Abstract
Small molecule inhibitors selectively targeting the immunoproteasome subunit β5i are currently being developed for the treatment of autoimmune disorders. However, patients carrying loss‐of‐function mutations in the gene encoding β5i (Psmb8) suffer from the proteasome‐associated autoinflammatory syndromes (PRAAS) emphasizing the need to study pharmacological inhibition of immunoproteasome function in human cells. Here, we characterized the immunomodulatory potential of the selective β5i inhibitor ONX 0914 and Bortezomib, a pan‐proteasome inhibitor, in human peripheral blood mononuclear cells (PBMCs). Both compounds efficiently blocked pro‐inflammatory cytokine secretion in human whole blood and PBMC cultures stimulated with toll‐like receptor (TLR) agonists. Furthermore, the compounds inhibited T cell cytokine production induced by recall antigen CMVpp65 or by polyclonal stimulation. The viability of PBMCs, however, was rapidly decreased in the presence of ONX 0914 and Bortezomib demonstrated by decreased residual cytosolic ATP and increased Annexin V surface binding. Interestingly, HLA‐DR + monocytes were rapidly depleted from the cultures in the presence of ONX 0914 as a β5i‐selective inhibitor and Bortezomib. In conclusion, the anti‐inflammatory potential of β5i‐selective inhibitors is correlating with a cytotoxicity increase in human PBMC subsets ex vivo. Our results provide important insights into the anti‐inflammatory mechanism of action of β5i‐inhibitors which currently hold the promise as a novel therapy for autoinflammatory diseases.
Keywords: human immune cells, immunoproteasome, monocytes, viability
Abbreviations
- C
concentration
- hWB
human whole blood
- LDH
lactate dehydrogenase
- LLOQ
lower limit of quantification
- LMP
low molecular mass polypeptide
- MECL‐1
multicatalytic endopeptidase complex subunit‐1
- MFI
mean fluorescence intensity
- MHC
major histocompatibility
- PBMCs
peripheral blood mononuclear cells
- PRAAS
proteasome‐associated autoinflammatory syndromes
- TCR
T cell receptor
- TLR
toll‐like receptor
1. INTRODUCTION
Proteasomes are specialized complexes degrading poly‐ubiquitylated proteins in eukaryotic cells. Proteasome‐dependent proteolysis occurs in the central 20S core particle, a four stacked ring of seven subunits each.1 The two inner rings are made of constitutive catalytic β‐subunits β1c, β2c and β5c which have caspase‐like, trypsin‐like and chymotrypsin‐like activity, respectively.2 In cells of hematopoietic origin or upon receipt of inflammatory signals such as IFN‐γ, nascent proteasome complexes incorporate the inducible catalytic subunits β1i (LMP2), β2i (MECL‐1), and β5i (LMP7) instead of the constitutive counterparts in the core particle to form the immunoproteasome.3, 4, 5 In particular monocytes and lymphocytes have been shown to contain immunoproteasome proteins.6, 7, 8 It is believed that cells upregulate immunoproteasomes to maintain protein homeostasis to cope with cellular stress thereby altering antigen processing for the generation of major histocompatibility (MHC) class I‐restricted epitopes.9, 10
Since the approval of proteasome inhibitor Bortezomib (Velcade®, PS‐341) by the US Food and Drug Administration (FDA) in 2003 for the treatment of therapy‐refractory multiple myeloma, many inhibitors targeting specific catalytic subunits have been developed.2, 11 Targeting the active β5i site of the immunoproteasome in particular appears a promising strategy for the treatment of autoimmune disorders.9, 12 Bortezomib targets the chymotrypsin‐like catalytic subunits β5c and β5i and, in addition, inhibits the other active sites β1c/β1i and β2c/β2i at higher concentrations.13, 14 Its applicability in inflammatory disorders is limited by neurotoxicity observed in clinical use.15 However, the irreversible immunoproteasome selective inhibitor ONX 0914 (formerly designated PR‐957), shows 20‐ to 40‐fold selectivity to β5i over its constitutive counterpart β5c16 and attenuated disease progression in experimental autoimmune and inflammatory models.16, 17, 18, 19 Because of its selectivity for β5i inhibition, ONX 0914 is supposed to exhibit an improved side effect profile compared to rather unselective immunoproteasome inhibition. In human PBMCs, ONX 0914 treatment blocked endotoxin‐induced cytokine production and polyclonal‐mediated IFN‐γ release in vitro16 suggesting that β5i regulates inflammatory cytokine production in human cells and that its function goes beyond MHC class I‐related antigen processing.
Naïve β5i‐deficient animals do not show an overt phenotype in the absence of pathogen challenge aside from an overall reduced MHC class I surface expression.20, 21 Interestingly, the recently discovered human type I IFN‐driven proteasome‐associated autoinflammatory syndromes (PRAAS) are caused by loss‐of‐function mutations in PSMB8, the gene encoding β5i, and/or other proteasome subunits22, 23, 24, 25 which results in an accumulation of oxidized and poly‐ubiquitylated proteins in patient tissues.22, 24 Cytokine‐induced oxidative stress can lead to accumulation of poly‐ubiquitylated‐proteins and their clearance by immunoproteasomes is essential for preservation of cell viability.10 Hence, it remains of high interest for the development of novel therapeutic agents targeting immunoproteasome catalytic subunits to study the relation of inhibiting immunoproteasome function in human cells, cellular viability, and cytokine production.
In the present study, we addressed this concept by characterizing cytokine production along with viability of human peripheral blood‐derived immune cells in the presence of proteasome inhibitors Bortezomib and ONX 0914. We demonstrate that TLR‐dependent and TCR‐triggered cytokine release was suppressed in PBMC cultures upon pharmacological inhibition of the immunoproteasome. Human monocyte subsets, however, were rapidly depleted in the presence of Bortezomib or ONX 0914 at least in part via apoptosis induction.
2. MATERIALS AND METHODS
2.1. Compounds
Bortezomib (Velcade®, PS‐341) was purchased from Synchem (Felsberg, Germany). ONX 0914 (formerly PR957) or (2S)‐3‐(4‐methoxyphenyl)‐N‐[(2S)‐1‐[(2R)‐2‐methyloxiran‐2‐yl]‐1‐oxo‐3‐phenylpropan‐2‐yl]‐2‐[[(2S)‐2‐[(2‐morpholin‐4‐ylacetyl)amino]propanoyl]amino]propanamide was synthesized as previously described in WO2007149512.
