Abstract
Background
Diabetic Foot Ulceration in patients with diabetes could be associated with high plantar pressure caused by diabetes neuropathy. Therefore, it seems that one of the ways of identifying high-risk legs in diabetic patients with neuropathy would be characterization of elevated plantar pressure distributions.
Objective
Comparing the plantar pressure distribution in diabetic patients who suffered neuropathy with those without neuropathy.
Methods and materials
Plantar pressure distribution was recorded in the following categories: 38 diabetic patients without neuropathy, 30, 40 and 34 patients with mild neuropathy, moderate and severe neuropathy respectively.
Results
Patients suffered from severe neuropathy suggested higher maximum peak plantar pressure at midfoot, heel, and medial forefoot. The peak pressure of midfoot was significantly different in the following categories as well: patient without neuropathy (32.3 ± 17.9 kPa), mild neuropathic (24.0 ± 17.9 kPa), moderate neuropathic (21.5 ± 12.6 kPa), and severe neuropathic (22.9 ± 10.7 kPa) groups (p = 0.02).
Conclusion
The progression of diabetic neuropathy would have been increased followed by the peak plantar pressure.
Keywords: Biomechanics, Diabetes mellitus, Neuropathy, Plantar pressure
Introduction
It is estimated that in 2030 the number of patients with diabetes worldwide will exceed 365 million [1]. Cardiovascular disease, neuropathy, nephropathy, and retinopathy are among various complications of diabetes mellitus. Peripheral neuropathy is a leading cause of morbidity in these patients. With increase in the prevalence of obesity and associated diabetes mellitus, the frequency of symptomatic diabetic neuropathy is growing. It is believed that about 50% of patients with diabetes will have diabetic peripheral neuropathy in 10 to 15 years [2]. The prevalence of diabetic neuropathy is also increased with poor diabetes control [3–5]. Patients with diabetes mellitus 1 may also suffer from severe diabetic neuropathy in the first years after diagnosis if their blood sugar is poorly controlled [6].
In the course of peripheral neuropathy, peripheral nerves are progressively degenerated especially in inferior extremities, which can lead to motor and sensory deficits affecting biomechanics of diabetic foot. One of these effects is on the plantar pressure distribution [7–9], which in combination with sensory defect are mainly responsible for development of ulcer in patients with diabetic neuropathy [2, 10]. Changes in ankle [2, 11, 12] and gait kinetics [13–15] are other consequences of diabetic neuropathy. Previous studies showed that increased plantar pressure in patients with diabetic neuropathy is related to foot ulceration [16–23], although some authors failed to show increased peak plantar pressure in patients with diabetic foot ulcer [24]. However, no study has evaluated plantar pressures in patients with different stages of neuropathy but without ulceration. Since worsening neuropathy could be a predictive of diabetic ulcer development study; the aim of this study was to investigate the distribution of plantar pressure in three groups of patients with different stages of neuropathy and to compare them with a control group of diabetic patients without neuropathy.
Methods and materials
After ethics committee approval, this cross-sectional study was carried out between November 2014 and June 2016 in Shariati Hospital, a university hospital in Tehran, Iran.
Study population and design
Patients with diabetes mellitus type 2 with or without peripheral neuropathy presenting to the Diabetes Clinic of Shariati Hospital were assessed for eligibility. The diagnosis of diabetes mellitus was made by reviewing medical records of patients and those between the ages 30 and 60 years with a history of more than 2 years since diagnosis of diabetes were enrolled into the study after obtaining informed consent about the study procedure. The exclusion criteria were the ages younger than 30 and older than 60 years, use of any kind of walking assist devices, history of foot ulcer or present ulcer, Charcot arthropathy confirmed by radiography, lower limb amputation, and use of medicines for treatment of neuropathy.
