Abstract
We have utilized patients' own oral mucosa as a cell source for the fabrication of transplantable epithelial cell sheets to treat limbal stem cell deficiency and mucosal defects after endoscopic submucosal dissection of esophageal cancer. Because there are abundant microbiotas in the human oral cavity, the oral mucosa was sterilized and 40 μg/mL gentamicin and 0.27 μg/mL amphotericin B were added to the culture medium in our protocol. Although an oral surgeon carefully checked each patient's oral cavity and although candidiasis was not observed before taking the biopsy, contamination with Candida albicans (C. albicans) was detected in the conditioned medium during cell sheet fabrication. After adding 1 μg/mL amphotericin B to the transportation medium during transport from Nagasaki University Hospital to Tokyo Women's Medical University, which are 1200 km apart, no proliferation of C. albicans was observed. These results indicated that the supplementation of transportation medium with antimycotics would be useful for preventing contamination with C. albicans derived from the oral mucosa without hampering cell proliferation.
Keywords: Amphotericin B, Candida albicans, Oral mucosal epithelial cell
Abbreviations: C. albicans, Candida albicans; DMEM, Dulbecco's modified Eagle's medium
Highlights
-
•
Normal human oral mucosal epithelial cells were cultured in a clinical setting.
-
•
Contamination with Candida albicans was detected in the culture.
-
•
The culture medium included 0.27 μg/mL amphotericin B.
-
•
Contamination was prevented by 1 μg/mL amphotericin B in the medium for transportation of the oral tissue.
Cultured oral mucosal epithelial cells have been utilized for sympatric and ectopic transplantation to reconstruct stratified epithelia such as the oral mucosa, skin, and cornea [1], [2], [3]. After optimizing culture medium containing autologous serum for fabricating autologous oral mucosal epithelial cell sheets, we have treated an esophageal ulcer resulting from endoscopic mucosal dissection of a mucosal tumor by performing endoscopic transplantation of autologous oral mucosal epithelial cell sheets fabricated on temperature-responsive cell culture surfaces to promote wound healing and prevent stenosis [4], [5], [6].
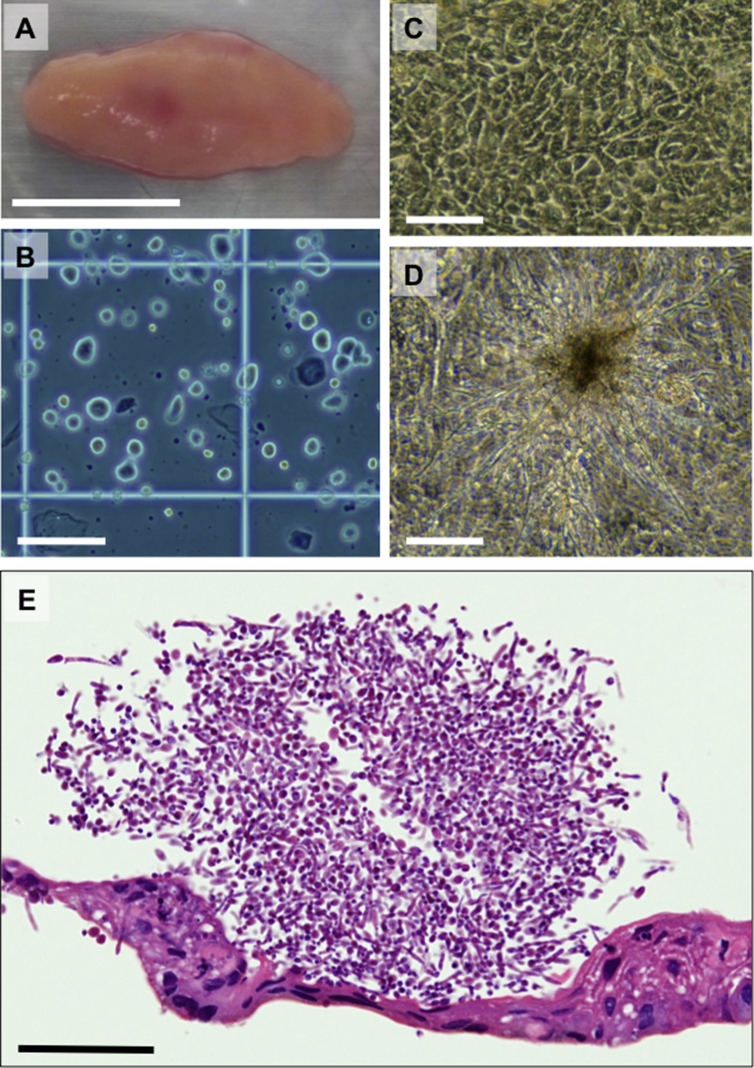
Because the human oral cavity contains abundant microbiota, biopsies of oral mucosa are treated with povidone-iodine. Furthermore, biopsies are stored in Dulbecco's modified Eagle's medium (DMEM) supplemented with 86 μg/mL ampicillin-sulbactam (Unasyn-S; Pfizer, NY, USA) and 100 μg/mL streptomycin (Meiji Seika Pharma, Tokyo, Japan) during transport from the oral surgery department to the cell culture facility. Moreover, the tissue is treated with povidone-iodine in the cell culture facility and is treated with dispase in DMEM including the same concentrations of ampicillin-sulbactam and streptomycin for epithelium separation. In addition, we add 40 μg/mL gentamicin (Gentacin; Schering-Plough, NJ, USA) and 0.27 μg/mL amphotericin B (Fungizone; Bristol-Myers Squibb, NY, USA) to the culture medium to maintain a sterile environment. Therefore, we have not experienced bacterial or fungal contamination in 8 