2.2. Enzymatic activity of human (immuno)proteasomes
Human purified immunoproteasome and constitutive proteasomes were kindly provided by Dr. Marcus Groettrup, University of Constance, and isolated from human cell lines LCL721.174 (containing constitutive proteasome), and LCL721.145 (containing immunoproteasome) as described.26 Compounds were serially diluted in dimethyl sulfoxide (DMSO; 34869‐1L, Sigma‐Aldrich, Munich, Germany), then diluted in reaction buffer consisting of 50 mmol/L BisTrisPropane pH 8.0, 10 mmol/L CaCl2, 0.5 mmol/L DTT, 0.001% (v/v) BSApf, and added to purified proteasome preparations for 15 minutes at room temperature. Final DMSO concentration did not exceed 1% (v/v). Following preincubation, chymotrypsin‐like activity was tested by adding the fluorogenic substrate Suc‐LLVY‐AMC (I‐1395, Bachem, Bubendorf, Switzerland) for 60 minutes at room temperature in a final reaction buffer consisting of 50 mmol/L BisTrisPropane pH 8.0, 10 mmol/L CaCl2, 0.5 mmol/L DTT, 0.001% (v/v) BSApf, 4% (v/v) ACN, 1% (v/v) DMSO. Caspase‐like and trypsin‐like activity were determined using a reaction buffer consisting of 50 mmol/L Tris‐HCl pH7.5, 25 mmol/L KCl, 10 mmol/L NaCl, 1 mmol/L MgCl2, 0.01 mmol/L EDTA, 1 mmol/L DTT, and the fluorogenic substrates Z‐LLE‐AMC and Bz‐VGR‐AMC, respectively. Measurement of fluorogenic product was performed at PHERAstar (BMG Labtech, Ortenberg, Germany) and data analyzed with Genedata Screener and Dotmatics Vortex. Percentage activity was calculated using the formula: 100 × (value – NC)/(IC – NC) whereby neutral control (NC) is the median of the response values for human immunoproteasome (no inhibitor control) and inhibitor control (IC) is the median of the response values for human immunoproteasome containing 10 μmol/L ONX 0914.
2.3. Human PBMC and whole blood cultures
Heparinized peripheral blood from healthy donors was derived from internal blood donation program of Grünenthal or from ClinPharmCologne, Cologne. Human PBMCs were isolated by density gradient centrifugation from buffy coats obtained from Aachen University. PBMCs were cultured in cell culture medium (RMPI 1640 supplemented with GlutaMAX, 10% (v/v) heat‐inactivated FCS; all Gibco, Life Technologies, Darmstadt, Germany) and treated overnight (approximately 20 hours) with Bortezomib or ONX 0914 at 0.1% DMSO (Sigma‐Aldrich, Munich, Germany) final concentration in the presence of 0.1 μg/mL LPS from E. coli 0127:B8 (Sigma‐Aldrich, Munich, Germany) when indicated. Supernatants were harvested and cytokine levels determined by ELISA (IL‐23 or IL‐8; R&D systems, Abingdon, UK) and AlphaLISA (TNF, IL‐6 or MCP‐1; Perkin Elmer, Velbert, Germany) according to the manufacturer's instructions. Absorption and luminescence were determined on a Powerwave HT340 and Synergy4 reader, respectively and analyzed with Gen5 software (all BioTek, Bad Friedrichshall, Germany). Cell pellets were analyzed for ATP content by using the CellTiter‐Glo luminescent cell viability kit (Promega, Mannheim, Germany). Human heparinized blood was treated overnight (approximately 20 hours) with compounds at final concentration of 0.1% (v/v) DMSO and 1 μg/mL LPS. Plasma was harvested following centrifugation, diluted 1:2 in FCS and analyzed for cytokine content as described. Percentage inhibition was calculated compared to no inhibitor control containing DMSO at a final concentration of 0.1% (v/v).
2.4. Human T cell cultures
Human PBMCs were cultured in cell culture medium in the presence of recombinant CMVpp65 (Miltenyi, Bergisch Gladbach, Germany) and compounds at a final concentration of 0.1% DMSO. After 7 days, supernatants were harvested and IFN‐γ cytokine content was determined using a time‐resolved fluorescence resonance energy transfer (HTRF) based assay (Cisbio, Codolet, France) measured on a SynergyNeo reader using Gen5 software (BioTek, Bad Friedrichshall, Germany). For polyclonal T cell activation, CD4+ T cells were enriched from PBMCs by negative selection using an antibody‐based magnetic bead system (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Enrichment procedure typically results into more than 90% purity as determined by flow cytometry. Isolated CD4+ T cells were cultured in cell culture medium and stimulated with Dynabeads™ Human T‐Activator CD3/CD28 (Life Technologies, Darmstadt, Germany) at a 1:2 ratio (T cell:bead) for 48 hours. Supernatants were harvested and IFN‐γ cytokine content analyzed by ELISA (R&D Systems, Abingdon, UK). Lactate dehydrogenase (LDH) levels in supernatant were determined by colorimetric assay kit (Life Technologies, Darmstadt, Germany) according to the instruction provided by the manufacturer. Absorption was determined on a Synergy4/H4 plate reader and analyzed with Gen5 software (BioTek, Bad Friedrichshall, Germany). Percentage inhibition was calculated compared to no inhibitor control containing DMSO at a final concentration of 0.1% (v/v).
2.5. Flow cytometry
Cells were stained in ice‐cold staining buffer consisting of PBS (Life Technologies, Darmstadt, Germany) supplemented with 0.5% (v/v) bovine serum albumin (BSA; Merck, Darmstadt, Germany), 2 mmol/L EDTA (Merck, Darmstadt, Germany), and 0.1% (v/v) sodium azide (Merck, Darmstadt, Germany). Cells were preincubated with human TruStain FcX™ Fc‐receptor blocking solution (BioLegend, Fell, Germany) followed by staining with the following fluorochrome labeled monoclonal antibodies for 30 minutes at 4°C in the dark: anti‐human CD16‐FITC (clone 3G8; BD Biosciences, Heidelberg, Germany), CD14‐PE (clone HCD14; BioLegend, Fell, Germany), HLA‐DR‐APC (clone G46‐6; BD Biosciences, Heidelberg, Germany). Samples were washed twice by centrifugation for 300g for 5 minutes, and cell pellet resuspended in ice‐cold PBS supplemented with Fixable Viability Dye eFluor 780 (Life Technologies, Darmstadt, Germany). Annexin V staining was performed according to the manufacturer's instructions (Life Technologies, Darmstadt, Germany). Briefly, cells were washed once in Annexin V binding buffer followed by incubation with Annexin V‐PECy7 (Life Technologies, Darmstadt, Germany) in binding buffer for 15 minutes at room temperature in the dark. Following incubation, cells were washed once with binding buffer and immediately analyzed by flow cytometry. Samples were measured on a BD Canto II flow cytometer (BD Biosciences, Heidelberg, Germany) and analyzed with FlowJo software (TreeStar, Ashland). Fluorescence minus one (FMO) controls were used for defining gates during analysis.
2.6. Data analysis
All data are expressed as mean ± SEM. Analysis was performed with GraphPad Prism software version 6. IC50 values were determined from three parameter fit graphs.
3. RESULTS
3.1. Selective inhibition of β5i catalytic subunit associated chymotrypsin‐like activity by ONX 0914 but not Bortezomib
To study the role of immunoproteasome in human PBMC function, we synthesized two inhibitor compounds, Bortezomib as a pan‐proteasome inhibitor27 and ONX 0914 as a β5i‐selective inhibitor16 and evaluated enzymatic inhibition of the immunoproteasome using subunit‐specific fluorogenic substrates in vitro.
