In 2015, favipiravir was administered on a compassionate-use basis to patients with Ebola virus disease in Guinea. This study reveals a trend toward improved survival in favipiravir-treated patients; however, the effect of treatment was not statistically significant, except for its influence on survival time.
Keywords: Filovirus, Ebola virus disease, Favipiravir, Guinea, epidemic, mobile laboratory
Abstract
Background
In 2015, the laboratory at the Ebola treatment center in Coyah, Guinea, confirmed Ebola virus disease (EVD) in 286 patients. The cycle threshold (Ct) of an Ebola virus–specific reverse transcription–polymerase chain reaction assay and 13 blood chemistry parameters were measured on admission and during hospitalization. Favipiravir treatment was offered to patients with EVD on a compassionate-use basis.
Methods
To reduce biases in the raw field data, we carefully selected 163 of 286 patients with EVD for a retrospective study to assess associations between potential risk factors, alterations in blood chemistry findings, favipiravir treatment, and outcome.
Results
The case-fatality rate in favipiravir-treated patients was lower than in untreated patients (42.5% [31 of 73] vs 57.8% [52 of 90]; P = .053 by univariate analysis). In multivariate regression analysis, a higher Ct and a younger age were associated with survival (P < .001), while favipiravir treatment showed no statistically significant effect (P = .11). However, Kaplan-Meier analysis indicated a longer survival time in the favipiravir-treated group (P = .015). The study also showed characteristic changes in blood chemistry findings in patients who died, compared with survivors.
Conclusions
Consistent with the JIKI trial, this retrospective study revealed a trend toward improved survival in favipiravir- treated patients; however, the effect of treatment was not statistically significant, except for its influence on survival time.
The 2013–2016 West African Ebola virus disease (EVD) outbreak originated in Guinea and rapidly spread to neighboring countries. Unprecedented in its duration and scale, the outbreak resulted in > 28 000 suspected, probable, and confirmed cases and >11 000 deaths in Guinea, Liberia, and Sierra Leone [1]. A fatal outcome of EVD is associated with age, a high viral load, severe acute kidney injury, and elevated liver enzyme levels [2–15]. Various investigational therapeutic agents, including the broad-spectrum antiviral drug favipiravir, monoclonal antibody cocktails, small interfering RNA, and interferon, have been evaluated in patients during the 2013–2016 EVD outbreak [16–19]. After completion of the JIKI clinical trial for favipiravir in Guinea [18], the drug was offered to patients on a compassionate-use basis.
In March 2014, a laboratory unit of the European Mobile Laboratory (EMLab) consortium was deployed to the EVD treatment center (ETC) in Guéckédou, Guinea, to provide laboratory diagnostic service [15]. In response to the increasing number of EVD cases in western Guinea, the unit was relocated to the ETC in Coyah, where it was operational from February to December 2015. During this period, EMLab tested 7000 samples by an Ebola virus (EBOV)–specific reverse transcription–polymerase chain reaction (RT-PCR) assay. Here, we report the analysis of the laboratory data generated in Coyah and the results of a retrospective study aiming to assess associations between potential risk factors, compassionate use of favipiravir, and EVD outcome.
METHODS
Patients and Treatment
Blood specimens were collected from individuals with suspected EVD attending an ETC and tested for EBOV by RT-PCR. Most suspected cases originated from the regions of Kindia (3945 [85%]), Boké (454 [9.8%]), and Conakry (43 [0.93%]). Laboratory-confirmed cases were admitted to an ETC and received supportive treatment, including (1) oral rehydration therapy and/or intravenous fluids; (2) correction of electrolyte abnormalities, based on blood chemistry findings; (3) symptomatic care with antipyretics, anti-emetics, and antidiarrheal agents; (4) preemptive treatment of concomitant infections, using drugs such as broad-spectrum antibiotics and antimalarials; (5) nutritional support; and (6) psychological care. Patients were also offered specific treatment with oral favipiravir on a compassionate-use basis. The favipiravir treatment team aimed to offer the drug to all patients with laboratory-confirmed EVD, and except for the eligibility criteria (>1 year of age, no pregnancy, and ability to take oral drug), no further selection criteria were applied. Reasons given by patients for not consenting to participation were not recorded, although concurrently conducted clinical trials (see below) interfered with recruitment. The dosing scheme was identical to that of the prior JIKI trial, with a loading dose of 6000 mg on the first day followed by 2400 mg/days for 9 days [18]. All favipiravir-treated patients completed the 10-day treatment course or died while receiving treatment. Interferon β1a (IFN-β1a) or a combination of favipiravir with ZMapp was given to a small number of patients in the framework of clinical trials, as reported previously [17, 19]. The majority of patients with EVD confirmed by the EMLab in Coyah were admitted to the ETC in Coyah. Throat swab specimens from individuals who died in the community were tested by EBOV RT-PCR.
