Abstract
To better understand the effects of ultrasonic treatment in the whole freezing process (UWF) and the maximum ice crystal formation zone (UMF) on the quality of frozen dough, the textural properties of dough and the structure of gluten were investigated. The results showed that the UWF and UMF treatments improved the textural properties of frozen dough and obtain the best effect at the 60 W/L power densities. Ultrasound-assisted freezing reduced the destructive effect of disulfide bonds on dough, and led to a state of dynamic equilibrium of hydrophobic groups. UWF treatment at 80 W/L and UMF treatment at 40 W/L had positive effects prevented the secondary structure from destruction by freezing. The network of gluten treated by ultrasound-assisted freezing was more uniform and smaller than that of traditional freezing samples, which was similar to the network structure of fresh protein. According to Pearson’s correlation analysis, there was a high correlation between SH, α-helix content and springiness. There was a significant positive correlation between β-turn and G′, G″, and there was a significant negative correlation between β-turn and hardness. These results suggest that ultrasound-assisted freezing improved the process quality of dough though reducing the damage to gluten structure caused by freezing.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03822-6) contains supplementary material, which is available to authorized users.
Keywords: Ultrasonic-assisted freezing, Textural properties, Gluten structure, Pearson’s correlation analysis
Introduction
Frozen dough is widely used in the food industry in China for the production of bread, Chinese steamed bread, dumplings, and several viennoiseries foods. This commodity can improve work efficiency, reduce the labor intensity of workers, increase the shelf-life of products, and facilitate the long-distance distribution of foods (Ban et al. 2016; Ma et al. 2016). However, water redistribution is triggered by the modification of the water-binding capacity of the dough’s constituents during the freezing process. The expansion pressures generated by the large ice crystals result in damage to the gluten network and deterioration of dough processing quality (Yadav et al. 2008). Therefore, control ice crystals size and reduce the proportion of large ice crystals in the freezing process were the key method to avoid the damage of water crystallization to the sample structure and improving the quality of processed dough.
Power ultrasound, as a green and pollution-free technology was applied in the fields of food preservation and extraction, which improved the quality of food without adding additives (Chamat et al. 2011; Kiani et al. 2012; Mandal et al. 2017; Mason et al. 1996; Monroy et al. 2018; Pingret et al. 2013; Simal et al. 1998). The cavitation bubbles can induce the formation of primary crystal nucleus or promote secondary nucleation through high pressure or microjet generation during fracture or movement, and lead the ice crystals sizes with smaller and uniform in the ultrasound-assisted freezing (UAF) process. (Kiani et al. 2011; James et al. 2015). The microjets produced by cavitation bubble motion can also enhance the heat transfer efficiency in the medium (Cheng et al. 2015), thus improving the freezing rate and product quality. Sun and Li reported that UAF reduced the effects of freezing on the microstructural changes of potatoes and found that it also reduced cell structure damage (Sun and Li 2003). Zhang et al. (2018a) reported that UAF at certain powers significantly increased the freezing rate of porcine longissimus muscles, and samples with UAF-180 had the shortest total freezing time, which was 212 s shorter.
It also reported that ultrasound-assisted freezing has a positive effect on the dough. Hu et al. (2013) reported that UAF reduced the total freezing time of dough by 11% and improved the microstructure of frozen dough at 288 and 360 W power level. Song et al. (2009) reported that UAF made the secondary structure of wet gluten more orderly, with smaller and more uniform ice crystals than traditional freezing. The texture quality of dough is the key factor affecting the quality of the subsequent processing of dough, and the texture quality of dough is determined by gluten protein. In the freezing process, the formation of ice crystals destroys the structure of wheat gluten, and then damage the texture characteristics of dough (Zhang et al. 2018c). Thus, the purpose of this paper was investigating the textural characteristics of frozen dough affected by the UAF and the relationship between textural characteristics of frozen dough and protein structure of wheat gluten.
Nearly 80% of the water in most food centers can be frozen to ice when the temperature drops from 0 to − 5 °C (the maximum ice crystal formation zone). Most ice crystals are formed at this zone, and the formation mode of ice crystals in the maximum ice crystal formation zone has a great influence on the quality of food. So, the study of the effect of ultrasound-assisted frozen in the maximum ice crystal formation zone is also extremely necessary. The primary objectives of this study were to evaluate (1) the effects of ultrasonic-assisted freezing on rheological and textural properties of frozen dough in the whole freezing process and maximum ice crystal generation zone, (2) the effects of ultrasonic-assisted freezing on wheat gluten structure in the whole freezing process and maximum ice crystal generation zone, and (3) the relationship between the textural properties of dough and protein structure of wheat gluten.
Materials and methods
Preparation of dough
Wheat flour (300 g, Henan Jinyuan Grain and Oil Co., Ltd., Zhengzhou, China) and water (135 mL) were added to an HA-3480AS dough maker (Clymeis Electromechanical Technology, Shenzhen, China), then kneaded for 10 min and molded into dough. Finally, the dough was pressed repeatedly four times with the press machine to make regular cuboids (14 × 7 × 4 cm3) and packed into double high-density polyethylene bags for use.