Subunit selectivity of the generated compounds (Figure 1A) was explored by measuring cleavage of fluorescent probe Suc‐LLVY‐AMC indicative for chymotrypsin‐like activity by purified human constitutive proteasomes (cPS) or immunoproteasome (iPS) preparations isolated as described.26 Bortezomib potently inhibited cleavage of the substrate of chymotrypsin‐like activity from both human cPS and iPS preparations at IC50 of 6.275 × 10−8 mol/L and 4.992 × 10−8 mol/L, respectively (Figure 1B,C). Chymotrypsin‐like activity of the human iPS was potently attenuated in the presence of ONX 0914 at IC50 value of 6.251 × 10−8 mol/L showing 14‐fold selectivity of attenuating iPS over cPS chymotrypsin‐like activity (Figure 1B,C). Using fluorogenic substrates containing caspase‐like (Z‐LLE‐AMC) and trypsin‐like (Bz‐VGR‐AMC) active sites, Bortezomib was able to inhibit other enzymatic activities of the human iPS preparation but at IC50 of 1.914 × 10−7 mol/L and 1.435 × 10−6 mol/L, respectively (Supporting Information Figure S1). Similarly, ONX 0914 inhibited cleavage of the substrate of trypsin‐like activity of human iPS at IC50 values of 1.183 × 10−6 mol/L, but showed low potency on caspase‐like activity (IC50 > 10 μmol/L; Supporting Information Figure S1).
Figure 1.
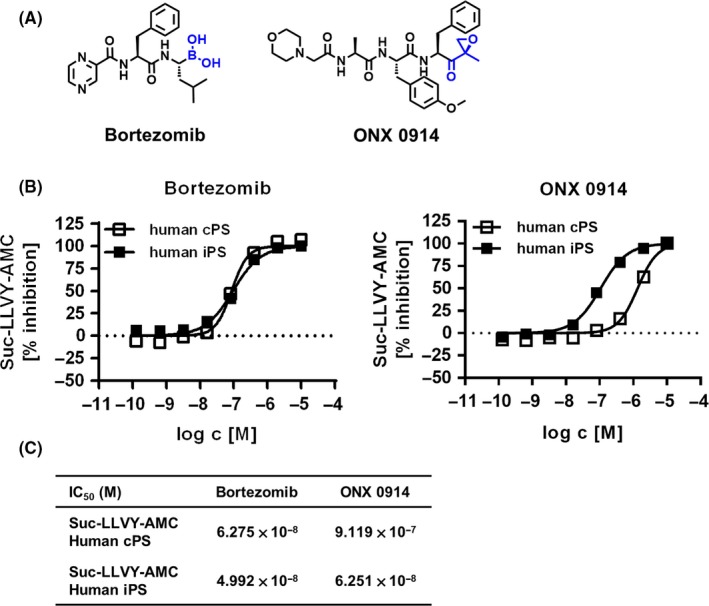
Small molecule inhibitors inhibit chymotrypsin‐like activity of human immunoproteasome. A, Chemical structures of Bortezomib and ONX 0914. B, Human constitutive proteasome or immunoproteasome preparations were incubated with fluorogenic substrate Suc‐LLVY‐AMC in the presence of various Bortezomib or ONX 0914 inhibitor concentrations as indicated. Data show percentage inhibition calculated as described in material and methods as mean ± SEM of one representative experiment (each concentration in duplicate). C, Table showing inhibitory potencies summarized from 12 (Bortezomib) and 33 (ONX 0914) number of tested (fitted) concentration response curves performed in duplicates for the human constitutive proteasomes and 23 (Bortezomib) and 111 (ONX 0914) number of tested (fitted) concentration response curves performed in duplicates for the human immunoproteasomes
In summary, Bortezomib inhibited chymotrypsin‐like activity of both cPS and iPS preparations suggesting broad‐spectrum proteasome inhibitory potential, whereas ONX 0914 selectively inhibited the chymotrypsin‐like activity associated with β5i enzymatic activity.
3.2. Immunoproteasome inhibitors suppress cytokine production but largely affect cellular viability
Earlier reports demonstrated that selective immunoproteasome inhibitors inhibit TLR‐triggered pro‐inflammatory cytokine production in human PBMCs.16 However, since proteasome inhibition might lead to protein aggregate formation and apoptosis induction,10 we investigated TLR‐induced cytokine production as well as viability of cells in the presence of Bortezomib and ONX 0914.
Bortezomib potently inhibited TNF (Figure 2A,E; IC50 2.047 × 10−8 mol/L) and IL‐23 (Figure 2A,E; IC50 1.854 × 10−9 mol/L) cytokine production in LPS‐stimulated PBMCs (Figure 2A). Interestingly, cell viability measured as residual cellular ATP content decreased rapidly upon increasing concentrations of Bortezomib (Figure 2A). The selective inhibitor ONX 0914 efficiently blocked pro‐inflammatory TNF and IL‐23 production (Figure 2A) as reported elsewhere.16 Similar to pan‐inhibitor Bortezomib, cellular viability decreased rapidly in the presence of ONX 0914 in a concentration‐dependent manner (Figure 2A,E; IC50 8.984 × 10−8 mol/L) which corresponded to the TNF secretion potency (Figure 2E; IC50 8.999 × 10−8 mol/L) but not to the potency of IL‐23 inhibition (IC50 5.964 × 10−9 mol/L). Both Bortezomib and ONX 0914 potently decreased LPS‐induced MCP‐1, IL‐6, and IL‐8 release of PBMC cultures suggesting that overall LPS‐induced cytokine release was affected (Supporting Information Figure S2a,b). Inhibition of pro‐inflammatory mediator production and viability of human PBMCs by ONX 0914 was also observed in the presence of TLR‐9 agonist CpG (data not shown). In addition, none of the inhibitors induced TNF secretion in the absence of TLR stimulation (data not shown). Short‐term drug exposure of PBMCs (2 hours) followed by washing and additional culture for 18 hours in drug‐free, LPS‐supplemented media resulted in reduced inhibition of LPS‐induced TNF and IL‐23 release for both Bortezomib and ONX 0914 (Supporting Information Figure S3a; TNF: IC50 9.383 × 10−8 mol/L and IC50 4.451 × 10−7 mol/L, respectively; IL‐23: IC50 5.370 × 10−9 mol/L and IC50 2.961 × 10−8 mol/L, respectively) compared to the continuous drug exposure set‐up (Figure 2A,E). Similarly, residual cellular ATP inhibitory potencies shifted 2.0‐fold and 2.6‐fold for Bortezomib and ONX 0914, respectively, for the 2‐hour drug exposure setup (Supporting Information Figure S3a) compared to continuous drug incubation (Figure 2A,E). Both inhibitors were equally potent in attenuating production of IL‐23 in LPS‐treated human whole blood (hWB) (Figure 2B,E; Bortezomib IC50: 2.916 × 10−8 mol/L and ONX 0914 IC50: 3.612 × 10−8 mol/L). Inhibition of TNF secretion of LPS‐treated hWB by Bortezomib and ONX 0914 was 16‐fold and 49‐fold less potent compared to IL‐23, respectively (Figure 2E). Human T cell activation as monitored by IFN‐γ release was potently suppressed by Bortezomib and ONX 0914 upon stimulation with recall antigen CMVpp65 (Figure 2C) or anti‐CD3/anti‐CD28 beads (Figure 2D, left panel). Similar to the TLR‐induced PBMC cytokine production setting, a decrease in viability of primary human T cell cultures was observed by both inhibitors as assessed by cellular ATP content (Figure 2C) or LDH release (Figure 2D, right panel). To simulate short‐term in vivo half‐life of ONX 0914, human PBMCs were treated with Bortezomib or ONX 0914 for 2 hours followed by setting to drug‐free media and stimulation with recall antigen CMVpp65. As observed for the continuous drug exposure setting (Figure 2C and Supporting Information Figure S3b, upper panel), short‐term drug exposure to Bortezomib or ONX 0914 reduced IFN‐γ release and cellular viability in a concentration‐dependent manner (Supporting Information Figure S3b, lower panel).
