Abstract
Purpose
To assess the association between diabetes mellitus (DM) and the incidence of cancer at different sites.
Methods
Data from the baseline and first three follow-up visits of the Atherosclerosis Risk in Communities (ARIC) study, an ongoing cohort study of adults from four American communities, were used in this study. Of 15,792 persons aged 45–64 years old who participated in the baseline visit, the data of 15,118 participants were available for this study. For each cancer site, a conditional stratified Poisson regression model was fitted to estimate the adjusted relative rate and 95% confidence interval (adj. RR, 95% CI) of its incidence in diabetics compared to non-diabetics.
Results
We excluded 850 participants with a history of cancer at baseline and 149 participants who developed cancer during 2 years after enrollment, leaving a total of 14,119 participants of whom 1721 were diabetics. Independent of age, body mass index, alcohol consumption, and physical activity, DM decreased the risk of all cancers combined (adj. RR: 0.77, 95% CI: 0.60, 0.98) and the risk of prostate cancer (adj. RR: 0.51, 95% CI: 0.27, 0.97) and increased the risk of colorectal cancer in non-menopausal women (adj. RR: 12.08, 95% CI: 2.06, 70.94).
Conclusions
In conclusion, DM may be associated with an increased risk of colorectal cancer in non-menopausal women and a decreased risk of prostate cancer and all cancers combined.
Keywords: Diabetes mellitus, Cancer, Cohort study, Poisson regression
Introduction
In 2016, an estimated 17.2 million new cancer cases were diagnosed across the world [1]. Cancer accounted for 8.93 million deaths in this year and remains to be the second leading cause of death globally, right behind cardiovascular diseases [2]. In addition, 415 million adults aged 20–79 years old were diagnosed with diabetes in 2015, which is projected to increase to 642 million by 2040 [3].
Some previous studies have suggested that DM might increase the risk of certain cancers; however, they have failed to provide robust evidence in this regard due to lack of taking confounding factors, latency period, and/or surveillance bias into consideration [4, 5]. It is noteworthy that because of the high prevalence of DM worldwide [3], even a small significant association could have important consequences on public health.
Hence, the purpose of this study was to assess the association between diabetes mellitus (DM) and risk of cancer at different sites after adjustment for relevant confounding factors considering a latency period.
Methods
Study design and participants
The data from the Atherosclerosis Risk in Communities (ARIC), an ongoing multicenter prospective cohort study in four U.S. communities, were used in this study. This study was originally designed to investigate the etiology of atherosclerosis and its consequences as well as variations in the cardiovascular risk factors, medical care, and diseases. The design, measurement methods, and sampling strategy of the study have been described in details elsewhere [6].
In brief, from each of the randomly selected ARIC field centers (Washington County, MD; Forsyth County, NC; Jackson, MS; and Minneapolis, MN), a sample of approximately 4000 individuals aged 45–64 was initially recruited. A total of 15,792 participants completed baseline examinations from 1987 to 1989 (visit 1). After the baseline examinations, the participants were invited for four follow-up visits in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). The data of 15,118 subjects collected during visit 1 (baseline) to visit 4 were available for use in this study.
The ARIC study protocol was approved by the Institutional Review Boards of the University of Minnesota, Johns Hopkins University, University of North Carolina, University of Mississippi Medical Center, and Wake Forest University. Written informed consent was obtained from participants at each clinical site [6]. Our study, which was part of a Ph.D. dissertation in epidemiology, was approved by the Graduate Council and the Ethics Committee of the School of Public Health (SPH), Tehran University of Medical Sciences.
Exposure and covariate measurements
Baseline characteristics, lifestyle data, medical history, and menopausal status (women) were obtained from visit 1 (baseline visit). In the ARIC study, the DM status was defined based on one or more of the following criteria: a fasting plasma glucose level of at least 126 mg/dl, a non-fasting plasma glucose level of at least 200 mg/dl, use of anti-diabetic medication(s) in the past 2 weeks, or self-report of a physician’s diagnosis of diabetes. The body mass index (BMI, kg/m2) was calculated using the participants’ weight and height. The participants were asked if they currently or formerly drank alcoholic beverages. For each participant, modified versions of the Baecke Physical Activity Questionnaire (PAQ) and the Willett Food Frequency Questionnaire (FFQ) were applied to measure the level of physical activity (during work, sport, and leisure time) and the amount of total energy intake, respectively. Women were also asked whether they had reached menopause (Yes or No).
In this study, the continuous variables of age, BMI, physical activity, total energy intake, fiber intake, and saturated fat intake as well as the multi-categorical variable of alcohol consumption were converted into binary variables using suitable cutoff points (Table 1).
Table 1.
