Introduction
Psoriasis is a chronic inflammatory T cell–mediated condition that affects approximately 1% of children. Of the risk factors, obesity is associated with higher frequency and severity of disease.1 Although most children have mild to moderate disease and generally respond well to topical treatment or phototherapy, those with severe disease require systemic treatment. Until recently in the United States, etanercept was the only approved biologic agent for pediatric patients aged 4 years and older with moderate to severe psoriasis; however, data from adults suggests that it is less efficacious than the other available biologic medications. In Europe, adalimumab is also approved for psoriasis for patients aged 4 years and older.
Ustekinumab was recently approved for the treatment of psoriasis in pediatric patients aged 12 and older, providing another on-label therapeutic option for adolescents.2, 3 In refractory cases, however, alternative treatment options often need to be pursued. Here we present a case of severe, recalcitrant psoriasis in a child that responded to guselkumab.
Case report
A 12-year-old obese Hispanic girl presented with a 6-month history of plaque psoriasis. Family history was notable for psoriasis in her maternal grandmother. Prior treatment with medium- and high-potency topical steroids had yielded only minimal improvement. Phototherapy using narrow-band ultraviolet B had been attempted but was discontinued due to difficulty attending treatment sessions.
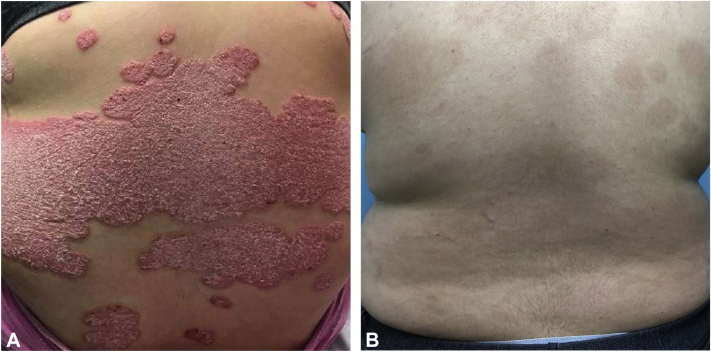
Physical examination revealed diffuse pink-red scaly plaques involving the scalp, forehead, trunk, buttocks, and upper and lower extremities involving >50% body surface area, with a Psoriasis Area Severity Index score of 31 (Fig 1, A). She had pitting of multiple fingernails but no dactylitis or joint swelling.
Fig 1.
Response to guselkumab. A, Back with sharply demarcated pink-red scaly plaques, which were persistent despite treatment with methotrexate, adalimumab, and ustekinumab. B, Back with postinflammatory hyperpigmentation. There has been near complete resolution of psoriasis after 5 months of treatment with guselkumab.
Given the extensive body surface area involvement, methotrexate, 15 mg (0.4 mg/kg) weekly, was initiated. After 4 months of treatment, there was minimal response, so she was switched to adalimumab for 10 weeks, followed by ustekinumab for 16 weeks, both without improvement. At this point, she transitioned back to methotrexate (20 mg weekly; dose of 0.3 mg/kg given interval weight gain) as other biologic agents were considered.
Among the remaining available options, we considered initiating an interleukin 17 (IL-17) inhibitor; however, there presently are no data regarding their use in children. Although guselkumab, an IL-23 inhibitor, also does not have a pediatric indication, its mechanism is similar to that of ustekinumab, which targets the shared p40 subunit of IL-12 and IL-23 and is approved for use in patients 12 and older.2, 3
Even though the patient had not improved on ustekinumab, we were optimistic that guselkumab would be more effective because a variety of evidence suggests that IL-23 is a more important mediator in psoriasis than is IL-12.4, 5, 6, 7 Furthermore, IL-12 has been shown to provide protective effects against inflammation, suggesting that targeting both IL-12 and IL-23 may actually be counterproductive.8
Given its specificity for IL-23, in conjunction with data demonstrating superior efficacy of guselkumab over ustekinumab and adalimumab in adults,9, 10 guselkumab (100 mg) was added to methotrexate. Results of initial screening laboratory analysis, including complete blood cell count, comprehensive metabolic panel, hepatitis B and C and HIV serologies, and QuantiFERON-TB Gold (QIAGEN, Germantown, MD) were all within normal reference ranges.
After loading doses at week 0 and week 4, the patient received guselkumab (100 mg subcutaneously) every 8 weeks. As early as week 4, the plaques had already started to thin. Given the dramatic improvement at 8 weeks, methotrexate was decreased to 10 mg (0.16 mg/kg) weekly. At 5 months, most plaques had resolved with postinflammatory hyperpigmentation (Fig 1, B) and her Psoriasis Area Severity Index score was reduced to 1. Methotrexate is currently being tapered. The patient did not experience any adverse effects or laboratory abnormalities during treatment.
