Abstract
Purpose
Type 2 diabetes is heterogeneous disease characterized by several conditions including hyperglycemia. It is estimated that over 350 million people worldwide are suffering from type 2 diabetes and this number is expected to rise. According to the CDC, African Americans were observed to have a 40% higher incidence of diabetes compared to European Americans. Epigenetic modulating mechanisms such as microRNAs (miRNAs), have recently been established as a massive regulatory machine in metabolic syndrome, obesity and type 2 diabetes. In the present study, we aimed to investigate the serum levels of circulating miRNA 17 (miR-17) of obese, African American women with elevated HbA1c.
Methods
We investigated miR-17 serum levels using qPCR. Then we used Pairwise Pearson Correlation Test to determine the relationship between clinical metabolic parameters and miR-17 serum levels.
Results
The results indicated that participants with elevated HbA1c exhibited a down regulation of serum miR-17 levels compared to participants with normal HbA1c. MiR-17 was also correlated with serum calcium in participants with normal HbA1c.
Conclusions
The results suggest that serum miR-17 is involved in the regulation of glucose and calcium homeostasis, which may contribute to the development of type 2 diabetes.
Keywords: African American, Calcium, Diabetes, HbA1C, miR-17, Obesity
Introduction
Type 2 diabetes is a heterogeneous disease characterized by hyperglycemia, insulin resistance, beta-cell malfunction, and obesity-induced deterioration of systemic insulin sensitivity [1, 2]. It is estimated that over 350 million people worldwide are suffering from type 2 diabetes and this number is expected to reach 600 million by 2030 [3, 4]. Type 2 diabetes has been identified as the most widespread metabolic disease worldwide and the prevalence is increasing exponentially [5]. Individual risk of developing type 2 diabetes is assessed by a combination of clinical metabolic parameters, physical characteristics, and lifestyle choices.
According to the CDC, African Americans (AA) are observed to have a 40% higher incidence of diabetes compared to European American (EA). AA women (58.6%) and men (38.8%) have a higher prevalence of obesity than EA women (33.4%) and men (36.4%) [6]. Thus, racial differences in type 2 diabetes incidence may be related to obesity. Hyperinsulinemia and insulin resistance are also more common in AA than EA, which may be due to correlation with obesity [7]. In a previous study, obese AA women had a 30% higher incidence of type 2 diabetes than EA women [8]. Interestingly, non-obese AA women also have a 2-fold higher chance of developing diabetes than EA women [8], which suggests additional etiologies and mechanisms may be driving type 2 diabetes, racial differences. Nutritional availability and distinct environmental factors may interact with AA genome and enhance susceptibility to altered epigenetic patterns. This may also influence metabolic disorders and adult chronic diseases [9]. Epigenetic modulating mechanisms such as miRNAs, have recently been established as a massive regulatory machine in metabolic syndrome, obesity and type 2 diabetes [10].
MiRNAs are short, noncoding sequences approximately 22 nucleotides in length. MiRNAs bind to the 3’UTR region of target mRNA to negatively regulate gene expression at the post-transcriptional level. MiRNAs are major controllers of numerous biological and pathological processes. Mature miRNAs may have hundreds of targets and can be released by the cells or integrated in the RNA-induced silencing complex to guide translational repression. MiRNAs can attach to proteins, lipoproteins, or are loaded inside vesicles that are discharged into the extracellular space [11]. Several miRNAs are found in bodily fluids such as blood, urine, saliva, amniotic fluid, and breast milk [12–15]. The function of circulating miRNAs remains to be established. This observation raises the intriguing possibility of the involvement of miRNAs in a novel cell-to-cell communication. Several miRNAs are involved with insulin-sensitive organs, including skeletal muscle, white adipose tissue, liver and insulin-producing pancreatic cells, all of which, are linked to diabetes [16]. Circulating miRNAs are very stable and may have advantages as potential biomarkers of type 2 diabetes in AA. Therefore, a better understanding of the mechanisms of serum miRNAs in type 2 diabetes pathogenesis in AA is of great clinical significance.
