Abstract
Evaluation of tender coconut water was done that was subjected to a nonthermal two stage microfiltration process that involved filtration through 0.8 µm and 0.45 µm pore size filters followed by addition of 200 mg/L citric acid, 180 mg/L ascorbic acid, orange honey at 5% (w/v) followed by packaging in glass bottles with headspace flushed with nitrogen. The effect of storage under refrigeration was studied. Microfiltration reduced the total simple sugars, protein and reducing sugars respectively by 13.4, 13.0 and 21.5% without significantly affecting the overall acceptability. Microfiltered samples did not show any signs of haemolytic activity. Addition of citric acid, ascorbic acid and honey was able to reduce activity of polyphenol oxidase and peroxidase and maintain product stability. Even though microfiltered samples were sterile for 190 days, the samples were acceptable for sensory attributes till day 90 of storage. Microfiltration and use of additives were thus found to increase the shelf life of tender coconut water.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03825-3) contains supplementary material, which is available to authorized users.
Keywords: Tender coconut water, Nonthermal technology, Microfiltration, Additives, Honey, Refrigerated storage
Introduction
Tender coconut water is the clear liquid extracted from unripe green coconuts. Tender coconut water industry has developed into a billion-dollar industry in the past 10 years because of its growing demand among athletes and also an increasingly health conscious middle class considers it as a natural health benefiting beverage. This demand is due to its rehydration property, medical benefits as well as its unique taste. The importance of processing and packaging of tender coconut water arises due its low shelf life as it gets contaminated by microbes easily and loses its sensory and nutritive properties, rendering it unsuitable for drinking within a day or two (Sucupira et al. 2017; Haseena et al. 2010; Reddy et al. 2005).
The unique flavour of tender coconut water is lost on thermal processing (Sucupira et al. 2017; Haseena et al. 2010). Non-thermal technologies viz. cold pasteurization, dense phase carbon dioxide technology, microfiltration etc. were applied to preserve tender coconut water (Mahnot et al. 2014; DasPurkayastha et al. 2012). DasPurkayastha et al. (2012) and Mahnot et al. (2014) reported a maximum shelf life of 21 days and 46 days, respectively for tender coconut water processed and packaged by microfiltration technique with added ascorbic acid and citric acid. Microfiltration alone was found to be effective in removal of spoilage causing microorganisms but could not maintain product characteristics for long owing to inherent deteriorative reactions in the water. Thus, considering the findings of previous works, the current study was taken up to further enhance the shelf life of tender coconut water by non-thermal means.
Additives are commonly added to maintain product characteristics for a sufficient time. Additives alone or in combination are mostly utilized for microbial inactivation, antioxidant activity, prevention of browning and increase in titratable acidity. Currently honey is being utilized as an added component to various fruits and vegetables juices, milk, and tea in the preparation of ready to serve beverages (Balogu and Towobola 2017; Bhama et al. 2013; Krushna et al. 2007). Honey is considered as a class I preservative and is well known for its medicinal value, antioxidant and antimicrobial properties (Israili 2014; Alvarez-Suarez et al. 2010). Thus utilization of natural food like honey and additives that are naturally present in foods in the processing of tender coconut water seemed to be viable for experimental studies. Further, the application of kinetic analysis would reveal how the product characteristics could be maintained. In the current work, a comparative study was carried out to observe the effects of microfiltration and additives viz. citric acid, ascorbic acid and honey on tender coconut water stability during storage at 4 °C and assess the changes in sensory attributes.
Materials and methods
Materials
Tender coconuts were procured as whole nuts from the market near Tezpur city, Assam, India. Cultivated honey sample form Sohra town (state of Meghalaya, India) was collected from orange plantation fields without any processing i.e. raw. All the chemicals were obtained from Merck except for citric acid and L-ascorbic acid that were obtained from Sigma, USA. Microbial media were obtained from HiMedia, India. The membrane filters with pore size of 0.8 µm and 0.45 µm were obtained from Merck Millipore.
Sensory evaluation of tender coconut water on honey addition
Tender coconut water was added with Sohra honey sample at concentration levels of 0, 5, 10 and 15% (w/v) and the samples were given to 15 semi-trained panellists for judging sensory attributes on a 9-point hedonic scale (9-like extremely, 8-like very much, 7- like moderately, 6-like slightly, 5-neither like nor dislike, 4-dislike slightly, 3-dislike moderately, 2-dislike very much and 1-dislike extremely). The sensory attributes taken into consideration were aroma, sweetness, colour and overall acceptability. Mean sensory score less than 5 (neither like nor dislike) was judged as non-acceptable.
Tender coconut water processing
The procured whole tender coconuts were sanitized by dipping for 10 min in 300 mg/L sodium hypochlorite solution and cut open with a clean stainless steel knife. The tender coconut water was strained into a beaker to remove large particles and then transferred inside a laminar hood for further processing. Inside the laminar hood the tender coconut water was filtered using a filter and clamp connection with a sterile Whatman No. 4 filter and the filtrate was divided into two portions. The filtrate was then filtered first through a 0.8 µm membrane using a sterile bacterial filtration unit and then again the filtration was carried out through a 0.45 µm membrane filter in a separate sterile unit. The final filtered product was transferred to a sterile heavy duty vacuum flask. The filtration processes were aided with a vacuum pump attached to the filtration unit. Calculated amount of citric acid (200 mg/L), ascorbic acid (180 mg/L) and honey (5%) were the additives added. Additives were added in different combination just before the 0.45 µm filtration step. The tender processed coconut water samples were bottled in sterile glass bottles in which the head space was flushed with nitrogen gas. The bottles were stored in refrigerator at 4 °C for monitoring of shelf life. The portion filtered only through Whatman filter paper is referred to as non-micofiltered tender coconut water and the portion filtered through 0.8 µm and 0.45 µm membrane filters are referred to as microfiltered samples in this paper.
Sample coding
The following samples with codes were processed for the comparative study.
- Non-Microfiltered samples (Whatman filtered only)
- TCW—Tender coconut water filtered with Whatman no. 4 filter
- TCWA—Tender coconut water added with additives (i.e. citric acid and ascorbic acid)
- TCWH—Tender coconut water added with honey
- TCWAH—Tender coconut water added with additives and honey
- Microfiltered samples (Whatman filtered followed by microfiltration through 0.8 and 0.45 µm membrane filters)
- TCWF—Microfiltered tender coconut water
- TCWFA—Microfiltered tender coconut water added with additives
- TCWFH—Microfiltered tender coconut water added with honey
- TCWFAH—Microfiltered tender coconut water added with additives and honey
Storage study
The storage study was designed for 190 days i.e., beyond 6 months. Physicochemical analysis and enzyme monitoring of the stored samples were carried out at an interval of 7 days till day 42 of analysis as rapid changes in the biochemical parameters were expected to occur. Thereafter the analyses of non-microfiltered samples were carried out every 15 days as large number of tests were required to be carried out. Non-microfiltered samples could be studied till day 115 only as the samples became microbiologically unacceptable thereafter. Microfiltered samples were analysed for microbial load after every 15 days till day 190 of storage as the samples were found to be sterile even on day 190. Sensory studies were also carried out for the samples during the storage study and haemolytic activity of the samples was checked. Sensory parameters (mentioned in Sect. 2.2) of the non-microfiltered stored samples were determined till day 30 as after this period, the microbial load became unacceptable. Microfiltered samples were analysed for sensory attributes after 30 days interval till 115 days. The study was stopped after 115 days because the overall acceptability of the samples was below 5 (neither like nor dislike).
