Abstract
Purpose
Degradation or decomposition of the chemical herbicides by natural reagents after using can lead to produce various types of harmful intermediates. Ultrafiltration by the mixed matrix membranes blended with the graphene oxide/TiO2 can remove the residual herbicides from aqueous solution.
Methods
Graphene oxide/TiO2x% (x = 10, 30, 50%) was prepared by solvothermal method and blended by polysulfone to prepare GO/TiO2/PSf membranes for dynamic rejection of aqueous solutions of glyphosate, 2,4-D, butachlor, and trifluralin in a dead-end flow system. The blended membranes were also applied for the adsorption of herbicides in batch experiments.
Results
Addition of GO/TiO2 nanocomposite increased water flux from 7.3 for pure membrane to 211–326 kg/m2 h for mixed matrix samples in order to increase of the membrane porosity and surface hydrophilicity. The herbicides rejections were found in the range of 50–70% related to GO/TiO2 content. It was found that the membrane blended with 0.5 wt.% of GO/TiO2(10%) demonstrated the most efficiency.
Conclusions
Details of dynamic filtration showed that the blended membrane acted based on the size exclusion mechanism. Adsorption experiments indicated that the strong attractions between H-bond donor sites of the herbicide and GO/TiO2 nanoparticles in membranes played a key role in the increase of adsorption of herbicides on the membrane.
Keywords: Herbicide, Graphene oxide, Titanium oxide, Membrane filtration, Polysulfone
Introduction
Chemical herbicides include a wide range of synthetic compounds which are used for prevention of weeds growth. Annual consumption of them in Iran is more than 6000 tons containing about 40 synthetic herbicides such as phenoxy acids (e.g. 2,4-D), organophosphorus compounds (e.g. glyphosate), dinitroaniline’s (e.g. trifluralin), acetanilide’s (e.g. butachlor), etc. [1]. Pimentel reported that less than 0.1% of a herbicide reaches to the target, so a large amount of it releases in the environment resulting pollution of the soil, surface, and ground waters [2]. Degradation or decomposition of the herbicides by natural reagents can lead to produce various types of harmful intermediates such as aniline derivatives, phenolic compounds, aromatics, etc.
Filtration by polymeric membranes is known as an effective method for the removal of contaminations from water in order to their easy preparation method, simple installation, and application in large scale treatment plants. In addition, modification of their structure by means of advanced nanocomposite materials in both forms of thin film composite (TFC) or mixed matrix membranes (MMM’s) has developed their applications in ultra/nanofiltration systems. Various types of nano-based composite materials have been applied for modifying surface or matrix of the polymers. Among them, graphene oxide (GO) and GO-assisted nanocomposites have been widely used for the removal of water pollutants in batch experiments [3–5] or membrane filtration [6–8]. In order to the existence of large amounts of hydroxyl, carboxyl, and epoxy groups on the surface of GO, it can be easily functionalized by the chemical compounds to prepare advanced nanocomposite adsorbents [9]. Modification of ultra/nanofiltration membranes by means of GO-assisted nanocomposites can improve their permeability, fouling resistance and removal efficiency. Several literatures have been published about synthesis of GO functionalized with the various types of metal/non-metal oxide nanoparticles such as ZnO [10], SiO2 [11], Fe3O4 [12], MoO2 [13], WO3 [13], etc. and their application as advanced modifier of the polymeric membranes.
GO/TiO2 nanocomposite is a good candidate for the enhancement of structural properties, fouling resistance, and efficiency of the membranes. A TFC nanofiltration containing rGO/TiO2 nanocomposite was made by interfacial polymerization of piperazine and trimesoyl chloride for salt and BSA rejections [14]. Results showed improvement of fouling resistance of membrane at the presence of nanocomposite. Safarpour et al. blended polyethersulfone (PES) with rGO/TiO2 for rejection of textile dyes [15]. They reported that the membrane by 0.1 wt.% of nanocomposite demonstrated the best fouling resistance and dye rejection in comparison of TiO2/PES and GO/PES membranes. TiO2/GO nanocomposite was also applied for modification of PSf membrane by Layer-by-Layer method and used for removal of methylene blue (MB) under photocatalytic experiments by UV/sunlight irradiation [16]. It was observed that the flux of modified membranes increased and MB photodegradation was faster than the pure PSf membrane. Emadzadeh et al. synthesized TFC membranes by use of TiO2 nanoparticles prepared with solvothermal method for improving antifouling properties of PSf membranes [17]. They found that addition of 0.01 w/v% of nanocomposite led to the lower flux decline. Photodecomposition of MB was also investigated by photocatalytic PSf membrane blended with nitrogen doped GO/TiO2 nanocomposite [18]. It was reported that photodecomposition of MB improved 20–50% and 30–80% under UV and sunlight irradiation, respectively. GO/TiO2 polycarbonate membranes were used for filtration of methyl orange and rhodamine B and adsorptive filtration mechanism was suggested for removal of dyes in aqueous solution [19].
In this study, GO/TiO2 nanocomposite has been synthesized and mixed with PSf membranes and applied for ultrafiltration of some commercial herbicides using a continuous filtration set up. The activities of membranes for removal of herbicide from aqueous solution have been also studied in a batch system and their adsorption capacities have been compared with some natural and synthetic adsorbents.
Experimental
Materials
Graphene oxide prepared by modified hammers method was provided from US Research Nanomaterials, Inc. (6–10 layers, thickness of 3.4–7 nm). Titanium tetra isopropoxide (TTIP), and N-methyl-2- pyrrolidone (NMP) were purchased from Merck in high purity form. PSf (MW = 60 kg/mol) was provided from BASF (Ultrason® S 6010). Trifluralin, butachlor, glyphosate, and 2,4-D were provided from HPC standards GmbH. In all experiments pure water was used. Modeling and quantum calculation of the herbicides were carried out by free Gaussian 98 package using RHF method and STO-3G basis set to calculate some geometrical and electronic properties shown in Table 1.
Table 1.
