Abstract
This study was aimed to investigate the effect of processing techniques on the characteristics of green and red chilli powder. Four samples, such as pretreated green chilli paste (PTGP), pretreated green chilli longitudinal slit (PTGL), pretreated whole red chilli (PTWR) and untreated green chilli paste (UTGP), were prepared and dried at 60 °C in a cabinet dryer. The pretreatment was blanching in acetic acid solution and soaking immediately in a combined solution of Na2S2O5 and CaCl2. Pretreated samples took a shorter drying time than the untreated sample in reducing moisture content from 86.31 to 8%. Pretreatment before drying resulted in retaining total chlorophyll (~ 86%), phenolic compounds (~ 32%), green color, and pungency of chilli. Analysis result indicated that more than 60% retention of β-carotene was found while retention of ascorbic acid was comparable. Conclusively, this research reveals a good nutritional profile in cabinet dried green chilli powder, which may open the scope for commercial production.
Keywords: Green chilli, Drying, Blanching, Chemical soaking, Chlorophyll, Ascorbic acid, Phenolic compounds
Introduction
Spices occupy an important group of agricultural commodities since ancient times, which naturally contain a significant amount of antioxidants and bioactive components (Dubey et al. 2015). Chilli (Capsicum annum L.) is the most widely cultivated vegetable cum spice over the world (Dubey et al. 2015). Apart from the rich source of ascorbic acid, it is a good source of flavonoids, carotenoids, phenols, vitamins, saponins, nitrogenous compounds and minerals (Sarker et al. 2012). Chilli is also associated with important health benefits, i.e. antioxidants, anti-inflammatory, anti-arthritic, anti-neoplastic, anticancer and antifungal characteristics. Besides, chilli extracts are reported as biochemical pest repellants and pesticides (Chinn et al. 2011). It is well-known due to the presence of pungent compounds, i.e. crystalline and harsh unstable alkaloid capsaicin having various prophylactic and beneficial uses as medicine (Dubey et al. 2015). Chilli is a non-climacteric and highly perishable fruit, which generally encounters postharvest complications, e.g. quality degradation, rapid weight loss, and color change (Edusei et al. 2012). Chilli contains more than 80% (wet basis, wb) moisture during harvest, which is very prone to insect and fungal attack during storage.
Drying is an essential step for the preservation and cost reduction of transport and storage of plant material (Shishir et al. 2018). It is one of the methods of reducing moisture content by which microbial actions can be prevented as well as the shelf-life of chilli can be extended (Pham et al. 2017). However, fresh chilli is generally dried in the ripened condition in the open sunlight without any pretreatment (Sarker et al. 2012). But, sun drying has some problems, such as prone to contamination, long drying time and weather dependence. Additionally, sun drying affects color and results in shrinkage of the product, which leads to a final unattractive product. This is because the outer layer of the fruit tissue impedes to transfer water from the inner surface (Ganiy et al. 2010). Although shade drying retains many important properties, it has some disadvantages as like occur in sun drying e.g. low energy efficiency, inconsistent quality standards, contamination problems, that are undesirable for the food industry (Dwivedy et al. 2012). In contrast, hot air drying or mechanical drying is a fast drying process, even though it is energy consuming. The product qualities particularly color, texture, flavor, ascorbic acid, β-carotene, phenolics and other nutrients are often deteriorated by thermal drying due to the development of browning pigment and direct contact with air and light (Wiriya et al. 2009). This is why, blanching and chemical pretreatments are used prior to the drying of many food products (Duarte et al. 2017; Take-Ajaykumar et al. 2012). Therefore, a suitable technology and better processing conditions are required for the production of green chilli powder in the present situation.
To date, several works have been reported on the manufacturing of green chilli powder (Sarker et al. 2012; Take-Ajaykumar et al. 2012; Jyothirmayi et al. 2008). According to their observation, several pretreatments, i.e., blanching and soaking in chemical solutions before drying have been used in order to preserve color and the nutritional quality during drying. However, the present study was the following work of the previous study (Sarker et al. 2012). The new approach of this study was blanching with acetic acid solution and soaking in a mixed chemical solution of Na2S2O5 and CaCl2 before proceeding for drying, which was not done in the previous studies (Sarker et al. 2012; Take-Ajaykumar et al. 2012; Jyothirmayi et al. 2008) on the production of green chilli powder. Literature supports that blanching with the acid solution before drying can alleviate the adverse effect of hot air drying, preserve color, and enhance the relative bioaccessibility of bioactive compounds (Hiranvarachat et al. 2012). Therefore, the aim of the study was to investigate the effect of different pretreatments under hot air drying on the drying characteristics, physicochemical properties and functional compounds of green chilli powder. The findings of this study can play a significant role in powder technology particularly in the production and commercialization of such spice.
