Abstract
Background
Phosphate (PO43−) is the main etiological factor of eutrophication in surface waters. Metal organic frameworks (MOFs) are novel hybrid materials with amazing structural properties that make them a prominent material for adsorption.
Methods
Zeolitic imidazolate framework 67 (ZIF-67), a water stable member of MOFs, with a truncated rhombic dodecahedron crystalline structure was synthesized in aqueous environment at room temperature and then characterized using XRD and SEM. PO43− adsorption from synthetic solutions using ZIF-67 in batch mode were evaluated and a polynomial model (R2: 0.99, R2adj: 0.98, LOF: 0.1433) developed using response surface methodology (RSM).
Results
The highest PO43− removal (99.2%) after model optimization obtained when ZIF-67 dose, pH and mixing time adjusted to 6.82, 832.4 mg/L and 39.95 min, respectively. The optimum PO43− concentration in which highest PO43− removal and lowest adsorbent utilization occurs, observed at 30 mg/L. PO43− removal eclipsed significantly in the presence of carbonate. The equilibrium and kinetic models showed that PO43− adsorbed in monolayer (qmax: 92.43 mg/g) and the sorption process controlled in the sorption stage. Adsorption was also more favorable at higher PO43− concentration, according to the separation factor (KR) graph. Thermodynamic parameters (minus signs of ∆G°, ∆H° of 0.179 KJ/mol and ∆S° of 44.91 KJ/mol.K) demonstrate the spontaneous, endothermic and physisorption nature of the process.
Conclusion
High adsorption capacity and adsorption rates, make ZIF-67 a promising adsorbent for PO43− removal from aqueous environment.
Keywords: Metal organic frameworks (MOFs), ZIF-67, Adsorption, Phosphate, RSM, Thermodynamic
Introduction
Metal–organic frameworks (MOFs) are hybrid compounds composed of organic molecules and metal ions that linked together by a controlled manner [1, 2]. These crystalline structures provide huge porosity and tunable surfaces that make them a promising target in widespread fields of biomedical, sensing, molecular separation, gas adsorption, chemical catalysis, and pharmacology [3, 4]. Moreover, their post synthesis modification and potential to be functionalized, make MOFs even more attractive among scientists [1].
Many types of MOFs had been made recently, but lately Zeolitic Imidazolate Frameworks (ZIFs) distinguished as one of the most prominent family of MOFs [2]. Exclusive features of ZIFs are high resistance against environmental situation such as temperature and chemicals, very high level of adsorption capacity due to their high specific surface area and large pores and simplicity of their synthesis route [3, 5–8]. These characteristics cause its reputation for removal of neonicotinoid insecticides [9], ciprofloxacin antibiotics [5], arsenic [10, 11], and 1-naphthol [12] from water medium. ZIF-67 is a subfamily of ZIFs that considered a novel sorbent for removal some pollutants from aqueous solution [3, 12]. Although some studies have shown that ZIF-67 is sensitive to strong acidic condition, the capacity of sorption reported much more from common sorbents such as activate carbon [5, 12].
The results of Pan et al. [13] showed that the capacity of ZIF-67 in removal of phenol was 378.89 mg/g at pH = 9 and 303 K. Huang et al. [14] reported that saturated adsorption capacities for ZIF-67 for removal Pb2+ and Cu2+ from wastewater were 1119.80 and 617.51 mg/g, respectively. Yan et al. [12] revealed that the maximum adsorption capacity of ZIF-67 in removal of 1-naphthol happened at pH = 10 which was 339 mg/g at 313 K. Few studies have been reported about the use of ZIF-67 absorbent for the removal of contaminants from the aqueous solution.
Phosphate (PO43−) is one of the important drinking water contaminants [15]. PO43− plays prominent role in human life as fertilizers, detergents, softeners in water processes, foods and beverages, but the existence of PO43− together with nitrogen stimulates algal bloom in surface water resources [16, 17]. When the concentration of phosphate exceeds 0.02 mg/L, the eutrophication phenomena and continuing water quality deterioration would happen [18]. Although some biological wastewater treatment techniques like can reduce the PO43− concentration to less than 0.02 mg L−1, resources of PO43− intrusion to water supply is different and sometimes is more than standard levels [19]. For this reason, many countries has established wastewater discharge standard to surface water [20]. In order to overcome these problems, it is recommended the concentration of PO43− should be diminished using different strategies, especially adsorption. Sorption process have widely used to remove pollutants from aqueous solutions [21–23].
Experimental designs usually give a lot of information about the experiments to researchers in short time and less energy. To reach these goals response surface methodology (RSM) has proposed. This method was developed to identify the interaction of variables and optimize the process. Central composite design (CCD), Doehlert matrix, and Box–Behnken design are the most famous RSM approaches. Among the proposed approach design, the CCD is a preferred and more welcoming RSM design [24–26]. Present work aimed to evaluate the application of a ZIF-67 as a water stable member of MOFs in the removal of PO43− from aqueous environments.
