Abstract
Background
Considering the aging population associated with higher osteoporotic fracture risk, high prevalence of diabetes and its effect on bone health along with lack of information on bone quality using common methods (BMD) the aim of present study was to determine the association between trabecular bone score (TBS) and diabetes in an elderly population participating in Bushehr Elderly Health (BEH) program.
Materials and methods
This cross-sectional study was performed on data collected during the BEH Program, stage II. Anthropometric indices were measured based on NHANES III protocol. Diabetes and pre-diabetes were defined according to ADA Guideline 2018. Bone density was measured using DXA method (DXA, Discovery WI, Hologic Inc., USA). A software installed on the same device (TBS iNsight® software) was applied to assess TBS values. Variables related to bone health were compared based on their glycemic status (participants with diabetes, participants with prediabetes, and normoglycemic) using analysis of variance. Univariate and multivariate linear and logistic regression models were used to determine the association between TBS values and bone density in different glycemic states.
Results
The data of 2263 participant aged 60 years and over were analyzed. Mean TBS values were significantly different between participants with diabetes, participants with prediabetes, and normoglycemic groups (P = 0.004;, however, P trend was not significant (0.400)). Mean BMD values at femoral neck and lumbar spine were significantly higher in diabetics compared with those diagnosed with pre-diabetes; the latter also had higher bone density compared with normoglycemic individuals (both P ANOVA test and P trends for means were < 0.01]. In univariate linear regression model, TBS values were negatively associated with pre-diabetes (β = −0.070; P < 0.001) but not with diabetes (β = −0.002, P = 0.915). This significant relationship disappeared when the results were adjusted for BMI. In fully adjusted multivariate logistic regression models, odds ratio linking pre-diabetes and diabetes with spinal osteoporosis was 0.861 (CI 95% 0.670–1.105) and 0.525 (CI 95% 0.392–0.701), respectively. As for femoral osteoporosis, odds ratio was 0.615 (CI 95% 0.407–0.928) and 0.968 (CI 95% 0.629–1.489), correspondingly. Moreover, for cumulative osteoporosis, the odds were 0.843 (CI 95% 0.676–1.106) and 0.551 (CI 95% 0.415–0.732), respectively.
Conclusion
Our findings suggest that subjects with pre-diabetes and diabetes have higher bone mineral density than normoglycemic subjects; the quality of bone, however, was not different between them. The discordance between BMD and TBS values in participants with diabetes suggest that although these patients have higher BMD values, their quality of bone microarchitecture may not be better than normoglycemic subjects.
Keywords: Pre-diabetes, Diabetes, Bone mineral density, Trabecular bone sore, Aged
Introduction
While diabetes and osteoporosis are two seemingly unrelated health conditions, recent studies have demonstrated osteoporotic fractures to be more prevalent among diabetic individuals [1–4].
Several studies have linked type 2 diabetes with higher bone mineral density (BMD) values in both men and women [5, 6]. This is while increased risk of fractures is seen in patients with type one and two diabetes, despite the reported high BMD values [1, 3, 7]. Conversely, others have indicated a significant decrease in femoral BMD values along with an increased risk of hip fracture in men suffering from diabetes mellitus [8]. This is while some studies have not failed to show any increment in fracture risk in this group [9]. These contradictory results confirm the shortcoming of BMD in predicting the risk of osteoporotic fractures, mainly due to the fact that it is not a good indicator of bone quality.
Trabecular bone score (TBS), on the other hand, is a measure of bone texture correlated with bone microarchitecture. Low TBS values are considered a marker of impaired bone quality and thus is linked with increasing prevalence and incidence of osteoporotic fractures [10]. A few studies have assessed the association between high blood glucose levels at pre-diabetes status with osteoporosis and low TBS values.
This study aimed to evaluate the association between diabetes and pre-diabetes and quantity and quality of bone microarchitecture evaluated by BMD and TBS, respectively, in a group of community dwelling older adults.
Materials and methods
Study population
The protocols of phase one and two of the Bushehr Elderly Health (BEH) program is described elsewhere [11, 12] . In summary, the BEH program is a prospective population-based longitudinal study with multistage stratified-cluster sampling aimed at determining the prevalence of non-communicable diseases (NCD)-related risk factors among a representative urban sample of older population in Bushehr, the capital city of a province located in South Iran.