Materials and methods
Raw materials and chemicals
Fresh, matured but not over-matured, and disease free green and red chilli (Capsicum annum L.) were collected from a local farmer’s field, near to Hajee Mohammad Danesh Science and Technology University, Dinajpur, Bangladesh. All the chemicals used for the experiments were of analytical grade and purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
Preparation of samples
Fresh green and red chilli were sorted and de-stalked manually and washed in running tap water to remove adhering dirt and dust particles. Then, the chilli was immediately wiped with a table cloth to remove superficial moisture. After cleaning, five sets of samples were prepared such as pretreated green chilli paste (PTGP), pretreated green chilli longitudinal slit (PTGL), pretreated whole red chilli (PTWR), untreated green chilli paste (UTGP), and untreated whole fresh green chilli (UTWG) (as control). 1000 g of each chilli sample was used for the study. Pretreatment of chilli was done by blanching in 2% acetic acid solution for 2 min at a temperature of 100 °C and by soaking immediately in the chemical solution of 0.3% Na2S2O5 combined with 1% CaCl2 for 10 min. The green chilli was made into a paste using a Laboratory Grinder (Jaipan CM/L-7360065, Japan) while the longitudinal slit was done by a sharp knife. All the prepared samples (except UTWG) were dried in a cabinet dryer (Model-136-120, Seoul, Korea) at 60 °C until a final moisture content of around 8% (w/w) was obtained. During drying, trays of the dryer were altered from time to time to facilitate uniform drying. Thereafter, dried chilli samples were pulverized to prepare powder by the grinder. Then the prepared powder was passed through a sieve (Sieve no. MIC-300) for obtaining fine chilli powder. The screened powder was weighed and finally packed in high-density polyethene (HDPE) pouches. The samples were stored at room temperature until further use.
Construction of drying characteristic curves
The loss of moisture from the samples was taken at every 1-h interval during the whole drying period. The drying curves were drawn by plotting the percent moisture content against the drying time. Drying rates for all the samples were calculated according to the formula applied by Wade et al. (2014) as follows:
| 1 |
where R—drying rate (g H2O/100 g dry sample/h); Wr—amount of moisture removed (g); Wd—total bone-dry weight of the sample (g) and t—drying time (h). The drying rate curves were drawn by plotting the drying rates against the percent moisture content.
Proximate analysis
The proximate composition (moisture, fat, protein, ash and carbohydrate contents) of the obtained chilli powder and fresh green chilli was determined as per the method mentioned by AOAC (2005).
Measurements of color parameters
The surface color of the samples was evaluated with a spectrophotometer (CM2500d, Konica, Minolta Optics Inc., Japan) based on the CIE L*a*b* color space. Values of L* represent brightness, a* correspond to the red-green color gradient while b* denotes to the yellow-blue color gradient. Three measurements were conducted in each sample. The Hue angle (h) and Chroma (C*) were calculated according to the formula (Wiriya et al. 2009) as follows:
| 2 |
| 3 |
Determination of ascorbic acid content
The content of ascorbic acid was determined according to the method described by Adebayo (2010) with slight modification. Two grams of each sample was mixed with 5 mL of 20% metaphosphoric acid solution and filtered through Whatman No. 1 filter paper. 1 mL of the filtrate was added to a small beaker and mixed with 10 mL de-ionized water. Then, 2 mL was transferred into a beaker, shaken with 2 drops of phenolphthalein solution, and titrated against 2,6-dichlorophenol indophenol until the pink color was developed. Ascorbic acid content was calculated according to the following equation:
| 4 |
Here, dye factor—the amount of 2,6-dichlorophenol indophenol required to neutralize a known volume (usually 5 mL) of standard ascorbic acid [Dye factor = 0.5/Titre, where 0.5 implies that 5 mL of the standard ascorbic acid solution contains 0.5 mg ascorbic acid].
Determination of chlorophyll and β-carotene content
Chlorophyll and β-carotene contents were determined using the method described by Nagata and Yamashita (1992) with little modification. Pigments in the sample were extracted with acetone-hexane (4:6) simultaneously, and the optical density of all the supernatants was measured at 663, 645, 505, and 453 nm through a UV/VIS-spectrometer (T80 UV/VIS Spectrometer, PG Instruments LTD.). The chlorophyll ‘a’ and chlorophyll ‘b’ contents were estimated in mg/100 g by using the following equations:
| 5 |
| 6 |
The β-Carotene content was estimated in mg/100 mL by using the following equation (Igbokwe et al. 2013):
| 7 |
where A663, A645, A505 and A453 are the absorbance at 663, 645, 505 and 453 nm, respectively.
Pungency test
The pungency of chilli samples was determined using the method described by Hossain and Bala (2007). 4 g of chilli powder was extracted with acetone until a colorless acetone solution was obtained. The volume was then made up to 100 mL with acetone. The extract was kept 3 h at room temperature. After 3 h, 5 mL of acetone was taken in a beaker and heated on a water bath until fully dry. To this, 5 mL of 0.1 N NaOH solution was added followed by 3 mL of 3% phosphomolybdic acid solution and was kept at room temperature for 1 h. Finally, optical density was measured at 650 nm using a spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments LTD.). The value of optical density was considered as the pungency index of chilli powder. Samples with higher optical density were considered to contain more capsaicin, and therefore more pungent.
Determination of total phenolic content
The total phenolic content was determined by the Folin–Ciocalteau method (Heimler et al. 2006) with minor modification. The extracted solution was obtained using 1 g sample mixed with 40 mL 100% methanol in a separate glass beaker and stirred for 4–5 min. Then the mixture was concentrated to 10 mL by heat using hotplate stirrer followed by adding 10 mL of 100% methanol to concentrated samples solution. From these mixtures, an aliquot of 1 mL of each sample was taken in glass test tubes to which 0.2 mL 10% Folin–Ciocalteau reagent was added. These mixtures were vortexed for 3 min. Then 0.8 mL of 7.5% Na2CO3 was added to the mixture and allowed to stand in a dark place for 1–2 h before measuring the absorbance at 760 nm using a spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments LTD.) against the blank (contained the same mixture solution without the sample extract). The total phenolic content was determined using the following formula by a comparison of the values obtained with the standard curve of gallic acid. The results were expressed as mg gallic acid/100 g of the sample.
| 8 |
Statistical analysis
Each experiment was repeated thrice, and results were expressed as mean ± standard error mean (SEM). The obtained data were analyzed by IBM SPSS Statistics, version 20 (SPSS Inc., Chicago, IL). One-way analysis of variance was done using ANOVA procedures. Significant differences among the means were determined by Duncan’s Multiple Range Test (DMRT) at the 95% confidence level.