Baseline characteristics of the participants in this study: 1987–1989
| Variables | Total | Non-diabetic | Diabetic | P | |
|---|---|---|---|---|---|
| (N: 14,119) | (N:12,398) | (N:1721) | |||
| Percent(n) or mean (SD) | Percent(n) or mean (SD) | Percent(n) or mean (SD) | |||
| Age | 45–54 | 53.3 (7524) | 54.8 (6800) | 42.1 (724) | <0.001 |
| 55–64 | 46.7 (6595) | 45.2 (5598) | 57.9 (997) | ||
| Sex | Female | 54.0 (7630) | 54.1 (6702) | 53.9 (928) | 0.92 |
| Male | 46.0 (6489) | 45.9 (5696) | 46.1 (793) | ||
| Race | White | 72.9 (10295) | 75.4 (9354) | 54.7 (941) | <0.001 |
| Black | 27.1 (3824) | 24.6 (3044) | 45.3 (780) | ||
| Alcohol consumption | Never | 55.9 (7852) | 58.4 (7213) | 37.4 (639) | <0.001 |
| Currently or Former | 44.1(6200) | 41.6 (5132) | 62.6 (1068) | ||
| Total energy intakea | As recommended | 87.4 (12048) | 87.2 (10565) | 88.6 (1483) | 0.11 |
| Excessive | 12.6 (1740) | 12.8 (1549) | 11.4 (191) | ||
| Body Mass Index (BMI)b | Normal | 32.8 (4609) | 35.5 (4385) | 13.1 (224) | <0.001 |
| Overweight or obese | 66.2 (9444) | 64.5 (7964) | 86.9 (1480) | ||
| Fiber intakec | At or above recommended | 39.3 (5414) | 39.1 (4747) | 40.0 (667) | 0.48 |
| Below recommended | 60.7 (8379) | 60.9 (7380) | 60.0 (999) | ||
| Saturated fat intaked | At or Below Recommended | 19.5 (2691) | 19.3 (2342) | 21.0 (349) | 0.11 |
| above recommended | 80.5 (11102) | 80.7 (9785) | 79.0 (1317) | ||
| Physical activitye | Non active | 48.4 (6798) | 46.7 (5764) | 60.5 (1034) | <0.001 |
| Active | 51.6 (7250) | 53.3 (6574) | 39.5 (676) | ||
| Menopausal status | Non-Menopausal | 29.0 (2201) | 30.3 (2025) | 19.1 (176) | <0.001 |
| Menopausal | 71.0 (5398) | 69.7 (4651) | 80.9 (747) | ||
N Number, SD Standard Deviation
aAs recommended: < 2000 kg per day for women and < 2500 kg per day for men / excessive: ≥ 2000 kcal per day for women and ≥ 2500 kcal per day for Men
bNormal: 18.5 < BMI < 25, overweight: 25 ≤ BMI <30, obese: 30 ≤ BMI
c At or above recommended fiber intake: ≥ 14 g/1000 kcal/day, below recommended fiber intake: < 14 g/1000 kcal/day
dAt or below recommended saturated fat intake: < 18 g/1000 kcal/day, above recommended Saturated fat intake: ≥ 18 g/1000 kcal/day
eThe median value of all subjects (7 scores) was used as a cutoff point for physical activity in this study; Active: score ≥ 7, and Non-Active: score < 7
Outcome measurements
A diagnosis of cancer (outcome), the cancer site, and the date of diagnosis were determined based on participants’ self-reported data obtained through interviews at baseline and follow-up visits. We excluded cancers diagnosed in 2 years after enrollment to consider the minimum latency period for DM-related cancers. Therefore, our study included new cases of cancer that were identified between 1987 and 1998 among those participants who did not report cancer at the baseline visit and in the first 2 years of the study.
The types of cancer included in this study are presented in Table 2. We classified cancers of the nasal cavity, middle ear, lip, oral cavity, pharynx, larynx, and sinuses as “head and neck” cancer (HNC) according to the ICD-10 codes (C00 to C14 and C30 to C32) [7].
Table 2.
The incidence rate, crude and adjusted relative rate of different cancer types in diabetics compared to non-diabetics in ARIC cohort study
| Site of cancer | All | Diabetic | Non-Diabetic | Crude relative rate; 95% CI | Adjusted* relative rate; 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| (N:14,119) | (N:1721) | (N:12,398) | ||||||
| N of new cases | Incidence rate (95% CI) | N of new cases | Incidence rate (95% CI) | N of new cases | Incidence rate (95% CI) | |||
| Skin | 287 | 2.13 (1.89, 2.39) | 27 | 1.67 (1.10, 2.43) | 260 | 2.20 (1.90, 2.50) | 0.76; 0.51,1.14 | 0.79; 0.51, 1.23 |
| Breast† | 169 | 2.30 (1.96,2.67) | 18 | 2.05 (1.22, 3.24) | 151 | 2.33 (1.97, 2.73) | 0.88; 0.54, 1.45 | 0.93; 0.54, 1.62 |
| Prostate | 162 | 2.60 (2.20, 3.01) | 12 | 1.62 (0.84, 2.84) | 150 | 2.77 (2.35, 3.25) | 0.59; 0.33, 1.06 | 0.51; 0.27, 0.97 ‡ |
| Colon & Rectum | 55 | 0.41 (0.31, 0.53) | 9 | 0.56 (0.25, 1.05) | 46 | 0.39 (0.28, 0.52) | 1.44; 0.71, 2.94 | 1.34; 0.60, 2.97 |
| Lung | 42 | 0.31 (0.22, 0.42) | 8 | 0.50 (0.21, 0.98) | 34 | 0.29 (0.20, 0.40) | 1.72; 0.80, 3.74 | 1.96; 0.83, 4.64 |
| Uterus | 38 | 0.52 (0.36, 0.71) | 7 | 0.80 (0.32, 1.64) | 31 | 0.48 (0.33, 0.68) | 1.67; 0.73, 3.79 | 1.47; 0.59, 3.69 |