Data collection
Demographic data were collected through interviews with the participants. Lifestyle information such as physical activity and smoking were collected using Global Physical Activity Questionnaire (QPAG) and a brief Tobacco Questionnaire. Anthropometric measures including height, weight, and waist circumference were measured based on the NHANES III anthropometric measurement protocol [13]. Height and weight were measured to the nearest 0.1 cm, and 0.1 kg, correspondingly. Body mass index (BMI) was calculated by dividing weight (kg) by squared height (m2). For laboratory measurements, samples were collected after 12 h overnight fasting. Fasting plasma glucose (FPG) FPG was measured by an auto-analyzer using Enzymatic (CHE & CHO) colorimetric method. Hemoglobin A1C (HbA1C) was measured using Boranate affinity methods.
Total body composition also BMD values at lumbar spine and total hip were measured using dual x-ray absorptiometry (DXA, Discovery WI, Hologic Inc., USA). TBS of L1-L4 was assessed using TBS iNsight® software installed on our DXA machine.
Definitions
Pre-diabetes and diabetes (DM)
In this study, we used the 2018 American Diabetes Association (ADA) definition for
Pre-diabetes: FBG ≥100 and < 126 or hemoglobin A1C ≥ 5.7% and < 6.5% in individuals with no history of diabetes, and
Diabetes: FPG ≥ 126 mg/dl or HbA1C ≥ 6.5%, or pharmacological treatment in patients with a positive history of diabetes [14].
Osteoporosis
Femoral neck and lumbar spine osteoporosis were defined when calculated T-scores were equal or less than −2.5. Cumulative osteoporosis was defined in the presence of osteoporosis in the two mentioned sites.
Statistical analysis
Normal distribution of continuous variables was assessed using Kolmogorov-Smirnov test. Mean and standard deviation was used to express descriptive statistics. Statistical significance was defined as a P value less than 0.05. Uni- and multivariable linear and logistic regression models were used to assess the association between different definitions of osteoporosis (based on BMD and TBS values) and glycemic status (pre-diabetes and diabetes). Any independent variables with a P value ≤0.20 in univariate model analysis along with those with a significant relationship with osteoporosis in literature were considered in the final multivariate model (in final multivariable linear and logistic regression models, adjustment was performed for age, sex, physical activity, current smoking, and BMI). All analyses were performed using STATA (Release 12, Statistical software. College Station, Texas: STATA Corp LP).
Ethical consideration
All the participants signed a written informed consent before taking part in the study that was approved by the Research Ethics Committee of Bushehr University of Medical Sciences and Ethical Board Committee of the Endocrinology & Metabolism Research Institute (Ethical Code: IR.TUMS.EMRI.REC.1394.0036).
Results
The mean age of the participants was 69.27 (SD: 6.32) years and 51.79% of them were female. General characteristics of the participants according to their glycemic status are illustrated in Table 1.
Table 1.
General characteristics of the participants based on their glycemic status
| Total subjects N = 2263 |
Subjects with diabetes N = 459 |
Subjects with pre-diabetes N = 620 |
Normoglycemic subjects N = 1184 |
P value | |
|---|---|---|---|---|---|
| Age year mean(SD) | 69.27 (6.32) | 68.22 (6.31) | 69.55 (6.53) | 69.55 (6.55) | <0.001 |
| Gender (female) No (%) | 1172 (51.79) | 245 (53.38) | 346 (55.81) | 581 (49.07) | 0.019 |
| BMI Kg/m2 mean (SD) | 27.52 (4.96) | 27.96 (4.76) | 28.81 (5.32) | 26.67 (4.66) | <0.001 |
| WC cm mean (SD) | 98.73 (12.10) | 100.85 (10.99) | 101.48 (12.21) | 96.48 (12.02) | <0.001 |
| Physical Inactivity No (%) | 75.25 (1703) | 358 (78.00) | 473 (76.29) | 73.65 (872) | 0.008 |
| Cigarette Smoking No (%) | 396 (17.50) | 71 (15.50) | 80 (12.90) | 245 (20.69) | 0.007 |
BMI, Body Mass Index; WC, Waist Circumference
As it could be seen in Table 2, mean BMD value at both L1-L4 and neck of femur was significantly higher in participants with diabetes (Figs. 1 and 2). This is while such as a correlation was not reported between mean TBS and glycemic status (P trend = 0.400) (Fig. 3).
Table 2.