Materials and methods
In the present work, the reagents and chemicals for the synthesis of ZIF-67 were purchased from Sigma-Aldrich (Steinheim, Germany). The morphology and structures of adsorbent crystals were characterized using scanning electronic microscopy (FE-SEM, MIRA3 TESCAN, Czech Republic), and X-ray diffraction (Unisantis S.A, XMD300 model, Geneva, Switzerland) with cu-kα as source radiation at wavelength 0.154 nm.
Sorption studies
Batch mode experiments were adopted to conduct the present work. When the samples agitated at 250 rpm in pre-adjusted condition, the solutions were filtered through Whatman No. 42 filter paper for determining residual PO43− using the stannous chloride method by a UNICO UV- 2100 spectrophotometer. With the exception of thermodynamic study, all the experiments carried out at room temperature (23 ± 2 °C) without controlling pH.
The removal percentage and adsorption capacity for PO43− were calculated by using the following expression:
| 1 |
| 2 |
In Eq. (1) and Eq. (2), Cinitialand Cfinalare PO43− concentrations before and after adsorption; V is the volume of the solution, and W is the weight of the adsorbent.
ZIF-67 preparation
ZIF-67 crystals were synthesized using 2-methylimidazole (Hmim) as organic linker for joining metallic cobalt ions with a Hmim/Co+2 ratio of 20. In short, 1.642 g of Hmim and 1 mmol of CoSO4 were dissolved in 10 mL of deionized water, separately, and stirred to obtain clear solutions. The metal solution then was added into the ligand solution while stirring continued for further 30 min. After the crystallization period, the purple crystals were separated using 3000 rpm centrifugation for ten min. Finally, the crystals were washed by deionized water several times and were ovened at 70 °C for 24 h [27].
Design of experiments, modeling and optimization
RSM technique helps the researchers to design the platform of experiments without spending too much time and energy. CCD as a standard RSM design can cover the interaction of variables by using less numbers of experiments. To specify optimum conditions and to graphically presentation the relationship between all study variables, response surface plots were adopted by Design Expert software. The model is represented by Eq. (3) [24, 25].
| 3 |
Where i express the number of studied factors; X1i and X2i express the input variables influencing the model outputs; b0 and b12 are the intercept and interaction constant coefficient, respectively, b1 to b2 and b11 to b22 are the linear and quadratic constant coefficients, respectively, and e1i is a random error. Analysis of variance (ANOVA) in the confidence interval of 95% was applied to evaluate of fitted model. Correlation coefficient, F-value, and P value were used to express the fitted polynomial mode [24, 28].
In this work, three factor CCD matrix at five levels was applied in order to analysis the variables and procure regression and their coefficients. Table 1 shows the variable level for coded and actual values of the study variables.
Table 1.
Five-level Three -factor response surface design of experiments
| Factor | Code | Variable level | ||||
|---|---|---|---|---|---|---|
| −1.525 | −1 | 0 | + 1 | +1.525 | ||
| pH | A | 4 | 5.38 | 8 | 10.62 | 12 |
| ZIF-67 dose (g/L) | B | 0.1 | 0.2548 | 0.55 | 0.8451 | 1 |
| Mixing time (min) | C | 10 | 25.49 | 55 | 84.51 | 100 |
Results and discussion
ZIF-67 structural characterization
To be sure about the structural and optical preciseness of ZIF-67 crystals, the adsorbent were characterized using scanning electronic microscopy and X-ray powder diffraction (XRD).
The sharp peaks at 2 theta values of 7.75, 10.54, 12.98, 14.87, 16.62, 18.21, 22.33, 24.69 and 26.73 and the good agreement of surface geometrical morphology and the uniform structure of synthesized crystals is entirely in accordance with those in the literature for ZIF-67. Moreover, the BET surface area and total pore volume of ZIF-67 according to the literature were 1375 m2/g and 0.62 cm3/g, respectively (Figs. 1 and 2).
Fig. 1.
XRD pattern of as-synthesized ZIF-67
Fig. 2.
FE-SEM micrograph of as-synthesized ZIF-67
Sorption modeling using RSM
RSM was applied to model the effect of the most important operating parameters including pH of the solution (A), adsorbent dose (b) and contact time (C). Throughout the modeling step, the initial PO43− concentration adjusted to 10 mg/L. As Table 2 shows the experimental matrix with a total of twenty experiments were used to evaluate the effect of independent variables on the PO43− removal efficiency (as response).
Table 2.