| Head and Neck (HNC) | 35 | 0.26 (0.18, 0.36) | 1 | 0.06 (1.5 × 10 −6, 0.34) | 34 | 0.29 (0.20, 0.40) | 0.22; 0.03, 1.58 | 0.23; 0.03, 2.04 |
| Bladder | 34 | 0.25 (0.17, 0.35) | 1 | 0.06 (1.5 × 10 −6, 0.34) | 33 | 0.28 (0.19, 0.39) | 0.22; 0.03, 1.63 | 0.24; 0.03, 2.16 |
| Kidney | 14 | 0.10 (0.06, 0.17) | 1 | 0.06 (1.5 × 10 −6, 0.34) | 13 | 0.11 (0.06, 0.19) | 0.56; 0.07, 4.32 | 0.47; 0.05, 4.43 |
| Lymphatic System | 13 | 0.09 (0.05, 0.16) | 1 | 0.06 (1.5 × 10 −6, 0.34) | 12 | 0.10 (0.05, 0.18) | 0.61; 0.08, 4.71 | 0.75; 0.08, 7.07 |
| Stomach | 12 | 0.09 (0.05, 0.15) | 3 | 0.19 (0.04, 0.54) | 9 | 0.08 (0.04, 0.14) | 2.45; 0.66, 9.06 | 2.01; 0.47, 8.56 |
| Cervix | 9 | 0.12 (0.06, 0.23) | 1 | 0.11 (2.9 × 10 −6, 0.64) | 8 | 0.12 (0.05, 0.24) | 0.92; 0.12, 7.38 | 0.64; 0.06, 6.44 |
| Blood | 8 | 0.06 (0.03, 0.11) | 0 | 0.00 (0.00, 0.22) | 8 | 0.07 (0.03, 0.13) | 4. 6 × 10 −8; 0, NE c | 7 × 10−6; 0.00, NE |
| Thyroid | 4 | 0.03 (8 × 10 −6, 0.07) | 0 | 0.00 (0.00, 0.22) | 4 | 0.03 (9× 10−6, 0.09) | 4. 6 × 10 −8; 0, NE | 7 × 10−6; 0.00, NE |
| Ovary | 4 | 0.05 (0.02, 0.14) | 0 | 0.00 (0.00, 0.42) | 4 | 0.06 (0.02, 0.16) | 4. 6 × 10 −8; 0, NE | 7 × 10−6; 0.00, NE |
| Testicle | 2 | 0.03 (4 × 10 −6, 0.11) | 0 | 0.00 (0.00, 0.49) | 2 | 0.04 (4× 10−6, 0.13) | 4. 6 × 10 −8; 0, NE | 7 × 10−6; 0.00, NE |
| Others§ | 15 | 0.11 (0.06, 0.18) | 0 | 0.00 (0.00, 0.22) | 15 | 0.13 (0.07, 0.21) | ||
| Unknown | 28 | 0.21 (0.14, 0.30) | 1 | 0.06 (1.5 × 10 −6, 0.34) | 27 | 0.23 (0.15, 0.33) | ||
| All | 931 | 6.89 (6.46,7.35) | 90 | 5.57 (4.47, 6.84) | 841 | 7.07 (6.60, 7.56) | 0.79; 0.63–0.98 ‡ | 0.77; 0.60, 0.98 ‡ |
N number, CI Confidence Interval, NE Non-Estimable
*Adjusted for age, Body Mass Index, Physical activity and alcohol consumption using stratified poisson regression; †For women only; ‡P < 0.05; §Eye (1 case) Vagina (2 cases) Penis (1 case) Bone marrow (2 cases) Bone (2 cases) Gallbladder (2 cases) Spleen (1 case)
Data analysis
Descriptive part
In diabetic and non-diabetic groups:
Baseline characteristics of the included participants are summarized and reported as mean (Standard Deviation (SD)) for continuous variables or frequency and percentage for categorical variables.
Person-years at risk was calculated from baseline to the date of cancer diagnosis, death, loss to follow-up, or to the end of the fourth visit (1998), whichever occurred first.
The crude incidence rate per 1000 person-years at risk and its 95% confidence interval were calculated for various cancer types.
Analytical part
To control for both main and interaction effects of confounding variables simultaneously, stratification on these variables is a main option. A useful approach for fitting stratified Poisson regression models is a ‘conditional’ Poisson analysis that avoids estimation of large numbers of stratum-specific parameters by conditions out their coefficients [8] . In this study, the following steps were taken to employ a conditional stratified Poisson regression model:
Of the known risk factors for cancer [9], we asseseed the asociassion of the variabels of age, alcohol consumption, dietary factors (daily intake of total energy, fiber and saturated fat), physical activity and BMI with diabetes; finally, based on these findings, four variables were considered as potential confounders.
A variable named strata (with 16 levels) was defined by cross-classification of the important confounding variables of age at enrolment (45–54 vs. 55–65 years), BMI (<25 vs. ≥25), physical activity (active vs. less active), and alcohol consumption (formerly or currently vs. never).
For each type of cancer, a dataset was generated that consisted of a total number of person-year and events, namely new cases of cancer cross-classified by the level of the exposure, DM, and the strata.
By fitting a conditional stratified Poisson regression model, an adjusted parameter (Ln Relative Rate) was estimated separately to describe the association of diabetes with the risk of each type of cancer.
All P values were two-sided. P value less than 0.05 were considered significant. All analyses were performed using the SAS 9.2 (SAS Institute Inc., Cary, NC) and Stata 11.0 (Stata Corp, College Station, Texas, USA).
Results
Baseline characteristics of participants
After excluding participants with a history of cancer at baseline and participants who were diagnosed with cancer in the first 2 years of follow-up, a total of 14,119 subjects remained in the study of whom 1721 (12.2%) were diabetic at baseline (Fig. 1).
Fig. 1.
