Abstract
Lycopene extracted from pink grapefruit was encapsulated on Ca(II)-alginate beads with the addition of trehalose and galactomannans to improve its stability against freezing and drying. Three galactomannans of different physicochemical properties were studied since their inclusion affects both loading efficiency and release of lycopene in wet beads; however, there is no information about their performance during freezing and dehydration operations. The remaining lycopene and its stability towards isomerization were analyzed in beads subjected to continuous freezing, freezing/thawing cycles and vacuum- and freeze-drying. Isothermal crystallization studies were conducted by LF-NMR and related to beads formulation and lycopene stability. In the absence of excipients, lycopene was severely affected by all the treatments, retaining less than 20% of the original content. Alginate beads containing trehalose with guar gum protected more than 80% of the lycopene regardless of the employed freezing or drying methods. These beads concomitantly showed higher solid fraction than the other two galactomannans-containing systems, displaying guar gum ability to associate water. On the other hand, the addition of vinal gum affected lycopene stability (between 40 and 60% were recovered after treatments), even compromising the positive effect of a well-established cryoprotectant as trehalose. Thus, the addition of secondary excipients should be carefully conducted. The differences among galactomannans could be related to the substitution degree of the polymer chains, affecting the overall systems interactions. These results can contribute to excipients selection for the encapsulation of labile biomolecules in Ca(II)-alginate beads subjected to freezing and drying.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03783-w) contains supplementary material, which is available to authorized users.
Keywords: Carotenoids, Hydrocolloids, Encapsulation, Freezing and thawing cycles, Freeze-drying
Introduction
Red-colored fruits and vegetables (tomatoes, watermelon, pink grapefruit, pink guava, and papaya) contain relevant quantities of carotenoids, particularly lycopene (Sinha and Dua 2015). Lycopene structure reveals 13 unsaturated bonds which are prone to be affected by oxidant agents, light and heat, damaging lycopene’s structure or causing it to rearrange into different geometric isomers (Pérez-Masiá et al. 2015). These changes may result in the deterioration or loss of its beneficial properties. Among the health benefits associated with lycopene, there is the maintenance of normal cardiovascular function, its antioxidant capacity that quenches free radicals (formed during normal metabolism) and potentially deactivates DNA chain-breaking agents which have been implicated in some cancers (Costa-Rodrigues et al. 2018; Stahl and Sies 1996). Since lycopene cannot be synthesized by animals or humans, its intake must come exclusively from the diet (Rodríguez Amaya and Kimura 2016).
The purpose of the preservation of foods is to reduce the rate at which detrimental changes occur and at the same time maintaining their properties during shelf life or storage. Thermal treatment is often a crucial step in the food industry. The exposition to severe temperature (both low or high temperatures) cause physical and chemical changes affecting food components, and therefore, product quality is compromised. For instance, high losses of nutritional content and functionality are observed when the raw materials containing lycopene are subjected to freezing or dehydration, since lycopene is released from matrices and then is easily degraded (Barankevicz et al. 2015; Dias et al. 2014). It has been proven that encapsulation of labile biomolecules is a good strategy to generate ingredients for foods that enhance functionality and stability (Quintanilla-Carvajal et al. 2010; Souza et al. 2018). Particularly, Ca(II)-alginate hydrogels were a suitable alternative to generate formulations with high lycopene levels (Aguirre Calvo et al. 2017; Aguirre Calvo and Santagapita 2017). Polysaccharides such as galactomannans (which consist of a linear backbone of (1 → 4)-linked β-D-mannopyranosyl residues with α-D-galactopyranosyl units attached at C-6) are often technologically used as wall materials to ensure and protect encapsulated biocompounds. Guar gum is widely used as stabilizer, thickener and/or emulsifier in foods such as syrup drinks, gummy candies and creams (Busch et al. 2017; Torres et al. 2014). Gums incorporation for encapsulation of biomolecules has shown improvements in the intrinsic transport properties of the initial hydrogel and stabilized its microstructure, even during freezing and dehydration processes (Traffano-Schiffo et al. 2017b, 2018). Non-conventional galactomannans such as vinal (Busch et al. 2018) and espina corona (Perduca et al. 2013) gums can also be incorporated successfully in a Ca(II)-alginate network (Aguirre Calvo et al. 2017). Both natural gums possess interesting physicochemical and rheological properties comparable with guar gum but gives different polymer characteristics related to the molecular weight, mannose/galactose ratio and physicochemical properties. It was previously established that galactomannans inclusion affects loading efficiency and release of lycopene on wet beads (Aguirre Calvo et al. 2017). Then, it is interesting to understand the performance of these systems during freezing and dehydration operations as protentional ingredients for functional foods, since the structural galactomannans differences may determine functionality in encapsulated systems subjected to those treatments.
