Abstract
In order to identify pigmented corn with nutraceutical potential, the secondary metabolite content, the antioxidant capacity and antimutagenic activity of red, and blue corn were analyzed. The ranges of total phenolic, flavonoid and anthocyanin contents of the corn samples were from 69.4 to 212.8 mg gallic ac. equiv./100 g DW, 0.07 to 12.19 mg (+) catechin eq./100 g DW and 3.89 to 34.17 mg cyanidin-3-O-glucoside eq./100 g DW, respectively. The phenolic extracts demonstrated the highest antioxidant capacity evaluated by the ABTS assay displaying values from 2.06 to 7.34 mmol Trolox/100 g DW. None of the extracts was toxic to the tested bacteria strains TA98 and TA100. For TA98 tester strain, percentage inhibition values against AFB1 mutagenicity from 61 to 93, and 38 to 75 for flavonoid and anthocyanin extracts were obtained. The total phenol and anthocyanin contents correlate with the observed antioxidant capacity. The most biological active corn samples were the blue color while the least actives were the red ones. The results show that the studied blue corn samples are good sources of antioxidant and antimutagenic compounds, which could use to develop products that contribute to human health.
Keywords: Antioxidant, Antimutagenic, Phenolics, Pigmented corn
Introduction
Pigmented corn has been proposed as a functional food considering that is rich in secondary metabolites that displayed biological activities. It is known that the purple and red colors of maize are due to the flavonoid content while the yellow color is produced by the presence of carotenoids. These compounds are mainly contained in the pericarp and aleurone layer of the corn. Several anthocyanins, flavonols and carotenoids have been identified in samples of pigmented corn, such as: cyanidin, pelargonidin, peonidin glucosides; protocatechuic, vanillic, p-coumaric, ferulic acids; quercetin and hesperetin derivatives, among others (Abdel-Aal et al. 2006; Del Pozo-Insfran et al. 2006; De la Parra et al. 2007; Mora-Rochín et al. 2016; Pedreschi and Cisneros-Zevallos 2006, 2007; Urias-Peraldí et al. 2013; Trehan et al. 2018). The antioxidant, antimutagenic and antiproliferative properties (Del Pozo-Insfran et al. 2006; Hagiwara et al. 2001; Pedreschi and Cisneros-Zevallos 2006) of purple corn extracts as well as the antiobesity and anti-hypertensive activities (Tsuda 2008; Shindo et al. 2007) have been the objective of several studies. A subchronic oral toxicity study with purple corn was performed on F344 rats; the study revealed that at the tested concentrations no adverse effects were observed (Nabae et al. 2008). Furthermore, Reynoso-Camacho et al. (2015) demonstrated that consumption of tortilla made with white and blue corns reduced 1,2-dimethyl hydrazine induced rat colon carcinogenesis.
Some of the biological properties displayed by purple corn have been assigned to the high concentration of anthocyanins. This fact has attracted the attention of the consumers since beneficial effects on human health such as protection to develop cancer, cardiovascular diseases, diabetes, allergy and mutagenesis have been demonstrated (Ghosh and Konishi 2007). However, pigmented corn also contains phenolic acids and flavonols that may as well contribute to the observed biological properties.
Systematic studies are important to identify corn genotypes with potential functional properties, and then the present study was aimed to chemically characterize pigmented genotypes of corn and to evaluate the antioxidant and antimutagenic properties.
Materials and methods
Chemicals
Solvents were either analytical or HPLC grade and were obtained from Baker (Mallinckrodt Baker, Inc., Phillipsburg, NJ, USA). 1,1-diphenyl-2-picrylhydrazyl (DPPH), Folin Ciocalteu reagent, 2,4,6-tripyridil-s-triazine (TPTZ), ferric chloride, FeSO4, 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), potassium persulfate, acetic acid, sodium acetate, (+)-catechin, gallic acid, ferulic acid, and cyanidine-3-glucoside were purchased from Sigma Chemical Co. (St. Louis MO, U.S.A.). The tester strains, TA100, TA98 and the microsomal fraction S9 were purchased from Molecular Toxicology Inc. (Annapolis, Md., USA).
Samples
Eleven genotypes of pigmented corn were collected at the localities of Huimilpan, San Juan del Río, El Márquez of the State of Querétaro, Mexico: 4 red and 7 purple. Dried kernels were separated and milled to a fine powder through a 60-mesh screen. All samples were store at − 20 °C and cover from light until use. Table 1 shows the sample nomenclature.
Table 1.