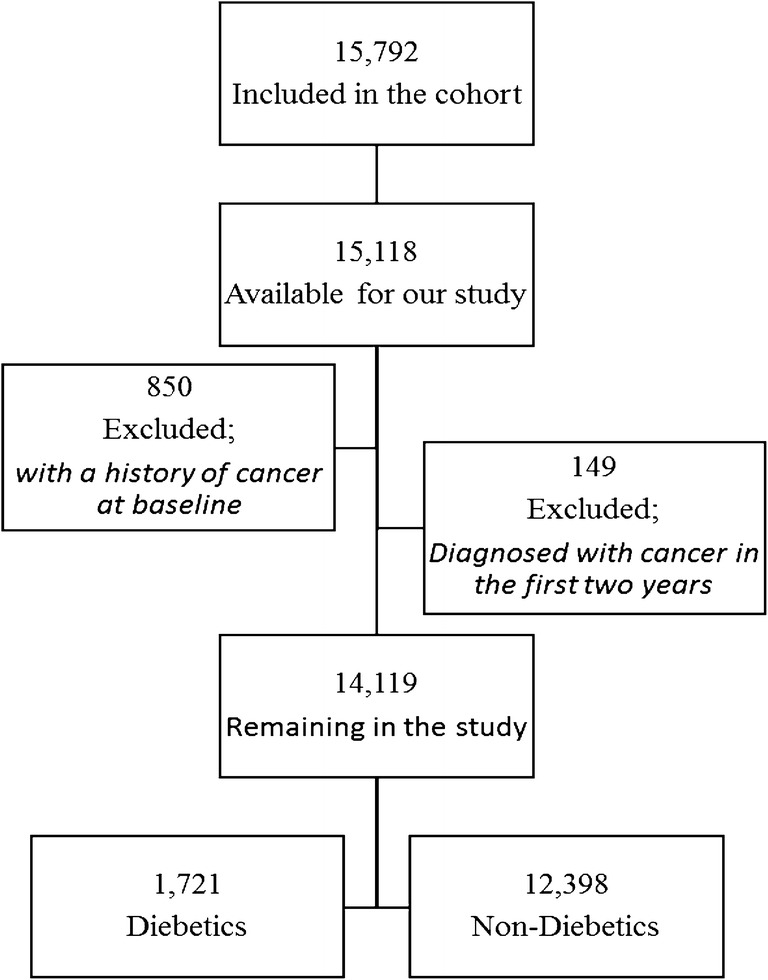
Flow-chart of the included participants
Table 1 presents the baseline characteristics of the included participants overall and by study group. Compared to non-diabetics, diabetic subjects were older, less physically active, and more overweight or obese. Moreover, they drank alcohol either at the time of the study or in the past more frequently.
The crude incidence rate of different cancers
Of 1080 various cancers detected between 1987 and 1998, 931 occurred after a minimum follow-up of 2 years, including 23 different types of cancers and 28 unidentified cases. The mean follow-up time (SD) was 9.6 (1.5) and 9.4 (1.9) years in diabetics and non-diabetics, respectively. Table 2 shows that the crude incidence rate of all cancer sites combined was significantly lower in diabetics compared with non-diabetics, while there was no significant difference in site-specific crude incidence rates between the two groups.
Subgroup analysis (not shown) showed that in non-menopausal women, diabetics had a higher crude incidence rate of colorectal cancer compared to non-diabetics (crude Relative Rate (RR): 7.02, 95% CI: 1.68, 29.38; P < 0.01). In menopausal women, the crude incidence rate of skin cancer was lower in diabetics versus non-diabetics (crude RR:0.31, 95% CI: 0.12, 0.86; P = 0.02). The crude incidence rate of all cancer sites combined was lower in diabetic men compared to their non-diabetic counterparts (crude RR:0.73, 95% CI: 0.54, 0.99; P = 0.04). No significant differences were observed in ethnical subgroups (white and black ethnicities). (All P values >0.05).
Adjusted association between DM and risk of different cancers
The relative rates of different types of cancer adjusted for age, BMI, alcohol consumption, and physical activity are presented in Table 2. DM had a significant inverse association with the incidence rate of all cancers combined and prostate cancer after adjusting for confounding factors. The association of DM with the incidence of ovarian, testicular, thyroid, and blood cancers were not estimable.
Subgroup analysis showed a significant inverse association between DM and the incidence of all cancers combined in men (adjusted RR: 0.66; 95% CI: 0.47, 0.93; P = 0.02). In non-menopausal women, a significant association was observed between DM and the incidence of colorectal cancer (adj. RR: 12.08, 95% CI: 2.06, 70.94; P < 0.01), while this association was not observed in post-menopausal women (adj. RR: 1.79, 95% CI: 0.42, 7.67; P = 0.41). No significant associations were seen in other subgroups. (All P values >0.05).
Discussion
This study was done to examine the adjusted association between DM and the incidence of cancer at several sites using a conditional stratified Poisson regression model on the data of a prospective cohort (ARIC) study. After adjusting for confounding factors of age, BMI, physical activity, and alcohol consumption, it was found that DM reduced the risk of all cancers combined significantly. DM was also associated with a reduced risk of prostate cancer and all cancers combined in men, and an increased risk of colorectal cancer in non-menopausal women.
In 2013, a review of published meta-analyses and systematic reviews on the association between DM and risk of cancer development at several sites showed robust, unbiased evidence only for endometrial, breast, and colorectal cancer and intrahepatic cholangiocarcinoma while there was substantial uncertainty about other cancers [4]. Our findings did not support an association between DM and the risk of endometrial and breast cancer. There was a lack of data about cholangiocarcinoma in this study. However, the results showed a decreased risk of prostate cancer in male diabetic patients and an increased risk of colorectal cancer in non-menopausal women.
DM and prostate cancer
Consistent with our findings, an inverse association has been reported between DM and risk of prostate cancer in earlier studies [4]. On the other hand, a few studies have observed a direct association [10–12]; however, these studies have two major limitations, including a short follow-up period and/or not controlling important confounding factors such as obesity. The most probable explanations proposed for this inverse association are hypoinsulinemia [13], a genetic link [14, 15], and decreased circulating testosterone levels in men with DM [16, 17]. However, it is unknown whether diabetes decreases the level of intraprostatic androgen, which is supposed to be a stronger predictor of the risk of prostate cancer compared to its circulating levels [13, 18].