The aim of the present work is to study the stability of an encapsulated lycopene extract towards freezing and drying in Ca(II)-alginate beads formulated with different galactomannans. Lycopene content and stability, and isothermal crystallization studied by LF-NMR were analyzed and related to beads formulation, searching for structural/functional relationships.
Materials and methods
Materials
Pink grapefruit (Citrus paradisi, Red variety, from Jujuy, Argentina) were obtained in the local market. Not-damaged or not-defective fruits were selected and stored at 25 °C.
Encapsulating agents were used, as listed below: sodium alginate from Cargill S.A. (San Isidro, Buenos Aires, Argentina), with mannuronate/guluronate ratio = 0.6 and MW = 1.97.105 g/mol; trehalose (T) dihydrate from Hayashibara Co., Ltd. (Cargill Inc., Minneapolis, Minnesota, USA); guar gum (GG) (Cordis S.A., Villa Luzuriaga, Buenos Aires, Argentina), of MW ~ 1.8.106 g/mol, protein content of 21 g/kg, and with mannose/galactose (M/G) ratio = 1.8; vinal gum (VG), extracted from Prosopis ruscifolia (Busch et al. 2018), of MW ~ 1.4.106 g/mol, protein content of 19 g/kg, and M/G ratio = 1.6; espina corona gum (ECG) from Idea Supply Argentina S.A (Chaco, Argentina), of MW ~ 1.4.106 g/mol, protein content of 22 g/kg, and M/G = 2.5 (Perduca et al. 2013). Extra-virgin olive oil (Molino Cañuelas SACIFIA, Mendoza, Argentina) was used for lycopene extraction.
Lycopene extraction and encapsulation
Lycopene was extracted from freeze-dried pulp by using edible oil (extra-virgin olive oil) in a green extraction process reported in Aguirre Calvo et al. (2017), obtaining an extract with 18 ± 1 µg lycopene/gd.b. sample.
Encapsulation was produced in a two-steps process: emulsification and ionotropic gelation. Emulsions were prepared by mixing sodium alginate solutions and lycopene extract in a 2:1 mass ratio in an Ultra-Turrax T18B (IKA ®-Werke GMBH & CO.KG, Staufen, Germany). A solution of 10 g/kg of sodium alginate with or without 200 g/kg of trehalose and 2.5 g/kg of biopolymers suspensions were previously prepared until complete dissolution (up to 12 h), following the same procedure reported on Aguirre Calvo et al. (2017). Table S1 (Supplementary File) summarizes the used emulsions and the composition of each system.
Beads were prepared by ionotropic gelation dropping the solutions with a peristaltic pump following the same procedure and parameters described in Aguirre Calvo et al. (2017). The gelling solutions contained 25 g/kg calcium chloride (Cicarelli S.A., Argentina) for preparing Ca(II)-alginate beads without excipients (A) and were supplemented with 200 g/kg trehalose for the rest of the systems. After generation, beads were stored at 4 °C and darkness till the correspondent treatments or determinations.
Thermal treatments
Freezing
Two freezing treatments were applied: (a) four freezing/thawing cycles, performed with liquid nitrogen (− 196 °C) for 1 min (freezing) and 30 min at 25 °C (thawing); (b) continuous freezing at − 18 °C for 1 month (conventional freezer).
For both treatments, beads (0.25–0.3 g) were placed in microcentrifuge tubes.
Dehydration
A thin layer of beads was arranged in a petri dish for both drying treatments: (a) vacuum drying (VD) at 25 °C for 48 h at 113.25 mbar on a vacuum oven (Fistreem International Ltd, Loughborough, UK); (b) freeze-drying (FD) for 48 h in an ALPHA 1–4 LD2 freeze-drier (Martin ChristGefriertrocknungsanlagen GMBH, Germany); beads were frozen at − 18 °C in a standard freezer for 24 h prior to FD.
After drying, dehydrated beads were kept in vacuum desiccators containing dried silica until analyses.
