Abstract
Mucormycosis is a rare but potentially mortal opportunistic infection caused by Mucorales. We present a case of rhinoorbital mucormycosis in an 11-year old male with neuroblastoma successfully treated with aggressive surgical debridement and antifungal combination of liposomal amphotericin B and posaconazole. Our patient developed signs of paranasal sinus infection and culture of fine needle biopsy grew Rhizopus arrhizus. Prompt treatment and drastic surgical resection led to complete clinical and radiological recovery without evidence of mucormycosis relapse.
Keywords: Mucormycosis, Zygomycosis, Mucorales, Pediatric oncology, Paranasal sinuses
1. Introduction
Mucormycosis is a potentially life-threatening invasive infection caused by the widespread saprophytic organisms Mucorales and was first described as Mycosis mucorina by Arnold Paltauf in 1885. It should be suspected in every granulocytopenic, otherwise immunocompromised or diabetic patient with signs or symptoms affecting the nose, paranasal sinuses, orbits or central nervous system. Mucormycosis is characterized by acute onset. The fungi penetrate the organs through orbital, oral, nasal, or skin pathways and produce granulomatous, thrombotic and necrotic lesions, while the hyphae penetrate all normal structures. Although the frequency of invasive mucormycosis in children is low, it is more common among patients with hematological malignancies [1,2,9] and not that common in patients with solid tumors. Specifically, in pediatric patients with neuroblastoma, there are only 3 cases of invasive mucormycosis reported making this case report an important addition to the literature [[3], [4], [5]]. We describe a case of rhinoorbital mucormycosis in an 11-year old male with neuroblastoma successfully treated with aggressive surgical debridement and antifungal combination of liposomal amphotericin B (L-AmB) and posaconazole.
2. Case
An 11-year-old boy on chemotherapy for recurrent neuroblastoma was severely neutropenic (ANC 0.1 × 109/lit) when presented with fever (38.8 °C) and started treatment with meropenem, amikacin, and teicoplanin. Two days later he developed simultaneous signs of paranasal sinus infection, such as oedema, nasal secretion and, severe headache. On day 4 he developed periocular swelling, accompanied by local sinus tenderness. An urgent CT scan showed sinusitis, especially of the left side, with no evidence of bone, orbital, or brain invasion. The symptoms progressively deteriorated with erythema and swelling over the left eye, nasal stuffiness, photophobia, and facial pain needed morphine on day 12. Liposomal amphotericin B 5mg/kg/d was initiated because of ongoing concern of a fungal infection. Serial CT scan and MRI showed findings compatible with mycosis. The CT scan showed a mass in the left ethmoidal sinus extending into the retrobulbar area of the left eye (Fig. 1, Fig. 2). Invasive mucormycosis was diagnosed by fungal cultures of nasal discharge after paracentesis, which revealed Rhizopus arrhizus as the causative agent. Nasal discharge was cultivated and R. arrhizus was identified by microscopic appearance of fungal elements. L-AmB dose was increased to 7 mg/kg/d and oral posaconazole 20 mg/kg/d was added on day 15, in view of clinical and radiological deterioration. The next few days the patient had clinical aggravation with incomplete eyelid closure due to dislocation of the left eye, ptosis, diplopia, and facial palsy. Surgical debridement of the infected tissue along with extended external ethmoidectomy and maxillectomy was performed on the 21st day of hospitalization. After surgery, the patient was stabilized. The disease resolved with complete clinical and radiological recovery and antifungal treatment was terminated 45 days after the initiation of therapy. As there was no apparent residual fungal infection, the patient was discharged from hospital. Oral posaconazole was prescribed for six months. No clinical evidence of mucormycosis relapse was observed during the subsequent follow-up visits and weekly CT and MRI scans (Fig. 3). Unfortunately, the patient passed away due to neuroblastoma progression two months following his discharge.
