Abstract
Purpose
Alexithymia, defined as the inability of a person to identify, describe and express emotions, has been found to influence glycemic control in type 2 diabetes patients (D2). The characteristics and influencing factors of alexithymia and the association of this psychological construct with D2 has not yet been studied in Lebanon where 14.6% of adults are diagnosed with the disease. This study aims at evaluating the prevalence of alexithymia and its relationship with glycemic control among Lebanese adults with D2.
Methods
Alexithymia was assessed in 104 patients diagnosed with D2 and 100 healthy controls using the 20-item Toronto Alexithymia Scale (TAS-20). The impact of alexithymia on glycemic control was evaluated using HbA1c values, fasting blood glucose levels, number of severe hyperglycemic episodes and hospitalizations for hyperglycemia within the past months.
Results
Alexithymia prevalence was significantly higher in D2 patients compared to controls (35.5% vs 15%). Patients with alexithymia showed higher levels of HbA1c and glucose in comparison to those without alexithymia. Consistently, significant positive correlations were found between the TAS-20 total and subscale scores and both HbA1c and glucose levels. Alexithymic patients had three times more severe hyperglycemic episodes and five times more hospitalizations for hyperglycemia compared to those without alexithymia. According to multivariate regression analysis, lifestyle factors alone were not found predictive of alexithymia in D2 patients.
Conclusion
Given the impact of alexithymia on D2 regulation, screening of alexithymia in case of D2 and appropriate psychological follow-up are important for a better prognosis, management and treatment of the disease.
Keywords: Alexithymia, Type 2 diabetes, Prevalence, Glycemic control, Lebanon
Background
Diabetes consists of a chronic metabolic and endocrine disorder characterized by a disturbance in glycemic control that may lead to microvascular and macrovascular complications in the absence of appropriate treatment. This disease is associated with an increased risk of mortality and morbidities, and thus constitutes a public health problem [1]. Type 2 diabetes (D2) is the most frequent type occurring particularly in adults older than 45 years of age [2]. In Lebanon, 585,400 cases of diabetes were reported in 2017, hence representing a rate of 14.6% among adults [3]. Overall, different lifestyle factors have been linked to an improved glycemic control among diabetic individuals including dietary modifications, reduction in alcohol and tobacco consumption, physical exercise and weight loss [4].
In regard to the psychological aspects of the regulation of glycemia, depression and stress were mainly investigated with a subsequent improvement in glycemia upon management of these conditions [5]. Alexithymia as a psychological construct related to difficulties in emotional skills has also been studied in this context [6]. It entails three dimensions: difficulty identifying feelings (DIF), difficulty describing feelings (DDF) and externally oriented thinking (EOT). This trait seems to impact the evolution of the disease through psychological, behavioral and biological effects such as adaptation capabilities or even the release of stress hormones [7, 8].
A recent study conducted by Ahmadieh et al. in 2018 has shown that depression was prevalent among 28.8% of Lebanese patients with diabetes mellitus, but without establishing any significant association between depression and glycemic control [9]. Alexithymia on the other hand has not yet been studied in Lebanon. Therefore, our research aims at exploring the relation between emotional skills as represented by alexithymia and glycemic control in terms of fasting glycemia, HbA1c, episodes of severe hyperglycemia, and hospitalizations for hyperglycemia in Lebanese adults presenting with D2. In this regard, the objectives of the study were to: (1) evaluate the prevalence of alexithymia in patients with D2 as well as in a group of healthy subjects constituting the control group; (2) specify the three dimensions of alexithymia and their association with glycemic regulation in D2, and (3) identify the relationship between lifestyle factors and alexithymia in diabetes control. Consequently, our work raises targeted recommendations in relation to emotional skills among subjects with type 2 diabetes and emotional difficulties.
Methods
Study design and population
The study was carried out between December 2017 and February 2018 in a hospital-based laboratory located in the Metn region of Lebanon. The research involved Lebanese outpatients older than 18 years, diagnosed by a specialist physician with D2, and admitted to the laboratory for routine blood testing. A total of 118 patients fulfilling the inclusion criteria were randomly selected on a daily basis over the entire period of the research. Patients excluded from the study were those diagnosed with type 1 diabetes mellitus or presenting any psychiatric or cognitive disorder based on their medical and psychiatric history as well as medication intake. Subjects having a visual impairment to such a degree that filling the questionnaire was impossible, and those who did not understand French or English were also excluded from the study.