MiR-17 belongs to a family of polycistronic miRNA genes containing 15 mature miRNA species (miR-17, 18a, 18b, 20a, 20b, 93, 106a, 106b) [17]. MiR17–92 cluster has been prevalent in many types of cancer, coronary artery disease and multiple sclerosis [18]. MiR-17 is also involved in the pathological progression of type 2 diabetes. Previously, miR-17 was linked to obesity-associated biological mechanisms in the pathology of type 2 diabetes [19].
In this study we aimed to investigate the expression of serum miR-17 levels and how they correlate with serum clinical metabolic parameters. We aimed to identify the role of miR-17 serum levels in metabolism and type 2 diabetes. This study aims provide new information on the development and potential biomarkers of type 2 diabetes.
Materials and methods
Serum samples from human patients
Blood samples were obtained from 69 African American women over 40 years old in a rural northeastern county in North Carolina. We selected for BMI over 30 in participants with elevated and normal HbA1c. Participants with HbA1c > 6.5 were classified as the experimental, diabetic group and participants with HbA1c < 6.4 were classified as the control, non-diabetic group. Serum was collected and immediately frozen and stored at −80 °C until use. The North Carolina Central University Institutional Review Board approved protocol, with that allowed written informed consent, was obtained from all participants prior to the collection of blood samples. The right to privacy was observed for all participants.
RNA isolation
Total RNA was isolated from 69 serum samples (23 normal HbA1c and 46 high HbA1c) using Exiqon miRCURY RNA isolation kit-Biofluids (Woburn, MA, USA) per the manufacturers’ protocols. The RNA quality and yield of each total RNA sample was obtained from A260 measurements using a NanoDrop 2000; Thermo Fischer (Waltham, MA, USA).
CDNA synthesis and qPCR analysis
For analysis of miR-17, cDNA was prepared from total RNA and amplified using the Taqman Advanced miRNA from Applied Biosystems (Cartsbad, CA, USA). Reverse transcription was carried out according to the manufacturers’ protocol. qPCR was performed using Taqman Advanced MicroRNA Assays from Applied Biosystems (Pleasanton, CA, USA) in combination with Taqman Fast Advanced Master Mix (Austin, TX, USA) from Applied Biosystems. qPCR was used to amplify miRNA target gene hsa-miR-17-5p and reference gene hsa-miR-221-3p was used for a control. MiR-221-3p is a marker for obesity. We used it as a control because the average BMI for both groups was <30, which is classified as obese. Samples were analyzed according to manufacturer’s instructions. Each sample was run in triplicate and averaged. Wells with Ct values >37 were excluded.
Statistical analysis
Descriptive statistics were computed for each variable for participants with elevated and normal HbA1c. Data are expressed as mean ± SEM. A series of unpaired t-test were computed to determine significant differences between participants with elevated and normal elevated HbA1c. Pairwise Pearson Correlation Test was computed for each variable and miR-17 ΔCt value.
Results
Serum parameters and circulating miR-17 levels
Circulating miRNAs function in various pathways such as metabolism. Metabolic dysregulation is caused by abnormally functioning miRNAs and is implicated in the development of type 2 diabetes. We analyzed miRNA serum levels in serum of 23 normal HbA1c participants and 44 high HbA1c participants. The basic demographic and clinicopathological properties of these participants were shown in Table 1. It shows that age is balanced between the two groups. There is a significant difference in BMI (p = 5.44* 10−13), HbA1c (p = 3.8* 10−13) and glucose level in the serum (p = 0.0001). We also observed a significant difference in HDL cholesterol (p = 0.02), VLDL cholesterol (p = 0.001) and triglycerides (p = 0.0001) between the two groups. Then, qPCR analysis determined that serum miR-17 was significantly downregulated in participants with elevated HbA1c (Fig. 1, p = 0.03).