Some molecular parameters of herbicides (from RHF method) and solubility in water
| Commercial name | Molar mass (g/mol) | Molecular diameter (nm) | Dipole moment (debye) | Eg (ev) | Number of H-bond donor | Solubility (mg/l) |
|---|---|---|---|---|---|---|
| Trifluralin | 335.28 | 143.6 | 0.2725 | 16.14 | 0 | 24 |
| Butachlor | 311.85 | 142.1 | 5.1457 | 14.1 | 0 | 23 |
| 2,4-D | 221.03 | 64.3 | 1.3586 | 16.31 | 1 | 677 |
| Glyphosate | 169.07 | 50.3 | 7.5323 | 19.31 | 4 | >1000 |
Preparation of GO/TiO2 nanocomposite
GO/TiO2 nanocomposite was synthesized by solvothermal method [15]. 0.5 g GO powder was dispersed and stirred (rpm = 600) in 50 cc ethanol in pH = 5 adjusted by nitric acid and sonicated 30 min by a Labsonic FALC ultrasonic bath (Frequency 50 kHz and absorbed power 100 W) at 40 °C. TTIP was diluted in 20 cc ethanol and then added dropwise by a homemade droplet generator to GO and stirred vigorously at 10 °C for 2 h. The mixture was then kept into a Teflon-lined stainless autoclave and heated to 100 °C for 12 h. The sample was then centrifuged, washed by ethanol and water and finally dried at 150 °C for 12 h. By this method, three samples of GO/TiO2 nanocomposite containing 10, 30, and 50% wt. of TiO2 were synthesized and named as GO/TiO2–10 (or A), GO/TiO2–30 (or B), and GO/TiO2–50 (or C), respectively.
Membrane casting
Phase inversion method was used for preparation of mixed matrix membrane [15]. 2 g PSf was dissolved in 12 g NMP (casting solution) and stirred at 60 °C for 18 h. A certain amount of GO/TiO2 was then dispersed in the casting solution and stirred again for 8 h. The solution was then kept at room temperature for one day and then sonicated 30 min at 40 °C for degassing. Membrane casting was performed by use of a homemade film applicator with the thickness of 200 μm and dimension of 15 × 30 cm. The sample was then immersed immediately in water bath (40 °C) to form membrane film during phase inversion process. The membrane was then kept in water bath for 24 h to remove the solvent from membrane film. By this method, 9 types of membranes were prepared by addition of 0.25, 0.5, or 1% wt. of three types of GO/TiO2 (A, B, or C) to the casting solution. The membranes were named as MA-0.25, MA-0.5, MA-1, MB-0.25, MB-0.5, MB-1, MC-0.25, MC-0.5, and MC-1, where the words and numbers show the type of GO/TiO2 and its weight percentage in the membrane, respectively.
Characterization
FTIR spectra of GO/TiO2 samples were obtained by means of AVATAR instrument (Thermo Co.) in order to observe functional groups of GO before and after coating by TiO2 nanoparticles. X-ray diffraction technique was applied to determine the christally phases in the nanocomposites using a Philips PW1730 X-ray diffractometer with Cu Kα radiation (1.5472 Å). FE-SEM micrographs of the prepared GO/TiO2 and membranes were performed by means of S-4160, HITACHI device. Surface roughness and roughness parameters of the membranes were determined by Aria Pajohesh AFM microscopy in non-contact mode by scanning 1 cm2 of membrane piece. Membrane porosity was determined by soaking a piece of dried membrane in water bath for 24 h according to the following equation:
| 1 |
where Wdry and Wwet are weights of dried membrane and wet membrane after immersing in water bath for 24 h, respectively. ρ, A, and h are density of water (0.998 g/cm3), area (cm2) and thickness of membrane (cm), respectively. Pure water flux (PWF) was determined after steady state condition in dynamic filtration set up (discussed in section 2.6) by Eq. (2):
| 2 |
Jw, V, A, and t are weight water flux (kg/m2h), weight of permeate water (kg), membrane area (m2), and time of experiment (h), respectively. Surface charge of the prepared membranes in pH = 7 was measured by determination of zeta potential from streaming potential measurements using electro kinetic analyzer (SurPASS, Anton-Paar, Austria). Contact angles of membranes were estimated by fitting Young-Laplace equation to the drop images by open source Image J v.1 software developed by MERCK Institute (Iran). 4 μl of deionized water was deposited on four different locations on the membrane surface and images were taken by color industrial camera (DFK 23U618 USB 3.0) with a 2X lens and contact angle was calculated by 0.01 of certainty. Determination of total pore volume, pore size distribution, and mean pore diameter of the membranes were carried out by BET measurement using BELSORP MINI II instrument (BEL Co.) and N2 adsorption/desorption technique after degassing at 85°C for 4 h.
Dynamic filtration and batch experiments
A dead-end flow system was applied for ultrafiltration of herbicides using mixed matrix membranes. A piece of membrane was held in a stainless steel holder with an effective area of 21 cm2. Four aqueous solutions containing trifluralin, butachlor, glyphosate, or 2,4-D with the initial concentration of 20 ppm were prepared. Each herbicide solution was pumped through the membrane in the holder at constant transmembrane pressures (TMP) of 1 bar monitored by a gauge and constant temperature of 25 °C. Sampling was done after steady state condition and determination of herbicide concentration was followed by direct injection of the aqueous samples into the total organic carbon (TOC) analyzer (Beckman 915A TOC analyzer). Filtration experiments of each herbicide were repeated for all 9 mixed matrix membranes. In order to determine variation of flux during filtration period, a long time experiment was performed and the value of flux for each herbicide solution was determined.
Study of adsorption behavior of herbicide on the membranes was investigated by batch experiments. 0.5 g membrane was fragmented to the very small pieces (1 × 1 mm) and mixed by 50 cc herbicide solution in different initial concentrations of 3, 6, 9, 12, 15, 18, and 21 ppm (7 solutions for each herbicide). Each mixture was shaken for 24 h at 25 °C and the membrane pieces were then removed by centrifuge. The sample was analyzed by HPLC for determination of residual herbicide using Agilent HPLC (1260 Infinity) with UV detector (EX 1600), column C18, and EX 1600 software. Quantitative analysis was performed by use of certified reference materials (CRM) for accurate calibration.
Results and discussion
Molecular characteristics of herbicides
According to the values of solubility and details of molecular modeling presented in Table 1, the herbicides can be divided to two different types: hydrophilic samples including glyphosate and 2,4-D characterized by high solubility in water and hydrophobic herbicides i.e. butachlor and trifluralin having low solubility. According to the results of computational modeling, three factors can affect the solubility: number of hydrogen bond donor sites, molecular size, and dipole moment. From details, the herbicides having more H-bond donor sites, smaller size, and higher dipole moment are easy soluble in water.