The ultrasound-assisted freezing method
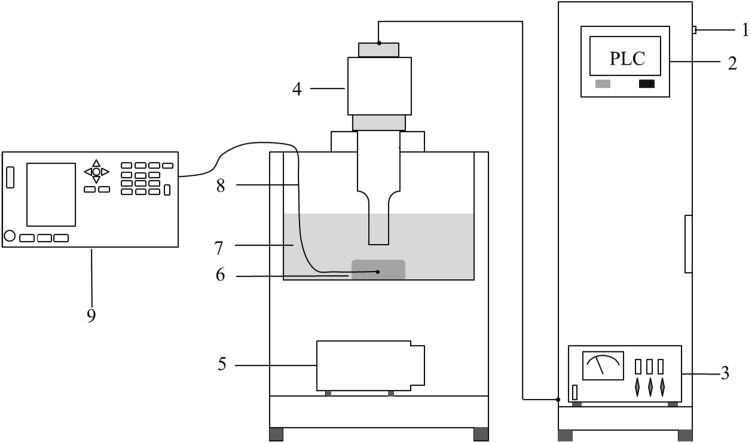
The ultrasound-assisted freezing method was a modification of the procedure by Hu et al. (2013). The 95% (v/v) ethanol solution was used as the coolant, and the temperature was maintained at − 30 °C by refrigeration (see Fig. 1). The ultrasonic equipment (Fig. 1, Shangjia Biotechnology Co., Ltd., Wuxi, China) used a frequency of 20 kHz, and the instrument could deliver a maximum power of 1000 W. The real-time temperature at the geometric center of each sample (i.e., the sample temperature) was measured via a K-type thermocouple, which was connected to a digi-sense eight channel scanning benchtop thermometer (Applent Instruments, Jiangsu, China). The sample temperatures were acquired at 2 s intervals and transmitted to a computer to obtain the internal temperatures of dough.
Fig. 1.
Ultrasound-assisted freezing system (1. Power supply, 2. Ultrasound parameter control panel, 3 Ultrasound generator, 4. Ultrasound probe, 5. Refrigeration compressor, 6. Dough sample, 7. Coolant storage tank, 8. K-type thermocouple, 9. Temperature testing instrument)
The dough was placed into the center of an ultrasonic reactor with 10 L coolant at − 30 °C. The ultrasonic probe was dipped to a depth of 2 cm in the ethanol and placed directly above the center of the dough. In this study, the whole freezing process was defined as the period from 4 to − 18 °C. The total freezing time was divided into three stages, from 4 to 0 °C is the pre-cooling stage, from 0 to − 5 °C is the phase transition stage, from − 5 to − 18 °C is sub-cooling stage (Hu et al. 2013).
In the ultrasound-assisted of whole freezing process (UWF) trials, the ultrasound begins to work at the center temperature of dough reached to 4 °C, stopped working at the center temperature of dough reached − 18 °C. In the ultrasound-assisted of maximum ice crystal generation zone (UMF) trials, the ultrasound begins to work at the center temperature of dough reached to 0 °C, stopped working at the center temperature of dough reached − 5 °C. The frequency of ultrasound was 20 kHz, on-time and off-time were 5 s, and the power density of ultrasound were 20, 40, 60, 80, or 100 W/L.
After freezing, each sample was stored at − 18 °C. Each freezing experiment was repeated in triplicate. The control samples were frozen in ethanol without ultrasound. The fresh samples were dough that was not stuffed during the freezing process.
Determination of the textural characteristics of dough
Rheological properties
The frozen dough pieces were defrosted for 150 min in an incubator at 30 °C, and then the rheological properties were measured according to the methods of Wang et al. (2018). In brief, the rheological behavior of the dough was analyzed by a controlled stress rheometer for small-amplitude oscillation tests (DSR200, Rheometric Scientific, Piscatawat, NJ, USA). The measurement system was equipped with parallel-plate geometry (20 mm diameter) with a smart swap Peltier plate temperature system to maintain the temperature at 25 °C. The dough was loaded between the parallel plates and compressed to obtain a gap of 1 mm. The dough was then rested between the plates (5 min) before measurements. Stress sweep from 0.001 to 100% at 1 Hz frequency was carried out to determine the linear viscoelastic zone. A frequency-sweep test from 0.1 to 40 Hz at a stress of 0.05% was carried out to determine the elastic modulus (G′), viscous modulus (G″), and loss tangent (tan δ) as a function of frequency. Each measurement was performed three times.
Texture profile analysis (TPA)
The TPA of steamed bread simulates hewing movements and has been accepted universally (Zhang et al. 2018c). The frozen dough was 0.7 cm thick and 3.0 cm in diameter. It was incubated in boiling water for 2 min, and then placed in a 500 mL container containing 25 °C distilled water for 1 min. The moisture on the surface of the dough was drained, and then the pieces were subjected to a TPA test using a tensile analyzer (TMS-Pro, Food Technology, Blacksburg, VA, USA). Five texture parameters were measured: hardness (g), springiness, cohesiveness (g/s), chewiness, and resilience. The experiment used a P/50 aluminum cylindrical probe. The compression test was set as follows: pretest speed, 1.00 mm/s, test speed, 0.80 mm/s, post-test speed, 0.80 mm/s, strain, 70.00%, and trigger force, 5 g, with a 3 s time interval between compressions. Each measurement was performed five times.
Extraction of gluten
The extraction method of gluten was slightly modified according to Wang et al. (2014a, b). Dough was washed with plenty of deionized water to remove starch granules until the deionized water was clear, and then the wet gluten was lyophilized. Dried gluten (20 g) was shaken with dichloromethane (300 ml) for 60 min at room temperature and then filtered through filtered paper. The above procedures were repeated three times. Finally the gluten protein was dried in the draught cupboard for 24 h.
Structural characterization of wheat gluten
Determination of free sulfhydryl (SH) content
The free SH content was determined according to the Ellman’s reagent method established by Patrick and Swaisgood (1976) with some modifications. 1.0 mL of protein solution was added to 5.0 mL of standard buffer (containing 8 M urea, 0.5% sodium dodecyl sulfate, 86 mM Tris, 90 mM glycine, pH 8.0) and 50 μL Ellman’s reagent (4.0 mg/mL 5,5′-Dithiobis-2-nitrobenzoic acid dissolved in standard buffer) with rapid mixing. The mixture was then put in a 30 °C water bath for 60 min and the absorbance was measured at 412 nm. The buffer instead of the protein solution was used as a reagent blank and 50 μL of buffer instead of Ellman’s reagent was used as the protein blank. The free sulfhydryl content was calculated using the following formula (Ellman et al. 1959; Yang et al. 2017):
where D is the dilution factor, C is the protein concentration (mg/mL), ΔAbs412 is the differential absorbance values of sample and the protein blank solutions at 412 nm.
