Abstract
The effects of heating (90 °C/30 min) or ultrasound (200/400/600 W) treatment on antioxidant and angiotensin-converting enzyme inhibitory (ACEI) activity of hydrolysates from hempseed protein isolates (HPI) were studied. The secondary structure, surface hydrophobicity, intrinsic fluorescence, scanning electron microscopy (SEM) and sodium dodecyl sulfate–polyacrylamide gel electrophoresis of HPI treated by heating or ultrasound were measured. The results showed that hydrolysate from HPI treated with ultrasound at 200 W showed higher hydrolysis degree, proportion of lower molecular mass components (1.0–3.0 kDa), antioxidant and ACEI activity than those from heating or high-power treated. The changes in secondary structure, surface hydrophobicity and intrinsic fluorescence indicated the unfolding of HPI after ultrasound. The SEM results showed that HPI treated with ultrasound at 200 W exhibited decrease in particle size and deformation and further increased in power caused the aggregates of HPI. In conclusion, the ultrasound treatment at low-power was superior to 90 °C/30 min treatment in facilitating enzymatic release of antioxidant and ACEI peptides from HPI.
Keywords: Hempseed protein, Antioxidant activity, ACE inhibitory activity, Ultrasound
Introduction
Hemp (Cannabis sativa L.) is widely cultivated plant of great industrial importance in China, and its seed is a valuable source of Chinese medicine and pharm food. The seed typically contains about 2.5 g kg−1 proteins which contain essential amino acids and can be easily digested than soy protein (Tang et al. 2006; Wang et al. 2008). However, seeds of hemp have been only used for the extract of oil for a long period in China resulting in abundant protein waste. Recently, hempseed protein isolates (HPI) have attracted more and more attention as an food ingredient, which is incorporated into many food (such as milk products and snack food) (House et al. 2010). In fact, functional properties of hempseed protein were poor compared to those of soy protein isolate, which limited greatly its application in industry (Tang et al. 2006). Therefore, it is necessary to employ modern technique to improve its undesirable properties in order to utilize this nutritional protein preferably.
Recently, HPI have been applied for preparation of bioactive peptides which exerted various activities, such as antioxidant, antihypertensive, α-glucosidase inhibitory (Girgih et al. 2014, 2011; Ren et al. 2016; Malomo et al. 2015). Six commercial proteases, including Flavourzyme, Protamex, Neutrase, Papain, Trypsin and Alcalase, were widely used to activate the enzymatic reactions. Alcalase was reported to possess the highest catalytic efficiency for HPI among above six proteases (Ren et al. 2016). Alcalase is known as a non-specific serine-type protease which has been used in the production of hydrolysates from different sources of protein (Žuža et al. 2017). In the process of enzymatic hydrolysis, degree of hydrolysis is a key parameter that could affect the bioactivity of peptides. However, proteins from plant seed are usually resistant to enzymatic hydrolysis due to their compact structures (Chen et al. 2011). Therefore, unfolding of protein is necessary step to expose more groups inside the molecules, improving the efficiency of enzymatic hydrolysis (Girgih et al. 2015). However, limited information is available concerning the unfolding treatment of HPI for increasing their accessibility to enzymatic hydrolysis.
The ultrasound has been widely used to modify protein structure and functional properties. Several papers have reported on the application of ultrasound treatment to increase the enzymatic hydrolysis of proteins (Chen et al. 2011; Li et al. 2018; Yang et al. 2017). It has been suggested that ultrasonic treatment could induce alterations in tertiary or secondary structure and more hydrolysis sites were then exposed (Gülseren et al. 2007; Güzey et al. 2006; Xue et al. 2017). However, no research has been reported about whether ultrasound treatment could induce the unfolding of HPI for improving the efficiency of enzymatic hydrolysis.
Hence, the objective of this work was to study whether the ultrasound treatment could improve the antioxidant and ACE inhibitory activity of hydrolysis from HPI. It was also hoped that by measuring changes in structure of HPI, the underpinning mechanisms of improved efficiency of Alcalase hydrolysis for HPI can be better understood.
Materials and methods
Materials and chemicals
The hempseed flour was purchased from a local supplier (Nanjing, China). Alcalase and Hip-His-Leu were purchased from Yuanye Biotechnology (Shanghai, China). 1,8-Anilinonaphthalenesulfonate (ANS) regent and angiotensin converting enzyme (ACE) were purchased from Sigma Aldrich Chemical Company (St Louis, MO, USA). Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Preparation of HPI
The 100 g hempseed flour was dispersed in 1200 mL n-hexane/ethanol (10:1, v/v) for oil extraction (for 1 h at 25 °C). The 100 g defatted flour was added into 1200 mL distilled water and the pH was adjusted to 8.0 for protein extraction (for 2 h at 25 °C), and then centrifuged at 4000 g for 30 min. The supernatant was adjusted to 4.5 for protein precipitation and then centrifuged at 4000 g for 30 min. The pellet was dispersed in distilled water and neutralized to pH 7.0, and then dried using FD-2B freezing dryer (Beijing Boyikang Experimental Instrument Co. Ltd., China). Protein content in the prepared HPI powder was 89.16 ± 3.01% (w/w) as determined with the biuret method (Gornall et al. 1949).