According to a study by Bacarin et al. [18], entitled “distribution of plantar pressure during walking in diabetic patients suffering neuropathy, considering α = 05.05, β = 0.02, SD1 = 78/4, SD2 = 118/5, d = 60 and regarding to the following formula, the sample size in each group was estimated to be about 20 patients.
In our study, the minimum number of samples in each group was 30 patients.
Subject selection was continued until the desired sample size was achieved. Total number of 71 patients enrolled into the study was divided into 4 groups: diabetic control patients without neuropathy (DC, n = 38), with mild neuropathy (Mi, n = 30), moderate neuropathy (Mo, n = 40), and severe neuropathy (S, n = 34) (Table 1). The diabetic control group was consisted of patients who were matched with neuropathic patients for anthropometric characteristic such as weight, body mass index, and age. Michigan Neuropathy Screening Instrument questionnaire (MNSI-q) was used for allocation of patients into the study groups. MNSI consists of two parts. The first part was related to the patient’s history including 15 self-administered questions on foot sensation. The second part was a following brief physical assessment which completed by health professionals: feet inspection (for deformities, dry skin, hair or nail abnormalities, callous or infection), vibration sensation screening at the dorsum of the great toe, ankle reflexes grading and monofilament testing.
Table 1.
Demographic features of study population
| No neuropathy | Mild neuropathy | Moderate neuropathy | Severe neuropathy | |
|---|---|---|---|---|
| n | 38 | 30 | 40 | 34 |
| Age (years) | 53.8 ± 8.5 | 52.7 ± 6.6 | 54.0 ± 6.8 | 54.4 ± 8.0 |
| M/F (% male) | 24/14 (63.2%) | 20/10 (66.7%) | 14/26 (35.0%) | 16/18 (47.1%) |
| BMI (kg/m2) | 27.0 ± 4.4 | 27.7 ± 3.2 | 28.0 ± 4.0 | 27.2 ± 5.8 |
| Diabetes duration (years) | 5.7 ± 2.9 | 7.6 ± 6.9 | 8.2 ± 5.0 | 10.8 ± 5.7* |
Data are means ± SD, unless otherwise indicated. *P < 0.001 vs non-neuropathic group (Bonferroni method)
Study procedure
Data collection began with anthropometric and demographic data and information on duration of the disease. All the clinical examinations were performed by a single examiner to preserve consistency and repeatability and to avoid inter-observational variations. All the patients were then asked to answer the MNSI-q, which includes questions about common sensory symptoms of diabetic neuropathy. …..Plain radiographs of foot were obtained from all subjects and reviewed by an expert radiologist to ensure absence of Charcot arthropathy.
Plantar pressure distribution was assessed using Foot Pressure SN GP MultiSence 4–2008-703 (Gebiom mbH munster). First, subjects stood on the device with barefoot to measure overall status of plantar pressure in a static fashion. Then, dynamic pressures were measured for both feet by instructing the patients to walk on the surface of the device. Peak plantar pressures in different areas of foot were then recorded. We divided the plantar surface into 5 areas: heel, midfoot, lateral forefoot, medial forfoot, and hallux (Fig. 1).
Fig. 1.
