Abstract
Background
Oxidative stress is strongly associated with development of diabetes mellitus. F. vulgaris, contains antioxidant ingredients. This study was designed in order to evaluate the effect of F. vulgaris on the damaged liver in diabetic rats.
Methods
In this study, hydroalcoholic extract of F. vulgaris was prepared. Sixty four male rats were divided into 8 groups (n = 8), including saline (normal control), streptozotocin (STZ) (diabetic control) (60 mg/kg), F. vulgaris (50, 100, 150 mg/kg), and STZ plus F. vulgaris (50, 100, 150 mg/kg) were administered through oral gavage on treated group once a day for 28 consecutive days. Serum nitrite oxide (NO) level, Ferric reducing/antioxidant power (FRAP), Malondialdehyde (MDA), liver weight, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), diameter of hepatocytes and central hepatic vein have been examined. Statistical analysis was performed using one-way analysis of variance and the post hoc test.
Result
The outcomes showed that administrating streptozotocin enhanced liver MDA, nitrite oxide, the mean diameter of central hepatic vein and hepatocyte, liver enzymes significantly and reduced liver weight compared to saline group (P < 0.05). Though, administrating F. vulgaris and F. vulgaris plus STZ enhanced liver weight and tissue FRAP level significantly and reduced liver enzymes, NO levels, liver MDA, the mean diameter of hepatocyte and central hepatic vein in entire doses were equal to STZ group (P < 0.05).
Conclusion
It seems that, were equal F. vulgaris might recover liver injuries in diabetic rats.
Keywords: F. vulgaris; Liver; Diabetes mellitus, antioxidants
Background
Diabetes is one of the leading causes of mortality and medical costs in various societies [1]. Liver plays a pivotal role in the metabolism of carbohydrates by regulating the blood glucose [2]. Diabetes-induced oxidative stress is one of the most important causes of histological impairments in diabetic patients [3]. Increased production of free radicals has been observed in diabetic patients [4]. Hyperglycaemia accelerates the production of Reactive Oxygen Species (ROS) in different tissues through glucose oxidation [5]. Although, the exact mechanism of diabetes mellitus is still not well-known, an increase in the production of free radicals is regarded as one of its major destructive mechanisms [6]. Production of free radicals in diabetic patients is one of the reasons for changes which occur in activity of liver enzymes [7]. Streptozotocin is a nitrosourea compound and a factor that induces diabetes in experimental animals [8]. STZ causes the death in beta cells through impairing their membrane [9]. Natural pharmaceutical compounds, have a more sustainable effect than other chemical drugs [10]. Falcaria vulgaris, a member of umbelliferae family which is found in the western and southern part of Iran, which is a wild, fast growing annual plant. In the western part of Iran, it is used as vegetable and in making salad, and it has been used in order to remove the kidney stones and liver problems in the past [11]. Phytochemical studies on this plant have shown that it contains tannin and saponin. This plant also contains vitamin C, phytosterol [12]. Choobkar et al. reported that there are various compounds in F. vulgaris, especially antioxidant and antimicrobial compounds in F. vulgaris [13]. Moreover, the analysis of antioxidant activity of F. vulgaris has shown that the main elements of different parts of this plant can have antioxidant activity [14]. The results of Soudamani et al. showed that, the tannin and saponin in are existed in F. vulgaris [15]. Given the various properties of F. vulgaris, especially containing antioxidants and since no study has ever investigated the effect of F. vulgaris on the diabetes-induced liver damages, this study was aimed to investigate the effects of F. vulgaris extract on the liver impairments which are induced by diabetes in the rats.
Subjects and methods
Preparation of F. vulgaris extract
F. vulgaris extract was collected from western part of Iran. Subsequently confirming by a botanist, the leaves and stems were dried and they were milled in order to prepare plant powders. Herbal powder (100 g) was mixed with ethanol 70% (in a1:4 ratios). The prepared solution was kept for 48 h inside a warm water bath in a condition including 35 °C, in darkness. The solution was filtered using a vacuum pump device. The solution was transferred to a rotary device in order to remove its extraneous solvent. The separation procedure continued till a highly concentrated extract was attained. The extract which was prepared in the process described above was dissolved in purified water [12].
Experimental design and treatments
In this experimental study, animals were divided into 8 groups randomly (n = 8). Group 1, which was normal control (saline) group, received normal saline, other groups like; Group 2, STZ (diabetic control) group was induced by STZ (60 mg/kg); groups 3 to 5, F. vulgaris groups (non-diabetic) were given 50, 100 and 150 mg/kg F. vulgaris respectively and groups 6 to 8, including STZ plus F. vulgaris groups, primarily became diabetic by STZ and then were treated by receiving (50, 100 and 150 mg/kg) F. vulgaris extract. In groups 3 to 5 F. vulgaris was administered through oral gavage once a day for 28 consecutive days. Animals in groups 6–8, received F. vulgaris extract daily for 28 consecutive days 4 weeks after induction of STZ [1, 16].
