Abstract
β-Carotene is a member of the carotenoid family and is a red–orange pigment abundantly present in many vegetables and fruits. As an antioxidant, it eliminates excessive reactive oxygen species generated in the body. Accordingly, it has potential to be used in the pharmaceutical, food, and cosmetic industries. β-Carotene has a very low water solubility and low bioavailability; thus, there is a need to develop techniques to overcome these issues. In this study, we aimed to enhance the water solubility of β-carotene by using hot-melt technology, a type of solid dispersions technology. When preparing β-carotene solid dispersion using this method, suitable conditions for the emulsifiers and mixing ratios were investigated using water solubility as an index. Setting the weight ratio of β-carotene:polyvinylpyrrolidone:sucrose fatty acid ester to 10%:70%:20% resulted in the poorly-water soluble β-carotene showing improved water solubility (120 μg/mL). The physicochemical properties of the optimized β-carotene solid dispersion were analyzed using field emission scanning electron microscopy, differential scanning calorimetry, and powder X-ray diffraction. The solid dispersion was found to have an amorphous structure. The improved solubility observed for β-carotene in the solid dispersions developed in this work may make these dispersions useful as additives in foods or in nutraceutical formulations.
Keywords: β-Carotene, Emulsifier, Water solubility, Hot-melt technology, Solid dispersion, Amorphous
Introduction
β-Carotene is a member of the carotenoid family and is a red–orange pigment abundantly present in many vegetables and fruits (Johnson 2002). It is known that β-carotene acts as provitamin A, a precursor of vitamin A in vivo (Weber and Grune 2012). Therefore, it is expected to exhibit the pharmacological actions of vitamin A. These include the maintenance of healthy skin and mucosa and improvement in vision. As an antioxidant, β-carotene eliminates excessive reactive oxygen species generated in the body and therefore contributes to the prevention of degenerative diseases such as cardiovascular diseases, diabetes, and several types of cancer (Fiedor and Burda 2014). It can be used as an effective pharmaceutical agent, in foods, and in cosmetics. However, its use is restricted owing to its very low water solubility and low bioavailability (Piorkowski and McClements 2014). It is also an unstable compound in the presence of oxygen, light, heat, and acid (Fiedor and Burda 2014). Therefore, when developing products using β-carotene, it is important to develop strategies to improve its water dispersibility, chemical stability, and bioavailability.
Various formulation processing techniques have been reported to increase the solubility and bioavailability of poorly water-soluble compounds (Aungst 2017). Specifically, the solid dispersion technique is a promising strategy for the enhancement of water solubility of hydrophobic molecules (DeBoyace and Wildfong 2018). Polymers such as polyvinylpyrrolidone (PVP), hydroxypropylmethyl cellulose (HPMC), and hydroxypropyl cellulose (HPC), and sugar alcohols such as mannitol are generally prepared as carriers for solid dispersions. For β-carotene, there are several reports of solid dispersions made with these carriers (Sutter et al. 2007). However, these studies evaluated the physicochemical properties mainly related to the stability of β-carotene without investigating its solubility in water. Improving the solubility of β-carotene in water is expected to be difficult even if a solid dispersion is prepared using conventional methods. To dissolve β-carotene, which has a very low water solubility, a new solid dispersion technique is required.
In this study, we aimed to enhance the water solubility of β-carotene using the hot-melt technology, a type of solid dispersion technology in which a solid dispersion is prepared by melting the compound and the polymer at a high temperature, followed by mixing (Repka et al. 2018; Maniruzzaman et al. 2012). Preparation via the hot-melt technology can result in an amorphous solid dispersion, and the solubility and dissolution rate of a poorly water-soluble compound may improve. Since present in a solid amorphous dispersion, a poorly water-soluble compound can freeze inside the polymer when dispersed, inhibiting nucleation and the crystallization process (Baghel et al. 2016; Theil et al. 2017). This method is also suitable for the food industry as large-scale production can be achieved without the use of organic solvents. Therefore, we devised a novel hot-melt technology where an emulsifier was added to the compound and polymer in an attempt to solubilize β-carotene. To prepare a β-carotene solid dispersion using this method, suitable conditions for the emulsifiers and mixing ratios were investigated using the solubility in water as an index.