Results and discussion
Effects of processing techniques on the drying characteristics
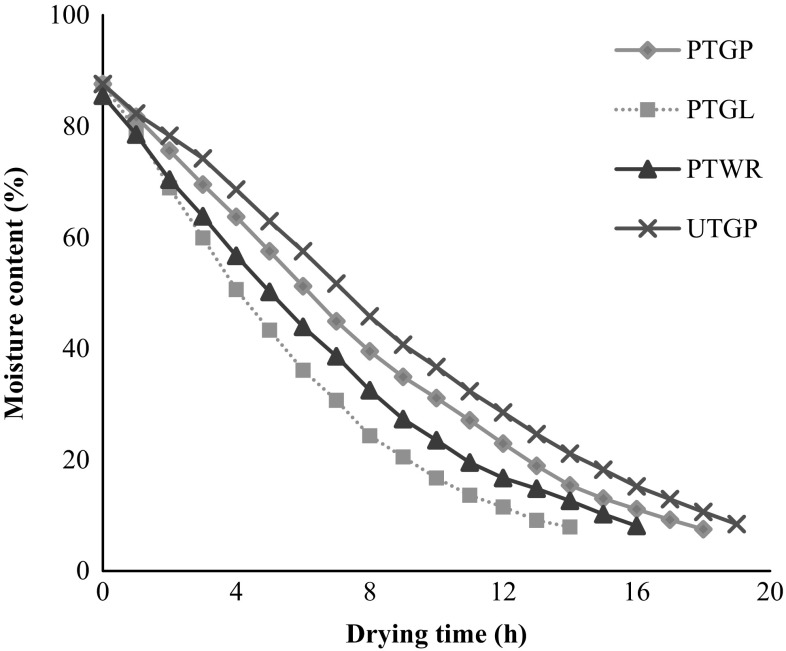
The moisture changing pattern during drying of chilli samples as a result of drying temperature at different time intervals is sketched in Figs. 1 and 2. It was observed from Fig. 1 that the moisture rapidly decreased at the initial stage and afterwards slowed down until equilibrium moisture level. The drying time required for lowering the initial moisture content of chilli samples (86.31%, wb) to the desired moisture content (~ 8%, wb) varied between 14 and 19 h. The reason of slow moisture removal during drying process could be attributed to the low diffusion of moisture within the chilli than that of evaporation of moisture from the surface, and therefore the overall drying process is diffusion-controlled mass transfer phenomena (Wiriya et al. 2010). Maximum drying time (19 h) was taken by untreated chilli sample (UTGP) while treated samples took comparatively shorter time from 14 to 18 h. The results clearly demonstrated that the pretreatments reduced the drying time of chilli samples (Fig. 1). Blanching in acetic acid solution and soaking in a combined solution of Na2S2O5 and CaCl2 helped to accelerate the drying rate of chilli samples. The blanching and chemical soaking softened the texture or membrane, which facilitated a faster drying process (Raja et al. 2017). The drying rate of chilli samples showed that the moisture removal occurred logarithmically during the drying process as shown in Fig. 2a–d. At the initial stage of drying, the moisture content of chilli was higher, and as a consequence of drying, more moisture was readily evaporated from the outer surface of chilli. As the drying process proceeded, the moisture of the chilli surface was decreased and the evaporation zone moved from the surface into the inside of chilli leading to the less water evaporation of chilli samples. Hence, the drying rate was reduced with the drying time. Further, the absolute constant-rate period was not observed during drying of chilli, while drying mostly took place in the falling-rate period. The following regression equations were obtained for drying rate curves of chilli samples. For PTGP:
| 9 |
For PTGL:
| 10 |
For PTWR:
| 11 |
For UTGP:
| 12 |
From the above regression equations, it is obvious that the most efficient and smooth drying rate was observed for PTWR (R2 = 0.964), followed by PTGL (R2 = 0.9477), PTGP (R2 = 0.9412) and UTGP (R2 = 0.9013). These results are corroborated by Hossain and Bala (2007) and Banout et al. (2011).
Fig. 1.
Drying curve obtained during drying of various chilli samples in a cabinet dryer at 60 °C (PTGP pretreated green chilli paste, PTGL pretreated green chilli longitudinal slit, PTWR pretreated whole red chilli, UTWG untreated green chilli paste)
Fig. 2.
