Abstract
Background
Chlamydia trachomatis can cause reproductive morbidities after ascending to the upper genital tract of women, and repeated infection can lead to worse disease. Data related to protective immune responses at the cervical mucosa that could limit chlamydial infection to the cervix and/or prevent reinfection inform vaccine approaches and biomarkers of risk.
Methods
We measured 48 cytokines in cervical secretions from women having chlamydial cervical infection alone (n = 92) or both cervical and endometrial infection (n = 68). Univariable regression identified cytokines associated with differential odds of endometrial infection and reinfection risk, and multivariable stepwise regression identified cytokine ratios associated with differential risk.
Results
Elevated interleukin (IL) 15/CXCL10 (odds ratio [OR], 0.55 [95% confidence interval {CI}, .37–.78]), IL-16/tumor necrosis factor–α (OR, 0.66 [95% CI, .45–.93]), and CXCL14/IL-17A (OR, 0.73 [95% CI, .54–.97]) cytokine ratios were significantly (P ≤ .05) associated with decreased odds of endometrial infection. A higher Flt-3L/IL-14 ratio was significantly (P = .001) associated with a decreased risk of reinfection (hazard ratio, 0.71 [95% CI, .58–.88]).
Conclusions
Cytokines involved in humoral, type I interferon, and T-helper (Th) 17 responses were associated with susceptibility to C. trachomatis, whereas cytokines involved in Th1 polarization, recruitment, and activation were associated with protection against ascension and reinfection.
Keywords: Chlamydia trachomatis, inflammatory disease, cytokines, risk factor
Cervical cytokine profiles were compared between women having chlamydial cervical infection alone or endometrial infection. Elevated IL-15/CXCL10, IL-16/TNF-α, and CXCL14/IL-17A ratios were associated with decreased odds of endometrial infection, and a higher Flt-3L/IL-14 ratio was associated with reduced reinfection risk.
Chlamydia trachomatis is the most prevalent sexually transmitted bacterium globally. It is an obligate intracellular pathogen with a complex developmental cycle that multiplies within a protective cytosolic vacuole. Replicating reticulate body progeny redifferentiate to infectious chlamydial elementary bodies (EBs) that are released to infect neighboring cells or hosts exposed through sexual contact. In women, chlamydial infection is initiated at the cervical mucosa, where it may be contained, but in approximately 50% of women, it ascends from the cervix to the endometrium and fallopian tubes. Infection and inflammation of upper genital tract tissues can lead to symptomatic or subclinical pelvic inflammatory disease (PID) that may result in chronic pelvic pain, ectopic pregnancy, and infertility. A better delineation of immune responses at the cervical mucosa associated with infection susceptibility or resistance in women is needed.
Immunoepidemiologic studies of women infected with C. trachomatis indicate T cells are important in defining susceptibility to infection [1–4]. In women with human immunodeficiency virus (HIV), low CD4 counts increased the risk for chlamydial PID [2]. In contrast, peripheral blood mononuclear cell interferon gamma (IFN-γ) responses to a chlamydial heat shock protein correlated with reduced incident infection in a large cohort of female sex workers [1]. We observed that frequencies of peripheral blood CD4 and CD8 T-cell IFN-γ responses to specific chlamydial antigens were associated with reduced incident and ascending infection, respectively, in a cohort of highly sexually active young women participating in the longitudinal T Cell Response Against Chlamydia (TRAC) study [3]. Parallel analyses of the study participants’ antibody responses to chlamydial EBs revealed that, although serum anti-EB immunoglobulin G correlated inversely with cervical burden, this was insufficient to prevent ascension to the upper genital tract [5].
Identifying cervical cytokines associated with reduced or enhanced susceptibility to chlamydial ascension or reinfection could provide clues regarding protective and harmful immune responses that would inform development of novel therapeutics or vaccines. Cytokine profiling could advance biomarker development to provide targeted screening to women at increased risk for upper reproductive tract pathology, and serve as a surrogate marker for induction of protective responses or as a predictor of upper tract infection in women enrolled in vaccine trials. Prior studies have analyzed a limited number of cytokines in cervical secretions and compared their relative abundance in infected and uninfected women. Arno et al demonstrated that women who were chlamydial culture positive at the cervix secreted higher levels of IFN-γ than culture-negative patients, but no correlation was found between IFN-γ levels and bacterial load [6]. Chlamydial infection was also associated with lower interleukin (IL) 2 and higher IL-12 levels when compared to uninfected controls in a predominantly HIV-infected cohort of adolescent and adult women (N = 396) [7], and IFN-γ, IL-12, IL-1, and IL-10 were increased in cervical secretions obtained from a small cohort of C. trachomatis–infected Indian women [8]. However, no study examined the relationship of cervical cytokines to endometrial ascension or reinfection.
Eradication of chlamydial infection from the endocervical epithelium requires T cells at this infected mucosal site [9–11]. Local production of chemokines and cytokines are important for trafficking of chlamydial-specific T cells from the blood to the endocervix and for induction of tissue resident memory T cells [12]. We hypothesized that cytokines shown to play significant roles in T-cell recruitment and activation would be increased in women with infection limited to their cervix compared to those with both cervical and endometrial infection, and that detection of higher levels of T-cell–promoting cytokines at baseline during an active infection would be associated with remaining uninfected after treatment during a year of follow-up.
Analysis of 48 cytokines and chemokines that were quantified in cervical secretions by bead-based array technology revealed that elevated IL-15/CXCL10, IL-16/tumor necrosis factor alpha (TNF-α), and CXCL14/IL-17A ratios were associated with decreased odds of endometrial infection. Furthermore, a higher FMS-like tyrosine kinase 3 ligand (Flt-3L)/IL-14 ratio was found to be significantly associated with reduced reinfection risk after adjusting for clinical and behavioral covariates previously identified in this cohort. These data reveal that cytokines involved in humoral, type I interferon, and T-helper (Th) 17 immune responses are associated with increased susceptibility, while cytokines involved in Th1 polarization, recruitment, and activation are associated with protection against endometrial ascension and reinfection.