Table 1.
Clinical characteristics of the study population
| Patient Characteristics | All | Diabetics | Non-Diabetics |
|---|---|---|---|
| (mean ± SEM) | (mean ± SEM) | (mean ± SEM) | |
| Age, (years) | 61.32 ± 1.08 | 62.27 ± 1.23 | 60.11 ± 1.89 |
| BMI, (kg/m2) | 34.914 ± 1.01 | 38.31 ± 1.27 | 30.55 ± 1.29 **** |
| HbA1c, (mmol/mol) | 56.80 ± 2.16 | 65.07 ± 2.41 | 40.26 ± 0.73 **** |
| HbA1C, (%) | 7.143 ± 0.18 | 8.14 ± 0.23 | 5.87 ± 0.05 **** |
| Glucose Serum, (mg/dl) | 123.375 ± 6.66 | 143.84 ± 10.71 | 97.06 ± 2.96 *** |
| Total Cholesterol, (mg/dl) | 179.25 ± 4.10 | 175.33 ± 5.05 | 184.29 ± 6.76 |
| Cholesterol HDL, (mg/dl) | 58.45 ± 2.32 | 53.31 ± 1.96 | 65.06 ± 4.46 * |
| Cholesterol LDL, (mg/dl) | 101.34 ± 3.43 | 98.76 ± 4.29 | 104.66 ± 5.58 |
| Cholesterol VLDL, (mg/dl) | 19.46 ± 1.24 | 23.27 ± 1.89 | 14.57 ± 0.94 *** |
| Triglycerides, (mmol/l) | 97.11 ± 6.15 | 115.93 ± 9.45 | 72.91 ± 4.68 ** |
| Creatinine, (mg/dl) | 0.87 ± 0.02 | 0.90 ± 0.03 | 0.83 ± 0.03 |
| BUN/ Creatinine, Ratio | 16.65 ± 0.54 | 17.24 ± 0.75 | 15.89 ± 0.74 |
| Sodium (Na+), (mmol/l) | 140.49 ± 0.29 | 140.11 ± 0.42 | 140.97 ± 0.37 |
| Calcium (Ca2+), (mg/dl) | 9.51 ± 0.05 | 9.49 ± 0.67 | 9.53 ± 0.08 |
| Potassium, (mmol/l) | 4.19 ± 0.04 | 4.23 ± 0.06 | 4.15 ± 0.06 |
| MIR17, (∆CT) | 1.33 ± 0.06 | 1.65 ± 0.20 | 0.92 ± 0.26 * |
*significant at p < 0.05; **significant at p < 0.005;
***significant at p < 0.001; ****significant at p < 0.0001
Fig. 1.
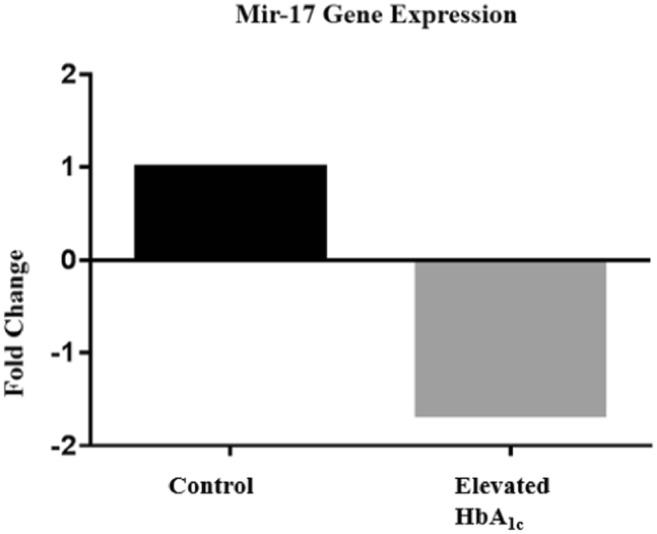
Circulating mir-17 was downregulated 1.66 fold (p = 0.029)
Correlations between miR-17 and serum parameters
We then investigated possible correlations between miR-17 serum levels and the parameters listed in Table 1. Positive correlations were observed between serum miR-17 in all participants with HbA1c (r = 0.22, p = 0.05), serum miR-17 in participants with normal HbA1c and calcium (r = 0.41, p = 0.02), and serum miR-17 in all participants with BUN/Creatinine (r = 0.23, p = 0.03) (Fig. 2). The other parameters listed in Table 1 were not significantly correlated.
