Abstract
Essential oils (EOs) are known for their antioxidant properties, and are widely employed in the food industry as preservatives. They can be used as condiments or as preservatives to achieve certain organoleptic effects for consumers. The aim of this research was to evaluate antioxidant activity in mixtures of three EOs: Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L., using the Simplex Lattice Mixture Design. Ultimately, a linear model was used, as it best adjusted to the experimental behavior, and it allowed the prediction of EOs mixtures antioxidant activity, determined by FRAP and ABTS techniques. The mixture of the three EOs that showed the best antioxidant activity and also had the highest synergistic effect, was composed of 66.7% of T. vulgaris, 16.7% of C. sativum and 16.7% of A. graveolens. The greatest contribution to the potentiation of antioxidant activity was shown by T. vulgaris followed by A. graveolens and then C. sativum.
Keywords: Food science, Aromatic crops, Spice, Natural antioxidants, Additives
1. Introduction
Essential oils (EOs) are volatile aromatic compounds generated from secondary metabolism in plants. Each EO is composed of between a dozen and several hundred components, which mainly consist of terpenes and terpenoids including oxygenated derivatives, such as aldehydes, ketones, alcohols, ethers, esters and epoxides (Bajpai and Baek, 2016). Some EOs are also known to contain allyl- and propenylphenols (Bajpai et al., 2012, 2013), as well as nitrogen, sulfur or chlorine atoms in their structure (Bakkali et al., 2008; Nagella et al., 2012). Moreover, EOs contain conjugated double bonds and phenolic functional groups. Their significant free radical scavenging properties have been extensively studied in recent years (Aruoma, 1998; Baj et al., 2018; Kamatou and Viljoen, 2010). These chemical characteristics give them their antioxidant properties (Miguel, 2010). Thymol, carvacrol, and eugenol are the most powerful antioxidants contained in the EOs examined, and their effects on food are among their benefits to human health (Amerah and Ouwehand, 2016; Elgndi et al., 2017; Firenzuoli et al., 2014). Antioxidant properties play a pivotal role in some EOs’ biological activity, related to oxidative stress pathologies (Valgimigli et al., 2000).
The antioxidant activity of the EOs extracted from A. graveolens, T. vulgaris and C. sativum could be attributed to their main components. These three species of plants are herbs which are widely used for culinary purposes, with their fresh or dried leaves often used as spices. EOs extracted from the leaves and flowers of these species may also be used as flavoring additives in food. The antioxidant activity and non-toxic properties of these EOs have been well established (Burdock and Carabin, 2009; Lee et al., 2005; Nagella et al., 2012). The major components of the A. graveolens volatile oil are 4-chloro-4.4-dimethyl-3-(1-imidazolyl)-valerophenone, 1-dodecanol, 9-octadecen-12-ynoic acid (Nagella et al., 2012), and limonene (Baananou et al., 2013; Rożek et al., 2016; Seo et al., 2015). T. vulgaris oil is composed of the chemical compounds α-pinene, thymol, caryophyllene (Al-Asmari et al., 2017) and carvacrol, depending on chemotype (Benzie and Strain, 1996; Mancini et al., 2015). The dominant compound of C. sativum is generally linalool (more than 70%) (Chahal et al., 2018). Studies about the antioxidant properties of EO mixtures are rare, and in case of A. graveolens, T. vulgaris and C. sativum, there are no previous studies using similar mixture design, nor demonstrating any type of synergy. ‘‘Synergy’’ is a popular concept in the field of herbal medicine, which suggests that plant extracts contain compounds which potentiate one another (Mancini et al., 2015).
Research regarding the synergistic effects of these plants in other mixtures is still limited. Thus, the aim of the present study was to evaluate the synergistic effect of mixed EOs by Simplex Lattice Mixture Design (SL-MD). In this study, the antioxidant properties depend only on the percentage of each component in the mixtures. SL-MD was preferred, as it is a tool widely used for evaluating the constituent factors and their response effects (Cornell, 2011). Recently, there have been research papers that showed use of the SL-MD for optimizing the antioxidant activity of EO mixtures (Baj et al., 2018).