METHODS
Study Population
The Institutional Review Boards for Human Subject Research at the University of Pittsburgh and the University of North Carolina approved the study and all participants provided written informed consent prior to inclusion. A total of 160 cervical secretion specimens were available for analysis from asymptomatic C. trachomatis–infected women (aged 15–30 years) participating in the previously described TRAC cohort [5] recruited from 3 urban sites in Pittsburgh, Pennsylvania, from 2011 to 2015 (Supplementary Table 1). Eligibility criteria for the TRAC Study included clinical evidence of mucopurulent cervicitis, diagnosis of chlamydial infection prior to treatment, or reported sexual contact with a male partner recently diagnosed with chlamydial urethritis or nongonococcal urethritis. Exclusion criteria included PID or pregnancy.
Demographic and clinical data were obtained using standardized questionnaire and examination at enrollment. Cervical and endometrial samples were obtained for microbiology using nucleic acid amplification tests (NAAT) and chlamydial cervical burden was estimated via quantitative polymerase chain reaction (PCR) [5]. All participants at enrollment received single-dose agents for gonorrhea (ceftriaxone, 250 mg intramuscularly) and C. trachomatis (azithromycin, 1 g orally). No contact tracing or expedited partner therapy was provided. Participants returned for follow-up visits at 1, 4, 8, and 12 months after enrollment. Microbiologic, clinical, and sexual exposure data were gathered at each follow-up visit but endometrial samples were not obtained. Women testing positively for chlamydial infection during follow-up were treated with azithromycin. Uninfected women tested negatively for C. trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium. Infected women testing positively for C. trachomatis were assigned to groups according to the extent of their infection at enrollment: women testing negatively for endometrial infection were defined as Endo-negative, while those testing positively for endometrial infection were defined as Endo-positive.
Quantification of Cytokines in Cervical Secretions
Cervical secretions collected at enrollment were eluted for multiplex protein assays as described with slight modifications [13]. Cryovials and ophthalmic sponges were weighed to estimate the volume of secretions absorbed onto the sponge. Corning Costar Spin-X centrifuge tubes containing 0.45-μm filters (Millipore Sigma) were equilibrated with 500 μL of blocking buffer (phosphate-buffered saline [PBS], 2% bovine serum albumin [BSA], and 0.05% Tween-20) for 30 minutes at room temperature. Filters were then washed 3 times with 100 μL of PBS. Sponges were equilibrated using 300 μL of elution buffer (PBS, 0.5% BSA, 0.05% Tween-20, and protease inhibitor) before being placed in Spin-X tubes where they were incubated on ice for 10 minutes. Spin-X tubes containing sponges were centrifuged at 10 000g for 1 hour at 4°C and eluted secretions were stored at –80°C. A dilution factor was calculated based on the estimated volume of the secretion and pre- and postweight of the collection tube: (x-y + 0.36 g of elution buffer) / (x-y), where x equals the weight of the sponge + cryovial tube and y is the average weight of the dry cryovial, based on independently weighing 3 dry cryovials.
Cytokine levels were determined using Milliplex Magnetic Bead Assay Kits (Millipore Sigma) at the Duke Regional Biocontainment Laboratory Immunology Core Unit, according to the manufacturer’s instructions, using a BioPlex 200 Luminex bead array reader (Bio-Rad). Assay kits included lyophilized standards that were reconstituted and diluted at 7 serial concentrations following manufacturers’ instructions to generate standard curves (pg/mL). Standards included all cytokines tested and were used as positive controls for the procedure. The levels of 96 cytokines in cervical sponge eluates from 160 infected and 85 uninfected women were assayed using 4 specific kits: (1) Milliplex Map Human Cytokine/Chemokine Magnetic Bead Panel-Premixed 41 Plex–Immunology Multiplex Assay; (2) Milliplex Map Human Cytokine/Chemokine Magnetic Bead Panel II–Immunology Multiplex Assay; (3) Milliplex Map Human Cytokine/Chemokine Magnetic Bead Panel III–Immunology Multiplex Assay; and (4) Milliplex Map Human Cytokine/Chemokine Magnetic Bead Panel IV–Immunology Multiplex. Panels 1–4 were used to analyze samples from 108 women; 39 cytokines were excluded, because values were missing for >60% of women. The remaining 137 women were screened using a customized 57-cytokine panel. An additional 9 cytokines were excluded from statistical analysis because >10% of participants had undetectable or missing values, leaving 48 for further statistical comparisons (Supplementary Table 2). Samples from women who were uninfected at enrollment (n = 86) were excluded from statistical analyses.
Cytokine levels above the upper limit of quantification (ULOQ) were set to the ULOQ, and values below the lower limit of quantification (LLOQ) were set to half the respective LLOQ [14]. Cytokine values were log2-transformed before being corrected for batch effect by the ComBat method [15]. Cytokine values that were missing in <10% of participants were generated by multiple imputation [16].
Statistical Analysis
Relationship Between Cervical Cytokines and Endometrial Infection
Cytokine concentration values were log2-transformed and chlamydial genome equivalents were log10-transformed, and Shapiro–Wilk tests were performed to test datasets for normal distribution. First, the correlation of cytokines with cervical chlamydial burden was conducted by Pearson correlation test (Supplementary Table 3). We then determined the association of individual cytokines with ascending infection (Endo-positive vs Endo-negative) by univariable logistic regression, which measured one cytokine at a time with adjustment of covariates that were previously found to be associated with enhanced odds of endometrial infection in this cohort (oral contraceptive use, gonorrhea coinfection, chlamydial burden) [5]. Cytokines with adjusted P values < .2 by univariable analysis were subsequently analyzed as relative ratios (Supplementary Table 4). Cytokines and ratios with P values < .2 from univariable analysis were advanced to stepwise multivariable logistic regression analysis with the above 3 covariates. A final multivariable parsimonious regression model was developed to determine the optimal subset of cytokines or ratios as risk factors for ascending infection with P ≤ .05. Statistical analyses were performed in R 3.4.2 software (R Foundation for Statistical Computing).
Relationship Between Cervical Cytokines and Reinfection
To measure the effect of cytokines on the risk of reinfection, the hazard ratio was determined using a univariable Wei–Lin–Weissfeld Cox model that accounts for multiple chlamydial infections per person and adjusts for within-participant correlations [17]. Previously identified clinical and behavioral risk factors for incident infection (age, gonorrhea, sex with new, uncircumcised, or infected partners; tissue site of infection, and chlamydial cervical burden) were included as covariates [5]. Individual cytokines and their relative ratios with adjusted P values < .2 in the univariable model (Supplementary Table 5) were advanced to stepwise multivariable Wei–Lin–Weissfeld Cox regression analysis with the above 7 covariates. A final multivariable parsimonious regression model was developed to determine the best subset of cytokines and ratios as risk factors for reinfection with P ≤ .05. The statistical analyses were performed in SAS version 9.4 software (SAS Institute).