Ultrasound or heating treatment
The 5 g HPI was dispersed in 100 mL phosphate buffer (0.05 M, pH 7.0) with stirring at 4 °C for at least 12 h. Ultrasound equipment (NingBo Scientz Biotechnology Co. Ltd., China) with a 0.636 cm diameter titanium probe was applied to treat the HPI samples. The HPI dispersions were treated at 20 kHz at different levels of power output (200, 400 or 600 W) for 20 min. The ultrasound power and intensity in this study were determined according to the previous research (Jiang et al. 2014). The ultrasonic intensity in this study was 79.40, 105.86 and 158.79 W cm−2, respectively. The sample from ultrasound treatment was defined as HPI treated with ultrasound. Meanwhile, the heating treatment (90 °C for 30 min) was used as comparison with ultrasound to induce the unfolding of protein. The sample from heating treatment was defined as 90 °C/30 min treated HPI. The freshly prepared HPI were also used as control. All samples were dried using FD-2B freezing dryer (Beijing Boyikang Experimental Instrument Co. Ltd., China) and stored at 4 °C for further study.
Enzymatic hydrolysis of HPI
The 100 mL slurries of freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound were adjusted to pH 9.0 and heated to 50 °C before addition of Alcalase (0.4 g kg−1, protein content basis). After 5 h of hydrolysis, the sample was immersed in water (100 °C) for 15 min to inactivate the Alcalase, cooled to 25 °C and adjusted to pH 4.5 for precipitation of unhydrolyzed protein by centrifugation (4000 × g for 30 min). The collection of supernatant was adjusted to pH 7.0 and freeze-dried for further study.
Determination of the degree of hydrolysis (DH)
The DH was estimated according to the 2,4,6-trinitrobenzenesulfonic acid (TNBS) methods described by previous study (Adler-Nissen 1979) with modifications. The 5 mg hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound were added into 100 mL phosphate buffer (0.2 M, pH 8.2). The 1 mL hydrolysates buffer was mixed with 1 mL TNBS to start the reaction. After incubation, 1 mL sodium sulfite (0.1 M) was added to stop the reaction and the absorbance values were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland). The leucine was used as standard.
Gel filtration high-performance liquid chromatography (GP-HPLC)
Molecular mass distribution (MWD) was determined according to the method of Nongonierma et al.(Nongonierma and Fitzgerald 2012) with modifications. The 0.01 g/kg freshly prepared HPI, hydrolysates from freshly prepared HPI, hydrolysates from 90 °C/30 min treated HPI or hydrolysates from HPI treated with ultrasound were suspended in mobile phase. The TSK G3000 SW column (Stuttgart, Germany) was used for separation on a HPLC (Waters, USA). The 45% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid was used as mobile phase. The standards of cytochrome C, trasylol, ovalbumin, glutathione, Vitamin B12, cytidine, Asp-Glu and Tyr were used to draw the standard curve.
Antioxidant properties of HPI hydrolysate
The scavenging activity of DPPH were determined by the method of Liu et al. (2009) with modifications. The 0.3 mL hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI, HPI treated with ultrasound or glutathione solution was mixed with 2.7 mL DPPH (0.10 mM) to give final protein concentration of 1 mg/mL. After incubation, the absorbance values of 200 μL samples were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland).
The scavenging activity of ABTS were determined by the method of Yikling et al. (2009) with modifications. The 0.3 mL hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI, HPI treated with ultrasound or glutathione solution was mixed with 2.7 mL ABTS (7 mM) to give final protein concentration of 1 mg/mL. After incubation, the absorbance values of 200 μL samples were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland).
The scavenging activity of hydroxyl radical were determined by the method of Kuda and Ikemori (2009) with modifications. The 3.0 mL sodium phosphate buffer (0.2 M, pH 7.4, containing 2 mM 1,10-phenanthroline and 2 mM FeSO4) and 1.0 mL H2O2 (0.001 g kg−1) were prepared. Thereafter, the 1 mL hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI, HPI treated with ultrasound or glutathione solution was added to give final protein concentration of 1 mg/mL. After incubation, the absorbance values of 200 μL samples were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland).
The reducing power were determined by the method of Escudero et al. (2013) with modifications. The 0.5 mL of hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI, HPI treated with ultrasound or glutathione solution was mixed with 2.0 mL of 0.2 M sodium phosphate buffer (0.2 M, pH 6.6, containing 0.05 g kg−1 potassium ferricyanide). After incubation, 2.5 mL trichloroacetic acid (1 g kg−1) was added to give final protein concentration of 1 mg/mL and the absorbance values were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland).
The metal ions chelating activity were determined by the method of Decker and Welch (1990) with modifications. The 1 mL of hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI, HPI treated with ultrasound or glutathione solution was mixed with 0.05 mL iron dichloride solution (2 mM) and 1.85 mL deionized water. Thereafter, 0.1 mL ferrozine solution (5 mM) were added to give final protein concentration of 1 mg/mL and the absorbance values were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland).
Angiotensin-converting enzyme inhibitory activity (ACEI)
ACEI was measured by the spectrophotometric assay according to the method of Cushman and Cheung (1971) with modifications. The hydrolysates (5 mg/mL, 50 μL) from freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound were incubated with 50 μL of ACE (0.4 U/mL) at 25 °C for 10 min. To this mixture, 200 μL of 8.3 mM Hip-His-Leu were added and incubated at 37 °C for 30 min. After incubation, 200 μL HCl (1 N) was added to stop the reaction. The hippuric acid released was extracted with 1.2 mL ethyl acetate. After removal of ethyl acetate by heat evaporation, hippuric acid was dissolved in 2 mL deionized water and the absorbance values were measured using Spark 10 M microplate spectrophotometer (Tecan, Switzerland).