Diagnostic Assays
All laboratory data were generated by the EMLab unit at the ETC in Coyah. Viral RNA was extracted with the QIAamp Viral RNA Mini Kit (Qiagen) from whole blood specimens mixed with EDTA, other body-fluid specimens, or swab specimens. EBOV RNA was detected using the RealStar Filovirus Screen RT-PCR kit, version 1.0 (until March 2015; Altona Diagnostics), or the RealStar Zaire Ebolavirus RT-PCR kit, version 1.0 (from March 2015 onward; Altona Diagnostics), using the SmartCycler II system (Cepheid; until June 2015) or the Rotor-Gene Q system (Qiagen; from March 2015 onward). Although these assays slightly differ in terms of sensitivity, they produce virtually identical cycle thresholds (Cts) [20]. Cts were reported to clinicians as a semiquantitative measure of viral load. Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale antigens in blood specimens were detected using the BinaxNow Malaria (Alere) rapid diagnostic test (RDT).
Blood Chemistry Analysis
Blood levels of alanine aminotransferase (ALT), albumin, amylase, aspartate aminotransferase (AST), calcium, C-reactive protein (CRP), creatine kinase (CK), creatinine, glucose, potassium, sodium, total bilirubin, and blood urea nitrogen (BUN) were analyzed in the EMLab unit, using a Piccolo Xpress Chemistry Analyzer with Amlyte 13 Reagent Discs (Abaxis) according to the manufacturer’s instructions.
Data Management
Demographic patient data was provided on the laboratory request forms accompanying the samples by hospital staff, contact-tracing teams, and other partners in the field. Patient name, age, sex, residence, ETC patient identifier, sample identifier, sample type, collection date, date of symptom onset, EBOV RT-PCR result (with the corresponding Ct), and malaria RDT result were captured in the EMLab database (Excel, Microsoft) and reported to national authorities and the World Health Organization (WHO) on a daily basis.
To facilitate the assignment of multiple samples to individual patients, validate the demographic information, and document the outcome, the EMLab database was manually merged with the Guinean database of patients with EVD, maintained at the WHO country office in Conakry. Patient names and sample identifiers recorded in both databases were used as primary identifiers for merging; additional variables were used to verify the match. Inconsistencies between the 2 databases and between sample entries for the same patient were resolved, and the data were cleaned using Stata 14 (StataCorp). Patients were classified into 4 main categories: (1) patients with suspected EVD who attended an ETC and had negative results of EBOV RT-PCR analysis, (2) patients with EBOV RT-PCR–confirmed EVD admitted to the ETC, (3) patients who had negative results of EBOV RT-PCR analysis and died in the community, and (4) patients with EBOV RT-PCR–confirmed EVD who died in the community (Table 1). Individuals who could not be assigned to any category because of missing or conflicting data (n = 151) were excluded from further analysis.
Table 1.
Characteristics of 4636 Patients Tested at the European Mobile Laboratory Unit in Coyah, Guinea
| Hospitalized Patients With Suspected EVD | Patients Who Died in the Community | |||||
|---|---|---|---|---|---|---|
| Characteristic | Overall | EBOV RT-PCR Positive | EBOV RT-PCR Negative | Overall | EBOV RT-PCR Positive | EBOV RT-PCR Negative |
| Patients | 813/813 (100) | 286/813 (35) | 527/813 (65) | 3823/3823 (100) | 84/3823 (2) | 3739/3823 (98) |
| Female sex | 372/813 (46) | 151/286 (53) | 221/527 (42) | 1815/3810 (48) | 58/84 (69) | 1757/3726 (47) |
| Age, y | 30 (18–45)a | 30 (20–43)b | 29 (13–45)c | 25 (2–55)d | 35.5 (23.5–51)e | 25 (2–56)f |
| Malaria RDT positive | 194/637 (30) | 33/213 (15) | 161/424 (38) | Not tested | Not tested | Not tested |
| Fatal outcome | 204/813 (25) | 149/286 (52) | 55/527 (10) | 3823/3823 (100) | 84/84 (100) | 3739/3739 (100) |
Data are no. of patients with the characteristic/no. evaluated (%) or median value (interquartile range), unless otherwise indicated.
Abbreviations: EBOV, Ebola virus; RT-PCR, reverse transcription–polymerase chain reaction; RDT, rapid diagnostic test.