RESULTS
Baseline Characteristics of Participants
Cytokine analysis was performed on cervical secretions from 160 C. trachomatis–infected women; 92 (57%) with cervical infection only (Endo-negative), and 68 (43%) who had both cervical and upper genital tract infection (Endo-positive). Supplementary Table 1 details their sociodemographic characteristics. Most were young (median age, 20 years [range, 18–35 years]), single (91%), and African American (66%). The majority (54%) reported previous chlamydial infection, and 23% reported ≥2 prior infections. Participants also reported past infections with gonorrhea (22%) and/or Trichomonas vaginalis (26%). No participants reported HIV infection. A total of 126 (79%) women completed at least 3 follow-up visits, and 92 (58%) completed 4 follow-up visits.
Association Between Cervical Cytokines and Endometrial Infection
Cytokines that were detected in cervical secretions of >90% of infected TRAC participants are listed in Supplementary Table 2. Cytokines involved in neutrophil chemotaxis, survival, and activation (YKL40, CXCL5, CXCL1, granulocyte-colony stimulating factor, IL-8, CXCL6) were highly abundant. Linear regression analysis was used to determine if chlamydial burden influenced cytokine levels and revealed that the concentrations of 38 of 48 (79%) cytokines detected in cervical secretions were positively correlated with burden (Supplementary Table 3). No cytokine displayed a significantly negative correlation with pathogen load. We previously demonstrated that oral contraceptive use, gonorrhea coinfection, and chlamydial cervical burden are associated with endometrial chlamydial infection [5]. Consequently, we adjusted for these 3 factors in a univariable logistic regression analysis and determined that CXCL10, TNF-α, IL-17A, CXCL9, CXCL11, CCL4, and CXCL13 were positively associated with endometrial infection, whereas Platelet-derived growth factor (PDGF)-AA, IL-15, CXCL14, PDGF-BB, IL-14, IL-4, and IL-16 were negatively associated with endometrial infection (adjusted P < .2) (Table 1). These cytokines were entered individually and as ratios (Supplementary Table 4) into stepwise regression analysis with chlamydial burden, oral contraceptive use, and gonorrhea coinfection to determine the least parsimonious final model for individual cytokines or relative ratios associated with endometrial infection. Elevated IL-15/CXCL10 (odds ratio [OR], 0.55 [95% confidence interval {CI}, .37–.78), IL-16/TNF-α (OR, 0.66 [95% CI, .45–.93]), and CXCL14/IL-17A (OR, 0.73 [95% CI, .54–.97]) ratios remained in the final multivariable model (adjusted P ≤ .05) and were associated with decreased odds of endometrial infection (Table 2).
Table 1.
Association of Cytokines With Endometrial Chlamydial Infection by Univariable Regression Analysis
| Cytokine | Unadjusted OR | (95% CI) | P Value | Adjusted ORa | (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Increased infection odds | ||||||
| CXCL10 (IP-10) | 1.8 | (1.40–2.38) | < .0001 | 1.49 | (1.12–2.02) | .008 |
| TNF-α | 1.59 | (1.25–2.06) | < .0001 | 1.32 | (1.00–1.77) | .053 |
| IL-17A | 1.68 | (1.27–2.27) | < .0001 | 1.37 | (.99–1.94) | .062 |
| CXCL9 | 1.62 | (1.30–2.07) | < .0001 | 1.29 | (.99–1.71) | .065 |
| CXCL11 | 1.48 | (1.20–1.86) | < .0001 | 1.2 | (.94–1.54) | .147 |
| CCL4 (MIP-1β) | 1.64 | (1.21–2.28) | .002 | 1.28 | (.90–1.86) | .177 |
| CXCL13 (BCA1) | 1.47 | (1.20–1.82) | .000 | 1.17 | (.93–1.49) | .183 |
| G-CSF | 1.49 | (1.00–2.30) | .058 | 1.26 | (.79–2.04) | .336 |
| YKL40 (CHI3L1) | 1.39 | (1.02–1.94) | .044 | 1.15 | (.79–1.69) | .466 |
| CCL3 (MIP-1α) | 1.27 | (.95–1.71) | .111 | 1.09 | (.76–1.55) | .617 |
| MPIF-1 | 1.27 | (.97–1.69) | .088 | 1.03 | (.75–1.43) | .838 |
| CXCL5 | 1.43 | (.93–2.26) | .110 | 1.03 | (.61–1.75) | .903 |
| sCD40L | 1.24 | (.89–1.74) | .214 | 1 | (.67–1.51) | .997 |
| Decreased infection odds | ||||||
| PDGF-AA | 0.88 | (.73–1.07) | .203 | 0.78 | (.61–0.97) | .034 |
| IL-15 | 0.97 | (.71–1.34) | .858 | 0.69 | (.46–1.01) | .059 |
| CXCL14 (BRAK) | 0.98 | (.79–1.23) | .877 | 0.77 | (.57–1.01) | .067 |
| PDGF-BB | 0.97 | (.79–1.17) | .720 | 0.81 | (.64–1.03) | .088 |
| IL-14 | 1.05 | (.81–1.34) | .726 | 0.79 | (.58–1.07) | .133 |
| IL-4 | 0.75 | (.46–1.19) | .225 | 0.66 | (.37–1.14) | .144 |
| IL-16 | 1.08 | (.84–1.39) | .558 | 0.81 | (.59–1.09) | .173 |