Bone health status of the individuals based on their glycemic status
| Total subjects N = 2263 |
Participants with diabetes N = 459 |
Participants with prediabetes N = 620 |
Normoglycemic subjects N = 1184 |
P value | P trend | |
|---|---|---|---|---|---|---|
| Neck of femur BMD mean (SD) gr/cm2 | 0.657 (0.142) | 0.677 (0.150) | 0.661 (0.146) | 0.648 (0.136) | <0.001* | 0.001 |
| Lumbar BMD mean (SD) gr/cm2 | 0.899 (0.182) | 0.936 (0.180) | 0.910 (0.180) | 0.879 (0.182) | <0.001* | <0.001 |
| TBS (L1-L4) mean (SD) | 1.296 (0.106) | 1.300 (0.109) | 1.284 (0.110) | 1.301 (0.103) | 0.004* | 0.400 |
| Neck of femur osteoporosis No (%) | 230 (10.16) | 39 (8.50) | 51 (8.23) | 140 (11.82) | 0.022** | 0.029 |
| Spinal osteoporosis No (%) | 653 (28.86) | 93 (20.26) | 168 (27.10) | 392 (33.11) | <0.001** | <0.001 |
| Cumulative osteoporosis No (%) | 700 (30.93) | 104 (22.66) | 180 (29.03) | 416 (35.14) | <0.001** | <0.001 |
*P value of ANOVA
**P value of Chi2
Fig. 1.
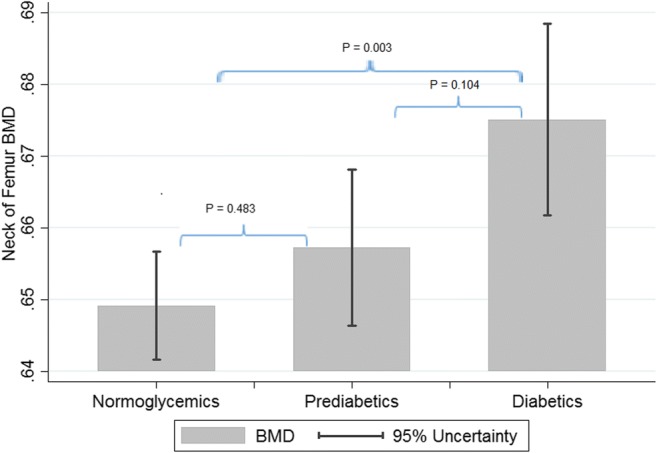
Mean BMD values at neck of femur based on glycemic status
Fig. 2.
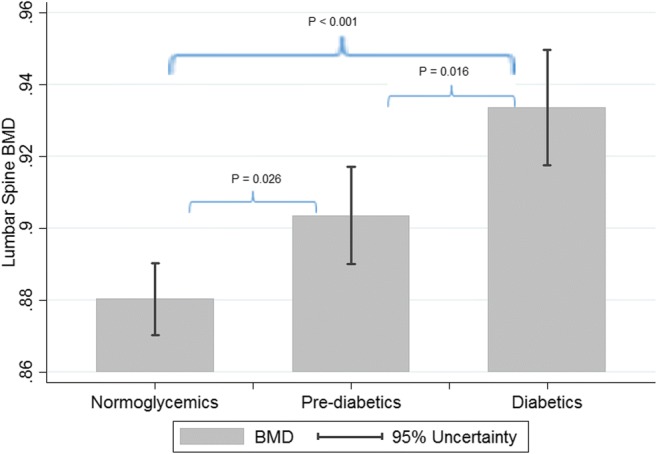
Mean BMD values at lumbar spines based on glycemic status
Fig. 3.