Structure of HPI
The 0.20 mg/mL freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound in phosphate buffer (10 mM, pH 7.0) were used to obtain spectra by Mos-450 CD spectropolarimeter (Biologic, Claix, France) for secondary structure analyze (Feng et al. 2017).
The freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound were dispersed into phosphate buffer (10 mM, pH 7.0) to give the final concentration of 0.05–2.00 mg/mL. The 20 µL ANS (8.0 mM) was added into to 4 mL protein buffer and fluorescence intensity was measured at 390 nm (excitation) and 470 nm (emission) using a Hitachi F-7000 fluorescence spectrophotometer (Tokyo, Japan). The surface hydrophobicity was calculated according to our previous study (Feng et al. 2017).
The 0.15 mg/mL freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound in phosphate buffer (10 mM, pH 7.0) was used to obtain fluorescence emission spectroscopy by using Hitachi F-7000 fluorescence spectrophotometer (Tokyo, Japan).
The microstructure of the freeze-dried freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound was observed with a SEM (Hitachi S3400, Japan) at an accelerating voltage of 20 kV.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was carried out on a Bio-rad Mini-Protein Tetra Electrophoresis System (Hercules, USA) with a 12% separating gel and a 5% stacking gel. The protein content of freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound was 2 mg/mL. After electrophoresis, gels were stained for protein with Coomassie R250 dye and analyzed by Bio-rad GelDoc XR System (Hercules, USA).
Statistical analysis
All the experiments were performed in triplicate and the data obtained were analyzed by one-way analysis of variance (One-Way ANOVA) using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). Values are expressed as mean ± SD. Duncan’s multiple range test was used to identify significant differences (p < 0.05) between means.
Results and discussion
DH of hydrolysates
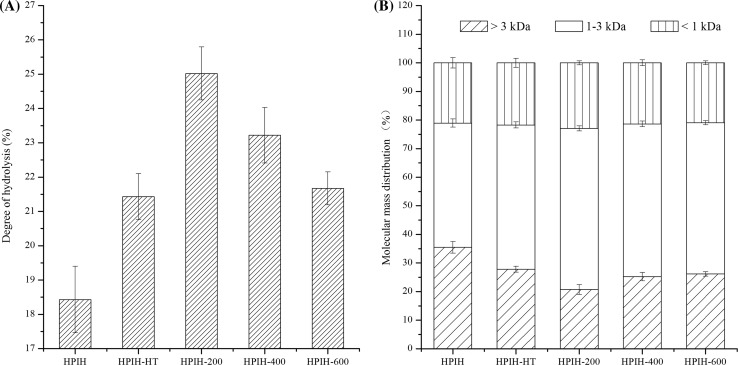
The effects of ultrasound or heating treatment on DH of hydrolysates are shown in Fig. 1a. As compared with freshly prepared HPI, the hydrolysates from both 90 °C/30 min treated HPI and HPI treated with ultrasound exhibited higher degree of hydrolysis. This finding indicated that both heating and ultrasound treatment could increase the accessibility of HPI to the Alcalase. However, HPI treated with ultrasound at low-power (200 W) showed higher degree of hydrolysis than those of high-power treated samples. This could be an indication that HPI treated with ultrasound at higher power could decrease the supporting of groups and regions for the process of enzymatic hydrolysis.
Fig. 1.
Degree of hydrolysis (a) and molecular mass distribution (b) of hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound. HPIH: hydrolysate obtained from freshly prepared HPI. HPIH-HT: hydrolysate obtained by heated to and held at 90 °C for 30 min prior to hydrolysis. HPIH-200/400/600: hydrolysate obtained by ultrasound at 20 kHz at different levels of power output (200 W, 400 W or 600 W) for 20 min prior to hydrolysis
MWD of hydrolysates
The effects of ultrasound or heating treatment on MWD of hydrolysates are shown in Fig. 1b. As compared with freshly prepared HPI, the hydrolysates from both 90 °C/30 min treated HPI and HPI treated with ultrasound possessed higher proportion of lower molecular mass (1.0–3.0 kDa) components. However, HPI treated with ultrasound at low-power (200 W) showed higher degree of hydrolysis than those of high-power treated samples. This result is consistent with the finding in DH (Sect. 3.1).