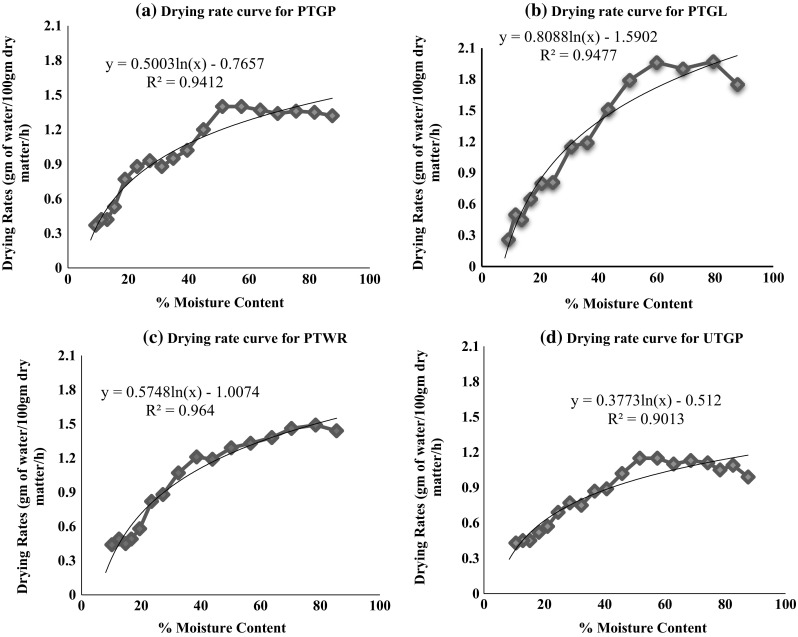
Drying rate curves showing the rate of moisture removal during drying of chilli samples (PTGP pretreated green chilli paste, PTGL pretreated green chilli longitudinal slit, PTWR pretreated whole red chilli, UTWG untreated green chilli paste)
Effects of processing techniques on physicochemical properties and functional compounds
Effect on proximate composition
The effects of processing methods on the proximate analysis of the chilli samples are summarized in Table 1. The moisture content of fresh green chilli (UTWG) was 86.31 g/100 g. However, the moisture contents of chilli powder were satisfactorily ranging from 7.50 to 8.45 g/100 g and statistically similar (P ≤ 0.05). This was a good indication of the stability of green chilli powder against oxidative and microbial deterioration (Toontom et al. 2012). Crude protein, fat, ash and carbohydrate contents of chilli powders ranged from 11.93 to 12.99 g/100 g, 6.07 to 7.40 g/100 g, 4.68 to 7.72 g/100 g and 64.81 to 68.08 g/100 g, respectively, which were significantly different at 95% level of confidence. Similar findings were reported by other studies (Sarker et al. 2012; Jyothirmayi et al. 2008). The changes in proximate composition among the chilli samples might be due to several factors e.g. processing techniques used, stage of maturity, drying temperature, time of drying, oxidation of important constituents, weather conditions including soil types and compositional differences (Singh et al. 2016; Sarker et al. 2012; Ahmed et al. 2010).
Table 1.
Proximate composition of fresh green chilli and chilli powder
| Sample | Proximate composition | ||||
|---|---|---|---|---|---|
| Moisture (g/100 g) | Total ash (g/100 g) | Fat (g/100 g) | Crude protein (g/100 g) | Carbohydrate (g/100 g) | |
| Chilli powder | |||||
| PTGP | 7.50 ± 0.15a | 4.68 ± 0.52c | 6.75 ± 0.09b | 12.99 ± 0.03a | 68.08 ± 0.54a |
| PTGL | 7.81 ± 0.06a | 4.84 ± 0.32c | 7.24 ± 0.06a | 12.74 ± 0.06b | 67.37 ± 0.24a |
| PTWR | 8.14 ± 0.03a | 7.72 ± 0.07a | 7.40 ± 0.07a | 11.93 ± 0.02c | 64.81 ± 0.05b |
| UTGP | 8.45 ± 0.22a | 6.06 ± 0.07b | 6.07 ± 0.05c | 12.81 ± 0.02b | 66.61 ± 0.33a |
| Fresh green chilli | |||||
| UTWG | 86.31 ± 0.61 | 0.73 ± 0.01 | 2.46 ± 0.21 | 5.49 ± 0.03 | 4.99 ± 0.78 |
All values are mean ± SEM of three replicates
PTGP pretreated green chilli paste, PTGL pretreated green chilli longitudinal slit, PTWR pretreated whole red chilli, UTGP untreated green chilli paste, UTWG whole fresh green chilli
a–cThe test values along the same column carrying different superscripts for each composition contents are significantly different (P < 0.05)
Effect on ascorbic acid
Ascorbic acid contents of all dried chilli samples significantly (P < 0.05) decreased and ranged from 69.56 to 74.19 mg/100 g (Table 2), whereas it was 127.09 mg/100 g in fresh chilli. Maximum ascorbic acid was recorded in sample PTGL (74.09 mg/100 g) followed by PTGP (73.46 mg/100 g), UTGP (71.14 mg/100 g) and PTWR (69.55 mg/100 g). These values are compatible with the report on fresh bell pepper (Sharma et al. 2014), green pepper (Igbokwe et al. 2013), fresh green chilli (Toontom et al. 2012), and dried green chilli powders (Sarker et al. 2012). According to the ‘Food Composition Table’ reported by the Rural Development Administration (RDA), the ascorbic acid content of dried chilli is about 26 mg/100 g (RDA 2001). However, this study exposed maximum ascorbic acid content which is 74 mg/100 g of dried chilli powder, which indicates a significant contribution in comparison with the ‘Food Composition Table’ reported by Rural Development Administration (RDA 2001).
Table 2.