Materials and methods
Material
β-Carotene was purchased from Nacalai Tesque, Inc. (Kyoto, Japan). PVP (Kollidon 25) was kindly provided by BASF Japan Ltd. (Tokyo, Japan). For the emulsifier, Mitsubishi Chemical Foods Co., Ltd. (Tokyo, Japan) provided polyglycerol fatty acid ester (L-7D) and sucrose fatty acid ester (S-1670). Sorbitan fatty acid ester (L-10V) and Tween 20 (L-120V) were provided by Kao Corporation (Tokyo, Japan).
Preparation of solid dispersion
β-Carotene, PVP, and emulsifier were mixed in the following proportions to yield 1 g of β-carotene: PVP: emulsifier: 10:90:0, 10:80:10, 10:70:20, 10:45:45, 10:20:70, or 10:0:90 (w/w). The mixture was then placed in a 20-mL glass vial and kneaded on a hotplate at 190 °C for 30 min using a microspatula. After the product was cooled in the dark at room temperature, it was pulverized in an agate mortar and passed through a 150-μm sieve. The prepared solid dispersion was placed in an amber bottle and stored in a desiccator at room temperature.
Solubility test and HPLC analysis
The prepared solid dispersion (100 mg; equivalent to 10 mg β-carotene) was added to 10 mL of phosphate buffer (pH 6.8). After shaking at 37 °C for 60 min, the sample was centrifuged and the supernatant passed through a 0.2-μm filter. The solution passed through the filter was used for HPLC analysis. The concentration of β-carotene was measured by HPLC on a system equipped with an LC-20AD pump and an SPD-M20 detector (Shimadzu, Japan). The reverse-phase column (CAPCELL PAK C18 MGII, 4.6 mm × 100 mm, 5 µm; Shiseido Co., Ltd., Tokyo) was operated at 35 °C. The mobile phase was prepared with 50 mg of dibutylhydroxytoluene, 20 mL of isopropyl alcohol, 0.2 mL of N-ethyldiisopropylamine, 25 mL of 0.2% ammonium acetate solution, and 500 mL of acetonitrile. The volume was then adjusted to 1 L using methanol and degassed with ultrasound prior to use (Schierle et al. 2004). The flow rate was 1 mL/min, and the detection wavelength was 450 nm. The HPLC system was calibrated with standard solutions of 62.5–4000 ng/mL of β-carotene dissolved in acetonitrile, methanol, and tetrahydrofuran (4:4:2, v/v). In this study, limits of detection and limits of quantification of β-carotene were 31.25 ng/mL and 62.5 ng/mL.
Field emission scanning electron microscope (FE-SEM) analysis
Particle morphology, size, and shape were analyzed by FE-SEM using a JSM-6335F scanning electron microscope (JEOL, Japan). All micrographs were the products of secondary electron imaging used for surface morphology identification at 2500 × magnification.
Differential scanning calorimetry (DSC)
Thermograms of samples were taken using a DSC calorimeter (ThermoPlusEVOII DSC8230, Rigaku, Japan). Samples accurately weighed (5 mg) and in a covered aluminum pan were heated from 25 to 240 °C at a rate of 20 °C/min under nitrogen atmosphere.
Powder X-ray diffraction (PXRD)
The PXRD patterns for samples were taken using an automated multipurpose X-ray diffractometer SmartLab (Rigaku, Japan). Ni-filtered, CuKα radiation with a wavelength of 1.54 Å as the X-ray source over the diffraction angle range (2θ) of 5–50° at 10°/min, voltage of 45 kV, and a current of 200 mA were employed.
Statistical analysis
All data are presented as means ± standard deviation (SD). Statistical analyses were performed using Dunnett’s test with Statcel version 3.0 (OMS Publishing Inc.).