Lycopene content and stability
Lycopene content was determined in triplicate before and after thermal treatments by UV/Vis measurements following the methodology previously employed (Aguirre Calvo et al. 2017). Control samples of beads without lycopene were also prepared to avoid content overestimation.
The spectral fine structure was studied as described by Aguirre Calvo and co-workers (2017) by analyzing %III/II. This ratio is defined as the height of the absorption peak at 503 nm, designated as III, and the height of the absorption peak at 472 nm, designated as II, taking the minimum between the two peaks as the baseline, multiplied by 100.
Lycopene content and spectral fine structure were normalized by the content of lycopene or the %III/II, respectively, of the extract used in the preparation of beads. Then, the remaining lycopene content or %III/II of the beads after thermal treatments were calculated as the percentage of each system obtained after a given treatment (freezing or drying) and the value of the same system before the treatment (wet samples without treatment).
Beads characterization
Water content (WC) was determined using three beads (in triplicate) by Karl Fischer titration following the same methodology described in Aguirre Calvo and Santagapita (2019). Size and shape were analyzed by image analysis (Aguirre Calvo and Santagapita 2016, 2019) using ImageJ software (NIH, USA) with a digital camera coupled to a binocular microscope. Area, perimeter, Feret’s diameter, and circularity for at least 60 beads were analyzed by applying the “analyze particle” command of the software.
Low-field proton nuclear magnetic resonance measurements (LF-1H-NMR)
The percentage of solid component was obtained in isothermal studies conducted at − 20.05, − 30.00 and − 70.00 °C (± 0.05) controlled by a BVT3000 unit (Bruker Biospin GmbH) using the FID (free induction decay) sequence in a Bruker Minispec mq20 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). Measurements were taken every 10 s during 5, 30 or 100 min, respectively. The FID sequence settings were: scans = 8, dummy shots = 0, recycle delay = 5 ms, gain = 81–83 dB.
Experimental data at − 20 and − 30 °C were fitted according to one-phase association equation (Eq. 1, obtaining R2 > 0.97) and those obtained at − 70 °C were fitted using the Johnson–Mehl–Avrami–Kolmogorov equation (Eq. 2, achieving R2 > 0.99), based on previous works (Aguirre Calvo and Santagapita 2019; Traffano-Schiffo et al. 2017b, respectively).
| 1 |
where Y0 is the Y value when time t is zero; Plateau is the Y value at infinite times; K is the rate constant, expressed in reciprocal of the time units.
| 2 |
where α is the solid crystal fraction obtained over time t; n is known as Avrami index, related to nucleation and dimension of crystal growing; and Kc [(time)−n], related to the isothermal crystallization rate (which mainly is temperature dependent).
Statistical analysis
1-way ANOVA with Tukey post-test using Prism 6 (GraphPad Software Inc., San Diego, CA, USA) was used to analyze statistical differences on each parameter.
Results and discussion
Freezing: lycopene stability, isothermal studies and bead characterization
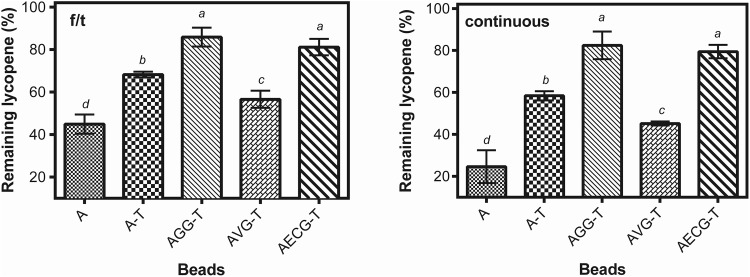
It was previously established that lycopene extracted from grapefruit pulp is prone to destabilization mediated by freezing and should be stabilized for further use in frozen products (Aguirre Calvo and Santagapita 2019; Shi 2000). Lycopene encapsulated in Ca(II)-alginate beads containing galactomannans were successfully produced achieving remaining lycopene values higher than 80% in presence of vinal gum (Aguirre Calvo et al. 2017). However, there is no available information on lycopene stability towards freezing or drying treatments in these systems. Hence, beads were frozen using two protocols: four cycles with liquid nitrogen for 1 min and 15 min of thawing (f/t) and continuous freezing at − 18 °C for 1 month. Figure 1 shows the remaining lycopene obtained for beads containing trehalose and galactomannans after freezing. As observed previously by Aguirre Calvo and Santagapita (2019) it is critical to include trehalose to retain more than 60% of initial lycopene content, since A-beads showed more than 60% of lycopene losses (Fig. 1) but is not the only determining factor that resulted in improved retention of the lycopene. Espina corona and guar gum additions significantly improved remaining lycopene. However, vinal gum reduced the recovery with respect to trehalose, revealing a negative effect on its inclusion. Therefore, the addition of a secondary excipient must be handled with extreme care, since the stability of the encapsulated lycopene during freezing processes can be highly compromised, even if a known cryoprotectant such as trehalose is included. It is known that gum characteristics such as molecular weight or polymer structure affects OH distribution and consequently both the H-bond capacity and molecular packing, producing an impact on labile biomolecules interactions and stability. Pushpamalar et al. (2016) reported that slight variations on biopolymers structure modified its physicochemical properties and encapsulation ability. Vinal gum possess the higher degree of substitution among these gums as well as the lower viscosity (Busch et al. 2018). The high substituted polymer chains reduced galactomannan–galactomannan interactions due to sterical factors, possibly promoting alginate-galactomannan and trehalose-galactomannan associations, leaving the encapsulated lycopene unprotected.