Figure 2.
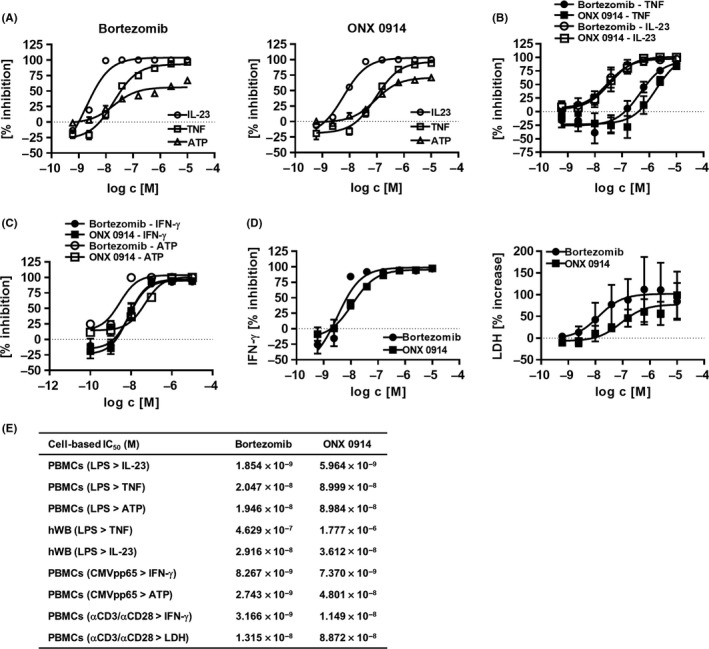
Immunoproteasome inhibitors inhibit inflammatory cytokine production and affect cell viability. A, Human PBMCs were treated with immunoproteasome inhibitors at concentrations indicated and stimulated overnight with LPS. TNF and IL‐23 cytokine content in cell culture supernatant was assessed by AlphaLisa/ELISA and ATP content determined via luminescence. Figure shows percentage inhibition normalized to no inhibitor control (0% inhibition depicted as dotted line) as a mean ± SEM from six independent donors. Absolute cytokine values: TNF 48 ± 50 pg/mL (unstimulated) vs 4976 ± 2137 pg/mL (LPS stimulated); IL‐23 <LLOQ (unstimulated; LLOQ = 125 pg/mL) vs 711 ± 362 pg/mL (LPS stimulated). B, Concentration response curves of immunoproteasome inhibitors on human heparinized whole blood stimulated overnight with LPS. Plasma was analyzed for TNF and IL‐23 content by AlphaLISA and ELISA, respectively. Data show percentage inhibition of TNF (four donors) and IL‐23 (three donors) as mean ± SEM normalized to no inhibitor control. Absolute cytokine values: TNF 281 ± 170 pg/mL (unstimulated) vs 2566 ± 1579 pg/mL (LPS stimulated); IL‐23 <LLOQ (unstimulated; LLOQ = 125 pg/mL) vs 372 ± 174 pg/mL (LPS stimulated). C, Human PBMCs were incubated with inhibitors at concentrations indicated and stimulated with recombinant CMVpp65 antigen for 7 days. IFN‐γ levels in cell culture supernatant were assessed by HTRF and ATP content of the cells determined via luminescence. Percentage inhibition was calculated using no inhibitor control (0% inhibition represented as dotted line) from two (ATP) to four (IFN‐γ) donors as mean ± SEM. D, Human CD4+ T cells were stimulated with anti‐CD3 and anti‐CD28 coated beads for 2 days in the presence of graded concentrations of inhibitors as indicated. IFN‐γ and LDH levels in the cell culture supernatant were determined using ELISA and a colorimetric assay kit, respectively. Data show percentage inhibition of IFN‐γ secretion (left panel) and percentage increase of LDH release (right panel) normalized to no inhibitor control as a mean ± SEM from four donors. E, Table summarizing inhibitory potencies of experiments described in A‐D
In sum, pan‐proteasome inhibition as well as specific pharmacological inhibition of β5i immunoproteasome activity in human primary immune cells led to potent inhibition of TLR‐induced cytokine production and TCR‐triggered IFN‐γ release. However, both inhibitors strongly affected cell viability in a similar potency range regardless of a continuous or short‐term drug exposure regimen.
3.3. Immunoproteasome inhibitors induce apoptosis in human PBMCs
To further elucidate how immunoproteasome inhibitors decreased cell viability, we investigated the cells by flow cytometry using the apoptosis marker Annexin V.
Upon treatment of human PBMCs with ONX 0914 or Bortezomib, cells decreased cellular size as indicated by lowered FSC and increased granularity reflected by high SSC (Figure 3A, left panels). Interestingly, following treatment of human PBMCs with Bortezomib and ONX 0914, the monocyte fraction identified by FSC/SSC properties were hardly detected (Figure 3A, left panels). Furthermore, adding ONX 0914 to the PBMC culture enhanced the total Annexin V positive fraction (Figure 3A, Annexin V+/Viability Dye‐ 54.9%; Annexin V+/Viability Dye+ 24.9%) compared to untreated control (Figure 3A, Annexin V+/Viability Dye‐ 27.1%; Annexin V+/Viability Dye+ 14.6%). ONX 0914 potently increased Annexin V binding in human PBMCs showing an EC50 of approximately 1.646 × 10−7 mol/L (Figure 3B) corresponding to the decrease in cellular ATP content (Figure 2A,E, IC50 8.984 × 10−8 mol/L). Similarly, a higher proportion of Annexin V+/Viability Dye‐ as well as Annexin V+/Viability Dye+ expressing PBMCs (Figure 3A, 47.1% and 24.6%, respectively) were detected in the presence of Bortezomib compared to untreated control (Figure 3A, right panels). Bortezomib induced Annexin V binding on human PBMC in a concentration‐dependent manner (Figure 3B). Similarly, short‐term drug exposure to Bortezomib or ONX 0914 resulted in increased Annexin V binding of human PBMCs after 46 hours of culture in drug‐free medium (Supporting Information Figure S3c, IC50 3.439 × 10−8 mol/L and 3.277 × 10−7 mol/L, respectively), corresponding to the cellular ATP inhibitory potencies for the 2‐hour drug exposure regimen (Supporting Information Figure S3a, IC50 3.937 × 10−8 mol/L and 2.301 × 10−7 mol/L, respectively).
Figure 3.
