Abstract
Background
As from last decade, the pollution of water bodies by chemical toxicants has become a topic of public discourse and concern in many countries. Polycyclic aromatic hydrocarbons (PAHs) are carcinogens and being ubiquitous in nature, are daily being released into water bodies as a result of anthropogenic sources.
Methods
The water samples were collected with plastic bottles/containers by dipping the sampler below the water surface to minimize the contamination of water sample by surface films and cocked below water surface to avoid air entrapment while the fish samples namely Clarias spp (Catfish) and Oreochromis spp (Tilapias) were purchased from fishermen at the bank of the river confluence. Three water samples each were collected from five sample points [A], [B], [C], [D] and [E] created for that purpose at each visit monthly for a period of six months and taken in an ice-cooler box to the laboratory. In all a total of 90 water samples and 20 fish samples were analysed. The water samples were preserved in a refrigerator below 4 °C prior to analysis. The concentrations of the sixteen US EPA priority polycyclic aromatic hydrocarbons (PAHs) were investigated using Gas chromatography coupled with Mass Spectrometer detector (GC-MS) after liquid-liquid and solid-liquid extractions.
Results
The concentrations of the six detected PAHs in water were of the following ranges: Nap(Not Detected {ND} to 0.543), Ph(ND to 0.083) Ant (ND to 0.083), BbF(0.080 to 0.093), BkF(0,083 to 0.093) and BaP(0.083 to 0.113) mg/L with distribution pattern of Nap>BaP > BbF=BkF > Ant = Ph. The mean concentration value of PAHs in Catfish and Tilapia were Nap(2.383 and 1.947), Ph(0.050 and 0.057), Ant(0.057 and 0.057), BbF(0.043 and ND), BkF(0.043 and ND) and BaP(0.050 and ND). The health risk assessment showed that the concentration of Benzo[a]pyrene, a known indicator of the presence of carcinogenic PAHs is of health risk concern. The PAHs were not significantly different in the water and fish respectively and the correlation studies showed that the PAHs were from the same source.
Conclusions
The study showed clearly that the levels of PAHs in the samples are of concern due to increasing pollution.
Keywords: PAHs, River Niger, Benue, Water, Fish, Risk assessment, Cancer risk
Introduction
Pollution of water bodies by chemical toxicants has been in public domain of recent because human beings can be exposed to toxic chemicals which bioaccumulate in aquatic organisms harvested from contaminated waters [1]. It has become of great importance to prevent agricultural and industrial contamination of water resources [2, 3].
Polycyclic aromatic hydrocarbons (PAHs) are compounds containing two or more fused aromatic rings in linear, angular or clustered arrangement which sixteen of them are classified by the U.S environmental protection agency as pollutant of high priority having characteristics of persistence in the environment [4, 5]. Polycyclic aromatic hydrocarbons (PAHs) are daily being leached into rivers, lakes and oceans from anthropogenic sources such as waste water, industrial effluents, and burning of fossil fuel and petroleum products incompletely. The pollutants are distributed in the rivers water, sediments and are bio-accumulated by the fishes and other aquatic animals in the water and this leads to bio-magnification of these pollutants in the food chain [6–10]. Polycyclic aromatic hydrocarbons (PAHs) have been reported to have carcinogenic, mutagenic and teratogenic effect on aquatic animals and humans who depends on the water and fishes of rivers for survival and humans who sometimes have direct encounter with the pollutants, especially with occupational exposure [11–13]. Polycyclic aromatic hydrocarbons (PAHs) are often found attracted to stable particles in the water which settles in the sediment. After sometimes, the deposited PAHs in the sediments are remobilized into water column and become available to fish and other aquatic organisms. PAHs have been implicated in many health aspects of fish such as adverse histopathologic and immunological response, hepatic lesions and liver neoplasm [12]. Aquatic organism bio-accumulate PAHs because they have membranes that are easily penetrated by PAHs due to their lipophilicity. Hence, dietary intake of PAHs via fish and water is a public health concern [14].
Like many other rivers across the world, Rivers Niger and Benue are faced with PAHs pollution due to precipitation and urban runoff which leach these pollutants from anthropogenic sources into the water bodies. The deterioration of water quality is a significant problem for the rivers Niger and Benue confluence due to urbanization, industrialization and trade growth along the bank of the confluence. Hence there is the rational for monitoring the levels and effect of such pollutants on the river body and the aquatic lives therein.
The objectives of this study were to estimate the concentrations of PAHs in water and popular fish samples and to assess the possible health hazard posed by the ingestion of fish and river waters with the hope that the results so generated will form a baseline for such similar studies in the future in this area of study.
Materials and methods
All chemicals used were of analytical grade. The chemicals used were: Distilled water (H2O), Dichloromethane (CH2Cl2) (JHD China), Anhydrous Sodium Sulphate (Na2SO4) (BDH Chemicals, Poole, England), 60–120 mesh Silica gel (Qualikems fine Chemicals, India), Potassium silicate (K2SiO3), Hexane (C6H14) (JHD China), Acetone (CH3COCH3), Standards of the sixteen priority PAHs (New Haven, USA) and Helium gas (Air liquid gas company, France). Analysis were performed with GC–MS (Agilen 7890, series A, USA).