Total phenol, β-carotene, chlorophyll (a and b) and ascorbic acid content of fresh green chilli and chilli powder
| Sample | Total phenol (mg/100 g) | β-Carotene (µg/100 g) | Ascorbic acid (mg/100 g) | Chlorophyll-a (mg/100 g) | Chlorophyll-b (mg/100 g) |
|---|---|---|---|---|---|
| Chilli powder | |||||
| PTGP | 958.34 ± 2.48a | 982.30 ± 1.15b | 73.46 ± 0.05b | 2.57 ± 0.003b | 3.82 ± 0.014ab |
| PTGL | 871.81 ± 2.35b | 989.46 ± 1.23a | 74.09 ± 0.09a | 2.87 ± 0.008a | 3.89 ± 0.256a |
| PTWR | 820.57 ± 3.17c | 122.63 ± 1.45c | 69.55 ± 0.04d | 0.39 ± 0.002d | 0.32 ± 0.002c |
| UTGP | 873.80 ± 3.10b | 978.70 ± 1.62b | 71.14 ± 0.01c | 2.18 ± 0.023c | 3.45 ± 0.056b |
| Fresh green chilli | |||||
| UTWG | 435.92 ± 3.23 | 241.50 ± 1.32 | 127.09 ± 0.10 | 0.405 ± 0.007 | 0.756 ± 0.007 |
All values are mean ± SEM of three replicates
PTGP pretreated green chilli paste, PTGL pretreated green chilli longitudinal slit, PTWR pretreated whole red chilli, UTGP untreated green chilli paste, UTWG whole fresh green chilli
a–dThe test values along the same column carrying different superscripts for each composition contents are significantly different (P < 0.05)
According to Table 3, it was found that the retentions of ascorbic acid in chilli powders were very low ranging from 8.37 to 8.69% on a solid mass basis. These findings are corroborated by the study of Gupta et al. (2013) who reported 4–8% retention (fresh weight basis) of ascorbic acid in dried green leafy vegetables except for dried Centella asiatica leafy vegetable retained 14% ascorbic acid. On the contrary, the retention of ascorbic acid was reported at around 13.36–37.53% (dry mass basis), in mustard, mint and spinach (Kaur et al. 2008). A number of studies strongly supported that ascorbic acid is highly sensitive to heat (Raja et al. 2017; Gupta et al. 2013; Sarker et al. 2012). The loss of ascorbic acid in this study can be a result of prolonged exposure of chilli samples to cabinet drying temperature (60 °C). In addition to drying temperature, oxygen, pH, metal and other parameters were reported to have a significant contribution to the loss of ascorbic acid (Wang et al. 2018). This phenomenon can be attributed to the destruction of cell structure as it can lead to ascorbic acid release and contribute to the rapid oxidation of ascorbic acid to dehydroascorbic acid (Wang et al. 2018). So, ascorbic acid content often used as an indicator to evaluate the nutrient loss of fruits and vegetables during processing and storage.
Table 3.
Retention of functional compounds in green chilli powders in comparison with fresh green chilli (solid mass basis)
| Sample | Total phenol (%) | β-Carotene (%) | Ascorbic acid (%) | Total chlorophyll (%) |
|---|---|---|---|---|
| PTGP | 32.39 ± 0.11a | 60.34 ± 0.15b | 8.58 ± 0.02b | 81.36 ± 1.34b |
| PTGL | 29.56 ± 0.07b | 60.79 ± 0.10a | 8.69 ± 0.01a | 86.43 ± 1.84a |
| UTGP | 29.87 ± 0.17b | 60.68 ± 0.25b | 8.37 ± 0.01c | 72.52 ± 1.19c |
All values are mean ± SEM of three replicates
PTGP pretreated green chilli paste, PTGL pretreated green chilli longitudinal slit, UTGP untreated green chilli paste
a–cThe test values along the same column carrying different superscripts for each composition contents are significantly different (P < 0.05)
Effect on β-carotene
Table 2 indicates significant (P ≤ 0.05) differences in β-carotene content of chilli powders. The β-carotene content ranged from 122.63 to 989.46 µg/100 g. Similar results were reported by Sharma et al. (2014) for bell pepper. The findings of the present study were considerably higher than the findings reported by Igbokwe et al. (2013) for green and red pepper; and Sarker et al. (2012) for both fresh green chilli and dried chilli. Moreover, Table 3 indicates that the retention of β-carotene content among chilli powders were greater than 60% on a solid mass basis. Gupta et al. (2013) reported about 49–73% retention of β-carotene (fresh weight basis) in five types of dried leafy vegetables, while Kaur et al. (2008) observed 22.26–55.16% retention β-carotene (dry weight basis) in three types of dried green leafy vegetables.
Effect on chlorophyll ‘a’ and chlorophyll ‘b’ content
Chlorophyll content of chilli samples significantly (P ≤ 0.05) reduced after drying (Table 2). The content of chlorophyll ‘a’ and chlorophyll ‘b’ were around 2.87–0.390 mg/100 g and 3.89–0.318 mg/100 g, respectively. It was previously reported that long dehydration times together with high temperatures can produce poor quality products due to caramelization, Maillard reactions, enzymatic reactions, pigment degradation and L-ascorbic acid oxidation (Kim et al. 2006). However, Table 3 clearly illustrates that retention of total chlorophyll content in treated chilli powders were more than 80% with maximum retention of 86.43% in PTGL, while untreated green chilli powder UTGP exhibited 72.52% retention of chlorophyll content. It can be inferred that pretreatment, i.e. blanching with chemical soaking before drying has a significant effect on the retention of total chlorophyll content in green chilli powders (Negi and Roy 2000).