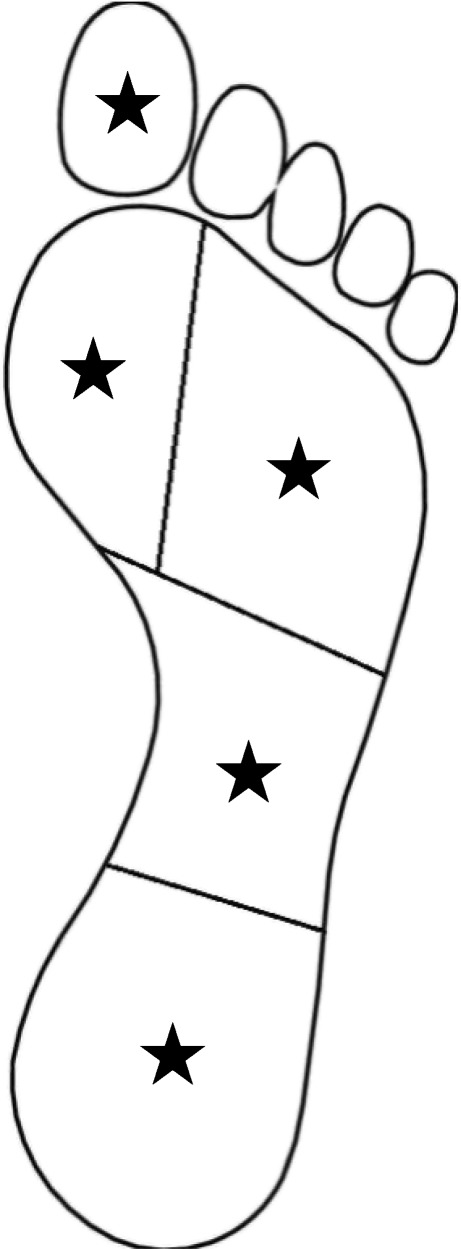
Areas of palmar surface: heel (27% of foot length), midfoot (28% of foot length), lateral forefoot (65% of foot width), medial forfoot (35% of foot width), and hallux (final 20% of length with 33% of foot width)
Before executing measurements, the subjects walked freely in the department in order to reproduce their typical gait for about 3 min. Several training runs were performed to familiarize subjects with the system.
Statistical analysis
IBM SPSS statistical software version 21 was used for the statistical analysis. Means and percentages were used to describe data. Distribution of data was evaluated by Kolmogrov-Smrinov test. For parametrically distributed data, one-way ANOVA was used followed by the Sheffer’s Post Hoc test. Moreover Fischer’s exact test used to identify differences among the various groups. Kruskal-Wallis test was used for non-parametrically distributed data. In the current study, the first type errors less than 0.05 were considered acceptable.
Results
The demographic features of study population are summarized in Table 1. The group with severe neuropathy had a longer duration of diabetes than their counterparts with no neuropathy. No statistically significant differences were found regarding other features between study groups.
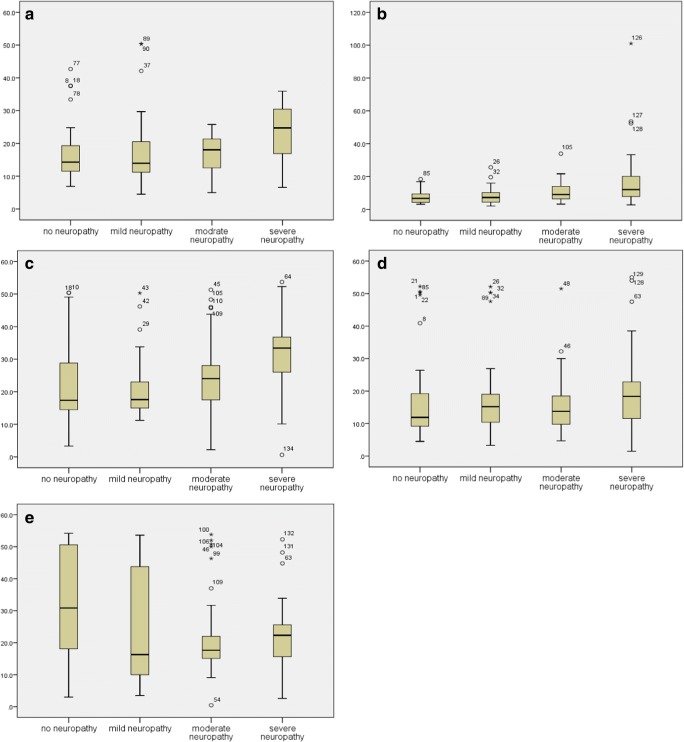
The peak plantar pressures are shown in Table 2. Peak pressure was significantly different between severe neuropathic group versus other groups for the midfoot (p < 0.05). Severe neuropathy group had higher values than diabetic patients without neuropathy in 3 of 5 areas: heel, midfoot, and medial forefoot (p < 0.05). In lateral forefoot, no difference was found among different groups. Figure 2 shows boxplot graphs of peak plantar pressure in diabetic patients without and with mild, moderate, and severe neuropathy.