Determination of surface hydrophobicity
Surface hydrophobicity (H0) of protein dispersions was determined using 1-anilino-8 naphthalene-sulfonate (ANS, Sigma-Aldrich, St. Louis, MO, USA) as a fluorescence probe according to the method of Kato et al. (1980). The prepared protein solution was subjected to gradient dilution to obtain a protein solution having final concentrations of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL. Then, 20 μL of ANS (8.0 mM in 0.01 M phosphate buffer, pH 7.0) was added to 4.0 mL of diluted protein solution, mixed, and kept in the dark for 15 min. The relative fluorescence intensity was measured at room temperature with a Hitachi F-7000 fluorescence spectrophotometer (Hitachi High-Technologies, Tokyo, Japan) at an excitation wavelength of 280 nm (5.0 nm slit) and emission wavelength of 350 nm (5.0 nm slit), with a scanning speed of 240 nm/min. The initial slope of the fluorescence intensity versus protein concentration (mg/mL) was calculated using linear regression analysis and was used as the index of H0.
Secondary structural characterization of wheat gluten in dough
Fourier transform infrared (FTIR) spectra of samples were prepared according to the method of Liao et al. (2010). 1.0 mg of protein powder was mixed with 200 mg of solid KBr powder. After homogenization using an agate mortar and pestle, the powder was formed into pellets (1–2 mm thick) using a 10 t hydraulic press. A 8210E FTIR spectrometer (Thermo Fisher, Waltham, MA, USA) equipped with a denudated triglycine sulfate detector was used for the readings. The raw spectra using 32 scans were obtained at 400–4000 cm−1 with a resolution of 4 cm−1.
The raw spectra and the overlapping amide I band (1600–1700 cm−1) were preprocessed and analyzed by OMNIC (Thermo Fisher) and Peakfit software, version 4.12 (SPSS, Chicago, IL, USA). The secondary structure was analyzed using the amide I band of protein, which was resolved by deconvolution, secondary derivation, and Gaussian curve fitting. The different amide I regions were assigned to protein secondary structures according to previous reports: β-sheet (1615–1637 cm−1), random coil (1637–1645 cm−1), α-helix (1645–1664 cm−1), β-turn (1664–1682 cm−1), and β-sheet (1682–1695 cm−1) (Long et al. 2015, Sun et al. 2017).
Scanning electron microscope (SEM)
The extraction procedures for gluten, glutenin, and gliadin were slightly modified according to Wang et al. (2018). Dough was washed with excess deionized water to remove starch granules until the deionized water was clear, and the wet gluten was lyophilized. Dried gluten (20.0 g) was shaken with dichloromethane (300 mL) at room temperature for 60 min and then passed through filter paper. The above procedures were repeated three times, and the resulting gluten protein was dried in a draught cupboard overnight. Gliadin was extracted in three steps from 20.0 g of gluten using two extractions with 60% ethanol (300 mL each) and one extraction with deionized water (300 mL). Before the second and third extraction steps, the cohesive glutenin was mechanically disrupted with a spatula. The extraction was conducted at 25 °C for 3 h and centrifuged (3000 × g, 4 °C 10 min) after each extraction. The supernatants were pooled and the remaining ethanol was removed using a rotary evaporator at 35 °C. The gliadin and glutenin (sediment after ethanol extraction) were freeze-dried.
Microstructural analyses of the gluten, glutenin and gliadin were according to Zhang et al. (2016) with minor modifications. Proteins under different treatment conditions were freeze-dried and ground through a 100-mesh sieve and coated with gold for 120 s. The microstructure was observed using a SEM (Hitachi, Tokyo, Japan) at a voltage of 20.0 kV.
Pearson’s correlation analysis
Analysis of Pearson’s correlation were performed at a significance level of α = 0.05 using SPSS Statistics software, version 17.0 (SPSS). Pearson’s correlation analysis was conducted to evaluate relationships between the textural characteristics of frozen dough (G′, G″, tan δ, hardness, springiness, cohesiveness, chewiness and resilience) and structure of gluten (H0, SH, α-helix, β-sheet, β-turn and random coil).
Statistical analysis
The results were analyzed by one-way analysis of variance using a significance level of P < 0.05 using SPSS Statistics software, version 17.0 (SPSS). The graphs were drawn using OriginPro8.5 (OriginLab, Northampton, MA, USA). The data are expressed as means and standard errors (n = 3).
Results and discussion
The effect of ultrasound-assisted freezing on the textural characteristics of dough
Rheological
The G values indicate the stiffness, viscoelastic properties, and rheological properties of dough. Measurements of G provide information on the polymer structure and may be related to the molecular weight distribution or cross-link. The effect of ultrasound-assisted frozen on the rheological behavior of dough was showed in supplement data and Table 1.
Table 1.