Number of individuals: a, 811; b, 286; c, 525; d, 3785; e, 84; f, 3701
To analyze the association between independent variables and outcome by using statistical methods, the cleaned EMLab/WHO database was manually merged with the favipiravir treatment database. The merged database comprised 286 patients with EVD, of whom 99 (35%) had been treated with favipiravir. Patients were selected for the retrospective study according to 6 inclusion/exclusion criteria, specified as follows (Supplementary Figure 1). First, children <1 year of age, who had not been eligible for compassionate use of favipiravir, were also excluded from the nontreatment group, to maintain comparability between favipiravir-treated and untreated patients (7 patients were excluded). Second, patients who received ZMapp in combination with favipiravir (12 patients) or were treated with IFN-β1a (9 patients) were excluded. Third, the EVD diagnosis including the Ct had to be confirmed by EMLab in the first specimen collected from the patient (40 patients were excluded because of missing EMLab test results for the first specimen). Fourth, the first specimen tested had to be a blood specimen (11 patients were excluded because testing was done on a swab specimen). Fifth, owing to the above criteria, favipiravir-treated patients from 2 centers only, ETC Coyah and ETC Forécariah, remained in the data set. To maintain comparability between favipiravir-treated and untreated patients, the analysis was confined to the ETCs in Coyah and Forécariah (20 untreated patients were excluded because of admission to various other centers). Sixth, EVD cases were admitted to the ETC on the same day the diagnostic sample was collected (median, day 0; interquartile range [IQR], days 0–0), and treatment with favipiravir was commenced the day after (median, day 1; IQR, days 0–1). The treatment group included only patients who survived the period between diagnosis and commencement of favipiravir treatment. To ensure that the observation of outcome began with comparable delay in both the treatment and nontreatment groups, all patients who did not survive day 1 (corresponding to the median delay between diagnosis and initiation of favipiravir treatment) were excluded from the nontreatment group (24 patients). This criterion was adopted to prevent allocation of patients to the nontreatment group among those who died too early for inclusion in the treatment group .
Based on these criteria, 163 patients were eligible for analysis (a line list of patient data is specified in the Supplementary Materials). The selection process hardly changed the mortality figures: the case-fatality rate (CFR) among favipiravir-treated patients included in and excluded from the study was 42.5% (31 of 73) and 42.3% (11 of 26), respectively; the CFR among nontreated patients included in and excluded from the study was 57.8% (52 of 90) and 56.7% (55 of 97), respectively.
We verified with the records that all eligible patients had the first specimen collected before treatment was commenced. In addition, all Cts were verified using data recorded by the real-time PCR machines.
Statistical Analysis
Statistical analyses were performed using Stata 14. Categorical variables are reported as numbers and percentages of patients. Percentages are based on all observations, excluding missing values. Continuous variables are reported as medians and IQRs for nonnormally distributed variables. Groups were compared using the χ2 test for categorical variables or the Mann-Whitney U test for continuous variables, with Benjamini-Hochberg correction for multiple testing. Kaplan-Meier curves and log-rank tests were used to assess differences in survival time between groups. Univariate associations between independent variables and a dichotomous outcome (survival or death) were analyzed using logistic regression and displayed with crude (unadjusted) odds ratios. To account for confounding factors, multivariate logistic regression was used to analyze the association between multiple independent variables and the dichotomous outcome.