Results
The prepared β-carotene solid dispersion had increased water solubility
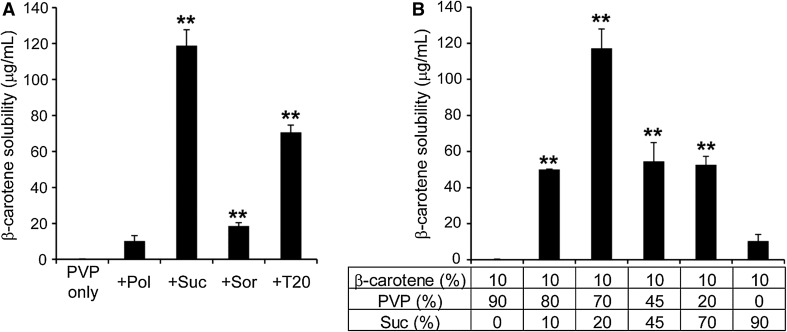
We prepared a solid dispersion composed of β-carotene and a macromolecular polymer, PVP, by the hot-melt technology, and its apparent solubility in water was investigated. As a result, the solubility of β-carotene, which has a very low water solubility in its raw form, increased to 300 ng/mL. To further improve the solubility of β-carotene, we attempted to develop a formulation method by adding an emulsifier to the two-component formulation of β-carotene and PVP. Four types of emulsifiers (Food grade), polyglycerol fatty acid ester (Pol), sucrose fatty acid ester (Suc), sorbitan fatty acid ester (Sor), and Tween 20 (T20) were used. Examination of the apparent solubility of the β-carotene solid dispersion showed that the solubility of β-carotene in water increased with all emulsion concentrations (Fig. 1a), and was significantly increased when Suc, Sor, or T20 was used. Compared to PVP, Suc showed a higher solubility. Therefore, in the subsequent experiments, the β-carotene solid dispersion was prepared using this emulsifier. The weight ratio of the three components was examined with β-carotene fixed at 10%. The weight ratio of PVP and Suc was adjusted as shown in Fig. 1b. As a result, it was found that when the weight ratio of β-carotene: PVP: Suc was set to 10%: 70%: 20%, the highest apparent solubility was observed. Since a solubility of 1 mg/mL was obtained for the complete dissolution of β-carotene, 12% of β-carotene was used in this formula. As the apparent solubility of β-carotene was high, further analysis was performed using this formulation.
Fig. 1.
Analysis of the apparent solubility of β-carotene solid dispersion prepared using the hot-melt technology. a β-Carotene solid dispersion consisting of β-carotene, PVP, and one of four emulsifiers was dissolved in phosphate buffer solution (pH 6.8). The two-component formulation of β-carotene and PVP was prepared with a weight ratio of 10%:90% β-carotene: PVP. Values are presented as mean ± SD (n = 3); **P < 0.01 versus PVP only. b β-Carotene solid dispersion consisting of β-carotene, PVP, and Suc in different weight ratios were dissolved in phosphate buffer solution (pH 6.8). The β-carotene solubility was analyzed by HPLC following centrifugation and filtration. Values are presented as mean ± SD (n = 3); **P < 0.01 versus a weight ratio of 10%:90% β-carotene: PVP
Surface morphology of β-carotene solid dispersion
The shape and surface morphology of the prepared solid dispersion were examined using FE-SEM. Pure β-carotene had irregular agglomerates of different shapes, with an average particle size ranging from 2 to 10 μm (Fig. 2a). PVP also showed irregular spherical particles while Suc showed square-like particles (Fig. 2b, c). Based on the formulation shown in Fig. 1, without heat, the physical mixture (PM) was comprised of β-carotene, PVP, and Suc, and this served as a control for β-carotene solid dispersion. In the PM, three components were observed in the mixed state (Fig. 2d). On the other hand, in the prepared β-carotene solid dispersion, an irregular angular-shaped particle with an average particle size ranging from 1 to 10 μm was observed (Fig. 2e). Compared to the PM, an obvious shape and morphology change was observed in the β-carotene solid dispersion.
Fig. 2.