Table 2.
Peak plantar pressure (kPa)
| No neuropathy | Mild neuropathy | Moderate neuropathy | Severe neuropathy | |
|---|---|---|---|---|
| Heel | 16.8 ± 8.5 | 18.0 ± 11.8 | 16.9 ± 5.5 | 23.5 ± 8.2*‡ |
| Midfoot | 7.8 ± 4.2 | 8.2 ± 5.3 | 10.8 ± 6.2 | 18.3 ± 19.0*†‡ |
| Lat. forefoot | 17.5 ± 13.4 | 18.6 ± 13.4 | 15.1 ± 8.6 | 19.0 ± 13.4 |
| Med. forefoot | 23.0 ± 12.6 | 20.7 ± 9.7 | 25.1 ± 11.0 | 12.1 ± 25.8*† |
| Hallux | 32.3 ± 17.9 | 24.0 ± 17.9* | 21.5 ± 12.6 | 22.9 ± 10.7 |
Data are means ± SD. *P < 0.05 vs non-neuropathic group, †P < 0.05 vs mild neuropathy group, ‡P < 0.05 vs moderate neuropathy group (Bonferroni method)
Fig. 2.
Peak plantar pressure in diabetic patients without and with mild, moderate, and severe neuropathy for different areas of palmar surface: a heel, b midfoot, c medial forefoot, d lateral forefoot, and e hallux
Discussion
The combination of high plantar pressure and sensory defect is responsible for development of ulcer in diabetic patients with neuropathy [2, 10]. Increased plantar pressure is described in neuropathic patients and has been showed to be related with foot ulcer [16, 17, 22]. Most studies describing foot pressure point towards peak plantar pressures without indicating the area under which pressure is measured. Many studies report peak plantar pressures in anterior region of foot, because neuropathic ulcers commonly occur in the area. Moreover, previous studies have not evaluated plantar pressures in diabetic patients with different stages of neuropathy yet without foot ulcer [16, 17, 21, 22, 25, 26].
Sensory defect, foot deformities, decreased joint mobility, presence of callus and decreased thickness of plantar tissue have been related to high plantar pressure [9, 27–29]. Proper foot design for reducing increased plantar pressure is an active area of research [30, 31]. The knowledge about different affecting variables including plantar pressure is necessary to achieve the best design in order to reduce probability of foot ulcer formation [32]. Considering the limitations of previous studies, the current study aims to evaluate peak plantar pressures for different areas of plantar surface in diabetic patients without and with mild, moderate, and severe neuropathy.
For the heel and medial forefoot areas, peak plantar pressures in severe neuropathic group was higher than non-neuropathic and mild neuropathic groups in the current study. The most significant differences among groups were found in the midfoot. In this area, severe neuropathy group had higher pressures than other groups of study. Interestingly, for the hallux area, plantar pressure was higher in non-neuropathic group than moderate neuropathy group. There was no significant difference regarding peak plantar pressure for lateral forefoot area.
The results of studies about plantar pressures are so different. The reasons for this variability includes how participating patients were selected regarding disease severity, presence or absence of peripheral neuropathy, history of foot ulcer, and the tool used for investigations. In 2002, Caselli et al. [20] showed that patients with diabetic neuropathy had higher plantar pressures than non-neuropathic patients with diabetes in both anterior and posterior areas of plantar surface. Later, some studies found that in patients with diabetic neuropathy the point of the maximum plantar pressure is matched with the maximum stress entered in inferior surface of foot in just 20% of occasions [33].