Animals
Studies on animals were conducted according to the guidelines for the care and handling of animals which are prepared by the Iranian Ministry of Health. Sixty four male wistar rats (weighing 220–250 g) were purchased from Pastur Institute in Iran. The animals were kept in standard cages (three rats in each cage) and control conditions were met including keeping temperature at 23 ± 2 °C and exposing rats to 12- h light/dark cycle, in animal care facilities of University of Medical Sciences for a week before testing and exposing to environmental and climatic conditions. The animals had free access to water and food during this period [16]. All investigations conformed with the ethical and humane principles of research and were approved by the Ethical Committee of Medical Sciences (ethics certificate No.1395.202).
Inducing diabetic with STZ
For creating an animal diabetic, a solitary dose of STZ which was dissolved in normal saline (60 mg/kg) was used intraperitoneally (the saline group was given the same volume of normal saline). Seventy-two h after STZ injection, through process of glucose oxidase enzyme (using glomer) blood glucose was measured using blood samples which were from the animal’s tail, and animals with serum glucoses̓ level which is greater than or equal to 300 mg/dl were considered as diabetic [1].
Collecting blood serum and measuring livers̓ weight
All animals were anesthetized with chloroform, sacrificed and bloods were taken through cardiac puncture from right ventricle. The samples were incubated at 37 °C to be accumulated. Then, the coagulated samples were centrifuged for 15 min at 4000 rpm until the serum was separated. The separated serum was kept at −18 °C until the measuring nitrite oxide and levels of biological factors of liver. Animals were slayed and livers were removed and weighed on a microbalance sensitive to 0.001 mg (Precisa 125A, Switzerland) and average weights regarding the livers of animals were calculated and documented [10].
Biological factors
The liver was fragmented and turned into a homogenous solution. In order to separate the biological enzymes, the prepared solution was centrifuged for 20 min at 10,000 rpm. The over part of the solution was separated to measure the enzymes. ALT, and AST actions were examined using the method of Reitman and Frankel [17]. ALP actions were determined pursuant to the technique which is defined in laboratory practical manual [2].
Histological and morphometric examinations
The lower one cm-long slice of the right lobe of the liver in horizontal sections was removed, washed in saline, then fixed in 10% formalin, was dehydrated in ascending concentration ethanol, then was cleared in xylene and was embedded by paraffin. Tinny sections (4 mm) were cut using a microtome (Germany) and became noticeable using haematoxylin and eosin. For each hepatocyte, the complete cellular area was measured. Outline of hepatocyte was measured subsequently by taking an image with a × 40 objective. The largest and smallest axis was measured in drawing each hepatocyte in order to estimate the mean axis. At least 50 hepatocytes from each region were measured in each liver. A separate measurement for central hepatic vein was performed, using the same examination. The planning was observed using an Olympus BX-51 T-32E01 research microscope which was connected to a DP12 Camera with 3.34-million pixel resolution and Olysia Bio software (Olympus Optical Co. LTD, Tokyo, Japan) [10].
NO analysis
Nitrite oxide was measured using Griess test through microplate method. In order to measure nitrite concentration, de-freezing was carried out on the serum samples subsequently, in this survey, supernatant (500 μl) was deproteinized t using zinc sulfate (9 mg, zinc sulphate powder was mixed with 500 μL serum and was mixed in vortex mixer for 1 min) using centrifugation. One hundred μl supernatant was taken and 100 μl vanadium chloride, 100 μl N-(1- naphthyl) ethylenediamine dihydrochoride and 100 μl sulfonamide solutions were used as supplementary. Standard solutions of sodium nitrate which was ready with various concentrations of nitrate and the standard curve of nitrite concentration is calculated. Samples’ optical density was assessed using ELISA reader at the wavelength of 560 nm [2].