Surface morphology of a crystalline β-carotene, b PVP, c Suc, d PM, and e β-carotene solid dispersion using FE-SEM
β-Carotene solid dispersion forms amorphous structure
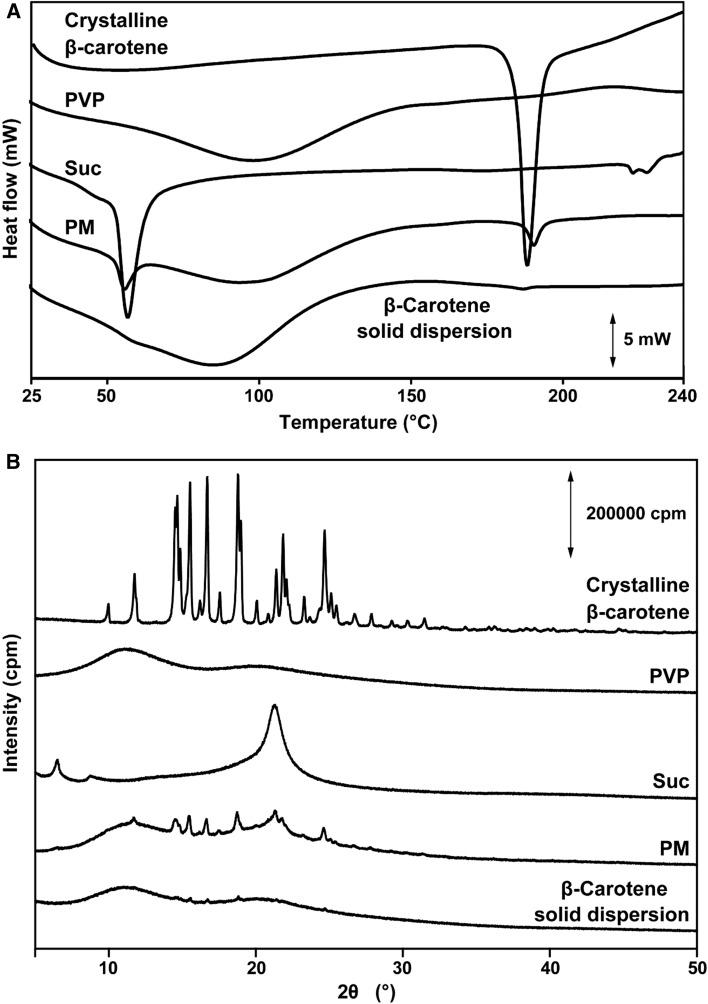
Figure 3a shows the DSC analysis of β-carotene, PVP, Suc, PM, and β-carotene solid dispersion. The DSC thermogram of pure β-carotene had a sharp endothermic peak at nearly 188 °C, which corresponded to its melting point of 183 °C (Lide 2005). In addition, PVP showed a broad endothermic peak at approximately 100 °C, due to water contained in PVP. Suc showed an endothermic melting point peak at approximately 57 °C, aligning with the melting point of 56 °C from its Safety Data Sheet. The PM of β-carotene displayed a weak endothermic peak, which indicated that peaks from each of the three component formulations in PM were still existent. However, in the thermogram of the β-carotene solid dispersion, the peak of the β-carotene crystalline structure almost disappeared.
Fig. 3.
DSC thermograms (a) and X-ray diffraction patterns (b) for crystalline β-carotene, PVP, Suc, PM, and β-carotene solid dispersion
To further confirm the crystalline transformation of β-carotene solid dispersion, PXRD analysis was performed. As shown in Fig. 3b, the diffraction pattern of pure β-carotene has a strong crystalline peak between 5° and 35°, indicating that it is in a crystalline form. PVP showed a diffused halo pattern, indicating its amorphous nature, while Suc showed peaks around 6° and 21°. For PMs, the characteristic peaks observed for β-carotene, PVP, and Suc exhibited small changes in their peak intensity. On the other hand, the β-carotene solid dispersion showed a diffused broad peak, with a very low intensity of the characteristic peak observed in PM. These results suggest that a phase transition of β-carotene from the crystalline form to the amorphous form occurred during the preparation of the solid dispersion.
Discussion
β-Carotene has various pharmacological activities and many health benefits (Johnson 2002; Weber and Grune 2012). Since β-carotene has a low solubility in water and low bioavailability, only a small pharmacological activity is exhibited when it is incorporated into foods, medicines, and cosmetics. In addition, it cannot exert pharmacological benefits in vivo to the extent expected. Once β-carotene is solubilized, the development of new products can be achieved. β-Carotene solid dispersion prepared using a novel formulation technology was soluble in water to a concentration of 117 ± 11 μg/mL (Fig. 1). This solubility greatly exceeds the 3 µM (approximately 2 μg/mL) demonstrated by Kaur et al. (2016) for the solubility of β-carotene-β-cyclodextrin inclusion complex in water. This solubility is also higher than when β-carotene is dissolved in organic solvents such as methanol (10 μg/mL), 2-propanol (40 μg/mL), and dimethyl sulfoxide (30 μg/mL) (Craft and Soares 1992). These findings indicate that β-carotene prepared by our method has a higher solubility in water than other previously prepared β-carotene products (Martini et al. 2010).
