ-32P]ATP and 66 μM ATP as indicated. Samples were treated as described in Materials and Methods. Download FIG S6, PDF file, 1.1 MB (1.2MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sequence alignment of GltX and TrpS. The alignment shows that S239 in GltX aligns to S197 in TrpS in the conserved sequence motifs KKLSKR and KKMSKS in GltX and TrpS, respectively. Download FIG S7, PDF file, 0.4 MB (461.9KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(p)ppGpp accumulation following overproduction of HipA, HipTO127, and RelE. The figure shows a repetition of the experiment whose results are shown in Fig. 4A. Cells of E. coli MG1655 carrying pAH1 (pNDM220::hipA), pSVN116 (pNDM220::hipTO127), or pAH2 (pNDM220::relE) were grown exponentially in low-phosphate MOPS minimal medium containing H32PO4. Samples were withdrawn before and 10, 30, and 60 minutes after induction of the toxin genes by the addition of IPTG (1 mM) and analyzed by TLC and phosphor imaging. The data contributed to the quantification shown in Fig. 4B. Materials and Methods gives additional experimental details. Download FIG S8, PDF file, 2.7 MB (2.7MB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Modelling of the HipBSTO127 complex structure. (A). Top, the crystal structure of E. coli HipBA (PDB ID 4YG7) colored according to a sequence alignment with HipBSTO127, with the part corresponding to HipBO127 in purple, HipSO127 in blue, and HipTO127 in yellow. Bottom, schematic overview of the sequence alignment with colors as described above. HipA of E. coli K-12 is shown in red. (B) The structure of HipBA bound to DNA shows that the part corresponding to HipSO127 (the HipA N-terminal subdomain) mediates complex dimerization in the DNA-bound form. (C) The N-terminal subdomain of HipA contains a hydrophobic core conserved in HipSO127 (green) but a divergent dimerization interface (red). Two high-persister mutations in the N-subdomain-1 of HipA conserved among HipS orthologs are shown in yellow. Download FIG S9, PDF file, 0.2 MB (243.1KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Representative mass spectra after in vitro kinase assay revealing HipTO127-mediated phosphorylation sites on TrpS (A, B) and autophosphorylation sites of HipTO127 (C, D). Download FIG S10, PDF file, 0.2 MB (177KB, pdf) .
Copyright © 2019 Nielsen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.