Discussion
Advances in understanding of the pathogenesis of psoriasis have led to development of a variety of targeted therapies with improved efficacy, ushering in an era in which it is possible for most patients to achieve complete or nearly complete clearance with treatment. Anecdotally, when treating psoriasis in adults, most dermatologists are comfortable moving freely from one biologic agent to the next; however, in the pediatric age group we are typically (and justifiably) more cautious and tend to follow a more traditional therapeutic ladder, first using treatments approved for use in children or those with a longer history of use.
In cases of particularly severe and recalcitrant disease, however, it may be necessary to move beyond these more widely accepted therapies to achieve results. Although it was a newer agent compared with other available options, such as IL-17 inhibitors, the rationale for choosing guselkumab in this case was not only its efficacy data but that it has a similar mechanism to ustekinumab, which is approved for use in the pediatric population. In addition, guselkumab is generally well tolerated, with infections being the most common type of adverse events, with incident rates similar to those associated with and adalimumab and ustekinumab.9, 10 Although larger studies are needed to explore the safety and efficacy of guselkumab in pediatric patients, the marked improvement seen in our patient with refractory psoriasis suggests that it may be a promising therapeutic option for this population.
Footnotes
Funding sources: none.
Conflicts of interest: Dr Craiglow has received honoraria and/or fees from Aclaris, LEO Pharma, Pfizer, Regeneron and Sanofi-Genzyme, and her spouse has received honoraria and/or fees from Regeneron, Sanofi-Genzyme, Concert Pharmaceuticals, Eli Lily, Pfizer, and Aclaris. Sa Rang Kim and Dr Kibbi have no conflicts of interest to declare.
References
- 1.Koebnick C., Black M.H., Smith N. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577–583. doi: 10.1016/j.jpeds.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Koebnick C., Black M.H., Smith N. et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011; 159:577-583 [DOI] [PMC free article] [PubMed]
- 2.Papp K.A., Langley R.G., Lebwohl M. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis:52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]; Papp, K.A., Langley, R.G., Lebwohl, M. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis:52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008; 371:1675-1684. [DOI] [PubMed]
- 3.Landells I., Marano C., Hsu M.C. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594–603. doi: 10.1016/j.jaad.2015.07.002. [DOI] [PubMed] [Google Scholar]; Landells, I., Marano, C., Hsu, M.C. et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015; 73:594-603. [DOI] [PubMed]
- 4.Lee E., Trepicchio W.L., Oestreicher J.L. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, E., Trepicchio, W.L., Oestreicher, J.L. et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004; 199:125-130 [DOI] [PMC free article] [PubMed]
- 5.Nair R.P., Ruether A., Stuart P.E. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nair, R.P., Ruether, A., Stuart, P.E. et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008; 128:1653-1661. [DOI] [PMC free article] [PubMed]
- 6.Nakajima K., Kanda T., Takaishi M. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011;186:4481–4489. doi: 10.4049/jimmunol.1000148. [DOI] [PubMed] [Google Scholar]; Nakajima, K., Kanda, T., Takaishi M, et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011; 186:4481-4489. [DOI] [PubMed]
- 7.Levin A.A., Gottlieb A.B. Specific targeting of interleukin-23p19 as effective treatment for psoriasis. J Am Acad Dermatol. 2014;70:555–561. doi: 10.1016/j.jaad.2013.10.043. [DOI] [PubMed] [Google Scholar]; Levin, A.A., Gottlieb, A.B. Specific targeting of interleukin-23p19 as effective treatment for psoriasis. J Am Acad Dermatol. 2014; 70:555-561 [DOI] [PubMed]
- 8.Kulig P., Musiol S., Freiberger S.N. IL-12 protects from psoriasiform skin inflammation. Nat Commun. 2016;7:13466. doi: 10.1038/ncomms13466. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kulig, P., Musiol, S., Freiberger, S.N. et al. IL-12 protects from psoriasiform skin inflammation. Nat Commun. 2016; 7:13466 [DOI] [PMC free article] [PubMed]
- 9.Langley R.G., Tsai T.F., Flavin S. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114–123. doi: 10.1111/bjd.15750. [DOI] [PubMed] [Google Scholar]; Langley, R.G., Tsai, T.F., Flavin, S. et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018; 178:114-123. [DOI] [PubMed]
- 10.Blauvelt A., Papp K.A., Griffiths C.E. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo-and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]; Blauvelt, A., Papp, K.A., Griffiths, C.E. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo-and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017; 76:405-417. [DOI] [PubMed]