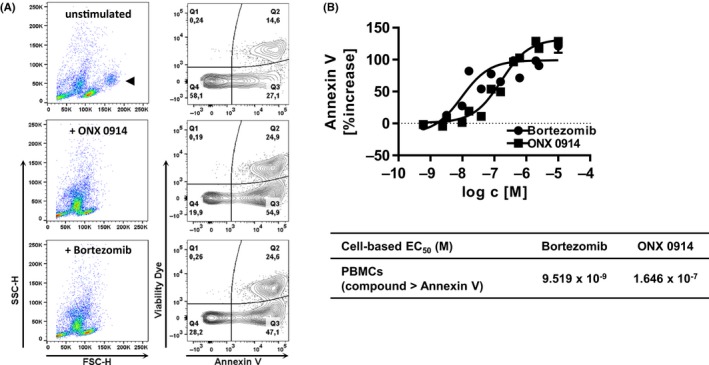
Immunoproteasome inhibitors induce cell death in human PBMCs. A, Human PBMCs were incubated overnight with inhibitors at a concentration of 625 μmol/L when indicated followed by flow cytometry detection of Annexin V binding and viability dye exclusion. Numbers in quadrant plots represent percentage cells. Cells were not gated to include all cells. One representative example from two independent experiments is shown. Monocytes population identified by FSC/SSC properties indicated in upper panel ( ). B, Human PBMCs were incubated overnight with inhibitors followed by flow cytometry analysis for Annexin V and viability dye expression. Data show percentage increase of Annexin V positive cells gated as described in supplementary Figure S4a as mean ± SEM from three donors. Percentage increase was calculated as total percentage of Annexin V‐positive cells in cell culture normalized to no inhibitor control (0% increase represented as dotted line). Table shows EC
50 values calculated from three donors
). B, Human PBMCs were incubated overnight with inhibitors followed by flow cytometry analysis for Annexin V and viability dye expression. Data show percentage increase of Annexin V positive cells gated as described in supplementary Figure S4a as mean ± SEM from three donors. Percentage increase was calculated as total percentage of Annexin V‐positive cells in cell culture normalized to no inhibitor control (0% increase represented as dotted line). Table shows EC
50 values calculated from three donors
Thus, immunoproteasome inhibitors decreased cellular viability of human PBMCs at least in part by induction of apoptosis as determined via Annexin V staining.
3.4. Rapid depletion of human monocyte subsets by immunoproteasome inhibitors
The observation that TLR‐induced cytokine production and monocyte numbers are significantly suppressed in the presence of immunoproteasome inhibitors prompted us to investigate monocyte subset frequencies by flow cytometry. Human PBMCs were directly isolated from peripheral blood to exclude the possibility that delayed processing from buffy coat preparations influenced viability outcome (as previously suggested28).
Both Bortezomib and ONX 0914 potently depleted the HLA‐DR+ monocyte population from human PBMC preparations in a concentration‐dependent manner within 15 hours (Figure 4a, upper panel and Supporting Information Figure S4c), and as previously reported for Bortezomib.29 Upon incubating human PBMCs with Bortezomib, cellular frequencies of total HLA‐DR+ monocytes as well as CD14+CD16‐ classical or CD14+CD16+ intermediate monocyte subsets decreased with similar potencies (Figure 4A). Downregulation of CD14 and CD16 surface marker expression could be excluded due to the absence of the monocyte population identified by FSC/SSC properties (Figure 3A). Interestingly, numbers of HLA‐DR+ monocytes decreased rapidly in the presence of ONX 0914 (IC50 9.010 × 10−8 mol/L) corresponding to the decrease in ATP content and TNF production induced by LPS stimulation of human PBMCs (IC50 8.984 × 10−8 mol/L and 8.999 × 10−8 mol/L, respectively, Figure 2A right panel and E). Addition of ONX 0914 to human PBMCs potently decreased the frequencies of CD14+CD16‐ classical monocytes subsets (Figure 3A; IC50 9.566 × 10−8 mol/L), whereas the CD14+CD16+ intermediate monocyte population was depleted from the human PBMC culture at a 4.6‐fold lower ONX 0914 concentration (Figure 3A lower panel; IC50 2.082 × 10−8 mol/L). Treatment of human PBMCs with Bortezomib strongly increased detection of Annexin V binding on HLA‐DR+ monocytes (Figure 4B, upper panel and C) and CD14+CD16‐ classical monocyte subsets (Figure 4B, lower panel and C), similar to the total PBMC culture as described (Figure 3B). Annexin V binding analysis showed increased Annexin V detection on HLA‐DR+ monocytes (Figure 4B, upper panel and C) and CD14+CD16‐ classical monocyte subsets (Figure 4B, lower panel and C) in the presence of ONX 0914.
Figure 4.
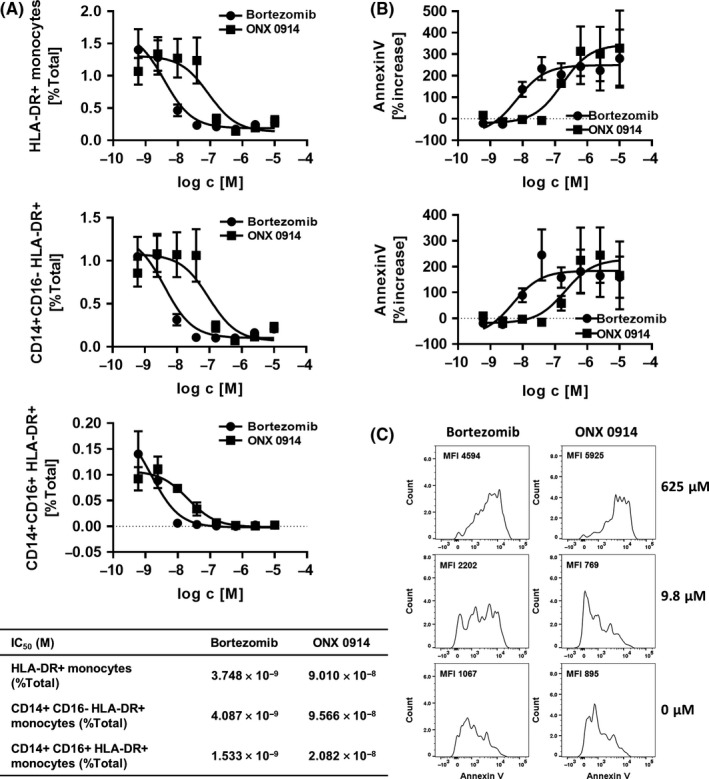
Early depletion of monocyte subsets by immunoproteasome inhibitor. A, Human PBMCs were incubated with inhibitors for approximately 15 hours followed by flow cytometry analysis of monocyte subset markers. Figure shows percentage of total culture of monocytes (HLA‐DR+), CD14+ CD16‐ classical monocytes, and CD14+ CD16+ intermediate monocyte subsets from four donors as mean ± SEM and gated as described in supplementary Figure S4b. No inhibitor control percentage of total culture of monocytes (HLA‐DR +), CD14+ CD16‐ classical monocytes, and CD14+ CD16+ intermediate monocyte subsets are depicted in supplementary Figure S5. Table summarizes IC 50 values calculated from four donors B. Human PBMCs were incubated with inhibitors for approximately 15 hours followed by flow cytometry analysis of Annexin V expression. Figure shows percentage increase of Annexin V expression of HLA‐DR + monocytes (upper panel) and CD14+ CD16‐ classical monocyte subsets (lower panel) as mean ± SEM from four donors and gated as described in supplementary Figure S4d. Percentage increase was calculated compared to no inhibitor control (0% increase represented as dotted line). C, Human PBMCs were incubated with inhibitors for approximately 15 hours followed by flow cytometry analysis as described in B. Representative example of the Annexin V expression on HLA‐DR + monocyte population is shown from one donor incubated with inhibitors at concentration indicated. Numbers depicted in plots represent MFI of Annexin V
In sum, immunoproteasome inhibitors potently depleted human monocyte subsets from untreated human PBMC cultures in vitro.