Study area
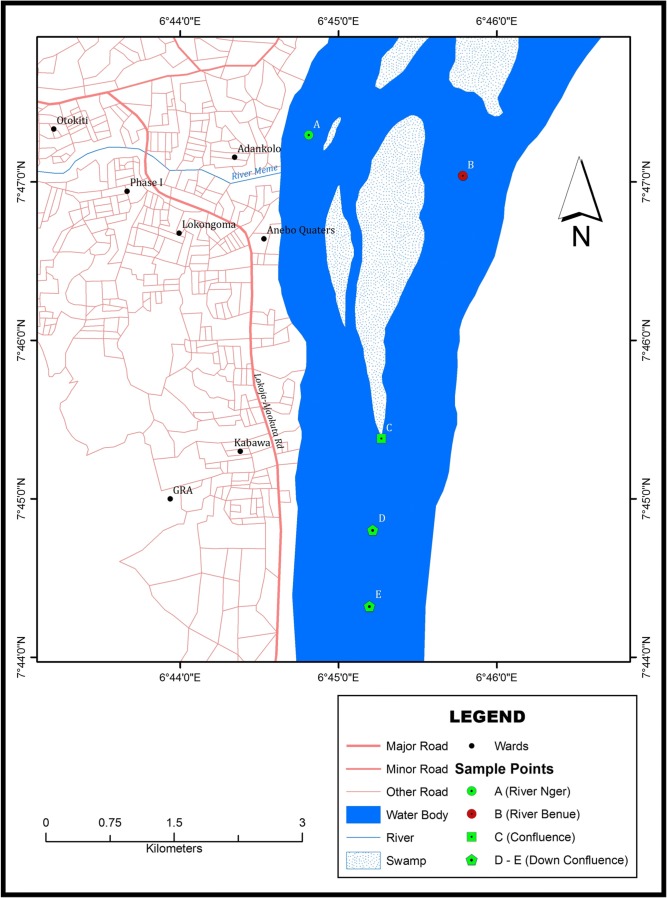
The study was carried out at the confluence of rivers Niger and Benue in Lokoja, Kogi State, Central Nigeria. The state lies between the coordinate of latitude 7o30’N/6o42’E and longitude 7.500oN/6.700°E and Lokoja lies between 7o45’N, 7o52’N of the equator and longitude 6o45’E of the Greenwich meridian. Lokoja is bounded in the west by the river Niger at an altitude of 45–125 m above sea level. The area has two climatic seasons known as wet (raining) season from May to October and dry season from December to April, with an annual rainfall of about 1000 mm [15]. Lokoja is the headquarters of Lokoja L.G.A. and the capital of Kogi State, having an area of 3180 km2 land and a population of 196,643 people at the 2006 census with majority of the local indigene involved in farming and fishing [16]. The map of the study area showing the sampling points is presented in Fig. 1.
Fig. 1.
Map of study area showing sampling points
Sample collection
All polyethylene sample containers were washed thoroughly with detergent, later treated with dilute Nitric acid (10% HNO3) and thoroughly rinsed with distilled water. Water samples were collected upstream and downstream of the confluence 2 km apart. Three samples each were collected from five sample points [A], [B], [C], [D] and [E] created for that purpose at each visit monthly for a period of six months and taken in an ice-cooler box to the laboratory. In all a total of 90 water samples and 20 fish samples were analysed. The water samples were preserved in a refrigerator below 4 °C prior to analysis. The water samples were collected with polyethylene containers by dipping the sampler below the water surface to minimize the contamination of water sample by surface films and cocked below water surface to avoid air entrapment. Ten fish samples each, namely Clarias spp (Catfish) and Oreochromis spp (Tilapias) were purchased from fishermen at the bank of the river and put in a polyethylene bags and transported in the ice-cooler box to the laboratory and stored below 4 °C in a refrigerator prior to analysis.. Some physicochemical parameters were determined in the water samples using appropriate digital readout meters in situ. These parameters include temperature, pH, electrical conductivity and total dissolved solids.
Samples preparation
The PAHs) in the water samples were extracted, purified and analyzed according to the United State Environmental Protection Agency (US EPA) Method 3510C [17]. One liter of water sample was extracted thrice with 100 mL dichloromethane. The extracts were added and put into a funnel containing anhydrous sodium sulfate. It was later evaporated to 2–3 ml on the rotary vacuum evaporator and cleaned using silica gel/potassium silicate column chromatography consisting of 2 cm anhydrous sodium sulfate (approx.2.0 g) overlaid with 10 cm of activated silica gel (approx 10.0 g) and topped with another 2 cm of anhydrous sodium sulfate. After rinsing the column with 30 mL of Hexane, the sample was added and a 50 mL mixture of hexane and dichloromethane (1:1 v/v). The eluted extract was concentrated using a rotary vacuum evaporator to 2.0 mL and analysed using GC-MS. 10 g of the Gill and muscles samples of the dissected fish samples were homogenized with 100 g of anhydrous sodium sulfate using a mortar and pestle and were transferred into the thimble of the Sohxlet apparatus and extracted using 300 ml mixture of acetone and n-hexane (1:1, v/v) in a water bath for 8 h. Extracts were reduced on a rotary evaporator to 1 ml and then passed through a silica gel column to remove lipids and interfering compounds as cleanup process. The fraction (containing PAHs) was eluted with 100 ml of acetone: hexane (3:2 v/v). The extract evaporated to a volume of less than 1 mL and was made up to a final volume of 1 mL for GC-MS analysis.