Fig. 1.
Remaining lycopene content from beads subjected to four cycles of freezing and thawing (− 196/25 °C) (left) and continuous freezing with 1 month at − 18 °C (right). Standard deviations values are included. The letters above the columns indicate significant differences between values of different beads for the same treatment with p value < 0.05
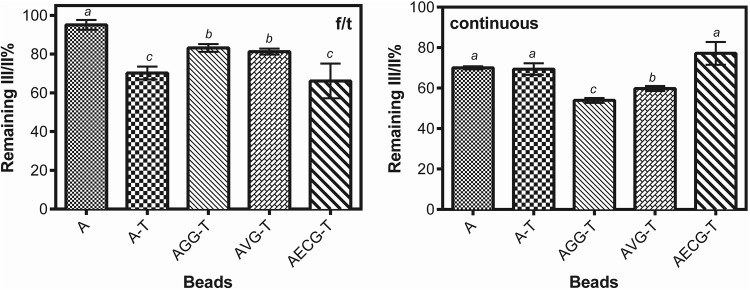
Freezing/thawing showed higher remaining lycopene values than continuous freezing. This could be related to the formation of larger crystals during the latter treatment, causing the destabilization of the encapsulated lycopene, but the difference in the time length of the freezing treatment could also have an influence that cannot be neglected. The III/II% index showed in Fig. 2 is indicative of lycopene stability, showing the extent of the lycopene structural damage, especially for isomerization and oxidation (Rodríguez Amaya and Kimura 2016). Continuous freezing drastically affected the lycopene structure compared to f/t cycles. Neither the inclusion of trehalose nor any gum improved lycopene stability, revealing some degree of isomerization in any of its 13 all-trans double bonds. Hence, it is possible to encapsulate lycopene in Ca(II)-alginate beads containing trehalose and galactomannans, but always a fraction of lycopene will be isomerized during freezing. However, cis-lycopene have higher bioactivity than its trans form according to Urbonaviciene and Viskelis (2017), which is possibly related to the higher solubility of the micelles formed with bile during digestion (Vitale et al. 2010).
Fig. 2.