Total phenolic content, ferulic acid content and antioxidant activity of phenolic extracts
| Code | Total phenolic content mg GAE/100 g DW |
mg FA/100 g DW | mmol Trolox/100 g DW | mmol FeSO4/100 g DW |
|---|---|---|---|---|
| 1R | 212.84 ± 0.42 | 7.45 ± 0.12 | 7.34 ± 0.11 | 0.83 ± 0.01 |
| 2R | 81.59 ± 1.74 | 28.22 ± 1.81 | 4.88 ± 0.07 | 0.56 ± 0.01 |
| 3R | 103.87 ± 2.14 | 40.40 ± 0.23 | 3.84 ± 0.07 | 0.37 ± 0.01 |
| 4R | 116.82 ± 2.05 | 32.91 ± 0.08 | 4.62 ± 0.11 | 0.50 ± 0.01 |
| 5B | 98.91 ± 0.09 | 27.92 ± 0.40 | 2.06 ± 0.10 | 0.46 ± 0.01 |
| 6B | 91.41 ± 1.59 | 32.14 ± 1.58 | 3.65 ± 0.09 | 0.49 ± 0.02 |
| 7B | 130.38 ± 0.16 | 19.08 ± 1.49 | 4.22 ± 0.12 | 0.47 ± 0.00 |
| 8B | 108.67 ± 1.66 | 42.63 ± 1.08 | 3.30 ± 0.02 | 0.32 ± 0.01 |
| 9B | 98.45 ± 0.42 | 24.65 ± 0.39 | 4.51 ± 0.04 | 0.54 ± 0.01 |
| 10B | 79.35 ± 0.23 | 41.84 ± 1.36 | 3.11 ± 0.07 | 0.45 ± 0.02 |
| 11B | 69.43 ± 1.07 | 24.83 ± 0.44 | 2.65 ± 0.03 | 0.32 ± 0.01 |
Values are means ± S.D. of three independent experiments
R red corn, B blue corn
Quantification of phenolics
100 g of corn powder were covered with 100 mL of MeOH:CHCl3 (1:1) and stirred for three h. The solvent was filtered and the extraction was repeated two times. The recovered extract was concentrated under vacuum to dryness. The total phenolic content was determined according to the Folin–Ciocalteu colorimetric method (Dewanto et al. 2002). Briefly, appropriate dilutions of the extracts were oxidized with 250 μL of 1 N Folin–Ciocalteu reagent, after 5 min, 1.25 mL of a 20% Na2CO3 solution was added to neutralize for 2 h. The absorbance was measured against a prepared blank at 760 nm Results were expressed as mg of gallic acid equivalents per 100 g dried weight (GAE/100 g DW). To determine ferulic acid by HPLC, the phenolic extract was hydrolyzed (20 mg sample of corn powder was mixed with 100 mL 1.2 M HCl of a solution MeOH:H2O (50:50) for 2 h at 90 °C under reflux). Chromatographic conditions: λmax 280 nm; isocratic elution of 20% (v/v) acetonitrile in water adjusted with trifluoroacetic acid at a pH = 2 with a flow rate of 0.6 ml/min. Results were expressed as mg of ferulic acid per 100 g dried weight (FA/100 g DW).
Quantification of flavonoids
100 g of corn powder were covered with 100 mL of MeOH:H2O (7:3) and stirred for 3 h. The solvent was filtered and the procedure was repeated two times. The solvent was evaporated under vacuum. 10 mg of extract was dissolved in 1 mL of H2O and the total flavonoid content was determined by the method described by Sun et al. (2002). Briefly, appropriate dilutions of the extracts were mixed with 75 μL of a 5% NaNO2 solution. After 6 min, 150 μL of a 10% AlCl3·6H2O solution was added, and the mixture was allowed to stand for another 5 min. Then, 0.5 mL of 1 M NaOH was added, and distilled water was added to a total volume of 2.5 mL. The solution was well mixed, and the absorbance was measured against a prepared blank at 510 nm. The results were expressed as mg of (+)-catechin equivalents per 100 g of DW (CAE/100 g DW).
Quantification of anthocyanins
100 g of corn powder were covered with 100 mL of MeOH:H2O:Acetic acid (50:45:5) and stirred for 24 h cover from light and under nitrogen atmosphere. Thereafter, the solvent was filtered and the procedure repeated. The extract was evaporated under vacuum at a volume of 10 mL and then it was freeze-dry. The total anthocyanin content was determined by a conventional spectrophotometric method. 10 mg of sample were dissolved in 4 mL of 10% acetic acid aqueous solution and the absorbances were recorded at 535 nm. Total anthocyanin content was calculated as mg of cyanidin-3-O-glucoside equivalents per 100 g DW (CGE/100 g DW) using the extinction coefficient of 25,965 cmM−1 and the molecular weigh of 449.2 g/mol. All data were reported as mean ± standard deviation of three repetitions. Chromatographic conditions to quantify cyanidin-3-O-glucoside: λmax 520 nm; gradient elution of (A) MeOH, (B) 10% (v/v) formic acid in water at a flow rate of 1 mL/min. The gradient was programmed as follows: 0–20 min, 5–60%A, 95–40% B, 20–25 min, 60–100% A, 40–0% B; 25–30 min, 100% A; 30–40 min, 100–5% A, 0–95% B; 40–45 min, 5% A, 95% B. The results were expressed as mg of Cyanidine glucoside per 100 g of DW (CG/100 g DW).