| CCL5 (RANTES) | 1.02 | (.88–1.18) | .821 | 0.9 | (.75–1.07) | .247 |
| IL-1α | 0.95 | (.77–1.17) | .636 | 0.87 | (.67–1.10) | .254 |
| FGF-2 | 1.04 | (.82–1.32) | .724 | 0.85 | (.63–1.13) | .276 |
| CX3CL1 (Fractalkine) | 1.01 | (.64–1.57) | .980 | 0.74 | (.43–1.27) | .284 |
| GM-CSF | 0.93 | (.66–1.31) | .698 | 0.81 | (.52–1.21) | .322 |
| IL-7 | 0.74 | (.40–1.35) | .330 | 0.71 | (.35–1.44) | .347 |
| MDC | 1 | (.71–1.41) | .996 | 0.84 | (.55–1.25) | .412 |
| EGF | 1.06 | (.76–1.50) | .726 | 0.85 | (.56–1.27) | .420 |
| IL-12p40 | 1.16 | (.74–1.82) | .516 | 0.81 | (.48–1.35) | .421 |
| CCL11 (Eotaxin) | 1.09 | (.73–1.63) | .660 | 0.81 | (.47–1.33) | .425 |
| IL-12p70 | 1 | (.63–1.58) | .986 | 0.81 | (.46–1.38) | .436 |
| IL-13 | 0.88 | (.57–1.33) | .546 | 0.84 | (.51–1.35) | .471 |
| IL-6 | 1.08 | (.90–1.30) | .385 | 0.92 | (.72–1.16) | .472 |
| TNFSF13 (APRIL) | 1.1 | (.79–1.54) | .567 | 0.87 | (.58–1.30) | .507 |
| MCP-1 | 1.02 | (.81–1.28) | .892 | 0.91 | (.69–1.19) | .514 |
| TGF-α | 1.21 | (.82–1.81) | .342 | 0.86 | (.54–1.37) | .517 |
| MCP-3 | 1.12 | (.72–1.75) | .606 | 0.84 | (.47–1.43) | .534 |
| GRO | 1.37 | (.74–2.63) | .328 | 0.8 | (.38–1.68) | .544 |
| IL-1RA | 1.08 | (.76–1.56) | .686 | 0.89 | (.59–1.33) | .551 |
| IFN-α | 1.1 | (.64–1.89) | .728 | 0.82 | (.42–1.61) | .563 |
| Flt-3L | 1 | (.64–1.54) | .982 | 0.87 | (.51–1.45) | .590 |
| TNFSF10 (TRAIL) | 1.21 | (.94–1.57) | .148 | 0.93 | (.69–1.27) | .656 |
| IFN-γ | 1.13 | (.84–1.53) | .414 | 0.93 | (.64–1.34) | .679 |
| VEGF | 1.19 | (.75–1.92) | .464 | 0.88 | (.49–1.59) | .682 |
| IL-8 | 1.02 | (.80–1.30) | .854 | 0.94 | (.70–1.26) | .693 |
| IL-1β | 1.09 | (.90–1.31) | .386 | 0.96 | (.76–1.20) | .746 |
| CXCL6 | 1.77 | (.89–3.68) | .113 | 0.94 | (.41–2.14) | .877 |
| IL-10 | 1.4 | (.99–2.02) | .063 | 0.97 | (.64–1.49) | .900 |
| IL-23 | 1.06 | (.81–1.38) | .678 | 0.99 | (.72–1.37) | .970 |
Abbreviations: CI, confidence interval; EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; Flt-3L, FMS-like tyrosine kinase 3 ligand; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO, growth related oncogene-alpha; IFN, interferon; IL, interleukin; IP, interferon-γ-inducible protein; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory proteins; MPIF, myeloid progenitor inhibitory factor; OR, odds ratio; PDGF, platelet-derived growth factors; RANTES, regulated on activation, normal T cell expressed and secreted; sCD40L, soluble CD40L; TGF, transforming growth factor; TNF, tumor necrosis factor; TNFSF, tumor necrosis factor superfamily; TRAIL, NF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
aAdjusted for oral contraceptive use, gonorrhea coinfection, and cervical chlamydial load.
Table 2.
Multivariable Stepwise Regression Analysis of Cytokines, Cytokine Ratios, and Clinical and Behavioral Factors Associated With Chlamydial Endometrial Infection
| Factor | OR | (95% CI) | P Value |
|---|---|---|---|
| IL-15/CXCL10 (IP-10) | 0.55 | (.37–.78) | .002 |
| IL-16/TNF-α | 0.66 | (.45–.93) | .023 |
| CXCL14/IL-17A | 0.73 | (.54–.97) | .036 |
| Gonorrheaa | 2.02 | (.61–6.75) | .244 |
| Oral contraceptive pillsa | 1.07 | (.22–5.58) | .936 |
| Cervical chlamydial load | 2.29 | (1.60–3.46) | < .001 |
Abbreviations: CI, confidence interval; IL, interleukin; IP-10, interferon-γ-inducible protein 10; OR, odds ratio; TNF-α, tumor necrosis factor alpha.
aPreviously identified risk factors for chlamydial endometrial infection [5]
Association Between Cervical Cytokines and Reinfection
We used univariable analysis to identify cytokines that were associated with reinfection, while adjusting for age, gonorrhea infection, sex with new/uncircumcised/infected partners, site of chlamydial infection, and chlamydial burden (Table 3). IL-14, CXCL11, CXCL9, and CXCL10 were associated with increased risk, whereas vascular endothelial growth factor (VEGF), Flt-3L, and TNF (ligand) superfamily, member 10 (TNF-related apoptosis-inducing ligand [TRAIL]) were associated with decreased risk of reinfection (adjusted P < .2). These cytokines and their relative ratios (Supplementary Table 5) were entered into multivariable analysis. An elevated Flt-3L/IL-14 ratio remained in the final multivariable model and was significantly associated (P = .001) with a decreased risk of reinfection after adjusting for other covariates (hazard ratio, 0.71 [95% CI, .58–.88]; Table 4).
Table 3.