Oxidative stress
In order to assess oxidative stress, the thiobarbituric acid reactive species were measured using MDA which examined the last product of lipid peroxidation in liver tissue by performing colorimetric analysis. In brief, 1400 μl of acetic acid (Sigma, USA), and 1400 μl of sodium dodecyl sulphate (Sigma, USA) were added to 100 μl of liver homogenate and the mixture was animated for 50 min. Four ml of 1-butanol (Sigma, USA) was added to the combination and was mixed in vortex mixer for 2 min through centrifugation at 5000 rpm for 15 min. The absorbance level of the higher layer was measured at 532 nm (Spectro; Germany) and sequential concentrations of tetraethoxypropane (Sigma, USA) were used as the external standard. The antioxidant capacity of the liver was measured using FRAP assay. The FRAP substance consisted of 30 ml of acetate buffer (Sigma, USA) and 1.5 ml chloride ferric (Sigma, USA). In brief, 60 μl of kidney homogenate was added to 1.5 ml of newly prepared one. FRAP substance (Sigma, USA) was put in a test tube and incubated at 37 °C for 10 min. The absorbance of the blue-colored complex was read against a blank at 593 nm. Sequential concentrations of FeSO4.7H2O (Sigma, USA) were used as an external standard [17].
Statistic
After obtaining the information, Kolmogorov–Smirnov test was first performed to confirm the data compliance of the normal distribution. One way analysis of variance was used for statistical analysis and Tukey post hoc test was used to determine the difference between the groups. SPSS 16 was used for data analysis, and the results were expressed as mean ± standard error, and p < 0.05 was considered significant.
Results
Weight of livers
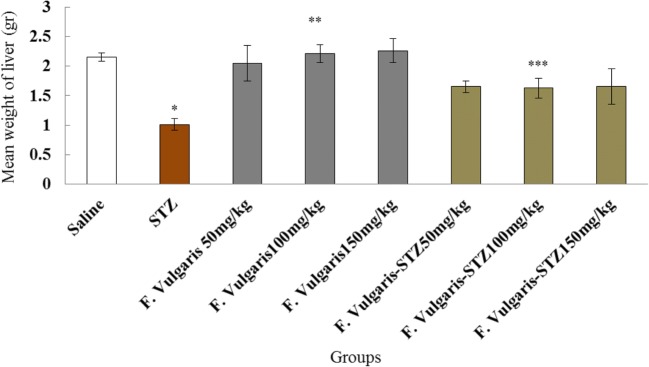
In the present study, STZ caused a significant decrease in livers weight compared to normal control group (p < 0.05). F. vulgaris improved livers weight in treated animals of all doses which were equated with the diabetic control group (p < 0.05). The mean livers weight was not significant in all F. vulgaris groups compared to the normal control group (P > 0.05). Also, livers weight was improved significantly in animals which were treated with F. vulgaris and F. vulgaris plus STZ in all doses compared to the diabetic control group (p < 0.05) (Fig. 1).
Fig. 1.
Result of administrating STZ, F. vulgaris and F. vulgaris plus STZ on weight of liver.*Significant decrease in weight in diabetic control group which is equated to normal control (saline) group (P < 0.05). **Significant growth in all doses of F. vulgaris groups compared to the diabetic control group (P < 0.05). ***Significant increase in all doses of F. vulgaris plus STZ groups compared to the diabetic control group (P < 0.05)
Biological factors
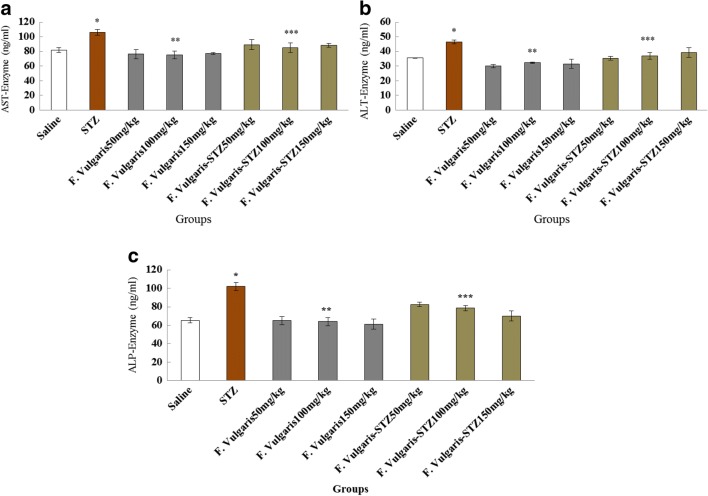
STZ caused a significant growth in the mean of ALT, AST and ALP enzymes which was equated to normal control group (p < 0.05). The mean ALT, AST and ALP enzymes concentration was not significant in all F. vulgaris groups compared to the normal control group (P > 0.05). Moreover, the mean of ALT, AST and ALP enzymes reduced significantly in all groups which received F. vulgaris and F. vulgaris plus STZ compared to the diabetic control group(p < 0.05) (Fig. 2).
Fig. 2.