Physicochemical analysis of tender coconut water during storage
The pH was measured using a pH meter (Eutech pH 700, Singapore). Hunter L, a, b parameters were taken using Hunter color lab (Ultrascan, VIS-Hunter Lab., USA). Total soluble solids (TSS) was measured using an Abbe refractometer (Atago DR-A1, Japan) at 20 °C as per AOAC (2005). Total simple sugars was measured by taking 0.1 mL of the sample and making the volume up to 1 mL with distilled water to which 4 mL of anthrone reagent (200 mg in 100 mL of 95% H2SO4) was added and the color developed after boiling for 8 min in a water bath was measured in a spectrophotometer (Cecil Aquarius 7400, England) at 630 nm (Mahnot et al. 2014). Titratable acidity (TA), free fatty acid content (FFA) were estimated as per Mahnot et al. (2014). For total reducing sugars that was estimated using Nelson Somogyi method (Sadasivam and Manickam 2007), 0.1 mL of the samples was diluted to 1 mL with distilled water from which 0.1 mL of aliquot was taken for analysis and absorbance was recorded at 620 nm. Total phenolic content was measured using Folin-Ciocalteu reagent (FCR) method wherein 0.5 mL of the sample was added with 7.9 mL of distilled water and 0.5 mL FCR and finally after 8 min 1.5 mL Na2CO3 was added. The solution was incubated for 30 min at 40 °C for reaction and the colour developed was measured in a spectrophotometer at 765 nm with gallic acid as standard (Saikia and Mahanta, 2013). For 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity i.e. antioxidant activity, 0.1 mL of sample was added to 1.4 mL of 10−4 M DPPH solution in methanol and after 30 min the absorbance was observed at 517 nm using 0.1 mL of methanol as blank (Saikia and Mahanta 2013). Soluble protein was estimated following the method given by Bradford (1976) wherein, precisely 0.1 mL of the sample was added with 5 mL of coomassine brilliant blue dye and vortexed after a 2 min incubation period and the absorbance was recorded at 595 nm using bovine serum albumin as standard.
Enzyme activity measurements
Activity of polyphenol oxidase (PPO) and peroxidase (PO) was measured according to the protocol given by Campos et al. (1996). For both PPO and PO, the data were reported as ∆A/min/mL × 100% (DasPurkayastha et al. 2012).
Sucrose neutral invertase (SNI) enzyme activity was measured using the method reported by Mao et al. (2007) with slight modifications. Briefly, 100 µL of 120 mM of sucrose was added to a mixture consisting of 50 µL of 1 M sodium acetate (pH 7.5) and 50 µL of the sample to be tested. The reaction was stopped after 60 min by boiling the mixture for 3 min. The concentration of glucose liberated was determined by Nelson Somogyi method (Sadasivam and Manickam 2007).
Microbiological analysis
Total plate count and total coliform count were performed using plate count agar and Rapid HiVeg coliform agar media, respectively. Briefly, 100 µL of the tender coconut water samples under study was taken and spread on the plates of respective media. The plates were than incubated at 37 °C for 24 h in an incubator for enumeration of colonies. The colony counts were carried out at 15 days’ interval till day 75 for non-microfiltered samples. The microfiltered samples were also analysed at 15 days’ interval till day 190. Those samples that showed coliform count above 10 CFU/mL were considered as non-acceptable as 10 CFU/mL of coliform is regarded as the critical limit in tender coconut water (FAO 2007).
Sensory analysis of tender coconut water samples during storage
The tender coconut water samples were also tested for sensory scores for the same parameters similar to that for standardizing honey concentration except for sweetness which was replaced by taste as a sensory attribute and was conducted by 10 semi trained panellist instead of 15 due to constrains of processed samples amount. During the storage studies the sensory analysis was carried out on day 0, 31, 61, 91 and 116 of storage just after getting the data for microbiological loads of the samples to ensure microbial safety to before consumption.
Haemolytic activity
The haemolytic activity assay was performed as per the protocol described by DasPurkayastha et al. (2014) briefly, fresh goat blood collected from a slaughter house in a centrifuge tube containing anticoagulant (3.2% trisodium citrate) was centrifuged at 700×g for 15 min at 4 °C (Remi C-30BL, India). The supernatant was discarded, and the red blood corpuscles (RBCs) were collected and washed three times with phosphate buffer saline (PBS, pH 7.4). A 10% (v/v) suspension of RBCs in PBS was prepared, to 1.9 mL of which 100 µL of sample was added and incubated for 1 h at 37 °C. Further, 1% Triton X-100 and PBS were taken as positive and negative controls, respectively. After incubation, the tubes were again centrifuged at 700×g for 15 min at 4 °C and the absorbance of the supernatant was measured in a UV–Visible spectrophotometer (Cecil Aquarious 7400, England) at 540 nm. The haemolytic activity was tested for the samples on day 0, 86 and 115. The percent haemolysis was calculated according to the formula
Statistics and kinetic studies
All experiments were carried out in triplicate except for experiments pertaining to sensory data and Hunter colour parameters where experiments were carried out with minimum of 10 readings and reported as mean ± standard deviation. Two factor factorial complete random design, Duncan’s multiple range tests and paired comparison t test were carried out on the data and the level of significance was tested at p ≤ 0.05 using IBM SPSS Statistic version 20 software. The storage studies data of the samples were subjected to kinetics studies by fitting zero order (Eq. 1) and first order (Eq. 2) reaction kinetics.
| 1 |
| 2 |
where P represents the parameter under study (pH, TSS, total soluble sugars, total reducing sugar, soluble protein, TA, FFA, antioxidant activity and phenolic content) at time ‘t’ and P0 are the parameters under consideration at time t = 0, and k0 and k1 are rate constants for zero order and first order reaction, respectively. The kinetic data was carried out to understand how reaction rate varied in the different samples during the storage study. Further co-efficient of determination (R2), and root mean square error (RMSE) were estimated for suitability of fit. R2 and RMSE were calculated using Windows Microsoft Excel (2007) package. RMSE was calculated as per the formula given below
where Pexp, i and Ppre, I are the ith experimental and predicted parameter respectively, ‘n’ is the number of observation. RMSE was calculated according to DasPurkayastha et al. (2012).