The research also included a group of control matched by age and sex involving 100 healthy subjects admitted to the laboratory for blood analysis. Eligible candidates had no established diagnosis of diabetes or any other chronic disease based on medical history as well as levels of glucose and HbA1c. After explaining the objectives and methods of the research to the candidates, a written informed consent was obtained prior to enrollment from all participants. Fourteen patients refused to take part in the research and were subsequently excluded from the study.
Questionnaire
A structured questionnaire including 19 items was filled to gather relevant information regarding socio-demographic data, family and personal medical history, dietary intake, and treatment and disease characteristics information. The latter included the age of diagnosis of D2, the frequency of glucose measurements, and the number of severe hyperglycemic episodes in the previous three months in relation to a significantly elevated hyperglycemic state involving signs such as dehydration, nausea and vomiting. Further assessment covered the number of hospitalizations for hyperglycemia during the past year, and questions about investigations for complications of D2 using sensitive methods such as tests evaluating blood pressure, renal function and funduscopic examination of the retina [10].
TAS-20
Alexithymia was measured by the 20-item Toronto Alexithymia Scale (TAS-20) developed by Bagby et al. [11]. The scale defined as a person’s inability to identify and describe his/her emotions consists of 20 statements rated from 1 to 5. It is divided into three subscales that assess three dimensions: Difficulty identifying feelings (DIF) subscale, difficulty describing feelings (DDF) subscale, and externally-oriented thinking (EOT) subscale. The first two match the emotional component of alexithymia whereas the third one is more linked to the cognitive component. Scores are calculated for each subscale, and the sum of scores is used as the total score which can range from 20 to 100. The cutoff point was determined as 61, and subjects whose TAS-20 score ≥ 61 were considered as presenting alexithymia. The reliability and validity of scores on the TAS-20 has been established among adults [12]. TAS-20 was proposed in both the English and French versions to participants [11, 13].
Blood analyses
Glycemia and HbA1c levels were assessed in patients and controls at recruitment day.
Glycemia
Fasting blood glucose levels were assessed by the automated analyzer COBAS INTEGRA 400 plus using the hexokinase/Glucose-6-Phosphate Dehydrogenase method [14].
HbA1c
Venous blood was collected in EDTA tubes to analyze HbA1c levels. The percentage of HbA1c in whole blood was determined by the cassette COBAS INTEGRA Hemoglobin A1c kit intended for use on COBAS INTEGRA systems according to the manufacturer’s instructions. Diabetes was considered balanced if HbA1c value was less than <7% according to the American Diabetes Association’s diagnostic criteria for D2 (2015) [15].
Statistical analysis
Study data were analyzed using the GraphPad Prism software version 6 (GraphPad Software, Inc., USA). Means and standard deviations for quantitative variables were calculated. The unpaired t test with Welch’s correction was used to compare means between two groups. The Pearson correlation test was used for correlations between alexithymia scores and HbA1c or glycemia levels. The search for variables associated with diabetes control was performed by Fisher’s exact test comparing frequencies between groups. In order to identify factors independently associated with alexithymia, a multivariate analysis using binary logistic regression was performed. Alexithymia was considered as a dependent variable, while sex, dietary intake, physical activity, tobacco and alcohol consumption, HbA1c levels, and glycemic control features as independent variables. Prior to the binary logistic regression analyses, a test of significance (omnibus test) and a goodness-of-fit test (Hosmer–Lemeshow test) were applied for the model used. P values <0.05 were considered statistically significant.
Results
Socio-demographic and clinical characteristics of D2 patients
This study included 104 patients between 27 and 90 years of age diagnosed with D2 and of whom 60.6% were males and 62.5% married. Patients had a mean age of 59.4 years ±14.2 and a body mass index (BMI) of 27.0 kg/m2 ± 3.8 on average.