Effect on total phenolic compounds
In addition to ascorbic acid and carotenoids, phenolic compounds are one of the principal antioxidant constituents of natural products, which composed of phenolic acids and flavonoids that are potent radical terminators. Data presented in Table 2 indicates that total phenolic content in chilli powders showed a significant difference (P ≤ 0.05) with a range of 820.57–958.34 mg/100 g, while it was 435.92 mg/100 g in fresh green chilli. These findings exhibited greater values of phenolic content in comparison with previous studies reported on dried green chilli (Wiriya et al. 2009) and sweet bell pepper (Sharma et al. 2014). Our study revealed 29.56–32.39% retention of total phenolic content (solid mass basis) in chilli powders (Table 3). The pretreatment enhanced the preservation of phenolic compounds in green chilli powder produced from green chilli paste (PTGP) compared to the untreated green chilli paste powder (UTGP). However, there was no effect of pretreatment in the preservation of phenolic compounds in chilli powder produced from PTGL (Table 3). These results were compatible with the findings of Wiriya et al. (2009). Moreover, all of the green chilli powders exhibited 2 times higher phenolic compounds in comparison with fresh chilli, if it is considered in wet mass basis (not solid mass basis). Previous studies corroborated that dried form (powder) can reveal a greater amount of phenolic compounds than that of fresh one (Raja et al. 2017). Furthermore, phenolic content in dried products can be increased in many reasons as such the conversion of flavonoids to secondary phenolic compounds (Barz and Hoesel 1977). Heating together with chemical treatment may reduce the enzyme activities and increase the free radical scavenging activities, which may enhance the presence of total phenolic content in dried chilli powder. Also, the significant increase in polyphenolic content in case of dried chilli might be due to the formation of polyphenolic substances due to the availability of precursors of polyphenolic molecules by non-enzymatic interconversion between polyphenolic molecules (Mehta et al. 2017). Alteration of the matrix during drying may also enhance the extractability of phenolic compounds (Raja et al. 2017).
Effect on color characteristics
The quality of dried chilli can also be characterized through its color and pungency as these properties reflects the presence of natural compounds, consumer’s acceptance, and therefore the market value. The results of this study showed significant differences (P ≤ 0.05) in color values of dried chilli samples (Table 4). Overall, the values of L*, a*, b*, hue and chroma of the dried chilli samples were higher than that of the fresh chilli. A clear effect of pretreatment on color preservation of dehydrated green chilli was observed as shown in Table 4. Pretreated chilli powders exhibited lower a* value and higher hue angle compared to untreated chilli powder (UTGP), which indicates better retention of the greenness of chilli in powder form. This is because the region of pure green color is 180°. The increase of hue angle near to 180° indicates that the conversion of light green color to dark green color. Similarly, lower values of a* refers to higher greenness. Moreover, the chemical compound chlorophyll is responsible for the greenness of chilli. The presence of higher amount of total chlorophyll (chlorophyll ‘a’ and chlorophyll ‘b’) in treated chilli powders also proves the availability of higher green color in treated chilli powders (Table 3) compared to untreated chilli powder (UTGP). Previous researchers reported that pretreatment, i.e. blanching of green chilli in acetic acid solution can prevent the enzyme reaction i.e. enzymatic browning reaction (responsible for discoloration of sample) induced by oxidase reaction of polyphenol groups, and also acts as the green color fixing agent (Wiriya et al. 2009; Hossain and Bala 2007). Nevertheless, the results of this study exerted that blanched chilli soaked in chemical solution (Na2S2O5 combined with CaCl2) also preserved the purity of color; because Na2S2O5 can inhibit the browning reaction by binding with the carbonyl group of reducing sugars and other compounds to retard the browning process (Take-Ajaykumar et al. 2012). In addition, CaCl2 improved the color stability as it may react with water molecules resulting in increased water mobility and reduced drying time (Wiriya et al. 2009). In contrast, higher color degradation in untreated samples may be the result of pigment oxidation and decomposition due to higher exposure to oxygen during drying as well as the Maillard reaction between reducing sugar and amino acid in the pericarp of chilli (Toontom et al. 2012).
Table 4.
Colour parameters and pungency index of fresh green chilli and chilli powder
| Sample | Color parameters | Pungency index | ||||
|---|---|---|---|---|---|---|
| L* | a* | B* | Hue Angle (h) | Croma (C*) | ||
| Chilli powder | ||||||
| PTGP | 38.99 ± 0.23a | 2.71 ± 0.04d | 30.81 ± 1.04b | 84.95 ± 0.11a | 30.93 ± 1.04b | 0.958 ± 0.002b |
| PTGL | 33.84 ± 0.17c | 4.38 ± 0.12c | 30.78 ± 0.61b | 81.88 ± 0.08b | 31.09 ± 0.62b | 0.945 ± 0.000bc |
| PTWR | 34.81 ± 0.30b | 32.96 ± 0.30a | 39.73 ± 0.30a | 50.32 ± 0.08d | 51.63 ± 0.42a | 2.425 ± 0.042a |
| UTGP | 18.32 ± 0.22d | 12.14 ± 0.54b | 24.03 ± 0.38c | 63.21 ± 0.95c | 26.93 ± 0.49c | 0.887 ± 0.004c |
| Fresh green chilli | ||||||
| UTWG | 29.19 ± 0.10 | − 5.21 ± 0.13 | 29.75 ± 1.25 | − 80.03 ± 0.44 | 30.20 ± 1.24 | 1.103 ± 0.006 |
All values are mean ± SEM of three replicates
PTGP pretreated green chilli paste, PTGL pretreated green chilli longitudinal slit, PTWR pretreated whole red chilli, UTGP untreated green chilli paste, UTWG whole fresh green chilli
L* = lightness; a* = red (+)/green (−); b* = yellow (+)/blue (−)
a–dThe test values along the same column carrying different superscripts for each parameter are significantly different (P < 0.05)
Effect on pungency index
The values of pungency index for all samples are shown in Table 4. A significant difference (P ≤ 0.05) in pungency index was observed between samples being the highest in PTWR (2.425 ± 0.042) and the lowest in UTGP (0.887 ± 0.004). Pungency index of pretreated samples was found higher than that of untreated samples. Capsaicinoid compounds, primarily capsaicin (8-Methyl-N-vanillyl-trans-6-nonenamide) and dihydrocapsaicin determine the pungency of chilli (Gangadhar et al. 2012). Previously it was reported in various studies that chilli genotype and environmental interactions (intensity of light, temperature, and age of the fruit etc.) also determine the level of capsaicin i.e. pungent flavor of chilli. These properties are also accorded due to the presence of phenolic compounds and carotenoids (Dubey et al. 2015).