Results and discussion
Characteristics of honey and selection of its concentration in tender coconut water
The honey from Sohra was characterized as unifloral orange honey based on pollen analysis and had a refractive index of 1.4982 at 20 °C, moisture content of 15.4%, pH of 3.9, total acidity of 23.14 meq/kg, soluble protein content of 1.7 g/kg, a carbohydrate content of 828.8 g/kg and hydroxymethylfurfural content of 49.87 mg/kg. This data is based on previous experimental results of our lab (Mahnot et al. 2018). The sample was acceptable as per codex standard (Codex Alimentarius 2001) for honey quality based on its moisture content, total acidity as well as hydroxymethylfurfural as a quality marker. The results of sensory analysis of tender coconut water added with different levels of Sohra honey to select the acceptable level of addition are given in supplementary data file (Table S1). The results suggested that sample with 5% w/v of honey did not significantly change the overall acceptability of tender coconut water. At concentration above 5%, there was significant reduction in the flavour, color and thereby overall acceptability of tender coconut water. Thus, honey at a 5% concentration level was selected for addition in tender coconut water.
Changes in physicochemical properties on refrigerated storage
Changes in pH
On storage the pH did not change significantly in any sample under study although a decreasing trend was observed. Statistical analysis showed a significant difference in the pH among the samples that were not microfiltered (TCW, TCWA, TCWH and TCWAH) while their corresponding microfiltered samples did not show any effect (Fig. 1a). A slight increase was noticed in microfiltered samples on day 115 of analysis. Thus, microfiltration technique helped to keep the pH stable during storage. Microfiltered samples on day 115 showed a rise in pH which might be due to greater protein denaturation (Mgaya-Kilima et al. 2014).
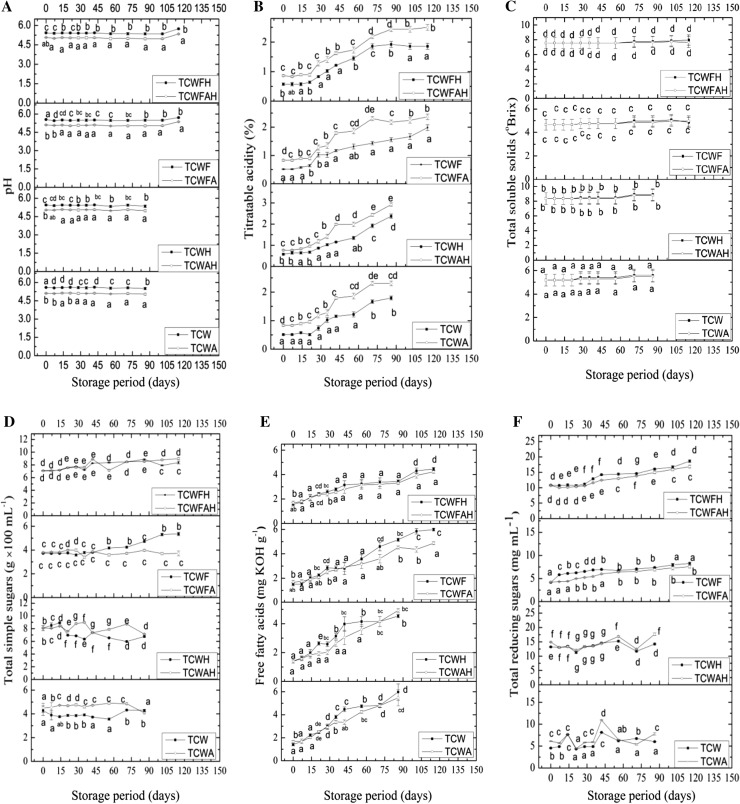
Fig. 1.
Comparative changes in physiochemical analysis of storage data of the non-microfiltered and microfiltered samples a pH, b titratable acidity, c Total soluble solids, d total simple sugars, e free fatty acid, f total reducing sugars. Different letters (a, b, c…) on data corresponding to a particular day of analysis states significant change between the different samples at p < 0.05 using Duncan’s mutilple range test
Changes in titratable acidity (TA)
The titratable acidity (TA) increased during storage in all the samples under study as shown in Fig. 1b. The increase in TA in non-microfiltered samples can be attributed to production of free acids due to microbial growth (DasPurkayastha et al. 2012; Durrani et al. 2010). On the other hand, the increase in TA in microfiltered samples might be due to hydrolysis or conversion of polysaccharide with time (Bhardwaj and Pandey 2011). The presence of additives significantly increased the TA by 35.8% as compared to TCW due to the H + ions present in them and this also decreased the pH. Microfiltration, on the other hand, did not have any significant effect on TA changes. After a gradual increase in microfiltered samples, TA had stabilised after day 75 of storage and remained stable up to day 115.
Changes in total soluble solids (TSS)
The change in TSS in the samples under study is shown in Fig. 1c. The TSS content of tender coconut water was 5.20 ± 0.50 oBrix whereas on addition of honey, it increased to 8.4 ± 0.80 oBrix. Microfiltration marginally changed the oBrix values owing to minimal changes in soluble components. Although there was no statistically significant change during storage of the samples, a slight decrease in the unfiltered samples was observed which can be attributed to microbial activity during the storage period.
Changes in total simple sugars
The changes in total simple sugars during the storage study of the samples are shown in Fig. 1d. Data on the initial day of analysis suggests that microfiltration caused a reduction of 13.4% as compared to non-microfiltered samples which may be due to retention on the microfiltering membrane. In comparison, Reddy et al. (2007) reported a 23% loss in sugar on microfiltration. The addition of honey caused a spike in total simple sugars in tender coconut water by 45.74%. The changes in total simple sugars during storage of non-microfiltered samples viz. TCW, TCWA, TCWH and TCWAH did not follow any order even though a decreasing trend in the total sugar content was expected. This might possibly be due to the presence of different sets of microbes naturally present in the samples as well as their uneven distribution during the bottle filling stage. In the filtered samples a definite trend could be observed. An increase in total soluble solids was noted in TCWF, TCWFH and TCWFAH, while in TCWFA the content remained more or less unaffected during the storage period which might be due to the stability provided by the added additives viz. citric acid and ascorbic acid. The nonspecific trend in non-microfiltered samples could be due to variable microbial activity in the samples.