The mean duration of illness since diagnosis was 10.3 years ±8.7. D2 patients exhibited a vast range of comorbidities including hypertension (47.2%), cardiovascular disease (26.0%), hypercholesterolemia (26.0%), and hypertriglyceridemia (12.5%). Moreover, 97.1% of patients had been on treatment for diabetes, 13.5% had a diabetes family history, 68.6% were following a specific dietary regimen adapted to the disease, 83.7% were followed regularly by a specialist physician, and 64.4% were getting routine laboratory and medical tests and checkups. Finally, 50.9% of patients were consuming alcohol while 43.3% of them were smokers.
Prevalence of alexithymia
Our results showed significantly higher scores in the TAS-20 scale as well as in the DDF and EOT components in D2 patients when compared to controls (Table 1). Accordingly, the prevalence of alexithymia in D2 patients was 35.5%, higher than that detected in the control group (15%) (p = 0.0093).
Table 1.
TAS-20 total and subscale scores in D2 patients and healthy controls
| D2 patients | Healthy controls | P value | |
|---|---|---|---|
| TAS-20 | 52.2 ± 16.3 | 45.7 ± 13.8 | 0.0085 (**) |
| DIF | 17.3 ± 7.2 | 16.9 ± 6.6 | 0.0651 (ns) |
| DDF | 13.6 ± 5.1 | 10.7 ± 3.5 | 0.0123 (*) |
| EOT | 21.3 ± 9.8 | 18.1 ± 8.3 | 0.0075 (**) |
TAS-20 20-item Toronto Alexithymia Scale, DIF Difficulty Identifying Feelings, DDF Difficulty Describing Feelings, EOT Externally-Oriented Thinking. Data are represented as mean ± SD. The unpaired t test with Welch’s correction was used to analyze differences between D2 patients and healthy controls
Ns: non-significant; *: p < 0.05. **: p < 0.01
Alexithymia characteristics of D2 patients
The mean TAS-20 total and subscale scores, namely DIF, DDF and EOT, were significantly higher in male patients with D2 in comparison to females (Table 2). Furthermore, results showed differences in the frequency distribution between the two sexes, with male participants being more likely to present alexithymia than females (46.0% vs 19.5%). In contrast, no significant difference was noted between D2 patients with alexithymia and those without alexithymia with regard to age and BMI (Table 3).
Table 2.
TAS-20 total and subscale scores in males and females
| Males | Females | P value | |
|---|---|---|---|
| TAS-20 | 56.2 ± 18.2 | 46.4 ± 15.1 | 0.0035 (**) |
| DIF | 19.3 ± 7.8 | 15.3 ± 5.6 | 0.0034 (**) |
| DDF | 14.5 ± 5.3 | 12.3 ± 4.5 | 0.0255 (*) |
| EOT | 22.4 ± 6.8 | 18.8 ± 6.5 | 0.0082 (**) |
TAS-20 20-item Toronto Alexithymia Scale, DIF Difficulty Identifying Feelings, DDF Difficulty Describing Feelings, EOT Externally-Oriented Thinking. Data are represented as mean ± SD. The unpaired t test with Welch’s correction was used to analyze differences between males and females
*: p < 0.05. **: p < 0.01
Table 3.
Sex, age and BMI distribution in patients with alexithymia and those without alexithymia
| Patients with alexithymia | Patients without alexithymia | P value | |
|---|---|---|---|
| Sex (%) | |||
| - Males | 46.0 | 54.0 | 0.0066 (**) |
| - Females | 19.5 | 80.5 | |
| Age (mean ± SD) | |||
| - Males | 66.0 ± 15.5 | 60.7 ± 14.7 | 0.1722 (ns) |
| - Females | 57.6 ± 12.2 | 52.5 ± 9.6 | 0.2958 (ns) |
| BMI (mean ± SD) | |||
| - Males | 28.4 ± 4.7 | 28.3 ± 2.3 | 0.9377 (ns) |
| - Females | 25.9 ± 2.3 | 24.8 ± 3.5 | 0.2941 (ns) |
Differences between patients with alexithymia and those without alexithymia with regard to sex were evaluated using Fisher’s exact test. The unpaired t test with Welch’s correction was used to analyze differences of age and BMI between the two groups
**: p < 0.01. Ns non-significant
Alexithymia and glycemic control in D2 patients
The interval of time since the diagnosis of D2 was comparable between patients with alexithymia (12.1 years ±7.6) and those without alexithymia (9.3 years ±3.1) (p = 0.11).