To improve the solubility of poorly water-soluble compounds, the preparation of an amorphous solid dispersion is an effective method (DeBoyace and Wildfong 2018). Analysis of the physicochemical properties of solid dispersion prepared from only β-carotene and PVP, using the hot-melt technology resulted in amorphization (data not shown). However, the solubility of β-carotene in water using this two-composition formulation was very low (Fig. 1). Adler et al. prepared an amorphous solid dispersion composed of a mixture of β-carotene, polymer, lipid, and silica by the hot-melt extrusion method and studied its physicochemical properties (Adler et al. 2016). They reported that the structure of this solid dispersion was influenced by H-bonding and ion–dipole interactions between lipid and silica. It is therefore suggested that a proper choice of material is important in the formation of a stable, amorphous structure, which will lead to an improvement in the solubility of the compound.
In our study, the solubility of β-carotene dramatically improved by adding an emulsifier to the formulation consisting of β-carotene and the hydrophilic polymer, PVP, prepared using the hot-melt technology (Fig. 1). An emulsifier is expected to have an effect on dispersal in water and oil; they are also difficult to mix with each other in the other solution (Alizadeh-Sani et al. 2018). Owing to the effect of PVP, drug stability can be improved by embedding the drug in the PVP matrix (Yen et al. 2010). It is also known that PVP suppresses crystallization by reducing the molecular mobility of a drug (Sethia and Squillante 2004). These effects of PVP can be exerted because PVP itself contains a proton acceptor group and can form a hydrogen bond with a proton donator group (Yen et al. 2010; Kazarian and Martirosyan 2002). Interestingly, in a recent study using lutein-loaded particles, an intermolecular hydrogen bond between PVP and an emulsifier was formed by adding an emulsifier during sample preparation (Zhao et al. 2014). Lutein was found to form a stable, amorphous structure with improved saturation solubility and stability. Although there is a difference between the rotary evaporation method used by Zhao et al. and our hot-melt technology, the existence of an emulsifier is considered to stabilize the amorphous structure, leading to an improvement in the solubility of β-carotene in this study (Vasconcelos et al. 2007; Feng et al. 2018). In the future, structural analysis of the developed β-carotene solid dispersion focusing on hydrogen bonding is important.
β-Carotene prepared in this study had improved solubility in water. Fan et al. (2017) reported that β-carotene formulation using organogel-based nanoemulsion has improved bioavailability in vivo, indicating that an increase in solubility is responsible for an increase in bioavailability. Thus, the β-carotene solid dispersion prepared in our study, exhibiting increased water solubility, is also expected to exhibit high bioavailability. In a future study, we will investigate the bioavailability of our prepared formulation.
Conclusion
In the present study, the water solubility of a very low water soluble β-carotene was successfully increased up to 120 μg/mL by preparing a solid dispersion using the hot-melt method. The solid dispersion of β-carotene showed obvious changes in shape and morphological characteristics compared to PM, and DSC and PXRD analyses showed that an amorphous structure was formed. The hot-melt method is effective for improving the solubility of poorly water-soluble functional food materials.
Acknowledgements
We thank BASF Japan Ltd. for technical advice. We thank Dr. Kiyohito Yagi (Osaka University) for technical support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kenji Ishimoto and Shohei Miki have contributed equally to this work.
Contributor Information
Mikihiko Nakamura, Phone: +81 6 6879 8176, Email: boundarylipid@yahoo.co.jp.
Shinsaku Nakagawa, Phone: +81 6 6879 8175, Email: nakagawa@phs.osaka-u.ac.jp.