Fig. 2.
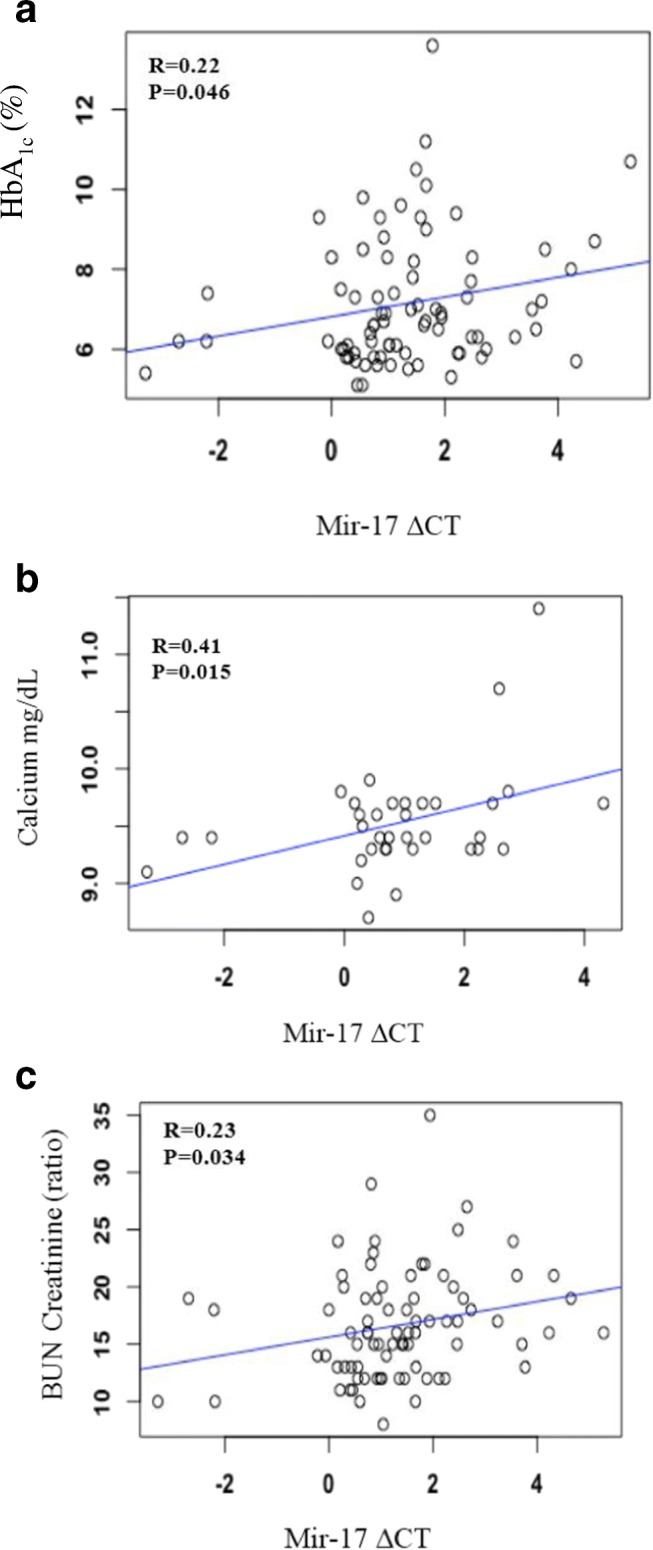
Mir-17 is associated with HbHBA1C, Calcium and Bun/Creatinine ratio. a In all participants, HbHbA1c correlates with mir-17 (b) In participants without diabetes, calcium correlates with mir-17 (c) In all participants, Bun/Creatinine ratio correlates with mir-17
Discussion
Type 2 diabetes is the most common metabolic disorder in the United States. It is estimated that over 350 million people worldwide are suffering from type 2 diabetes. The number of individuals affected by type 2 diabetes is increasing exponentially each year. It has been reported that African Americans are affected at a disproportional rate [3, 4]. MiRNAs, an epigenetic modulating mechanism, has recently been established as a massive regulatory machine in obesity and metabolic disease. Therefore, more effective therapeutic strategies are needed immediately to control the incidence and progression of type 2 diabetes.