Remaining III/II% index from beads subjected to four cycles of freezing and thawing (− 196/25 °C) (left) and continuous freezing with 1 month at − 18 °C (right). The index III/II% is obtained from the percentage ratio of absorbance values of peak III (503 nm) and peak II (472 nm), being standardized by the indices of the respective initial time (zero time) of each bead. Standard deviations values are included. The letters above the columns indicate significant differences between values of different beads for the same treatment with p value < 0.05
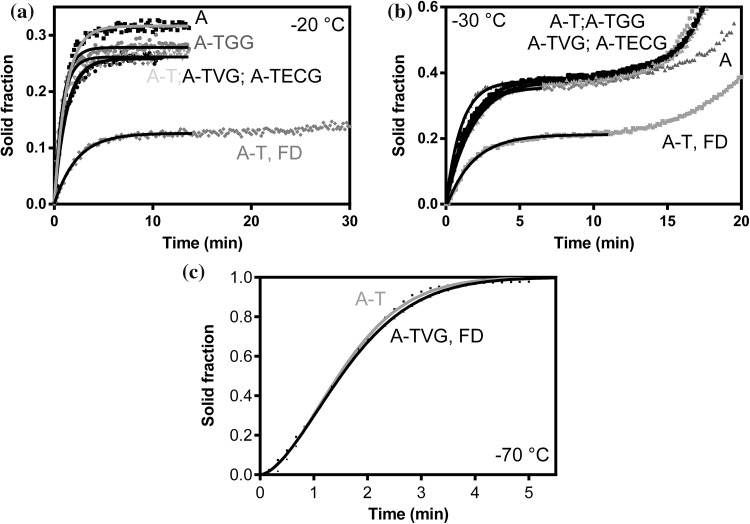
Since the stability of lycopene is affected by freezing, and in turn with the formation of crystals, crystallization was studied by LF-NMR (Aguirre Calvo and Santagapita 2019; Traffano-Schiffo et al. 2017b). Figure 3 shows the isothermal crystallization of selected wet and freeze-dried beads as a function of time at three different temperatures. It is important to keep in mind that both water and oil can crystallize in beads. Crystallization was followed by analyzing the increase of the percentage of the solid component, which is defined as the ratio of the signal from the solid component divided by the total NMR signal. This methodology relies on the assumption that the signal from the solid component decays very quickly with respect to the one of the liquid components (either oil or water, both having longer transversal relaxation times with respect to solids). The crystallization of freeze-dried (FD) beads was also included for comparative purposes. Curves at − 20 and − 30 °C showed a similar trend: a plateau is reached before proceeding to a second stage of crystallization. These results were comparable to those obtained for Ca(II)-alginate beads containing chitosan, pectin and/or sugars (Aguirre Calvo and Santagapita 2019). The addition of trehalose reduced the percentage of solid crystallized at − 20 °C with respect to Ca(II)-alginate plain beads as it can be observed in Fig. 3a. Table 1 shows the solid fraction at the first plateau at − 20 and − 30 °C, and the time employed to achieve 50% of crystallization at − 30 °C.
Fig. 3.
Isothermal LF-NMR studies of wet and freeze-dried (FD) beads. Crystallization is displayed as a function of time at − 20 (a) − 30 (b) − 70 °C (c). Experimental values were fitted by the association single phase equation (at − 20 and − 30 °C) and the Avrami equation (− 70 °C)
Table 1.
Percentage of crystallization at the plateau at − 20 and − 30 °C and the time at which 50% of crystallization was achieved at − 30 °C (t50) for beads of different formulations containing lycopene
| Beads | Solid fraction (%) | t50 (min) | |
|---|---|---|---|
| − 20 °C | − 30 °C | − 30 °C | |
| A | 31.8 ± 0.1A | 37.5 ± 0.2B | 19.3 ± 0.1A |
| A-T | 25.5 ± 0.9C | 37.9 ± 0.2A,B | 17.1 ± 0.1B |
| AGG-T | 28.0 ± 0.2B | 38.1 ± 0.2A | 16.9 ± 0.1B |
| AVG-T | 25.6 ± 0.3C | 35.8 ± 0.3C | 16.8 ± 0.1B |
| AECG-T | 25.7 ± 0.3C | 36.7 ± 0.6A,B,C | 16.7 ± 0.4B |
The letters in the same column indicate significant differences between values of different beads for the same temperature with p value < 0.05
The difference observed for the plateau between wet and freeze-dried beads frozen at − 20 and − 30 °C was related to water crystallization since FD beads do not possess freezable water, as will be analyzed in the next section. Comparing the curves at these temperatures, it was possible to observe a higher lipid crystallization for FD beads, in accordance to the values reported by Hammer (2008) in DSC scans, and by Aguirre Calvo and Santagapita (2019) for NMR relaxograms. Table 1 shows that each bead system has differences in the amount of crystallized water. Trehalose significantly reduced the obtained values for both temperatures as a consequence of the formation of an amorphous matrix, which retains a larger volume of water whose crystallization is kinetically inhibited/delayed (Traffano-Schiffo et al. 2017a, b). Besides, t50 values were also reduced, and no significant differences were observed by the addition of galactomannans. Guar gum containing beads showed higher solid fraction at both temperatures (28.0 and 38.1% ± 0.2, respectively) than the other two employed galactomannans. Traffano-Schiffo and co-workers (2017a) reported that guar gum produced an increase in the onset temperatures of ice melting (Tm’), revealing its ability to associate water. At − 70 °C, crystallization was completed. Avrami equation (Aguirre Calvo and Santagapita 2019; Traffano-Schiffo et al. 2017b) was applied to model the kinetics of crystallization. Two parameters were obtained: the Avrami index (n), related to the nucleation mechanism and crystal growth, and the isothermal crystallization constant (Kc). A two-dimensional growth can be proposed since n values were 1.66 and 1.41 (both ± 0.02) for wet and freeze-dried A-TVG beads, respectively, with similar Kc values around 0.35 (both ± 0.01), even though if freeze-dried beads showed some restrictions in the structure imposed by the drying.