Study design and experimental and predicted responses by the model for PO43− removal by ZIF-67
| Run No | Coded variable | Response (% removal) |
Run No | Coded variable | Response (% removal) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Observed | Predicted | A | B | C | Observed | predicted | ||
| 1 | −1.525 | 0 | 0 | 98.6 | 97.1 | 11 | 0 | 0 | 0 | 96.7 | 94.9 |
| 2 | 1 | −1 | 1 | 71.8 | 72.2 | 12 | 1 | −1 | −1 | 48 | 45.4 |
| 3 | −1 | −1 | −1 | 74.75 | 74.5 | 13 | 0 | 0 | 0 | 95.87 | 94.9 |
| 4 | 0 | 0 | −1.525 | 69.8 | 72.5 | 14 | 0 | 0 | 0 | 96.4 | 94.9 |
| 5 | 0 | 0 | 1.525 | 97.6 | 95.3 | 15 | −1 | −1 | 1 | 88.3 | 88.9 |
| 6 | 0 | 0 | 0 | 93.4 | 94.9 | 16 | 0 | −1.525 | 0 | 71.4 | 72.5 |
| 7 | −1 | 1 | −1 | 99.38 | 98.7 | 17 | 0 | 0 | 0 | 93.1 | 94.9 |
| 8 | 1.525 | 0 | 0 | 55.2 | 57.0 | 18 | 0 | 0 | 0 | 94.3 | 94.9 |
| 9 | 0 | 1.525 | 0 | 96.2 | 95.5 | 19 | 1 | 1 | 1 | 78.2 | 78.2 |
| 10 | 1 | 1 | −1 | 63.6 | 62.8 | 20 | −1 | 1 | 1 | 99.37 | 101.7 |
The proposed quadratic equation obtained by RSM, in term of coded values, was given below (Eq. (4)):
| 4 |
From the above equation, the effect of any independent variable as well as their interactions on the PO43− removal could readily understand. Having the highest negative coefficient among the individual variables, pH is the most important operating parameter that influences the adsorption efficiency, indirectly. Moreover, the level of influence by experimental variables decreases from adsorbent dose and then mixing time. The positive and negative signs of interactive variables in the Eq. (4) imply their synergic and antagonistic effects of the variables on PO43− adsorption, respectively.
The ANOVA test was used to obtain the best model to describe the responses, precisely. The adequacy of the developed model evaluated by statistical parameters including correlation factor (R2), adjusted R2 (R2adj), lack of fit and prediction R2. These parameters are summarized in Table 3.
Table 3.
Coefficients estimated by the polynomial model for PO43− adsorption by ZIF-67
| Source | Sum of Squares | df | Mean Square | F Value | p value Prob > F |
|---|---|---|---|---|---|
| Model | 4894.989 | 9 | 543.8876 | 117.4753 | < 0.0001 |
| A-pH | 2188.209 | 1 | 2188.209 | 472.6353 | < 0.0001 |
| B-Dose | 721.1897 | 1 | 721.1897 | 155.7711 | < 0.0001 |
| C-Time | 703.3896 | 1 | 703.3896 | 151.9264 | < 0.0001 |
| AB | 23.46125 | 1 | 23.46125 | 5.067438 | 0.0481 |
| AC | 77.25245 | 1 | 77.25245 | 16.6859 | 0.0022 |
| BC | 64.7522 | 1 | 64.7522 | 13.98595 | 0.0038 |
| A2 | 635.2194 | 1 | 635.2194 | 137.2022 | < 0.0001 |
| B2 | 238.563 | 1 | 238.563 | 51.52766 | < 0.0001 |
| C2 | 242.9516 | 1 | 242.9516 | 52.47557 | < 0.0001 |
| Residual | 46.29805 | 10 | 4.629805 | ||
| Lack of Fit | 34.03997 | 5 | 6.807993 | 2.77694 | 0.1433 |
| Pure Error | 12.25808 | 5 | 2.451617 | ||
| Cor Total | 4941.287 | 19 | |||
| Std. Dev. | 2.15 | R-Squared | 0.9906 | ||
| Mean | 84.1 | Adj R-Squared | 0.9822 | ||
| C.V. % | 2.56 | Pred R-Squared | 0.9429 | ||
| PRESS | 282.31 | Adeq Precision | 37.017 | ||
According to the literature, a R2 value higher than 0.8, ΔR [R2- R2adj] value below 0.2, and lack of fit value higher than 0.05 prove the statistically adequacy of the model. The R2, R2adj, prediction R2, and lack of fit in this study are 0.99, 0.98, 0.94 and 0.1433 which implies a rigorous model with a high level of response estimation preciseness. The predicted values of PO43− removals based on the developed model are given in Table 2. Figure 3 simply shows the uniform distribution of experimental PO43− removal next to the regression line (predicted removal).
Fig. 3.