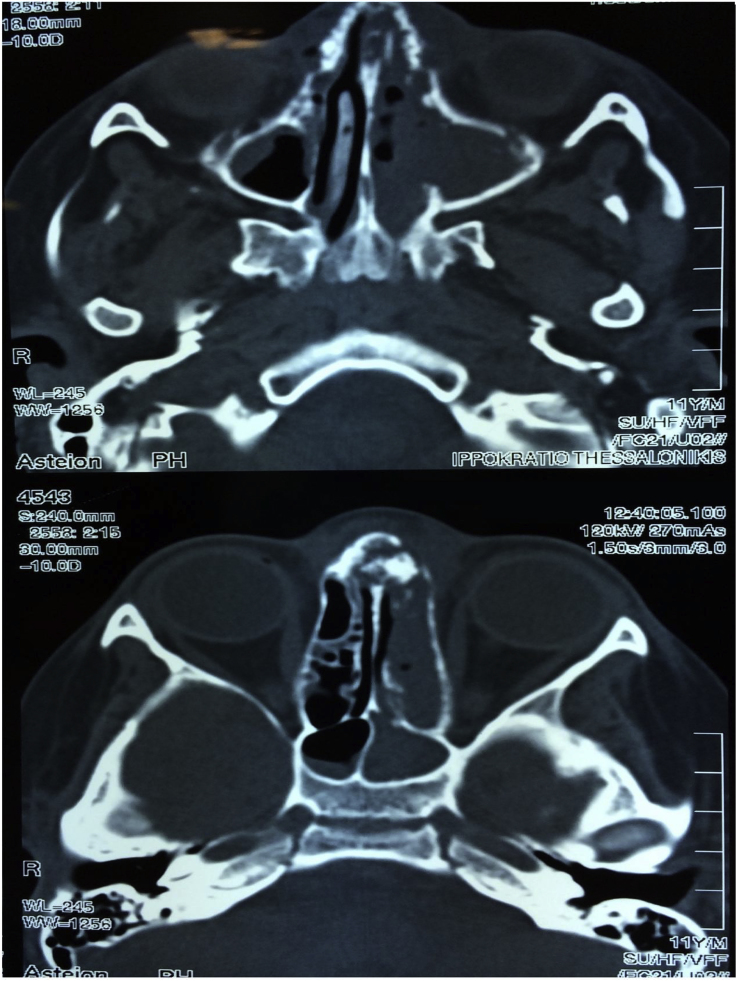
Fig. 1.
A) Computed tomography (CT scan) demonstrated a soft tissue density in the left maxillary sinus extending to the left nasal cavity. In addition, erosions of the left nasal septum were observed. B) Sphenoid sinuses lesions, more prominent at the left side.
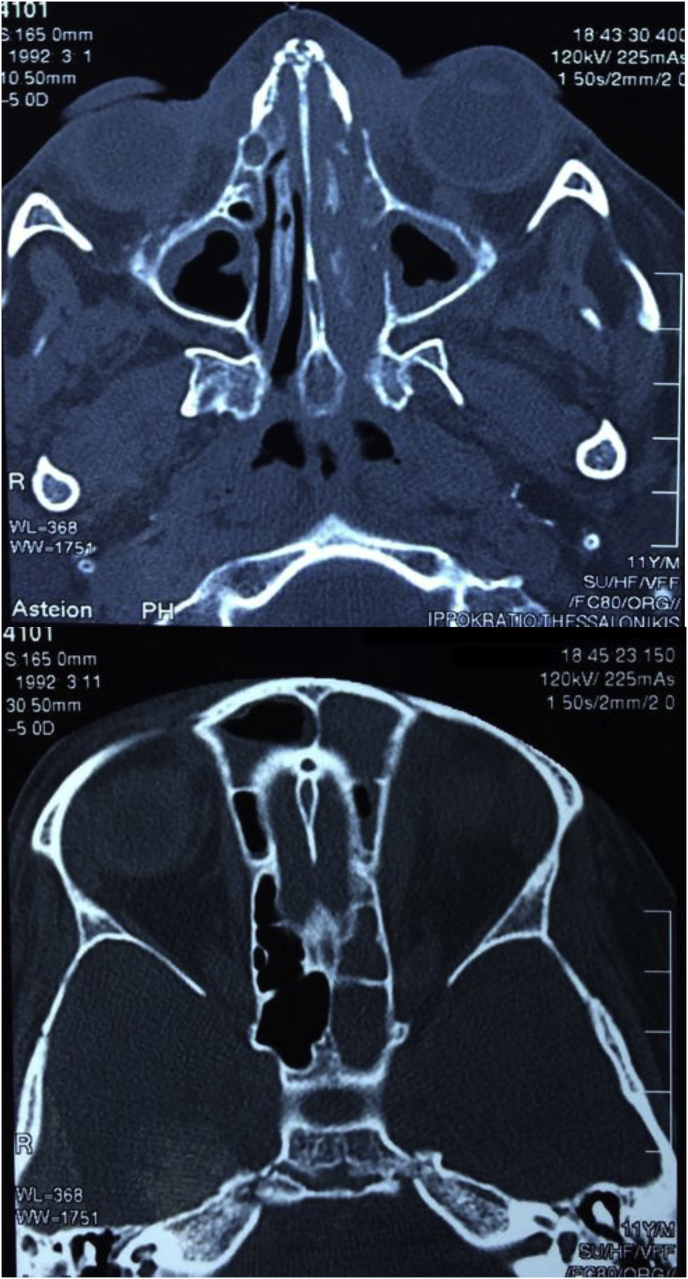
Fig. 2.