The results of a study carried out by Bacarin et al. [18] showed that plantar pressure in the group with neuropathy but without ulcer is higher than diabetic controls without neuropathy for the midfoot and rearfoot areas. The most significant difference was for the midfoot, a result that has been confirmed by our study. Charcot arthropathy in neuropathic patients has been ruled out from the current study and absence of the condition was confirmed by performing plain radiographies. Thus, higher peak pressures for the midfoot in patients with more severe neuropathy cannot be associated with Charcot condition or longitudinal arch descend in neuropathic patients. The explanation for higher plantar pressure in this area can be made by loading pattern shift from lateral part of the foot to more medial parts, which is more obvious in patients with more severe neuropathy [34]. In the study performed by Sinacore et al. [35], authors found changes in plantar pressure of the midfoot in patients with neuropathy. They suggested the possibility of presence of polymorphisms not detectable with plain radiographs which can lead to increased load under the midfoot [35].
Under the hallux, more severe neuropathy groups showed no higher peak pressures than less severe neuropathy groups, but surprisingly, patients without neuropathy had higher pressures than moderate neuropathy group. Bacarin et al. [18] found that in the hallux area, there was no difference between diabetic controls, non-neuropathics and patients with diabetic foot ulcer regarding both peak pressure and pressure-time integral. The authors showed the less differences among groups in the area [18]. In our study, the less differences among groups was also found in 2 areas: hallux and lateral forefoot.
Neuromuscular compensatory mechanisms that develop in neuropathic patients to compensate their sensory deficit lead to changes in roll-over mechanisms of foot [36]. Fang et al. [37] suggested that peripheral neuropathy plays an important role in the disturbance of plantar pressure distribution in patients with diabetic foot. Presence of abnormality in foot physical features due to neuropathy is associated with disorder of sensory conduction of sural nerve, motor conduction of common peroneal nerve, and involvement of extensor muscles. Reversely, it can be stated that disturbance of plantar pressure distribution is an indicator of development of neuropathic disorder in patients with diabetes [37]. Syed et al. [38] also showed that in the absence of neuropathy, there is no significant difference regarding plantar pressures between patients with diabetes and healthy controls.
Age, duration of diabetes, increased blood sugar, retinopathy and history of plantar ulcer are among risk factors for formation of peripheral neuropathy [39]. The prevalence and pattern of peripheral neuropathy is different in various countries and varies from 1.5 to 100% in patients with type 2 diabetes mellitus. In general, there is a positive and direct relationship between the severity of peripheral neuropathy and duration of DM type 2 [40]. Although there was no statistically significant difference among study groups, but the means for diabetes duration were higher in patients with more severe neuropathy. It’s known for years that diabetic neuropathy is a chronic outcome of diabetes mellitus and the risk of ulcer formation increases with time [2].
In our study, no significant difference was found among the groups regarding body mass index. Previous studies showed higher body mass indices in diabetic patients with neuropathy than non-neuropathic controls [20]. Shen et al. [41] reported that each unit increase in body mass index of patients with diabetes leads to 5.96 kPa increase of plantar pressure. Ethnic differences can be the probable explanation for dissimilarity of previous studies with the current study.
It is important to point out other factors that can interfere with plantar pressure distribution. These factors include foot deformities [7] and movements of foot and ankle joint complex [9, 21, 42]. Diabetic neuropathy leads to progressive changes in muscular trophism, especially in foot and ankle intrinsic muscles, increase in joint rigidity, and changes in collagenous structures in fasciae and tendons due to cross-connection and non-enzymatic glycosylation of creatine. This means that the structures of muscles, cartilages, tendons, and ligaments are being altered which results in limitations of foot mobility [9, 42, 43]. The study did not evaluate factors related to join mobility. Although, we excluded subjects with obvious foot deformity or Charcot arthropathy confirmed by readiographs. Moreover, studies regarding the relationship between less passive or dynamic range of motion of foot joint and higher plantar pressure are not so clear. In a study carried out by Turner et al. [44] the author showed that despite significant decrease in passive range of motion of ankle joint complex in diabetic patients, the range of motion at the time of walking in patients with diabetes is not distinguishable from healthy subjects and was not related to the plantar pressure parameters [44].