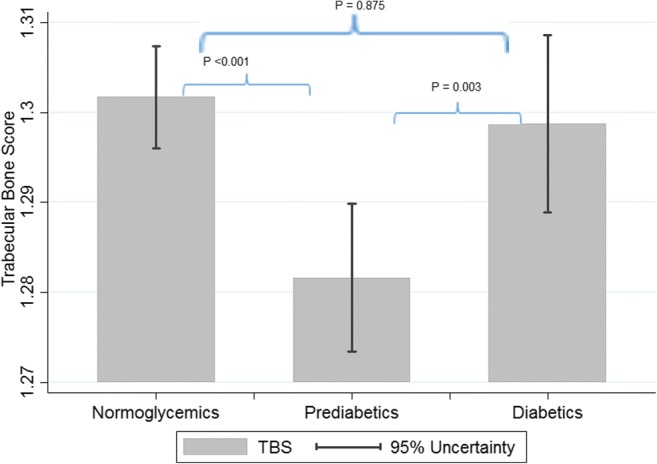
Mean TBS values based on glycemic status
Univariate linear regression results revealed pre-diabetes status had a significant but negative association with TBS values (β = −0.070, P = 0.001); no such an association was reported between diabetes status and TBS values (β = −0.002, P = 0.915). Diabetes status, however, was significantly associated with BMD values at neck of femur and spine (β = 0.083, and β = 0.125, respectively; Ps <0.05) (Table 3). Moreover, there was a significant association between BMD at L1-L4 and HbA1c values in multivariable regression model even after adjusting for diabetes status (β standardized = 0.051 and P = 0.025). This association is stronger for BMD at neck of femur after full adjustment (after adjustment for age, gender, physical activity, smoking, and BMI) (β standardized = 0.075 and P = 0.001). However, there was no such an association between TBS and HbA1c values (β standardized = 0.031, P = 0.197).
Table 3.
Linear association between TBS and BMD spine and neck of femur with pre-diabetes and diabetes
| Trabecular Bone Score | BMD Lumbar Spine | BMD Neck of Femur | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | P Value | B | β | P Value | B | β | P Value | |
| Univarate Model | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes |
−0.017 (−0.017 - -0.006) |
−0.071 (−0.115 - -0.028) |
0.001 |
0.030 (0.013–0.048) |
0.075 (0.031–0.119) |
0.001 |
0.013 (−0.001–0.027 |
0.042 (−0.001–0.085) |
0.056 |
| Participants with diabetes |
−0.001 (−0.012–0.011) |
−0.003 (−0.536–0.048) |
0.915 |
0.056 (0.037–0.076) |
0.148 (0.096–0.200) |
<0.001 |
0.030 (0.015–0.045) |
0.100 (0.045–0.151) |
<0.001 |
| First Multivariate Model** | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes |
−0.010 (−0.018 - -0.001) |
−0.041 (−0.077 - -0.004) |
0.030 |
0.042 (0.027–0.057) |
0.105 (0.067–0.142) |
<0.001 |
0.023 (0.011–0.034) |
0.067 (0.033–0.102) |
<0.001 |
| Participants with diabetes |
0.001 (−0.001–0.011) |
0.003 (−0.041–0.046) |
0.839 |
0.059 (0.042–0.075) |
0.154 (0.100–0.198) |
<0.001 |
0.027 (0.015–0.040) |
0.088 (0.045–0.130) |
<0.001 |
| Second Multivariate Model*** | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes |
−0.011 (−0.020 - -0.002) |
−0.048 (−0.085 - -0.011) |
0.016 |
0.038 (0.022–0.053) |
0.093 (0.055–0.131) |
<0.001 |
0.020 (0.011–0.034) |
0.067 (0.033–0.102) |
0.001 |
| Participants with diabetes |
<0.001 (−0.001–0.001) |
−0.003 (−0.0468–0.040) |
0.938 |
0.055 (0.038–0.072) |
0.145 (0.100–0.190) |
<0.001 |
0.026 (0.014–0.039) |
0.088 (0.045–0.130) |
<0.001 |
| Final Multivariate Model**** | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes |
−0.004 (−0.013–0.004) |
−0.021 (−0.058–0.016) |
0.466 |
0.017 (0.001–0.030) |
0.037 (0.001–0.073) |
0.043 |
0.011 (−0.001–0.022) |
0.032 (0.002–0.067) |
0.043 |
| Participants with diabetes |
0.004 (−0.006–0.013) |
0.013 (−0.031–0.056) |
0.417 |
0.044 (0.028–0.060) |
0.115 (0.072–0.157) |
<0.001 |
0.021 (0.001–0.033) |
0.067 (0.026–0.108) |
0.002 |
B = beta coefficient
β = beta standardized
**Adjusted for age and sex
***Adjusted for age, sex, physical activity, and current smoking
****Adjusted for age, sex, physical activity, current smoking, and BMI
Univariate logistic regression analysis revealed a reverse association between glycemic status and osteoporosis (Table 4). On the other word, in multivariable models, the risk of femoral osteoporosis was about 38% less in participants with prediabetes in compare to normoglycemic individuals. Furthermore, in participants with diabetes and participants with prediabetes the risk for spinal osteoporosis is near to half and third fourth in compare to normal glycemic subjects, respectively. In the fully adjusted model (after adjustment for age, gender, physical activity, smoking, and BMI) a significant association was reported between diabetes and spinal osteoporosis (odds ratio = 0.524; P < 0.001) as well as pre-diabetes and femoral osteoporosis (odds ration = 0.615; P = 0.021). The association between pre-diabetes and spinal osteoporosis or that of diabetes and femoral osteoporosis was significant (Table 4).