Ferric reducing antioxidant power (FRAP)
FRAP values were obtained according to the method reported by Firuzi et al. (2005). Briefly, 25 μL of each extract (2.5 mg of extract was dissolved in 1 mL of MeOH except for the anthocyanin extracts which were dissolved in DMSO) was place in quadruplicate in a 96 well microplate (Nalge Nunc International, NY, USA). Then 175 μL of freshly prepared and warm (37 °C) FRAP solution was added to three of the wells and the same volume of acetate buffer was added to the fourth one. The absorbances at 595 nm were monitored by a Spectra Max Tunable Microplate Reader (Molecular Devices Co., Sunnyvale, CA, USA) at 0, 4, 10, 30 and 60 min. Blanks were prepared and a standard curve of FeSO4 was obtained. The results at 30 min were expressed as equivalents of FeSO4 per gram of extract. All data are reported as means ± standard deviation.
Trolox equivalent antioxidant capacity (TEAC)
The estimation of TEAC was performed using the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay described by Nenadis et al. (2004). The method was modified for 96 well microplates (Nalge Nunc International, NY, USA). Briefly, a 20 μL aliquot of the extracts was mixed with 230 μL of previously prepared ABTS•+ solution. The controls contained all the reaction reagents except the extract or Trolox. The absorbance was recorded at 730 nm at 0 and 6 min in a Spectra Max Tunable Microplate Reader (Molecular Devices Co., Sunnyvale, CA, USA). The radical scavenging activities were expressed as mmol Trolox/100 g DW.
Mutagenicity and antimutagenicity assay
The Kado microsuspension assay was used to evaluate the mutagenic and antimutagenic effect of the extracts (Kado et al. 1983). TA100 and TA98 Salmonella typhimurium strains and 0.25 µg/test tube of aflatoxin B1 (AFB1) mutagen were used. Extract solutions were tested (7 and 138 µM CAE for flavonoid extracts, 1.6 and 3.2 µM CGE for anthocyanin extracts). Samples were tested in triplicate for each independent experiment performed.
Statistical analyses
Results about active compounds in samples are given as means ± standard deviation of repeated measurements. To compare antimutagenic and antioxidant activities among the studied samples, statistical significance was determined by one-way variance analysis (ANOVA) (JMP v. 10.0.0). Star plots was created using package R version 2.15 (2010 The R Foundation for Statistical Computing www.project-r.org). Besides to explore nutraceutical potential, the so-called Partial Least Squares regression (Wold et al. 2001) was used to evaluate associations among biological effects and chemical contents.
Results and discussion
Phenolic extracts
The free phenolic extracts were assayed for total phenol content as well as the antioxidant activity, while the acid-hydrolyzed extracts were submitted to HPLC analysis for identification and quantification of ferulic acid, the most abundant phenolic acid in maize (Table 1). The ranges of total phenolic content and ferulic acid of the corn samples were from 69.4 to 212.8 mg GAE/100 g DW and 7.4–42.6 mg FA/100 g DW, respectively. The sample with the highest total phenol content, 1R, demonstrated the lowest content of ferulic acid. ABTS and FRAP assays were performed to measure the free radical scavenging activity and the total reducing power of the extracts, respectively (Table 1). The extract of sample 1R exerted the strongest free radical scavenging activity. FRAP values indicate the capacity of the extract to reduce Fe3+ to Fe2+ ions and the values ranged from 0.32 to 0.83 mmol FeSO4/100 g DW. Statistical speaking there is a significant correlation among phenolic components and antioxidant activity (p = 0.0033 and 0.0068 for ABTS and FRAP, respectively). Considering that the antioxidant evaluation was performed with the free phenolic extract of the sample, it is expected that the bounded phenols contribute to enhance the antioxidant activity of the studied samples.
It can be found a wide range of reported values for total phenolic content and antioxidant activity for pigmented corn samples. Del Pozo-Insfran et al. (2006) reported a total polyphenolic content of 45.1 mg/100 g DW and 29 µmol Trolox eq./g DW of antioxidant activity, evaluated by the ORAC method, for a Mexican blue corn genotype. Lopez-Martínez et al. (2009) demonstrated that Mexican red and blue corn genotypes had total phenol values of 283–617 and 465–3400 mg gallic ac. eq/100 g, respectively. These phenolic extracts at concentration of 0.01 mM of gallic ac. eq. demonstrated 25 to 100% and 40 to 100% free radical inhibition evaluated by DPPH and ABTS assay, respectively. Trehan et al. (2018) reported for purple corn samples contents of total phenols of 122–184 mg gallic ac. eq/100 g with antioxidant capacity of 0.40–0.48 μM Trolox/mg and 3.92–4.43 μM Trolox/mg for DPPH and ABTS, respectively.