We only used peak plantar pressures to compare different groups regardless of measuring pressure-time integral. The reason was as follows: first, assessment of pressure-time integral was not feasible with the system used in our study; second, Waaijman et al. [45] investigated the correlation between the variables peak plantar pressure and pressure-time integral in diabetic patients with different footwear and showed that the 2 parameters are directly correlated in common areas for development of diabetic foot ulcer. They also reported that peak plantar pressure is clinically more relevant parameter and value of reporting pressure-time integral added to peak pressure is low.
Conclusion
Our investigations showed that groups with more severe neuropathy had different plantar pressure distribution than less severe neuropathy groups. The peak plantar pressures were higher in the midfoot, heel, and medial forefoot areas in patients with severe neuropathy and the most significant differences among groups were seen in the midfoot. Evaluation of plantar pressure in patients with diabetic neuropathy is of importance and the results of such assessments can lead to the detection of points at risk for development of ulcer in the plantar surface of foot to take proper measures for preventing ulcer formation.
Acknowledgements
This project was supported by Endocrinology and Metabolism Research Institute. We gratefully acknowledge the substantial contribution of all scientific and executive personnel of this institution. We would also like to extend our sincere thanks to all the diabetic patients who participated in this research project.
Compliance with ethical standards
Conflict of interest
None declared.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh PR, Simoneau GG, Ulbrecht JS. Ulceration, unsteadiness, and uncertainty: the biomechanical consequences of diabetes mellitus. J Biomech. 1993;26:23–40. doi: 10.1016/0021-9290(93)90077-R. [DOI] [PubMed] [Google Scholar]
- 3.Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29(2):340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Control D, Group CTR The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978;1(3):168–188. doi: 10.2337/diacare.1.3.168. [DOI] [Google Scholar]
- 6.Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007;3(6):331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 7.Bus SA, Maas M, de Lange A, Michels RP, Levi M. Elevated plantar pressures in neuropathic diabetic patients with claw/hammer toe deformity. J Biomech. 2005;38(9):1918–1925. doi: 10.1016/j.jbiomech.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Payne C, Turner D, Miller K. Determinants of plantar pressures in the diabetic foot. J Diabetes Complicat. 2002;16(4):277–283. doi: 10.1016/S1056-8727(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 9.Fernando DJ, Masson EA, Veves A, Boulton AJ. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Diabetes Care. 1991;14(1):8–11. doi: 10.2337/diacare.14.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Cavanagh PR, Ulbrecht JS. Disorders of the foot and ankle: medical and surgical management. 1991. Biomechanics of the diabetic foot: a quantitative approach to the assessment of neuropathy, deformity and plantar pressure; pp. 1864–1907. [Google Scholar]
- 11.Kwon O-Y, Minor SD, Maluf KS, Mueller MJ. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture. 2003;18(1):105–113. doi: 10.1016/S0966-6362(02)00166-2. [DOI] [PubMed] [Google Scholar]