Table 4.
Association between osteoporosis and diabetes and pre-diabetes in female participants
| Spinal Osteoporosis | Neck of Femur Osteoporosis | Cumulative Osteoporosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI odds ratio | P value | Odds ratio | 95% CI odds ratio | P value | Odds ratio | 95% CI odds ratio | P value | |
| Univaraiable Model | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes | 0.751 | 0.606–0.931 | <0.001 | 0.667 | 0.477–0.934 | 0.018 | 0.755 | 0.612–0.932 | 0.009 |
| Participants with diabetes | 0.513 | 0.397–0.664 | <0.001 | 0.691 | 0.476–1.003 | 0.052 | 0.541 | 0.422–0.694 | <0.001 |
| First Multivariable Model** | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes | 0.651 | 0.517–0.818 | <0.001 | 0.511 | 0.349–0.748 | 0.001 | 0.644 | 0.513–0.809 | <0.001 |
| Participants with diabetes | 0.487 | 0.371–0.639 | <0.001 | 0.812 | 0.540–1.223 | 0.319 | 0.511 | 0.392–0.666 | <0.001 |
| Second Multivariable Model*** | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes | 0.699 | 0.550–0.888 | 0.003 | 0.538 | 0.360–0.804 | 0.003 | 0.690 | 0.544–0.875 | 0.002 |
| Participants with diabetes | 0.494 | 0.372–0.856 | <0.001 | 0.898 | 0.586–1.376 | 0.621 | 0.521 | 0.395–0.687 | <0.001 |
| Final Multivariable Model**** | |||||||||
| Normoglycemic | Reference | Reference | Reference | ||||||
| Participants with prediabetes | 0.861 | 0.670–1.105 | 0.240 | 0.615 | 0.407–0.928 | 0.021 | 0.843 | 0.676–1.106 | 0.176 |
| Participants with diabetes | 0.524 | 0.392–0.701 | <0.001 | 0.968 | 0.629–1.489 | 0.881 | 0.551 | 0.415–0.732 | <0.001 |
**Adjusted for age and gender
***Adjusted for age, gender, current smoking, and physical activity
****Adjusted for age, gender, current smoking, physical activity, and BMI
Discussion
In this community-based study, the relationship between osteoporosis and glycemic status was assessed in an Iranian population. The prevalence of spinal and cumulative osteoporosis was significantly lower in participants with diabetes. Moreover, osteoporosis at both of the studied sites was more prevalent in normoglycemic subjects than the participants with prediabetes. Whiles, the frequency of femoral osteoporosis were less common in participants with diabetes also in participants with prediabetes. Furthermore, bone mineral density at both spinal and neck of femur sites were higher in people with diabetics than group with prediabetes. In addition, the BMD at these two sites in subjects with pre-diabetes were higher than normal glycemic individuals.
On the contrary, mean lumbar TBS values did not differ among patients suffering from diabetes and non-diabetic individuals. This is while TBS values were significantly lower among participants with prediabetes compared with normoglycemic subjects. The association between TBS value and glycemic status became statistically non-significant after adjusting for BMI. Higher BMI values in subjects with pre-diabetes can explain lower values of TBS in this population, suggesting that glycemic condition has no effect on TBS spontaneously. Compared with normoglycemic individuals, spinal and cumulative osteoporosis were about 45% less common among participants with diabetes; neck of femur osteoporosis, conversely, was less significantly common in people with pre-diabetes.
The relation between diabetes and BMD values is controversial, with inconsistent results regarding the influence of increased serum glucose levels on bone health [8, 15–17]. Von et al. reported an inverse association between serum glucose levels and bone health in men; the association was positive in women [18]. Rotterdam study revealed that serum glucose levels were positively associated with BMD values at femoral neck in both genders independent of their glycemic status. Our study showed higher BMDs in diabetics that is in consistent with the results of the Rotterdam [19] and NHANS III studies [16].