Association of Cytokines With Chlamydial Reinfection During 1 Year Of Follow-up by Univariable Regression Analysis
| Cytokinea | HR | (95% CI) | P Value | Adjusted HRb | (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Increased risk of reinfection | ||||||
| IL-14 | 1.1 | (.91–1.33) | .333 | 1.19 | (.97–1.45) | .093 |
| CXCL11 | 1.01 | (.86–1.19) | .860 | 1.14 | (.96–1.34) | .138 |
| CXCL9 | 1.04 | (.91–1.2) | .535 | 1.12 | (.96–1.31) | .147 |
| CXCL10 (IP-10) | 1.01 | (.84–1.2) | .949 | 1.12 | (.95–1.31) | .174 |
| MCP-3 | 1.14 | (.79–1.63) | .486 | 1.18 | (.83–1.68) | .356 |
| CCL11 (Eotaxin) | 1.07 | (.79–1.45) | .662 | 1.17 | (.83–1.64) | .375 |
| CCL5 (RANTES) | 1.07 | (.96–1.2) | .220 | 1.05 | (.93–1.19) | .396 |
| MDC | 1.04 | (.78–1.38) | .788 | 1.09 | (.84–1.44) | .512 |
| CXCL13 (BCA1) | 1.01 | (.87–1.18) | .870 | 1.04 | (.91–1.2) | .540 |
| IL-13 | 1.08 | (.71–1.66) | .715 | 1.12 | (.77–1.63) | .542 |
| PDGF-BB | 1.03 | (.88–1.2) | .724 | 1.04 | (.88–1.23) | .625 |
| FGF-2 | 1.1 | (.92–1.32) | .285 | 1.05 | (.85–1.29) | .674 |
| IL-10 | 0.99 | (.75–1.3) | .938 | 1.04 | (.83–1.32) | .717 |
| IL-17A | 0.96 | (.78–1.17) | .659 | 1.03 | (.85–1.24) | .786 |
| IL-4 | 0.9 | (.6–1.34) | .596 | 1.03 | (.68–1.57) | .885 |
| PDGF-AA | 1.01 | (.87–1.18) | .869 | 1.01 | (.87–1.16) | .910 |
| IL-1RA | 1.15 | (.86–1.54) | .350 | 1 | (.76–1.33) | .979 |
| Decreased risk of reinfection | ||||||
| VEGF | 0.77 | (.56–1.05) | .099 | 0.71 | (.49–1.03) | .069 |
| Flt-3L | 0.9 | (.66–1.23) | .526 | 0.8 | (.6–1.07) | .128 |
| TNFSF10 (TRAIL) | 0.92 | (.76–1.13) | .441 | 0.89 | (.76–1.05) | .154 |
| IL-8 | 0.81 | (.66–.99) | .042 | 0.88 | (.71–1.08) | .228 |
| IL-7 | 0.64 | (.4–1.01) | .056 | 0.79 | (.52–1.21) | .279 |
| MCP-1 | 0.89 | (.75–1.05) | .177 | 0.92 | (.77–1.09) | .320 |
| sCD40L | 0.93 | (.73–1.19) | .574 | 0.88 | (.69–1.13) | .328 |
| IFN-γ | 0.92 | (.72–1.17) | .501 | 0.9 | (.73–1.12) | .355 |
| TGF-α | 1.04 | (.77–1.41) | .796 | 0.87 | (.63–1.19) | .388 |
| IL-1α | 0.93 | (.79–1.09) | .358 | 0.93 | (.79–1.1) | .400 |
| CCL4 (MIP-1β) | 0.81 | (.64–1.03) | .085 | 0.92 | (.73–1.15) | .439 |
| G-CSF | 0.84 | (.63–1.12) | .236 | 0.88 | (.64–1.22) | .457 |
| IL-6 | 0.91 | (.78–1.06) | .233 | 0.94 | (.79–1.12) | .464 |
| GM-CSF | 0.87 | (.62–1.21) | .411 | 0.89 | (.63–1.27) | .534 |
| IL-23 | 0.98 | (.78–1.24) | .880 | 0.94 | (.75–1.16) | .549 |
| IL-1β | 0.91 | (.8–1.04) | .174 | 0.96 | (.84–1.11) | .608 |
| CXCL14 (BRAK) | 0.98 | (.82–1.17) | .790 | 0.95 | (.78–1.16) | .627 |
| MPIF-1 | 0.85 | (.68–1.05) | .138 | 0.95 | (.75–1.2) | .654 |
| CXCL6 | 0.96 | (.59–1.54) | .851 | 0.92 | (.56–1.49) | .724 |
| YKL40 (CHI3L1) | 0.85 | (.67–1.08) | .193 | 0.96 | (.77–1.21) | .753 |
| IL-15 | 0.97 | (.78–1.2) | .777 | 0.98 | (.77–1.25) | .882 |
| IL-12p40 | 0.96 | (.68–1.36) | .834 | 0.98 | (.7–1.37) | .907 |
| EGF | 1.01 | (.75–1.36) | .944 | 0.98 | (.71–1.36) | .911 |
| IL-16 | 1.02 | (.84–1.24) | .867 | 0.99 | (.8–1.23) | .922 |
| CCL3 (MIP-1α) | 0.9 | (.73–1.11) | .316 | 0.99 | (.8–1.23) | .926 |
| TNFSF13 (APRIL) | 0.95 | (.76–1.2) | .668 | 0.99 | (.79–1.25) | .946 |
| IL-12p70 | 0.9 | (.61–1.32) | .576 | 0.99 | (.68–1.43) | .957 |
| GRO | 0.76 | (.51–1.13) | .171 | 0.99 | (.54–1.81) | .968 |
| CX3CL1 (Fractalkine) | 0.93 | (.65–1.32) | .676 | 0.99 | (.67–1.48) | .976 |
| CXCL5 | 0.89 | (.62–1.28) | .524 | 0.99 | (.68–1.47) | .979 |
| TNF-α | 0.91 | (.78–1.06) | .209 | 1 | (.84–1.19) | .980 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aPlease see the Table 1 footnote for cytokine names.
bAdjusted for cervical chlamydial load, age, gonorrhea coinfection, chlamydial infection sites at enrollment, and sex with new, uncircumcised, or infected partners.
Table 4.
Multivariable Stepwise Regression Analysis of Cytokines, Cytokine Ratios, and Clinical and Behavioral Factors Associated With Incident Chlamydial Infection
| Factor | HR | (95% CI) | P Value |
|---|---|---|---|
| Flt-3L/IL-14 | 0.71 | (.58–.88) | .001 |
| Agea | 0.91 | (.83–.99) | .038 |
| Gonorrhea infection during follow-upa | 3.88 | (1.92–7.87) | < .001 |
| Site of chlamydial infection at enrollmenta | |||
| Endometrial infection negative | Reference | Reference | … |
| Endometrial infection positive | 0.70 | (.45–1.10) | .121 |
| Chlamydial infection diagnosis received by partner during follow-upa | 7.46 | (3.53–15.76) | < .001 |
| Sex exposurea | |||
| Sex with uncircumcised male in last 3 mo | 3.36 | (1.50–7.51) | .003 |
| New male partner(s) since last visit | 1.83 | (1.06–3.17) | .031 |
| Cervical chlamydial load | 0.97 | (.8–1.17) | .745 |
Abbreviations: CI, confidence interval; Flt-3L, FMS-like tyrosine kinase 3 ligand; HR, hazard ratio; IL-14, interleukin 14.
aPreviously identified risk factors for incident chlamydial infection [5].