Effect of ultrasound-assisted freezing on the rheological properties and texture characteristics of dough
| Power density (W/L) | G′ (Pa) | G″ (Pa) | Tan δ | Hardness (g) | Springiness (g/s) | Cohesiveness | Chewiness | Resilience |
|---|---|---|---|---|---|---|---|---|
| Fresh dough | 108,871 ± 208b | 36,010 ± 131b | 0.331 ± 0.001b | 23,320 ± 97e | 0.597 ± 0.004a | 0.636 ± 0.004b | 8849 ± 55b | 0.243 ± 0.004ab |
| Control | 74,352 ± 100g | 24,638 ± 139d | 0.331 ± 0.002b | 25,706 ± 99a | 0.582 ± 0.004bc | 0.632 ± 0.004c | 9454 ± 168bc | 0.245 ± 0.002a |
| UWF | ||||||||
| 20 | 85,121 ± 125e | 28,369 ± 79e | 0.333 ± 0.001b | 25,724 ± 53a | 0.587 ± 0.009bc | 0.637 ± 0.006c | 9615 ± 149ab | 0.240 ± 0.004a |
| 40 | 93,089 ± 301c | 30,798 ± 182c | 0.331 ± 0.001b | 24,104 ± 88b | 0.627 ± 0.012ab | 0.667 ± 0.011a | 10,070 ± 55ab | 0.243 ± 0.006a |
| 60 | 123,251 ± 168a | 40,903 ± 149a | 0.332 ± 0.001b | 23,118 ± 10e | 0.580 ± 0.011bc | 0.640 ± 0.008bc | 8571 ± 273d | 0.240 ± 0.013a |
| 80 | 91,459 ± 298d | 29,431 ± 263d | 0.322 ± 0.003c | 24,308 ± 19b | 0.625 ± 0.004ab | 0.664 ± 0.001ab | 10,087 ± 40ab | 0.241 ± 0.006a |
| 100 | 80,624 ± 264f | 26,615 ± 158f | 0.330 ± 0.001b | 25,613 ± 77a | 0.553 ± 0.005c | 0.626 ± 0.001c | 8960 ± 28cd | 0.245 ± 0.002a |
| UMF | ||||||||
| 20 | 78,986 ± 145e | 26,093 ± 133c | 0.330 ± 0.006b | 25,016 ± 81b | 0.581 ± 0.001b | 0.612 ± 0.001c | 8883 ± 32b | 0.251 ± 0.002a |
| 40 | 81,819 ± 428d | 26,953 ± 595c | 0.329 ± 0.006b | 25,089 ± 89b | 0.574 ± 0.003b | 0.647 ± 0.004a | 9319 ± 189a | 0.245 ± 0.004ab |
| 60 | 91,002 ± 291b | 30,127 ± 45b | 0.331 ± 0.004b | 23,996 ± 97d | 0.563 ± 0.002c | 0.635 ± 0.004b | 8571 ± 14b | 0.238 ± 0.001b |
| 80 | 87,113 ± 167c | 29,843 ± 460b | 0.343 ± 0.005a | 24,547 ± 69c | 0.576 ± 0.001b | 0.626 ± 0.003b | 8843 ± 4b | 0.245 ± 0.001ab |
| 100 | 81,374 ± 376d | 26,708 ± 412c | 0.328 ± 0.007b | 24,925 ± 34b | 0.546 ± 0.002d | 0.649 ± 0.003a | 8820 ± 11b | 0.250 ± 0.004b |
The G′, G″, Tan δ here was determined by a controlled stress rheometer and were obtained at a frequency of 1 Hz. The hardness, springiness, cohesiveness, chewiness and resilience were measured by a tensile analyzer, TPA mode. Mean ± SD, Means in same column with different letters are significantly different (P < 0.05)
Table 1 showed that G′ and G″ of the dough were significantly increased by the ultrasound-assisted frozen treatment when compared to the control (P < 0.05). These results suggested that ultrasound-assisted freezing promoted the elasticity and viscosity of dough, which resulted in an improvement in the quality of the subsequent processed products (Soliman et al. 2007). With the increase of power density, G′ and the G″ were increased at first and then decreased, obtaining their maximum values at a power density of 60 W/L. Compared to the control samples, the UWF treatment increased the G′ and G″ by 65.77% and 66.01%, and the UMF treatment increased the G′ and the G″ by 22.37% and 22.28%, respectively. For the improvement effect of G′ and G″ at 60 W/L, the UWF samples were superior to the UMF samples. The tan δ value was significantly decreased by the UWF treatment at 80 W/L. The opposite result occurred in the UMF treatment, the tan δ value was significantly increased compared with the fresh samples. The tan δ value is another important indicator of dough quality. A lower tan δ value implied the dough tended to the nature of the fluid, its plasticity was better, and result in the processing properties of the dough improved (Dreese et al. 1987; Wang et al. 2016).
Dough is a heterogeneous mixture system with the coexist of solids (gluten and starch) and fluids. The freezing process is also from the outside to the center point. The acoustic cavitation and acoustic streaming arising from the propagation of power ultrasound waves into a fluid may cause an acceleration of mass-transfer and heat-transfer. The improvement of heat transfer during the dough-freezing process by ultrasound can also shorten the freezing time for each stage. However, the mechanical vibration effect of ultrasound rapidly breaks large ice crystal particles and reduces the proportion of large ice crystals in frozen dough (Kontogiorgos et al. 2007; Bin et al. 2008). Therefore, the destruction of the molecular structure of the components in the dough subject to the freezing process was alleviated by ultrasound treatment.
It was therefore proposed that the decreases of G′ and G″ were mainly due to the destruction of the gluten protein network structure by ice crystals. However, ultrasound cavitation and microjets induced nucleus formation and breakage of large ice crystals into smaller ice crystals, thus reducing the proportion of large ice crystals and increasing the proportion of small ice crystals, to reduce the destruction of gluten network structure, and improve the quality of frozen dough.
TPA analysis
Table 1 also shows that the hardness of control samples was significantly (P < 0.05) increased compared to the fresh samples. The springiness of control samples was significantly (P < 0.05) decreased compared to the fresh samples. These results meant that the taste of dough became worse, especially in noodles and dumplings after the traditional freezing process (Zhang et al. 2018c). The cohesiveness was significantly (P < 0.05) decreased by the traditional freezing process, resulting in inferior dough after it was cooked. The lower cohesiveness meant that the macromolecular polymers in dough were reduced, resulting in an increased rate of cooking loss.
However, ultrasound-assisted freezing resulted in a positive change in the hardness, springiness, cohesiveness, and chewiness. For the UWF samples, the hardness was decreased first and then increased with an increase of power density, when compared to the control. It obtained the lowest hardness value of 23,118 g at the power density of 60 W/L, there was no significant difference for the fresh dough, which was not subjected to any freezing process. With the power density increased, the springiness, cohesiveness and chewiness were all increased first and then declined. There was no significant difference between the resilience in different UMF samples. Together, the results showed that the UWF treatment improved the masticatory characteristics of frozen dough.