DM and colorectal cancer
Similar to our findings, Neilson et al. (2001) [19], in a population-based 12-year follow-up study, found a positive association between DM and the risk of colorectal cancer only in women (RR: 1.55, 95% CI: 1.04, 2.31) and not in men (RR: 0.66, 95% CI: 0.35, 1.24). On the other hand, a recent retrospective cohort study of over 34,000 diabetics and non-diabetics in each group showed an increased risk of colon cancer only in men (HR: 1.6, 95% CI: 1.2, 2.2) [20] although the observed association was not adjusted for important confounding factors such as obesity and alcohol consumption. Overall, large meta-analyses of observational studies have reported an average of 20–30% increase in the risk of colon cancer in both genders [21–26].
The probable mechanisms of this association are genetic links [27], slower colonic transit time [4, 28], and elevated serum insulin levels in diabetics [29–31]. However, it should be noted that metformin is likely to lower the risk of colorectal cancer [27].
DM and breast cancer
Similar to our findings, a pooled analysis of 182,542 Japanese women participating in 8 prospective cohort studies showed no significant association between DM and risk of breast cancer [32]. Moreover, a large case-control study conducted in 2017 found no significant association between DM and all breast cancer stages combined [33]; however, similar to other studies investigating the association of DM with cancer stage [33–35], a significant direct association was observed with higher stages. In a meta-analysis in 2013, De Bruijn et al. found a weakly significant association (HR: 1.23, 95% CI: 1.12, 1.34) between DM and breast cancer [36]; nonetheless, the effect of confounders was only controlled in one of the studies included in this meta-analysis. A possible site-specific mechanism is hyperinsulinemia. Insulin can affect the development and progress of breast cancer through various mechanisms, but it is more involved in the promotion and progression stages of breast tumorgenesis rather than the initiation stage of this process [37].
DM and uterus cancer
Consistent with our findings, Luo et al. conducted a cohort study of more than 88,000 post-menopausal women followed for an average of 11 years and reported a non-significant elevated risk of uterus cancer in diabetics adjusted for BMI when they only focused on the prevalence of DM at enrolment (HR: 1.16, 95% CI: 0.90, 1.48). However, this elevated risk became statistically significant after considering new DM cases (HR: 1.31, 95% CI: 1.08, 1.59). The reason may be high levels of insulin in newly diagnosed DM cases [38].
A meta-analysis of 16 studies including 13 case-control studies and 3 cohorts showed an elevated risk of uterine cancer in diabetic women [39]; however, most of the studies included in this meta-analysis were only adjusted for age.
It has been proposed that insulin can biologically develop this association through a direct effect on the uterine epithelial lining [5] and an indirect effect on the levels of insulin-like growth factors, sexual hormones, and adipokines [6].
Methodological issues
In this study, a conditional stratified Poisson regression model was fitted as a novel approach to adjust the confounding and interaction effects through applying a stratification method. This model was also fitted to reduce the number of parameters that need to be estimated by conditioning out the coefficients of stratum-specific parameters simultaneously [8].
After adjusting for the potential confounding variables, important changes were noticed; the non-significant crude rate ratio of prostate cancer became significant in people with DM compared to nondiabetics, while the crude rate ratio of skin cancer in women lost its significance. In this regard, Bonovas et al. performed a subgroup meta-analysis of the association between DM and risk of prostate cancer. The results showed that studies that controlled at least two potential confounders yielded a stronger summary relative risk (sRR) compared to studies with poor control (0 or 1) (sRR = 0.86, 95% CI: 0.73, 0.99 vs. sRR = 0.91. 95% CI: 0.88, 0.95) [40]. Similarly, Luo et al. reported that the significant association between DM (prevalent cases) and the risk of endometrial cancer became non-significant after adjusting for BMI [38].
The latency period for cancer development is considered one of the main methodological challenges in observational studies exploring the impact of DM on cancer incidence [5]. To address this consideration, we only included the new cases of cancer identified after 2 years of follow-up.
Strengths and limitations
In the ARIC study, to determine the DM status of the participants at baseline, fasting and non-fasting blood glucose tests were used in all subjects in addition to self-reports, minimizing the possibility of misclassification bias in exposure (DM).
One of the limitations was that the conversion of continuous and polytomous variables to dichotomous ones might have led to residual confounding although the likelihood ratio tests did not show this phenomenon. In addition, the type of DM was unknown in our study, but the majority of them had DM type 2 given the age of the participants. Furthermore, we could not control the effect of DM duration and the use of glucose-lowering treatments because of lack of sufficient data.
Conclusion
According to our findings, after adjustment for age, BMI, alcohol consumption, and physical activity, DM may only be associated with a reduced risk of all cancers combined and prostate cancer and an increased risk of colorectal cancer in menopausal women.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN2682011 00007C, HHSN268201100008C, HHSN268201100009C, HHSN268 201100010C, HHSN268201100011C, and HHSN268201100012C). NHLBI has provided public data access to the cohort raw data.
The authors thank the staff and participants of the ARIC study for their important contributions, and also appreciate NHLBI for providing access to the raw public data of the cohort.