Drying: lycopene stability and bead characterization
Beads were also dried by vacuum (VD) and freeze-drying (FD). The water content (WC) values after dehydration shown in Table S2 (Supplementary File) revealed that beads were successfully dehydrated. The inclusion of trehalose and gums provoked higher water content, related to the strong interactions established between excipients and water. In accordance with Traffano-Schiffo and coworkers (2017b), VD produced higher WC values than FD in Ca(II)-alginate beads containing trehalose and gums.
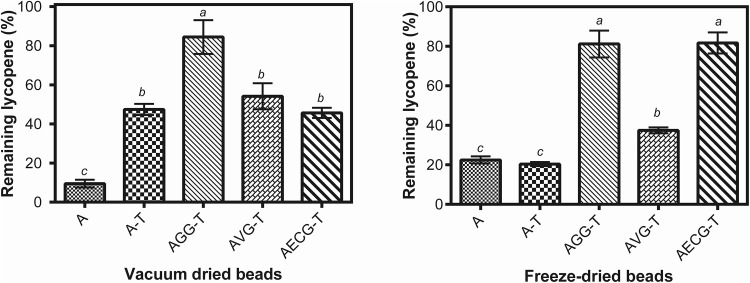
The remaining lycopene content for different beads after vacuum and freeze-drying is showed in Fig. 4. The concentration of lycopene decreased by almost 90% during the VD treatment and almost 80% after FD. Alginate by its own was not able to protect lycopene towards dehydration, confirming previous results (Aguirre Calvo and Santagapita 2019; Traffano-Schiffo et al. 2017b). The addition of trehalose and of secondary biopolymers improved lycopene retention after both drying treatments. Nevertheless, it is interesting to analyze that the lycopene values of beads containing trehalose after FD (20 ± 1%) were even lower than after VD (47 ± 3%) since lycopene already was damaged during freezing, a necessary step for FD. Only if effective interactions between lycopene and trehalose are established in the emulsions, the lycopene could be stabilized, since trehalose can increase stability through time and towards flocculation (Álvarez Cerimedo et al. 2010). Besides, the presence of interphases (a crystallized phase is observed for FD beads in Fig. 3a) can enhance lycopene deterioration. Beads containing guar gum were the best among all systems, for both drying treatments. Espina corona inclusion also provokes high lycopene retention for freeze-dried beads. Present results are also in accordance to those obtained for freezing (Fig. 1).
Fig. 4.
Remaining lycopene content from beads subjected to vacuum drying (left) and freeze-drying (right). Standard deviations values are included. The letters above the columns indicate significant differences between values of different beads for the same treatment with p value < 0.05
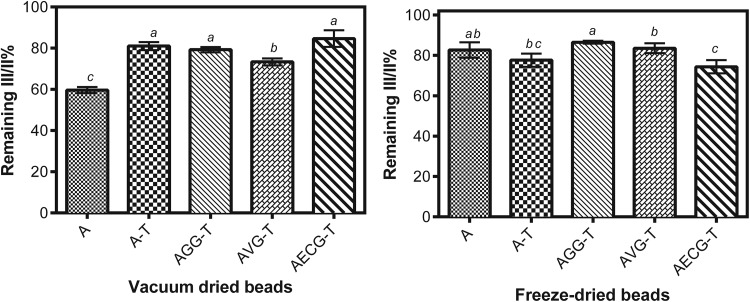
Lycopene structural changes were once again analyzed through the III/II% index, as shown in Fig. 5. All beads were unable to fully protect lycopene from isomerization, observing higher changes for VD beads than for the FD ones. GG containing beads were the ones with higher III/II% index conservation during both treatments. Thus, it can be considered an excellent alternative for lycopene stabilization taking altogether both Figs. 4 and 5. It is necessary to contemplate that lycopene degradation is dependent of the balance between its interaction, distribution and migration, since its exposure to O2, temperature changes and light increase isomerization or may even cause degradation (Rodríguez Amaya and Kimura 2016).
Fig. 5.