Changes in total free fatty acids (FFA)
Figure 1e. shows that the FFA gradually increased in all the samples during storage. On comparing the data for non-microfiltered samples (TCW, TCWA, TCWH and TCWAH) between day 0 and the last day of analysis i.e. day 86, it was inferred that the presence of additives helped in reducing the increase of FAA during storage. The increase in FFA between zero and last day of storage in TCW was 4.26 ± 0.60 fold; for TCWA was 3.25 ± 0.54 fold; for TCWH was 3.23 ± 0.02 fold; and for TCWAH was 3.51 ± 0.04 fold. Microfiltration affected the initial FFA content in TCWF and TCWFA on day 0 as compared to TCW and TCWA. There was an initial increase of 1.72 ± 0.03 fold and 1.68 ± 0.12 fold in TCWFH and TCWFAH, respectively as compared to the corresponding unfiltered samples (TCWH and TCWAH). This might be due to the longer processing time taken for microfiltration with the addition of honey. The extent of increase in FFA in microfiltered samples was less as compared to non-microfiltered samples. The increase in FFA on day 0 and day 115 in TCWF was 3.95 ± 0.45 fold; in TCWFA was 2.86 ± 0.32 fold; in TCWFH was 1.84 ± 0.16 fold; and in TCWFAH was 1.79 ± 0.14 fold. Thus use of ascorbic acid and citric acid as additives in tender coconut water was found to be effective in reducing the FFA generation during storage.
The higher increase in FFA in unfiltered samples might be due to microbial activity as well as due to changes in the headspace environment of the bottles. Due to longer storage time, oxygen might have seeped into the headspace which was initially filled with nitrogen. In the microfiltered samples it was observed that there was a slower increase in FFA till 45 days of storage but after that there was an abrupt increase in FFA which might be due to the changes in headspace environment on prolonged storage.
Total reducing sugars
The changes in total reducing sugars in the non-microfiltered and microfiltered samples are shown in Fig. 1f. It was observed that in unfiltered samples (TCW, TCWA, TCWH, and TCWAH) there was a variable change in reducing sugar content which might be due to differences in the kind of microbial activity. However, as compared to the level at day 0 there was a significant increase on the final day of analysis for unfiltered samples. On analysing the effect of additives i.e. citric acid, ascorbic acid and honey it was observed that they caused an increase in the reducing sugar content for unfiltered samples. Addition of honey increased the reducing sugars by 58.4%. Microfiltration, on the other hand caused an overall 21.5% reduction in total reducing sugars. On comparison of the micro-filtered samples, it was observed that when only citric acid and ascorbic were added together the extent of increase in the reducing sugar content was reduced. Honey alone caused an increase in the reducing sugar content on storage but in samples having honey, citric acid and ascorbic acid the extent of increase in reducing sugar was reduced. The increase in reducing sugars is attributed to the presence of natural enzyme like invertase in coconut water (Rosario et al. 1979) as well as in honey (Sporns 1992). Presence of sucrose neutral invertase (SNI) activity in the studied samples (Fig. 3c) caused an increase in the reducing sugar content in all the samples. Similar increase in reducing sugar content due to SNI has been reported by Mao et al. (2007) in their studies conducted on sugarcane juice.
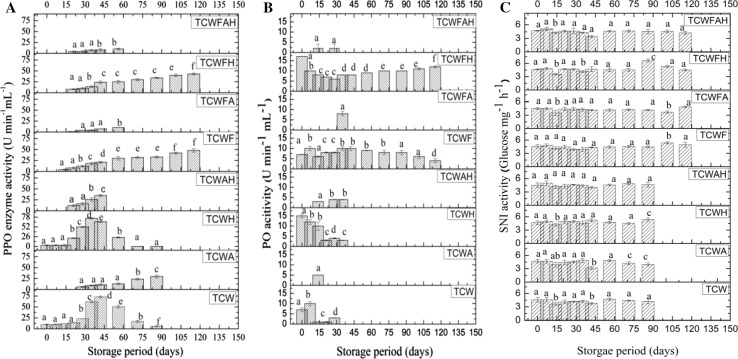
Fig. 3.
Monitoring activity of enzymes a Polyphenol oxidase, b Peroxidase and c Sucrose neutral invertase activity during storage of non-microfiltered and microfiltered tender coconut water samples during storage. Different letters (a, b, c…) on data corresponding to a particular sample states significant change with time at p < 0.05 using Duncan’s multiple range test
Changes in soluble protein
Figure 2a shows the changes in soluble protein in the samples under study. A loss of 13.06% of soluble protein was noted in the microfiltered samples as compared to non-microfiltered samples. Addition of honey caused 22% increase of soluble protein in samples as compared to samples without added honey. A decrease in protein content was observed with storage time in all the samples although an initial rise on addition of honey was observed. A significant decrease in protein was observed on microfiltration in all the samples compared to unfiltered samples initially which might be due to retention on the microfiltering membranes. Microfiltration and additives were observed to delay the degradation process. The decrease in content might be due to oxidation of proteins by lipid degradation products like peroxides (Davies 2016), due to interaction of proteins with phenolic compounds (Cheynier 2005), and also due to hydrolysis of protein during storage (Kulkarni and Aradhya 2005).
Fig. 2.
Comparative changes in a soluble protein, b antioxidant activity, c Phenolic content, d Hunter L value, e Hunter a value, f Hunter b value in non-microfiltered and microfiltered of variously processed tender coconut water samples during storage. Different letters (a, b, c…) on data corresponding to a particular day of analysis states significant change between the different samples at p < 0.05 using Duncan’s multiple range test
DPPH radical scavenging activity
Figure 2b shows the changes in antioxidant activity of the samples under study. TCW had a low antioxidant activity of 6.18 ± 2.0%. As expected, samples with ascorbic acid and honey (TCWA, TCWH, TCWFA and TCWFH) exhibited higher DPPH scavenging activity than TCW. The antioxidant activities of the non-microfiltered samples were: TCWA 52.71 ± 3.41%; TCWH 8.77 ± 2.23% and TCWAH 47.52 ± 2.24%. Further microfiltration did not significantly cause changes in antioxidant activity and the corresponding values of the microfiltered samples were: TCWF 7.71 ± 2.00%; TCWFA 51.56 ± 5.50%; TCWFH 9.18 ± 2.10%; and TCWFAH 45.49 ± 3.21%. There was no significant difference between TCWAH and TCWFAH as compared to samples without honey i.e. TCWA and TCWFA. During the storage period no significant changes were observed in antioxidant activity of TCW, TCWH, TCWF and TCWFA. In TCWA and TCWAH, an irregular change in antioxidant activity was observed which could be attributed to the growth of microbes. The microbial fermentation might have cause the release of molecules which have good antioxidant properties like peptides, amino acids or due to release of some bound phenolics and further fermentation and storage period could have caused hydrolysis of the generated peptides or conversion of phenolics causing lowering in antioxidant activity (Porto et al. 2017; Chen et al. 2018) or degradation of ascorbic acid which might have also effected the lowering of antioxidant activity. In the micro-filtered TCWA, TCWAH samples, a steady decline in the antioxidant activity was observed. Also the antioxidant activity in sample TCWFAH was more stable than that of TCWA which might be due to presence of phenolic compounds from honey.