DISCUSSION
Chlamydial ascension to the endometrium inherently increases the risk for PID and chronic complications, and repeated chlamydial infections are associated with heightened risk for disease [18]. This study revealed that cytokines involved in humoral, type I interferon, and Th17 responses were associated with susceptibility to C. trachomatis, whereas cytokines involved in Th1 polarization, recruitment, and activation were associated with protection against ascension and reinfection. Previous analyses of messenger RNA transcriptional profiles revealed that women with endometrial chlamydial infection and endometritis express elevated levels of type I and type II IFN genes in their peripheral blood compared to women with cervical infection only, while increased levels of the type I IFN–induced chemokine CXCL10 were detected in cervical secretions of women with endometrial infection [19]. These findings prompted an expanded analysis of cervical cytokines to identify whether they were associated with endometrial ascension, and to investigate if cytokine levels detected during infection were associated with a decreased or increased risk of subsequent reinfection. Previously determined clinical and behavioral factors associated with increased ascension and risk of incident infection in this cohort were adjusted for using multivariable parsimonious regression analyses [5].
We observed that chlamydial burden positively correlated with levels of most detectable cytokines. This was unsurprising, because greater bacterial abundance will lead to increased activation of pathogen recognition receptors and promote enhanced cytokine production. Our prior multivariable analysis of clinical and behavioral variables also revealed that gonorrhea coinfection and oral contraceptive pills were associated with increased risk of endometrial infection [5]. Consequently, all 3 variables were included as candidate cofactors in a multivariable analysis of cervical cytokines. Elevated IL-15/CXCL10, IL-16/TNF-α, and CXCL14/IL-17A cytokine ratios were negatively associated with endometrial infection, whereas cervical chlamydial load remained positively associated with increased odds of endometrial infection after adjustment of all other variables. Oral contraceptive pills and gonorrhea coinfection were not retained in the final model, likely because of their collinearity with cytokines and burden. We also adjusted for chlamydial load and previously identified cofactors for incident infection determined in the entire cohort that included age, gonorrhea infection, endometrial chlamydial infection, and sex with new, uncircumcised, or infected partners. Older age and an elevated Flt-3L/IL-14 ratio was associated with a significantly reduced risk of reinfection, whereas gonorrhea infection and sex with new, uncircumcised, or infected partners remained positively associated with reinfection in the multivariable model.
CXCL10, TNF-α, and IL-17A were cytokines associated with increased odds of endometrial infection. We previously demonstrated that type I IFN signaling in mice drives CXCL10 production, prolonged chlamydial infection, and increased oviduct pathology [20]. This is consistent with the type I IFN blood transcriptional signature observed in women with endometrial chlamydial infection and endometritis [19]. TNF-α has been shown to cause oviduct pathology in mice after Chlamydia muridarum infection [21], and studies have linked TNF-α to infertility in C. trachomatis–infected women [8, 22]. The susceptibility associated with IL-17A may represent the development of a nonprotective Th17 response. IL-17A has been shown to be either expendable or pathological during chlamydial infection in mice [23, 24].
IL-16, IL-15, and CXCL14 were cytokines associated with a decreased susceptibility to endometrial infection. IL-16 is a potent chemoattractant that has been directly correlated with the abundance of infiltrating CD4 T cells in asthmatic lung epithelium [25] and pleural effusions in tuberculosis patients [26]. IL-16 can also prime recruited Th1 cells for IL-2 and IL-15 responsiveness [27]. Since Th1 cells are a well-characterized correlate of immune protection against chlamydial infection [28, 29], cervical IL-16 may reflect infiltration of effector Th1 cells capable of eliminating infection at the cervix and preventing endometrial infection. After tissue infiltration, IL-15 may help promote T-cell survival [30] and effector function by upregulating IFN-γ production [31] and decreasing IL-17A expression [32]. Priming of these effector Th1 responses may be aided by CXCL14 that is chemotactic for dendritic cells [33] and transports CpG oligodeoxynucleotides into endosomes for Toll-like receptor 9 stimulation [34], which subsequently leads to the development of Th1 responses [35]. This is consistent with the increased frequency of effector memory Th1 cells reported in CXCL14-transgenic mice [36]. Furthermore, multiple studies have demonstrated that CXCL14 has direct antimicrobial activity via bacterial membrane depolarization and rupture [37, 38].
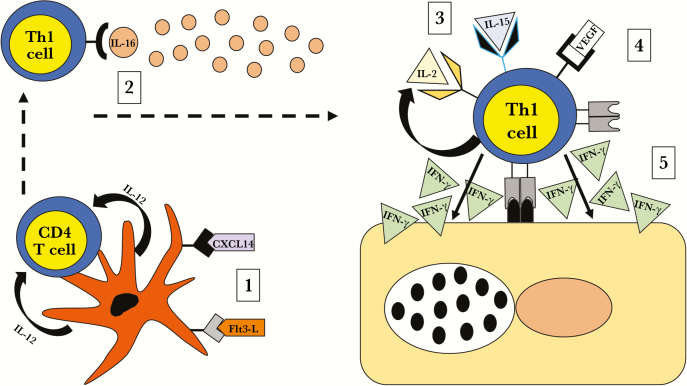
Higher VEGF/IL-14 and Flt-3L/IL-14 ratios were significantly associated with a decreased risk of reinfection by univariable analysis, but only the Flt-3L/IL-14 ratio was maintained in the final multivariable model. VEGF is secreted by T cells after stimulation by cognate antigen or IL-2 in rats [39]. VEGF increased IFN-γ and inhibited IL-10 production by T cells during antigen stimulation, suggesting VEGF can enhance a Th1 phenotype. VEGF has also been shown to enhance IFN-γ production and chemotaxis of human memory CD4 T cells [40]. In mice, VEGF amplifies effector T-cell recruitment and activation via Notch signaling [41] and T-cell VEGF receptor (KDR) expression [42]. Flt-3L is a dendritic cell hematopoietin that regulates dendritic cell levels in tissues and draining lymph nodes [43]. It is secreted during active infection, where it can activate natural killer cell [44] and T-cell responses [45]. Flt-3L dendritic cell activation is important for IL-12 and IFN-γ production [46], and enhances protein vaccine efficacy through global T-cell and B-cell immunity [47]. In contrast, the association of the B-cell growth factor IL-14 with an increased risk of reinfection may represent a nonprotective humoral response. IL-14–transgenic mice demonstrated increased numbers of B1, B2, and germinal center B cells, along with enhanced antibody responses to T-dependent and T-independent antigens, compared with littermate controls [48]. Figure 1 depicts these cervical immune response data in a model that relates mechanisms for their potential roles in protection from ascension and repeated infection.