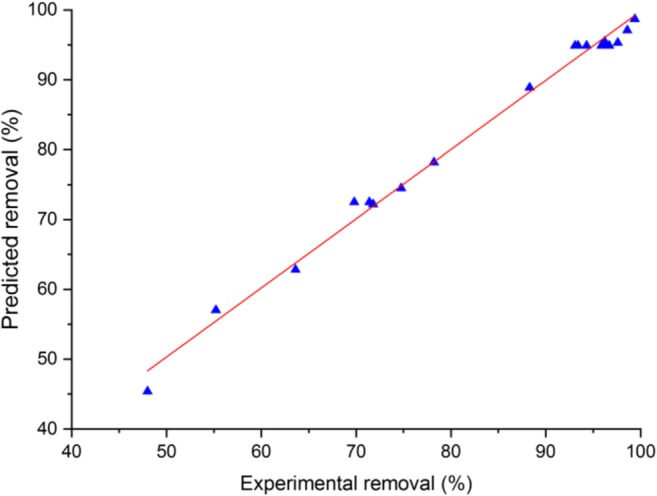
Experimental vs predicted removal for PO43− adsorption by ZIF-67
Response surfaces plots and the effect of independent variables
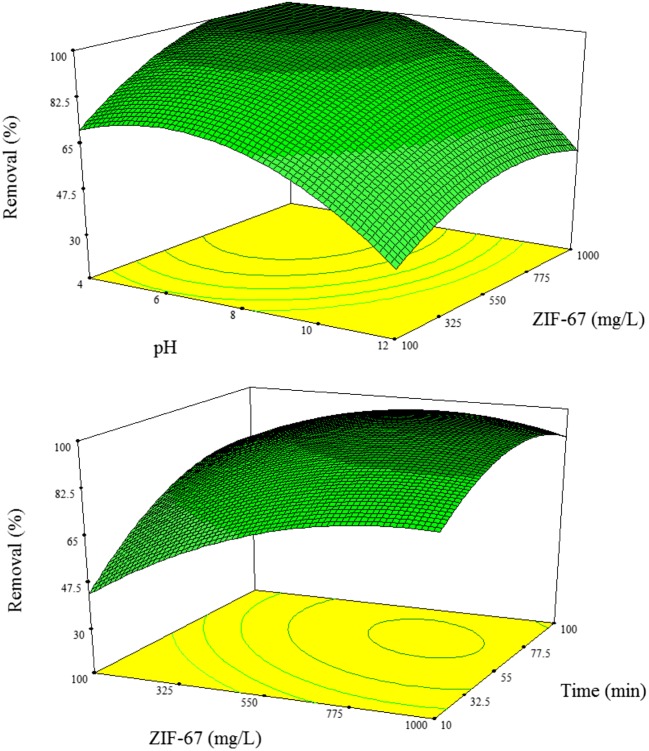
Response surfaces plots are very useful in visualization of the effects of independent variables and their possible interactions. Herein, the adsorption efficiency according to the developed model is presented as a function of pH, adsorbent dose and contact time.
Figure 4 shows the mutual effects of pH and ZIF-67 dose on PO43− removal efficiency. As presented, the PO43− removal was increased by adsorbent dose from 0.1 g/L to 1 g/L and this trend continued to an optimum level. Increasing ZIF-67 crystals beyond this level negatively affect the PO43− removal efficiency. According to the literature [29], the pHZPC for the ZIF-67 is about 9.8 which means the crystals could increase the pH of the solution they are within to this level. In this case, the increases of the solution pH would lead to dominate the negative charge of the ZIF-67 crystals, which in turn produce repelling electrostatic force for the negatively charged PO43− ions. Such phenomena could explain the effect of solution pH as an independent variable. Moreover, at higher pH, the hydroxyl ions could also interact with the PO43− ions to attach the ZIF-67 surface.
Fig. 4.
The response surface plot of the effect of (a) pH and ZIF-68 dose and (b) adsorbent dose and time
The PO43− removal as a function of contact time is presented in Fig. 4. The plot reveals that the PO43− removal could highly is influenced by the contact time. The higher removal efficiency at higher mixing time is due to that more PO43− ions would have time to penetrate into ZIF-67 pores and attach to the surfaces.
The effect of PO43− concentration was studied in the ideal sorption condition obtained during the model optimization. The effect of PO43− level on sorption efficiency and capacity of ZIF-67 plotted in Fig. 5. As expected, the PO43− removal efficiency decreased by the concentration of PO43− ions. This is a routine response to the increasing contaminant level which is related to the competition of the adsorbate molecules to adsorb on the limited sorption sites and also the repelling force between them [30, 31]. As the plot shows, the ZIF-67 capacity for PO43− on the other hand was increased by PO43− concentration. In general, a cost benefit analysis for the utilization of adsorbent capacity and minimization of contaminant level in the effluent usually applied for sorption systems.
Fig. 5.

Effect of initial PO43− concentration on sorption efficiency and capacity of ZIF-67
The intersection point in the Fig. 5, which is corresponding to about 30 mg/L PO43−, gives the optimal PO43− concentration in terms of both ZIF-67 capacity utilization and its removal efficiency.
Model optimization
Finding the best level of independent variables is the final goal in the process modeling. To obtain these conditions, the range of variables set as the same of those applied in the study. The optimum condition in which the removal predicted to be 100%, were calculated to be 6.82, 832.4 mg/L and 39.95 min for pH, ZIF-67 dose and time, respectively. These conditions were finally simulated in the laboratory to evaluate the accuracy of the model optimization. The average removal efficiency for PO43− with three replicates was 99.2%.
Kinetic study
To find the kinetic model that the PO43− adsorption process obey, batch mode experiment was carried out at the optimum pH and adsorbent dose obtained in the previous step. Solutions with the different initial PO43− concentration agitated and the removal efficiencies were determined as a function of time. The Pseudo- first order (Eq. (5)), Pseudo- second order (Eq. (6)), and Intra-particle diffusion (Eq. (7)), which are the most widely used kinetic models, are given in the following equations:
| 5 |
| 6 |
| 7 |
Where, k1 and k2 are the first and the second order rate constants, and qe, qt, and t are equilibrium capacity, capacity at any time, and time, respectively. Table 4 summarized the kinetic parameters obtained from correlating experimental data with the linear form of kinetic models. According to the R2 values in the Table 4, Pseudo- second order model describe the data well. This behavior proves that the chemisorption controls the rate of PO43− sorption onto ZIF-67.