Antioxidant properties of hydrolysates
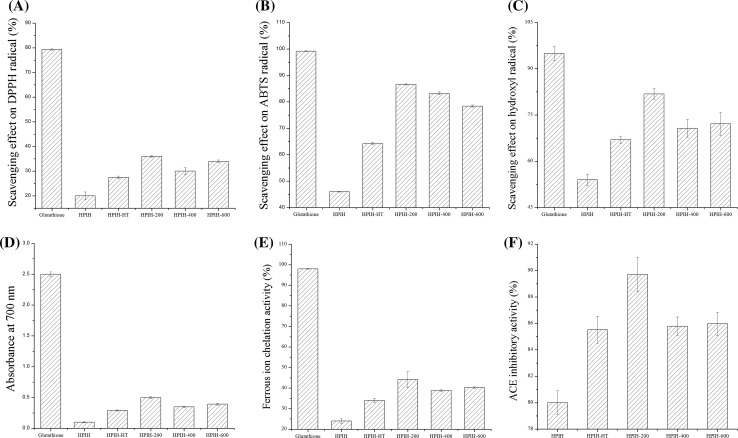
As shown in Fig. 2a–e, the antioxidant properties of hydrolysates from HPI are much lower than the glutathione at the concentration of 1 mg/mL, but higher than hydrolysates from pea protein isolates (Girgih et al. 2015) and hydrolysates from Quinoa protein (Li et al. 2018) at the similar concentration. This result indicated that HPI can be used as sources of antioxidant peptides. As compared with freshly prepared HPI, the hydrolysates from both 90 °C/30 min treated HPI and HPI treated with ultrasound showed the better antioxidant activities. However, hydrolysates from HPI treated with ultrasound at low-power (200 W) showed higher radical scavenging activity, reducing powder and metal chelating activity than those of high-power treated samples. The better antioxidant properties might be related to the degree of hydrolysis (as shown in Sect. 3.1) and higher proportion of lower molecular mass components (as shown in Sect. 3.2).
Fig. 2.
Antioxidant and ACEI activity of hydrolysates from freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound. a DPPH radical scavenging activity; b ABTS radical scavenging activity; c hydroxyl radical scavenging activity; d reducing power; e metal ion chelating activity; f ACEI activity. HPIH: hydrolysate obtained from freshly prepared HPI. HPIH-HT: hydrolysate obtained by heated to and held at 90 °C for 30 min prior to hydrolysis. HPIH-200/400/600: hydrolysate obtained by ultrasound at 20 kHz at different levels of power output (200 W, 400 W or 600 W) for 20 min prior to hydrolysis
ACEI of hydrolysates
It is well known that food protein-derived peptides can be used as alternative natural sources for ACEI (Hartmann and Meisel 2007). For example, the hydrolysates obtained from squid pen chitosan extraction effluent showed the ACEI activity (Shavandi et al. 2017). As shown in Fig. 2f, the ACE was inhibited by HPI hydrolysate to the extent of 80–92% at the final concentration of 0.5 mg/mL in test tube. The similar study about peanut protein hydrolysate has also shown that the final concentration for ACEI to the extent of 90–97% was about 0.7 mg/mL in test tube (Jamdar et al. 2010). As compared with freshly prepared HPI, the hydrolysates from both 90 °C/30 min treated HPI and HPI treated with ultrasound showed higher ACEI activity. As expected, hydrolysates from HPI treated with ultrasound at low-power (200 W) showed better ACEI activity than those of high-power treated samples, which is consistent with the results of antioxidant properties (Sect. 3.3).
Structure of HPI
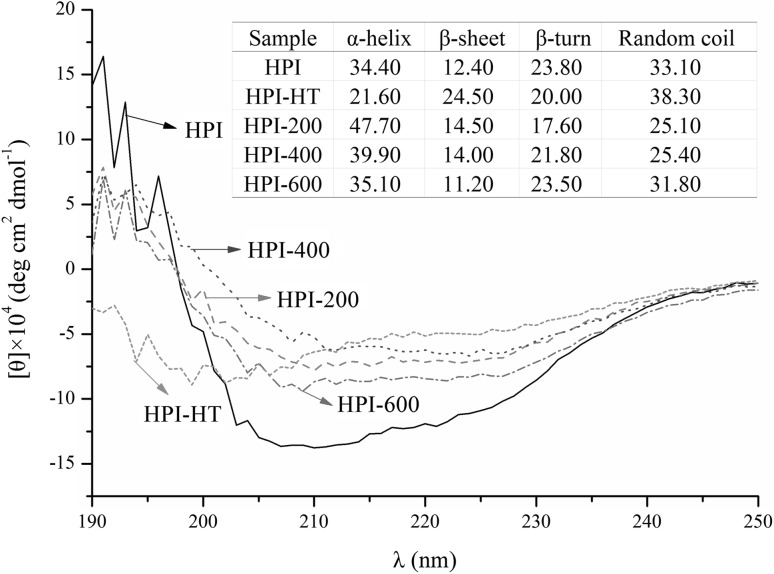
The secondary structure of samples was determined using CD spectroscopy and results were shown in Fig. 3. According to the calculation from website (http://dichroweb.cryst.bbk.ac.uk/html/process.shtml), both heating and ultrasound treatments could change the proportions of α-helix, β-sheet, β-turn and random coil comparing with freshly prepared HPI. In general, HPI treated with ultrasound showed increase in the proportions of α-helix, decrease in the proportions of β-turn and random coil. This result is inconsistent with previous studies. Li et al. (2018) showed that ultrasound treatment decreased the proportion of α-helix in Quinoa protein. Hu et al. (2013) also found that ultrasound treatment decreased the α-helix in soy protein. However, study about peanut protein showed that ultrasound treatment could not change the secondary structure of protein (Jiang et al. 2014). The contradictory results are probably due to the differences in protein fractions. Furthermore, HPI treated with ultrasound at low-power (200 W) showed higher proportions of α-helix than those of high-power treated samples. The decrease in α-helix is probably related to the exposure of the hydrophobic regions in protein (Sun et al. 2013). Therefore, the surface hydrophobicity of HPI was further analyzed.