Figure 1.
Potential roles for protective cytokines against chlamydial ascension and reinfection. (1) Dendritic cells are recruited and activated by CXCL14 and FMS-like tyrosine kinase 3 ligand (Flt-3L) in the cervix. (2) Activated dendritic cells migrate to the draining lymph node where they release interleukin (IL) 12 and present chlamydial antigen to cognate CD4 T cells promoting differentiation of antigen-specific CD4 T-helper (Th) 1 cells. (3) Th1 cells egress from the lymph node into the blood and are chemotactically recruited to the infected cervix by IL-16. (4) IL-16 enhances Th1 responsiveness to IL-2 and IL-15, which promote T-cell survival and accelerate vascular endothelial growth factor (VEGF)–mediated Th1 activation; IL-15 and VEGF costimulation leads to enhanced production of interferon gamma (IFN-γ) by Th1 cells and targeting of chlamydia-infected epithelial cells.
Limitations of our study include the inability to determine if cytokine responses change with infection duration, how long after infection acquisition the sample was obtained in each participant, or their relationship to potential changes in bacterial burden that might occur over time. Additionally, since cytokine levels were measured in cervical sponge eluates, we were not able to determine their cellular sources. Commercial NAATs are highly sensitive but nonquantitative, while the quantitative PCR assay used here to determine chlamydial abundance is precise but relatively insensitive because of the small fraction of clinical sample analyzed. Neither assay discriminates infectious chlamydiae from dead and/or degraded bacteria, which have no potential for ascension or transmission. Quantitation of infectious chlamydiae as a reflection of active bacterial replication would be useful to help confirm the association of cervical burden with endometrial infection.
The cytokine ratios determined to be associated with chlamydial susceptibility can be prospectively examined in an independent cohort to test their ability to predict endometrial infection. Incorporation of host gene expression signatures [19, 49], and other potential factors associated with chlamydial susceptibility such as the vaginal microbiome [50] could improve the predictive accuracy and may also allow for prediction of risk for repeat infection. In addition, our determination of protective responses that are detectable at the cervical mucosa during natural infection have provided important information for vaccine development, regarding specific chemokines and cytokines that might be incorporated as adjuvants to drive Th1 responses necessary for protection against this highly prevalent intracellular pathogen.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All coauthors contributed to the final manuscript; T. D., C. M. O., H. C. W., S. L. H., and X. Z. conceived and designed the study; H. C. W. and S. L. H. recruited the study participants and performed their clinical evaluations; T. D., H. C. W., and S. L. H. coordinated the study logistics, including the fulfillment of all ethical and legal requirements of the study; cervical cytokines were eluted by D. E. L. and measured by multiplex assay under the supervision of T. D. and G. D. S.; D. E. L., W. Z., and L. D. performed the statistical analyses with the supervision of X. Z; T. B. P., T. D., C. M. O., D. E. L., and X. Z. wrote the manuscript, which was reviewed, edited, and approved by all authors.
Acknowledgments. The authors thank the women who agreed to participate in this study; Ingrid Macio, Melinda Petrina, Carol Priest, Abi Jett, and Lorna Rabe for their efforts in the clinic and the microbiology laboratory; and the staff at the Allegheny County Health Department Sexually Transmitted Disease Clinic for their support. The authors also acknowledge Dr Andrew Macintyre, Manager and Lead Investigator in the Immunology Unit of the Duke Regional Biocontainment Laboratory.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant numbers R01 AI119164, U19 AI084024, U19 AI113170, and T32 AI007001). The Duke Regional Biocontainment Laboratory received partial support for construction from the NIAID (grant number UC6-AI058607).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cohen CR, Koochesfahani KM, Meier AS, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. J Infect Dis 2005; 192:591–9. [DOI] [PubMed] [Google Scholar]
- 2. Kimani J, Maclean IW, Bwayo JJ, et al. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J Infect Dis 1996; 173:1437–44. [DOI] [PubMed] [Google Scholar]
- 3. Russell AN, Zheng X, O’Connell CM, et al. Identification of Chlamydia trachomatis antigens recognized by T cells from highly exposed women who limit or resist genital tract infection. J Infect Dis 2016; 214:1884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakshi RK, Gupta K, Jordan SJ, et al. An adaptive Chlamydia trachomatis-specific IFN-γ-producing CD4+ T cell response is associated with protection against Chlamydia reinfection in women. Front Immunol 2018; 9:1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell AN, Zheng X, O’Connell CM, et al. Analysis of factors driving incident and ascending infection and the role of serum antibody in Chlamydia trachomatis genital tract infection. J Infect Dis 2016; 213:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arno JN, Ricker VA, Batteiger BE, Katz BP, Caine VA, Jones RB. Interferon-gamma in endocervical secretions of women infected with Chlamydia trachomatis. J Infect Dis 1990; 162:1385–9. [DOI] [PubMed] [Google Scholar]
- 7. Wang C, Tang J, Crowley-Nowick PA, Wilson CM, Kaslow RA, Geisler WM. Interleukin (IL)-2 and IL-12 responses to Chlamydia trachomatis infection in adolescents. Clin Exp Immunol 2005; 142:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy BS, Rastogi S, Das B, Salhan S, Verma S, Mittal A. Cytokine expression pattern in the genital tract of Chlamydia trachomatis positive infertile women—implication for T-cell responses. Clin Exp Immunol 2004; 137:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol 1997; 158:3344–52. [PubMed] [Google Scholar]
- 10. Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun 2000; 68:6979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun 1991; 59:1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilk MM, Mills KHG. CD4 TRM cells following infection and immunization: implications for more effective vaccine design. Front Immunol 2018; 9:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rohan LC, Edwards RP, Kelly LA, Colenello KA, Bowman FP, Crowley-Nowick PA. Optimization of the weck-Cel collection method for quantitation of cytokines in mucosal secretions. Clin Diagn Lab Immunol 2000; 7:45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar D, Ferreira VH, Blumberg E, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018; 67:1322–9. [DOI] [PubMed] [Google Scholar]