Changes in phenolic content
The changes in phenolic content in the samples are shown in Fig. 2c. The data shows that the total phenols show a gradual pattern in decrease during storage in the microfiltered samples TCWF, TCWFA, TCWFH and TCWFAH. A similar decrease in the phenolic content during storage have been also noticed in other clarified juices using microfiltration like banana and pineapple juices (Lee et al. 2007; Laorko et al. 2013) While an irregular pattern was observed for non microfiltered samples which probably might be due to microbial activity causing release of bound phenolics causing an initial increase such results have also been demonstrated in fermentation of grape juice (Khanniri et al. 2018) while further microbial activity lowered the phenolic content except for TCW where the phenolic content increased on the last day of analysis i.e. day 88. The phenolic content (Fig. 2c) could be related to the total antioxidant activity (Fig. 2b) of TCWA and TCWH samples as both followed the same pattern.
Hunter Lab parameters
Figure 2d, e and f respectively shows the changes in Hunter color L, a, b values for the samples under study during storage. The L values for the sample showed a gradual increasing trend with time in both microfiltered and unfiltered samples but the change was not significant. An initial increase in the non-microfiltered samples was noted which might be due to turbidity in the samples caused by microbial growth. The addition of citric acid, ascorbic acid and honey did not have a significant effect on the changes in L values. Very slight changes occurred in the Hunter a values during storage period of the microfiltered as well as unfiltered samples. In the unfiltered sample the addition of citric acid and ascorbic acid did not significantly induce any changes while addition of honey slightly increased the a values suggesting a slight redness in samples. In microfiltered samples TCWFA and TCWFAH the a values remained in the negative region possibly due to degradation of ascorbic acid during storage which is also supposed to cause color changes due to non-enzymatic browning reaction (Li et al. 2016) thereby shifting more towards the green region while samples TCWF and TCWFH the a values did not show a significant change during storage and remained in the positive region i.e. towards the red region. A gradual decrease in the Hunter b values was noted for all the samples during storage. The colour data suggested that colour of tender coconut water is preserved better without the addition of ascorbic acid and citric acid.
Polyphenol oxidase (PPO), peroxidase (PO) and sucrose neutral invertase (SNI) activities
Figure 3a–c, respectively depicts the activity of polyphenol oxidase (PPO), peroxidase (PO) and sucrose neutral invertase (SNI) during the period of study. A significant decrease in PPO activity was observed in samples where additives viz. citric acid, ascorbic acid and honey were added. On comparing TCW and TCWF, it is evident that microfiltration was able to reduce PPO activity possibly due to retaining of enzymes on the membrane evident from the loss of proteins on microfiltration. With increase storage period higher PPO activity was noticed in unfiltered samples viz. TCW, TCWH and TCWAH which can be attributed due to residual oxygen present in the bottles while further microbial growth caused utilization of all the oxygen in the bottle causing lowered PPO activity. While in unfiltered samples TCWA degradation of ascorbic acid might have caused an increase in PPO activity during storage. In the microfiltered sample viz. TCWF and TCWFH, initially the PPO activity was not detected but with time the activity increased probably due to changes in the headspace environment. In TCWFA and TCWFAH, citric acid and ascorbic acid controlled the PPO activity. Citric acid and ascorbic acid also controlled the PO activity in TCWA, TCWAH, TCWFA and TCWFAH. Similar results of lowering of PPO and PO activity on addition of ascorbic acid and citric acid has been reported for coconut water as well as sugarcane juice processing (DasPurkayastha et al. 2012; Mao et al. 2007). On the other hand microfiltration and honey addition did not have any effect on the activity of peroxidase enzyme but citric acid and ascorbic acid addition were able to reduce the activity of the enzymes. SNI activity did not change much during the storage study in TCW. Even microfiltration as well as the addition of additives viz. ascorbic acid, citric acid and honey did not significantly affect SNI activity. This can be related to the increase in reducing sugar during storage (Fig. 1f) Mao et al. (2007) observed reduction in SNI activity in sugarcane juice on addition of ascorbic acid but in the current study the concentration of ascorbic acid used was not sufficient enough to cause change in the SNI activity.
Microbial analysis
Microbial load of non- microfiltered samples increased with storage (Table 1). Taking into consideration the microbial quality criteria (FAO 2007), all the unfiltered samples (TCW, TCWA, TCWH and TCWAH) were at par with the quality standards for 30 days. The total coliform count exceeded 10 CFU/mL but the total plate count was well below 5000 CFU/mL till day 75 of storage. No further analysis was carried out beyond day 75 of storage. In the microfiltered samples, no microbial count was detected both on the total plate count agar as well as on coliform plates till day 190 of storage (period of storage study). Thus microfiltration of the samples under study did help in obtaining microbe free products ensuring its quality and proved to be an effective processing technique.
Table 1.
Total plate counts and total coliform counts in non microfiltered tender coconut water samples during the storage period
| Storage (days) | Total plate count (CFU/mL) | Total coliforms (CFU/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| TCW | TCWA | TCWH | TCWAH | TCW | TCWA | TCWH | TCWAH | |
| 0 | 48 ± 4.0a | 40 ± 8.0a | 38 ± 6a | 43 ± 5a | ND | ND | ND | ND |
| 15 | 72 ± 6.0b | 53 ± 7.0b | 44 ± 2b | 68 ± 4b | ND | ND | ND | ND |
| 30 | 97 ± 9.0c | 86 ± 2.0c | 67 ± 7c | 91 ± 9c | ND | ND | ND | ND |
| 45 | 126 ± 11d | 118 ± 12d | 121 ± 9d | 145 ± 16d | 13 ± 3d | 9 ± 1d | 17 ± 2d | 22 ± 3d |
| 60 | 168 ± 12e | 144 ± 18e | 159 ± 11e | 174 ± 8e | 22 ± 6e | 20 ± 4e | 31 ± 2e | 39 ± 7e |
| 75 | 193 ± 17f | 182 ± 22f | 194 ± 19f | 211 ± 24f | 52 ± 9f | 45 ± 5f | 67 ± 8f | 74 ± 8f |
Different letters (a, b, c…) in the column suggests significant difference at p < 0.05significance level at different days of storage using Duncan’s multiple range tests
ND indicates below detection limit i.e. non detectable
Sensory analysis
The data of sensory analysis of tender coconut water samples during storage (Table 2) shows an overall decrease in sensory scores. No significant changes in sensory scores for unfiltered samples (TCW, TCWA, TCWH and TCWAH) as compared to microfiltered sample (TCWF, TCWAF, TCWFH, TCWFAH) on day 0. The unfiltered samples underwent significant loss (based on paired sample t-test) in sensory scores on day 31 as compared to day 0 owing to microbial growth. As the total coliform count exceeded 10 CFU/mL (Table 1), the sensory experiments were not carried out for unfiltered samples after 31 days of storage. No significant difference in sensory scores was observed among the microfiltered samples while the storage time had a significant effect on the sensory scores. TCWF did not have a significant decrease in aroma, taste and color at the end of day 61 but a significant change in overall acceptability was observed for the same storage time with further loss in sensory score till the end of day 116 making it unacceptable. Similar was the case with TCWFA, TCWFH and TCWFAH. The colour on the other hand for the microfiltered samples did change significantly though not drastic i.e. the colour of the samples were still accepted by the panelist even at the end of day 116. The data suggested that the sensory properties of the microfiltered samples were acceptable up to day 91 of storage.