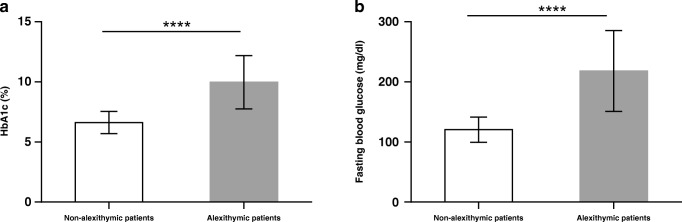
The mean levels of HbA1c and fasting blood glucose in our sample were respectively 7.8% ± 2.2 and 155.2 mg/dL ± 63.8. Interestingly, patients with alexithymia showed significantly higher levels of HbA1c (9.9% ± 2.2) and glucose (218.1 mg/dL ± 67.4) when compared to those without alexithymia (HbA1c: 6.6% ± 0.9; glucose: 120.5 mg/dL ± 20.9) (p < 0.0001) (Fig. 1).
Fig. 1.
HbA1c and fasting blood glucose levels in non-alexithymic and alexithymic patients. HbA1c and fasting blood glucose are important indicators to assess glycemic control in diabetic patients. Diabetes is considered balanced if HbA1c value is less than <7%. Data are reported as mean ± SD. ****p < 0.0001 based on Welch’s t-test for differences between groups
These results were further supported by the strong positive significant correlations of the TAS-20, DIF and DDF scores with HbA1c values, on one hand, and fasting blood glucose levels, on the other hand, as well as the moderate positive significant correlation between the EOT score and both glycemic control indicators (Table 4). In other terms, patients having greater difficulty in expressing emotions verbally tend to have higher HbA1c and blood glucose levels, and subsequently a less controlled glycemia.
Table 4.
Correlation of TAS-20 total and subscale scores with HbA1c and fasting blood glucose levels
| HbA1c | Fasting blood glucose | |||
|---|---|---|---|---|
| R | P | R | P | |
| Alexithymia scores | ||||
| - TAS-20 | 0.75 | <0.0001 | 0.77 | <0.0001 |
| - DIF | 0.72 | <0.0001 | 0.72 | <0.0001 |
| - DDF | 0.73 | <0.0001 | 0.73 | <0.0001 |
| - EOT | 0.63 | <0.0001 | 0.67 | <0.0001 |
TAS-20 20-item Toronto Alexithymia Scale, DIF Difficulty Identifying Feelings, DDF Difficulty Describing Feelings, EOT Externally-Oriented Thinking
R correlation coefficients and p values are calculated by Pearson correlation test
In a second attempt, we explored the possible association between alexithymia and the number of severe hyperglycemic episodes in the previous three months as well the number of hospitalizations for hyperglycemia during the past year. In agreement with previous data, patients having difficulties in emotional skills as represented by alexithymia were three times more susceptible to have severe hyperglycemic episodes in the previous three months compared to the non-alexithymic group (75% vs 25%) (p < 0.0001). Moreover, the frequency of hospitalizations for hyperglycemia during the past year was approximately five times more important in patients with alexithymia than in those without alexithymia (82.9% vs 17.1%) (p < 0.0001).
Lifestyle factors and alexithymia in D2 patients
The relationship between lifestyle factors and alexithymia in D2 patients is represented in Table 5. Indeed, in the group of D2 individuals having alexithymia, results showed higher frequencies of alcohol consumption (p = 0.0002), lower rates of physical activity (p = 0.032) and fewer self-reports of adequate dietary intake related to diabetes (0.0031) in comparison to patients without alexithymia. In contrast, no significant association was observed between tobacco consumption and alexithymia among D2 patients.
Table 5.