Remaining III/II% index from beads subjected to vacuum drying (left) and freeze-drying (right). Standard deviations values are included. The letters above the columns indicate significant differences between values of different beads for the same treatment with p value < 0.05
Finally, size was also affected by dehydration as well as freezing. Both Feret’s diameter and circularity diminished around 20% after drying (data not shown), as expected for similar systems (Aguirre Calvo and Santagapita 2019). The observed reduction was less marked than in similar systems (Traffano-Schiffo et al. 2017b) due to the oil presence, allowing to partially preserve the size, but in a not homogeneous way. All formulations showed similar trends, and no significant differences were observed between freeze- and vacuum-dried beads.
Conclusion
The use of Ca(II)-alginate beads without excipients cause a significant decrease on lycopene content after freezing and drying, revealing the need to include other stabilizers. The search for better excipient combinations is a challenging field. Galactomannans inclusion not only affects loading efficiency and release of lycopene but also its stability against thermal and hydric stresses. Guar gum inclusion proved to be a very good alternative towards freezing and dehydration processes, since in all cases more than 80% of the biocompound was protected, preserving mostly the integrity of trans-lycopene. Isothermal studies revealed that frozen water was reduced at − 20 and − 30 °C, mainly affected by trehalose. Differences among galactomannans could be related to the substitution degree of the polymer chains, affecting the overall systems interactions, modifying isothermal crystallization kinetics. The addition of some excipients affected lycopene stability towards freezing, even compromising the positive effect of a well-established cryoprotectant as trehalose, revealing that the selection of secondary excipients should be carefully conducted. The results of this work can contribute to the selection of excipients to formulate Ca(II)-alginate beads containing not only lycopene but also other labile biomolecules, being a starting point for technological applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the financial support of ANPCYT (PICT 2017-0569), CIN-CONICET (PDTS 2015 n° 196) and UBA (Project UBACyT 20020130100610BA). We also acknowledge Dr. Verónica Busch for vinal gum donation. TRAC acknowledge CONICET for the Ph.D. scholarship. PRS is member of CONICET.
Compliance with ethical standards
Conflict of interest
The author declares that there is no conflict of interests regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguirre Calvo TR, Santagapita PR. Physicochemical characterization of alginate beads containing sugars and biopolymers. J Qual Reliab Eng. 2016 [Google Scholar]
- Aguirre Calvo TR, Santagapita PR. Encapsulation of a free-solvent extract of lycopene in alginate-Ca(II) beads containing sugars and biopolymers. Chem Biol Technol Agric. 2017;4:16. doi: 10.1186/s40538-017-0099-3. [DOI] [Google Scholar]
- Aguirre Calvo TR, Santagapita PR. Pink grapefruit’ lycopene encapsulated in alginate-based beads: stability towards freezing and drying. Int J Food Sci Technol. 2019;54:368–375. doi: 10.1111/ijfs.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre Calvo TR, Busch VM, Santagapita PR. Stability and release of an encapsulated solvent-free lycopene extract in alginate-based beads. LWT Food Sci Technol. 2017;77:406–412. doi: 10.1016/j.lwt.2016.11.074. [DOI] [Google Scholar]
- Álvarez Cerimedo MS, Iriarte CH, Candal RJ, Herrera ML. Stability of emulsions formulated with high concentrations of sodium caseinate and trehalose. Food Res Int. 2010;43:1482–1493. doi: 10.1016/j.foodres.2010.04.008. [DOI] [Google Scholar]
- Barankevicz GB, Novello D, Resende JT, Schwarz K, Santos EF. Physical and chemical characteristics of tomato hybrids pulp during frozen storage. Hortic Bras. 2015;33:7–11. doi: 10.1590/S0102-053620150000100002. [DOI] [Google Scholar]