Table 4.
Kinetic constants and parameters for PO43− adsorption by ZIF-67
| C0 [mg/L] |
qe, exp [mg/g] |
Pseudo- first order | Pseudo- second order | Intra-particle diffusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| qe,cal [mg/g] |
K1 [min−1] |
R2 | qe,cal [mg/g] |
K2 [min−1] |
R2 | Kp [mg/g. min-0.5] |
R2 | ||
| 10 | 20.1 | 5.24 | 0.04 | 0.74 | 19.88 | 0.03 | 0.99 | 0.48 | 0.89 |
| 20 | 39.1 | 10.1 | 0.04 | 0.96 | 39.2 | 0.01 | 0.99 | 1.21 | 0.99 |
| 30 | 54.96 | 38.85 | 0.09 | 0.85 | 56.9 | 0.006 | 0.99 | 2.41 | 0.98 |
Equilibrium study
Equilibrium studies are valuable part of sorption studies that provide information on the sorption mechanisms, adsorbent surface, and its affinity to specific contaminant. Isotherm equations model the mobility of adsorbate molecules on the surface of adsorbent under constant environmental condition. The optimum level of 6.82 and 832.4 mg/L for pH and ZIF-67 dose which obtained by optimization were used in performing the equilibrium experiments. Four theoretical isotherm models (Langmuir (Eq. (8)), Freundlich (Eq. (9)), Temkin (Eq. 10), and Dubinin-Radushkevich (Eq. (11)) were used to fit the equilibrium data. The linear form of these models is given as follow:
| 8 |
| 9 |
| 10 |
| 11 |
Where, b, KF, , B1, kt, β, and ε are Langmuir constant, Freundlich’s sorption capacity (mg/g), sorption intensity, Temkin constant, Temkin isotherm constant (L/g), Dubinin-Radushkevich constant, and Polanyi potential, respectively. ε can calculated from the Eq. (12).
| 12 |
Where, R, T, and Ce as defined earlier. The model parameters and coefficients for the isotherms are presented in Table 5. According to the R2 values, the data were simulated by model in the order of Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich.
Table 5.
Isotherm parameters for the equilibrium of PO43− adsorption by ZIF-67
| Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| q max (mg/g) |
KL (L/mg) |
R2 | KF (mg/g(L/mg)1/n |
n | R2 | k t (L/mg) |
B1 | R2 | q max (mg/g) |
β | R2 |
| 92.43 | 1.56 | 0.984 | 44.5 | 2.93 | 0.978 | 0.698 | 11.4 | 0.87 | 49.68 | 1.3E-8 | 0.73 |
Owing to the better conformity of the sorption with the Langmuir isotherm, we can deduce that PO43− covered a monolayer on ZIF-67. Results also confirm that the ZIF-67 surface composed of a uniformly distributed sorption sites. A dimensionless parameter named separation factor (KR) proposed to show the essential features of the Langmuir isotherm as unfavorable (KR > 1), linear (KR = 1), favorable (0 < KR < 1) or irreversible (KR = 0). KR can obtain using the Eq. (13).
| 13 |
The plot of KR versus C0 at Fig. 6 shows a decreasing trend for KR which indicates that the adsorption is more favorable at higher PO43− concentration.
Fig. 6.

Plot of Kr versus initial PO43− concentration
The qmax in the Langmuir model provides a useful tool for comparing the capacity of adsorbents toward specific contaminant, and hence it is an important indicator in the economy of the sorption process. Table 6, compared the qmax for ZIF-67 with the other adsorbents in used for PO43−. The Table 6 demonstrates that the ZIF-67 has a superior capacity for PO43− compared to many adsorbents reported in the literature.
Table 6.
Comparison of monolayer adsorption capacity of different adsorbents for PO43−
| Adsorbent | *qmax (mg/g) |
Reference |
|---|---|---|
| Ferric hydroxide | 65 | [32] |
| Iron-zirconium modified activated carbon nanofiber | 26.3 | [33] |
| ZrO2@Fe3O4 | 15.98 | [34] |
| Fe3O4@SiO2 | 27.8 | [35] |
| La3+(ion)/La(OH)3−W/La(OH)3-EW-loaded magnetic cationic hydrogel composite | 88.3 | [36] |
| Date palm fibers | 4.35 | [37] |
| La(OH)3-modified exfoliated vermiculites | 79.6 | [38] |
| MIL-101(Fe)/ NH2-MIL-101 | 107.7/ 124.38 | [39] |
| ZrO2 particles | 67.3 | [40] |
| Zirconium-modified zeolite | 5.96 | [41] |
| Cubic ZIF-8 | 38.22 | [3] |
| ZIF-67 | 92.43 | Current study |
*Based on Langmuir model
Thermodynamic study
Thermodynamic parameters describe the feasibility of the process and the behavior of the sorption system under different temperatures. Standard enthalpy (∆H°), standard entropy (∆S°) and Gibb’s free energy (∆G°) witch are the most important parameters in thermodynamic study were calculated from the following equations:
| 14 |
| 15 |
Where, R and T stand for universal gas constant (8.314 J/mol. K) and temperature (k), respectively. The Thermodynamic parameters obtained from Van’t Hoff plot (Ln k0 vs 1/T) are summarized in Table 7. The negative values of Gibbs energies (∆G°) in the Table 7 demonstrate that the process continued spontaneous. The positive sign of ∆H° and ∆S° on the other hand, is an indication of endothermic nature of adsorption. Furthermore, the absolute ∆G° in the Table 7 is smaller than 18 kj/mol, indicates that physisorption is the predominant mechanism for PO43− adsorption.