Compliance with ethical standards
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burden G, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, Adetokunboh O, Afshin A, Agrawal A, Ahmadi A, Ahmed MB, Aichour AN, Aichour MTE, Aichour I, Aiyar S, Alahdab F, al-Aly Z, Alam K, Alam N, Alam T, Alene KA, al-Eyadhy A, Ali SD, Alizadeh-Navaei R, Alkaabi JM, Alkerwi A', Alla F, Allebeck P, Allen C, al-Raddadi R, Alsharif U, Altirkawi KA, Alvis-Guzman N, Amare AT, Amini E, Ammar W, Amoako YA, Anber N, Andersen HH, Andrei CL, Androudi S, Ansari H, Antonio CAT, Anwari P, Ärnlöv J, Arora M, Artaman A, Aryal KK, Asayesh H, Asgedom SW, Atey TM, Avila-Burgos L, Avokpaho EFG, Awasthi A, Babalola TK, Bacha U, Balakrishnan K, Barac A, Barboza MA, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Baune BT, Bedi N, Beghi E, Béjot Y, Bekele BB, Bell ML, Bennett JR, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beuran M, Bhatt S, Biadgilign S, Bienhoff K, Bikbov B, Bisanzio D, Bourne RRA, Breitborde NJK, Bulto LNB, Bumgarner BR, Butt ZA, Cahuana-Hurtado L, Cameron E, Campuzano JC, Car J, Cárdenas R, Carrero JJ, Carter A, Casey DC, Castañeda-Orjuela CA, Catalá-López F, Charlson FJ, Chibueze CE, Chimed-Ochir O, Chisumpa VH, Chitheer AA, Christopher DJ, Ciobanu LG, Cirillo M, Cohen AJ, Colombara D, Cooper C, Cowie BC, Criqui MH, Dandona L, Dandona R, Dargan PI, das Neves J, Davitoiu DV, Davletov K, de Courten B, Defo BK, Degenhardt L, Deiparine S, Deribe K, Deribew A, Dey S, Dicker D, Ding EL, Djalalinia S, Do HP, Doku DT, Douwes-Schultz D, Driscoll TR, Dubey M, Duncan BB, Echko M, el-Khatib ZZ, Ellingsen CL, Enayati A, Ermakov SP, Erskine HE, Eskandarieh S, Esteghamati A, Estep K, Farinha CSS, Faro A, Farzadfar F, Feigin VL, Fereshtehnejad SM, Fernandes JC, Ferrari AJ, Feyissa TR, Filip I, Finegold S, Fischer F, Fitzmaurice C, Flaxman AD, Foigt N, Frank T, Fraser M, Fullman N, Fürst T, Furtado JM, Gakidou E, Garcia-Basteiro AL, Gebre T, Gebregergs GB, Gebrehiwot TT, Gebremichael DY, Geleijnse JM, Genova-Maleras R, Gesesew HA, Gething PW, Gillum RF, Giref AZ, Giroud M, Giussani G, Godwin WW, Gold AL, Goldberg EM, Gona PN, Gopalani SV, Gouda HN, Goulart AC, Griswold M, Gupta R, Gupta T, Gupta V, Gupta PC, Haagsma JA, Hafezi-Nejad N, Hailu AD, Hailu GB, Hamadeh RR, Hambisa MT, Hamidi S, Hammami M, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Hareri HA, Hassanvand MS, Havmoeller R, Hay SI, He F, Hedayati MT, Henry NJ, Heredia-Pi IB, Herteliu C, Hoek HW, Horino M, Horita N, Hosgood HD, Hostiuc S, Hotez PJ, Hoy DG, Huynh C, Iburg KM, Ikeda C, Ileanu BV, Irenso AA, Irvine CMS, Islam SMS, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayaraman SP, Jeemon P, Jha V, John D, Johnson CO, Johnson SC, Jonas JB, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kamal R, Karch A, Karimi SM, Karimkhani C, Kasaeian A, Kassaw NA, Kassebaum NJ, Katikireddi SV, Kawakami N, Keiyoro PN, Kemmer L, Kesavachandran CN, Khader YS, Khan EA, Khang YH, Khoja ATA, Khosravi MH, Khosravi A, Khubchandani J, Kiadaliri AA, Kieling C, Kievlan D, Kim YJ, Kim D, Kimokoti RW, Kinfu Y, Kissoon N, Kivimaki M, Knudsen AK, Kopec JA, Kosen S, Koul PA, Koyanagi A, Kulikoff XR, Kumar GA, Kumar P, Kutz M, Kyu HH, Lal DK, Lalloo R, Lambert TLN, Lan Q, Lansingh VC, Larsson A, Lee PH, Leigh J, Leung J, Levi M, Li Y, Li Kappe D, Liang X, Liben ML, Lim SS, Liu PY, Liu A, Liu Y, Lodha R, Logroscino G, Lorkowski S, Lotufo PA, Lozano R, Lucas TCD, Ma S, Macarayan ERK, Maddison ER, Magdy Abd el Razek M, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malhotra R, Malta DC, Manguerra H, Manyazewal T, Mapoma CC, Marczak LB, Markos D, Martinez-Raga J, Martins-Melo FR, Martopullo I, McAlinden C, McGaughey M, McGrath JJ, Mehata S, Meier T, Meles KG, Memiah P, Memish ZA, Mengesha MM, Mengistu DT, Menota BG, Mensah GA, Meretoja TJ, Meretoja A, Millear A, Miller TR, Minnig S, Mirarefin