Occupied nasal cavity, sphenoid and ethmoid sinuses. Frontal sinus lesions.
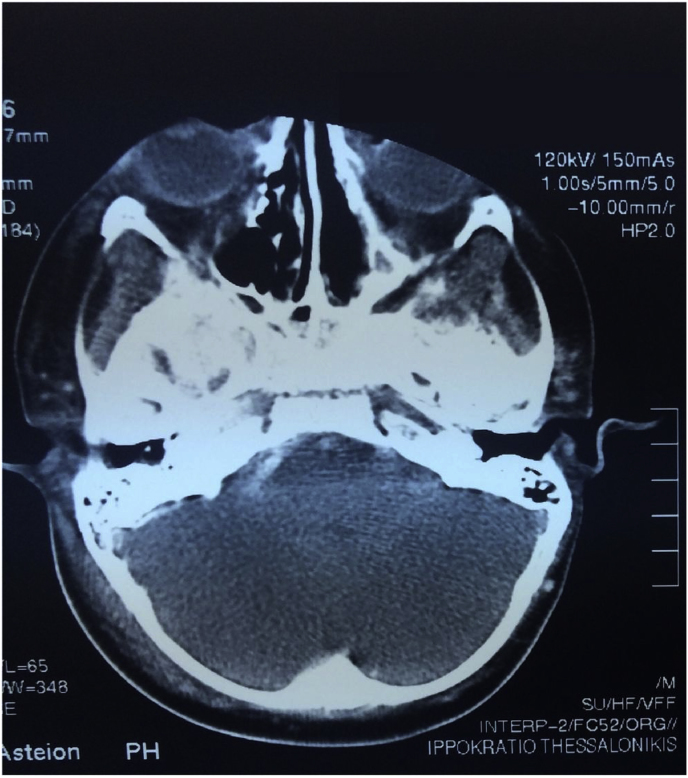
Fig. 3.
CT scan post-surgery and antifungal treatment reveals complete radiological recovery of mucormycosis.
3. Discussion
Mucormycosis is a life-threatening infection primarily caused by the fungi orders Mucorales and Entomophthorales. More specifically, Mucorales order entails the genera Rhizopus, Lichtheimia and Mucor, Rhizomucor, Apophysomyces, and Cunninghamella. Entomophthorales include Basidiobolus and Conidiobolus genera [6]. Rhizopus arrhizus is the most frequently identified pathogen and is responsible for 65% of all mucormycosis cases. Mucormycosis in oncologic patients is usually seen in hematological malignancies, such as acute leukemia, lymphoma, or chronic leukemia. Roden et al. [7] in a study of 929 mucormycosis cases, state that only 7 out of 154 patients with malignancy-related mucormycosis had non-hematological malignancy (NHM).
Neuroblastoma represents the underlying condition of 3% of mucormycosis cases in adult patients with NHM [8]. However, in the pediatric population, at the literature review that we conducted, the incidence was even lower, as there were only 3 cases of children that matched the criteria of neuroblastoma-related mucormycosis. To our knowledge, this is the first case of rhinoorbital mucormycosis in a pediatric patient with neuroblastoma reported in the literature, as the other three cases were related to mandibular, splenopancreatic, and disseminated mucormycosis. At Table 1 we present the characteristics of these 4 known cases of neuroblastoma-related mucormycosis.
Table 1.
Neuroblastoma-related mucormycosis cases referred in the literature.
| Year of publication | Country | Type of mucormycosis | Age (gender) | Antifungal treatment | Surgery | Outcome |
|---|---|---|---|---|---|---|
| 1997 | Italy | disseminated (gastrointestinal tract, liver, kidneys) | 3y (male) | amphotericin B | yes (bowel debridement) | stabilization- 10 months post-surgery: liver and kidney lesions unchanged |
| 2012 | Turkey | mandibular | 6y (male) | amphotericin B | yes (excision of necrotic lesions) | mucormycosis resolved (died from neuroblastoma progression 3 months later) |
| 2013 | Iran | splenopancreatic | 18 m (male) | liposomal amphotericin B, posaconazole | yes (splenectomy) | did not respond to treatment (died 2 weeks later) |
| 2018 | Greece | rhinoorbital | 11 (male) | liposomal amphotericin B, posaconazole | yes (ethmoidectomy and maxillectomy) | mucormycosis resolved (died from neuroblastoma progression 2 months later) |
Other pediatric malignancies complicated with mucormycosis referred in the literature are osteosarcoma, thoracic and brain tumour [8]. Furthermore, the mortality rate in systemic mucormycosis is greater than 40%, while in cerebral involvement, it exceeds 80%, and reaches 100% without treatment [1,10]. In a large European study of 230 cases total mortality was estimated 47% with the most chances for survival in immunocompetent patients [11].