Fig. 3.
Circular dichroism spectra of freshly prepared hempseed protein isolates (HPI), 90 °C/30 min treated HPI or HPI treated with ultrasound. HPI-HT: protein obtained by heated to and held at 90 °C for 30 min. HPI-200/400/600: protein obtained by ultrasound at 20 kHz at different levels of power output (200 W, 400 W or 600 W) for 20 min
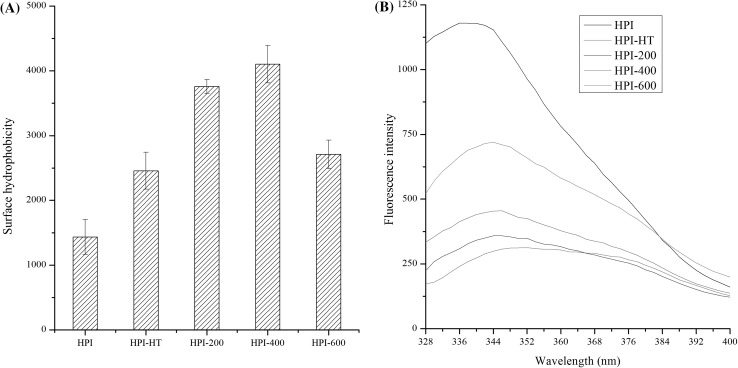
As shown in Fig. 4a, both heating and ultrasound treatment could increase the surface hydrophobicity as compared with freshly prepared HPI, which indicated that both treatments could induce the exposure of hydrophobic groups initially buried in the interior of protein. This finding is consistent with previous studies about soy, peanut and Quinoa protein (Hu et al. 2013; Jiang et al. 2014; Li et al. 2018). Furthermore, HPI treated with ultrasound at higher-power (600 W) showed lower values of surface hydrophobicity than those of lower-power treated samples. The exposure of hydrophobic groups might result in the change in tertiary structure of protein. Therefore, the intrinsic fluorescence emission spectra of HPI were further investigated.
Fig. 4.
Surface hydrophobicity (a) and intrinsic fluorescence emission spectra (b) of freshly prepared hempseed protein isolates (HPI), 90 °C/30 min treated HPI or HPI treated with ultrasound. HPI-HT: protein obtained by heated to and held at 90 °C for 30 min. HPI-200/400/600: protein obtained by ultrasound at 20 kHz at different levels of power output (200 W, 400 W or 600 W) for 20 min
As shown in Fig. 4b, both heating and ultrasound treatment could decrease the relative fluorescence intensity (λmax) as compared with freshly prepared HPI, which indicated that both treatments could induce the unfolding of protein, then caused the more chromophores exposed to the solvent (Pallarès et al. 2004). Furthermore, the values of λmax decreased as ultrasound power decreased, which indicated that HPI treated with ultrasound at higher-power might result in aggregates. The aggregates were probably due to the increase of surface hydrophobicity (Fig. 4a).
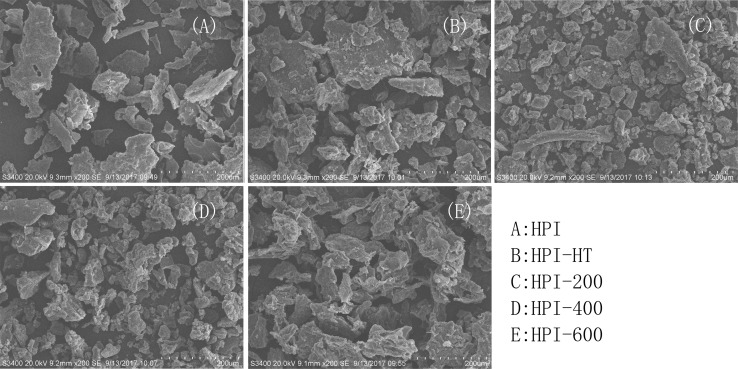
The microstructures of freshly prepared HPI, 90 °C/30 min treated HPI or HPI treated with ultrasound were shown in Fig. 5. The freshly prepared HPI showed the very tight structure and smooth surface. After the heating or ultrasound treatment, the samples showed more disordered structures and irregular fragments. This result is consistent with previous study about jackfruit seed protein isolate in which ultrasound treatment disrupted the microstructure of protein (Resendiz-Vazquez et al. 2017). Furthermore, the aggregates were observed in 90 °C/30 min treated HPI or HPI treated with ultrasound at higher-power (600 W), which is consistent with the results of surface hydrophobicity and relative fluorescence intensity (Fig. 4).
Fig. 5.