M, Mirrakhimov EM, Misganaw A, Mishra SR, Mohamed IA, Mohammad KA, Mohammadi A, Mohammed S, Mokdad AH, Mola GLD, Mollenkopf SK, Molokhia M, Monasta L, Montañez JC, Montico M, Mooney MD, Moradi-Lakeh M, Moraga P, Morawska L, Morozoff C, Morrison SD, Mountjoy-Venning C, Mruts KB, Muller K, Murthy GVS, Musa KI, Nachega JB, Naheed A, Naldi L, Nangia V, Nascimento BR, Nasher JT, Natarajan G, Negoi I, Ngunjiri JW, Nguyen CT, Nguyen QL, Nguyen TH, Nguyen G, Nguyen M, Nichols E, Ningrum DNA, Nong VM, Noubiap JJN, Ogbo FA, Oh IH, Okoro A, Olagunju AT, Olsen HE, Olusanya BO, Olusanya JO, Ong K, Opio JN, Oren E, Ortiz A, Osman M, Ota E, PA M, Pacella RE, Pakhale S, Pana A, Panda BK, Panda-Jonas S, Papachristou C, Park EK, Patten SB, Patton GC, Paudel D, Paulson K, Pereira DM, Perez-Ruiz F, Perico N, Pervaiz A, Petzold M, Phillips MR, Pigott DM, Pinho C, Plass D, Pletcher MA, Polinder S, Postma MJ, Pourmalek F, Purcell C, Qorbani M, Quintanilla BPA, Radfar A, Rafay A, Rahimi-Movaghar V, Rahman MHU, Rahman M, Rai RK, Ranabhat CL, Rankin Z, Rao PC, Rath GK, Rawaf S, Ray SE, Rehm J, Reiner RC, Reitsma MB, Remuzzi G, Rezaei S, Rezai MS, Rokni MB, Ronfani L, Roshandel G, Roth GA, Rothenbacher D, Ruhago GM, SA R, Saadat S, Sachdev PS, Sadat N, Safdarian M, Safi S, Safiri S, Sagar R, Sahathevan R, Salama J, Salamati P, Salomon JA, Samy AM, Sanabria JR, Sanchez-Niño MD, Santomauro D, Santos IS, Santric Milicevic MM, Sartorius B, Satpathy M, Schmidt MI, Schneider IJC, Schulhofer-Wohl S, Schutte AE, Schwebel DC, Schwendicke F, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shahraz S, Shaikh MA, Shamsipour M, Shamsizadeh M, Sharma J, Sharma R, She J, Sheikhbahaei S, Shey M, Shi P, Shields C, Shigematsu M, Shiri R, Shirude S, Shiue I, Shoman H, Shrime MG, Sigfusdottir ID, Silpakit N, Silva JP, Singh JA, Singh A, Skiadaresi E, Sligar A, Smith DL, Smith A, Smith M, Sobaih BHA, Soneji S, Sorensen RJD, Soriano JB, Sreeramareddy CT, Srinivasan V, Stanaway JD, Stathopoulou V, Steel N, Stein DJ, Steiner C, Steinke S, Stokes MA, Strong M, Strub B, Subart M, Sufiyan MB, Sunguya BF, Sur PJ, Swaminathan S, Sykes BL, Tabarés-Seisdedos R, Tadakamadla SK, Takahashi K, Takala JS, Talongwa RT, Tarawneh MR, Tavakkoli M, Taveira N, Tegegne TK, Tehrani-Banihashemi A, Temsah MH, Terkawi AS, Thakur JS, Thamsuwan O, Thankappan KR, Thomas KE, Thompson AH, Thomson AJ, Thrift AG, Tobe-Gai R, Topor-Madry R, Torre A, Tortajada M, Towbin JA, Tran BX, Troeger C, Truelsen T, Tsoi D, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Updike R, Uthman OA, Uzochukwu BSC, van Boven JFM, Vasankari T, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Vos T, Wakayo T, Wallin MT, Wang YP, Weiderpass E, Weintraub RG, Weiss DJ, Werdecker A, Westerman R, Whetter B, Whiteford HA, Wijeratne T, Wiysonge CS, Woldeyes BG, Wolfe CDA, Woodbrook R, Workicho A, Xavier D, Xiao Q, Xu G, Yaghoubi M, Yakob B, Yano Y, Yaseri M, Yimam HH, Yonemoto N, Yoon SJ, Yotebieng M, Younis MZ, Zaidi Z, Zaki MES, Zegeye EA, Zenebe ZM, Zerfu TA, Zhang AL, Zhang X, Zipkin B, Zodpey S, Lopez AD, Murray CJL. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011;54:2263–2271. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 6.Aric Investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objects. Am J Epidemiol. 1989;129:687–702. doi: 10.1093/oxfordjournals.aje.a115184. [DOI] [PubMed] [Google Scholar]
- 7.World HealthOrganization . International statistical classification of disease and related health problems, tenth revision (ICD-10) Geneva: World Health Organization; 1992. [Google Scholar]
- 8.Richardson DB, Langholz B. Background stratified Poisson regression analysis of cohort data. Radiat Environ Biophys. 2012;51:15–22. doi: 10.1007/s00411-011-0394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute (NCI). Risk Factors for Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk. Accessed 16 Mar 2018.