Table 2.
Sensory scores for attributes of tender coconut water samples during the storage period
| Days of storage | Sensory parameters | TCW | TCWA | TCWH | TCWAH | TCWF | TCWFA | TCWFH | TCWFAH |
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Aroma | 7.8 ± 1.2a,x | 8.1 ± 0.5a,x | 7.9 ± 0.6a,x | 7.8 ± 0.6a,x | 7.4 ± 1.3a,x | 7.8 ± 0.4a,x | 7.6 ± 0.5a,x | 7.7 ± 0.7a,x |
| Taste | 7.7 ± 1.2a,x | 8.3 ± 0.7a,x | 7.4 ± 1.3a,x | 7.7 ± 1.1a,x | 7.4 ± 1.3a,x | 8.4 ± 0.7a,x | 7.3 ± 1.2a,x | 8.0 ± 0.9a,x | |
| Appearance | 8.5 ± 0.5ab,x | 8.5 ± 0.5ab,x | 8.1 ± 0.8b,x | 8.1 ± 0.87b,x | 8.8 ± 0.4a,x | 8.8 ± 0.4a,x | 8.4 ± 0.7ab,x | 8.3 ± 0.7ab,x | |
| Overall acceptability | 8.1 ± 0.7ab,x | 8.7 ± 0.5b,x | 7.8 ± 1.03a,x | 7.9 ± 0.9a,x | 7.8 ± 0.6a,x | 8.2 ± 0.6ab,x | 7.9 ± 0.9a,x | 8.2 ± 0.6ab,x | |
| Day 30 | Aroma | 4.9 ± 1.1a,x | 4.1 ± 1.3b,x | 3.2 ± 1.3cb,x | 2.3 ± 1.2c,x | 7.1 ± 1.3d,x | 7.6 ± 0.5d,x | 7.5 ± 0.5d,x | 7.6 ± 0.7d,x |
| Taste | 3.3 ± 1.4a,x | 2.8 ± 1.4a,x | 2.5 ± 0.8a,x | 1.5 ± 0.7b,x | 7.0 ± 1.2c,x | 8.2 ± 0.8d,x | 7.2 ± 1.2 cd,x | 7.8 ± 0.9 cd,x | |
| Appearance | 5.4 ± 0.7a,x | 4.0 ± 1.6b,x | 3.4 ± 1.3b,x | 1.3 ± 0.5c,x | 8.7 ± 0.5d,x | 8.7 ± 0.7d,x | 8.3 ± 0.7d,x | 8.4 ± 0.7d,x | |
| Overall acceptability | 3.7 ± 1.5a,x | 2.1 ± 1.2b,x | 4.0 ± 2.1a,x | 1.6 ± 0.7b,x | 8.0 ± 0.7c,x | 8.0 ± 0.8c,x | 8.0 ± 0.8c,x | 8.0 ± 0.6c,x | |
| Day 60 | Aroma | – | – | – | – | 6.4 ± 1.4a,x | 6.3 ± 0.7a,y | 6.3 ± 1.1a.y | 5.9 ± 1.4a,y |
| Taste | – | – | – | – | 6.6 ± 1.1a,x | 6.2 ± 1.2a,y | 6.5 ± 0.8a,y | 7.8 ± 0.9a,y | |
| Appearance | – | – | – | – | 8.7 ± 0.5a,x | 8.6 ± 0.8a,y | 8.2 ± 0.6a,x | 6.0 ± 1.3a,x | |
| Overall acceptability | – | – | – | – | 6.6 ± 1.3a,y | 6.7 ± 0.9a,y | 6.9 ± 1.7a,y | 7.7 ± 0.7a,x | |
| Day 90 | Aroma | – | – | – | – | 4.8 ± 1.7a,y | 4.7 ± 2.3a,z | 4.0 ± 0.7a.z | 4.2 ± 1.9a,z |
| Taste | – | – | – | – | 4.9 ± 1.6a,y | 4.9 ± 1.4a,z | 5.5 ± 0.8a,z | 5.2 ± 1.2a,z | |
| Appearance | – | – | – | – | 8.5 ± 0.5a,y | 8.5 ± 0.8a,z | 8.3 ± 0.7a,y | 7.6 ± 1.1a,y | |
| Overall acceptability | – | – | – | – | 4.9 ± 0.7a,y | 5.0 ± 1.3a,z | 5.3 ± 1.0a,z | 4.9 ± 1.4a,y | |
| Day 115 | Aroma | – | – | – | – | 2.6 ± 1.5a,z | 2.6 ± 1.4a,z | 2.1 ± 0.9a,w | 2.9 ± 1.3a,w |
| Taste | – | – | – | – | 3.7 ± 1.5a,y | 2.5 ± 1.1b,z | 3.9 ± 1.3a,z | 2.6 ± 1.2a,z | |
| Appearance | – | – | – | – | 8.0 ± 0.8a,y | 7.4 ± 1.1a,w | 7.9 ± 0.7b,y | 6.9 ± 1.2b,y | |
| Overall acceptability | – | – | – | – | 3.1 ± 1.3a,w | 3.8 ± 1.1a,w | 2.7 ± 1.4a,w | 3.2 ± 1.4a,z |
Number of panellists = 10
Different letters (a, b, c, d) in the rows suggests significant difference in attributes between samples at a particular storage day and different letter (w, x, y, z) in the columns suggests significant in attributes difference between samples at different days of storage at significance p < 0.05 level using Duncan’s multiple range tests. For non-microfiltered samples between 0 and 30th day paired comparison t-test was performed to ascertain significance at p < 0.05
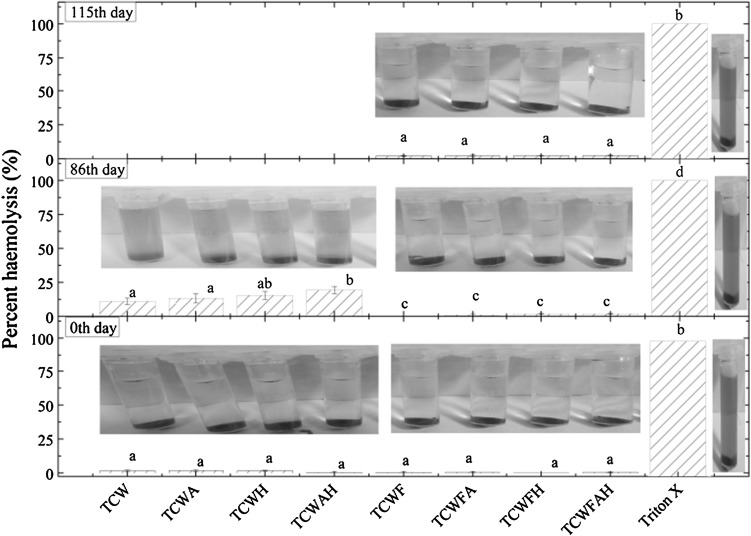
Haemolysis
Tender coconut water is supposed to be sterile and has been used in remote locations or during armed conflicts as a short-term intravenous hydration fluid (Campbell-Falck et al. 2000). Thus, measuring the haemolytic activity of the processed tender coconut water samples seemed important. Figure 4 shows the haemolytic activity of the samples on day 0, 86 and 115 of storage. The results suggested that both microfiltered and non-microfiltered tender coconut water did not have any haemolytic effect initially even with the addition of honey, citric acid and ascorbic acid. TCW, TCWA, TCWH and TCWAH on day 86 showed an increase in haemolytic activity which might be attributed to the presence of microbes causing chemical changes to the samples and formation of toxic compounds. In comparison, TCWF, TCWFA, TCWFH and TCWFAH did not show any significant haemolytic activity after day 115 of storage. Thus it could be inferred that processing conditions and storage of microfiltered samples did not cause toxicity.