biopsies from healthy volunteer donors in a preclinical study or in 10 biopsies from patients suffering from esophageal cancer treated at Tokyo Women's Medical University [6], [7]. We have performed another clinical research study to examine the safety of long-distance transport of fabricated cell sheets between Tokyo Women's Medical University and Nagasaki University Hospital, which are approximately 1200 km apart, with transport taking 5–7 h by air and train. The protocol for oral mucosal epithelial cell sheet transplantation into patients was approved by the Ethical Committees and Internal Review Boards of Nagasaki University and Tokyo Women's Medical University. Approval of this clinical study by the Health, Labour and Welfare Ministry was gained on March 29th, 2013. Unfortunately, we experienced contamination with a yeast-like fungus in the culture supernatant of a patient's oral mucosal epithelial cells, so we abandoned the fabricated cell sheets for transplantation. We then performed sterilization tests to identify the source of the contamination and the strain of the fungus. Supernatants from each sample were cultured in soybean-casein digest broth (Wako Pure Chemical Industries, Osaka, Japan) and alternative thioglycollate medium (Wako Pure Chemical Industries). The strain of the cultured fungus was identified using CHROMagar Candida (Becton, Dickinson and Company, NJ, USA) and API 20C AUX (bioMérieux, Lyon, France). The obtained results revealed that the patient's oral mucosa was the source of C. albicans (C. albicans), as described below (Table 1). The oral mucosal tissue appeared macroscopically healthy (Fig. 1A), and there was no Candida antigen or infection with C. albicans in the patient's serum, which was added to the culture medium (Table 1). In addition, the cultured oral mucosal epithelial cells exhibited normal cell morphology (Fig. 1B,C). However, contaminating C. albicans and hyphal formation were detected during epithelial cell culture (Fig. 1D,E). It should be noted that hyphal formation by C. albicans was inhibited under anaerobic conditions [8].
Table 1.
The results of quality control tests.
| Sample | Items | Result | |
|---|---|---|---|
| Cell culture supernatant (1st trial)a | Sterilization test | Bacteria | Negative |
| Fungi | Candida albicans | ||
| Mycoplasmal culture | Negative | ||
| Mycoplasma test (PCR)b | Negative | ||
| Endotoxin | 0.062 EU/mL | ||
| Reagents for cultivation | Sterilization test | Bacteria | Negative |
| Fungi | Negative | ||
| Serum (patient) | Sterilization test | Bacteria | Negative |
| Fungi | Negative | ||
| Candida antigen | Negative | ||
| Oral surface (patient) | Sterilization test | Fungi | Candida albicans |
| Oral surface (operator 1) | Sterilization test | Fungi | Negative |
| Oral surface (operator 2) | Sterilization test | Fungi | Negative |
| Cell culture supernatant (2nd trial)a | Sterilization test | Bacteria | Negative |
| Fungi | Negative | ||
| Mycoplasmal culture | Negative | ||
| Mycoplasma test (PCR)b | Negative | ||
| Endotoxin | 0.136 EU/mL | ||
| Oral surface (patient) | Sterilization test | Fungi | Candida albicans |
Cell culture supernatants were routinely used for quality control tests.