- 15. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8:118–27. [DOI] [PubMed] [Google Scholar]
- 16. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009; 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei LJ, Lin DY, Weissfeld L. Regression-analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc 1989; 84:1065–73. [Google Scholar]
- 18. Hillis SD, Owens LM, Marchbanks PA, Amsterdam LF, Mac Kenzie WR. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol 1997; 176:103–7. [DOI] [PubMed] [Google Scholar]
- 19. Zheng X, O’Connell CM, Zhong W, et al. Discovery of blood transcriptional endotypes in women with pelvic inflammatory disease. J Immunol 2018; 200:2941–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagarajan UM, Prantner D, Sikes JD, et al. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect Immun 2008; 76: 4642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manam S, Thomas JD, Li W, et al. Tumor necrosis factor (TNF) receptor superfamily member 1b on CD8+ T cells and TNF receptor superfamily member 1a on non-CD8+ T cells contribute significantly to upper genital tract pathology following chlamydial infection. J Infect Dis 2015; 211:2014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Srivastava P, Jha R, Bas S, Salhan S, Mittal A. In infertile women, cells from Chlamydia trachomatis infected sites release higher levels of interferon-gamma, interleukin-10 and tumor necrosis factor-alpha upon heat-shock-protein stimulation than fertile women. Reprod Biol Endocrinol 2008; 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scurlock AM, Frazer LC, Andrews CW Jr, et al. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun 2011; 79:1349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrew DW, Cochrane M, Schripsema JH, et al. The duration of Chlamydia muridarum genital tract infection and associated chronic pathological changes are reduced in IL-17 knockout mice but protection is not increased further by immunization. PLoS One 2013; 8:e76664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laberge S, Ernst P, Ghaffar O, et al. Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am J Respir Cell Mol Biol 1997; 17:193–202. [DOI] [PubMed] [Google Scholar]
- 26. Qin XJ, Shi HZ, Huang ZX, Kang LF, Mo WN, Wu C. Interleukin-16 in tuberculous and malignant pleural effusions. Eur Respir J 2005; 25:605–11. [DOI] [PubMed] [Google Scholar]
- 27. Parada NA, Center DM, Kornfeld H, et al. Synergistic activation of CD4+ T cells by IL-16 and IL-2. J Immunol 1998; 160:2115–20. [PubMed] [Google Scholar]
- 28. Gondek DC, Roan NR, Starnbach MN. T cell responses in the absence of IFN-gamma exacerbate uterine infection with Chlamydia trachomatis. J Immunol 2009; 183:1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Murthy AK, Guentzel MN, et al. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol 2008; 180:3375–82. [DOI] [PubMed] [Google Scholar]
- 30. Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med 2007; 204:951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borger P, Kauffman HF, Postma DS, Esselink MT, Vellenga E. Interleukin-15 differentially enhances the expression of interferon-gamma and interleukin-4 in activated human (CD4+) T lymphocytes. Immunology 1999; 96:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pandiyan P, Yang XP, Saravanamuthu SS, et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J Immunol 2012; 189:4237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salogni L, Musso T, Bosisio D, et al. Activin A induces dendritic cell migration through the polarized release of CXC chemokine ligands 12 and 14. Blood 2009; 113:5848–56. [DOI] [PubMed] [Google Scholar]
- 34. Tanegashima K, Takahashi R, Nuriya H, et al. CXCL14 acts as a specific carrier of CpG DNA into dendritic cells and activates Toll-like receptor 9-mediated adaptive immunity. EBioMedicine 2017; 24:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med 1997; 3:849–54. [DOI] [PubMed] [Google Scholar]
- 36. Chen L, Guo L, Tian J, et al. Overexpression of CXC chemokine ligand 14 exacerbates collagen-induced arthritis. J Immunol 2010; 184:4455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maerki C, Meuter S, Liebi M, et al. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol 2009; 182:507–14. [DOI] [PubMed] [Google Scholar]
- 38. Dai C, Basilico P, Cremona TP, et al. CXCL14 displays antimicrobial activity against respiratory tract bacteria and contributes to clearance of Streptococcus pneumoniae pulmonary infection. J Immunol 2015; 194:5980–9. [DOI] [PubMed] [Google Scholar]
- 39. Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol 2004; 172:4618–23. [DOI] [PubMed] [Google Scholar]
- 40. Basu A, Hoerning A, Datta D, et al. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol 2010; 184:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen Z, Shen Y, Berry G, et al. The microvascular niche instructs T cells in large vessel vasculitis via the VEGF-Jagged1-Notch pathway. Sci Trans Med 2017; 9. doi: 10.1126/scitranslmed.aal3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edelbauer M, Datta D, Vos IH, et al. Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo. Blood 2010; 116:1980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996; 184:1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eidenschenk C, Crozat K, Krebs P, et al. Flt3 permits survival during infection by rendering dendritic cells competent to activate NK cells. Proc Natl Acad Sci U S A 2010; 107:9759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guermonprez P, Helft J, Claser C, et al. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med 2013; 19:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dupont CD, Harms Pritchard G, Hidano S, et al. Flt3 ligand is essential for survival and protective immune responses during toxoplasmosis. J Immunol 2015; 195:4369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anandasabapathy N, Feder R, Mollah S, et al. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med 2014; 211:1875–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shen L, Zhang C, Wang T, et al. Development of autoimmunity in IL-14alpha-transgenic mice. J Immunol 2006; 177:5676–86. [DOI] [PubMed] [Google Scholar]
- 49. Zheng X, O’Connell CM, Zhong W, et al. Gene expression signatures can aid diagnosis of sexually transmitted infection–induced endometritis in women. Front Cell Infect Microbiol 2018; 8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Veer C, Bruisten SM, van der Helm JJ, de Vries HJ, van Houdt R. The cervicovaginal microbiota in women notified for Chlamydia trachomatis infection: a case-control study at the sexually transmitted infection outpatient clinic in Amsterdam, the Netherlands. Clin Infect Dis 2017; 64:24–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.