Ethics
The use of patient data was approved by the National Committee of Ethics in Medical Research of Guinea, as well as by the Ethics Committee of the Medical Association of Hamburg (permits 11/CNERS/14 and PV4910). Compassionate use of favipiravir was approved by the National Committee of Ethics in Medical Research of Guinea (permit 30/CNERS/15). Written informed consent was obtained from all patients who received favipiravir.
RESULTS
Characteristics of the EVD Epidemic Around Coyah
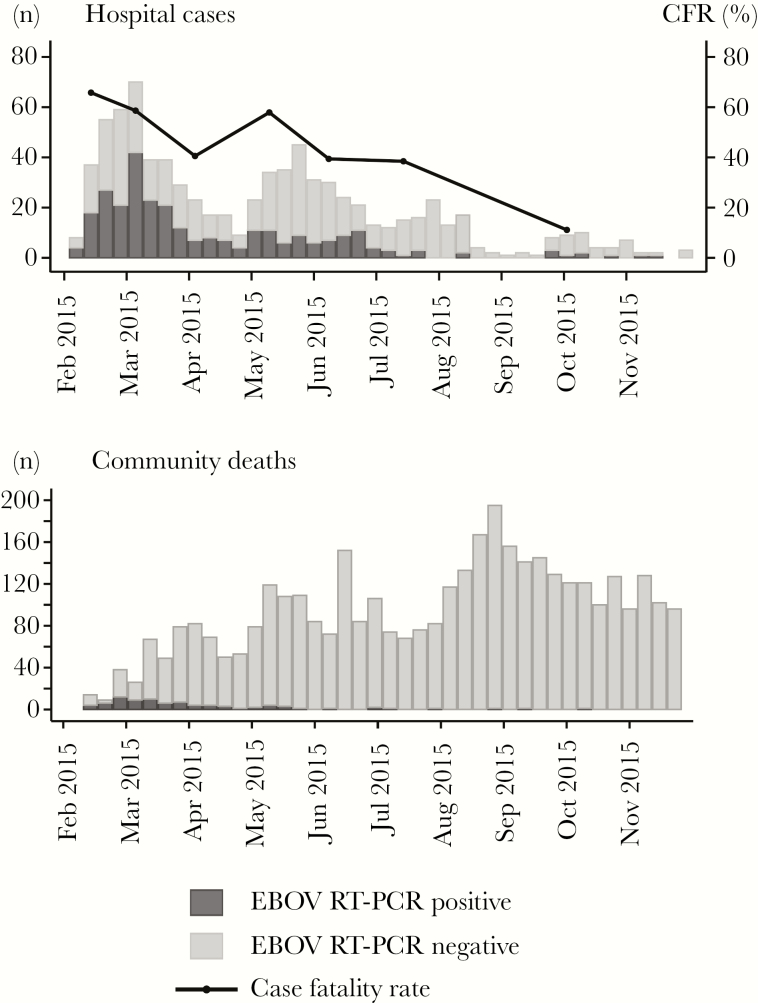
The EMLab unit in Coyah confirmed EVD in 286 of 813 individuals with suspected EVD (35%) who attended an ETC and in 84 of 3823 (2%) who died in the community (Table 1). The epidemic curve showed a major wave in February–April and a minor wave in May–July (Figure 1). The disease mainly affected adults aged 15–54 years (Supplementary Figure 2). The overall CFR in the ETC was 52%, with a downward trend over time (Figure 1). The CFR was highest in children aged <5 years and adults aged >50 years (Supplementary Figure 3). A malaria RDT was performed for 637 individuals (78%) with suspected EVD, of whom 194 (30%) tested positive for malaria. Patients with a positive malaria RDT result were younger than those with a negative malaria RDT result (median age, 18.5 years [IQR, 6–30 years] vs 35 years [IQR, 21–46 years]). Coinfection of EBOV with malaria parasites was diagnosed in 33 patients (15%) and was most frequent among children <15 years of age (Supplementary Figure 3). No major change in the virus load as measured via the Ct was observed during the course of the epidemic (Supplementary Figure 4). The Cts followed a bell-shaped distribution within the measurement range of the real-time RT-PCR assay (Supplementary Figure 5). Favipiravir monotherapy was administered to 87 of 286 patients with EVD (30%); 12 of 286 (4%) received a combination of favipiravir and ZMapp, and 9 of 286 (3%) received IFN-β1a [17, 19].
Figure 1.
Epidemic curve of Ebola virus disease (EVD) cases diagnosed at the European Mobile Laboratory unit during the study period. A, Ebola virus (EBOV)–specific reverse transcription–polymerase chain reaction (RT-PCR) results for 813 patients with suspected EVD who attended an Ebola treatment center, and case-fatality rates (CFRs) among hospitalized patients with EVD over time. B, EBOV RT-PCR results for 3823 individuals who died in the community.
Analysis of Risk Factors Associated With Outcome
We selected 163 (57%) of 286 patients with laboratory-confirmed EVD for a retrospective observational study to assess associations between potential risk factors, the effect of favipiravir treatment, and outcome. The criteria for selecting the study subjects aimed to eliminate or reduce various biases in the raw data collected under nonstandardized conditions in the field (Supplementary Figure 1). The CFR among study patients was 51% (83 of 163) and thus representative of the whole data set (52%). In addition to Ct and age, which were available for all patients, clinical chemistry parameters had been measured on admission in a fraction (40%–44%) of the 163 patients (Supplementary Figure 10). Fatal outcome was associated with a lower Ct (P < .001), a higher BUN level (P = .002), a higher creatinine level (P < .001), a higher ALT level (P < .001), a higher AST level (P < .001), a lower calcium level (P = .013), and a higher CRP level (P = .006; Supplementary Figure 10). Age and Ct showed a clear positive and negative correlation, respectively, with the risk of fatal outcome (Supplementary Figures 6 and 7) and were further analyzed by regression modeling (discussed below). Malaria was not considered in the evaluation because the number of coinfected patients was too small for a meaningful analysis.