Gas chromatography-mass spectrometry conditions
Analysis was carried out with an Agilen 7890, series A, gas chromatography (Agilent, Avondale, USA) interfaced to a mass selective detector (5975 series MSD, Agilent, Avondale, USA). Separation of PAHs was retaken using a 5% Phenyl-methyl-silicone (DB-5MS) bonded-phase fused-silica capillary column (30 m X 250 μm ID, film thickness 0.25 μm) (Agilent, USA) with part number 19019J_413 and a temperature limit of −60 to 350 °C. The injector port was run in splitless mode. The oven temperature program was 65 °C for 1 min, and raised finally to 290 °C at a rate of 10 °C/min and maintained at this temperature for 11 min. The transfer line was maintained at 300 °C. Retention time of each PAHs analyte was established using standard stock solution. PAHs were detected using scan mode, designed for preselected ion peaks. Helium was used as the GC carrier gas. The carrier gas helium was maintained at a constant pressure of 9.0855 Psi with a linear flow rate of 37.604 cm/s.
Statistical data analysis
Triplicate determinations were done and the results reported as mean ± standard deviation. Analysis of variance (ANOVA) at value less than 0.05 (P < 0.05) level of significance, Principal Component Analysis (PCA) based on the Pearson Correlation matrix analysis and component plot in rotated space statistics were performed using SPSS version 20 for Windows.
Quality assurance
Limit of detection (LOD) and limit of quantitation (LOQ)
Standard reference material (SRM 822–275,872-11) was obtained and analyzed for the 16 priority PAHs in other to check the instrument recovery, consistency and efficiency. Limit of detection (LOD) and limit of quantitation (LOQ) were estimated according to the following equations:
where Xb1 is the mean concentration of the blank and Sb1 is the standard deviation of the blank [18].
Human health risk assessment
The risk assessment model follows the carcinogenic health risk methodology recommended by the USEPA [19]. The carcinogenic risk uses the slope factor to estimate the upper-bound lifetime probability of an individual developing cancer as a result of exposure to a particular level of potential carcinogen. It was estimated by the multiplication of the pollutant oral slope factor (SF) with estimated daily intake (EDI) or daily exposure doses averaged over a life time shown in the equation:
where SF = oral slope factor (in mg of toxin per kg of body weight) every day for a lifetime. The environmental protection agency had proved that when the level of carcinogenic health effects is at 10−6 for a pollutant, this will result in a relatively negligible cancer risks. The water (EDIw) or fish (EDIf) ingestion per day or estimated daily intake of PAHs were calculated using the following equations
where CMw is the PAHs concentration in water (μg/L.), IRw is the daily water ingestion rate (L/day/person), CMf is the PAHs concentration in fish (μg/kg), IRf is the daily fish food ingestion rate (mg /day/person) EF = the exposure factor = 1(unitless) and BW is the Body weight (Kg) of the individual [14]. Therefore, the PAHs Cancer Risk (CR) for ingestion of water and fish were estimated using the following equations,
where SF is the oral slope factor for the PAHs. US EPA has proved that when the level of carcinogenic health risk is estimated to be 1.0E-6 for individual toxic metal or pollutant, it will result to negligible cancer risk of a person per one million persons. For the ease of comparing the calculated cancer risk with the standard carcinogenic health risk, cancer risk index (CRI) was introduced. It is the ratio of the calculated cancer risk and the US EPA standard acceptable standard risk of 1.0E-6 [20].
The non-carcinogenic risk was estimated using the hazard quotient model. The hazard quotient (HQ) is the ratio of daily intake (ED I) of pollutant to the oral reference dose (RfD) of that pollutant. If the value of HQ is less than 1, then, the exposed population (consumers) is said to be safe but, if HQ is equal to or higher than 1, human health is at risk. However, HQ parameter does not estimate the risks; it only indicates a risk level associated with pollutants exposure. The HQ estimated using the equation:
EDI is = estimated daily intake exposure dose and RfD is the oral reference dose [21].
The average rate of intake of fish for one hundred and sixty five persons was used to estimate fish intake by an adult and one quarter of it was used as the estimate for a child (Table 1). A Child is taken as human being between the age of three to eleven years while an adult is of the age of 18 years and above for the purposes of this study. Table 2 shows the values of oral reference dose and cancer slope factor for PAHs used for the risk assessment model calculations.
Table 1.
Calculation of exposure model for PAHs
| Parameter | Child | Adult | Reference |
|---|---|---|---|
| Ingestion rate of fish (IRf) (kg/person/ day) | 0.0163 | 0.049 | This study |
| Body weight (BW) (kg) | 15.00 | 70.00 | 21 |
| Ingestion rate of surface water (IRw) (liter/exposure/day) | 50 × 10–3 | 50 × 10–3 | 21, 33 |
| Conversion fsctor of fish from fresh weight to dry weight (Cf) | 0.208 | 0.208 | 34 |
Table 2.
Values of oral reference dose and cancer slope factor for PAHs used for risk assessment model
| PAHs | RfD | CSF |
|---|---|---|
| Naphthalene | 2.0E-2 | – |
| Phenanthrene | 3.0E-1 | – |
| Anthracene | 3.0E-1 | – |
| Benzo[b]fluoranthene | – | 7.3E-1 |
| Benzo[k]fluoranthene | – | 7.3E-2 |
| Benzo[a]pyrene | 3E-4 | 7.3 |
| Ref | 35 |
Results
The recovery study result ranged from 99.90 to 104% for the PAHs as shown in Table 3 .The results of the limit of detection ranges 0.0001–0.0002 μg/kg while limit of quantitation ranges 0.0003–0.0007 μg/kg of the polycyclic aromatic hydrocarbons. The result of the water physicochemical parameters is presented in Table 4. The water pH was low indicating acidity during the dry season but alkaline in the rainy season. However, these values were within the recommended limit World Health Organization (WHO) [22]. Also, there was slight variation in the values of the other physicochemical parameters assessed which could definitely be due to seasonal variation. The TDS values were low despite sources of contamination because those sources provide mainly organics which are mainly insoluble in water. Hence most of the solid wastes remain in the water body as suspended materials.