Alexithymia status in D2 patients in terms of lifestyle factors
| Glycemic control characteristics | Patients with alexithymia N (%) | Patients without alexithymia N (%) | P value | |
|---|---|---|---|---|
| Adequate dietary intake | Yes | 15 (40.5) | 48 (71.6) | 0.0031 (**) |
| No | 22 (59.5) | 19 (28.4) | ||
| Physical activity | Yes | 10 (27.0) | 34 (50.7) | 0.0320 (*) |
| No | 27 (73.0) | 33 (49.3) | ||
| Tobacco consumption | Yes | 21 (56.8) | 23 (34.3) | 0.0621 (ns) |
| No | 16 (43.2) | 44 (65.7) | ||
| Alcohol consumption | Yes | 28 (75.7) | 25 (37.3) | 0.0002 (***) |
| No | 9 (24.3) | 42 (62.7) | ||
N number of individuals
Ns non-significant; *p < 0.05; *p < 0.01; ***p < 0.001 based on Fisher’s exact test for differences between patients with alexithymia and those without alexithymia
Alexithymia predictive factors in D2 patients
Factors found to be significantly associated with alexithymia through the univariate analysis were studied in a multivariate model to calculate adjusted odds ratios measuring the specific role of each factor. D2 patients having HbA1c levels >7%, experiencing severe hyperglycemic episodes in the previous three months, or hospitalized for hyperglycemia during the previous 12 months were more likely to have alexithymia in comparison to their counterparts (OR = 2.1; p = 0.008; OR = 1.8; p = 0.027 and OR = 1.5; p = 0.018 respectively). In contrast, lifestyle factors alone were not found predictive of alexithymia in D2 patients (p > 0.05) (Table 6).
Table 6.
Multivariate analysis of alexithymia predictive factors in D2 patients
| Variables | β | SE | P value | OR (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| - Male | 1.00 | |||
| - Female | 0.323 | 0.308 | 0.254 | 0.69 (0.37–1.29) |
| Dietary intake | ||||
| - No | 1.00 | |||
| - Yes | 0.248 | 0.315 | 0.416 | 0.87 (0.45–1.81) |
| Physical activity | ||||
| - No | 1.00 | |||
| - Yes | 0.458 | 0.284 | 0.145 | 0.65 (0.34–2.15) |
| Tobacco consumption | ||||
| - No | 1.00 | |||
| - Yes | 0.147 | 0.289 | 0.613 | 0.98 (0.59–2.05) |
| Alcohol consumption | ||||
| - No | 1.00 | |||
| - Yes | 0.660 | 0.392 | 0.107 | 1.48 (0.71–2.37) |
| HbA1c level (%) | ||||
| - < 7 | 1.00 | |||
| - > 7 | 0.745 | 0.281 | 0.009 | 2.08 (1.20–3.61) |
| Severe hyperglycemic episodes in the previous three months | ||||
| - No | 1.00 | |||
| - Yes | 1.256 | 0.274 | 0.005 | 2.67 (1.86–4.12) |
| Hospitalizations for hyperglycemia during the previous 12 months | ||||
| - No | 1.00 | |||
| - Yes | 1.221 | 0.376 | 0.007 | 2.18 (1.64–3.18) |
Omnibus test: 0.000; Hosmer–Lemeshow test: 0.58; Nagelkerke R2: 0.772
β: beta coefficient; SE: standard error; CI: confidence interval; OR: odds ratio
Discussion
Our research explores the relationship between alexithymia in its three dimensions and D2 mainly through investigating biochemical markers and decompensation episodes reflecting glycemic control and evolution of the disease in a sample of Lebanese subjects with D2. It also contributes to assessing lifestyle variables such as dietary intake, physical activity and alcohol consumption and their relation to alexithymia in the context of D2.