Analysis of Association Between Favipiravir Treatment and Outcome
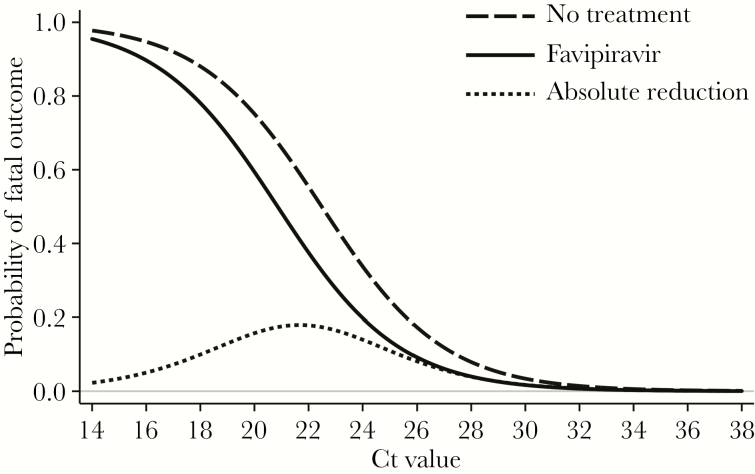
The 163 patients with EVD who were eligible for the retrospective study (Supplementary Figure 1) included 73 favipiravir-treated patients (45%) and 90 (55%) who had not been treated with the drug. The characteristics of the 2 groups are summarized and compared in Table 2. The CFR among favipiravir-treated patients was lower than among untreated patients (42.5% [31 of 73] vs 57.8% [52 of 90]). We used logistic regression models to assess a potential association between favipiravir treatment and EVD outcome. Besides drug treatment, the Ct on admission and age were included as continuous covariates in the regression model, owing to their strong correlation with the risk of fatal outcome (Supplementary Figures 6 and 7). Clinical chemistry parameters could not be considered because of large numbers of missing values. In both unadjusted and multivariate analyses, a higher Ct on admission (P < .001 in both analyses) and a younger age (P = .002 and P < .001, respectively) were statistically significant predictors of survival (Table 3). The positive effect of favipiravir treatment had borderline significance in the unadjusted model (P = .053) and lost statistical significance in the multivariate model (P = .11; Table 3). The corresponding regression curves for the association between Ct and the probability of a fatal outcome indicated an up to 18% lower risk of death among favipiravir-treated patients as compared to untreated patients (Figure 2). This effect reached its maximum in the Ct range of 20–24, depending on age (Supplementary Figure 8).
Table 2.
Characteristics on Admission of 163 Patients With Ebola Virus Disease, by Favipiravir Treatment Status
| Treated Patients (n = 73) | Nontreated Patients (n = 90) | P a | ||||
|---|---|---|---|---|---|---|
| Characteristic | Reference Range | Value | No. | Value | No. | |
| Ebola treatment center, patients, no. | 73 | 90 | <.001 | |||
| Forécariah, Guinea | NA | 18 | 2 | |||
| Coyah, Guinea | NA | 55 | 88 | |||
| Age, y | NA | 30 (23–40) | 73 | 29 (18–40) | 90 | .170 |
| Female sex | NA | 41 (56.2) | 73 | 55 (61.1) | 90 | .523 |
| Malaria RDT positive | NA | 10 (14.5) | 69 | 10 (12.7) | 79 | .745 |
| Ct | NA | 22.3 (18.8–26.5) | 73 | 20.2 (17.8–24.1) | 90 | .171 |
| Blood chemistry parameter | ||||||
| Glucose level, mmol/L | 4.1–6.6 | 4.8 (3.6–6.5) | 44 | 4.1 (3–5) | 27 | .209 |
| BUN level, mmol/L | 2.5–7.9 | 6.3 (3.8–11.8) | 44 | 12.4 (4.1–27.3) | 27 | .201 |
| Creatinine level, µmol/L | 53–106 | 104 (79–153) | 44 | 216 (89–713) | 27 | .201 |
| Total bilirubin level, µmol/L | 3.4–27.4 | 9 (8–12) | 43 | 13 (8–18) | 26 | .201 |
| Albumin level, g/L | 33–55 | 32 (28–36) | 44 | 30 (26–35) | 27 | .209 |
| ALT level, U/L | 10–47 | 115 (67–356) | 44 | 277 (108–444) | 27 | .209 |
| AST level, U/L | 11–38 | 502 (154–1290) | 42 | 1058 (323–2000) | 25 | .201 |
| CK level, U/L | 30–380 | 917 (323–2032) | 44 | 1443 (718–2534) | 25 | .209 |
| Amylase level, U/L | 14–97 | 98 (64–161.5) | 44 | 120 (62–164) | 27 | .639 |
| Sodium level, mmol/L | 128–145 | 130 (126–135) | 44 | 130 (128–131) | 26 | .765 |
| Potassium level, mmol/L | 3.6–5.1 | 4 (3.5–4.5) | 41 | 4.5 (3.7–5.1) | 24 | .209 |
| Calcium level, mmol/L | 2.00–2.58 | 2.15 (2.03–2.21) | 44 | 2.05 (1.85–2.19) | 27 | .209 |
| CRP level, mg/L | <7.5 | 20.3 (8.3–58.7) | 44 | 24.5 (13.9–68.4) | 24 | .525 |
Data are median value (interquartile range) or no. (%) of patients, unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; CRP, C-reactive protein; ETC, Ebola treatment center; NA, not applicable; RDT, rapid diagnostic test.
aBy the χ2 test (for categorical variables) and the Mann-Whitney U test (for continuous variables), with the Benjamini-Hochberg correction for multiple testing.