Table 3.
LOD, LOQ and recovery analysis
| PAHs | Matrix | LOD (μg/kg) | LOQ (μg/kg) | Recovery Range % | RSD r % (n = 3) |
|---|---|---|---|---|---|
| Nap | Fish | 0.0001 | 0.0003 | 100–104 | 1.96 |
| Ph | Fish | 0.0002 | 0.0007 | 99.90–100.50 | 0.30 |
| Ant | Fish | 0.0002 | 0.0007 | 100–101 | 0.49 |
| BbF | Fish | 0.0001 | 0.0003 | 101–103 | 0.98 |
| BkF | Fish | 0.0001 | 0.0003 | 99.90–102 | 1.17 |
| BaP | Fish | 0.0001 | 0.0003 | 101–104 | 1.48 |
Table 4.
Results of physicochemical parameters reported as mean ± Standard deviation
| Season | Parameters | Station | Max. Permissible Limit. WHO [22] | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Dry | Temperature (°C) | 29.97 ± 0.21 | 28.77 ± 0.81 | 30.57 ± 0.32 | 30.80 ± 0.10 | 31.47 ± 0.38 | 20–33 |
| pH | 6.70 ± 0.26 | 6.73 ± 0.15 | 6.80 ± 0.10 | 6.73 ± 0.06 | 6.83 ± 0.06 | 6–8 | |
| Elect. Cond. μS/cm | 15.15 ± 0.08 | 14.51 ± 0.38 | 14.58 ± 0.38 | 14.63 ± 0.26 | 15.07 ± 0.25 | 1000 | |
| TDS (mg/L) | 9.80 ± 0.61 | 9.50 ± 0.50 | 9.77 ± 0.25 | 9.80 ± 0.17 | 10.07 ± 0.15 | 500 | |
| Rainy | Temperature (°C) | 23.77 ± 0.23 | 22.83 ± 0.06 | 24.40 ± 0.46 | 24.63 ± 0.46 | 26.40 ± 1.31 | 20–33 |
| pH | 7.03 ± 0.15 | 7.30 ± 0.10 | 7.27 ± 0.31 | 7.29 ± 0.27 | 7.27 ± 0.12 | 6–8 | |
| Elect. Cond. μS/cm | 13.02 ± 0.80 | 13.06 ± 0.76 | 13.31 ± 0.40 | 14.56 ± 0.90 | 13.56 ± 0.53 | 1000 | |
| TDS (mg/L) | 11.40 ± 0.53 | 11.43 ± 0.51 | 11.60 ± 0.26 | 12.43 ± 0.60 | 11.77 ± 0.35 | 500 | |
Six out of the sixteen United States Environmental Protection Agency (US EPA) priority polycyclic aromatic hydrocarbons were detected in the water and fish samples (Tables 5 and 6). The PAHs detected were naphthalene, phenanthrene, anthracene, benzo [b] fluoranthene, benzo [k] fluoranthene and benzo [a] pyrene. Napthalene was detected in stations B and D with mean concentration (μg/L) ranging 0.420 to 0.543 while phenanthrene and anthracene were detected in only station D with mean concentration 0.083 each. Benzo[b]fluoranthene and benzo[k] fluoranthene mean concentrations were 0.080 to 0.093 μg/L across station A to E and Benzo[a]pyrene had values that ranged from 0.083 to 0.113 μg/L with Benzo[a]pyrene having the highest concentration in station E. The mean concentration of naphthalene (2.383 mg/kg) in catfish was higher than 1.947 mg/kg found in Tilapia. The mean concentrations of phenanthrene and anthracene in catfish is similar to values obtained in the tilapia fish (0. 050 and 0.057 μg/kg). Benzo[b] fluoranthene, Benzo[k]fluoranthene and Benzo[a]pyrene were not detected in the Tilapia but ranges from 0.043–0.05 μg/kg in catfish. Summarily, the distribution pattern of the PAHs in water across locations is in the order: Nap>BaP > BbF=BkF > Ant = Ph while the pattern for fish is Nap>BbF > BkF > Ph = Ant = Bap. The World Health Organization set 0.2 μg/L as the maximum permissible limit for total PAHs in drinking water while that of benzo(a) pyrene (0.1 μg/L) [22]. The PAHs values in Fish were below the EC recommended limit of 12 μg/kg [23].
Table 5.