Effect of administrating STZ (diabetes), F. vulgaris and F. vulgaris plus STZ on liver enzymes of Sixty four rats which were similarly separated into 8 groups. a AST enzyme, b ALT enzyme and c ALP enzyme. *Significant growth in enzymes in diabetic control group compared to the normal control group (P < 0.05). **Significant decrease in all doses of F. vulgaris group compared to the diabetic control group (P < 0.05). ***Significant decrease in all F. vulgaris plus STZ groups compared to the diabetic control group (P < 0.05)
Morphometric measurements
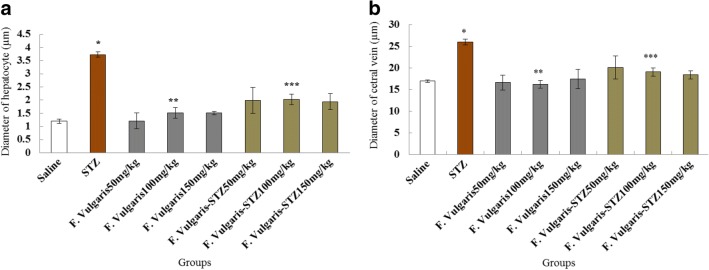
The investigation of the mean diameter of hepatocytes and central hepatic veins in experimental groups revealed a significant alteration among normal control and diabetic and STZ plus F. vulgaris groups (p < 0.05). The mean diameter of hepatocytes and central hepatic veins was not significant in all F. vulgaris groups compared to the normal control group (P > 0.05). Further, F. vulgaris and F. vulgaris plus STZ caused a significant decrease in the mean diameter of hepatocytes and central hepatic vein in all of treated groups compared to the diabetic control group (p < 0.05) (Fig. 3).
Fig. 3.
Effect of administrating STZ, F. vulgaris and F. vulgaris plus STZ on the mean diameter of hepatocytes (a) and central diameter of hepatic veins (b). *Significant increase in the mean hepatocytes and central hepatic vein diameters in diabetic control group which is equated to saline group (P < 0.05). **Significant decrease in all doses of group which were treated with F. vulgaris compared to the diabetic control group (P < 0.05). ***Significant decrease in all F. vulgaris plus STZ groups compared to the diabetic control group (P < 0.05)
Histopathological changes
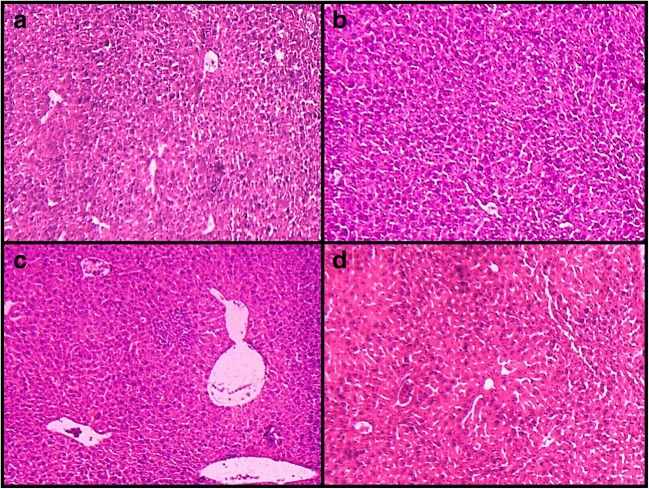
Histological analysis showed normal livers̓ structure in the normal control and F. vulgaris treatment group. After treating with STZ in diabetic control group, the liver showed evident changes and injury. These anomalies included increased white blood cells (Inflammation), increased irregularities, sinusoidal dilatation and the vacuolization hepatocyte (necrosis). Treating with F. vulgaris plus STZ at all doses reduced the liver damage which was caused by STZ toxicity (Fig. 4).
Fig. 4.
Microscopic images of liver tissue in mature rats in different groups (Five-micron thick sections, H&E staining, magnification×100). Micrograph of the liver section in the control normal groups (a), normal liver structure. Micrograph of the liver section in F. vulgaris (150 mg/kg) group (b), normal liver structure. Micrograph of the liver section in diabetic control group (c), increased white blood and macrophage cells (Inflammation) (black arrows), and central hepatic vein dilatation (blue arrows) and hyperemia (yellow arrows), due to the oxidative stress which is caused by STZ. Micrograph of liver section in F. vulgaris plus STZ (150 mg/kg) group (d), normal liver structure
NO analysis
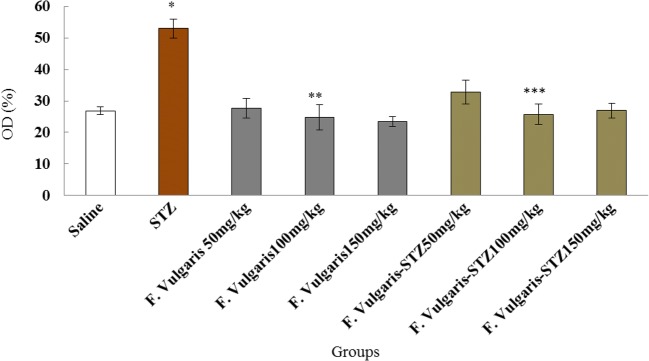
The results of blood serum NO analysis showed a significant growth in STZ group which was equated to normal control group (p < 0.05). The mean nitrite oxide in the blood serum was not significant in all F. vulgaris groups compared to the normal control group (P > 0.05). Moreover, the mean of NO in serum reduced significantly in F. vulgaris and F. vulgaris plus STZ in all doses compared to diabetic control group (p < 0.05) (Fig. 5).