- 12.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74(4):299–308. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 13.Maluf K, Mueller M, Strube M, Engsberg J, Johnson J. Tendon Achilles lengthening for the treatment of neuropathic ulcers causes a temporary reduction in forefoot pressure associated with changes in plantar flexor power rather than ankle motion during gait. J Biomech. 2004;37(6):897–906. doi: 10.1016/j.jbiomech.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JE, Van Schie C, Carrington AL, Abbott CA, Boulton A. An analysis of dynamic forces transmitted through the foot in diabetic neuropathy. Diabetes Care. 1998;21(11):1955–1959. doi: 10.2337/diacare.21.11.1955. [DOI] [PubMed] [Google Scholar]
- 15.Sacco I, Amadio A. Influence of the diabetic neuropathy on the behavior of electromyographic and sensorial responses in treadmill gait. Clin Biomech. 2003;18(5):426–434. doi: 10.1016/S0268-0033(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 16.Stess RM, Jensen SR, Mirmiran R. The role of dynamic plantar pressures in diabetic foot ulcers. Diabetes Care. 1997;20(5):855–858. doi: 10.2337/diacare.20.5.855. [DOI] [PubMed] [Google Scholar]
- 17.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21(10):1714–1719. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- 18.Bacarin TA, Sacco IC, Hennig EM. Plantar pressure distribution patterns during gait in diabetic neuropathy patients with a history of foot ulcers. Clinics. 2009;64(2):113–120. doi: 10.1590/S1807-59322009000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokes I, Faris I, Hutton W. The neuropathic ulcer and loads on the foot in diabetic patients. Acta Orthopaedica Scandinavica. 2009. [DOI] [PubMed]
- 20.Caselli A, Pham H, Giurini JM, Armstrong DG, Veves A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care. 2002;25(6):1066–1071. doi: 10.2337/diacare.25.6.1066. [DOI] [PubMed] [Google Scholar]
- 21.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23(5):606–611. doi: 10.2337/diacare.23.5.606. [DOI] [PubMed] [Google Scholar]
- 22.Veves A, Murray H, Young M, Boulton A. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35(7):660–663. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- 23.Rich J, Veves A. Forefoot and rearfoot plantar pressures in diabetic patients: correlation to foot ulceration. WOUNDS-A COMPENDIUM OF CLINICAL RESEARCH AND PRACTICE. 2000;12(4):82–87. [Google Scholar]
- 24.Reiber GE, Smith DG, Wallace C, Sullivan K, Hayes S, Vath C, et al. Effect of therapeutic footwear on foot reulceration in patients with diabetes: a randomized controlled trial. JAMA. 2002;287(19):2552–2558. doi: 10.1001/jama.287.19.2552. [DOI] [PubMed] [Google Scholar]
- 25.Lavery LA, Vela SA, Fleischli JG, Armstrong DG, Lavery DC. Reducing plantar pressure in the neuropathic foot: a comparison of footwear. Diabetes Care. 1997;20(11):1706–1710. doi: 10.2337/diacare.20.11.1706. [DOI] [PubMed] [Google Scholar]
- 26.Sarnow MR, Veves A, Giurini JM, Rosenblum BI, Chrzan JS, Habershaw GM. In-shoe foot pressure measurements in diabetic patients with at-risk feet and in healthy subjects. Diabetes Care. 1994;17(9):1002–1006. doi: 10.2337/diacare.17.9.1002. [DOI] [PubMed] [Google Scholar]
- 27.Abouaesha F, van Schie CH, Griffths GD, Young RJ, Boulton AJ. Plantar tissue thickness is related to peak plantar pressure in the high-risk diabetic foot. Diabetes Care. 2001;24(7):1270–1274. doi: 10.2337/diacare.24.7.1270. [DOI] [PubMed] [Google Scholar]
- 28.Schoenhaus H, Wernick E, Cohen R. Biomechanics of the diabetic foot. The high risk foot in diabetes mellitus. 1991;125.