4. DISCUSSION
Our results indicate that the pan‐proteasome inhibitor Bortezomib and the β5i‐selective inhibitor ONX 0914 are highly potent in suppressing human TLR‐induced cytokine production and TCR‐triggered T cell activation, but largely impact cell viability evaluated via cellular metabolic activity and phosphatidylserine externalization. The reduction in cell viability observed for Bortezomib, the only compound for which clinical pharmacokinetic data are available, occurs at IC50 concentrations of 2.7 nmol/L up to 20 nmol/L in the different cellular settings which is even below the range of free plasma peak concentrations detected in adults.30
The cytotoxicity of human PBMCs detected upon incubation with ONX 0914 corresponded to the TNF, but not to IL‐23 secretion inhibitory potency suggesting that β5i‐selective inhibition might reduce TLR‐induced IL‐23 production without impairing cellular viability.16 The regulation of IL‐23 by immunoproteasomes deserves further investigation;16, 31 however, our results raise the possibility that differential susceptibility of immune cell subsets to cytotoxicity may contribute to the anti‐inflammatory effect observed for β5i‐selective inhibitors such as ONX 0914. Inhibitors used in this study triggered depletion of the intermediate CD14+CD16+ monocyte subset in particular. A putative explanation might be that the CD16+ human monocyte subset seems to be less protected against oxidative stress‐induced apoptosis than CD16‐ monocyte populations.32 Oxidant‐damaged proteins are degraded by immunoproteasomes in particular and cells lacking β5i subunits are more susceptible to apoptosis induced by IFN.10 Interestingly, earlier reports indicated that primarily CD16‐ monocyte population seem to produce IL‐23 following TLR activation.33 Alternatively, although Bitzer and colleagues demonstrated that deficiencies in immunoproteasome subunits do not lead to defects in canonical NF‐κB signaling,34 it remains to be determined whether impairment in alternative signal transduction pathways following immunoproteasome inhibition might result into aberrant IL‐23 expression. LPS activation of human immune cells can not only trigger myeloid differentiation marker 88 (MyD88) but also Toll‐interleukin‐1 receptor (TIR) domain–containing adaptor‐inducing interferon‐β (TRIF)‐dependent pathways leading to interferon regulatory factor 3 (IRF3) activation,35 which is dependent on ubiquitination processes36 and leads to type I interferon, and also IL‐23 production.37, 38
Our study confirmed that the β5i‐selective inhibitor ONX 0914 and Bortezomib potently inhibited human T cell activation induced by polyclonal activation (previously demonstrated by others16, 39, 40) and by antigen‐specific stimulation.41 Presentation of CMVpp65‐derived epitopes to responder T cells is dependent on antigen‐presenting cells and hence, the cytotoxicity induced in monocytes as observed in this study upon immunoproteasome inhibition, will largely impact the CMVpp65‐induced IFN‐γ production. Reduced IFN‐γ, IL‐17A, and IL‐6 production of ONX 0914‐treated human PBMCs stimulated with heat‐killed C.albicans was reported previously,41 supporting the notion that the T cell activation process rather than alternate MHC class I epitope generation is impacted by ONX 0914. Furthermore, ONX 0914 treatment of polyclonally stimulated purified CD4+ human T cells is sufficient to inhibit IFN‐γ secretion with similar potencies compared to CMVpp65‐stimulated PBMCs. Treatment of human polyclonally stimulated CD4+ T cells with ONX 0914 also enhanced secretion of LDH indicative of necrosis or secondary apoptosis induction. Despite the approximate 8‐fold potency shift of the ONX 0914‐induced IFN‐γ secretion block and the LDH release of CD4+‐stimulated T cells, the therapeutic window for ONX 0914 in suppressing T cell function without impairing cell viability is most likely limited. Indeed, LDH release is detectable upon membrane damage only and other tools detecting early variations in mitochondrial function or appearance of phosphatidylserine in the outer leaflet of the cell membrane should be considered.42 Similarly, human CD4+ T cells polyclonally activated with allogeneic dendritic cells or phytohemagglutinin undergo apoptosis in the presence of Bortezomib.39, 40 Nevertheless, for its application in inflammatory or autoimmune disorders it will be of great interest to study whether ONX 0914 or analogs differentially affect human T cell subpopulations, for example, effector vs regulatory T cells, as suggested in a rodent setting.43
Although ONX 0914 was studied in great detail,16, 19, 44 we are—to our knowledge—the first to demonstrate its cytotoxicity effects in human peripheral blood‐derived immune cells alongside its reduction of TLR‐ or TCR‐triggered pro‐inflammatory cytokine production. Our present study, however, comes with some limitations. First of all, PBMCs from healthy volunteers most likely have a mixture of intermediate or fully assembled immunoproteasomes in their subsets6 and despite making significant progress in detecting β5i activity in human cell lysates,45 no detailed characterization of the subpopulation has been performed so far. Given that cells upregulate immunoproteasomes upon receiving inflammatory signals, it would be worthwhile to investigate the immunoproteasome subtypes present in patient samples and study the cell death susceptibility in the presence of proteasome inhibitors compared to healthy donors. Second, although blood samples were immediately processed after venipuncture for analysis of early apoptosis markers, it is known that T cell and innate immune cell function is affected by blood isolation parameters such as separation method and medium.46, 47 Hence, it remains to be shown if cells show similar sensitivity for cytotoxicity upon pharmacological inhibition of β5i, in vivo.
Safety, pharmacokinetics, and pharmacodynamics of KZR‐616, an analog of ONX 0914, are currently being addressed in a phase I study in healthy volunteers.48 Some subjects within multiple ascending dose group receiving 60 mg KZR‐616 reported systemic drug reactions; however, the repeated dosing at 30 and 45 mg KZR‐616 were overall well‐tolerated. Reductions in cytokine release were detected in whole blood from subjects receiving repeated administrations of 45 mg KZR‐616 and stimulated ex vivo with LPS or phytohemagglutinin. Interestingly, LPS‐induced IL‐12/23p40 production was almost completely inhibited, whereas only partial inhibition of TNF release was observed in that study group compared to placebo.49 Similar observations were made with our continuous ONX 0914 drug exposure setup in LPS‐stimulated whole blood comparing IL‐23 and TNF secretion. Our study supports the notion that ONX 0914 as a selective β5i inhibitor is a potent immunosuppressive agent with great potential as a blood cancer or autoimmune therapeutic. High dose glucocorticoid treatment has been shown to deplete the nonclassical monocyte subpopulations from human peripheral blood which may contribute to the immunosuppression profile of glucocorticoid therapy.50 Dimethyl fumarate used for the treatment of relapsing‐remitting multiple sclerosis induced cell death in T cell populations in vivo, among several other mechanisms of actions proposed.51, 52 Based on our results, however, we propose to monitor monocyte and T cell subpopulations in clinical trial subjects receiving ONX 0914 or analogs to explore whether immune cell susceptibility to apoptosis contributes to the efficacy profile of immunoproteasome inhibitors in vivo.