Results of PAHs concentration in water (μg/L) reported as range and (X ± SD)
| Location | |||||
|---|---|---|---|---|---|
| PAHs | A | B | C | D | E |
| Nap | ND | 0.380–0.440 | ND | 0.190–0.800 | ND |
| 0.420 ± 0.035 | 0.543 ± 0.316 | ||||
| Ph | ND | ND | ND | 0.08–.009 | ND |
| 0.083 ± 0.006 | |||||
| Ant | ND | ND | ND | 0.08–0.09 | ND |
| 0.083 ± 0.006 | |||||
| Bbf | 0.080–0.100 | 0.070–0.090 | 0.060–0.110 | 0.040–0.13 | 0.060–012 |
| 0.093 ± 0.012 | 0.080 ± 0.010 | 0.087 ± 0.025 | 0.083 ± 0.045 | 0.090 ± 0.030 | |
| Bkf | 0.080–0.100 | 0.080–0.090 | 0.060–0.110 | 0.040–0.130 | 0.060–0.120 |
| 0.093 ± 0.012 | 0.087 ± 0.006 | 0.087 ± 0.025 | 0.083 ± 0.045 | 0.090 ± 0.030 | |
| Bap | 0.08–0.100 | 0.07–0.130 | 0.100–0.110 | 0.050–0.110 | 0.100–0.120 |
| 0.090 ± 0.010 | 0.097 ± 0.031 | 0.103 ± 0.006 | 0.083 ± 0.031 | 0.113 ± 0.012 | |
| ∑PAHs | 0.276 | 0.264 | 0.271 | 0.958 | 0.293 |
| ∑LMW-PAHs | 0.0005* | 0.420 | 0.0005* | 0.709 | 0.0005* |
| ∑HMW-PAHs | 0.276 | 0.264 | 0.271 | 0.249 | 0.293 |
| LMW/HMW-PAHs | 0.002 | 1.59 | 0.002 | 2.85 | 0.002 |
*detection limit values used to calculate total LMW-PAHs
Table 6.
Results of PAHs concentration in fish (μg/kg)
| PAHs | Catfish | Tilapia |
|---|---|---|
| Nap | 2.340–2.420 | 1.200–2.310 |
| 2.383 ± 0.040 | 1.947 ± 0.647 | |
| Ph | 0.040–0.060 | 0.050–0.070 |
| 0.050 ± 0.010 | 0.057 ± 0.012 | |
| Ant | 0.040–0.080 | 0.050–0.070 |
| 0.057 ± 0.021 | 0.057 ± 0.012 | |
| Bbf | 0.040–.0.050 | ND |
| 0.043 ± 0.006 | ||
| Bkf | 0.040–0.050 | ND |
| 0.043 ± 0.006 | ||
| Bap | 0.040–0.060 | ND |
| 0.050 ± 0.010 | ||
| ∑PAHs | 2.626 | 2.061 |
| ∑LMW-PAHs | 2.497 | 2.061 |
| ∑HMW-PAHs | 0.143 | – |
| LMW/HMW-PAHs | 17.46 | 2.061 |
Discussion
The occurrence of P AHs in fish is an indication of PAH contamination in river waters. Both low and high molecular weight PAHs were observed in Synodontis clarias (cat fish) while only low molecular weight PAHS were found in Tilapia guineensis. S.calrias live in sandy area of water while T. guineensis are bottom feeders as they live in the muddy bottom of water. During PAHs transport, high molecular weight PAHs could be degraded to lower ones and are buried in the sediments [24]. Thus, S. clarias which are shallow feeders could accumulate both HMW and LMW PAHs released from anthropogenic activities in the water while T. guineensis accumulate mostly the LMW PAHs. Some sources of PAH in water bodies include oil spillage or discharge, runoffs from contaminated soils and dumping of refuse containing oily materials. The aquatic organisms get exposed to these contaminants which bioconcentrate in their bodies [25–27]. PAHs being lipophilic, accumulate in the fatty tissues of fish. The concentrations of all the PAHs examined in water, and fish species were below the WHO maximum permissible limit of 10 mg/l in water and 0.001 μg/g in fish and shell fish respectively [22] and EC regulation in fish.
The PAHs concentrations obtained from water in this study was higher than 0.009 to 0.347 μg/L obtained in an estuary in Mexico [28]. The total PAHs levels obtained in fish from this study were lower than 45.9–171.9 μg/kg reported in seafood from Niger Delta coastal waters [29]. Also, our values were lower than the total PAHs (48.75–166.79 μg/kg) reported in fish from Ghana coastal waters [30].
The concentration values of PAHs in the two media (water and fish) showed no significant difference within and between the groups with values less than 0.05 (p < 0.05) level of significance. The ANOVA showed that the exact probability (Sig.) value of 0.338 and 0.856 generated from the analysis of water and fish for PAHs were greater than 0.05 (p < 0.05) significant level which means that the data were not different significantly.
Correlation analysis and source identification
The mean concentration values of PAHs in water and fish samples were subjected to correlation analysis to understand if there is any hidden trend between the data. A multivariate technique, Principal Component Analysis (PCA) was applied to correlate PAHs that may have similar behavior and implies they could have common origin [31]. The correlation matrix of the PAHs in water and fish ranged from −0.970 to 1.000 at 0.05 and 0.01 levels of significance. At 0.05 level of significance, there is correlation between Phenanthrene in water and Naphthalene in water (0.632), Anthracene in water and Naphthalene in water (0.632) as well as a very strong, positive and perfect correlation with Phenanthrene in water (1.000) at 0.01 level of significance, there is also a very strong positive correlation between Benzo[k]fluoranthene in water and Benzo[b]fluoranthene in water (0.976) at 0.01 level of significance. Benzo[k]fluorantene and Benzo[a]pyrene in fish showed strong negative correlations with Naphthalene in water (−0.984, −0.984 and − 0.970). While Benzo[a]pyrene in fish strongly positively correlated with Benzo[b]fluoranthene and Benzo[k]fluoranthene in fish (0.963), Benzo[k]fluoranthene was strongly, positively and perfectly correlated to Benzo[b]fluoranthene at 0.01 level of significance. This shows that these PAHs behaviour similarly and may have same source or origin. Benzo[a]pyrene presence is mostly an indicator of PAHs originating from combustion. The correlation of Benzo[a]pyrene with other PAHs indicates that combustion is the primary source of the PAHs in the water body.