Table 3.
Logistic Regression of the Association of Independent Variables With a Fatal Outcome of Ebola Virus Disease
| Unadjusted Model | Multivariate Model | ||||
|---|---|---|---|---|---|
| Variable | Deaths/Total Cases, No. (%) | OR (95% CI) | P | OR (95% CI) | P |
| EBOV RT-PCR Ct (continuous; n = 163) | 83/163 (50.9) | .68 (.61–.77) | <.001 | .64 (.56–.74) | <.001 |
| Age, y (continuous; n = 163) | 83/163 (50.9) | 1.03 (1.01–1.05) | .002 | 1.06 (1.03–1.09) | <.001 |
| Favipiravir treatment (n = 163) | |||||
| No | 52/90 (57.8) | 1 (reference) | 1 (reference) | ||
| Yes | 31/73 (42.5) | .54 (.29–1.01) | .053 | .48 (.20–1.18) | .11 |
Abbreviations: CI, confidence interval; Ct, cycle threshold; EBOV, Ebola virus; OR, odds ratio; RT-PCR, reverse transcription–polymerase chain reaction.
Figure 2.
Multivariate logistic regression model for the association between the cycle threshold (Ct), age, favipiravir treatment status, and probability of a fatal outcome. The association between the Ct and the probability of death, depending on favipiravir treatment status, is displayed by regression curves. The curves were calculated for a mean age of 31.5 years among patients in the analysis. The estimated improvement in the chance of survival due to favipiravir treatment (absolute reduction in the probability of dying is defined as the difference between the “No treatment” curve and the “Favipiravir” curve) is shown. The curves were calculated from the parameters of the multivariate logistic regression analysis of data from the 163 patients with Ebola virus disease (EVD) included in the retrospective study (Table 3). For the age dependency of the curves, see Supplementary Figure 8.
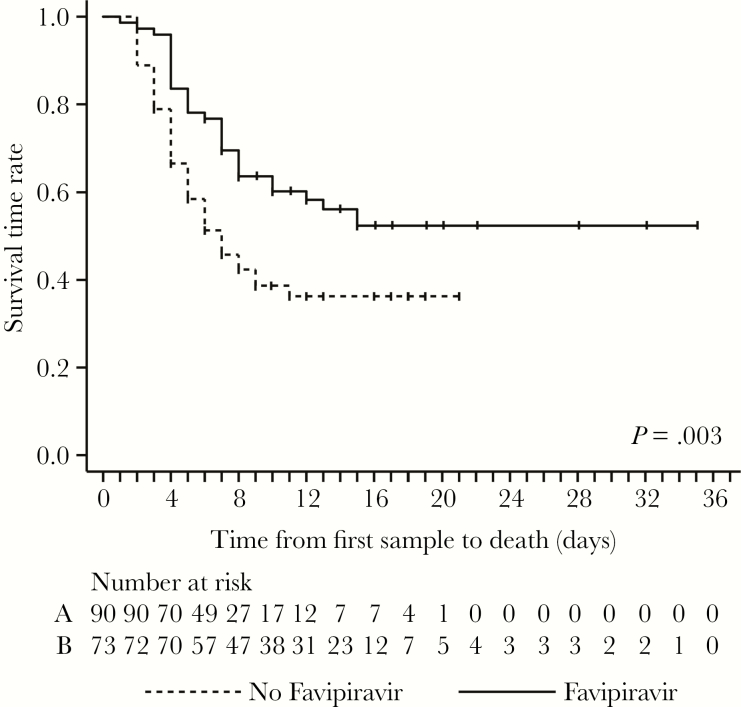
Kaplan-Meier analysis of survival time was performed under 2 assumptions. First, we assumed that survivors were lost to follow-up on discharge, and therefore we censored them at that day. This analysis revealed a statistically significant longer survival time in the favipiravir-treated group (P = .003; Figure 3). However, favipiravir-treated survivors stayed significantly longer in the ETC than untreated survivors, owing to the 10-day treatment course (median time between sampling and discharge, 14 days [IQR, 10–17 days] vs 9 days [IQR, 6–12 days]; P < .001), which introduces a bias in the number of patients at risk. Because the available data did not suggest that a survivor died from EVD after discharge (although this possibility cannot be firmly excluded), we performed a second analysis that assumed a 30-day observation period for all patients. This analysis also indicated a longer survival time, although with a lower statistical significance (P = .015; Supplementary Figure 9).
Figure 3.
Kaplan-Meier analysis of survival time among patients in the retrospective study. The analysis was performed under the assumption that survivors were lost to follow-up on discharge. Therefore, survivors were censored at discharge (small vertical ticks on the curves). Numbers of patients at risk are indicated for the nontreatment (A) and treatment (B) groups. For Kaplan-Meier analysis without censoring at the time of discharge, see Supplementary Figure 9.