Microstructure of freshly prepared hempseed protein isolates (HPI), 90 °C/30 min treated HPI or HPI treated with ultrasound. HPI-HT: protein obtained by heated to and held at 90 °C for 30 min. HPI-200/400/600: protein obtained by ultrasound at 20 kHz at different levels of power output (200 W, 400 W or 600 W) for 20 min
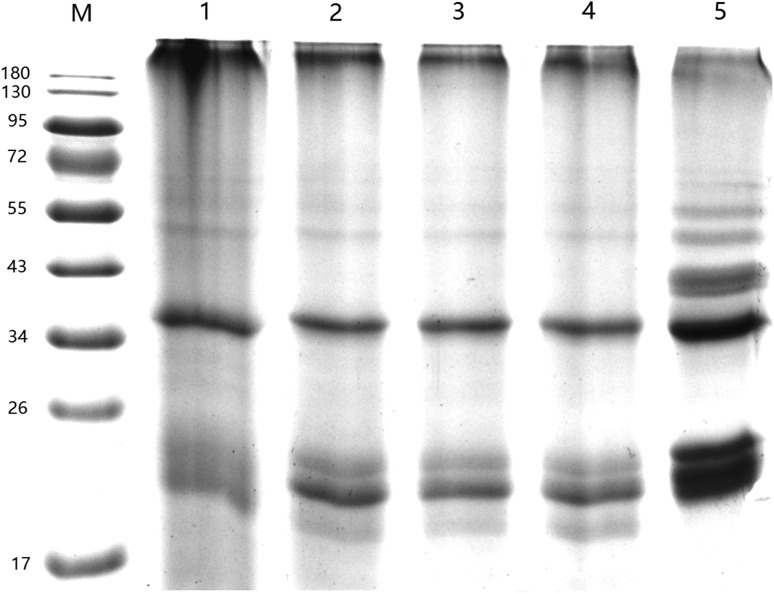
As shown in Fig. 6, comparing freshly prepared HPI, HPI treated with ultrasound did not show any changes in protein electrophoretic patterns. This finding is consistent with previous study in which ultrasound treatment only caused physical modifications of the protein macroscopically, while the native protein themselves remained unchanged (Zisu et al. 2011). In contrast, Resendiz-Vazquez et al. (2017) observed that ultrasound treatment changed the primary structure of jackfruit seed protein. The inconsistent results indicated that effects of shear stress and turbulence of ultrasound on molecular structure of the protein are probably related to the type of protein. Furthermore, no aggregation was observed in SDS-PAGE, which indicated that formation of aggregates observed in SEM (Fig. 5) is a noncovalent bond. However, the new band about 43 kDa was observed in high temperature treated HPI, which suggested that aggregates observed in SEM is covalent bond.
Fig. 6.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of freshly prepared hempseed protein isolates (HPI), 90 °C/30 min treated HPI or HPI treated with ultrasound. M: marker (kDa); 1: freshly prepared HPI; 2-4: HPI obtained by ultrasound at 20 kHz at different levels of power output (200 W, 400 W or 600 W) for 20 min; 5: HPI obtained by heated to and held at 90 °C for 30 min
Supposed mechanism for the effect of heating or ultrasound treatment with different power output on antioxidant and ACEI activities of hydrolysate
More recently, proteins from hempseed are recognized as an excellent alternative plant protein because it can exert a range of functionalities, such as emulsification, gelation, foaming properties and film formation (Hadnađev et al. 2018; Dapčević-Hadnađev et al. 2018). Moreover, bioactive peptides derived from HPI have also shown many health effects (Ren et al. 2016). Usually, preheating is used to induce the unfolding of protein and enhance efficiency of enzymatic protein hydrolysis. For instance, Ren et al. (2016) used the preheating (at 90 °C for 30 min) to treat the protein before the enzymatic hydrolysis. Girgih et al. (2015) used the preheating (30 min at 100 °C) as comparison with high pressure pretreatment to produce the antioxidant peptides from pea protein. In this study, the high-intensity ultrasound with different output power was used to facilitate the release of bioactive peptides from HPI. The results showed that the hydrolysate from HPI treated with ultrasound have better antioxidant and ACEI activities than those from 90 °C/30 min treated HPI. The better antioxidant and ACEI activities might be related to the higher degree of hydrolysis. Previous studies have shown positive correlation between radical scavenging activities and hydrolysis degree in both porcine collagen hydrolysate (Li et al. 2007) and peanut protein hydrolysate (Jamdar et al. 2010). The higher degree of hydrolysis can also be proved by the higher proportion of lower molecular mass components in HPI treated with ultrasound (Fig. 1b). The higher degree of hydrolysis is probably due to the unfolding of HPI induced by ultrasound treatment. This result is consistent with previous study that thermal, mechanical and chemical effects of ultrasound could induce the unfolding of protein and provide more groups for the process of enzymatic hydrolysis (Li et al. 2018). The unfolding of HPI treated with ultrasound can be proved by increase in the proportions of α-helix (Fig. 3), increase in H0 (Fig. 4a) and decrease in fluorescence intensity (Fig. 4b). On the other hand, the improvement in degree of hydrolysis might be also related to the cracked microstructure (Fig. 5) of HPI treated with ultrasound in which protein could provide more surface area and chances to contact with Alcalase. Moreover, the lower degree of hydrolysis in 90 °C/30 min treated HPI is probably due to the formation of covalent bond (Fig. 5).