There is no standard therapy established for mucormycosis in children yet, thus the therapeutic strategy followed is the same as in adults. Amphotericin B (AmB) is the first-line antifungal agent, with its liposomal formulation widely used in cerebral mucormycosis, due to higher permeability in the CNS. Liposomal amphotericin B is less nephrotoxic, can be administered for a longer period of time in higher doses and is associated with higher survival rate comparing to conventional AmB [10]. Clinical suspicion of mycosis led us to start immediate administration of L-AmB, in order to maximize cure and survival rates. In view of poor response to L-AmB alone and probable refractory disease, we added posaconazole, an alternative oral treatment, which inhibits ergosterol synthesis in the mycetes. Posaconazole is used as salvage therapy in cases of intolerance (renal toxicity of L-AmB) or treatment failure to previously used antifungal agents. It is a well-tolerated drug with a generally safe pharmacologic profile that can be used in immunocompromised patients with minimal adverse effects (nausea, vomiting) [12]. However, variable oral bioavailability of posaconazole poses some limitations in its use [13]. Burik et al. [14] reported 60% response rates to posaconazole (45% partial response, 15% complete response) in a study of 91 cases.
In the ECMM registry, the survival rate of mucormycosis in patients receiving posaconazole is 72% [15]. In our case, the combination of L-AmB and posaconazole had excellent results on resolving mucormycosis, while L-AmB alone failed. However, there are limited data in the literature that support the effectiveness of posaconazole and L-AmB combination. Rodriguez et al. [16] state that the combination is not superior to L-AmB alone, although it reduced the fungal burden in the brain. Another study [17] supports that the addition of posaconazole does not alter the clinical outcome compared to L-AmB monotherapy at full therapeutic doses. Rickerts et al. [18] reported a case of disseminated mucormycosis due to Rhizomucor, which was successfully treated exclusively with L-AmB and posaconazole combination, without surgical debridement.
Additionally, the use of granulocyte colony-stimulating factor (G-CSF) is promising and suggested in patients with mucormycosis and neutropenia, according to the latest ESMID guidelines [15].
Lately, a second-generation triazole, isavuconazole, has been approved by the FDA and the EMA for the treatment of invasive mucormycosis in adults and in cases where the use of amphotericin B is inappropriate, respectively [19]. Although there is limited clinical experience, isavuconazole seems to be a safe and effective drug that may be used as an alternative to amphotericin B. However, the drug is not approved for use in children yet.
Surgical debridement is strongly recommended to be combined with the antifungal agents, in order to optimize survival rates. Extensive resection of all the infected and necrotized tissues is essential in order to restrict the dissemination of mucormycosis. In Petrikkos' study [20] all patients with mucormycosis who did not undergo surgery died. During the procedure, it is urgent to ensure the drainage of paranasal sinuses, in case of their involvement. In our case repeated resections from the margins of the wound were performed in order to remove the residual fungus foci. Frequent nasal wash-outs with liposomal amphotericin B were performed as well. Post-operatively, functional and aesthetic sequelae may exist (especially in the rhinocerebral forms), but reconstructive surgical intervention is usually deferred until the infection is completely controlled.
Due to the life-threatening nature of the infection mucormycosis requires high level of clinical suspicion and needs to be added to the differential diagnosis of pediatricians caring of immunocompromised patients in view of specific clinical signs.
4. Conclusion
The successful outcome in our patient was probably related to early diagnosis combined with prompt initiation of antifungal treatment and complete surgical resection. Our patient's infection was totally resolved, despite the rhinoorbital type of mucormycosis and its expected poor outcome. Hence, this case suggests a role for posaconazole in the treatment of mucormycosis in immunocompromised children. It is important that our patient survived two months free of disease before passing away from neuroblastoma progression.
Conflict of interest
There are none.
Acknowledgements
There are none.