Fig. 4.
Percent haemolysis of variously processed tender coconut water samples during storage. Different letters (a, b, c, d) denotes significant difference between samples (p < 0.05) using Duncan Multiple range tests
Statistics
A two factor factorial complete random design was carried out on the data pertaining to non-microfiltered and microfiltered samples with and without honey and additives. It was observed that among the parameters studied, filtration significantly (p ≤ 0.05) affected the changes in total soluble sugars, total soluble solids, free fatty acids, reducing sugars, total soluble protein, total phenolics and Hunter a and b values. Further, it was also observed that the addition of honey and additives led to significant (p ≤ 0.05) changes in pH, total titratable acidity, total soluble solids, reducing sugars, antioxidant activity, total phenolics content and Hunter L, a and b values. The activities of enzymes PO and PPO changed significantly (p ≤ 0.05) for both factors, i.e., filtration as well as addition of honey and additives. On the other hand, no significant (p ≥ 0.05) changes in SNI activity were observed for both filtration and addition of honey and additives. Statistical data are given as Supplementary data.
Kinetic analysis
Mathematical analysis of the data as shown in supplementary data (Table S2) suggests that changes in TA, FFA and reducing sugars followed a zero order reaction while total soluble solids, protein and antioxidant followed a first order reaction during storage due to better coefficient of determination and RMSE values. (Supplementary data 2). In microfiltered samples, honey alone or in combination with ascorbic and citric acid was able to reduce the rate constants in the microfiltered samples for changes in TA, FFA, total soluble solids and antioxidant activity. Addition of additives increased the rate for changes in reducing sugar. Addition of ascorbic acid and citric acid reduced the reaction rates for soluble protein changes while honey caused an increase. Further the kinetic data was inconclusive to ascertain reaction order for changes in pH and soluble sugars probably due to insufficient changes. Even though changes in phenolic content could not be ascertained probably because of very large RMSE value, first order reaction was apparent. Rate of change was difficult to ascertain in non-microfiltered samples due to variable microbial activity and reactions orders.
Conclusion
In conclusion, the current study proved that microfiltration along with addition of additives, honey and packaging in glass bottles in nitrogen flushed atmosphere helped in obtaining a sterile product from tender coconut water up to day 90 of refrigerated storage. The supplements ascorbic acid (180 mg/L), citric acid (200 mg/L) and honey (5%) were were the effective additives. Microfiltration caused minimal impact on the physicochemical properties of the different samples while addition of orange honey caused an increase in soluble protein in tender coconut water. The microfiltered products were sterile for a period of 190 days with no haemolytic activity after packaging during storage. The addition citric acid and ascorbic acid showed to provide stability and effectively reduced browning enzyme activity thereby increased shelf life. Also addition of orange honey had a sweetening effect and was acceptable to the panel members. The microfiltered samples had a shelf life of 115 days but was acceptable for sensory properties till day 91 of refrigerated storage. Honey alone or with acids helped to maintain antioxidant activity during storage. Sensory attributes are important for a marketable product and additional research on externally added flavour delivery strategies may be helpful for further enhancing shelf life along with acceptable sensory properties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The author is thankful to Department of Science and Technology, New Delhi for grant of DST INSPIRE fellowship (DST/INSPIRE Fellowship/2013/[482]). The authors thank the faculty members, lab members and other research scholars of the department of Food Engineering and Technology, Tezpur University for participating in sensory studies. The equipment facilities provided by AICTE-NEQIP and DST-FIST are acknowledged.
Compliance with ethical standards
Conflict of interest
We declare there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nikhil Kumar Mahnot, Email: nikhil.mahnot@gmail.com.
Kuldeep Gupta, Email: guptak@tezu.ernet.in.
Charu Lata Mahanta, Phone: +91-3712-267008 (5702), Email: charu@tezu.ernet.in.