Irrespective of BMD values, risk of fracture is reported to be higher in diabetics compared with non-diabetics [20]. As a result, the use of TBS is suggested to improve the diagnostic value of lumbar spine DXA in diabetic patients. While BMD is typically normal or higher in diabetic patients, TBS appears to be lower in diabetics compared with non-diabetic subjects [21]. Leslie et al. showed that lumbar spine TBS can predict osteoporotic fractures irrespective of the glycemic status [21]. In addition, TBS is shown to be positively associated with good glycemic control [22, 23]. In our study, the significant relationship between diabetes and TBS disappeared after adjustment for BMI. Similar results were observed in a cohort study conducted in Germany. In this study, no significant difference was noted in TBS values between diabetics and non-diabetics. However, TBS and HbA1c values were independently associated with fracture risk, with TBS values lower than 1.42 predictive for fracture [24]. Nevertheless, the Manitoba cohort study on 29,407 postmenopausal women over 50 in Canada and the Ansung cohort on 1229 men and 1529 postmenopausal women over 50 in Korea, lumbar TBS was lower in diabetic patients. Similar to the present study, these two studies revealed higher mean BMD values in diabetic patients compared with non-diabetics [21, 23]. TBS was also inversely associated with HbA1c, FBS, fasting insulin and HOMA-IR1 [23]. While these results are of major clinical interest, it should be noted that TBS illustrates an indirect picture of bone microarchitecture with low resolution and reduced image quality.
The reason behind increased risk of fracture in diabetic patients is multifactorial and includes changes in bone component and structural abnormalities [25]. High levels of serum glucose in diabetic patients causes the accumulation of advanced glycosylation end-products in the bone matrix, leading to the formation of a more brittle bone [26–28]. Increased cortex porosity reported in women suffering from diabetes mellitus [29] could result in decreased bone strength, which is not detectable by DXA [30]. Furthermore, there are several evidences reporting potential defect in the trabecular bone microstructure in patients suffering from type 1 and 2 diabetics [31, 32]. This could explain low-impact fractures.
High insulin levels are another mechanism explaining higher fracture rates in diabetic patients, regardless of their higher BMD values. Insulin is an anabolic hormone that stimulates bone formation. It increases osteoblast proliferation, promoting collagen synthesis. Insulin growth factor-1 (IGF-1) affects bone in similar ways. Studies have reported a positive correlation between IGF-1 and BMD values, and a negative association between IGF-1 and fracture [33, 34].
On the other hand, obesity is a potential confounder in the relationship between diabetes and bone health. Recently, obesity has also been reported as a risk factor for fracture [35–37]. Trabecular and cortical microarchitectural abnormalities have been reported in obese participants; their diabetic status, however, was not studied [38]. Moreover, lower bone formation, elevated serum sclerostin, increased adipocyte markers, and abnormal bone marrow fat composition have been reported in obese individuals [39, 40]. It is likely that the effect of diabetes on bone health, due to known and unknown factors such as body biochemical balance and lifestyle, is stronger in certain ages. Also, medications used by diabetics may affect the results differently.
This paper has several strengths, as it was based on a population-based study conducted on the elderly population with specific attention paid to bone health. Moreover, bone indicators such as BMD and TBS were evaluated together.
This study, however, suffered from several limitations. BMD and TBS were assessed only in certain bone areas; this is while the relationship between diabetes with BMD and TBS may differ in various regions. Moreover, considering the cross-sectional nature of this study, it is not possible to determine the causal relationship between diabetes and BMD values. A longitudinal study would be more suitable for this purpose. In this study, participants with diabetes were analyzed regardless of the diabetes type. However, different etiologies of type 1 and type 2 diabetes along with their varied therapeutic intervention could affect the outcome.
Conclusion
Our findings suggest that osteoporosis as measured by BMD at lumbar and femoral site is less common among participants with diabetess compared with participants without diabetes. It seems that diabetes, regardless of background factors such as BMI, is associated with increased of bone density. This is while an individual’s glycemic condition does not influence bone quality and thus bone health. This was confirmed by TBS. Longitudinal studies are needed to determine the effect of pre-diabetes and diabetes on bone density. More information is needed regarding the pathogenesis of high blood glucose levels on bone health and the higher risk of fracture in diabetic patients.