Characterization of GO/TiO2 nanocomposite
Figure 1 demonstrates FT-IR spectra of GO and GO/TiO2 samples. The main vibrational bands of GO appear at 1056, 1228, 1625, 1733, and 3446 cm−1 corresponding to the stretching bands of alkoxy (C-O), epoxy (C-O-C), phenyl (C=C), carboxyl (C=O), and hydroxyl (O-H) groups in GO sheets, respectively [20]. The broad band at the range of 450–750 cm−1 in the spectra of GO/TiO2 sample can be assigned to the stretching band of Ti-O-Ti groups [21]. It can be seen that the intensity of this band increases by TiO2 content in the nanocomposite. It is also observed that the intensity of vibrational band at the range of 3000–3500 cm−1 increases by increase of TiO2 content in GO/TiO2 samples. It can be related to the stretching vibrations of hydroxyl groups on the surface of TiO2 nanoparticles (Ti-OH)as hydrophilic groups playing a key role for hydrophilicity and permeability of membrane discussed in section 3.3.
Fig. 1.
FTIR spectra of graphene oxide (GO) and graphene oxide/TiO2 nanocomposites
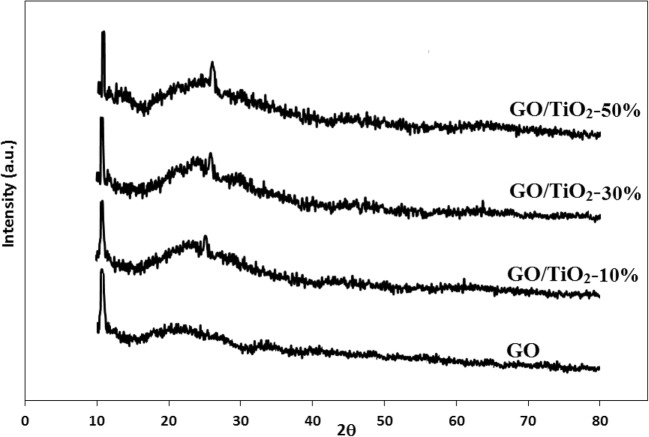
XRD patterns of the prepared samples are given in Fig. 2. The sharp peak at 10.5o corresponds to d001 reflection in GO as the characteristic peak of graphene oxide. The small peak at 25o in GO/TiO2 samples indicates formation of TiO2 particles assigning d101 reflection in anatase form of titanium dioxide [22].
Fig. 2.
XRD patterns of GO and GO/TiO2 nanocomposites
Figure 3 shows SEM micrographs of GO and GO/TiO2–50% sample. Grapheme oxide exhibits a flaked shape as the original structure of graphene oxide sheets. In addition, the nanoparticles of titanium dioxide are carefully dispersed on the graphene oxide in spherical form with the nano sized diameter less than 10 nm. Carefully dispersion and small particle size provide large amounts of active sites on the external surface of the nanocomposite that can interact with the polymer matrix during the membrane casting and enhance membrane porosity and permeability.
Fig. 3.
SEM micrographs of GO (a) and GO/TiO2–50% (b)
Characterization of GO/TiO2/PSf membranes
Porosity, pure water flux (PWF), and surface charge of the membranes are given in Table 2. The blended membranes exhibit higher porosity, PWF, surface charge in comparison of pure PSf membrane. The porosity of the membrane rises from 39% in M0 to 67% in MC-1. Moreover, the value of PWF increases from 7.3 kg/m2 h for M0 to 326.6 kg/m2 h for MC-1. According to the nature of the nanocomposite, existence of GO/TiO2 particles in the structure of membrane increases hydrophilic sites in the membrane structure and enhance diffusion rate of water molecules through the membrane resulting higher hydrophilicity and permeability. In addition, it is seen that the value of PWF increase by TiO2 content in nanocomposite. Coating GO by TiO2 nanoparticles leads to an increase the number of hydrophilic functional groups (Ti-OH) on the surface of GO and facilitates interaction of water molecules by GO/TiO2 particles in membrane resulting better diffusion and solution. Moreover, they increase negative charge in surface of membrane (zeta potential) according to the chemical nature hydroxyl groups as polar sites with electron pairs. These negative groups can easily interact with the protons in water molecules, adsorb them and increase diffusion and solution of water in the membrane [23].
Table 2.
Composition of casting solutions for the membrane samples
| Membrane | PSF (%) | NMP (%) | GO/TiO2(x)(%) | Porosity (%) | PWF (kg/m2h)a | ζ (mV)b |
|---|---|---|---|---|---|---|
| M0 | 12 | 88 | 0 | 39 | 7.3 | −11.6 |
| MA-0.25 | 11.75 | 88 | x = 10 (0.25) | 53 | 211.7 | −12.8 |
| MA-0.5 | 11.5 | 88 | x = 10 (0.5) | 62 | 278.1 | −15.3 |
| MA-1 | 11 | 88 | x = 10 (1) | 63 | 301.0 | −19.0 |
| MB-0.25 | 11.75 | 88 | x = 30 (0.25) | 49 | 237.3 | −13.0 |
| MB-0.5 | 11.5 | 88 | x = 30 (0.5) | 61 | 285.0 | −15.8 |
| MB-1 | 11 | 88 | x = 30 (1) | 64 | 310.5 | −20.4 |
| MC-0.25 | 11.75 | 88 | x = 50 (0.25) | 56 | 261.9 | −16.5 |
| MC-0.5 | 11.5 | 88 | x = 50 (0.5) | 63 | 290.3 | −19.4 |
| MC-1 | 11 | 88 | x = 50 (1) | 67 | 326.6 | −24.7 |
aTMP = 1 bar
bpH = 7
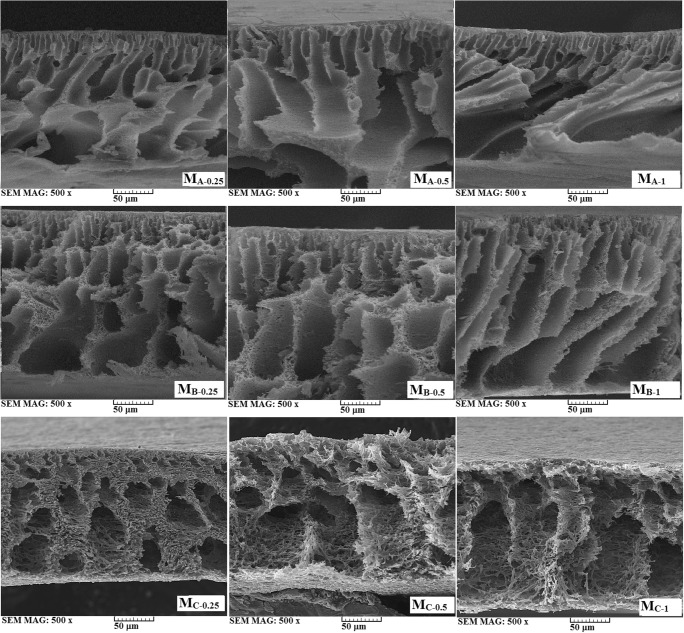
SEM micrographs of the prepared membranes are shown in Fig. 4. The membranes exhibit sponge like structure with the interconnected macrovoids in the sub-layer region. This structure can increase membrane permeability and flux in comparison of non-filled membrane (not shown here). In addition, it is observed that the macrovoids formed along the cross section of membrane as asymmetric structure can facilitate diffusion of water and increase water flux. It is also observed that by increase of TiO2 content in nanocomposite the porosity of membrane increase in order to growth of sponge-like structure with more interconnected macrovoids. Table 3 shows details of BET measurements for the membranes M0, MA-1, MB-1, and MC-1. It is seen that mean pore diameter increases from 8.1 nm for the pure PSf membrane to 27.6, 40.3, and 47.9 nm for MA-1, MB-1, and MC-1, respectively. Rise of pore diameter confirms this fact that increase of pore size and porosity of the membrane by GO/TiO2 content rise PWF of the blended samples.