Table 7.
Thermodynamic parameters for PO43− adsorption by ZIF-67
| Temperature K |
Ce mg/L |
-∆G° kJ/mol |
∆H° KJ/mol |
∆S° KJ/mol.K |
|---|---|---|---|---|
| 288 | 2.4 | 6.43 | 0.179 | 44.91 |
| 293 | 1.76 | 7.38 | ||
| 303 | 0.86 | 9.56 | ||
| 313 | 0.44 | 11.68 |
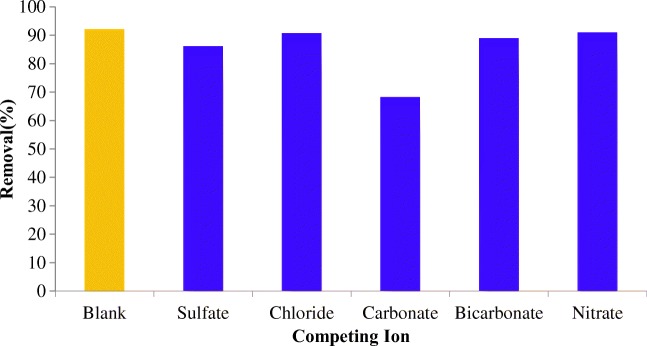
Effect of coexisting ions
In a real treatment system, the presence of competing ions in the water matrix could eclipse the removal efficiencies obtained on distilled water samples. To understand the inhibitory effect of interfering ions, the PO43− removal examined in the presence of 1 mMol sulfate, nitrate, chloride, carbonate, and bicarbonate. Figure 7 shows that the PO43− removal could strongly is prevented by CO3 and the level of inhibition decreases in the presence of sulfate, bicarbonate, chloride, and nitrate. Except than carbonate, which decreased the PO43− removal from about 92% to 68.3%, no significant change observed when adsorption performed in the presence of coexisting ions. These findings are in accordance with the previously published study for ZIF-8, which revealed that the change in pH underlies the effect of co-occurrence ions.
Fig. 7.
PO43− removal in blank sample and at the presence of coexisting ions
Conclusion
Contamination of surface waters with phosphate poses a great treat to the quality of water and aquatic life. Among the possible techniques available for PO43− removal, adsorption attracted high attention and studies conducted on exploring new and efficient adsorbents. ZIF-67, a subclass of MOFs, was synthesized and characterized. A precise (R2: 0.99, R2adj: 0.98, LOF: 0.1433) polynomial model then developed by performing the experiments according to RSM. The highest PO43− removal (99.2%) was determined to occur at pH of 6.82, ZIF-67 dose of 832.4 mg/L and 39.95 min. Furthermore, the optimum PO43− concentration in which the removal efficiency and the ZIF-67 usage capacity both are highest was 30 mg/L. The equilibrium data were simulated by Langmuir> Freundlich> Temkin and then Dubinin–Radushkevich models. Changes in separation factor by PO43− concentration shows that the adsorption is more favorable at higher PO43− concentration. Comparison of qmax (92.43 mg/g) for ZIF-67 proved its good position among adsorbent reported in the literature.
The minus signs of ∆G° and positive sign of ∆H° (0.179) and ∆S°(44.91) in thermodynamic study, demonstrate the spontaneous and endothermic nature of the process. The values of ∆G° indicated that physisorption is the predominant mechanism for PO43−. PO43− removal in the presence of coexisting ions revealed a high level of inhibitory effect by carbonate ions. According to the results, the study could conclude with proposing ZIF-67 as a promising adsorbent for PO43−.
Acknowledgements
The authors would like to appreciate the financial support provided by Ilam University of Medical Science, Iran (Grant Number: 974001-14).
Compliance with ethical standards
Conflict of interest
The authors of this article declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun Y, Sun Q, Huang H, Aguila B, Niu Z, Perman JA, Ma S. A molecular-level superhydrophobic external surface to improve the stability of metal–organic frameworks. J Mater Chem A. 2017;5(35):18770–18776. [Google Scholar]
- 2.Stock N, Biswas S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev. 2011;112(2):933–969. doi: 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- 3.Shams M, Dehghani MH, Nabizadeh R, Mesdaghinia A, Alimohammadi M, Najafpoor AA. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: modeling, mechanical agitation versus sonication. J Mol Liq. 2016;224:151–157. [Google Scholar]
- 4.Yin Z, Wan S, Yang J, Kurmoo M, Zeng M-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. J Coord Chem. 2017;In Press.