The effect of ultrasound-assisted freezing on the molecular structure of wheat gluten
Free sulfhydryl groups
As a functional group of proteins, free sulfhydryl groups are usually oxidized to form disulfide bonds, which are essential for protein folding and conformational stability. Glutenin can form a highly networked structure through intra-and extra chain disulfide bonds, thus constituting the framework of gluten (Patrick et al. 1976). At the same time, gliadin was filled into the glutenin network through the formation of spherical proteins via the chain disulfide bonds. Figure 2a shows that the sulfhydryl content of frozen dough was increased by the traditional freezing process, which meant that the disulfide bonds were destroyed. This may be due to the formation of ice crystals in wheat gluten, which changes the hydration environment of wheat gluten protein, destroying the force used to maintain protein structural balance, and changing the structure of wheat gluten protein (Wang et al. 2015). In addition, the concentration effect induced by freezing may lead to an increase in intramolecular and intermolecular disulfide bond exchange reactions of proteins.
Fig. 2.
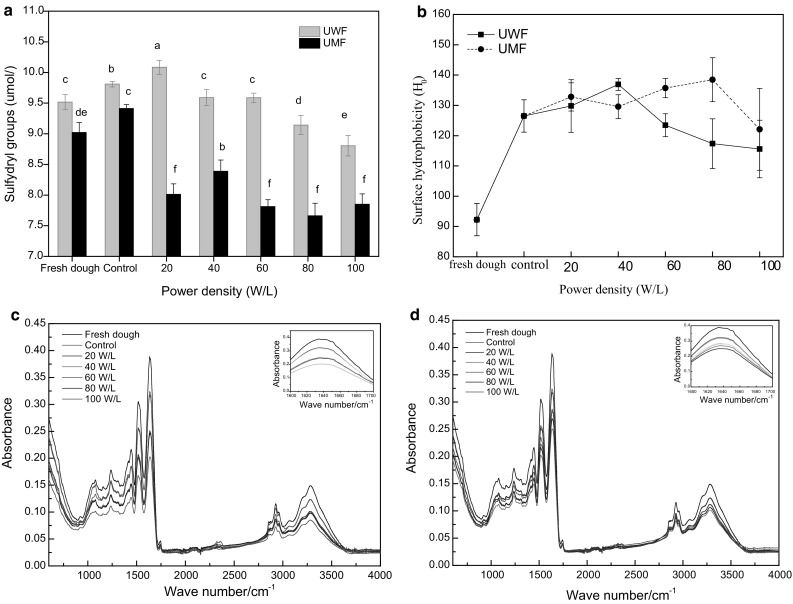
The effect of ultrasound-assisted freezing on sulfhydryl groups and surface hydrophobicity and infrared spectrum of wheat gluten (a sulfhydryl groups, b surface hydrophobicity, c infrared spectrum of UWF, d infrared spectrum of UMF)
The free sulfhydryl content of frozen dough was significantly increased by UWF treatment at 20 W/L, and then decreased with an increased power density when compared with the control. The sulfhydryl content of frozen dough was significantly decreased by the UMF treatment and obtained the lowest value at 80 W/L. Our previous study showed that the sulfhydryl and disulfide bond content of gluten protein was in a dynamic change during ultrasound treatment. Under the action of high intensity ultrasound, the region is destroyed, while the low intensity tends to be synthesized (Zhang et al. 2018b, Zhang et al. 2016). The increase of sulfhydryl content of dough by ultrasound-assisted freezing resulted from the destruction of disulfide bonds by ultrasound. The decrease of sulfhydryl content was attributed to the enhancement of disulfide bonds by the chemical action of ultrasound. The improvement of the freezing process by ultrasound, which prevented the dough from destroying large ice crystals, also contributed to the decrease of sulfhydryl content.
In total, ultrasound-assisted freezing reduced the destructive effect of disulfide bonds on dough, and strengthened the formation of disulfide bonds, leading to an improvement in the quality of the frozen dough.
Surface hydrophobicity of wheat gluten
Hydrophobic interaction is the main force maintaining protein tertiary structure, which is very important for protein conformation, structural stability, and functional characteristics (Wang et al. 2014a). The surface hydrophobicity is an indication of the number of hydrophobic groups on the surface of proteins connected with the external polar water environment (Hou et al. 2004). Figure 2b showed that the control group had greater surface hydrophobicity than fresh dough. During UWF treatment process, the surface hydrophobicity reached the maximum when the power density was 40 W/L and then decreased. During UMF treatment process, the surface hydrophobicity reached the maximum of 80 W/L, and then decreased. The ultrasound-assisted samples obtained the minimum value at 100 W/L. It revealed that freezing exposed a large number of hydrophobic groups, and the effect of ultrasound on hydrophobic groups was in a state of dynamic equilibrium. The ultrasound effect reduced the damage of gluten structure caused by freezing.
Secondary structure of wheat gluten
FTIR is widely used to analyze the secondary structure of all forms of samples. The raw FTIR spectra of wheat gluten were shown in Fig. 2c, d. We studied the effect of ultrasound-assisted freezing on the wheat gluten amide I band. The results after resolving by deconvolution, secondary derivation, and Gaussian curve fitting are summarized in Table 2.
Table 2.