Fig. 4.
SEM micrographs of the prepared mixed matrix membranes
Table 3.
BET parameters of some prepared membranes
| Membrane | Pore size distribution (nm) | Total pore volume (cm3/g) | Mean pore diameter (nm) |
|---|---|---|---|
| M0 | 5.9–15.2 | 0.0375 | 8.1 |
| MA-1 | 21.0–55.5 | 0.1167 | 27.6 |
| MB-1 | 20.7–63.9 | 0.1816 | 40.3 |
| MC-1 | 30.4–69.9 | 0.2114 | 44.9 |
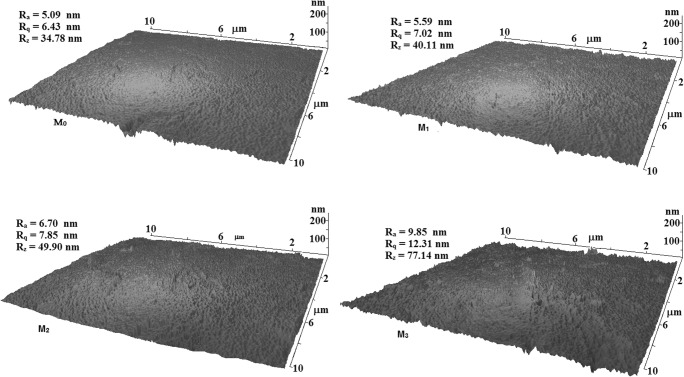
AFM micrographs of MC-x membranes are given in Fig. 5. Dark and light points represent nodes and peaks on the membrane surface, respectively. It is seen that by increase of nanocomposite content in the membrane structure the surface roughness increases. Roughness parameters i.e. Ra, Rq, and Rz rises from 5.09, 6.43, and 37.48 nm for M0 to 9.85, 12.31, and 77.14 nm for MC-1, respectively. It can be concluded that addition of GO/TiO2 nanocomposite to the casting solution increases the rate of solvent (NMP) replacement by non-solvent (water) during phase inversion and produces more porous structure and rougher surface in the membrane [24]. Rougher surface can provide more effective area for water molecules to pass through the membrane and increases interactions of permeate and active sites located on the surface of membrane.
Fig. 5.
3D-AFM images of non-filled membrane (M0) and the membranes mixed by GO/TiO2–50% nanocomposite
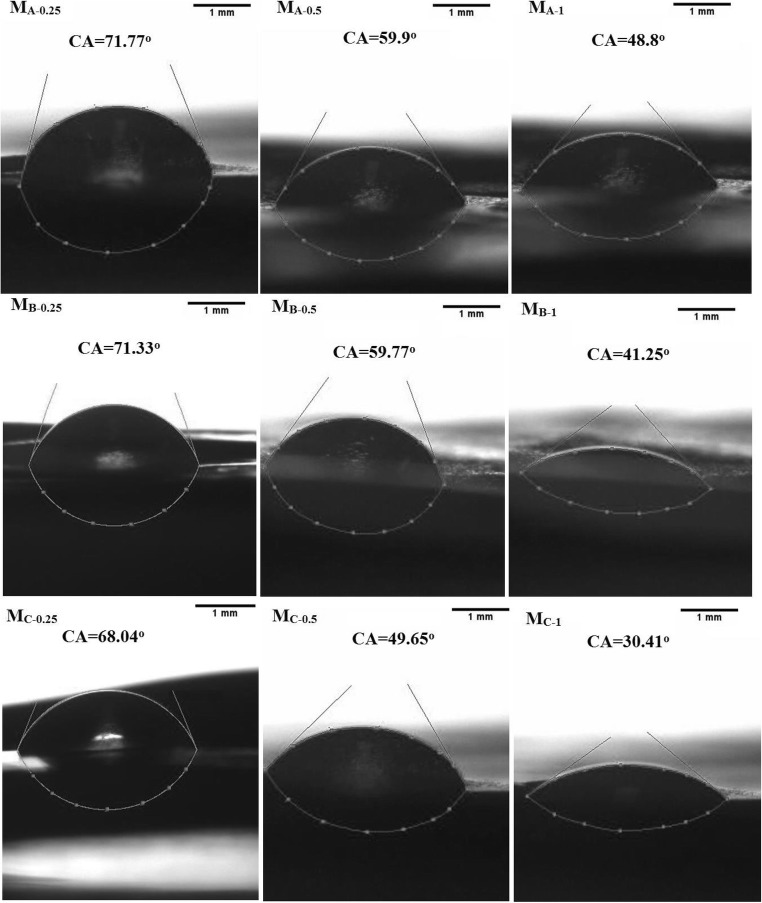
Figure 6 shows the sessile drops of water on the membranes and values of contact angle. It is observed that by addition of GO/TiO2 to the membrane the value of contact angle reduces in order to the increase of surface hydrophilicity of membrane. It is also observed that by increase of TiO2 in nanocomposite content the contact angle decreases. It is clear that at the presence of hydrophilic sites of Ti-OH the surface wettability enhances.
Fig. 6.