The acid hydrolysis allowed the acids to be free from its ester form. Ferulic acid has been reported as one of the major phenolic compound in maize. Vanillic, p-hydroxybenzoic, protocatechuic, syringic, p-coumaric, caffeic and sinapic acids have been also identified in pigmented corn (Del Pozo-Insfran et al. 2006; Pedreschi and Cisneros-Zevallos 2007; Trehan et al. 2018), then it can be expected that sample 1R may contain some other phenolic acids that contribute with the observed antioxidant activity.
Although in this research the phenolic extracts were not assayed to evaluate the antimutagenic activity, Pedreschi and Cisneros-Zevallos (2006) have reported an 89.2% inhibition of mutagenic activity against Trp-P-1 on TA98 for an ethyl acetate phenolic fraction from Andean purple corn.
Flavonoid extracts
Table 2 shows the total flavonoid content values, which range from 0.07 to 12.19 mg CE/100 g DW. All blue corn samples showed higher flavonoid content compared to red corn samples. Regarding antioxidant capacity, most blue corn samples displayed higher antioxidant activity values for both assays, ABTS and FRAP. Sample 5B showed the highest antioxidant activity (0.47 ± 0.007 mmol Trolox/100 g DW and 0.19 ± 0.004 mmol FeSO4/100 g DW). The flavonoid extracts displayed lower antioxidant activity than the phenolic extracts. The total flavonoid content showed mild association with the observed antioxidant capacity for the FRAP assay (p = 0.0103).
Table 2.
Total flavonoid content, antioxidant and antimutagenic activities of flavonoid extracts
| Code | Total flavonoid content mg CE/100 g DW |
mmol Trolox/100 g DW | mmol FeSO4/100 g DW | TA98 | TA100 | ||
|---|---|---|---|---|---|---|---|
| Revertants/plate | Inhibition (%) | Revertants/plate | Inhibition (%) | ||||
| 1R | 0.32 ± 0.05 | 0.25 ± 0.00 | 0.12 ± 0.01 | 263 ± 14 | 71 | 492 ± 20 | 53 |
| 2R | 0.07 ± 0.01 | 0.24 ± 0.00 | 0.08 ± 0.00 | 65 ± 5 | 93 | 296 ± 17 | 72 |
| 3R | 0.58 ± 0.07 | 0.21 ± 0.01 | 0.09 ± 0.00 | 208 ± 11 | 77 | 451 ± 21 | 57 |
| 4R | 2.31 ± 0.30 | 0.28 ± 0.02 | 0.13 ± 0.00 | 83 ± 6 | 90 | 296 ± 15 | 69 |
| 5B | 5.55 ± 1.25 | 0.47 ± 0.01 | 0.19 ± 0.00 | 90 ± 15 | 89 | 314 ± 23 | 67 |
| 6B | 7.91 ± 0.27 | 0.29 ± 0.01 | 0.15 ± 0.00 | 189 ± 27 | 78 | 547 ± 13 | 43 |
| 7B | 10.71 ± 0.60 | 0.34 ± 0.01 | 0.16 ± 0.00 | 309 ± 19 | 64 | 526 ± 23 | 45 |
| 8B | 8.44 ± 0.25 | 0.38 ± 0.01 | 0.15 ± 0.00 | 172 ± 40 | 80 | 230 ± 13 | 76 |
| 9B | 12.19 ± 0.63 | 0.35 ± 0.00 | 0.16 ± 0.00 | 250 ± 10 | 71 | 359 ± 21 | 62 |
| 10B | 8.56 ± 0.46 | 0.28 ± 0.00 | 0.18 ± 0.00 | 194 ± 28 | 77 | 326 ± 11 | 66 |
| 11B | 5.72 ± 0.16 | 0.24 ± 0.01 | 0.11 ± 0.01 | 135 ± 21 | 84 | 310 ± 12 | 67 |
Revertant/plate of AFB1 mutagen 849 ± 57 and 942 ± 46 for TA98 and TA100 strains
R red corn, B blue corn
None of the flavonoid rich extracts was toxic to the tested bacteria since the spontaneous mutation was 19 ± 1 revertants/plate for tester strain TA98 and 131 ± 4 revertants/plate for tester strain TA100. A concentration of 138 µM eq. (+)-catechin of flavonoid extracts was used to test the inhibitory effects on AFB1 mutagenicity in both TA98 and TA100 tester strains (Table 2). Due to solubility constrains a concentration of 7 µM eq. (+)-catechin was used to evaluate samples 1R, 2R and 3R. For these samples, the extracts showed 61 to 93% inhibition against AFB1 mutagenicity on TA98 strain and 50 to 72% for TA100 strain. Among these three samples, sample 2R showed the highest antimutagenic effect on both bacteria strains. Samples evaluated at a concentration of 138 µM eq. (+)-catechin demonstrated inhibitory mutagenicity of 64% to 90% and 43% to 76% for TA98 and TA100, respectively. These results suggest that the extracts have higher potential to inhibit frameshift mutations in the genome of the tasted microorganism (TA98) than base changes mutations (TA100). Samples 4R and 8B showed the best antimutagenic effect for TA98 and TA100, respectively.