Therapy with Bortezomib is associated with severe neurologic and hematologic adverse events such as thrombocytopenia and anemia in patients with relapsed or refractory multiple myeloma.53 Reduced platelet life span54 and increased B cell and CD14+ monocyte death29, 55 were reported after treatment of human cells isolated from fresh blood with Bortezomib. Typical clinical manifestations of PRAAS such as skin lesions and lipodystrophy, however, are not observed in multiple myeloma patients receiving treatment with Bortezomib or carfilzomib and dexamethasone as a co‐medication.53 Studies in HeLa cells have elegantly shown that at concentrations of Bortezomib leading to 75% inhibition of chymotrypsin‐like activity, which effectively induces apoptosis in sensitive myeloma cells,56 only small inhibition of total protein breakdown is reached.57 Furthermore, the number of surviving primary neurons was strongly affected in the presence of Bortezomib, whereas ONX 0914 did not affect neuronal survival at concentrations up to 0.1 μmol/L,58 a concentration which depletes human monocyte subsets from PBMC cultures as demonstrated in this study. Hence, different sensitivities of cells toward (immuno)proteasome inhibition probably influence the side effect profile observed with these inhibitors. More importantly, some mutations in PSMB8 described to induce PRAAS not only attenuate the chymotrypsin‐like activity of the immunoproteasome but also inhibit assembly or maturation of the immunoproteasome.59 Nevertheless, based on the enzymatic inhibitory profile, it is to be expected that ONX 0914 and analogs might inhibit different cell types in vivo41 leading to different toxicity profile as reported for pan‐proteasome inhibitor Bortezomib.
5. CONCLUSION
Here, we demonstrated that Bortezomib and ONX 0914 as a β5‐selective immunoproteasome inhibitor induced human immune cell death along with anti‐inflammatory efficacy in human PBMC cultures in vitro. Although our study provides mechanistic insights for these immunoproteasome inhibitors, the development of non‐covalent and/or reversible β5‐selective inhibitors as well as improved subunit‐selective immunoproteasome inhibitors will further help to elucidate the therapeutic potential of the immunoproteasome.
DISCLOSURE
Katrien Pletinckx, Silke Vaßen, Ilka Schlusche, Sonja Nordhoff, Gregor Bahrenberg, Torsten R. Dunkern are all employees of Grünenthal GmbH.
Supporting information
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Pletinckx K, Vaßen S, Schlusche I, Nordhoff S, Bahrenberg G, Dunkern TR. Inhibiting the immunoproteasome's β5i catalytic activity affects human peripheral blood‐derived immune cell viability. Pharmacol Res Perspect. 2019;e00482 10.1002/prp2.482
REFERENCES
- 1. Huber EM, Basler M, Schwab R, et al. Immuno‐ and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell. 2012;148:727‐738. [DOI] [PubMed] [Google Scholar]
- 2. Kisselev AF, van der Linden WA, Overkleeft HS. Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol. 2012;19:99‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aki M, Shimbara N, Takashina M, et al. Interferon‐gamma induces different subunit organizations and functional diversity of proteasomes. J Biochem. 1994;115:257‐269. [DOI] [PubMed] [Google Scholar]
- 4. Groettrup M, Kraft R, Kostka S, Standera S, Stohwasser R, Kloetzel PM. A third interferon‐gamma‐induced subunit exchange in the 20S proteasome. Eur J Immunol. 1996;26:863‐869. [DOI] [PubMed] [Google Scholar]
- 5. Yang Y, Waters JB, Fruh K, Peterson PA. Proteasomes are regulated by interferon gamma: implications for antigen processing. Proc Natl Acad Sci USA. 1992;89:4928‐4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guillaume B, Chapiro J, Stroobant V, et al. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc Natl Acad Sci USA. 2010;107:18599‐18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hensley SE, Zanker D, Dolan BP, et al. Unexpected role for the immunoproteasome subunit LMP2 in antiviral humoral and innate immune responses. J Immunol. 2010;184:4115‐4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murata S, Sasaki K, Kishimoto T, et al. Regulation of CD8+ T cell development by thymus‐specific proteasomes. Science (New York, NY). 2007;316:1349‐1353. [DOI] [PubMed] [Google Scholar]
- 9. Basler M, Kirk CJ, Groettrup M. The immunoproteasome in antigen processing and other immunological functions. Curr Opin Immunol. 2013;25:74‐80. [DOI] [PubMed] [Google Scholar]
- 10. Seifert U, Bialy LP, Ebstein F, et al. Immunoproteasomes preserve protein homeostasis upon interferon‐induced oxidative stress. Cell. 2010;142:613‐624. [DOI] [PubMed] [Google Scholar]
- 11. Huber EM, Groll M. Inhibitors for the immuno‐ and constitutive proteasome: current and future trends in drug development. Angew Chem Int Ed Engl. 2012;51:8708‐8720. [DOI] [PubMed] [Google Scholar]
- 12. Kisselev AF, Groettrup M. Subunit specific inhibitors of proteasomes and their potential for immunomodulation. Curr Opin Chem Biol. 2014;23:16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Bruin G, Xin BT, Kraus M, et al. A set of activity‐based probes to visualize human (immuno)proteasome activities. Angew Chem Int Ed Engl. 2016;55:4199‐4203. [DOI] [PubMed] [Google Scholar]
- 14. Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR‐171, a novel irreversible inhibitor of the proteasome. Can Res. 2007;67:6383‐6391. [DOI] [PubMed] [Google Scholar]
- 15. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib‐induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593‐1599. [DOI] [PubMed] [Google Scholar]
- 16. Muchamuel T, Basler M, Aujay MA, et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781‐787. [DOI] [PubMed] [Google Scholar]
- 17. Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol. 2010;185:634‐641. [DOI] [PubMed] [Google Scholar]
- 18. Basler M, Mundt S, Muchamuel T, et al. Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis. EMBO Mol Med. 2014;6:226‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ichikawa HT, Conley T, Muchamuel T, et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody‐secreting cells. Arthritis Rheum. 2012;64:493‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fehling HJ, Swat W, Laplace C, et al. MHC class I expression in mice lacking the proteasome subunit LMP‐7. Science (New York, NY). 1994;265:1234‐1237. [DOI] [PubMed] [Google Scholar]
- 21. Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10:73‐78. [DOI] [PubMed] [Google Scholar]
- 22. Arima K, Kinoshita A, Mishima H, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo‐Nishimura syndrome. Proc Natl Acad Sci USA. 2011;108:14914‐14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brehm A, Liu Y, Sheikh A, et al. Additive loss‐of‐function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest. 2015;125:4196‐4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitamura A, Maekawa Y, Uehara H, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121:4150‐4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Ramot Y, Torrelo A, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64:895‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basler M, Groettrup M. Immunoproteasome‐specific inhibitors and their application. Methods Mol Biol (Clifton, NJ). 2012;832:391‐401. [DOI] [PubMed] [Google Scholar]