Risk assessment
Tables 7 and 8 show the result of the estimated daily intake (EDI) of PAHs by the ingestion of water using the parameters in Tables 1 and 2. The Hazard quotients and the cancer risk for the ingestion of Benzo[b]flouranthene, Benzo[k]fluoranthene, Benzo[a]pyrene were estimated. The results of these parameters that were above the acceptable limits were indicated in bold fonts. Only the cancer risk for Catfish were computed since there was no detections for the carcinogenic PAHs (Benzo[b] fluoranthene (B[b]F), Benzo[k]fluoranthene (B[k]F) and Benzo[a]pyrene (B[a]P) in Tilapia.
Table 7.
Estimated daily intake (EDI), Hazard quotient (HQ) and Cancer risk (CR) of PAHs via ingestion of water
| Location | Nap | Ph | Ant | B[b]F | B[k]F | B[a]P |
|---|---|---|---|---|---|---|
| A | ND | ND | ND |
EDIAdult6.6E-8 EDIChild3.1E-7 CRAdult4.8E-8 CRChild2.3E-7 RIAdult0.05 RIChild0.23 |
EDIAdult6.6E-8 EDIChild3.1E-7 CRAdult4.8E-9 CRChild2.3E-8 RIAdult0.005 RIChild0.02 |
EDIAdult6.4E-8 EDIChild3.0E-7 CRAdult4.8E-7 CR Child 2.2E-6 RIAdult0.48 RI Child 2.2 |
| B |
EDIAdult 3.0E-7 EDIChild 1.4E-6 HQAdult1.5E-5 HQChild 7.0E-5 |
ND | ND |
EDIAdult5.7E-8 EDIChild2.7E-6 CRAdult4.2E-8 CR Child 1.9E-6 RIAdult0.04 RI Child 1.9 |
EDIAdult6.2E-8 EDIChild2.9E-7 CRAdult4.5E-9 CRChild2.1E-8 RIAdult0.05 RIChild0.02 |
EDIAdult6.9E-8 EDIChild3.2E-7 CRAdult5.1E-7 CR Child 2.4E-6 RIAdult0.51 RI Child 2.4 |
| C | ND | ND | ND |
EDIAdult6.2E-8 EDIChild2.9E-7 CRAdult4.5E-8 CRChild2.1E-7 RIAdult0.05 RIChild0.21 |
EDIAdult6.2E-8 EDIChild2.9E-7 CRAdult4.5E-9 CRChild2.1E-8 RIAdult0.005 RIChild0.02 |
EDIAdult7.4E-8 EDIChild3.4E-7 CRAdult5.4E-7 CR Child 2.5E-6 RIAdult0.54 RI Child 2.5 |
| D |
EDIAdult 3.9E-7 EDIChild 1.8E-6 HQAdult 1.9E-5 HQChild 9.1E-5 |
EDIAdult 5.9E-8 EDIChild 2.8E-7 HQAdult1 2.0E-7 HQChild 9.2E-7 |
EDIAdult 5.9E-8 EDIChild 2.8E-7 HQAdult1 2.0E-7 HQChild 9.2E-7 |
EDIAdult5.9E-8 EDIChild2.8E-7 CRAdult4.3E-8 CRChild2.0E-7 RIAdult0.04 RIChild 0.2 |
EDIAdult5.9E-8 EDIChild2.8E-7 CRAdult4.3E-9 CRChild2.0E-8 RIAdult0.004 RIChild0.02 |
EDIAdult5.9E-8 EDIChild2.8E-7 CRAdult4.3E-7 CR Child 2.0E6 RIAdult0.43 RI Child 2.0 |
| E | ND | ND | ND |
EDIAdult6.4E-8 EDIChild3.0E-7 CRAdult4.7E-8 CRChild2.2E-7 RIAdult0.05 RIChild0.22 |
EDIAdult6.4E-8 EDIChild3.0E-7 CRAdult4.7E-9 CRChild2.2E-8 RIAdult0.005 RIChild0.02 |
EDIAdult8.1E-8 EDIChild3.8E-7 CRAdult5.9E-7 CR Child 2.7E-6 RIAdult0.59 RI Child 2.7 |
Table 8.