The prevalence of alexithymia found in our study among D2 subjects (35.5%) was comparable to that reported in a Turkish study published in 2016 registering a rate of 37.7% of alexithymia in 326 patients diagnosed with D2 [16]. In Tunisian studies from 2010 and 2014 the corresponding prevalence was respectively 24% and 45% among participants with D2 [17, 18]. The differences in prevalence in comparison to our research may be due to variations of the characteristics of the recruited population with a majority of female participants enrolled in the Tunisian studies. Moreover, a Turkish research from 2006 using TAS-26 (Toronto Alexithymia Scale- 26, 26 items) to evaluate alexithymia among 193 patients with diabetes demonstrated a prevalence of 65% [7]. Indeed, alexithymia prevalence varies across researches and may reach up to 75% depending on study designs and measurement tools, as well as socio-demographic characteristics, geographic determinants and cultural traits of participants [7, 19, 20].
Nevertheless, according to most studies, the prevalence of alexithymia among subjects with D2 appears to be generally higher than that in non-diabetic subjects [7, 19]. Our results support these findings since a significant difference in the prevalence of alexithymia was observed when comparing participants with D2 to controls (35.5% vs 15%, p = 0.0093). Additionally, individuals with D2 scored higher than controls on the total TAS-20 score, specifically on the DDF and EOT subscales (Table 1). These results are concordant with those reported by Topsever et al. in 2006 who observed a significantly more elevated prevalence of alexithymia in a Turkish diabetic sample in comparison to a control group [7]. Similarly, a German research published in 2018 demonstrated higher alexithymia characteristics in diabetic patients than in healthy subjects [21]. These results raise the importance of considering this psychological construct and its impact among this specific population.
Concerning alexithymia characteristics among D2 subjects, higher scores of DIF, DDF and EOT were observed in males compared to females (Table 2). In addition, male participants were found to be more likely to present alexithymia features than females (Table 3). Data related to the association between gender and alexithymia features are variable and sometimes contradictory [7, 18, 20]. However, our results are consistent with Levant et al.’s (2006) hypothesis suggesting that men tend to score higher than women on average, which may be explained by the pattern of restrictive emotionality in men due to socio-cultural influencing factors [22].
As for the relationship between difficulties in analyzing or verbalizing emotions and D2 regulation, significantly higher levels of HbA1c and fasting blood glucose were obtained in D2 patients with alexithymia in comparison to those without alexithymia (Fig. 1). Furthermore, our research demonstrated significant positive correlations between all scores of alexithymia and both HbA1c and fasting blood glucose levels (Table 4). A multivariate regression analysis corroborated these results. Consistently, Topsever et al. demonstrated a positive association between poor postprandial glycemic control and alexithymia scores in individuals with diabetes [7]. Luca et al. also evaluated the relationship between HbA1c and alexithymia through a logistic regression, thus finding a significant association between the two variables [6], while Avci & Kelleci reported more alexithymic features among subjects with D2 with elevated HbA1c (HbA1c >7.0%) [16].
Interestingly, in our research, D2 participants with alexithymia also experienced more episodes of severe hyperglycemia in the previous three months and a higher number of hospitalizations due to hyperglycemia during the past year. Hence, our findings emphasize on quality of life related to D2 control and evolution, and on the degree of impact of alexithymia on D2 not only in terms of biochemical markers but effectively in the necessity for additional management. The association between alexithymia and glycemic control in D2 may be attributed to both biological and psychological effects of alexithymia in relation to awareness, disease evolution and compliance with management [21, 23]. Alexithymia seems to impact coping strategies in diabetes in regard to disease knowledge and capabilities of self-care [7]. The influence of emotions on diabetes evolution and regulation may be linked to the release of stress hormones such as catecholamines, cortisol and glucagon thus affecting insulin action. From another point of view, it is noteworthy to consider the effects of poor glycemic control in diabetes on body functions, particularly cognitive ones, through vascular and neuronal consequences, as well as via poor glucose utilization in the intracellular milieu. According to most recent studies, long-term hyperglycemia seems to alter functions such as information processing, memory, attention, and emotional competencies as represented by alexithymia [24, 25]. To be noted that from the perspective of emotional states, mainly depression and anxiety, research found them as comorbidities to diabetes occurring in parallel without a cause-effect relationship. Depression may appear in reaction to lifestyle changes while anxiety can be related to an adaptive problematic in the context of diabetes [24].