Ultrasound power output was one important parameter for its application to induce the unfolding of protein. Many studies have focused on the effects of ultrasound treatment at different levels of power output (200, 400 or 600 W) on the structure and functional properties of soy protein (Hu et al. 2013), jackfruit seed protein isolate (Resendiz-Vazquez et al. 2017), Quinoa protein (Li et al. 2018) and plum seed protein (Xue et al. 2018). The results showed that effects of ultrasound power output on unfolding of protein are related to the type of protein. Previous study showed that hydrolysate from Quinoa protein treated with ultrasound at 400 W possessed higher degree of hydrolysis than 200 W or 400 W (Li et al. 2018). Resendiz-Vazquez et al. (2017) reported that jackfruit seed protein treated with ultrasound at 200 W and 600 W showed higher degree of hydrolysis than those from 400 W. These results are inconsistent with our findings in which the sample from HPI treated with ultrasound at 200 W showed higher degree of hydrolysis and degree of hydrolysis decreased with further increase of power output. The inconsistent results might be due to different protein fractions being used. The decrease of hydrolysis degree in HPI treated with ultrasound at higher-power (400 W or 600 W) might be related to the formation of aggregates which is probably due to the exposure of hydrophobic groups (as shown in Fig. 4) and free SH groups (Hu et al. 2013). On the other hand, attenuation of ultrasound occurs at high ultrasonic power (Sutkar and Gogate 2009), which might decrease shear stress and turbulence effects in protein solution. In order to study this mechanism, further study should be focus on the pure protein or protein subunits and more parameters, such as different frequencies, working modes and time. Moreover, it has been proved that ultrasound field in liquids can produce reactive radicals via a cascade effect of the cavitation (Misra et al. 2018). However, little work has been undertaken for analysis of effects of free radicals produced by ultrasound on the structure and functional properties of protein.
Conclusion
In summary, this study found that both heating and ultrasound treatment could improve the antioxidant and ACEI activity of HPI hydrolysates as compared with freshly prepared HPI. Ultrasound power could significantly affect the enzymatic hydrolysis of HPI and hydrolysates from HPI treated with ultrasound at low-power (200 W) showed higher activities than those of high-power treated or 90 °C/30 min treated samples. The molecular mechanism of ultrasound treatment includes the changes in secondary/tertiary structure and microstructure of HPI and then enhances the accessibility of HPI to Alcalase.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC31501529), Natural Science Foundation of Jiangsu Province (BK20150950, BK20171066) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (035062002002A).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979;27:1256. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen JS, Ren JY, Zhao MM. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J Agric Food Chem. 2011;59:2600–2609. doi: 10.1021/jf103771x. [DOI] [PubMed] [Google Scholar]
- Cushman DW, Cheung HS. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochimica et Biophysica Acta (BBA)-Enzymology. 1971;250:261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- Dapčević-Hadnađev T, Hadnađev M, Lazaridou A, Moschakis T, Biliaderis CG. Hempseed meal protein isolates prepared by different isolation techniques. Part II. gelation properties at different ionic strengths. Food Hydrocoll. 2018;81:481–489. doi: 10.1016/j.foodhyd.2018.03.022. [DOI] [Google Scholar]
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- Escudero E, Mora L, Fraser PD, Aristoy MC, Toldrá F. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013;138:1282–1288. doi: 10.1016/j.foodchem.2012.10.133. [DOI] [PubMed] [Google Scholar]
- Feng X, Wu Z, Tong J, Zheng J, Chen L. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci Biotechnol Biochem. 2017;81:1891–1898. doi: 10.1080/09168451.2017.1361805. [DOI] [PubMed] [Google Scholar]
- Girgih AT, Udenigwe CC, Aluko RE. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J Am Oil Chem Soc. 2011;88:381–389. doi: 10.1007/s11746-010-1686-7. [DOI] [Google Scholar]
- Girgih AT, Alashi AM, He R, Malomo SA, Raj P, Netticadan T, Aluko RE. A novel hemp seed meal protein hydrolysate reduces oxidative stress factors in spontaneously hypertensive rats. Nutrients. 2014;6:5652. doi: 10.3390/nu6125652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgih AT, Chao D, Lin L, He R, Jung S, Aluko RE. Enzymatic protein hydrolysates from high pressure-pretreated isolated pea proteins have better antioxidant properties than similar hydrolysates produced from heat pretreatment. Food Chem. 2015;188:510–516. doi: 10.1016/j.foodchem.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Gülseren I, Güzey D, Bruce BD, Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason Sonochem. 2007;14:173–183. doi: 10.1016/j.ultsonch.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Güzey D, Gülseren İ, Bruce B, Weiss J. Interfacial properties and structural conformation of thermosonicated bovine serum albumin. Food Hydrocoll. 2006;20:669–677. doi: 10.1016/j.foodhyd.2005.06.008. [DOI] [Google Scholar]
- Hadnađev M, Dapčević-Hadnađev T, Lazaridou A, Moschakis T, Michaelidou AM, Popović S, Biliaderis CG. Hempseed meal protein isolates prepared by different isolation techniques. Part I: physicochemical properties. Food Hydrocoll. 2018;79:526–533. doi: 10.1016/j.foodhyd.2017.12.015. [DOI] [Google Scholar]