Images of sessile drops and contact angles of the mixed matrix membranes
Table 4 shows a comparison between the results of herbicide rejection and PWF for different mixed matrix membranes. It is seen that the prepared membranes in this study exhibits higher permeability compared to the other samples. Similarly, it was reported that size exclusion was the dominant mechanism of herbicide rejection [26–30] and the chemical structure of herbicide played a key role in membrane efficiency [26].
Table 4.
Comparison of rejection of some commercial herbicides by different mixed matrix membranes
| Membrane | Modifier/Filler | Jmax (kg/m2h) | CA (o) | Herbicide | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|
| PSf | PANi/HNT | 40.15 a | 55.0 | atrazine | 50 | [25] |
| PES | H3P12O40 | 26.1 b | 61.1 | trifluralin | > 90 | [26] |
| NF200 | NA | 17.8 c | 26.0 | atrazine | > 80 | [27] |
| PES | NA | 433.1d | – | isoproturone | 50 | [28] |
| PS | polyamide | 7 e | – | glyphosate | > 90 | [29] |
| PES | Sulfonated PEK | – | – | glyphosate | > 90 | [30] |
| PSf | GO/TiO2 | 278.1 f | 59.9 | trifluralin, butachlor, 2,4-D, glyphosate | 73,69,61,53 | present study |
a, b, c, d, e, f TMP = 2, 0.45, 5, 10, 2.5, and 1 bar, respectively
Dynamic filtration
Figure 7 shows results of dynamic filtration of herbicide solutions by different membranes. From details, the value of rejection for trifluralin rises from 70% for MA-0.25 to 73% for MA-0.5 and then reduces to 61% for MC-1. Similarly, the rejection of butachlor by means of MA-0.25 and MA-0.5 are 67 and 69%, respectively while it decreases to 58% using MC-1. The rejection of 2,4-D also reduces from maximum value of 61% for MA-0.5 to 56% and for glyphosate, the rejection decreases from 53% for MA-0.25 to 50% for MC-1, respectively. Comparison of rejection details and molecular properties of herbicides shown in Table 1 indicates that the herbicides by larger size and lower solubility have higher rejection. It can be concluded that the size exclusion is the dominant mechanism for herbicides rejection. Trifluralin and butachlor have larger size (143.6 and 142.1 nm, respectively), zero H-bond donor site, and lower solubility. Based on this mechanism, glyphosate and trifluralin have smaller size, so their rejections decrease. In addition, existence of H-bond donor sites in their structure leads to more interactions with hydrophilic surface of mixed matrix membrane and better diffusion resulting lower rejection.
Fig. 7.
Details of herbicides rejections by prepared mixed matrix membranes
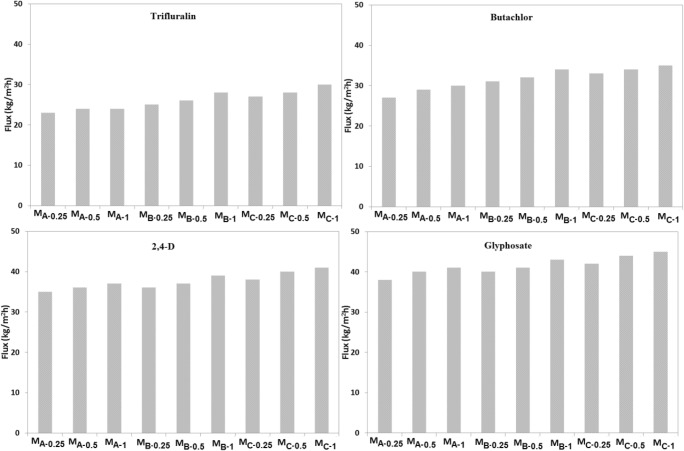
Figure 8 represents details of flux for herbicide solutions. It is observed that by use of MC-1 as the sample with the most values, flux of glyphosate, 2,4-D, butachlor, and trifluralin are 45.4, 41.8, 35.6, and 30.0 kg/m2 h, respectively. As discussed, the higher solubility and smaller size of glyphosate and 2,4-D lead to an increase of diffusion and solution rates of herbicide molecules and rise of the flux. Figure 9 shows variation of flux of herbicide solution by time of filtration for MA-0.5 as the sample with the higher rejection. The values of flux reduce from 24.2 and 29.4 to 18.7 and 23.5 kg/m2 h, respectively while the flux decline for 2,4-D and glyphosate are from 37.3 and 40 to 27.8 and 30.0 kg/m2 h, respectively. More reduction of flux for 2,4-D and glyphosate can be related to faster diffusion and adsorption of herbicide molecules on the active sites of GO/TiO2 nanocomposite and blockage of membrane pores resulting membrane fouling and reduction of flux.
Fig. 8.
Flux of herbicides solutions for different membranes
Fig. 9.
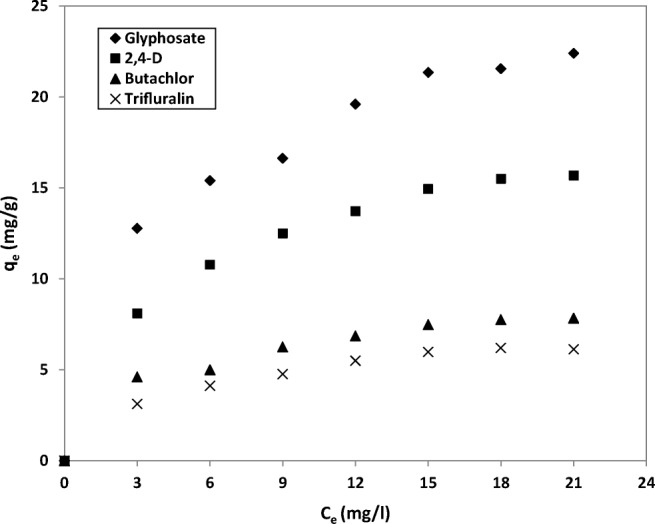
Adsorption isotherm of herbicides over MC-1 sample
Adsorption experiments
The results of removed values of each herbicide during adsorption experiments were fitted by Langmuir isotherm in order to calculate maximum adsorption capacity for each membrane. Details for adsorption of the herbicides on the various types of membranes are given in Table 5 based on Langmuir isotherm fitting. It is clear that MC-1 contains the most number of active sites in GO/TiO2 particles dispersed in the membrane structure and demonstrates highest value of qm in comparison of other membranes. Figure 9 demonstrates adsorption behavior of herbicides by means of MC-1 sample in different concentrations. It is shown that the results are carefully fitted by Langmuir isotherm. Maximum adsorption capacity, qm, for glyphosate, 2,4-D, butachlor, and trifluralin by use of MC-1 are 26.59, 19.08, 9.44, and 7.69 mg/g, respectively. Glyphosate and trifluralin demonstrate maximum and minimum adsorption values on the membrane pieces, respectively. It can be concluded that the chemical nature plays key role in the adsorption capacity of herbicide. Glyphosate contains four H-bond donor sites that can interact by hydrophilic functional groups of GO/TiO2 nanocomposite in membrane structure. Furtheremore, small size of glyphosate and 2,4-D molecules enhances diffusion of herbicide molecules on the pores and channels of the membranes, resulting strong adsorption of glyphosate and 2,4-D on the active sites of GO/TiO2 particles. On the other hand, hydrophobic nature of butachlor and trifluralin and their large sizes reduce interactions by hydrophilic GO/TiO2 nanoparticles and diffusion rate of herbicides molecules, so the adsorption capacities of these herbicides decrease.