- 27. Adams J. Proteasome inhibition in cancer: development of PS‐341. Semin Oncol. 2001;28:613‐619. [DOI] [PubMed] [Google Scholar]
- 28. Bull M, Lee D, Stucky J, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen‐specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arpinati M, Chirumbolo G, Nicolini B, Agostinelli C, Rondelli D. Selective apoptosis of monocytes and monocyte‐derived DCs induced by bortezomib (Velcade). Bone Marrow Transplant. 2009;43:253‐259. [DOI] [PubMed] [Google Scholar]
- 30. Leveque D, Carvalho MC, Maloisel F. Review: clinical pharmacokinetics of bortezomib. In Vivo. 2007;21:273‐278. [PubMed] [Google Scholar]
- 31. Basler M, Maurits E, de Bruin G, Koerner J, Overkleeft HS, Groettrup M. Amelioration of autoimmunity with an inhibitor selectively targeting all active centres of the immunoproteasome. Br J Pharmacol. 2018;175:38‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao C, Tan YC, Wong WC, et al. The CD14(+/low)CD16(+) monocyte subset is more susceptible to spontaneous and oxidant‐induced apoptosis than the CD14(+)CD16(‐) subset. Cell Death Dis. 2010;1:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manuzak JA, Dillon SM, Lee EJ, Dong ZM, Hecht DK, Wilson CC. Increased Escherichia coli‐induced interleukin‐23 production by CD16+ monocytes correlates with systemic immune activation in untreated HIV‐1‐infected individuals. J Virol. 2013;87:13252‐13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bitzer A, Basler M, Krappmann D, Groettrup M. Immunoproteasome subunit deficiency has no influence on the canonical pathway of NF‐kappaB activation. Mol Immunol. 2017;83:147‐153. [DOI] [PubMed] [Google Scholar]
- 35. Goriely S, Molle C, Nguyen M, et al. Interferon regulatory factor 3 is involved in toll‐like receptor 4 (TLR4)‐ and TLR3‐induced IL‐12p35 gene activation. Blood. 2006;107:1078‐1084. [DOI] [PubMed] [Google Scholar]
- 36. Zhong B, Liu X, Wang X, et al. Ubiquitin‐specific protease 25 regulates TLR4‐dependent innate immune responses through deubiquitination of the adaptor protein TRAF3. Sci Signal. 2013;6:ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith S, Gabhann JN, Higgs R, et al. Enhanced interferon regulatory factor 3 binding to the interleukin‐23p19 promoter correlates with enhanced interleukin‐23 expression in systemic lupus erythematosus. Arthritis Rheum. 2012;64:1601‐1609. [DOI] [PubMed] [Google Scholar]
- 38. Weighardt H, Jusek G, Mages J, et al. Identification of a TLR4‐ and TRIF‐dependent activation program of dendritic cells. Eur J Immunol. 2004;34:558‐564. [DOI] [PubMed] [Google Scholar]
- 39. Berges C, Haberstock H, Fuchs D, et al. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology. 2008;124:234‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blanco B, Perez‐Simon JA, Sanchez‐Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575‐3583. [DOI] [PubMed] [Google Scholar]
- 41. Mundt S, Basler M, Buerger S, Engler H, Groettrup M. Inhibiting the immunoproteasome exacerbates the pathogenesis of systemic Candida albicans infection in mice. Sci Rep. 2016;6:19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim H, Yoon SC, Lee TY, Jeong D. Discriminative cytotoxicity assessment based on various cellular damages. Toxicol Lett. 2009;184:13‐17. [DOI] [PubMed] [Google Scholar]
- 43. Kalim KW, Basler M, Kirk CJ, Groettrup M. Immunoproteasome subunit LMP7 deficiency and inhibition suppresses Th1 and Th17 but enhances regulatory T cell differentiation. J Immunol. 2012;189:4182‐4193. [DOI] [PubMed] [Google Scholar]
- 44. Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. A comparative analysis between proteasome and immunoproteasome inhibition in cellular and humoral alloimmunity. Int Immunopharmacol. 2017;50:48‐54. [DOI] [PubMed] [Google Scholar]
- 45. Winter MB, La Greca F, Arastu‐Kapur S, et al. Immunoproteasome functions explained by divergence in cleavage specificity and regulation. eLife 2017;6:e27364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mallone R, Mannering SI, Brooks‐Worrell BM, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen‐reactive T cell responses: position statement of the T‐Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163:33‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meier A, Fisher A, Sidhu HK, et al. Rapid loss of dendritic cell and monocyte responses to TLR ligands following venipuncture. J Immunol Methods. 2008;339:132‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lickliter J, Anderl J, Kirk C, Wang J, Bomba D. KZR‐616, a selective inhibitor of the immunoproteasome, shows a promising safety and target inhibition profile in a phase I, double‐blind, single (SAD) and multiple ascending dose (MAD) study in healthy volunteers [abstract]. Arthritis Rheumatol. 2017;69(suppl 10). [Google Scholar]
- 49. Lickliter J, Bomba D, Anderl J, Fan A, Kirk CJ, Wang J. AB0509 Kzr‐616, a selective inhibitor of the immunoproteasome, shows a promising safety and target inhibition profile in a phase i, double‐blind, single (SAD) and multiple ascending dose (MAD) study in healthy volunteers. Ann Rheum Dis. 2018;77:1413‐1414.29980576 [Google Scholar]
- 50. Fingerle‐Rowson G, Angstwurm M, Andreesen R, Ziegler‐Heitbrock HW. Selective depletion of CD14+ CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol. 1998;112:501‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diebold M, Sievers C, Bantug G, et al. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J Autoimmun. 2018;86:39‐50. [DOI] [PubMed] [Google Scholar]
- 52. Ghadiri M, Rezk A, Li R, et al. Dimethyl fumarate‐induced lymphopenia in MS due to differential T‐cell subset apoptosis. Neurol Neuroimmunol Neuroinflammat 2017;4:e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open‐label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327‐1337. [DOI] [PubMed] [Google Scholar]
- 54. Nayak MK, Kulkarni PP, Dash D. Regulatory role of proteasome in determination of platelet life span. J Biol Chem. 2013;288:6826‐6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mulder A, Heidt S, Vergunst M, Roelen DL, Claas FH. Proteasome inhibition profoundly affects activated human B cells. Transplantation. 2013;95:1331‐1337. [DOI] [PubMed] [Google Scholar]
- 56. Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS‐341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Can Res. 2001;61:3071‐3076. [PubMed] [Google Scholar]
- 57. Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582‐8590. [DOI] [PubMed] [Google Scholar]
- 58. von Brzezinski L, Saring P, Landgraf P, Cammann C, Seifert U, Dieterich DC. Low neurotoxicity of ONX‐0914 supports the idea of specific immunoproteasome inhibition as a side‐effect‐limiting, therapeutic strategy. Eur J Microbiol Immunol. 2017;7:234‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Torrelo A. CANDLE syndrome as a paradigm of proteasome‐related autoinflammation. Front Immunol. 2017;8:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