Kinetics of Laboratory Parameters During the Course of EVD
Ct and clinical chemistry parameters were measured during hospitalization in a fraction of the 163 study patients. Survivors showed a decline in virus load, and negative results of RT-PCR analysis of blood specimens usually began to appear between day 3 and day 18 (Supplementary Figure 11). Patients who died maintained a high virus load until death, although some patients who died at later stage (ie, after day 10) showed a moderate decline in virus load. There were no apparent differences in the virus load kinetics between favipiravir-treated and untreated patients. Follow-up blood chemistry findings were only available for favipiravir-treated patients (Supplementary Figure 11). Characteristic pathologic patterns in fatal cases included (1) substantial alterations in glucose levels, with hyperglycemia and hypoglycemia; (2) hyperkalemia; (3) increasing creatinine, BUN, bilirubin, CK, and CRP values; (4) decreasing calcium and albumin values; and (5) increasing ALT and AST values, followed by a decreasing trend. Similar although mostly less prominent alterations were observed in survivors. However, survival was basically associated with normalization of values around 10 days after commencement of favipiravir treatment.
DISCUSSION
We present an analysis of laboratory, demographic, treatment, and outcome data collected during the 10-month EMLab operation in Coyah. Compared with the first site of EMLab operation in Guéckédou, in the region of Forest Guinea, the much higher number of samples tested from patients who died in the community is apparent, with 3823 tests in Coyah versus 563 in Guéckédou [15]. This resulted from intensified active surveillance and contact-tracing efforts that were implemented during the later phase of the epidemic. However, although just 2% of patients (84 of 3823) who died in the community had EVD diagnosed, this number still accounted for 36% (84 of 233) of all fatal EVD cases diagnosed by the laboratory in Coyah (149 in the ETC plus 84 in community; Table 1). Thus, more than a year after the outbreak started in Guinea, these estimates suggest that one third of all patients with EVD in this region did not attend the ETC and died in the community, despite major efforts to increase awareness in communities. On one hand, these patients could not benefit from supportive care or even specific treatment, and on the other hand, they maintained the transmission chains in the community. These observations underline the importance of community engagement and trust building in outbreak situations.
The findings on risk factors for a fatal outcome—age, virus load (Ct), and blood chemistry findings on admission—largely confirm results of previous studies [2–15]. However, the kinetics of blood chemistry parameters provide further insight into the pathophysiology of EVD and may guide supportive management of patients. A key manifestation is a progressive impairment of renal function, as evidenced by increasing creatinine, BUN, and potassium values until death. Markers of cell damage, such as ALT, AST, and CK, may tend to normalize before death and are therefore not reliable prognostic marker during patient management. Alterations in glucose and electrolyte levels are amenable to supportive treatment.
Before favipiravir was offered to patients on a compassionate-use basis, it had been evaluated in a clinical trial, the so-called JIKI trial in Guinea [18]. The trial was performed at 4 ETCs supported by different laboratories and relied on historical controls. It revealed a trend of efficacy in patients with a low virus load as defined by a Ct ≥ 20. However, a statistically significant effect in this subgroup could not be demonstrated. The retrospective study presented here on compassionate use of the drug features some favorable methodological conditions for analysis of outcome predictors. First, only 2 ETCs were included; second, a single laboratory generated all Cts; and third, patients who were treated with favipiravir and those who were not treated were managed at the same time. On the other hand, favipiravir was not evaluated in the controlled setting of a clinical trial. While we have made great efforts to reduce biases in the raw field data by using a set of criteria, the retrospective study design still has serious limitations, and data arising from the study have to be interpreted with care. A key limitation is the lack of randomization. Patients who were treated are clearly identifiable, while retrospectively defining an unbiased nontreatment (ie, control) group is difficult. The field database is likely to include nontreated patients who were too sick to provide consent, who were not able to take oral drugs, or who died before they could provide consent or before treatment could be commenced; these aspects are not recorded. We tried to mitigate this bias by excluding all patients who died during day 0 and day 1 from the nontreatment group. However, this approach is arbitrary and does not guarantee an unbiased control group. The Kaplan-Meier curves still show higher mortality in the nontreatment group on day 2 and day 3, which might be by chance, due to a residual bias, or due to a higher virus load on admission (Ct ≤ 20, 59% in the nontreatment group vs 42% in the treatment group; Supplementary Figure 6). The latter is taken into account in the multivariate analysis. Despite these limitations, the obtained data complement the results of the JIKI trial. The regression model reveals a trend toward improved survival, with an estimated 18% reduction in the CFR depending on Ct and age. However, the effect did not reach statistical significance, similar to the JIKI trial data. The positive trend is supported by the Kaplan-Meier analysis, which suggested a prolongation of the survival time.