- Busch VM, Pereyra-Gonzalez A, Segatin N, Santagapita PR, Poklar Ulrih N, Buera MP. Propolis encapsulation by spray drying: characterization and stability. LWT Food Sci Technol. 2017;75:227–235. doi: 10.1016/j.lwt.2016.08.055. [DOI] [Google Scholar]
- Busch VM, Delgado JF, Santagapita PR, Wagner JR, Buera MP. Rheological characterization of vinal gum, a galactomannan extracted from Prosopis ruscifolia seeds. Food Hydrocoll. 2018;74:333–341. doi: 10.1016/j.foodhyd.2017.08.010. [DOI] [Google Scholar]
- Costa-Rodrigues J, Pinho O, Monteiro PRR. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018;245:1148–1153. doi: 10.1016/j.foodchem.2017.11.055. [DOI] [PubMed] [Google Scholar]
- Dias MG, Camões MFG, Oliveira L. Carotenoid stability in fruits, vegetables and working standards—effect of storage temperature and time. Food Chem. 2014;156:37–41. doi: 10.1016/j.foodchem.2014.01.050. [DOI] [PubMed] [Google Scholar]
- Hammer A. The characterization of olive oils by DSC. Therm Anal UserCom. 2008;28:6–8. [Google Scholar]
- Perduca MJ, Spotti MJ, Santiago LG, Judis MA, Rubiolo AC, Carrara CR. Rheological characterization of the hydrocolloid from Gleditsia amorphoides seeds. LWT Food Sci Technol. 2013;51:143–147. doi: 10.1016/j.lwt.2012.09.007. [DOI] [Google Scholar]
- Pérez-Masiá R, Lagaron JM, Lopez-Rubio A. Morphology and stability of edible lycopene-containing micro-and nanocapsules produced through electrospraying and spray drying. Food Bioprocess Technol. 2015;8:459–470. doi: 10.1007/s11947-014-1422-7. [DOI] [Google Scholar]
- Pushpamalar J, Veeramachineni AK, Owh C, Loh XJ. Biodegradable polysaccharides for controlled drug delivery. ChemPlusChem. 2016;81:504–514. doi: 10.1002/cplu.201600112. [DOI] [PubMed] [Google Scholar]
- Quintanilla-Carvajal MX, Camacho-Díaz BH, Meraz-Torres LS, Chanona-Peréz JJ, Alamilla-Beltrán L, Jimenéz-Aparicio A, Gutiérrez-López GF. Nanoencapsulation: a new trend in food engineering processing. Food Eng Rev. 2010;2:39–50. doi: 10.1007/s12393-009-9012-6. [DOI] [Google Scholar]
- Rodríguez Amaya DB, Kimura M (2016) Harvestplus handbook for carotenoid analysis. In: HarvestPlus
- Shi J. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Biotechnol. 2000;20:293–334. doi: 10.1080/07388550091144212. [DOI] [PubMed] [Google Scholar]
- Sinha N, Dua D. Lycopene: most potent antioxidant with endless benefits. Int J Pharma Bio Sci. 2015;6:838–846. [Google Scholar]
- Souza AL, Hidalgo-Chávez DW, Pontes SM, Gomes FS, Cabral LM, Tonon RV. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: characterization and stability. LWT Food Sci Technol. 2018;91:286–292. doi: 10.1016/j.lwt.2018.01.053. [DOI] [Google Scholar]
- Stahl W, Sies H. Lycopene: A biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- Torres MD, Hallmark B, Wilson DI. Effect of concentration on shear and extensional rheology of guar gum solutions. Food Hydrocoll. 2014;40:85–95. doi: 10.1016/j.foodhyd.2014.02.011. [DOI] [Google Scholar]
- Traffano-Schiffo MV, Aguirre Calvo TR, Castro-Giraldez M, Fito PJ, Santagapita PR. Alginate beads containing lactase: stability and microstructure. Biomacromol. 2017;18:1785–1792. doi: 10.1021/acs.biomac.7b00202. [DOI] [PubMed] [Google Scholar]
- Traffano-Schiffo MV, Castro-Giraldez M, Fito PJ, Santagapita PR. Encapsulation of lactase in Ca(II)-alginate beads: effect of stabilizers and drying methods. Food Res Int. 2017;100:296–303. doi: 10.1016/j.foodres.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Traffano-Schiffo MV, Castro-Giraldez M, Fito PJ, Perullini M, Santagapita PR. Gums induced microstructure stability in Ca(II)-alginate beads containing lactase analyzed by SAXS. Carbohydr Polym. 2018;179:402–407. doi: 10.1016/j.carbpol.2017.09.096. [DOI] [PubMed] [Google Scholar]
- Urbonaviciene D, Viskelis P. The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. LWT Food Sci Technol. 2017;85:517–523. doi: 10.1016/j.lwt.2017.03.034. [DOI] [Google Scholar]
- Vitale AA, Bernatene EA, Pomilio AB. Carotenoides en quimioprevención: Licopeno. Acta Bioquím Clín Latinoam. 2010;44:195–238. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.