Estimated daily intake (EDI), Hazard Quotient (HQ) and Cancer risk (CR) of PAHs in catfish and tilapia via ingestion
| Location | Nap | Ph | Ant | B[b]F | B[k]F | B[a]P |
|---|---|---|---|---|---|---|
| Catfish |
EDIAdult3.5E-7 EDIChild 5.4E-7 HQAdult 1.7E-5 HQChild2.7–5 |
EDIAdult 7.3E-9 EDIChild 1.1E-8 HQAdult 2.4E-8 HQChild 3.8E-8 |
EDIAdult8.3E-9 EDIChild1.3E-8 HQAdult 2.8E-8 HQChild4.3E-8 |
EDIAdult3.0E-8 EDIChild4.7E-8 CRAdult2.2E-8 CRChild3.4E-8 RIAdult0.02 RIChild 0.03 |
EDIAdult3.0E-8 EDIChild4.7E-8 CRAdult2.2E-9 CRChild3.4E-9 RIAdult 0.002 RIChild 0.003 |
EDIAdult3.5E-8 EDIChild5.4E-8 CRAdult2.6E-7 CRChild3.9E-7 RIAdult0.26 RIChild0.39 |
| Tilapia |
EDIAdult 2.8E-7 EDIChild4.4E-7 HQAdult1.4E-5 HQChild2.2E-5 |
EDIAdult8.3E-9 EDIChild 2.8E-9 HQAdult× 2.8E-8 HQChild9.3E-9 |
EDIAdult 8.3E-9 EDIChild 2.8E-9 HQAdult× 2.8E-8 HQChild9.3E-9 |
ND | ND | ND |
The carcinogenic PAHs detected in the water, and fish in this research were Benzo[b]fluoranthene (BbF), Benzo[k]fluoranthene (BkF) and Benzo[a]pyrene (BaP). Hazard quotient model was used for the non-carcinogenic health risk estimation for the other PAHs termed non-carcinogenic which include Naphthalene, Phenanthrene, and Anthracene determined in this work. From the result, the cancer risk of Benzo[b]flouranthene, Benzo[k]floranthene Benzo[a]pyrene were within or below limit except that for Benzo[a]pyrene at location E which gave cancer risk value of 3 person per one million people and the cancer risk index of 2.7 being above the limit of 1 per a million persons. The concentration of PAHs in cat fish showed cancer risk and cancer risk index value within the acceptable limit and the three carcinogenic PAHs (Benzo[b]flouranthene, Benzo[k]floranthene Benzo[a]pyrene) were not detected in the Tilapia fishes. Pollution recorded at location D and E could be because, between location C and D on the Lokoja river bank, mini or local port exist where people from Shintaku in Bassa local government area as well as neighboring local government area of the state enter and leave Lokoja leading to high vehicular and boats movement and usage of petroleum products where boats engines were repaired and maintained on the banks. This observation is in tandem with a study in Ghana that asserted that canoe landing site is more polluted than the inner fishing harbor due to the numerous anthropogenic activities that go on there as well as the activities of fishmongers who smokes fish at the canoe landing site and therefore introducing more PAHs [32]. Benzo[a]pyrene concentration values in water and Catfish was high comparing with the concentration limit of 0.01 μg/L in water by Environment Canada and been a common marker for PAHs originating from combustion, it presence above the set limit indicates that threat of PAHs exist for the rivers Niger and Benue confluence.
Conclusion
The research study profiled and estimated the concentration and human health and risk of PAHs water samples and two fish species in the confluence of rivers Niger and Benue. The result showed that the concentration of PAHs in the water and fish samples were of concern. Hence, study area need immediate attention to reduce improper waste disposal and improve on measures against indiscriminate dumping of petroleum products and domestic waste in the water. Also, burning of tyres, organic and petroleum products which are the major sources of petrogenic and pyrogenic PAHs should be minimized.
Acknowledgements
The authors wish to acknowledge the assistance given by our Laboratory staff. Though they wish to remain at the background, we appreciate their assistance.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest as regards this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Svensson BG, Nilsson A, Josson E, Schtüz A, Åkesson B, Hagmar L. Fish consumption and exposure to persistent organochlorine compounds, mercury, selenium and methylamines among Swedish fisherman. Scand J Work Environ Health. 1995;21:96–105. doi: 10.5271/sjweh.16. [DOI] [PubMed] [Google Scholar]
- 2.UNFPA (United Nations Population Fund). Global Population and water access and Sustainability, Population and Development Strategies Series. New York, NY 10017. 2003.
- 3.Avishai N, Rabin OWHZ C, Moiseeva E, And Rinkeveh B. Genotoxicity of the Kishon River, Israel: The Application of an in vitro cellular assey. Mutation Res. 2002;518:21–37. doi: 10.1016/s1383-5718(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 4.Mzoughi N, Chouba L. Heavy metals and PAH assessment based on mussel caging in the north coast of Tunisia (Mediterranean Sea) Int J Environ Res. 2012;6(1):109–118. [Google Scholar]
- 5.Cheng-Di D, Chih-Feng C, Chiu-Wen C. determination of polycyclic aromatic Hydrocarbons in industrial harbor sediments by GC-MS. Int J Environ Res Public Health. 2012;9(6):2175–2188. doi: 10.3390/ijerph9062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opuene K, Okafor EC. Preliminary assessment of trace metals and polycyclic aromatic hydrocarbons in sediments. Int J Environ Sci Technol. 2007;4(2):233–240. [Google Scholar]
- 7.Bhupander K, Mukherjee DP, Sanjey K, Mishra M, Prakash D, Kingh SK, Sharma CS. Bioaccumulation of heavy metals in muscle tissue of fishes from selected aquaculture ponds in East Kolkata wetlands. Ann Biol Res. 2011;2(5):125–134. [Google Scholar]
- 8.Zafer A, Guler E, Sedat V, Murat O. Heavy metal accumulation in water sediments and fishes of Nallihan bird paradise, Turkey. J Environ Biol. 2007;28(2):545–549. [PubMed] [Google Scholar]
- 9.Ajiboye OO, Yakubu AF. Adams TE. A review of polycyclic aromatic Hydrocarbons and heavy metal contamination of fish from fish farms. J. Appl. Sci. Environ Manag. 2011;15(1):235–238. [Google Scholar]
- 10.Babatunde AM, Waidi OA, Adeolu AA. Bioaccumulation of heavy metals in fish(hydrocynusforskahlii, hyperopisusbebeOccidentalisand clariasgariepinus) organs indownstream Ogun coastal water, Nigeria. Transnational J Sci Technol. 2012;2(5):119–133. [Google Scholar]
- 11.Olubunmi EF, Edward OO. Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyls (PCBs) in soils of Agbabu , Nigeria. Proceedings of 1st Annual International Interdisciplinary Conference, Azones Portugal. 2013:849–56.