Pharmacokinetics data for patients with EVD enrolled in the JIKI trial revealed that drug concentrations were lower than targeted levels [21]. Therefore, it is conceivable that administering higher doses of favipiravir, increasing its bioavailability, or modulating its metabolism could enhance its therapeutic effect. However, case reports on patients with Lassa fever or norovirus infection who received favipiravir at similar doses as in the JIKI trial and the present study describe putative adverse events along with symptomatic responses [22, 23]. In conclusion, the observations made so far with favipiravir, including those presented here, call for further preclinical and clinical studies to better understand pharmacokinetics, pharmacodynamics, and adverse events at various dosing schemes and pharmaceutical forms.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the Guinean health authorities, the World Health Organization (WHO) field teams, and the medical teams deployed by the Cuban government and the African Union in Coyah, for their commitment and excellent cooperation. The EMLab is a technical partner of the WHO Emerging and Dangerous Pathogens Laboratory Network and the Global Outbreak Alert and Response Network (GOARN), and the deployments in West Africa have been coordinated and supported by the GOARN Operational Support Team at WHO headquarters.
Financial support. This work was supported by the European Union’s Horizon 2020 Research and Innovation Program (grant agreements 666100 [to the EVIDENT project] and 666092 [to the REACTION! project]), the Directorate-General for International Cooperation and Development (contract IFS/2011/272–372), the German Research Foundation (GU883/5-1 to S. G.), and the German Center for Infection Research (TTU 01.702).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Ebola situation report—30 March 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016. Accessed 23 December 2018. [Google Scholar]
- 2. Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 2004; 78:4330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis 2007; 196(Suppl 2):S364–71. [DOI] [PubMed] [Google Scholar]
- 4. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schieffelin JS, Shaffer JG, Goba A, et al. ; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis 2015; 15:1292–9. [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Rong Y, Sun L, et al. Prognostic analysis of patients with Ebola virus disease. PLoS Negl Trop Dis 2015; 9:e0004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bah EI, Lamah MC, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med 2015; 372:40–7. [DOI] [PubMed] [Google Scholar]
- 9. de La Vega MA, Caleo G, Audet J, et al. Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J Clin Invest 2015; 125:4421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faye O, Andronico A, Faye O, et al. Use of viremia to evaluate the baseline case fatality ratio of Ebola virus disease and inform treatment studies: a retrospective cohort study. PLoS Med 2015; 12:e1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lanini S, Portella G, Vairo F, et al. ; INMI-EMERGENCY EBOV Sierra Leone Study Group Blood kinetics of Ebola virus in survivors and nonsurvivors. J Clin Invest 2015; 125:4692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu HJ, Qian J, Kargbo D, et al. Ebola virus outbreak investigation, Sierra Leone, September 28-November 11, 2014. Emerg Infect Dis 2015; 21:1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fitzpatrick G, Vogt F, Moi Gbabai OB, et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Medecins Sans Frontieres Ebola Case Management Centre, Kailahun, Sierra Leone, June-October 2014. J Infect Dis 2015; 212:1752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Wit E, Kramer S, Prescott J, et al. Clinical chemistry of patients with Ebola in Monrovia, Liberia. J Infect Dis 2016; 214:303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kerber R, Krumkamp R, Diallo B, et al. Analysis of diagnostic findings from the European Mobile Laboratory in Gueckedou, Guinea, March 2014 through March 2015. J Infect Dis 2016; 214:S250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunning J, Sahr F, Rojek A, et al. ; RAPIDE-TKM trial team Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med 2016; 13:e1001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davey RT Jr, Dodd L, Proschan MA, et al. ; PREVAIL II Writing Group, Multi-National PREVAIL II Study Team. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med 2016; 375:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sissoko D, Laouenan C, Folkesson E, et al. ; JIKI Study Group Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konde MK, Baker DP, Traore FA, et al. ; European Mobile Laboratory Consortium Interferon β-1a for the treatment of Ebola virus disease: a historically controlled, single-arm proof-of-concept trial. PLoS One 2017; 12:e0169255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rieger T, Kerber R, El Halas H, et al. Evaluation of RealStar reverse transcription-polymerase chain reaction kits for filovirus detection in the laboratory and field. J Infect Dis 2016; 214:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen TH, Guedj J, Anglaret X, et al. ; JIKI study group Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis 2017; 11:e0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruis C, Brown LK, Roy S, et al. Mutagenesis in norovirus in response to favipiravir treatment. N Engl J Med 2018; 379:2173–6. [DOI] [PubMed] [Google Scholar]
- 23. Raabe VN, Kann G, Ribner BS, et al. ; Emory Serious Communicable Diseases Unit Favipiravir and ribavirin treatment of epidemiologically linked cases of lassa fever. Clin Infect Dis 2017; 65:855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.