Fig. 5.
Effects of administrating F. vulgaris, STZ and F. vulgaris plus STZ on the mean NO levels *Significant increase in NO in diabetic control group compared to the normal control group (P < 0.05). **Significant decrease in all F. vulgaris groups compared to the diabetic control group (P < 0.05). ***Significant decrease in administrating all doses of F. vulgaris plus STZ groups compared to the diabetic control group (P < 0.05)
Oxidative stress
The results of testing oxidative stress in the groups showed that the liver MDA level increased significantly in the diabetic control group compared to the normal control group (P < 0. 05). The liver MDA level decreased significantly in all F. vulgaris plus STZ groups compared to the diabetic control group (P < 0.05). Similarly, diabetes decreased the liver tissue FRAP level of the diabetic control group significantly compared to the normal control group (P < 0.05). Administrating F. vulgaris increased the FRAP level in the liver tissue in all F. vulgaris plus STZ groups significantly compared to the diabetic control group (P < 0.05). The effect of Treating with F Vulgaris on the liver tissue FRAPS and liver MDA levels in all groups, was not significant compared to the normal control group (p > 0.05) (Fig. 6).
Fig. 6.
Comparison of STZ, saline and F. vulgaris groups of: a liver MDA level; b tissue FRAP level. *Significant increase in diabetic control group compared to normal control group (p < 0.05). **Significant decrease in all F. vulgaris groups compared to the diabetic control group (p < 0.05). ***Significant decrease in all F. vulgaris plus STZ groups compared to the diabetic control group (p < 0.05). ¶Significant decrease in diabetic control group compared to the normal control group (p < 0.05). ††Significant increase in all F. vulgaris groups compared to the diabetic control group (p < 0.05). ‡Significant increase in all F. vulgaris plus STZ groups compared to the diabetic control group (p < 0.05)
Discussion
Diabetes is a chronic illness, Hyperglycaemia is considered as one of the major characteristics of this disease. Hyperglycaemia can influence the performance of different organs, including liver in long term [1]. Liver dysfunction is seen frequently in the diabetic patients, especially those whose blood glucose is not controlled well [2]. Although the exact mechanism of diabetes mellitus is still not well-known, it seems that, increased production of free radicals is one of its major destructive mechanisms [3]. The present study examined the effect of hydroalcoholic extract of F. vulgaris on the improvement of liver damage in diabetic rats. The results of histopathology analysis of the diameter of hepatocytes and central vein in study groups indicated a significant increase in the size of hepatocytes and central vein in the diabetic control group compared to the normal control group. Also, all groups receiving F. vulgaris extract showed a significant decrease in the diameter of hepatocytes and central vein compared to the diabetic control group. Oxidative stress can cause cell damage and pathologic conditions such as liver cirrhosis and fibrosis in the body by producing free radicals like superoxide anions and hydroxyl radical [18]. The STZ-induced diabetes in the current study, could cause the increased size and swelling in hepatocytes and central vein. It seems that, depletion of antioxidant resources and reduced activity of enzymes in antioxidant defence system in diabetic patients impaires the antioxidant defence mechanism of the body, activating the intracellular signalling pathways which are susceptible to oxidative stress and increasing the expression of genes which are involved in inflammation, thereby increasing inflammation and tissue damage [19]. The findings of Nakagawa et al. revealed that diabets caused apoptosis and liver cells̓ death by inducing inflammation and oxidative stres in endoplasmic reticulum, which is in line with the results of the present study [20]. The antioxidant properiries of F. vulgaris seem to be the main reason for neutralizing the effect of diabetes on liver in the present study. Further, saponin which is extracted from the plants can have pharmacologic effects in the treatment and control of diseases like liver disorders [21]. The study which is conducted by Qu et al. indicated that, saponin which is extracted from actinidia valvate dunn has antioxidant properties, protecting the liver against inflammation induced by carbon tetrachloride, which is similarity to the results of the present research [22]. The results of weight analysis of rats at the beginning and end of the experiment showed that, their weight was reduced significantly at the end of experiment in diabetic control group compared to the normal control group. Moreover, compared to diabetic control group, all groups receiving F. vulgaris extract showed a significant increase in the weight. Apparently, during suffering from type I diabetes, because the body cannot use the glucose in blood due to dysfunction in insulin