Table 5.
Maximum adsorption capacity of herbicides for different membranes based on Langmuir isotherm
| Membrane | Glyphosate | 2,4-D | Butachlor | Trifluralin | ||||
|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | R2 | qm (mg/g) | R2 | qm (mg/g) | R2 | qm (mg/g) | R2 | |
| MA-0.25 | 17.64 | 0.981 | 12.45 | 0.985 | 5.49 | 0.984 | 5.00 | 0.995 |
| MA-0.5 | 18.50 | 0.983 | 14.69 | 0.980 | 6.01 | 0.987 | 5.37 | 0.993 |
| MA-1 | 21.88 | 0.987 | 16.00 | 0.987 | 7.13 | 0.980 | 5.90 | 0.990 |
| MB-0.25 | 18.10 | 0.965 | 14.80 | 0.985 | 6.60 | 0.987 | 5.45 | 0.997 |
| MB-0.5 | 20.94 | 0.980 | 15.45 | 0.983 | 7.07 | 0.985 | 6.06 | 0.993 |
| MB-1 | 22.37 | 0.980 | 17.79 | 0.981 | 7.93 | 0.985 | 6.49 | 0.990 |
| MC-0.25 | 19.06 | 0.967 | 16.00 | 0.980 | 7.51 | 0.989 | 5.44 | 0.993 |
| MC-0.5 | 21.18 | 0.980 | 17.97 | 0.987 | 8.90 | 0.990 | 6.80 | 0.995 |
| MC-1 | 26.59 | 0.979 | 19.08 | 0.985 | 9.44 | 0.989 | 7.69 | 0.995 |
Table 6 demonstrates comparison of qm of the herbicides over different adsorbents and GO/TiO2/PSf membrane (MC-1) used in this work. It can be seen that GO/TiO2/PSf membrane exhibits comparable value of qm regardless of adsorbent dosage. For example, qm for adsorption of glyphosate on GO/TiO2/PSf membrane is bigger than chitosan/alginate membrane and smaller than advanced nanoadsorbents such as polyaniline nanoparticles coated on ZSM-5. qm for adsorption of 2,4-D on GO/TiO2/PSf membrane is also bigger than modified activated carbon and lower than magnetic GO/Mg3Al-OH nanocomposite. In the case of trifluralin, qm is more than activated carbon and organo-clays and less than chitosan.
Table 6.
Comparison of maximum adsorption capacity of GO/TiO2/PSf membrane with different adsorbents
| Adsorbent | Herbicide | Dosage (g/l) | qmax (mg/g) | ref. |
|---|---|---|---|---|
| Fe-humic acid | Glyphosate | 10 | 4.05 | [31] |
| chitosan/alginate membranes | Glyphosate | 0.008 | 8.70 | [32] |
| GO/TiO2/PSf membrane | Glyphosate | 1 | 26.59 | This work |
| PANi/ZSM-5 | Glyphosate | NA | 61.9 | [33] |
| Alum sludge | Glyphosate | 5 | 113.6 | [34] |
| Resin D301 | Glyphosate | 11.1 | 169.49 | [35] |
| Modified activated carbon | 2,4-D | 4 | 0.688 | [36] |
| magnetic ion exchange resin | 2,4-D | 1 | NA | [37] |
| GO/TiO2/PSf membrane | 2,4-D | 1 | 19.08 | This work |
| Modified jute | 2,4-D | 10 | 37.5 | [38] |
| PAN@carbon fibers | 2,4-D | 0.625 | 164.47 | [39] |
| Magnetic GO/Mg3Al-OH | 2,4-D | 1 | 198.26 | [40] |
| GO/TiO2/PSf membrane | Butachlor | 1 | 9.44 | This work |
| Activated bentonite | Butachlor | 0.05 | 29.30 | [41] |
| Organo-clays | Trifluralin | 25 | <0.1 | [42] |
| Activated carbon | Trifluralin | 50 | 0.684 | [43] |
| GO/TiO2/PSf membrane | Trifluralin | 1 | 7.69 | This work |
| PS-DVB copolymer resin | Trifluralin | NA | 15.55 | [44] |
| Chitosan | Trifluralin | 6 | 31.8 | [45] |
Conclusion
Addition of GO/TiO2 prepared by solvothermal procedure to polysulfone membranes significantly increased water permeability and surface hydrophilicity via the existence of titanol groups dispersed on the nano scale TiO2 particles in nanocomposite. It was found that the blended membrane were efficient to rejection of herbicides based on the size exclusion mechanism. The membrane having 0.5 wt.% of GO/TiO2(10%) exhibited the most efficiency for herbicide rejection (73, 69, 61, and 53% for trifluralin, butachlor, 2,4D, and glyphosate, respectively). Addition of GO/TiO2 more than this value decreased membrane rejection, although it was observed the membrane as an adsorptive filter of herbicide by formation of porous sponge like structure in membrane. Results of adsorption experiments indicated that the membranes by more nanocomposite content, TiO2 percentage in GO/TiO2 structure, and porosity demonstrated higher adsorption capacity of the herbicides. The adsorption capacities of Glyphosate, 2,4-D, butachlor, and trifluralin over the membrane having 1 wt.% of GO/TiO2(50%) were 26.59, 19.08, 9.44, and 7.69 mg/g, respectively. More adsorption of glyphosate and 2,4-D resulted according to their smaller size and faster diffusion through the membrane pores. Moreover, strong attractions between H-bond sites and hydrophilic GO/TiO2 nanoparticles dispersed in membrane channels led to more adsorption of glyphosate and 2,4-D.