Concerning lifestyle variables among subjects with D2, when comparing those with alexithymia to subjects without alexithymia, our research registered significant differences in alcohol consumption, physical activity and dietary intake (Table 5) which may impact glycemic regulation. In other terms, D2 subjects with alexithymia seem to have higher frequencies of alcohol consumption than those without alexithymia. Globally, they also appear to exercise less and subjectively report less adequate dietary choices in regard to diabetes. However, according to the multivariate regression analysis, these lifestyle variables were not found to be independently related to alexithymia (Table 6). In literature, alexithymia has already been linked to overall substance use in various studies [26]. Moreover, a longitudinal Polish research including 36 female patients with adolescent idiopathic scoliosis showed that physical activity coexisted with lower scores of alexithymia [27].
Limitations of the study
First of all, participants who did not understand French or English were not included in our study since TAS-20 was proposed in these languages. Furthermore, our research focused on outpatients with D2 and thus did not assess the particularities of alexithymia among hospitalized D2 patients. Finally, the evaluation of lifestyle variables, specifically dietary choices, was subjective and based on self-reported habits.
Conclusions
D2 can be affected by psychological aspects pertaining to emotional expression and recognition. On the basis of our results in the context of current literature, individuals with D2 may benefit from screening for difficulties in emotional skills specifically in relation to identification and description of feelings. Psychological approaches can include individual or group work not only on emotional dimensions but also on behavioral effects and coping strategies. Bodily awareness techniques may also be used with focus on disease adaptation. Further research is needed in this field including larger samples and investigation of biological and psychological interaction mechanisms. A study of psychological techniques and their efficacy may also contribute in the understanding of the disease treatment and management.
Acknowledgments
The authors thank the students Carla Chlela and Setrida Kallass for their support, particularly in the sampling procedure.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
A written informed consent was obtained from all participants prior to enrollment.
Ethical approval
This study was approved by the Ethical Committee of the Lebanese German University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chirine Fares, Email: fares.chirine@gmail.com.
Robert Bader, Email: robertbader@live.com.
José-Noel Ibrahim, Phone: +961 70 68 31 79, Email: jn.ibrahim@lgu.edu.lb.
References
- 1.Papatheodorou K, Banach M, Edmonds M, Papanas N, Papazoglou D. Complications of diabetes. J Diabetes Res. 2015;2015:189525. doi: 10.1155/2015/189525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017 p. 20. Available from: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 15 Sept 2018.
- 3.The Intenational Diabetes Federation (IDF). Members. Available from: https://www.idf.org/our-network/regions-members/middle-east-and-north-africa/members/39-lebanon.html. Accessed 15 Sept 2018.
- 4.Chen L, Pei J-H, Kuang J, Chen H-M, Chen Z, Li Z-W, Yang HZ. Effect of lifestyle intervention in patients with type 2 diabetes: a meta-analysis. Metabolism. 2015;64:338–347. doi: 10.1016/j.metabol.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Whitworth SR, Bruce DG, Starkstein SE, Davis WA, Davis TME, Bucks RS. Lifetime depression and anxiety increase prevalent psychological symptoms and worsen glycemic control in type 2 diabetes: the Fremantle diabetes study phase II. Diabetes Res Clin Pract. 2016;122:190–197. doi: 10.1016/j.diabres.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Luca A, Luca M, Di Mauro M, Palermo F, Rampulla F, Calandra C. Alexithymia, more than depression, influences glycaemic control of type 2 diabetic patients. J Endocrinol Investig. 2015;38:653–660. doi: 10.1007/s40618-015-0238-2. [DOI] [PubMed] [Google Scholar]