production, it uses other sources like lipids and sometimes proteins; therefore, the diabetic animals become very thin [23]. Saponin can have protective effects against pancreatic beta cells and increase insulin secretion. Furthermore, the properties of saponin in reducing blood glucose [24] as well as presence of vitamin C, protein and phytosterol in F. vulgaris extract can be the reasons for the increased weight in diabetic animals following treatment with F. vulgaris extract [13]. In addition, it seems that, flavonoids exert their anti-diabetic effects through insulin secretion, reduced apoptosis and increased proliferation of pancreatic beta cells, improved hyperglycemia through regulating glucose metabolism in hepatocytes and increased glucose absorption from skeletal muscles and fatty tissue [25]. The findings of Nimenibo-Uadia et al. are in line with the results of the present study regarding administrating vernomia amygdalina extract (alkaloids, saponin and flavonoids) to diabetic rats which improved their lost weight [26]. The current study showed serum NO level in diabetic control group was significantly increased compared to control normal group. Also, all groups receiving F. vulgaris extract showed a significant decrease in NO compared to the diabetic control group. NO is an active nitrogenous type of free radicals. Increased NO production leads to cascade reaction and production of free radicals consequently [27]. NO can act like a double-edged sword, when it has both very low and very high pathologic concentrations (10). It seems that high NO production impairs the performance and reduces the vitality of beta cells producing insulin [28]. Different flavonoids have numerous anti-inflammatory and anti-analgesic effects. They can reduce intracellular calcium and activity of NO-synthesizing enzyme by inhibiting the activity of n-methyl-d-aspartate [29]. On the other hand, saponin seems to inhibit induced nitrite oxide synthase (iNOS) [30]. The results of Kang et al.s̓ study indicated that saponin in soybean regulated the reduced expression of iNOS mRNA protein, which is in line with the findings of the present study [31]. The analysis of serum levels of liver enzymes in this study showed that STZ administrating in diabetic control group increased the serum level of ALT, AST and ALP enzymes significantly compared to the normal control group. Furthermore, the groups receiving the extract showed a significant decrease in serum levels of liver enzymes compared to the diabetic control group. In evaluating liver damage, the levels of enzymes such as ALP, ALT and AST are assessed extensively. Necrosis or cell membrane damage leads to the release of these enzymes into blood circulation [2]. It seems that, diabetes-induced hyperglycemic, through increasing the glycation of antioxidant, super-oxidase dismutase, glutathione, peroxidase and catalase enzymes, reduces their activity in order to eliminate ROS, and an increase in ROS is the most important factor which is involved in the secondary disorders of diabetes, including destruction of liver cells̓ structure and increasing the activity of AST enzyme [32]. Perhaps the antioxidant properties of F. vulgaris were the most significant factor in reducing the effects of diabetes on the liver cells in the present study [13]. The findings of Uluisik et al.s̓ study indicated that, administrating ginseng (which is rich in saponin) reduced the serum levels of ALT, AST and ALP enzymes significantly in the male rats having high-fat diet, which is in line with to the results of the present study [33]. The results of histopathology analysis in the current study showed that, lobular and cellular structure of liver was normal in the group receiving F. vulgaris extract and saline (normal control) group, and impairment regarding histopathology was seen in the diabetic control group. Apparently, the invasion of free radicals to liver cells causes necrosis in parenchymal cells, and these cells induce inflammatory responses in the liver and consequently induce the invasion of mononuclear inflammatory cells to the liver damaged tissue. On the other hand, the necrotic cells of liver release the proinflammatory mediators, which intensify the liver impairments [34]. The results of current study also showed that F. vulgaris is able to reduce lipid peroxidation (decreased MDA) and increase anti-oxidant capacity (increased FRAP) of liver tissue, thus reducing oxidative stress. Consistent with these findings, a large body of studies has shown anti-oxidant properties for F. vulgaris (11, 12, 35). Thus, it appears that F. vulgaris could reduce MDA and increase FRAPS through its anti-oxidant properties in the treatment groups by inhibiting the production of Reactive Oxygen Species. Based on the results of the present study, it seems that, using F. vulgaris extract at the doses which were used in this study can have positive effects due to the presence of saponin and other antioxidant compounds in it, which reduce liver damage in diabetic rats.