References
- Adler C, Schönenberger M, Teleki A, Kuentz M. Molecularly designed lipid microdomains for solid dispersions using a polymer/inorganic carrier matrix produced by hot-melt extrusion. Int J Pharm. 2016;499:90–100. doi: 10.1016/j.ijpharm.2015.12.057. [DOI] [PubMed] [Google Scholar]
- Alizadeh-Sani M, Hamishehkar H, Khezerlou A, et al. Bioemulsifiers derived from microorganisms: applications in the drug and food industry. Adv Pharm Bull. 2018;8:191–199. doi: 10.15171/apb.2018.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst BJ. Optimizing oral bioavailability in drug discovery: an overview of design and testing strategies and formulation options. J Pharm Sci. 2017;106:921–929. doi: 10.1016/j.xphs.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Baghel S, Cathcart H, O’Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105:2527–2544. doi: 10.1016/j.xphs.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Craft NE, Soares JH. Relative solubility, stability, and absorptivity of lutein and β-carotene in organic solvents. J Agric Food Chem. 1992;40:431–434. doi: 10.1021/jf00015a013. [DOI] [Google Scholar]
- DeBoyace K, Wildfong PLD. The application of modeling and prediction to the formation and stability of amorphous solid dispersions. J Pharm Sci. 2018;107:57–74. doi: 10.1016/j.xphs.2017.03.029. [DOI] [PubMed] [Google Scholar]
- Fan Y, Gao L, Yi J, Zhang Y, Yokoyama W. Development of β-carotene-loaded organogel-based nanoemulsion with improved in vitro and in vivo bioaccessibility. J Agric Food Chem. 2017;65:6188–6194. doi: 10.1021/acs.jafc.7b02125. [DOI] [PubMed] [Google Scholar]
- Feng D, Peng T, Huang Z, et al. Polymer–surfactant system based amorphous solid dispersion: precipitation inhibition and bioavailability enhancement of itraconazole. Pharmaceutics. 2018;10:53. doi: 10.3390/pharmaceutics10020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EJ. The role of carotenoids in human health. Nutr Clin Care. 2002;5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- Kaur M, Bawa M, Singh M. β-carotene-β-cyclodextrin inclusion complex: towards enhanced aqueous solubility. J Glob Bio. 2016;5:3665–3675. [Google Scholar]
- Kazarian SG, Martirosyan GG. Spectroscopy of polymer/drug formulations processed with supercritical fluids: in situ ATR-IR and Raman study of impregnation of ibuprofen into PVP. Int J Pharm. 2002;232:81–90. doi: 10.1016/S0378-5173(01)00905-X. [DOI] [PubMed] [Google Scholar]
- Lide RD. CRC handbook of chemistry and physics. Boca Raton: CRC; 2005. [Google Scholar]
- Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm. 2012;2012:436763. doi: 10.5402/2012/436763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini S, D’Addario C, Bonechi C, et al. Increasing photostability and water-solubility of carotenoids: synthesis and characterization of β-carotene-humic acid complexes. J Photochem Photobiol B. 2010;101:355–361. doi: 10.1016/j.jphotobiol.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Piorkowski DT, McClements DJ. Beverage emulsions: recent developments in formulation, production, and applications. Food Hydrocoll. 2014;42:5–41. doi: 10.1016/j.foodhyd.2013.07.009. [DOI] [Google Scholar]
- Repka MA, Bandari S, Kallakunta VR, et al. Melt extrusion with poorly soluble drugs—an integrated review. Int J Pharm. 2018;535:68–85. doi: 10.1016/j.ijpharm.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierle J, Pietsch B, Ceresa A, Fizet C, Waysek EH. Method for the determination of beta-carotene in supplements and raw materials by reversed-phase liquid chromatography: single laboratory validation. J AOAC Int. 2004;87:1070–1082. [PMC free article] [PubMed] [Google Scholar]
- Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272:1–10. doi: 10.1016/j.ijpharm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Sutter SC, Buera MP, Elizalde BE. beta-carotene encapsulation in a mannitol matrix as affected by divalent cations and phosphate anion. Int J Pharm. 2007;332:45–54. doi: 10.1016/j.ijpharm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Theil F, Anantharaman S, Kyeremateng SO, et al. Frozen in time: kinetically stabilized amorphous solid dispersions of nifedipine stable after a quarter century of storage. Mol Pharm. 2017;14:183–192. doi: 10.1021/acs.molpharmaceut.6b00783. [DOI] [PubMed] [Google Scholar]
- Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–1075. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Weber D, Grune T. The contribution of β-carotene to vitamin A supply of humans. Mol Nutr Food Res. 2012;56:251–258. doi: 10.1002/mnfr.201100230. [DOI] [PubMed] [Google Scholar]
- Yen FL, Wu TH, Tzeng CW, Lin LT, Lin CC. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J Agric Food Chem. 2010;58:7376–7382. doi: 10.1021/jf100135h. [DOI] [PubMed] [Google Scholar]
- Zhao C, Cheng H, Jiang P, Yao Y, Han J. Preparation of lutein-loaded particles for improving solubility and stability by polyvinylpyrrolidone (PVP) as an emulsion-stabilizer. Food Chem. 2014;156:123–128. doi: 10.1016/j.foodchem.2014.01.086. [DOI] [PubMed] [Google Scholar]