- 29.Young M, Cavanagh P, Thomas G, Johnson M, Murray H, Boulton A. The effect of callus removal on dynamic plantar foot pressures in diabetic patients. Diabet Med. 1992;9(1):55–57. doi: 10.1111/j.1464-5491.1992.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 30.Mueller MJ. Application of plantar pressure assessment in footwear and insert design. J Orthop Sports Phys Ther. 1999;29(12):747–755. doi: 10.2519/jospt.1999.29.12.747. [DOI] [PubMed] [Google Scholar]
- 31.Cavanagh PR, Owings TM. Nonsurgical strategies for healing and preventing recurrence of diabetic foot ulcers. Foot Ankle Clin. 2006;11(4):735–743. doi: 10.1016/j.fcl.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Am Podiatr Med Assoc. 2010;100(5):360–368. doi: 10.7547/1000360. [DOI] [PubMed] [Google Scholar]
- 33.Yavuz M, Erdemir A, Botek G, Hirschman GB, Bardsley L, Davis BL. Peak plantar pressure and shear locations relevance to diabetic patients. Diabetes Care. 2007;30(10):2643–2645. doi: 10.2337/dc07-0862. [DOI] [PubMed] [Google Scholar]
- 34.Giacomozzi C, Caselli A, Macellari V, Giurato L, Lardieri L, Uccioli L. Walking strategy in diabetic patients with peripheral neuropathy. Diabetes Care. 2002;25(8):1451–1457. doi: 10.2337/diacare.25.8.1451. [DOI] [PubMed] [Google Scholar]
- 35.Sinacore DR, Bohnert KL, Hastings MK, Johnson JE. Mid foot kinetics characterize structural polymorphism in diabetic foot disease. Clin Biomech. 2008;23(5):653–661. doi: 10.1016/j.clinbiomech.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacco I, Amadio A. A study of biomechanical parameters in gait analysis and sensitive cronaxie of diabetic neuropathic patients. Clin Biomech. 2000;15(3):196–202. doi: 10.1016/S0268-0033(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 37.Fang F, Wang Y, Gu M, Chen H, Wang D, Xiao K, et al. Pedobarography-a novel screening tool for diabetic peripheral neuropathy. Eur Rev Med Pharmacol Sci. 2013;17(23):3206–3212. [PubMed] [Google Scholar]
- 38.Syed N, Maiya A, Hanifa N, Goud S. Plantar pressures in diabetes with no known neuropathy. Journal of diabetes. 2013;5(3):302–308. doi: 10.1111/1753-0407.12016. [DOI] [PubMed] [Google Scholar]
- 39.Adler A. Risk factors for diabetic neuropathy and foot ulceration. Current diabetes reports. 2001;1(3):202–207. doi: 10.1007/s11892-001-0035-5. [DOI] [PubMed] [Google Scholar]
- 40.Taylor AJ, Menz HB, Keenan A-M. Effects of experimentally induced plantar insensitivity on forces and pressures under the foot during normal walking. Gait Posture. 2004;20(3):232–237. doi: 10.1016/j.gaitpost.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Liu F, Zeng H, Wang J, Zhao J-G, Zhao J, et al. Vibrating perception threshold and body mass index are associated with abnormal foot plantar pressure in type 2 diabetes outpatients. Diabetes Technol Ther. 2012;14(11):1053–1059. doi: 10.1089/dia.2012.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salsich GB, Mueller MJ, Sahrmann SA. Passive ankle stiffness in subjects with diabetes and peripheral neuropathy versus an age-matched comparison group. Phys Ther. 2000;80(4):352–362. doi: 10.1093/ptj/80.4.352. [DOI] [PubMed] [Google Scholar]
- 43.Gefen A. Plantar soft tissue loading under the medial metatarsals in the standing diabetic foot. Med Eng Phys. 2003;25(6):491–499. doi: 10.1016/S1350-4533(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 44.Turner D, Helliwell PS, Burton AK, Woodburn J. The relationship between passive range of motion and range of motion during gait and plantar pressure measurements. Diabet Med. 2007;24(11):1240–1246. doi: 10.1111/j.1464-5491.2007.02233.x. [DOI] [PubMed] [Google Scholar]
- 45.Waaijman R, Bus S. The interdependency of peak pressure and pressure–time integral in pressure studies on diabetic footwear: no need to report both parameters. Gait Posture. 2012;35(1):1–5. doi: 10.1016/j.gaitpost.2011.07.006. [DOI] [PubMed] [Google Scholar]