Our study had certain limitations; the lack of detecting antioxidant levels in the plant. There was a lack of references about the plant or extract. The plant or its extract was not available in the market and we had to wait until the planting season (spring). Death of some animals due to STZ administration in this study was the most important limitations. Hence prospective studies should be taken for detailed association of the molecular interaction between F. vulgaris and liver in diabetic rats.
Conclusion
The current study indicated that F. vulgaris extract can recover some liver injuries significantly contrary to the destructive properties of STZ which induce diabetes in rats. The results also proved the potential effects of F. vulgaris extract particularly antioxidant properties against toxic effects of diabetes. The antioxidant effects of F. vulgaris extract might be a main cause for its positive effect. Though, further experiments are needed to explain its exact mechanism of action.
Acknowledgements
We gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences (no: 95202) for the financial support. This work was performed in partial fulfillment of the requirements for MD of Nazanin Najari in faculty of medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Authors’ contributions
MRS and NN carried out the experiments, analyzed and interpreted the data, and drafted the manuscript. MMM and SR designed the study and participated in analysis and interpretation of data. CJ coordinated the study, revised the manuscript and approved the final version to be submitted for publication and helped in the analysis and interpretation of data. All authors read and approved the final manuscript.
Funding
This study was funded by Kermanshah University of Medical Sciences (no: 95202).
Data availability
The data analyzed and materials used in this study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Reza Salahshoor, Phone: 0098-09188360349, Email: reza.salahshoor@yahoo.com.
Mohammad Mehdi Mohammadi, Phone: 0098-09187212470, Email: mehdi20.mohamadi@gmail.com.
Shiva Roshankhah, Phone: 0098-09122263729, Email: roshankhah@yahoo.com.
Nazanin Najari, Phone: 0098-09128242476, Email: c20.kums@gmail.com.
Cyrus Jalili, Phone: 0098-09188317220, Email: c20.jalili@gmail.com.
References
- 1.Ghorbani R, Mokhtari T, Khazaei M, Salahshoor MR, Jalili C, Bakhtiari M. The effect of walnut on the weight, blood glucose and sex hormones of diabetic male rats. Int J Morphol. 2014;32:858–863. doi: 10.4067/S0717-95022014000300015. [DOI] [Google Scholar]
- 2.Salahshoor MR, Mojtaba K, Roshankhah S, Seyran K, Cyrus J. Protective effect of crocin on liver toxicity induced by STZ. Res Pharm Sci. 2016;11(2):120–129. [PMC free article] [PubMed] [Google Scholar]
- 3.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 4.Maritim A, Sanders A, Watkins J., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 5.Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann N Y Acad Sci. 2005;1043:440–451. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- 6.Babizhayev MA. Advanced glycation end products: free radical generation by early glycation products as a mechanism for long-term complications of diabetes mellitus: toxicity, regulation, function and role in health, nutrition and disease. Qual Prim Health Care. 2017;1:1–001. [Google Scholar]
- 7.Eze E, Dawud F, Zainab A, Jimoh A, Malgwi I, Isa A. Preliminary studies of effects of vitamin C and zinc on some liver enzymes in alloxan-induced diabetic wistar rats. Asian J Med Sci. 2012;4:17–22. [Google Scholar]
- 8.zkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 9.Jung EH, Ran Kim S, Hwang IK, Youl Ha T. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 10.Salahshoor MR, Mohamadian S, Kakabaraei S, Roshankhah S, Jalili C. Curcumin improves liver damage in male mice exposed to nicotine. J Tradit Complement Med. 2016;6:176–183. doi: 10.1016/j.jtcme.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaberian H, Piri K, Nazari J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013;136:237–244. doi: 10.1016/j.foodchem.2012.07.084. [DOI] [PubMed] [Google Scholar]
- 12.Khazaei M, Salehi H. Protective effect of Falcaria vulgaris extract on ethanol induced gastric ulcer in rat. Iran J Pharmacol Ther. 2006;5:43–46. [Google Scholar]
- 13.Choobkar N, Kakoolaki S, Mohammadi F. The biological effects of herbal medicine, Falcaria vulgaris: an article review. Iran J Aquat Anim Health. 2017;3:74–81. [Google Scholar]