PCR for detecting Mycoplasma pneumoniae was performed in accordance with method shown by Jensen JS et al. [12].
Fig. 1.
Candida albicans (C. albicans) proliferating in the cell culture supernatant of human oral mucosal epithelial cells in this clinical study. (A) Human oral mucosal tissue of the patient. Bar = approximately 1 cm. (B) Oral mucosal epithelial cells derived from the patient after cell preparation. Bar = 100 μm. (C) Cellular morphology of the cultured human oral mucosal epithelial cells. Bar = 100 μm. (D) C. albicans observed on the cultured epithelial cells in a culture vessel. Bar = 100 μm. (E) Histological observation of the C. albicans adhering to a cultured epithelial cell sheet harvested from a temperature-responsive culture insert. The cell sheet and C. albicans were stained with hematoxylin and eosin. Bar = 50 μm.
We then tested the susceptibility of the C. albicans strain obtained from the conditioned medium and the oral surface of the patient to antimycotic agents using a commercially prepared colorimetric microdilution panel (ASTY; Kyokuto Pharmaceutical Industrial, Tokyo, Japan) [9]. The proliferation of the strain was completely inhibited by 0.5 μg/mL amphotericin B. In comparison, in previous susceptibility testing, the proliferation of nearly all Candida species was inhibited by 1.0 μg/mL amphotericin B [10], and a higher concentration of amphotericin B often hampers mammalian cell proliferation [11]. Therefore, we changed our protocol for the transport of oral mucosal biopsies from Nagasaki University Hospital to Tokyo Women's Medical University. The DMEM used for the transportation was supplemented with 1.0 μg/mL amphotericin B, and the concentration of amphotericin B in the culture medium was kept at 0.27 μg/mL, with no modification.
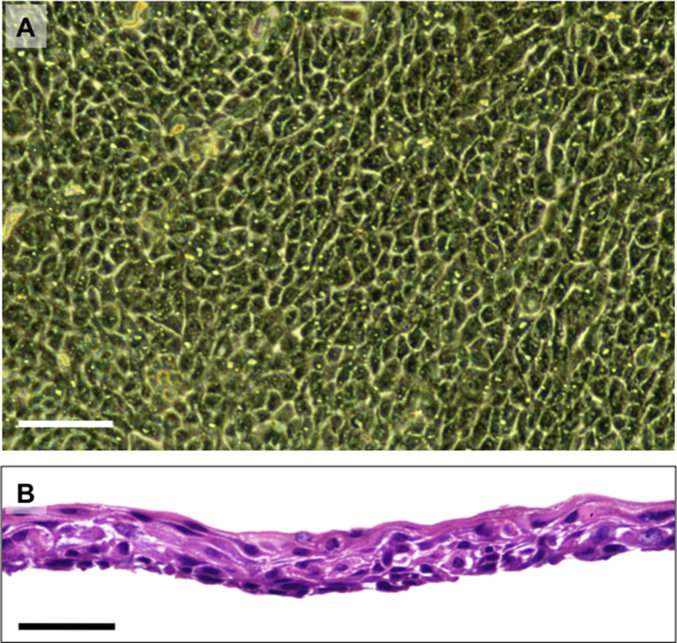
It took approximately 6 h to transport the biopsy by air and train, and then the transported biopsy was subjected to harvesting of the oral mucosal epithelial cells using dispase treatment for 2 h at 37 °C in DMEM supplemented with the same concentration of amphotericin B. As a result, no contamination with C. albicans was observed in the supernatant of the culture medium used for the fabrication of transplantable epithelial cell sheets from the same patient, and the cultured epithelial cells were successfully harvested as cell sheets (Table 1, Fig. 2). To maintain a sterile environment, the temperature-responsive cell culture inserts to which the cultured epithelial cell sheets adhered were placed in transportable containers while in the safety cabinet of a clean room specialized for fabricating transplantable cell sheets for a clinical setting. The containers were then transported to Nagasaki University Hospital in the transportation box, which was mounted on a hot plate to keep the temperature at 37 °C. After transport, the epithelial cell sheets were finally transplanted onto the esophageal ulcer of the patient after endoscopic dissection to remove esophageal cancer.