Conclusion
This study exhibited the effect of processing techniques on green chilli powder characteristics and its functional compounds. It was found that pretreatment before drying not only reduced the drying time but also improved the preservation of functional compounds and green color with high pungency index. This study exhibited the highest chlorophyll retention of around 86% (solid mass basis) with the acceptable retention of β-carotene (~ 60%) and phenolic compounds (~ 32%). Even though ascorbic acid retention was very low (> 8%, solid mass basis), the amount of retention still shows an excellent contribution to chilli powder. Therefore, the findings of cabinet drying of green chilli at 60 °C with pretreatment added valuable information to the current knowledge of the nutritional properties in green chilli powder, which might open the scope for commercial production of green chilli powder with adequate nutritive value.
Acknowledgements
We thankfully acknowledged the laboratory facilities of the Department of Food Processing and Preservation, Hajee Mohammad Danesh Science and Technology University, Dinajpur, Bangladesh.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adebayo AOA. Effect of processing methods on chemical and consumer acceptability of kenaf and corchorus vegetables. J Am Sci. 2010;6:165–169. [Google Scholar]
- Ahmed M, Akter MS, Eun JB. Peeling, drying temperatures, and sulphite-treatment affect physicochemical properties and nutritional quality of sweet potato flour. Food Chem. 2010;121:112–118. doi: 10.1016/j.foodchem.2009.12.015. [DOI] [Google Scholar]
- AOAC . Official method of analysis. 17. Washington, DC: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Banout J, Ehl P, Havlik J, Lojka B, Polesny Z, Verner V. Design and performance evaluation of a double-pass solar drier for drying of red chilli (Capsicum annum L.) Sol Energy. 2011;85:506–515. doi: 10.1016/j.solener.2010.12.017. [DOI] [Google Scholar]
- Barz W, Hoesel W. Metabolism and degradation of phenolic compounds in plants. Phytochemistry. 1977;12:339–369. [Google Scholar]
- Chinn MS, Sharma-Shivappa RR, Cotter JL. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod Process. 2011;89:340–345. doi: 10.1016/j.fbp.2010.08.003. [DOI] [Google Scholar]
- Duarte Y, Chaux A, Lopez N, Largo E, Ramírez C, Nuñez H, Vega O. Effects of blanching and hot air drying conditions on the physicochemical and technological properties of yellow passion fruit (Passiflora edulis Var. Flavicarpa) by-products. J Food Process Eng. 2017;40(3):e12425. doi: 10.1111/jfpe.12425. [DOI] [Google Scholar]
- Dubey RK, Singh V, Upadhyay G, Pandey AK, Prakash D. Assessment of Phytochemical composition and antioxidant potential in some indigenous chilli genotypes from North East India. Food Chem. 2015;188:119–125. doi: 10.1016/j.foodchem.2015.04.088. [DOI] [PubMed] [Google Scholar]
- Dwivedy S, Rayaguru K, Sahoo GR. Effect of drying methods on quality characteristics of medicinal Indian borage (Coleus aromaticus) leaves. J Food Process Technol. 2012;3(11):1–6. doi: 10.4172/2157-7110.1000188. [DOI] [Google Scholar]
- Edusei VO, Ofosu-Anim J, Johnson PNT, Cornelius EW. Extending postharvest life of green chilli pepper fruits with modified atmosphere packaging. Ghana J Hort. 2012;10:131–140. [Google Scholar]
- Gangadhar BH, Mishra RK, Pandian G, Park SW. Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. Hort Sci. 2012;47(12):1729–1735. [Google Scholar]
- Ganiy ORA, Kolawole OF, Fadeke WA. Effect of sucrose and binary solution on osmotic dehydration of bell pepper (chilli) (Capsicum spp.) varieties. J Food Sci Technol. 2010;47:305–309. doi: 10.1007/s13197-010-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Gowri BS, Lakshmi AJ, Prakash J. Retention of nutrients in green leafy vegetables on dehydration. J Food Sci Technol. 2013;50(5):918–925. doi: 10.1007/s13197-011-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimler D, Vignolin P, Dini MG, Vincieri FF, Romani A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006;99:464–469. doi: 10.1016/j.foodchem.2005.07.057. [DOI] [Google Scholar]
- Hiranvarachat B, Devahastin S, Chiewchan N. In vitro bioaccessibility of b-carotene in dried carrots pretreated by different methods. Int J Food Sci Technol. 2012;47(3):535–541. doi: 10.1111/j.1365-2621.2011.02874.x. [DOI] [Google Scholar]