References
- 1.Zaoutis T., Roilides E., Chiou C., Buchanan W., Knudsen T. Zygomycosis in Children : a systematic review and analysis of reported cases. Pediatr. Infect. Dis. J. 2007;26(8):723–727. doi: 10.1097/INF.0b013e318062115c. [DOI] [PubMed] [Google Scholar]
- 2.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012;54(Suppl 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 3.Aras M.H., Kara M.I., Erkiliç S., Ay S. Mandibular mucormycosis in immunocompromised patients: report of 2 cases and review of the literature. J. Oral Maxillofac. Surg. 2012;70(6):1362–1368. doi: 10.1016/j.joms.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Ebadi M., Alavi S., Ghojevand N., Aghdam M.K., Yazdi M.K., Zahiri A. Infantile splenorenopancreatic mucormycosis complicating neuroblastoma. Pediatr. Int. 2013;55(6):e152–e155. doi: 10.1111/ped.12182. [DOI] [PubMed] [Google Scholar]
- 5.Villani A., Vacca P., Onofri A., Cori M. Disseminated mucormycosis. A rare case in pediatric intensive care. Minerva Anestesiol. 1997;63(7–8):249–252. [PubMed] [Google Scholar]
- 6.Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012;54(Suppl 1):1–7. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L. Epidemiology and outcome of Zygomycosis : a review of 929 reported cases. Clin. Infect. Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 8.Shadrivova O., Khostelidi S., Borzova Y., Desyatik E., Volkova A., Popova M. P1233 Combination of invasive aspergillosis and mucormycosis in oncohematological patients : prospective study results. J. Med. Microbiol. Diagn. 2017;103(7) [Google Scholar]
- 9.Pana Z.D., Seidel D., Skiada A., Groll A.H., Petrikkos G., Roilides E. Invasive mucormycosis in children : an epidemiologic study in European and non- European countries based on two registries. BMC Infect. Dis. 2016;16(1):667. doi: 10.1186/s12879-016-2005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spellberg B., Ibrahim A.S. Recent advances in the treatment of mucormycosis. Curr. Infect. Dis. Rep. 2010;12(6):423–429. doi: 10.1007/s11908-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skiada A., Pagano L., Groll A., Zimmerli S., Dupont B., Lagrou K. Zygomycosis in Europe : analysis of 230 cases accrued by the registry of the European confederation of medical mycology ( ECMM ) working group on zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2007;17(12):1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 12.Raad I.I., Graybill J.R., Bustamante A.B. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 2006;42(12):1726–1734. doi: 10.1086/504328. [DOI] [PubMed] [Google Scholar]
- 13.Jancel T., Shaw P.A., Hallahan C.W., Kim T., Freeman A.F., Holland S.M. Therapeutic drug monitoring of posaconazole oral suspension in paediatric patients younger than 13 years of age: a retrospective analysis and literature review. J. Clin. Pharm. Ther. 2017;42(1):75–79. doi: 10.1111/jcpt.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burik JH Van, Hare R.S., Solomon H.F., Corrado M.L., Kontoyiannis D.P. Posaconazole is effective as salvage therapy in Zygomycosis : a retrospective summary of 91 cases. Clin. Infect. Dis. 2006;42:61–65. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 15.Cornely O.A., Dannaoui E., Groll A.H., Lagrou K., Chakrabarti A., Lanternier F. ESCMID † and ECMM ‡ joint clinical guidelines for the diagnosis and management of mucormycosis 2013. 2014;20(Suppl 3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez M.M., Serena C., Pastor F.J., Guarro J. Posaconazole combined with amphotericin B , an effective therapy for a murine disseminated infection caused by Rhizopus oryzae. Antimicrob. Agents Chemother. 2008;52(10):3786–3788. doi: 10.1128/AAC.00628-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim A.S., Gebremariam T., Schwartz J.A., Jr J.E.E., Spellberg B. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob. Agents Chemother. 2009;53(2):772–775. doi: 10.1128/AAC.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickerts V., Atta J., Herrmann S., Jacobi V., Lambrecht E., Bialek R. Successful treatment of disseminated mucormycosis with a combination of liposomal amphotericin B and posaconazole in a patient with acute myeloid leukaemia. Mycoses. 2006;49(Suppl 1):27–30. doi: 10.1111/j.1439-0507.2006.01299.x. [DOI] [PubMed] [Google Scholar]
- 19.Barg A.A., Malkiel S., Bartuv M., Greenberg G., Toren A., Keller N. Successful treatment of invasive mucormycosis with isavuconazole in pediatric patients. Pediatr. Blood Canc. 2018;65(10) doi: 10.1002/pbc.27281. [DOI] [PubMed] [Google Scholar]
- 20.Petrikkos G., Skiada A., Sambatakou H., Toskas A., Vaiopoulos G., Mucormycosis M.G. Ten-year experience at a tertiary-care center in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22(12):753–756. doi: 10.1007/s10096-003-1035-y. [DOI] [PubMed] [Google Scholar]