There are few reports regarding flavonoid information of corn samples. It has been reported concentrations of 2.14 mg CE/100 g DW for Mexican blue corn genotype (Del Pozo-Insfran et al. 2006) and quercetin and hesperitin derivatives were identified by HPLC from Andean purple corn (Pedreschi and Cisneros-Zevallos 2006). The antimutagenic activity of flavonoids depends on steric and electronic properties to penetrate into bacteria and bind specific membrane regions (Borges et al. 2010). Flavonoid glycosides exerted lower antimutagenicity than the corresponding aglycones and highly hydroxylated flavonoids observed strong antimutagenic properties against the mutagenicities induced by tetracyclic nitroarenes for Salmonella typhimurium TA98 (Edenharder and Tang 1997). More investigation is needed to determine the type and properties of the flavonoids contained in each extract to explain its antimutagenic potential.
Anthocyanin extracts
The total anthocyanin content of red and blue corn ranged from 3.89 to 34.17 mg CGE/100 g DW, respectively (Table 3). Sample 1R showed the lowest value for total anthocyanin content, while the sample 9B demonstrated the highest value. In addition, cyanidin-3-O-glucoside was determined by HPLC, finding trace of it on the red corn extracts. The blue corn extracts showed contents from 1.38 to 2.73 mg cyanidin-3-O-glucoside/100 g DW. The corn anthocyanin extracts demonstrated antioxidant capacity, values ranging from 0.05 to 0.17 mmol Trolox/100 g DW and 0.13 to 0.53 mmol FeSO4/100 g DW for ABTS and FRAP assay were observed. Samples 5B and 9B showed the highest antioxidant capacity. Statistically, the total content of anthocyanine showed significant association to antioxidant capacity for the FRAP assay (FRAP: p = 0.0011).
Table 3.
Total anthocyanin content, CG content, antioxidant and antimutagenic activities of anthocyanin extracts
| Code | Total anthocyanine content mg CGE/100 g DW | mg CG/100 g DW | mmol Trolox/100 g DW | mmol FeSO4/100 g DW | TA98 | TA100 | ||
|---|---|---|---|---|---|---|---|---|
| Revertants/plate | Inhibition (%) | Revertants/plate | Inhibition (%) | |||||
| 1R | 3.89 ± 0.03 | Tr | 0.15 ± 0.00 | 0.25 ± 0.01 | 436 ± 8 | 53 | 385 ± 33 | 54 |
| 2R | 10.77 ± 0.05 | Tr | 0.14 ± 0.00 | 0.37 ± 0.01 | 304 ± 13 | 67 | 338 ± 10 | 60 |
| 3R | 4.96 ± 0.07 | Tr | 0.05 ± 0.00 | 0.13 ± 0.00 | 366 ± 21 | 60 | 415 ± 6 | 50 |
| 4R | 12.09 ± 0.17 | Tr | 0.15 ± 0.01 | 0.34 ± 0.00 | 528 ± 18 | 43 | 532 ± 41 | 37 |
| 5B | 23.66 ± 0.21 | 2.73 ± 0.10 | 0.16 ± 0.01 | 0.53 ± 0.03 | 306 ± 14 | 67 | 498 ± 21 | 41 |
| 6B | 10.24 ± 0.10 | 1.38 ± 0.06 | 0.10 ± 0.00 | 0.26 ± 0.01 | 351 ± 5 | 62 | 420 ± 20 | 50 |
| 7B | 14.39 ± 0.08 | 1.57 ± 0.05 | 0.09 ± 0.00 | 0.27 ± 0.00 | 360 ± 18 | 61 | 597 ± 17 | 29 |
| 8B | 17.24 ± 0.19 | 1.66 ± 0.05 | 0.16 ± 0.00 | 0.32 ± 0.01 | 289 ± 13 | 69 | 419 ± 21 | 50 |
| 9B | 34.17 ± 0.19 | 6.29 ± 0.16 | 0.17 ± 0.01 | 0.52 ± 0.04 | 314 ± 19 | 66 | 512 ± 22 | 39 |
| 10B | 20.24 ± 0.07 | 3.51 ± 0.13 | 0.13 ± 0.00 | 0.35 ± 0.01 | 217 ± 14 | 76 | 467 ± 38 | 44 |
| 11B | 15.69 ± 0.15 | 1.89 ± 0.05 | 0.16 ± 0.00 | 0.28 ± 0.01 | 231 ± 12 | 75 | 426 ± 18 | 49 |
Revertant/plate of AFB1 mutagen 921 ± 14 and 838 ± 14 for TA98 and TA100 strains
R red corn, B blue corn, Tr trace
For the antimutagenic evaluation, red and blue corn extracts were evaluated at a concentration of 3.2 µM cyanidin-3-O-glucoside. % inhibitory values ranged from 43 to 76% and 29 to 60% for TA98 and TA100 strains, respectively. As in the case of the flavonoid rich extracts, these results suggest that the anthocyanine extracts have higher potential to inhibit frameshift mutations in the genome than base changes mutations. Extracts of samples 10B and 11B showed the highest antimutagenic effect for TA98 while sample 2R demonstrated the highest antimutagenic activity for TA100.