- Hossain MA, Bala BK. Drying of hot chilli using solar tunnel drier. Sol Energy. 2007;81:85–92. doi: 10.1016/j.solener.2006.06.008. [DOI] [Google Scholar]
- Igbokwe GE, Aniakor GC, Anagonye CO. Determination of β–Carotene and vitamin C content of fresh green pepper (Capsicum annnum) fresh red pepper (Capsicum annum) and fresh tomatoes (Solanum lycopersicum) fruits. Bioscientist. 2013;1:89–93. [Google Scholar]
- Jyothirmayi T, Rao GN, Rao DG. Physicochemical changes during processing and storage of green chili (Capsicum annuum) powders. J Food Process Preserv. 2008;32:868–880. doi: 10.1111/j.1745-4549.2008.00219.x. [DOI] [Google Scholar]
- Kaur A, Kaur D, Oberoi DPS, Gill BS, Sogi DS. Effect of dehydration on physicochemical properties of mustard, mint and spinach. J Food Process Preserv. 2008;32:103–116. doi: 10.1111/j.1745-4549.2007.00168.x. [DOI] [Google Scholar]
- Kim S, Lee KW, Park J, Lee HJ, Hwang IK. Effect of drying in antioxidant activity and changes of ascorbic acid and color by different drying and storage in Korean red pepper (Capsicum annuum L.) Int J Food Sci Technol. 2006;41:90–95. doi: 10.1111/j.1365-2621.2006.01349.x. [DOI] [Google Scholar]
- Mehta D, Prasad P, Bansal V, Siddiqui W, Sharma A. Effect of drying techniques and treatment with blanching on the physicochemical analysis of bitter-gourd and capsicum. LWT Food Sci Technol. 2017 [Google Scholar]
- Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J Jpn Soc Food Sci Technol. 1992;39:925–928. doi: 10.3136/nskkk1962.39.925. [DOI] [Google Scholar]
- Negi PS, Roy SK. Effect of blanching and drying methods on β-carotene, ascorbic acid and chlorophyll retention of leafy vegetables. LWT Food Sci Technol. 2000;33:295–298. doi: 10.1006/fstl.2000.0659. [DOI] [Google Scholar]
- Pham HNT, Vuong QV, Bowyer MC, Scarlett CJ. Effect of extraction solvents and thermal drying methods on bioactive compounds and antioxidant properties of Catharanthus roseus (L.) G. Don (Patricia White cultivar) J Food Process Preserv. 2017;41:e13199. doi: 10.1111/jfpp.13199. [DOI] [Google Scholar]
- Raja KS, Taip FS, Azmi MMA, Shishir MRI. Effect of pre-treatment and different drying methods on the physicochemical properties of Carica papaya L. leaf powder. J Saudi Soc Agric Sci. 2017 [Google Scholar]
- RDA . Food composition table. 6. Korea: National Rural Living Science Institute; 2001. [Google Scholar]
- Sarker MSH, Hasan SMK, Aziz MG, Islam MT, Azam SMR, Roy S, Ibrahim MN. The effect of processing treatments on the shelf life and nutritional quality of green chilli (Capsicum annuum L.) powder. Pertani J Trop Agric Sci. 2012;35:843–852. [Google Scholar]
- Sharma R, Joshi VK, Kaushal M. Effect of pre-treatments and drying methods on quality attributes of sweet bell-pepper (Capsicum annum) powder. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishir MRI, Taip FS, Saifullah M, Yong SY, Aziz NA, Talib RA. Changes in quality attributes of pink guava (Psidium guajava) powder with respect to different drying techniques and maltodextrin concentrations. Int Food Res J. 2018;25(4):1625–1632. [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-Ajaykumar M, Jadhav SL, Bhotmange MG. Effect of pretreatments on quality attributes of dried green chilli powder. ISCA J Eng Sci. 2012;1:71–74. [Google Scholar]
- Toontom N, Meenune M, Posri W, Lertsiri S. Effect of drying method on physical and chemical quality hotness and volatile flavour characteristics of dried chilli. Int Food Res J. 2012;19:1023–1031. [Google Scholar]
- Wade NC, Wane SS, Kshirsagar SM. Comparative study of drying characteristics in chillies. Indian J Sci Res Technol. 2014;2:105–111. [Google Scholar]
- Wang J, Yang XH, Mujumdar AS, Fang XM, Xiao HW. Effects of high-humidity hot air impingement blanching (HHAIB) pretreatment on the change of antioxidant capacity, the degradation kinetics of red pigment, ascorbic acid in dehydrated red peppers during storage. Food Chem. 2018 doi: 10.1016/j.foodchem.2018.03.123. [DOI] [PubMed] [Google Scholar]
- Wiriya P, Paiboon T, Somchart S. Effect of drying air temperature and chemical pretreatments on quality of dried chilli. Int Food Res J. 2009;16:441–454. [Google Scholar]
- Wiriya P, Somchart S, Paiboon T. Chemical pretreatments affecting drying characteristics of chilli. Dry Technol. 2010;28:1466–1476. doi: 10.1080/07373937.2010.482684. [DOI] [Google Scholar]