Effect of ultrasound-assisted freezing on secondary structure of gluten
| Samples | α-helix (%) | β-sheet (%) | β-turn (%) | Random coil (%) |
|---|---|---|---|---|
| Fresh dough | 30.09 ± 0.014a | 38.59 ± 0.028d | 14.45 ± 0.014e | 16.88 ± 0.007a |
| Control | 29.34 ± 0.021b | 38.28 ± 0.042e | 15.72 ± 0.014c | 16.66 ± 0.014a |
| UWF-20 | 28.50 ± 0.014c | 41.64 ± 0.014b | 14.45 ± 0.007e | 15.43 ± 0.007c |
| UWF-40 | 28.25 ± 0.014d | 42.05 ± 0.028a | 14.23 ± 0.014f | 15.47 ± 0.007c |
| UWF-60 | 28.90 ± 0.014c | 36.64 ± 0.042g | 18.30 ± 0.014a | 16.15 ± 0.007b |
| UWF-80 | 30.05 ± 0.014a | 37.25 ± 0.028f | 16.14 ± 0.007b | 16.57 ± 0.007a |
| UWF-100 | 29.90 ± 0.021ab | 38.81 ± 0.035c | 14.62 ± 0.007e | 16.69 ± 0.007a |
| UMF-20 | 29.73 ± 0.021c | 37.72 ± 0.035f | 15.81 ± 0.007a | 16.75 ± 0.014b |
| UMF-40 | 30.15 ± 0.007a | 38.24 ± 0.035e | 15.09 ± 0.007c | 16.53 ± 0.007d |
| UMF-60 | 28.43 ± 0.014e | 41.50 ± 0.028c | 14.86 ± 0.007d | 15.22 ± 0.007g |
| UMF-80 | 28.27 ± 0.021f | 41.73 ± 0.014b | 14.71 ± 0.007e | 15.30 ± 0.007e |
| UMF-100 | 28.01 ± 0.014g | 42.21 ± 0.028a | 14.52 ± 0.007f | 15.26 ± 0.007f |
Mean ± SD, Means in same column with different letters are significantly different (P < 0.05)
Table 2 shows that the α-helix and β-sheet contents were significantly decreased by the traditional freezing process. The β-sheet was significantly increased and there was no significant change in random coil. The decrease of α-helix structures indicated weaker hydrogen bond interactions and the adhesion of dough (Wang et al. 2014b). UWF and UMF treatments had a significant effect on the secondary structure of wheat gluten. During UWF treatment, the α-helix structure of the gluten presented irregular phenomenon with an increase of ultrasound power density, but it increased significantly at 80 W/L compared with the control. During UMF treatment, the α-helix first increased and then decreased with an increase of ultrasonic power density, with its maximum value at 40 W/L. The random coil increased with increased ultrasonic power density during UWF treatment, however, the results of UMF treatment were the opposite.
The effects of ultrasound-assisted freezing on the structure of wheat gluten were numerous. Under the combined effects of ultrasonic wave improving gluten protein structure and refining ice crystal, UWF treatment at 80 W/L and UMF treatment at 40 W/L had a positive effect to maintain the secondary structure from destruction by the freezing process.
Microstructure
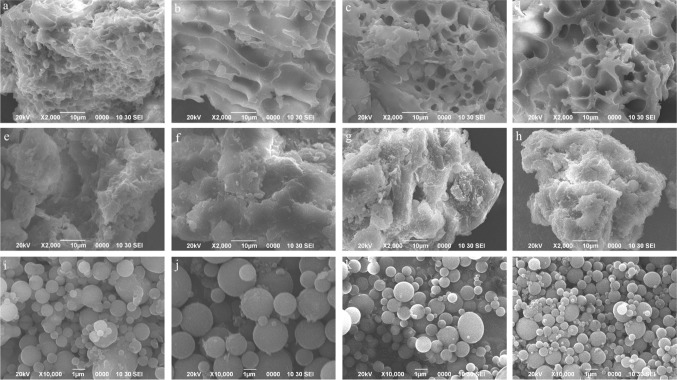
Figure 3 shows that the network structure of the gluten protein was destroyed by the traditional freezing process. The pore structure of the gluten network structure was larger, and the boundary of gluten was incomplete (Fig. 3b) when compared to the samples that were not subjected to freezing (Fig. 3a). However, the protein network of gluten subjected to ultrasound-assisted treatment was more uniform and smaller than that of traditional freezing samples (Fig. 3b), which was similar to the network structure of fresh protein (Fig. 3a). The ultrasound treatment can therefore inhibit the destruction of the protein network structure by ice crystals during the freezing process. There was no significant difference in the SEM data of glutenin with different samples. Figure 3i shows gliadin particles of fresh dough with intact granules and a smooth surface. The gliadin particles of dough after impregnation freezing changed to larger particles with damaged edges (Fig. 3g). However, the gliadin particles of dough after ultrasound-assisted freezing maintained the integrity of the gliadin granules and of the surface. Because of the crystallization of water in the freezing process, large ice crystals formed and damaged the biological tissue, thereby altering the processability. This result showed that ultrasound-assisted freezing can effectively avoid the destruction of gluten structures during freezing.
Fig. 3.
Effect of ultrasound-assisted freezing on SEM of wheat gluten (a gluten of fresh dough, b gluten of control, c gluten of UWF, d gluten of UMF, e glutenin of fresh dough, f glutenin of control, g glutenin of UWF, h glutenin of UMF, i gliadin of fresh dough, j gliadin of control, k gliadin of UWF, l gliadin of UMF)
However, the enhancement of heat and mass transfer by ultrasound enhanced the speed of freezing, accelerated the process of freezing, and promoted the rapid formation of ice crystals. The faster ice crystals are formed, the smaller their particles, and the less destructive effect on dough (Su et al. 2005). However, the mechanical effect of ultrasound breaks large ice crystals and increases the proportion of small ice crystals in dough. These effects produced by ultrasound made the internal structure of frozen dough more uniform, and avoided the damage caused by the freezing process.
Correlation analysis and principal component analysis (PCA) between textural characteristics and the gluten structure of frozen dough
Pearson’s coefficients (Table 3) showed a significant correlation between texture characteristics of dough and the protein structure of wheat gluten. The results indicated that the SH content and the relative percentage content of α-helix significantly contributed to the springiness of dough, the correlation coefficient were 0.50 and 0.55, respectively. The relative percentage content of β-turn structure was significantly contributed by G′ and G″, the correlation coefficient were 0.72 and 0.71, respectively. The relative percentage content of the β-turn also had a significant negative correlation on Pearson’s coefficients with the hardness of dough, the correlation coefficient were 0.59. These results showed that ultrasound-assisted freezing improved the rheological properties and texture characteristics of dough by changing the free sulfhydryl content and secondary structure of gluten.