- 12.Pruski AM, Dixon DR. Effects of cadmium on nuclear integrity and DNA repair efficiency in the gill cells of Mytilus Edulis. Aquat Toxicol. 2002;57:127–137. doi: 10.1016/s0166-445x(01)00192-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee RF, Steinert S. Use of the single cell gel electrophoresis/comet Assey for detecting DNA damage in aquatic (marine and freshwater) animals. Mutation Res. 2003;544:43–64. doi: 10.1016/s1383-5742(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 14.Obiakor MO, Okonkwo JC, Ezeonyejiaku CD, Okonkwo CN. Polycyclic aromatic Hydrocarbons (PAHs) in freshwater media: factorial effects and human dietary exposure risk assessment. Resource Environ. 2014;4(6):247–259. [Google Scholar]
- 15.Audu EB. An analytical view of temperature in Lokoja, Kogi state. Nigeria Int J Sci Technol. 2012;2(12):856–859. [Google Scholar]
- 16.Afolabi TA, Ogbnuneke CC, Ogunkunle OA, Bamiro FO. Comparative assessment of the potable quality of water from industrial, urban and rural parts of Lagos. Nigeria Ife J Sci. 2012;14(2):221–232. [Google Scholar]
- 17.United State Environmental Protection Agency (US EPA) methodic description of procedure for isolating organic compounds from aqueous samples and concentration techniques suitable for preparing extract for appropriate determinative methods. Revision. 1996;3:1–8. [Google Scholar]
- 18.Shrivastava A, Gupta VB. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. ChronYoung Sci. 2011;2(1):21–25. [Google Scholar]
- 19.USEPA Field sampling guidance Document N1215: Sediment sampling. U.S. EPA region 9 laboratory Richmond, Califorrnia, N1215, Rev. I. 1997.:1–10.
- 20.Ofosu FG, Afrifa CG, Bamford SA, Atiemo SM, IJK A, Gyampo O, Ahiamadjie H, Adeti JP, Arthur JK. Health risk assessment of heavy metal exposure from soil dust at selected fuel filling stations in Accra. Int J Sci Technol. 2015;l4(7):289–296. [Google Scholar]
- 21.Ekere NR, Ihedioha JN, Eze IS, Agbazue VE. Health risk assessment in relation to heavy metals in water sources in rural regions of south East Nigeria. Int J Phys Sci. 2014;9(6):109–116. [Google Scholar]
- 22.World Health Organization . Polynuclear aromatic hydrocarbons in drinking-water. Background document for development of WHO guidelines for drinking-water quality. Geneva: World Health Organization; 2003. [Google Scholar]
- 23.European Commission. EC regulation 2015/1933 amending EC regulation 1881/2006 regarding maximum levels of PAHs in foodstuffs, Torino Italy. 2015.
- 24.Berto D, Cacciatore F, Ausili A, Sunseri G, Luca G, Bellucci LG, Frignani M, Albertazzi S, Giani M. Polycyclic aromatic Hydrocarbons (PAHs) from diffuse sources in coastal sediments of a not industrialised Mediterranean Island. Wat Air Soil Pollut. 2009;200:199–209. [Google Scholar]
- 25.Gobas FAPC, Wilcockson JB, Russell RW. Haffner GD mechanism of biomagnification in fish under laboratory and field conditions. Envir Sci Technol. 1999;33:133–141. [Google Scholar]
- 26.Fowler SW, Knauer GA. Role of large particles in the transport of elements and organic compounds through the oceanic water column. Prog Oceanog. 1986;16:147–194. [Google Scholar]
- 27.Raoux C, Bayona JM, Miquel JC, Teyssie JL, Fowler SW, Labaiés J. Particulate fluxes of aliphatic and aromatic hydrocarbons in near shore waters to the north - western Mediterranean Sea, and the effect of continental runoff. Estuar Coast Shelf Sci. 1999;48:605–616. [Google Scholar]
- 28.Jaward FM, Alegria HA, Galindo Reyes JG, Hoare A. Sci World J. 2012. Levels of PAHs in the waters, sediments, and shrimps of Estero de Urias, an estuary in Mexico, and their toxicological effects; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwaichia EO, Ntorgbo SA. Assessment of PAHs levels in some fish and seafood from different coastal waters in the Niger Delta. Toxicol Rep. 2016;3:167–172. doi: 10.1016/j.toxrep.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyarko E, Botwe BO, Klubi E. Polycyclic aromatic Hydrocarbons (P AHs) levels in T wo commercially important fish species from the coastal waters of Ghana and their carcinogenic health risks. West African J Appl Ecol. 2011;19:53–65. [Google Scholar]
- 31.Afshin Q, Farid M. Statistical analysis of accumulation and source of heavy metals occurrence in agricultural soils of Khoshk River banks, shiraz, Iran. American-Eurasian J Agric Environ Sci. 2007;2(5):565–573. [Google Scholar]
- 32.Gorleku MA, Carboo D, Palm LMN, Quasie WJ, Armah AK. Polycyclic aromatic hydrocarbons (PAHs) pollution in marine waters and sediments at the Tema Harbor, Ghana. Acad J Environ Sci. 2014;2(7):108–115. [Google Scholar]