References
- Alvarez-Suarez JM, Tulipani S, Díaz D, Estevez Y, Romandini S, Giampieri F, Damiani E, Astolfi P, Bompadre S, Battino M. Antioxidant and antimicrobial capacity of several monofloral cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem Toxicol. 2010;48(8–9):2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- AOAC (2005) Official methods of analysis. In: Association of official analytical chemists, 18th, edn. AOAC, Washington, DC
- Balogu T, Towobola O. Production and quality analysis of wine from honey and coconut milk blend using Saccharomyces cerevisiae. Fermentation. 2017;3(2):16. doi: 10.3390/fermentation3020016. [DOI] [Google Scholar]
- Bhama S, Karthikeya T, Ramesh T, Gopinathan S. Development and nutritional impact of ready to serve (rts) juice from selected edible resources including indigenous fruits and vegetables of Indian origin. Am J Food Technol. 2013;8(2):102–113. doi: 10.3923/ajft.2013.102.113. [DOI] [Google Scholar]
- Bhardwaj RL, Pandey S. Juice blends—a way of utilization of under-utilized fruits, vegetables, and spices: a review. Crit Rev Food Sci Nutr. 2011;51(6):563–570. doi: 10.1080/10408391003710654. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell-Falck D, Thomas T, Falck TM, Tutuo N, Clem K. The intravenous use of coconut water. Am J Emerg Med. 2000;18(1):108–111. doi: 10.1016/S0735-6757(00)90062-7. [DOI] [PubMed] [Google Scholar]
- Campos CF, Souza PEA, Coelho JV, Glória MBA. Chemical composition, enzyme activity and effect of enzyme inactivation on flavor quality of green coconut water. J Food Process Preserv. 1996;20(6):487–500. doi: 10.1111/j.1745-4549.1996.tb00761.x. [DOI] [Google Scholar]
- Chen R, Chen W, Chen H, Zhang G, Chen W. Comparative evaluation of the antioxidant capacities, organic acids, and volatiles of papaya juices fermented by Lactobacillus acidophilus and Lactobacillus plantarum. J Food Qual. 2018;2018:1–12. [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(1):223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission (2001) Revised Codex Standard for Honey Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001) Codex Standard, vol 12. 1981, pp 1–7. https://teca.fao.org/sites/default/files/resources/Annex%20A%20Codex%20Alimentarius%20Honey%20Standard.pdf. Accessed 10 June 2017
- DasPurkayastha M, Kalita D, Mahnot NK, Mahanta CL, Mandal M, Chaudhuri MK. Effect of l-ascorbic acid addition on the quality attributes of micro-filtered coconut water stored at 4 °C. Innov Food Sci Emerg Technol. 2012;16:69–79. doi: 10.1016/j.ifset.2012.04.007. [DOI] [Google Scholar]
- DasPurkayastha M, Manhar AK, Das VK, Borah A, Mandal M, Thakur AJ, Mahanta CL. Antioxidative, hemocompatible, fluorescent carbon nanodots from an “End-of-Pipe” agricultural waste: exploring its new horizon in the food-packaging domain. J Agric Food Chem. 2014;62(20):4509–4520. doi: 10.1021/jf500138f. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Protein oxidation and peroxidation. Biochem J. 2016;473(7):805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrani Y, Ayub M, Muhammad A. Physicochemical response of apple pulp to chemical preservatives and antioxidant during storage. Int J Food Safety. 2010;12:20–28. [Google Scholar]
- FAO (2007) How to bottle coconut water. Spotlight. http://www.fao.org/ag/magazine/0701sp1.htm. Accessed 16 May 2017
- Haseena M, Kasturi Bai KV, Padmanabhan S. Post-harvest quality and shelf-life of tender coconut. J Food Sci Technol. 2010;47(6):686–689. doi: 10.1007/s13197-010-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israili ZH. Antimicrobial Properties of Honey. Am J Ther. 2014;21(4):304–323. doi: 10.1097/MJT.0b013e318293b09b. [DOI] [PubMed] [Google Scholar]
- Khanniri E, Sohrabvandi S, Mortazavian AM, Khorshidian N, Malganji S. Effect of fermentation, cold storage and carbonation on the antioxidant activity of probiotic grape beverage. Curr Nutr Food Sci. 2018;14(4):335–340. doi: 10.2174/1573401313666170614100418. [DOI] [Google Scholar]
- Krushna NSA, Kowsalya A, Radha S, Narayanan RB. Honey as a natural preservative of milk. Indian J Exp Biol. 2007;45(5):459–464. [PubMed] [Google Scholar]
- Kulkarni AP, Aradhya SM. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;93(2):319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- Laorko A, Tongchitpakdee S, Youravong W. Storage quality of pineapple juice non-thermally pasteurized and clarified by microfiltration. J Food Eng. 2013;116(2):554–561. doi: 10.1016/j.jfoodeng.2012.12.033. [DOI] [Google Scholar]
- Lee WC, Yusof S, Hamid NSA, Baharin BS. Effects of fining treatment and storage temperature on the quality of clarified banana juice. LWT: Food Sci Technol. 2007;40(10):1755–1764. doi: 10.1016/j.lwt.2006.12.008. [DOI] [Google Scholar]
- Li Y, Yang Y, Yu AN. Effects of reaction parameters on generation of volatile compounds from the Maillard reaction between L-ascorbic acid and glycine. Int J Food Sci Technol. 2016;51(6):1349–1359. doi: 10.1111/ijfs.13106. [DOI] [Google Scholar]
- Mahnot NK, Kalita D, Mahanta CL, Chaudhuri MK. Effect of additives on the quality of tender coconut water processed by nonthermal two stage microfiltration technique. LWT: Food Sci Technol. 2014;59(2):1191–1195. doi: 10.1016/j.lwt.2014.06.040. [DOI] [Google Scholar]
- Mahnot NK, Sangeeta S, Mahanta CL. Quality characterization and effect of sonication time on bioactive properties of honey from North East India. J Food Sci Technol. 2018;10:11–12. doi: 10.1007/s13197-018-3531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LC, Xu YQ, Que F. Maintaining the quality of sugarcane juice with blanching and ascorbic acid. Food Chem. 2007;104(2):740–745. doi: 10.1016/j.foodchem.2006.09.055. [DOI] [Google Scholar]
- Mgaya-Kilima B, Remberg SF, Chove BE, Wicklund T. Influence of storage temperature and time on the physicochemical and bioactive properties of roselle-fruit juice blends in plastic bottle. Food Sci Nutr. 2014;2(2):181–191. doi: 10.1002/fsn3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto M, Okina V, Pimentel T, Prudencio S. Physicochemical stability, antioxidant activity, and acceptance of beet and orange mixed juice during refrigerated storage. Beverages. 2017;3(3):36. doi: 10.3390/beverages3030036. [DOI] [Google Scholar]
- Reddy KV, Das M, Das SK. Filtration resistances in non-thermal sterilization of green coconut water. J Food Eng. 2005;69(3):381–385. doi: 10.1016/j.jfoodeng.2004.08.029. [DOI] [Google Scholar]
- Reddy KV, Das M, Das SK. Nonthermal sterilization of green coconut water for packaging. J Food Qual. 2007;30(4):466–480. doi: 10.1111/j.1745-4557.2007.00136.x. [DOI] [Google Scholar]
- Rosario RR, Aldaba R, Teodoro E. Biochemical changes in the developing coconut fruit (Cocos nucifera) Res Bull. 1979;34(2):107–130. [Google Scholar]
- Sadasivam S, Manickam A. Biochemical Methods. 3. New Delhi: New Age International Publishers; 2007. [Google Scholar]
- Saikia S, Mahanta CL. Effect of steaming, boiling and microwave cooking on the total phenolics, flavonoids and antioxidant properties of different. Int J Food Nutri Sci. 2013;2:47–53. [Google Scholar]
- Sporns P. Honey analysis. In: Hui YH, editor. Encyclopedia of food science and technology. 1. New York: Wiley; 1992. pp. 1417–1422. [Google Scholar]
- Sucupira NR, Alves Filho EG, Silva LMA, de Brito ES, Wurlitzer NJ, Sousa PHM. NMR spectroscopy and chemometrics to evaluate different processing of coconut water. Food Chem. 2017;216(1):217–224. doi: 10.1016/j.foodchem.2016.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.