Table 3.
Pearson analysis between texture characteristics of dough and protein structure of wheat gluten
| Texture characteristics | SH | H0 | α-helix | β-sheet | β-turn | Random coil |
|---|---|---|---|---|---|---|
| G′ | 0.28 | − 0.38 | 0.07 | − 0.28 | 0.72 ** | 0.02 |
| G″ | 0.25 | − 0.33 | 0.04 | − 0.27 | 0.71 ** | 0.01 |
| Tan α | − 0.25 | 0.42 | − 0.28 | 0.16 | − 0.07 | − 0.14 |
| Hardness | 0.01 | 0.29 | − 0.07 | 0.16 | − 0.59* | 0.05 |
| Springiness | 0.50 * | − 0.04 | 0.55 * | − 0.17 | 0.17 | 0.16 |
| Cohesiveness | 0.27 | − 0.04 | 0.12 | 0.15 | − 0.08 | − 0.23 |
| Chewiness | 0.48 | 0.11 | 0.43 | 0.04 | − 0.28 | 0.07 |
| Resilience | − 0.41 | 0.03 | − 0.14 | − 0.01 | − 0.21 | 0.22 |
*Significant correlation at 0.05 level, **Significant correlation at 0.01 level
Conclusion
In this study, the effects of the ultrasound-assisted whole freezing process and maximum ice crystal generation zone on texture characteristics and gluten structure of frozen dough were investigated. The relationship between texture characteristics and gluten structure was evaluated by Pearson’s correlation analysis. The rheological properties and texture properties of dough were improved by the UWF and UMF treatments. The protein secondary structure, content of SH, surface hydrophobicity, and microstructure all significantly changed. There was a significant correlation between the rheological properties and texture properties of dough and protein structures. However, practical application of this knowledge necessitates further study to determine the correlation between the textural and rheological properties of dough and the conformation of gluten protein. The results suggested that the ultrasound-assisted freezing could improve the rheological properties and texture properties by changes in the gluten structure and by reducing the damage of large ice crystals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31701541), the National Key R&D Program of China (No. 2018YFD0400604-02). The Key Research Projects of High Educational Institution (No. 18A550016).
Compliance with ethical standards
Conflict of interest
We declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yinli Li and Yanyan Zhang these two authors have contributed equally to this paper.
References
- Ban C, Yoon S, Han J, Kim SO, Han JS, Lim S, Choi YJ. Effects of freezing rate and terminal freezing temperature on frozen croissant dough quality. LWT Food Sci Technol. 2016;73:219–225. doi: 10.1016/j.lwt.2016.05.045. [DOI] [Google Scholar]
- Bin J, Vassilis K, Stefan K, Douglas GH. Rheological investigation and molecular architecture of highly hydrated gluten networks at subzero temperatures. J Food Eng. 2008;89:42–48. doi: 10.1016/j.jfoodeng.2008.04.001. [DOI] [Google Scholar]
- Chemat F, Huma Z, Khan MK. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Cheng XF, Zhang M, Xu BG, Adhikari B, Sun JC. The principles of ultrasound and its application in freezing related processes of food materials: a review. Ultrason Sonochem. 2015;27:576–585. doi: 10.1016/j.ultsonch.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Dreese PC (1987) Rheological Studies of flour and gluten dough. PhD dissertation. Kansas State University: Manhattan
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Hou DH, Chang SK. Structural characteristics of purified glycinin from soybeans stored under various conditions. J Agric Food Chem. 2004;52:3792–3800. doi: 10.1021/jf035072z. [DOI] [PubMed] [Google Scholar]
- Hu SQ, Liu G, Li L, Li ZX, Hou Y. An improvement in the immersion freezing process for frozen dough via ultrasound irradiation. J Food Eng. 2013;114:22–28. doi: 10.1016/j.jfoodeng.2012.07.033. [DOI] [Google Scholar]
- James C, Purnell G, James SJ. A review of novel and innovative food freezing technologies. Food Bioprocess Tech. 2015;8:1616–1634. doi: 10.1007/s11947-015-1542-8. [DOI] [Google Scholar]
- Kato A, Nakai S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta. 1980;624:13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Kiani H, Zhang ZH, Delgado A, Sun DW. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res Int. 2011;44:2915–2921. doi: 10.1016/j.foodres.2011.06.051. [DOI] [Google Scholar]
- Kiani H, Sun DW, Delgado A, Zhang ZH. Investigation of the effect of power ultrasound on the nucleation of water during freezing of agar gel samples in tubing vials. Ultrason Sonochem. 2012;19:576–581. doi: 10.1016/j.ultsonch.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Kontogiorgos V, Goff HD, Kasapis S. Effect of aging and ice structuring proteins on the morphology of frozen hydrated gluten networks. Biomacromol. 2007;8:1293–1299. doi: 10.1021/bm0610471. [DOI] [PubMed] [Google Scholar]
- Liao L, Zhao MM, Ren JY, Zhao HF, Cu C, Hu X. Effect of acetic acid deamidation-induced modification on functional and nutritional properties and conformation of wheat gluten. J Sci Food Agric. 2010;90:409–417. doi: 10.1002/jsfa.3830. [DOI] [PubMed] [Google Scholar]
- Long GH, Ji YH, Pan BZ, Sun W, Li YT, Qin JX. Characterization of thermal denaturation structure and morphology of soy glycinin by FTIR and SEM. Int J Food Prop. 2015;18:763–774. doi: 10.1080/10942912.2014.908206. [DOI] [Google Scholar]