Cyanidin-3-O-glucoside has been identified as the major anthocyanin in blue corn samples, pelargonidin-3-O-glucoside and peonidin-3-O-glucoside have also identified (Mora-Rochín et al. 2016; Pedreschi and Cisneros-Zevallos 2006; Yang and Zhai 2010). The total anthocyanin content values of the studied samples are similar to the reported values of 9.75 and 36.87 mg CGE/100 g DW for red and blue corn samples, respectively (De la Parra et al. 2007) and 32.1 mg CGE/100 g DW for a Mexican blue corn genotype. However, the total anthocyanin values were lower compared to the reported values of Mexican red and blue corn genotypes of 85–154 mg/100 g 93–851 mg CGE/100 g, respectively (Lopez-Martinez et al. 2009).
The antioxidant properties of some anthocyanin extracts from corn samples have been addressed. Chinese purple corn demonstrated antioxidant activity with an IC50 = 40.1 and 38.6 µg/mL for DPPH and ABTS assays, respectively, and a FRAP value of 18.7 mmol FeSO4/g extract (Yang and Zhai 2010). Furthermore, a rich anthocyanin water fractions from Andean purple corn showed antioxidant activity of 1.019 µg of Trolox eq./µg of phenolics and a dose-dependent antimutagenic effect against Trp-P-1 with an IC50 value of 321.7 µg chlorogenic ac. eq./plate (Pedreschi and Cisneros-Zevallos 2007). A Mexican blue corn with 96.3 mg CGE/100 g showed 56% and 62% antimutagenic activity against the effect of 2-aminoanthracene on Salmonella typhimurium TA98 and TA100, respectively (Mendoza-Díaz et al. 2012). Here, the blue corn anthocyanine extracts (10.24–34.17 mg CGE/100 g DW) showed higher % antimutagenic properties against AFB1 on TA98 (61–75%) than TA100 (29–50%), the differences could be attributed to the method used to determine the antimutagenic activity as well as the different secondary metabolic content of the extract.
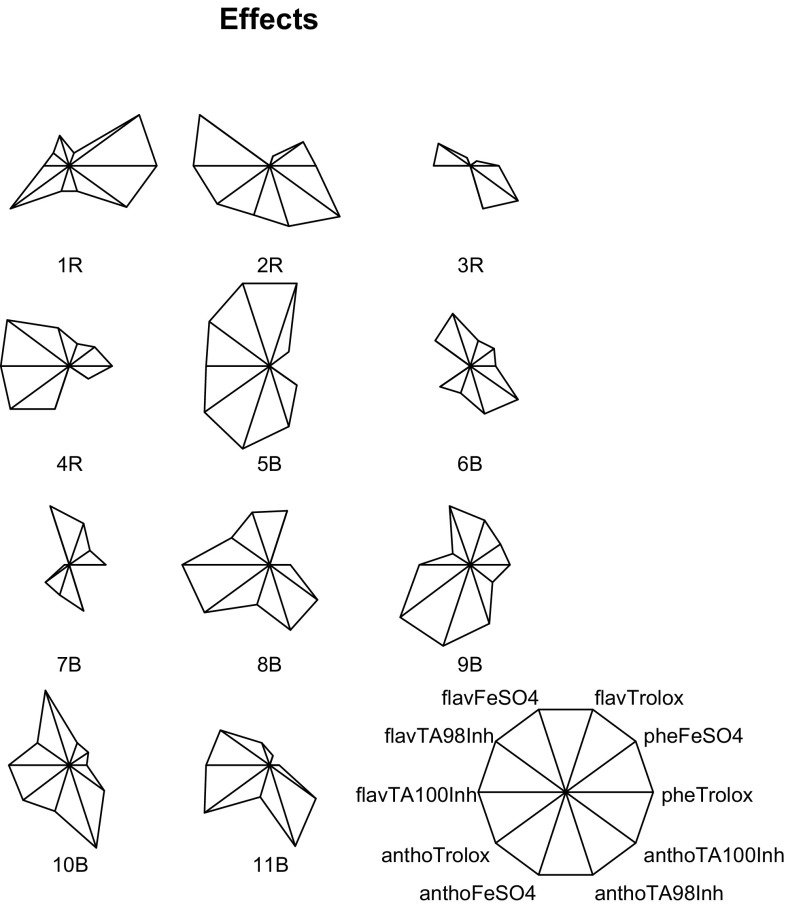
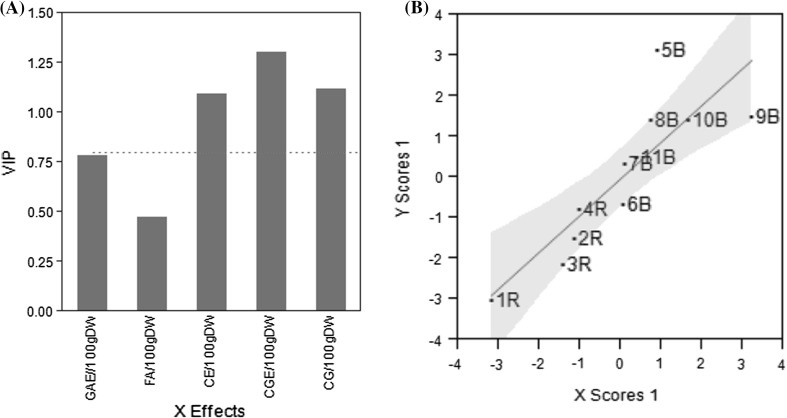
For purposes of having a multivariate perspective, descriptive schemes that include the secondary metabolite determinations (Fig. 1), the antioxidant capacity and antimutagenic activity (Fig. 2) of the corn samples, were developed. In a star plot, the length of each ratio-like line represents the relative value with respect to the observed maximum of a measured parameter. For example, in Fig. 1, line “FA/100 g DW” represents the ferulic acid content and it is the largest one for sample 10B and the smallest one for sample 1R. It means that samples 10B and 1R showed the highest and the lowest ferulic acid contents, respectively. It can be noticed that among the studied samples, sample 9B contains the richest secondary metabolite profile. Regarding biological activity, antioxidant and antimutagenic properties, sample 5B is the more biologically active (Fig. 2). The Partial Least Squares (PLS) regression was used to identify any association among the secondary metabolites of the corn samples and the biological evaluated effects. From the 5 evaluated chemical contents, only flavonoid and anthocyanine contents have impact on the biological activity (Fig. 3a), and the most biological active samples are 5B, 9B, and 10B while sample 1R is the least active (Fig. 3b).
Fig. 1.
Representative schemes of the secondary metabolite content of the extracts from pigmented corn
Fig. 2.