- 10.Li Q, Kuriyama S, Kakizaki M, Yan H, Sone T, Nagai M, Sugawara Y, Ohmori-Matsuda K, Hozawa A, Nishino Y, Tsuji I. History of diabetes mellitus and the risk of prostate cancer: the Ohsaki Cohort Study. Cancer Causes Control. 2010;21:1025–1032. doi: 10.1007/s10552-010-9530-9. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care. 2011;34:616–621. doi: 10.2337/dc10-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MY, Lin KD, Hsiao PJ, Shin SJ. The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism. 2012;61:242–249. doi: 10.1016/j.metabol.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Tsilidis KK, Allen NE, Appleby PN, Rohrmann S, Nöthlings U, Arriola L, Gunter MJ, Chajes V, Rinaldi S, Romieu I, Murphy N, Riboli E, Tzoulaki I, Kaaks R, Lukanova A, Boeing H, Pischon T, Dahm CC, Overvad K, Quirós JR, Fonseca-Nunes A, Molina-Montes E, Gavrila Chervase D, Ardanaz E, Khaw KT, Wareham NJ, Roswall N, Tjønneland A, Lagiou P, Trichopoulos D, Trichopoulou A, Palli D, Pala V, Tumino R, Vineis P, Bueno-de-Mesquita HB, Malm J, Orho-Melander M, Johansson M, Stattin P, Travis RC, Key TJ. Diabetes mellitus and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. 2015;136:372–381. doi: 10.1002/ijc.28989. [DOI] [PubMed] [Google Scholar]
- 14.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PCY, Ng MCY, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CNA, Rotimi C, Chan JCN, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 15.Machiela MJ, Lindström S, Allen NE, Haiman CA, Albanes D, Barricarte A, et al. Association of type 2 diabetes susceptibility variants with advanced prostate cancer risk in the breast and prostate cancer cohort consortium. Am J Epidemiol. 2012;176:1121–1129. doi: 10.1093/aje/kws191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson D, Garmo H, Bill-Axelson A, Mucci L, Holmberg L, Stattin P. Use of 5α-reductase inhibitors for lower urinary tract symptoms and risk of prostate cancer in Swedish men: nationwide, population based case-control study. BMJ. 2013;346:f3406. doi: 10.1136/bmj.f3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 18.Miulescu RD, Dănoiu S, Margină D, Păun S, Poiană C. Dynamics of prostate-specific antigen levels during treatment with testosterone undecanoate in patients with type 2 diabetes mellitus. Rom J Diabetes Nutr Metab Dis. 2012;19:397–403. [Google Scholar]
- 19.Nilsen TL, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose, and BMI: exploring the hyperinsulinemia hypothesis. Br J Cancer. 2001;84:417–422. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong RG, Burden AM, de Kort S, van Herk-Sukel MP, Vissers PA, Janssen PK, et al. Impact of detection bias on the risk of gastrointestinal cancer and its subsites in type 2 diabetes mellitus. Eur J Cancer. 2017;79:61–71. doi: 10.1016/j.ejca.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Yu C, Jiang H, Tang J, Huang HL, Gao J, Zhang X. Diabetes mellitus and the occurrence of colorectal cancer: an updated meta-analysis of cohort studies. Diabetes Technol Ther. 2013;15:419–427. doi: 10.1089/dia.2012.0263. [DOI] [PubMed] [Google Scholar]
- 23.Deng L, Gui Z, Zhao L, Wang J, Shen L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig Dis Sci. 2012;57:1576–1585. doi: 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 24.Krämer HU, Schöttker B, Raum E, Brenner H. Type 2 diabetes mellitus and colorectal cancer: a meta-analysis on sex-specific differences. Eur J Cancer. 2012;48:1269–1282. doi: 10.1016/j.ejca.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Luo W, Cao Y, Liao C, Gao F. Diabetes mellitus and the incidence and mortality of colorectal cancer: a meta-analysis of 24 cohort studies. Color Dis. 2012;14:1307–1312. doi: 10.1111/j.1463-1318.2012.02875.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Yu S. Diabetes mellitus is an independent risk factor for colorectal cancer. Dig Dis Sci. 2012;57:1586–1597. doi: 10.1007/s10620-012-2059-x. [DOI] [PubMed] [Google Scholar]
- 27.González N, Prieto I, del Puerto-Nevado L, Portal-Nuñez S, Ardura JA, Corton M, et al. 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms, and therapeutic implications. Oncotarget. 2017;8:18456. doi: 10.18632/oncotarget.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Will JC, Galuska DA, Vinicor F, Calle EE. Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol. 1998;147:816–825. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 29.Strickler HD, Wylie-Rosett J, Rohan T, Hoover DR, Smoller S, Burk RD, Yu H. The relation of type 2 diabetes and cancer. Diabetes Technol Ther. 2001;3:263–274. doi: 10.1089/152091501300209633. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 31.Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, et al. Plasma insulin, IGF-binding proteins-1 and-2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003;107:89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 32.Sasazuki S, Charvat H, Hara A, Wakai K, Nagata C, Nakamura K, Tsuji I, Sugawara Y, Tamakoshi A, Matsuo K, Oze I, Mizoue T, Tanaka K, Inoue M, Tsugane S, for the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan Diabetes mellitus and cancer risk: a pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104:1499–1507. doi: 10.1111/cas.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schairer C, Gadalla SM, Pfeiffer RM, Moore SC, Engels EA. Diabetes, abnormal glucose, dyslipidemia, hypertension and risk of inflammatory and other breast cancer. Cancer Epidemiol Biomark Prev. 2017;26:862–868. doi: 10.1158/1055-9965.EPI-16-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipscombe LL, Fischer HD, Austin PC, Fu L, Jaakkimainen RL, Ginsburg O, Rochon PA, Narod S, Paszat L. The association between diabetes and breast cancer stage at diagnosis: a population-based study. Breast Cancer Res Treat. 2015;150:613–620. doi: 10.1007/s10549-015-3323-5. [DOI] [PubMed] [Google Scholar]
- 35.Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J. Clinical, pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat. 2013;137:807–816. doi: 10.1007/s10549-012-2404-y. [DOI] [PubMed] [Google Scholar]
- 36.De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, Van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 37.Rose DP, Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19:R225–R241. doi: 10.1530/ERC-12-0203. [DOI] [PubMed] [Google Scholar]
- 38.Luo J, Beresford S, Chen C, Chlebowski R, Garcia L, Kuller L, Regier M, Wactawski-Wende J, Margolis KL. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014;111:1432–1439. doi: 10.1038/bjc.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 40.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47:1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]