- Ma S, Li L, Wang XX, Zheng XL, Bian K, Bao QD. Effect of mechanically damaged starch from wheat flour on the quality of frozen dough and steamed bread. Food Chem. 2016;202:120–124. doi: 10.1016/j.foodchem.2016.01.075. [DOI] [PubMed] [Google Scholar]
- Mandal D, Nath N, Sahoo PK (2017) Effect of ultrasonic pretreatment on the osmotic drying of ash gourd during Murabba processing. Computer, Communication and Electrical Technology, pp 233–238
- Mason TJ, Paniwnyk L, Lorimer JP. The uses of ultrasound in food technology. Ultrason Sonochem. 1996;3(3):S253–S260. doi: 10.1016/S1350-4177(96)00034-X. [DOI] [Google Scholar]
- Monroy Y, Rivero S, García MA. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason Sonochem. 2018;42:795–804. doi: 10.1016/j.ultsonch.2017.12.048. [DOI] [PubMed] [Google Scholar]
- Patrick PS, Swaisgood HE. Sulfhydryl and disulfide groups in skim milk as affected by direct ultra-high-temperature heating and subsequent storage 1. J Dairy Sci. 1976;59:594–600. doi: 10.3168/jds.S0022-0302(76)84246-4. [DOI] [Google Scholar]
- Pingret D, Fabiano-Tixier AS, Chemat F. Degradation during application of ultrasound in food processing: a review. Food Control. 2013;31(2):593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- Simal S, Benedito J, Sánchez ES, Rosselló C. Use of ultrasound to increase mass transport rate during osmotic dehydration. J Food Eng. 1998;36(3):323–336. doi: 10.1016/S0260-8774(98)00053-3. [DOI] [Google Scholar]
- Soliman OII, Krenning BJ. A comparison between QLAB and tom tec full volume reconstruction for real time three-dimensional echocardiographic quantification of left ventricular volumes. Echocardiography. 2007;24:967–974. doi: 10.1111/j.1540-8175.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- Song GS, Hu SQ, Li L, Chen P, Shen X. Structural and physical changes in ultrasound-assisted frozen wet gluten. Cereal Chem. 2009;86:333–338. doi: 10.1094/CCHEM-86-3-0333. [DOI] [Google Scholar]
- Su DM, Ding CH, Li LT, Su DH, Zheng XY. Effect of end oxylanases on dough properties and making performance of Chinese steamed bread. Eur Food Res Technol. 2005;220:540–545. doi: 10.1007/s00217-005-1170-z. [DOI] [Google Scholar]
- Sun DW, Li B. Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. J Food Eng. 2003;57:337–345. doi: 10.1016/S0260-8774(02)00354-0. [DOI] [Google Scholar]
- Sun JY, Qian F, Jiang SJ, Tuo YF, Mu GQ. Effect of heat treatments on the secondary structure of milk proteins analyzed by Fourier transform infrared spectroscopy. Food Sci. 2017;38:82–86. [Google Scholar]
- Wang L, Zhu XQ, Jia DL, Hui XU, Zheng HY, Wu HB. Effect of cryopreservation on surface hydrophobicity and disulfide bonds of soybean protein isolate liquid dispersion. Food Sci. 2014;35:28–32. [Google Scholar]
- Wang P, Xu L, Nikoo M, Ocen D, Wu FF, Yang N, Jin ZY, Xu XM. Effect of frozen storage on the conformational, thermal and microscopic properties of gluten: comparative studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014;35:238–246. doi: 10.1016/j.foodhyd.2013.05.015. [DOI] [Google Scholar]
- Wang P, Jin ZY, Xu XM. Physicochemical alterations of wheat gluten proteins upon dough formation and frozen storage-A review from gluten, glutenin and gliadin perspectives. Trends Food Sci Technol. 2015;46:189–198. doi: 10.1016/j.tifs.2015.10.005. [DOI] [Google Scholar]
- Wang JJ, Liu G, Huang YB, Zeng QH, Song GS, Hou Y, Li L, Hu SQ. Role of N-terminal domain of HMW 1Dx5 in the functional and structural properties of wheat dough. Food Chem. 2016;213:682–690. doi: 10.1016/j.foodchem.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Wang YS, Wei ZY, Li Y. Toughening polylactide with epoxidized styrene–butadiene impact resin: mechanical, morphological, and rheological characterization. J Appl Polym Sci. 2018;135:1–8. [Google Scholar]
- Yadav DN, Patki PE, Khan MA, Sharma GK, Bawa AS. Effect of freeze-thaw cycles and additives on rheological and sensory properties of ready to bake frozen chapatis. Int J Food Sci Tech. 2008;43:1714–1720. doi: 10.1111/j.1365-2621.2008.01763.x. [DOI] [Google Scholar]
- Yang X, Li YL, Li SY, Oladejo AO, Wang YC, Huang SF, Zhou CS, Wang Y, Mao L, Zhang YY, Ma HL, Ye XF. Effects of low power density muti-frequency ultrasound pretreatment on the enzymolysis and the structure characterization of defatted wheat germ protein. Ultrason Sonochem. 2017;38:410–420. doi: 10.1016/j.ultsonch.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Ma H, Wang B, Qu WJ, Asif W, Zhou CS. Relationships between the structure of wheat gluten and ace inhibitory activity of hydrolysate: stepwise multiple linear regression analysis. J Sci Food Agr. 2016;96:3313–3320. doi: 10.1002/jsfa.7509. [DOI] [PubMed] [Google Scholar]
- Zhang MC, Niu HL, Chen Q, Xia XF, Kong BH. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci. 2018;136:1–8. doi: 10.1016/j.meatsci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Li YL, Li SY, Zhang H, Ma HL. In situ monitoring of the effect of ultrasound on the sulfhydryl groups and disulfide bonds of wheat gluten. Molecules. 2018;23:1376. doi: 10.3390/molecules23061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YY, Li YL, Liu Y, Zhang H. Effects of multiple freeze-thaw cycles on the quality of frozen dough. Cereal Chem. 2018;95:499–507. doi: 10.1002/cche.10053. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.