Representative schemes of the antimutagenic and antioxidant assays of the extracts from pigmented corn
Fig. 3.
A one factor solution by PLS was obtained. a Variable importance plot, bars greater than dotted red line are the factors that contribute to biological effects. b Sorting of samples by the PLS one factor solution (color figure online)
Conclusion
In this work, some red and blue pigmented corns are described with regard to their secondary metabolite content, antioxidant and antimutagenic properties. High concentrations of ferulic acid were found for both red and blue corn while cyanidin-3-O-glucoside content was prominent for blue corn. Furthermore, blue corn samples demonstrated to be good sources of antioxidant and antimutagenic compounds. These pigmented corns can be considered for scaling production in order to obtain natural colorants, bioactive extracts for pharmaceutical and cosmetic application and maize based products that contribute to human health.
Acknowledgements
This work was supported by the Mexican Council for Science and Technology (CONACyT), projects GTO-04-C02-68 and P50596-Q. The authors thank CONACyT for a graduate fellowship to M. Neri.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Aal ESM, Young JC, Rebalski I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J Agric Food Chem. 2006;54:4696–4704. doi: 10.1021/jf0606609. [DOI] [PubMed] [Google Scholar]
- Borges E, Ataide JP, Marinho TC, Couto M, Castro MM. Multivariate QSAR study on the antimutagenic activity of flavonoids against 3-NFA on Salmonella typhimurium TA98. Eur J Med Chem. 2010;45:4562–4569. doi: 10.1016/j.ejmech.2010.07.017. [DOI] [PubMed] [Google Scholar]
- De la Parra C, Serna Saldivar SO, Hai Liu R. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J Agric Food Chem. 2007;55:4177–4183. doi: 10.1021/jf063487p. [DOI] [PubMed] [Google Scholar]
- Del Pozo-Insfran D, Brenes CH, Serna Saldivar SO, Talcott ST. Polyphenolic and antioxidant content of white and blue corn (Zea mays L.) products. Food Res Int. 2006;39:696–703. doi: 10.1016/j.foodres.2006.01.014. [DOI] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Edenharder R, Tang X. Inhibition on the mutagenicity of 2-nitrofluorene, 3-nitrofluoranthene and 1-notropyrene by flavonoids, coumarins, quinones and other phenolic compounds. Food Chem Toxicol. 1997;35:357–372. doi: 10.1016/S0278-6915(97)00125-7. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys. 2005;1721:174–184. doi: 10.1016/j.bbagen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16:200–208. [PubMed] [Google Scholar]
- Hagiwara A, Miyashita K, Nakanishi T, Sano M, Tamano S, Kadota T, Koda T, Nakamura M, Imaida K, Ito N, Shirai T. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1,2-dimethylhydrazine. Cancer Lett. 2001;171:17–25. doi: 10.1016/S0304-3835(01)00510-9. [DOI] [PubMed] [Google Scholar]
- Kado NY, Langley D, Eisenstadt E. A simple modification of the Salmonella liquid-incubation assay. Increased sensitivity for detecting mutagens in human urine. Mutat Res. 1983;121:25–32. doi: 10.1016/0165-7992(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez LX, Oliart-Ros RM, Valerio-Alfaro G, Lee CH, Parkin KL, Garcia HS. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci Technol. 2009;42:1187–1192. doi: 10.1016/j.lwt.2008.10.010. [DOI] [Google Scholar]