- 7.Topsever P, Filiz TM, Salman S, Sengul A, Sarac E, Topalli R, Gorpelioglu S, Yilmaz T. Alexithymia in diabetes mellitus. Scott Med J. 2006;51:15–20. doi: 10.1258/RSMSMJ.51.3.15. [DOI] [PubMed] [Google Scholar]
- 8.Bastin P, Luminet O, Buysschaert M, Luts A. Contrôle du diabète et alexithymie : le rôle de l’identification et de la verbalisation des émotions. Louvain Méd. 2004;123:252. [Google Scholar]
- 9.Ahmadieh H, Itani H, Itani S, Sidani K, Kassem M, Farhat K, Jbeily M, Itani A. Diabetes and depression in Lebanon and association with glycemic control: a cross-sectional study. Diabetes Metab Syndr Obes Targets Ther. 2018;11:717–728. doi: 10.2147/DMSO.S179153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Care CTF on PH Recommendations on screening for type 2 diabetes in adults. CMAJ. 2012;184:1687–1696. doi: 10.1503/cmaj.120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto alexithymia scale—I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Parker JDA, Taylor GJ, Bagby RM. The 20-item Toronto alexithymia scale. III. Reliability and factorial validity in a community population. J Psychosom Res. 2003;55:269–275. doi: 10.1016/S0022-3999(02)00578-0. [DOI] [PubMed] [Google Scholar]
- 13.Loas G, Parker JDA, Otmani O, Verrier A, Fremaux D. Confirmatory factor analysis of the French translation of the 20-item Toronto alexithymia scale. Percept Mot Skills. 1997;85:1018. doi: 10.2466/pms.1997.85.3.1018. [DOI] [PubMed] [Google Scholar]
- 14.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20:586–590. [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical Care in Diabetes—2015 abridged for primary care providers. Clin Diabetes Publ Am Diabetes Assoc. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avci D, Kelleci M. Alexithymia in patients with type 2 diabetes mellitus: the role of anxiety, depression, and glycemic control. Patient Prefer Adherence. 2016;10:1271–1277. doi: 10.2147/PPA.S110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damak R, Mnif L, Masmoudi J, Halwani N, Mnif F, Baati I, Jaoua A. P02-299 - alexithymia in diabetes type2. Eur Psychiatry. 2010;25:1008. doi: 10.1016/S0924-9338(10)70998-6. [DOI] [Google Scholar]
- 18.Mnif L, Damak R, Mnif F, Ouanes S, Abid M, Jaoua A, et al. Alexithymia impact on type 1 and type 2 diabetes: a case-control study. Ann Endocrinol. 2014;75:2013–2019. doi: 10.1016/j.ando.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Hintistan S, Cilingir D, Birinci N. Alexithymia among elderly patients with diabetes. Pak J Med Sci. 2013;29:1344–1348. doi: 10.12669/pjms.296.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salminen JK, Saarijärvi S, Aärelä E, Toikka T, Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. J Psychosom Res. 1999;46:75–82. doi: 10.1016/S0022-3999(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 21.Stingl M, Naundorf K, vom Felde L, Bernd H. Alexithymia in type I and type II diabetes. Interv Obes Diabetes. 2018;1:1–4. [Google Scholar]
- 22.Levant RF, Hall RJ, Williams CM, Hasan NT. Gender differences in alexithymia. Psychol Men Masculinity. 2009;10:190–203. doi: 10.1037/a0015652. [DOI] [Google Scholar]
- 23.Abramson L, McClelland DC, Brown D, Kelner S. Alexithymic characteristics and metabolic control in diabetic and healthy adults. J Nerv Ment Dis. 1991;179:490–494. doi: 10.1097/00005053-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Contreras CM, Gutiérrez-García AG. Cognitive impairment in diabetes and poor glucose utilization in the intracellular neural milieu. Med Hypotheses. 2017;104:160–165. doi: 10.1016/j.mehy.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Jones DT. Neural networks, cognition, and diabetes: what is the connection? Diabetes. 2012;61:1653–1655. doi: 10.2337/db12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morie KP, Yip SW, Nich C, Hunkele K, Carroll KM, Potenza MN. Alexithymia and addiction: a review and preliminary data suggesting neurobiological links to reward/loss processing. Curr Addict Rep. 2016;3:239–248. doi: 10.1007/s40429-016-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misterska E, Glowacki M, Adamczyk K, Glowacki J, Harasymczuk J. A longitudinal study of alexithymia in relation to physical activity in adolescent females with scoliosis subjected to cheneau brace treatment: preliminary report. Spine. 2014;39:E1026–E1034. doi: 10.1097/BRS.0000000000000426. [DOI] [PubMed] [Google Scholar]