Circulating miRNAs are ideal biomarker contenders because of stability in serum, even in freeze/thaw conditions, resistance against ribonuclease degradation, and other extreme conditions [20]. Several miRNAs are released into blood circulation to regulate specific gene function by binding of 3’UTR of target mRNA. MiRNAs play a role in modifying normal physiology and acting as mediators of disease, including type 2 diabetes. We investigated the expression of serum miR-17 and how it correlates with several clinical metabolic parameters. Previous studies have shown that obesity alters miRNA serum levels in organs involved in metabolism [17, 21–27]. The average BMI of participants with elevated HbA1c was greater than 30. MiRNAs have been found to regulate multiple pathways linked to diabetes including insulin signaling, immune-mediated inflammation, adipokine expression, adipogenesis, lipid metabolism, and food intake regulation [28–34]. Serum miR-17 is downregulated in participants with elevated HbA1c, which causes an upregulation in genes targeted by this miRNA (Fig. 1). The abnormal upregulation of serum miR-17 target genes may contribute to disease manifestation.
In previous studies, it has been shown that type 2 diabetes patients have lower serum levels of miR-17 in adipose tissue in comparison to normal glucose tolerance patients [35]. In our study, the participants with elevated HbA1c were all obese. We observed a positive correlative between HbA1c and serum miR-17 across all patients (Fig. 2). This suggests that serum miR-17 may regulate cellular metabolism. In addition, there are multiple reports linking plasma levels to cardio-metabolic disease, which also suggest circulating miR-17 may be a useful biomarker in multiple disease [36–38]. We also observed a 1.66 fold downregulation in serum levels of miR-17 in participants with elevated HbA1c in comparison to participants with normal HbA1c (Fig. 1). Calcium metabolism is also impaired in participants with elevated HbA1c. This dysregulation has been observed in many cell types including: erythrocytes, cardiac muscle, platelets, skeletal muscle, kidney, aorta, adipocytes, liver, osteoblasts, arties lens, peripheral nerves, brain synaptosomes, retinal tissue and pancreatic beta cells [39–44]. This suggests that abnormalities in cell calcium metabolism are basic pathology associated with diabetes.
The most common abnormality found in participants with elevated HbA1c is increased intracellular calcium levels [39]. Although we did not find a difference in serum calcium levels between the two groups (Table 1), we did observe a positive correlation between serum miR-17 and serum calcium in participants with normal HbA1c. This suggests that serum miR-17 may regulate normal maintenance of serum calcium levels. Abnormalities in cell calcium metabolism may be significant for the observed pathologies in insulin secretion in diabetes. In previous studies, it has been found that pancreatic islets from mice with induced diabetes lacked the initial reduction of intracellular calcium levels and subsequent calcium oscillations in response to glucose [45–47]. Oscillations are important for pulsatile insulin secretion. We believe that decreased serum levels of miR-17 may play a role in the dysfunction of intracellular calcium levels. This phenomenon may also impair insulin secretion and contribute to diabetes. Calcium also regulates glucose homeostasis mechanisms such as glycogen synthesis, glycolysis, gluconeogenesis, and glycogenolysis. In previous studies, early stage participants with diabetes have decreased serum miR-17 levels are associated with the upregulation of a calcium-activated potassium channel subunit alpha 1. MiR-17 binds to the 3’UTR and regulates the gene expression of this channel protein [48]. This calcium activated potassium channel has also been linked to obesity [49].