Footnotes
Homeostatic model assessment- Insulin resistance
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwartz AV, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 2.Strotmeyer ES, et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: the health, aging, and body composition study. J Bone Miner Res. 2004;19(7):1084–1091. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 3.Bonds DE, et al. Risk of fracture in women with type 2 diabetes: the Women’s health initiative observational study. J Clin Endocrinol Metab. 2006;91(9):3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 4.Choi YJ, Chung Y-S. Type 2 diabetes mellitus and bone fragility: special focus on bone imaging. Osteoporos Sarcopenia. 2016;2(1):20–24. doi: 10.1016/j.afos.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley D, et al. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-sectional results from the Canadian multicentre osteoporosis study. J Bone Miner Res. 2003;18(4):784–790. doi: 10.1359/jbmr.2003.18.4.784. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone? Diabetes Care. 1996;19(12):1388–1392. doi: 10.2337/diacare.19.12.1388. [DOI] [PubMed] [Google Scholar]
- 7.Janghorbani M, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 8.Yaturu S, et al. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med Sci Monit. 2008;15(1):CR5–CR9. [PubMed] [Google Scholar]
- 9.Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 10.Harvey N, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–224. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostovar A, et al. Bushehr Elderly Health (BEH) Programme, phase I (cardiovascular system) BMJ Open. 2015;5(12):e009597. doi: 10.1136/bmjopen-2015-009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafiee G, et al. Bushehr Elderly Health (BEH) programme: study protocol and design of musculoskeletal system and cognitive function (stage II) BMJ Open. 2017;7(8):e013606. doi: 10.1136/bmjopen-2016-013606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. National Health Statistics Reports. 2009;13:1–8. [PubMed] [Google Scholar]
- 14.Association, A.D. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Supplement 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi T, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45(2):174–179. doi: 10.1016/j.bone.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based US sample. J Clin Endocrinol Metab. 2007;92(11):4161–4164. doi: 10.1210/jc.2007-0757. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg AH, et al. The association between hyperglycemia and fracture risk in middle age. A prospective, population-based study of 22,444 men and 10,902 women. J Clin Endocrinol Metab. 2008;93(3):815–822. doi: 10.1210/jc.2007-0843. [DOI] [PubMed] [Google Scholar]
- 18.Von Muhlen D, et al. Associations between the metabolic syndrome and bone health in older men and women: the rancho Bernardo study. Osteoporos Int. 2007;18(10):1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 19.Muka T, et al. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: the Rotterdam study. PLoS One. 2015;10(6):e0129116. doi: 10.1371/journal.pone.0129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oei L, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam study. Diabetes Care. 2013;36(6):1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie WD, et al. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98(2):602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 22.Dhaliwal R, et al. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25(7):1969–1973. doi: 10.1007/s00198-014-2704-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100(2):475–482. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 24.Neumann T, et al. Trabecular bone score in type 1 diabetes—a cross-sectional study. Osteoporos Int. 2016;27(1):127–133. doi: 10.1007/s00198-015-3222-y. [DOI] [PubMed] [Google Scholar]
- 25.Leslie WD, et al. Type 2 diabetes and bone. J Bone Miner Res. 2012;27(11):2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 26.Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5(2):62–66. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 27.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci. 1984;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang S, et al. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20(6):887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burghardt AJ, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–5055. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzer G, et al. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009;24(3):468–474. doi: 10.1359/jbmr.081108. [DOI] [PubMed] [Google Scholar]
- 31.Pritchard JM, et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res. 2012;64(1):83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armas LA, et al. Trabecular bone histomorphometry in humans with type 1 diabetes mellitus. Bone. 2012;50(1):91–96. doi: 10.1016/j.bone.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu T, Rosen CJ. The insulin-like growth factor-I gene and osteoporosis: a critical appraisal. Gene. 2005;361:38–56. doi: 10.1016/j.gene.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Garnero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet. 2000;355(9207):898–899. doi: 10.1016/S0140-6736(99)05463-X. [DOI] [PubMed] [Google Scholar]
- 35.Compston JE, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielson CM, et al. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2011;26(3):496–502. doi: 10.1002/jbmr.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson H, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–233. doi: 10.1002/jbmr.2017. [DOI] [PubMed] [Google Scholar]
- 38.Sornay-Rendu E, et al. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des femmes de Lyon (OFELY) study. J Bone Miner Res. 2013;28(7):1679–1687. doi: 10.1002/jbmr.1880. [DOI] [PubMed] [Google Scholar]
- 39.Bredella MA, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48(4):748–754. doi: 10.1016/j.bone.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen A, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98(6):2562–2572. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