Acknowledgements
This research was financially supported by Qaemshahr Branch of Islamic Azed University (IAU) and the authors gratefully thank Dr. Sadeqi and Dr. Tayebi for providing the herbicides samples.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morteza Z, Mousavi SB, Baghestani MH, Aitio A. An assessment of agricultural pesticide use in Iran 2012-2014. J Environ Health Sci Eng. 2017;15:10–17. doi: 10.1186/s40201-017-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimentel D, Burgess M. Small amounts of pesticides reaching target insects. Environ Dev Sustain. 2012;14:1–2. doi: 10.1007/s10668-011-9325-5. [DOI] [Google Scholar]
- 3.Yang X, Li J, Wen T, Ren X, Huang Y, Wang X, Yang X, Li J, Wen T, Ren X, Huang Y, Wang X. Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloids Surf A: Physicochem Eng Aspects. 2013;422:118–125. doi: 10.1016/j.colsurfa.2012.11.063. [DOI] [Google Scholar]
- 4.Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA, Travlou N, Kyzas G, Lazaridis N, Deliyanni E. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir. 2013;29:1657–1668. doi: 10.1021/la304696y. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Wang J, Jiao C, Wang C, Wu Q, Wang Z. Graphene oxide framework: an adsorbent for solid phase extraction of phenylurea herbicides from water and celery samples. J Chromatography A. 2016;1469:17–24. doi: 10.1016/j.chroma.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 6.Hwang T, Oh J, Yim W, Nam J, Bae C, Kim H, Kim K. Ultrafiltration using graphene oxide surface-embedded polysulfone membranes. Sep Purif Technol. 2016;166:41–47. doi: 10.1016/j.seppur.2016.04.018. [DOI] [Google Scholar]
- 7.Rezaee R, Nasseri N, Mahvi AH, Nabizadeh R, Mousavi SA, Rashidi A, Jafari A, Nazmara S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J Environ Health Sci Eng. 2015;13:61–71. doi: 10.1186/s40201-015-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegab HM, Zou L. Graphene oxide-assisted membranes: fabrication and potential applications in desalination and water purification. J Membrane Sci. 2015;484:95–106. doi: 10.1016/j.memsci.2015.03.011. [DOI] [Google Scholar]
- 9.Sherlala AIA, Raman AAA, Bello MM, Asghar A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere. 2018;193:1004–1017. doi: 10.1016/j.chemosphere.2017.11.093. [DOI] [PubMed] [Google Scholar]
- 10.Chung YT, Mahmoudi E, Mohammad AW, Benamor A, Johnson D, Hilal N. Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination. 2017;402:123–132. doi: 10.1016/j.desal.2016.09.030. [DOI] [Google Scholar]
- 11.Wu H, Tang B, Wu P. Development of novel SiO2–GO nanohybrid/polysulfone membrane with enhanced performance. J Membrane Sci. 2014;451:94–102. doi: 10.1016/j.memsci.2013.09.018. [DOI] [Google Scholar]
- 12.Chai P, Mahmoudi E, Teow Y, Mohammad A. Preparation of novel polysulfone-Fe3O4/GO mixed-matrix membrane for humic acid rejection. J Water Process Eng. 2017;15:83–88. doi: 10.1016/j.jwpe.2016.06.001. [DOI] [Google Scholar]
- 13.Thebo KH, Qian X, Wei Q, Zhang Q, Cheng H-M, Ren W. Reduced graphene oxide/metal oxide nanoparticles composite membranes for highly efficient molecular separation. J Mater Sci Technol. 2018;34:1481–1486. doi: 10.1016/j.jmst.2018.05.008. [DOI] [Google Scholar]
- 14.Safarpour M, Vatanpour V, Khataee A, Esmaeili M. Development of a novel high flux and fouling-resistant thin film composite nanofiltration membrane by embedding reduced graphene oxide/TiO2. Sep Purif Technol. 2015;154:96–107. doi: 10.1016/j.seppur.2015.09.039. [DOI] [Google Scholar]
- 15.Safarpour M, Vatanpour V, Khataee A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination. 2016;393:65–78. doi: 10.1016/j.desal.2015.07.003. [DOI] [Google Scholar]
- 16.Gao Y, Hu M, Mi B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J Membrane Sci. 2014;455:349–356. doi: 10.1016/j.memsci.2014.01.011. [DOI] [Google Scholar]
- 17.Emadzadeh D, Ghanbari M, Lau W, Rahbari-Sisakht M, Rana D, Matsuura T, Ismail A. Surface modification of thin film composite membrane by nanoporous titanate nanoparticles for improving combined organic and inorganic antifouling properties. Mater Sci Eng C. 2017;75:463–470. doi: 10.1016/j.msec.2017.02.079. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Ding M, Chen W, Li Y, Wang K. Nitrogen–doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep Purif Technol. 2018;195:70–82. doi: 10.1016/j.seppur.2017.12.003. [DOI] [Google Scholar]
- 19.Xu C, Cui A, Xu Y, Fu X. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon. 2013;62:465–471. doi: 10.1016/j.carbon.2013.06.035. [DOI] [Google Scholar]
- 20.Naghdi S, Jaleh B, Shahbazi N. Reversible wettability conversion of electrodeposited graphene oxide/titania nanocomposite coating: investigation of surface structures. Appl Surf Sci. 2016;368:409–416. doi: 10.1016/j.apsusc.2016.01.193. [DOI] [Google Scholar]
- 21.Cabir B, Yurderi M, Caner N, Agirtas MS, Zahmakiran M, Kaya M. Methylene blue photocatalytic degradation under visible light irradiation on copper phthalocyanine-sensitized TiO2 nanopowders. Mater Sci Eng B. 2017;224:9–17. doi: 10.1016/j.mseb.2017.06.017. [DOI] [Google Scholar]
- 22.Goei R, Lim T-T. Ag-decorated TiO2 photocatalytic membrane with hierarchical architecture: photocatalytic and anti-bacterial activities. Water Res. 2014:59207–18. [DOI] [PubMed]
- 23.Toosi MR, Sarmasti Emami MR, Hajian H. Dynamic filtration and static adsorption of lead ions in aqueous solution by use of blended polysulfone membranes with nano size MCM-41 particles coated by polyaniline. Environ Sci Pollution Res. 2018;25:20217–20230. doi: 10.1007/s11356-018-2236-3. [DOI] [PubMed] [Google Scholar]
- 24.Tabar Y, Toosi MR. Adsorptive filtration of azo dyes by polysulfone membranes blended with polyaniline based MCM-48 mesopore prepared from rice husk. Desalin Water Treat. 2018. 10.5004/dwt.2018.22495.