In normal physiology, calcium metabolism is regulated through hormonal control of a three-tissue axis of intestine, kidney, and bone to tightly control serum ionized calcium within a narrow range [50]. The kidneys play a critical role in the balance between the internal milieu and external environment. Kidney failure is known to disrupt several homeostatic mechanisms that control serum calcium and normal bone metabolism [50]. We observed that serum miR-17 is positively correlated with the BUN/Creatinine in all participants. BUN/Creatinine is a measure of kidney damage. Type 2 diabetes may significantly affect the kidneys; approximately 40% of all patients requiring routine dialysis therapy suffer from diabetes [51]. Downregulation of serum miR-17 serum levels in participants with elevated HbA1c may affect normal calcium maintenance and attribute to kidney disease.
In the present study, we investigated the role of serum miR-17 in participants with elevated HbA1c. This study is one of few discussions on AA women although AA women have the highest prevalence of obesity and type 2 diabetes [6, 8]. Further investigation is required to elucidate the mechanisms behind these phenomena. Although serum miR-17 is not unique to AA women, in future studies, we plan to conduct larger studies inclusive to other ethnicities to help validate the role of serum miR-17 in glucose and calcium metabolism in AA women. MiR-17 has been suggested to regulate stk11 which would down regulate anabolic metabolism through LKB1 [52]. Hormonal regulation of calcium homeostasis would also assist in a better understanding of serum miR-17 and calcium. More recently, miR-17 has been implicated in calcium metabolism in bone stem cells, in a reverse relationship. This suggests that calcium serum levels could lower as calcium is absorbed and utilized by bone and induce a direct relationship with serum mirR-17 [53]. Though limited, the role of miR-17 in metabolism is emerging and evident is multiple cell types including tumor biology and metabolism.
This study adds to the exploration of miRs in metabolic disorders and may be, useful in establishing MIR biomarkers for metabolic syndrome. With the addition of newly developed of technologies, the treatment of metabolic syndrome with miRNAs and appropriate delivery system could offer a new arena of treatment in this area.
Acknowledgements
Thank you Laboratory Corporation of America for performing serum panel assessment.
Availability of data and material
Please contact author for data requests.
Abbreviations
- AA
African American
- EA
European American
- mRNA
messenger RNA
- miRNA
microRNA
- miR-17
microRNA 17
Author’s contribution
AW was responsible for RNA isolation, cDNA synthesis, qPCR experiment, sample management, data management, data interpretation, and drafting the manuscript. DM was responsible for RNA isolation, cDNA synthesis, qPCR experiment, and sample management. WJ was responsible for statistical analysis and data interpretation. NG was responsible for the experimental design, sample acquisition and manuscript editing. CWD was responsible for statistical analysis, data interpretation, and manuscript editing. KSK was responsible for experimental design, data interpretation, manuscript editing. KSK is the Guarantor.