- 14.Torrico F, Cepeda M, Guerrero G, Melendez F, Blanco Z, Canelón DJ, Diaz B, Compagnone RS, Suárez AI. Hypoglycaemic effect of Croton cuneatus in streptozotocin-induced diabetic rats. Rev Bras. 2007;17:166–169. [Google Scholar]
- 15.Soudamani S, Yuvaraj S, Malini T, Balasubramanian K. Experimental diabetes has adverse effects on the differentiation of ventral prostate during sexual maturation of rats. Anat Rec A: Discov Mol Cell Evol Biol. 2005;287:1281–1289. doi: 10.1002/ar.a.20250. [DOI] [PubMed] [Google Scholar]
- 16.Najafi HO, Ashtiyani SC, Madani SH, Fakhri SA, Yarijani ZM, Hazem ME. Therapeutic effects of curcumin on renal tissue damages induced by ischemia reperfusion in rat. koomesh. 2015;16:273–281. [Google Scholar]
- 17.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Jalili C, Tabatabaei H, Kakaberiei S, Roshankhah S, Salahshoor MR. Protective role of Crocin against nicotine-induced damages on male mice liver. Int J Prev Med. 2015;6:92. doi: 10.4103/2008-7802.165203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangvarasittichai S. Serum levels of malondialdehyde in type 2 diabetes mellitus Thai subjects. Siriraj Med J. 2017;61:20–23. [Google Scholar]
- 20.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q, Zhang S, Zheng L, He M, Huang R, Lin X. Hepatoprotective effects of total saponins isolated from Taraphochlamys affinis against carbon tetrachloride induced liver injury in rats. Food Chem Toxicol. 2012;50:713–718. doi: 10.1016/j.fct.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Qu L, Xin H, Zheng G, Su Y, Ling C. Hepatoprotective activity of the total saponins from Actinidia valvata dunn root against carbon tetrachloride-induced liver damage in mice. Evid Based Complement Alternat Med. 2012;2012:6. doi: 10.1155/2012/216061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashraf H, Heydari R, Nejati V, Ilkhanipoor M. Preventive effect of Berberis integerrima on the serum levels of glucose and lipids in streptozotocin (stz)-induced diabetes in rats. J Fasa Univ Med Sci. 2012;2:148–155. [Google Scholar]
- 24.Choi MR, Kwak SM, Bang SH, Jeong JE, Kim DJ. Chronic saponin treatment attenuates damage to the pancreas in chronic alcohol-treated diabetic rats. J Ginseng Res. 2017;41:503–512. doi: 10.1016/j.jgr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavle N, Shukla P, Panchal A. Role of flavonoids and saponins in the treatment of diabetes mellitus. J Pharm. 2016;6:41–53. [Google Scholar]
- 26.Nimenibo-Uadia R. Effects of Vernonia amygdalina in alloxan-induced diabetic albino rats. J Med Lab Sci. 2003;12:25–31. [Google Scholar]
- 27.Jalili C, Salahshoor MR, Jalili F, Kakaberaei S, Akrami A, Sohrabi M, et al. Therapeutic effect of F. vulgaris on STZ-induced damage in male reproductive system of mice by reducing nitrite oxide serum level. Int J Morphol. 2017;35:1342–1347. doi: 10.4067/S0717-95022017000401342. [DOI] [Google Scholar]
- 28.Salahshoor MR, Mozafari F, Gholami MR, Roshankhah SH, Jalili C. Thymoquinone prevents mice pancreas injuries against STZ. Pharmacophore. 2017;8:24–31. [Google Scholar]
- 29.Cho H, Yun C-W, Park W-K, Kong J-Y, Kim KS, Park Y, Lee S, Kim BK. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol Res. 2004;49:37–43. doi: 10.1016/S1043-6618(03)00248-2. [DOI] [PubMed] [Google Scholar]
- 30.Starec M, Waitzova D, Elis J. Evaluation of the analgesic effect of RG-tannin using the “hot plate” and “tail flick” method in mice. Cesk Farm. 1988;37:319. [PubMed] [Google Scholar]
- 31.Kang J-H, Sung M-K, Kawada T, Yoo H, Kim Y-K, Kim J-S, et al. Soybean saponins suppress the release of proinflammatory mediators by LPS-stimulated peritoneal macrophages. Cancer Lett. 2005;230:219–227. doi: 10.1016/j.canlet.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 33.Uluısık D, Keskin E. Hepatoprotective effects of ginseng in rats fed cholesterol rich diet. Acta Sci Vet. 2016;44:01–05. [Google Scholar]
- 34.Raushan HR, Seth PK. Bihavioral neurochemical and neuromorphological effects of deltamethrin in adult rats. J Toxicol Environ Health. 2010;48:515–516. doi: 10.1080/009841096161212. [DOI] [PubMed] [Google Scholar]
- 35.Jalili C, Kamani M, Roshankhah S, Sadeghi H, Salahshoor MR. Effect of Falcaria vulgaris extracts on sperm parameters in diabetic rats. Andrologia. 2018;50:e13130. doi: 10.1111/and.13130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed and materials used in this study are available from the corresponding author on reasonable request.