- Hartmann R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol. 2007;18:163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- House JD, Neufeld J, Leson G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J Agric Food Chem. 2010;58:11801–11807. doi: 10.1021/jf102636b. [DOI] [PubMed] [Google Scholar]
- Hu H, Wu J, Zhu L, Zhang F, Xu X. Effects of ultrasound on structural and physical properties of soy;protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. doi: 10.1016/j.foodhyd.2012.08.001. [DOI] [Google Scholar]
- Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Arun S. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- Jiang L, Wang J, Li Y, Wang Z, Liang J, Wang R, Chen Y, Ma W, Qi B, Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res Int. 2014;62:595–601. doi: 10.1016/j.foodres.2014.04.022. [DOI] [Google Scholar]
- Kuda T, Ikemori T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem. 2009;112:575–581. doi: 10.1016/j.foodchem.2008.06.008. [DOI] [Google Scholar]
- Li B, Chen F, Wang X, Ji B, Wu Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization–mass spectrometry. Food Chem. 2007;102:1135–1143. doi: 10.1016/j.foodchem.2006.07.002. [DOI] [Google Scholar]
- Li X, Da S, Li C, Xue F, Zang T. Effects of high-intensity ultrasound pretreatment with different levels of power output on the antioxidant properties of alcalase hydrolyzates from Quinoa (Chenopodium quinoa Willd.) protein isolate. Cereal Chem. 2018;95:518–526. doi: 10.1002/cche.10055. [DOI] [Google Scholar]
- Liu SC, Lin JT, Wang CK, Chen HY, Yang DJ. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009;114:577–581. doi: 10.1016/j.foodchem.2008.09.088. [DOI] [Google Scholar]
- Malomo SA, Onuh JO, Girgih AT, Aluko RE. Structural and antihypertensive properties of enzymatic hemp seed protein hydrolysates. Nutrients. 2015;7:7616–7632. doi: 10.3390/nu7095358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra NN, Martynenko A, Chemat F, Paniwnyk L, Barba FJ, Jambrak AR. Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies. Crit Rev Food Sci Nutr. 2018;58:1832–1863. doi: 10.1080/10408398.2017.1287660. [DOI] [PubMed] [Google Scholar]
- Nongonierma AB, Fitzgerald RJ. Tryptophan-containing milk protein-derived dipeptides inhibit xanthine oxidase. Peptides. 2012;37:263–272. doi: 10.1016/j.peptides.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Pallarès I, Vendrell J, Avilés FX, Ventura S. Amyloid fibril formation by a partially structured intermediate state of alpha-chymotrypsin. J Mol Biol. 2004;342:321–331. doi: 10.1016/j.jmb.2004.06.089. [DOI] [PubMed] [Google Scholar]
- Ren Y, Liang K, Jin Y, Zhang M, Chen Y, Wu H, Lai F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J Funct Foods. 2016;26:439–450. doi: 10.1016/j.jff.2016.07.024. [DOI] [Google Scholar]
- Resendiz-Vazquez JA, Ulloa JA, Urías-Silvas JE, Bautista-Rosales PU, Ramírez-Ramírez JC, Rosas-Ulloa P, González-Torres L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason Sonochem. 2017;37:436–444. doi: 10.1016/j.ultsonch.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Shavandi A, Hu Z, Teh SS, Zhao J, Carne A, Bekhit A, Bekhit EDA. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent. Food Chem. 2017;227:194–201. doi: 10.1016/j.foodchem.2017.01.099. [DOI] [PubMed] [Google Scholar]
- Sun W, Zhou F, Sun DW, Zhao M. Effect of Oxidation on the Emulsifying Properties of Myofibrillar Proteins. Food Bioprocess Technol. 2013;6:1703–1712. doi: 10.1007/s11947-012-0823-8. [DOI] [Google Scholar]
- Sutkar VS, Gogate PR. Design aspects of sonochemical reactors: techniques for understanding cavitational activity distribution and effect of operating parameters. Chem Eng J. 2009;155:26–36. doi: 10.1016/j.cej.2009.07.021. [DOI] [Google Scholar]
- Tang CH, Ten Z, Wang XS, Yang XQ. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein Isolate. J Agric Food Chem. 2006;54:8945–8950. doi: 10.1021/jf0619176. [DOI] [PubMed] [Google Scholar]
- Wang XS, Tang CH, Yang XQ, Gao WR. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008;107:11–18. doi: 10.1016/j.foodchem.2007.06.064. [DOI] [Google Scholar]
- Xue F, Wu Z, Tong J, Zheng J, Li C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci Biotechnol Biochem. 2017;81:1891–1898. doi: 10.1080/09168451.2017.1361805. [DOI] [PubMed] [Google Scholar]
- Xue F, Zhu CS, Liu F, Wang SY, Liu HZ, Li C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J Sci Food Agric. 2018;98:5690–5699. doi: 10.1002/jsfa.9116. [DOI] [PubMed] [Google Scholar]
- Yang X, Li Y, Li S, Oladejo AO, Ruan S, Wang Y, Huang S, Ma H. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason Sonochem. 2017;38:19–28. doi: 10.1016/j.ultsonch.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Yikling C, Jookheng G, Yauyan L. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009;116:13–18. doi: 10.1016/j.foodchem.2009.01.091. [DOI] [Google Scholar]
- Zisu B, Lee J, Chandrapala J, Bhaskaracharya R, Palmer M, Kentish S, Ashokkumar M. Effect of ultrasound on the physical and functional properties of reconstituted whey protein powders. J Dairy Res. 2011;78:226–232. doi: 10.1017/S0022029911000070. [DOI] [PubMed] [Google Scholar]
- Žuža MG, Milašinović NZ, Jonović MM, Jovanović JR, Kalagasidis Krušić MT, Bugarski BM, Knežević-Jugović ZD. Design and characterization of alcalase—chitosan conjugates as potential biocatalysts. Bioprocess Biosyst Eng. 2017;40:1713–1723. doi: 10.1007/s00449-017-1826-7. [DOI] [PubMed] [Google Scholar]