- 5.Li S, Zhang X, Huang Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J Hazard Mater. 2017;321:711–719. doi: 10.1016/j.jhazmat.2016.09.065. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Su Z, Jiang F, Yang L, Qian J, Zhou Y, Li W, Hong M. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale. 2014;6(12):6590–6602. doi: 10.1039/c4nr00348a. [DOI] [PubMed] [Google Scholar]
- 7.Chaikittisilp W, Hu M, Wang H, Huang H-S, Fujita T, Wu KC-W, Chen LC, Yamauchi Y, Ariga K. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem Comm. 2012;48(58):7259–7261. doi: 10.1039/c2cc33433j. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Zhu Q-L, Xu Q. Pd nanoparticles supported on hierarchically porous carbons derived from assembled nanoparticles of a zeolitic imidazolate framework (ZIF-8) for methanol electrooxidation. Chem Comm. 2015;51(54):10827–10830. doi: 10.1039/c5cc03008k. [DOI] [PubMed] [Google Scholar]
- 9.Hao L, Wang C, Wu Q, Li Z, Zang X, Wang Z. Metal–organic framework derived magnetic nanoporous carbon: novel adsorbent for magnetic solid-phase extraction. Anal Chem. 2014;86(24):12199–12205. doi: 10.1021/ac5031896. [DOI] [PubMed] [Google Scholar]
- 10.Jian M, Liu B, Zhang G, Liu R, Zhang X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf A Physicochem Eng Asp. 2015;465:67–76. [Google Scholar]
- 11.Liu B, Jian M, Liu R, Yao J, Zhang X. Highly efficient removal of arsenic (III) from aqueous solution by zeolitic imidazolate frameworks with different morphology. Colloids Surf A Physicochem Eng Asp. 2015;481:358–366. [Google Scholar]
- 12.Yan X, Hu X, Chen T, Zhang S, Zhou M. Adsorptive removal of 1-naphthol from water with Zeolitic imidazolate framework-67. J Phys Chem Solids. 2017;107(8):50–54. [Google Scholar]
- 13.Yong P, Zhi L, Zhe Z, Xiong-Shi T, Hai L, Chong-Zhi J, et al. Adsorptive removal of phenol from aqueous solution with zeolitic imidazolate framework-67. J Environ Manag. 2016;169(169):167–173. doi: 10.1016/j.jenvman.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Zeng X, Guo L, Lan J, Zhang L, Cao D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep Purif Technol. 2018;194:462–469. [Google Scholar]
- 15.Liu J, Wu Y, Wu C, Muylaert K, Vyverman W, Yu H-Q, Muñoz R, Rittmann B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresour Technol. 2017;241:1127–1137. doi: 10.1016/j.biortech.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Mahvi AH. Ebrahimi SJA-d, Mesdaghinia a, Gharibi H, Sowlat MH. Performance evaluation of a continuous bipolar electrocoagulation/electrooxidation–electroflotation (ECEO–EF) reactor designed for simultaneous removal of ammonia and phosphate from wastewater effluent. J Hazard Mater. 2011;192(3):1267–1274. doi: 10.1016/j.jhazmat.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Rafati L, Nabizadeh R, Mahvi AH, Dehghani MH. Removal of phosphate from aqueous solutions by iron nano-particle resin Lewatit (FO36) Korean J Chem Eng. 2012;29(4):473–477. [Google Scholar]
- 18.Khalil AM, Eljamal O, Amen TW, Sugihara Y, Matsunaga N. Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem Eng J. 2017;309:349–365. [Google Scholar]
- 19.Khazaei M, Nabizadeh R, Mahvi AH, Izanloo H, Ansari Tadi R, Gharagazloo F. Nitrogen and phosphorous removal from aerated lagoon effluent using horizontal roughing filter (HRF) Desalin Water Treat. 2016;57(12):5425–5434. [Google Scholar]
- 20.Yousefi N, Fatehizedeh A, Ghadiri K, Mirzaei N, Ashrafi SD, Mahvi AH. Application of nanofilter in removal of phosphate, fluoride and nitrite from groundwater. Desalin Water Treat. 2016;57(25):11782–11788. [Google Scholar]
- 21.Malakootian M, Yousefi N, Fatehizadeh A, Van Ginkel SW, Ghorbani M, Rahimi S, et al. Nickel (II) removal from industrial plating effluent by Fenton process. Environ Eng Manag J. 2015;14(4):837–842. [Google Scholar]
- 22.Ahmadian M, Yosefi N, Toolabi A, Khanjani N, Rahimi S, Fatehizadeh A. Adsorption of Direct Yellow 9 and Acid Orange 7 from Aqueous Solutions by Modified Pumice. Asian J Chem. 2012;24(7).