- Mendoza-Díaz S, Ortíz-Valerio Ma C, Castaño-Tostado E, Figueroa-Cárdenas JD, Reynoso-Camacho R, Ramos-Gómez M, Loarca-Piña G. Antioxidant capacity and antimutagenic activity of anthocyanin and carotenoid extracts from nixtamalized pigmented creole maize races (Zea mays L.) Plant Food Hum Nutr. 2012;67:442–449. doi: 10.1007/s11130-012-0326-9. [DOI] [PubMed] [Google Scholar]
- Mora-Rochín S, Gaxiola-Cuevas N, Gutiérrez-Uribe JA, Milán-Carrillo J, Milán-Noris EM, Reyes-Moreno C, Serna-Saldivar SO, Cuevas-Rodríguez EO. Effect of traditional nixtamalization on anthocyanin content and profile in Mexican blue maize (Zea mays L.) landraces. LWT Food Sci Technol. 2016;68:563–569. doi: 10.1016/j.lwt.2016.01.009. [DOI] [Google Scholar]
- Nabae K, Hayashi SM, Kawabe M, Ichihara T, Hagiwara A, Tamano S, Tsushima Y, Uchida K, Koda T, Nakamura M, Ogawa K, Shirai T. A 90-day oral toxicity study of purple corn color, a natural food colorant, in F344 rats. Food Chem Toxicol. 2008;46:774–780. doi: 10.1016/j.fct.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Nenadis N, Wang LF, Tsimidou M, Zhang HY. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J Agric Food Chem. 2004;52:4669–4674. doi: 10.1021/jf0400056. [DOI] [PubMed] [Google Scholar]
- Pedreschi R, Cisneros-Zevallos L. Antimutagenic and antioxidant properties of phenolic fractions from Andean purple corn (Zea mays L.) J Agric Food Chem. 2006;54:4557–4567. doi: 10.1021/jf0531050. [DOI] [PubMed] [Google Scholar]
- Pedreschi R, Cisneros-Zevallos L. Phenolic profiles of Andean purple corn (Zea mays L.) Food Chem. 2007;100:956–963. doi: 10.1016/j.foodchem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Reynoso-Camacho R, Guerrero-Villanueva G, Figueroa J, Gallegos-Corona MA, Mendoza S, Loarca-Piña G, Ramos-Gomez M. Anticarcinogenic effect of corn tortilla against 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis in Sprague Dawley rats. Plant Food Hum Nutr. 2015;70:146–152. doi: 10.1007/s11130-015-0471-z. [DOI] [PubMed] [Google Scholar]
- Shindo M, Kasai T, Abe A, Kondo Y. Effects of dietary administration of plant-derived anthocyanin-rich colors to spontaneously hypertensive rats. J Nutr Sci Vitaminol. 2007;53:90–93. doi: 10.3177/jnsv.53.90. [DOI] [PubMed] [Google Scholar]
- Sun J, Chu YF, Wu XZ, Liu RH. Antioxidant and antiproliferative activities of vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Trehan S, Singh N, Kaur A. Characteristics of white, yellow, purple corn accessions: phenolic profile, textural, rheological properties and muffin making potential. J Food Sci Technol. 2018;55:2334–2343. doi: 10.1007/s13197-018-3171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agric Food Chem. 2008;56:642–646. doi: 10.1021/jf073113b. [DOI] [PubMed] [Google Scholar]
- Urias-Peraldí M, Gutiérrez-Uribe JA, Preciado-Ortiz RE, Cruz-Morales AS, Serna-Saldívar SO, García-Lara S. Nutraceutical profiles of improved blue maize (Zea mays) hybrids for subtropical regions. Field Crops Res. 2013;141:69–76. doi: 10.1016/j.fcr.2012.11.008. [DOI] [Google Scholar]
- Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst. 2001;58:109–130. doi: 10.1016/S0169-7439(01)00155-1. [DOI] [Google Scholar]
- Yang Z, Zhai W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.) Innov Food Sci Emerg Technol. 2010;11:169–176. doi: 10.1016/j.ifset.2009.08.012. [DOI] [Google Scholar]