Funding
KSK, CWD, and NG were supported by the National Institutes of Health [P20MD00175; U54MD012392] grants.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mita T, Goto H, Azuma K, Jin W, Nomiyama Y, et al. Impact of insulin resistance on enhanced monocyte adhesion to endothelial cells and atherosclerogenesis independent of LDL cholesterol level. BBRC. 2010;395:477–483. doi: 10.1016/j.bbrc.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Dai X, Zhang H, Zeng S, Xi W. Profiling peripheral microRNAs in obesity and type 2 diabetes mellitus. APMIS. 2015;123:580–585. doi: 10.1111/apm.12389. [DOI] [PubMed] [Google Scholar]
- 3.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Scully T. Diabetes in numbers. Nature. 2012;485:S2–S3. doi: 10.1038/485S2a. [DOI] [PubMed] [Google Scholar]
- 5.Wang M. miR-433 protects pancreatic β cell growth in high-glucose conditions. Mol Med Rep. 2017;16:2604–2610. doi: 10.3892/mmr.2017.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Staiano AE, Harrington DM, Johannisen NM, Newton RL, Sarzynski MA, et al. Uncovering physiological mechanisms for health disparities in type 2 diabetes. Ethn Dis. 2015;25:31–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults the atherosclerosis risk in communities’ study, 1987–1998. Diabetes Care. 2002;25:1358–1364. doi: 10.2337/diacare.25.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feil R. Environmental and nutritional effects on the epigenetic regulation of genes. Mutat Res. 2006;600:46–57. doi: 10.1016/j.mrfmmm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Junien C. Impact of diets and nutrients/ drugs on early epigenetic programming. J Inherit Metab Dis. 2006;29:359–365. doi: 10.1007/s10545-006-0299-7. [DOI] [PubMed] [Google Scholar]
- 11.Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- 12.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 15.Vickers KC, Palmisano B, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Ding Y, Liang B, Lin J, Kim Kim T.K., Yu H., Hang H., Wang K. (2017) A systematic study of dysregulated microRNA in type 2 diabetes mellitus. Int J Mol Sci 18:456. [DOI] [PMC free article] [PubMed]
- 17.Deiuliis JA. MicroRNAa as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes. 2016;40:88–101. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on it genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;220:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Qian D, Zhao H, Yu P, Sun Z. Mir17 improves insulin sensitivity through inhibiting serum levels of ASK-1 and anti-inflammation of macrophages. Biomed Pharmacother. 2018;100:448–454. doi: 10.1016/j.biopha.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Kroh EM, Parkin RK, Mitchell P, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRTPCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MJ, Park DH, Hang JH. Exosomes as the source of biomarkers of metabolic diseases. Ann Pediatr Endocrinol Metab. 2016;21:119–125. doi: 10.6065/apem.2016.21.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diabetes Rep. 2016;16:35. doi: 10.1007/s11892-016-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Natarajan R. MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci. 2015;1353:72–88. doi: 10.1111/nyas.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price NL, Ramirez CM, Fernandez-Hernando C. Relevance of microRNA in metabolic diseases. Crit Rev Clin Lab Sci. 2014;51:305–320. doi: 10.3109/10408363.2014.937522. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Lan HY, Roukos DH, Cho WC. Application of microRNAs in diabetes mellitus. J Endocrinol. 2014;222:1–10. doi: 10.1530/JOE-13-0544. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33:178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNA-related disease? Transl Res. 2011;157:253–264. doi: 10.1016/j.trsl.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik (1993) A tumor necrosis factor- alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem 268:26055–26058. [PubMed]
- 30.Vatner DF, Majumdar SK, Kumashiro N, Petersen MC, Rahimi Y, Gattu AK, Bears M, Camporez JPG, Cline GW, Jurczak MJ, Samuel VT, Shulman GI. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci. 2015;112:1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai D, Yuan M, Frantz D, Melendez P, Hansen L, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira D, Simao A, Rodrigues C, Castro R. Revisiting the metabolic syndrome and paving the way for microRNAs in non-alcoholic fatty liver disease. FEBS J. 2014;281:2503–2524. doi: 10.1111/febs.12806. [DOI] [PubMed] [Google Scholar]