- 23.Pourfadakari S, Yousefi N, Mahvi AH. Removal of reactive red 198 from aqueous solution by combined method multi-walled carbon nanotubes and zero-valent iron: equilibrium, kinetics, and thermodynamic. Chin J Chem Eng. 2016;24(10):1448–1455. [Google Scholar]
- 24.Rezaee R, Maleki A, Jafari A, Mazloomi S, Zandsalimi Y, Mahvi AH. Application of response surface methodology for optimization of natural organic matter degradation by UV/H2O2 advanced oxidation process. J Environ Health Sci Eng. 2014;12(1):67. doi: 10.1186/2052-336X-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arslan A, Topkaya E, Bingöl D, Veli S. Removal of anionic surfactant sodium dodecyl sulfate from aqueous solutions by O3/UV/H2O2 advanced oxidation process: process optimization with response surface methodology approach. Sustain Environ Res. 2017;28(2):65–71. [Google Scholar]
- 26.Im J-K, Cho I-H, Kim S-K, Zoh K-D. Optimization of carbamazepine removal in O3/UV/H2O2 system using a response surface methodology with central composite design. Desalination. 2012;285:306–314. [Google Scholar]
- 27.Guo X, Xing T, Lou Y, Chen J. Controlling ZIF-67 crystals formation through various cobalt sources in aqueous solution. J Solid State Chem. 2016;235:107–112. [Google Scholar]
- 28.Qu J, Meng X, You H, Ye X, Du Z. Utilization of rice husks functionalized with xanthates as cost-effective biosorbents for optimal cd (II) removal from aqueous solution via response surface methodology. Bioresour Technol. 2017;241:1036–1042. doi: 10.1016/j.biortech.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 29.Du X-D, Wang C-C, Liu J-G, Zhao X-D, Zhong J, Li Y-X, et al. Extensive and selective adsorption of ZIF-67 towards organic dyes: performance and mechanism. J Colloid Interface Sci. 2017;506:437–441. doi: 10.1016/j.jcis.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 30.Dehghan A, Zarei A, Jaafari J, Shams M, Khaneghah AM. Tetracycline removal from aqueous solutions using zeolitic imidazolate frameworks with different morphologies: a mathematical modeling. Chemosphere. 2018. [DOI] [PubMed]
- 31.Qasemi M, Afsharnia M, Zarei A, Najafpoor AA, Salari S, Shams M. Phenol removal from aqueous solution using Citrullus colocynthis waste ash. Data Brief. 2018;18:620–628. doi: 10.1016/j.dib.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon H-S, Chung KW, Kim C-J, Kim J-H, Lee H-S, Kim S-J, Lee SI, Yoo SJ, Lim BC. Characteristics of phosphate adsorption on ferric hydroxide synthesized from a Fe 2 (SO 4) 3 aqueous solution discharged from a hydrometallurgical process. Korean J Chem Eng. 2018;35(2):470–478. [Google Scholar]
- 33.Xiong W, Tong J, Yang Z, Zeng G, Zhou Y, Wang D, Song P, Xu R, Zhang C, Cheng M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: performance and mechanism. J Colloid Interface Sci. 2017;493:17–23. doi: 10.1016/j.jcis.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Fang L, Wu B, Lo IM. Fabrication of silica-free superparamagnetic ZrO2@ Fe3O4 with enhanced phosphate recovery from sewage: performance and adsorption mechanism. Chem Eng J. 2017;319:258–267. [Google Scholar]
- 35.Lai L, Xie Q, Chi L, Gu W, Wu D. Adsorption of phosphate from water by easily separable Fe3O4@SiO2 core/shell magnetic nanoparticles functionalized with hydrous lanthanum oxide. J Colloid Interface Sci. 2016;465:76–82. doi: 10.1016/j.jcis.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 36.Dong S, Wang Y, Zhao Y, Zhou X, Zheng H. La3+/La (OH)3 loaded magnetic cationic hydrogel composites for phosphate removal: effect of lanthanum species and mechanistic study. Water Res. 2017;126:433–441. doi: 10.1016/j.watres.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 37.Riahi K, Thayer BB, Mammou AB, Ammar AB, Jaafoura MH. Biosorption characteristics of phosphates from aqueous solution onto Phoenix dactylifera L. date palm fibers. J Hazard Mater. 2009;170(2–3):511–519. doi: 10.1016/j.jhazmat.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Huang W-Y, Li D, Liu Z-Q, Tao Q, Zhu Y, Yang J, Zhang YM. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La (OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem Eng J. 2014;236:191–201. [Google Scholar]
- 39.Xie Q, Li Y, Lv Z, Zhou H, Yang X, Chen J, Guo H. Effective adsorption and removal of phosphate from aqueous solutions and eutrophic water by Fe-based MOFs of MIL-101. Sci Rep. 2017;7(1):3316. doi: 10.1038/s41598-017-03526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, Wang X, Zhan Y. Effect of precipitation pH and coexisting magnesium ion on phosphate adsorption onto hydrous zirconium oxide. J Environ Sci. 2018(In Press). [DOI] [PubMed]
- 41.Lin J, Zhang Z, Zhan Y. Effect of humic acid preloading on phosphate adsorption onto zirconium-modified zeolite. Environ Sci Pollut Res. 2017;24(13):12195–12211. doi: 10.1007/s11356-017-8873-0. [DOI] [PubMed] [Google Scholar]