Fig. 2.
Second trial of cultivation of human oral mucosal epithelial cells derived from the same patient, without contamination with bacteria or fungi. (A) Cellular morphology of the cultured human oral mucosal epithelial cells derived from the patient. Bar = 100 μm. (B) Histological observation of a cultured human oral mucosal epithelial cell sheet harvested from a temperature-responsive culture insert. The cell sheet was stained with hematoxylin and eosin. Bar = 50 μm.
Here, we have reported our experience of contamination with C. albicans during the fabrication of transplantable oral mucosal epithelial cell sheets derived from a patient who was not suffering from candidiasis. By adding 1 μg/mL amphotericin B to the transportation medium, fungal proliferation was completely inhibited and esophageal mucosal regeneration was successfully observed. Therefore, the method described in this report should be useful for preventing contamination with C. albicans without increasing the concentration of amphotericin B in the culture medium.
Disclosure statement
Teruo Okano is a founder and director of the board of CellSeed Inc., licensing technologies and patents from Tokyo Women's Medical University. Teruo Okano and Masayuki Yamato are shareholders of CellSeed Inc. Tokyo Women's Medical University is receiving research funding from CellSeed Inc.
Acknowledgments
This work was supported by the Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology ‘Cell Sheet Tissue Engineering Center (CSTEC)’ and by the Global Center of Excellence Program's Multidisciplinary Education and Research Center for Regenerative Medicine (MERCREM) established by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Ueda M., Hata K., Horie K., Torii S. The potential of oral mucosal cells for cultured epithelium: a preliminary report. Ann Plast Surg. 1995;35:498–504. doi: 10.1097/00000637-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hata K., Ueda M. Fabrication of cultured epithelium using oral mucosal cells and its clinical applications. Hum Cell. 1996;9:91–96. [PubMed] [Google Scholar]
- 3.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 4.Murakami D., Yamato M., Nishida K., Ohki T., Takagi R., Yang J. Fabrication of transplantable human oral mucosal epithelial cell sheets using temperature-responsive culture inserts without feeder layer cells. J Artif Organs. 2006;9:185–191. doi: 10.1007/s10047-006-0342-3. [DOI] [PubMed] [Google Scholar]
- 5.Ohki T., Yamato M., Murakami D., Takagi R., Yang J., Namiki H. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–1710. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Takagi R., Yamato M., Murakami D., Kondo M., Ohki T., Sasaki R. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology. 2011;78:311–319. doi: 10.1159/000322575. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T., Ogasawara A., Mikami T., Matsumoto T. Hyphal formation of Candida albicans is controlled by electron transfer system. Biochem Biophys Res Commun. 2006;348:206–211. doi: 10.1016/j.bbrc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller M.A., Arikan S., Lozano-Chiu M., Chen Y., Coffman S., Messer S.A. Clinical evaluation of the ASTY colorimetric microdilution panel for antifungal susceptibility testing. J Clin Microbiol. 1998;36:2609–2612. doi: 10.1128/jcm.36.9.2609-2612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y.L., Li S.Y., Cheng H.H., Lo H.J., TSARY Hospitals Susceptibilities to amphotericin B and fluconazole of Candida species in TSARY 2002. Diagn Microbiol Infect Dis. 2005;51:179–183. doi: 10.1016/j.diagmicrobio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Uribe C.C., Dos Santos de Oliveira F., Grossmann B., Kretzmann N.A., Reverbel da Silveria T., Giugliani R. Cytotoxic effect of amphotericin B in a myofibroblast cell line. Toxicol In Vitro. 2013;27:2105–2109. doi: 10.1016/j.tiv.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J.S., Søndergård-Andersen J., Uldum S.A., Lind K. Detection of Mycoplasma pneumoniae in simulated clinical samples by polymerase chain reaction. Brief report. APMIS. 1989;97:1046–1048. doi: 10.1111/j.1699-0463.1989.tb00516.x. [DOI] [PubMed] [Google Scholar]