- 33.Shoelson S, Lee J, Goldfine A. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaler J, Yi C, Schur E, Guyenet S, Hwang B, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloting N, Berthold S, Kovacs P, Schon M, Fasshauer M, et al. MicroRNA serum levels in human omental and subcutaneous adipose tissue. PLoS One. 2009;4:e4699. doi: 10.1371/journal.pone.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 37.Heneghan H, Miller N, McAnena O, O’Brien T, Kerin M. Differential miRNA serum levels in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J Clin Endocrinol Metab. 2011;96:E846–E850. doi: 10.1210/jc.2010-2701. [DOI] [PubMed] [Google Scholar]
- 38.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 39.Levy J, Gavin IIIJ, Sowers J. Diabetes mellitus: a disease of abnormal cellular calcium metabolism? Am J Med. 1994;96:260–273. doi: 10.1016/0002-9343(94)90152-X. [DOI] [PubMed] [Google Scholar]
- 40.Roe M, Philipson L, Frangakis C, Kuznestov A, Mertz R, et al. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem. 1994;269:279–282. [PubMed] [Google Scholar]
- 41.Janicki P, Horn J, Singh G, Franks W, Franks J. Diminished brain synaptic plasma membrane Ca2+-ATPase activity in rats with streptozocin-induced diabetes: association with reduced anesthetic requirements. Life Sci. 1994;55:359–364. doi: 10.1016/0024-3205(94)00761-6. [DOI] [PubMed] [Google Scholar]
- 42.Tomizawa H, Yamazaki M, Kunika K, Itakura M, Yamashita K. Association of elastin glycation and calcium deposit in diabetic rat aorta. Diab Res Clin Pract. 1993;19:1–8. doi: 10.1016/0168-8227(93)90138-U. [DOI] [PubMed] [Google Scholar]
- 43.Kern T, Kowluru R, Engerman R. Abnormalities of retinal metabolism in diabetes or galactosemia: ATPase and glutathione. Invest Ophthalmol Vis Sci. 1994;35:2962–2967. [PubMed] [Google Scholar]
- 44.Levy J, Zhu Z, Dunbar J. The effect of glucose and calcium on CA2+-ATPase in pancreatic islets isolated from normal and NIDDM rat models. Metabolism. 1998;47:185–189. doi: 10.1016/S0026-0495(98)90218-9. [DOI] [PubMed] [Google Scholar]
- 45.Leahy J. Natural history of beta-cell dysfunction in NIDD. Diabetes Care. 1990;13:992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- 46.Juntti-Berggren L, Larson O, Rorsman P, Ammala C, Bokvist K, et al. Increased activity of L-type Ca2+ channels exposed to serum from patients with type 1 diabetes. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- 47.Hellman B, Gylfe E, Bergsten P, Grapengiesse E, Lund P, et al. Glucose induces oscillatory Ca2+ signaling and insulin release in human pancreatic beta cells. Diabetologia. 1994;37(Suppl 2):S11–S20. doi: 10.1007/BF00400821. [DOI] [PubMed] [Google Scholar]
- 48.Cheng Y, Wright C, Kirschner M, Williams M, Sarin K, et al. KCa1.1, a calcium activated potassium channel subunit alpha 1, is targeted by mir-17-5p and modulates cell migration in malignant pleural mesothelioma. Mol Cancer. 2016;15:44. doi: 10.1186/s12943-016-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jioa H, Arner P, Hoffstedt J, Brodin D, Dubern B, et al. Genome wide association study identifies KCNMA1 contributing to human obesity. BMC Med Genet. 2011;4:51. doi: 10.1186/1755-8794-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallant K, Speiegel D. Calcium balance in chronic kidney disease. Curr Osteoporos Rep. 2017;15:214–221. doi: 10.1007/s11914-017-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mima A. Diabetic nephropathy: protective factors and a new therapeutic paradigm. J Diabetes Complicat. 2013;27:526–530. doi: 10.1016/j.jdiacomp.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Izreig S, Ssamborska B, Johnson R, Artyomov M, Duchaine T, Jones R. The miR-17~92 microRNA cluster is a global regulator of tumor metabolism. Cell Rep. 2016;16:1915–1928. doi: 10.1016/j.celrep.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Liu W, Hu C, Xue Z, Wang G, Dang B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29:1804–1816. doi: 10.1002/stem.728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.