- 25.Babu VS, Padakia M, D'Souza LP, Déon S, Balakrishna RG, Ismail AF. Effect of hydraulic coefficient on membrane performance for rejection of emerging contaminants. Chem Eng J. 2018;334:2392–2400. doi: 10.1016/j.cej.2017.12.027. [DOI] [Google Scholar]
- 26.Ghaemi N, Nasirmanesh F. Synthesis of a hybrid organic-inorganic polyethersulfone membrane incorporated with phosphotungstic acid: controversial performance in removal of dinitroaniline herbicides from water. J Cleaner Production. 2018. 10.1016/j.jclepro.2018.02.069.
- 27.Plakas KV, Karabelas AJ, Wintgens T, Melin T. A study of selected herbicides retention by nanofiltration membranes-the role of organic fouling. J Membrane Sci. 2006;284:291–300. doi: 10.1016/j.memsci.2006.07.054. [DOI] [Google Scholar]
- 28.Acero JL, Benitez FJ, Real FJ. Carolina García, removal of phenyl-urea herbicides in natural waters by UF membranes: permeate flux, analysis of resistances and rejection coefficients. Sep Purif Technol. 2009;65:322–330. doi: 10.1016/j.seppur.2008.11.003. [DOI] [Google Scholar]
- 29.Yuan J, Duan J, Saint CP, Mulcahy D. Removal of glyphosate and aminomethylphosphonic acid from synthetic water by nanofiltration. Environmental Technol. 2017. 10.1080/09593330.2017.1329356. [DOI] [PubMed]
- 30.Song J, Li X-M, Figoli A, Huang H, Pan C, He T, Jiang B. Composite hollow fiber nanofiltration membranes for recovery of glyphosate from saline wastewater. Water Res. 2013;47:2065–2074. doi: 10.1016/j.watres.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Piccolo A, Celano G, Pietramellara G. Adsorption of the herbicide glyphosate on a metal-humic acid complex. Sci Total Environ. 1992;123:77–82. doi: 10.1016/0048-9697(92)90134-E. [DOI] [Google Scholar]
- 32.Carneiro RT, Taketa TB, Neto RJ, Oliveira JL, Campos EV, Moraes MA, Fraceto LF. Removal of glyphosate herbicide from water using biopolymer membranes. J Environ Manag. 2015;151:353–360. doi: 10.1016/j.jenvman.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Milojević-Rakić M, Janošević A, Krstić J, Vasiljević BN, Dondur V, Ćirić-Marjanović G. Polyaniline and its composites with zeolite ZSM-5 for efficient removal of glyphosate from aqueous solution. Micropor Mesopor Mater. 2013;180:141–155. doi: 10.1016/j.micromeso.2013.06.025. [DOI] [Google Scholar]
- 34.Hu Y, Zhao Y, Sorohan B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination. 2011;271:150–156. doi: 10.1016/j.desal.2010.12.014. [DOI] [Google Scholar]
- 35.Chen F, Zhou C, Li G, Peng F. Thermodynamics and kinetics of glyphosate adsorption on resin D301. Arab J Chem. 2016;9:s1665–s1669. doi: 10.1016/j.arabjc.2012.04.014. [DOI] [Google Scholar]
- 36.Dehghani M, Nasseri S, Karamimanesh M. Removal of 2,4-Dichlorophenolyxacetic acid (2,4-D) herbicide in the aqueous phase using modified granular activated carbon. J Environ Health Sci Eng. 2014;12:28–37. doi: 10.1186/2052-336X-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Lu X, Li S, Zhong M, Shi X, Luo G, Ding L. Investigation of 2,4-dichlorophenoxyacetic acid adsorption onto MIEX resin: optimization using response surface methodology. J Taiwan Institute Chem Eng. 2014;45:1835–1841. doi: 10.1016/j.jtice.2014.02.012. [DOI] [Google Scholar]
- 38.Manna S, Saha P, Roy D, Sen R, Adhikari B. Removal of 2,4-dichlorophenoxyacetic acid from aqueous medium using modified jute. J Taiwan Institute Chem Eng. 2016;67:292–299. doi: 10.1016/j.jtice.2016.07.034. [DOI] [Google Scholar]
- 39.Zhao R, Li X, Sun B, Ji H, Wang C. Diethylenetriamine-assisted synthesis of amino-rich hydrothermal carbon-coated electrospun polyacrylonitrile fiber adsorbents for the removal of Cr(VI) and 2,4-dichlorophenoxyacetic acid. J Colloid Interface Sci. 2017;487:297–309. doi: 10.1016/j.jcis.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F, Song Y, Song S, Zhang R, Hou W. Synthesis of magnetite–graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-Dichlorophenoxyacetic acid from aqueous solutions. ACS Appl Mater Interface. 2015;7:7251–7263. doi: 10.1021/acsami.5b00433. [DOI] [PubMed] [Google Scholar]
- 41.Pal O, Vanjara A. Removal of malathion and butachlor from aqueous solution by clays and organoclays. Sep Purif Technol. 2001;24:167–172. doi: 10.1016/S1383-5866(00)00226-4. [DOI] [Google Scholar]
- 42.Leovac A, Vasyukova E, Ivančev-Tumbas I, Uhl W, Kragulj M, Tričković J. Dalmacija B sorption of atrazine, alachlor and trifluralin from water onto different geosorbents. RSC Adv. 2015;5:8122–8133. doi: 10.1039/C4RA03886J. [DOI] [Google Scholar]
- 43.Lule GM, Atalay MU. Comparison of Fenitrothion and Trifluralin adsorption on Organo-zeolites and activated carbon. Part I: pesticides adsorption isotherms on adsorbents. Particulate Sci Tech. 2014;32:418–425. doi: 10.1080/02726351.2014.890687. [DOI] [Google Scholar]
- 44.Kyriakopoulos G, Doulia D, Anagnostopoulos E. Adsorption of pesticides on porous polymeric adsorbents. Chem Eng Sci. 2005;60:1177–1186. doi: 10.1016/j.ces.2004.09.080. [DOI] [Google Scholar]
- 45.Melo AM, Valentim IB, Goulart MO, Abreu FC. Adsorption studies of trifluralin on chitosan and its voltammetric determination on a modified chitosan glassy carbon electrode. J Brazilian Chem Soc. 2008;19:704–710. doi: 10.1590/S0103-50532008000400014. [DOI] [Google Scholar]