2. Materials and methods
2.1. Plant material
Four kilograms of fresh aerial parts for each herbs A. graveolens, T. vulgaris and C. sativum, harvested before flowering and belonging to only one batch, were purchased from a local market in Puyo, Pastaza in the Amazon region of Ecuador in June, 2018.
All the herbs were taken to the laboratory and air-dried at a temperature range between 20 - 25 °C, for 48 hours (additional airflow was not used). The equilibrium moisture content, as percent moisture, for each of the 3 herbs after drying under the conditions referenced here were: 7.72 ± 0.04% for A. graveolens, 4.25 ± 0.02% for T. vulgaris and 6.59 ± 0.03% for C. sativum.
2.2. Essential oil extraction by steam distillation
A FIGMAY brand (manufacturing in Argentina, www.figmay.com.ar) essential oil extraction apparatus was used to extract volatile oil from the three plant species.
The apparatus comprises: heating system made of transparent quartz and polytetrafluoroethylene (PTFE) provided with an internal electrical resistance inside a borosilicate glass boiler, as well as borosilicate glass condenser, loading and unloading lid, and stainless steel basket (capacity 2 L).
A sample of 500 grams of air-dried coarsely powdered, using a Thomas Scientific Model 4 Wiley mill, aerial parts with a particle size <0.5 mm, obtained by RETSCH test sieves with round hole, were placed in the distiller. Distillation with a continuous flow of water vapor (near to 100 °C due to the altitude) was stopped after 40 minutes when it was observed that successive readings of the volume of oil remained constant.
Multiple runs were carried out to obtain the EOs and only one fraction was collected for each individual herb. The receiver vessel was not cooled, it remained at room temperature (<20 °C). The oil was then separated and passed over anhydrous sodium sulfate. The oil was filtered using filter paper and kept at 4 °C in sealed vials in the dark, up to 24 hours with the aim of ensuring that oxidative degradation has not occurred, for later use. Test were done immediately after the extraction in order to avoid degradation or changes in the samples’ composition. EOs were obtained in a yield of 0.13%, 0.33% and 0.23% w/w for A. graveolens., T. vulgaris and C. sativum, respectively.
2.3. Sample preparation
Each individual essential oil was put in plastic tube in the required proportion (Table 1), and afterwards, a dilution of 1:100 v/v with methanol was applied using a micropipette Eppendorf (SIGMA-ALDRICH), and finally, a vortex stir (Scientific Industries Vortex Genie2 Vortex Mixer G560E) was carried out for one minute. The final volume was 1 mL.
Table 1.
Experimental conditions of mixture design. Antioxidant experimental activity and synergistic effect calculated from individual ingredients.
| Mixturea | Ingredient proportions |
Measured antioxidant |
Synergistic effect (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FRAP (g Eq. Trolox L−1) (Y1) |
ABTS (% Inhibition) (Y2) |
||||||||||
| A. graveolens (X1) | T. vulgaris (X2) | C. sativum (X3) | Actual value | Predicted value | Residual | Actual value | Predicted value | Residual | FRAP | ABTS | |
| 1 | 1/6 | 1/6 | 2/3 | 61.5 | 61.31 | 0.26 | 19.0 | 18.69 | 0.35 | 10.1 | 8.0 |
| 2 | 1/2 | 1/2 | 0 | 169.2 | 163.49 | 5.72 | 52.4 | 51.77 | 0.7 | 8.9 | 4.9 |
| 3 | 1 | 0 | 0 | 19.9 | 23.24 | −3.33 | 3.2 | 3.89 | −0.68 | 0 | 0 |
| 4 | 0 | 1/2 | 1/2 | 169.5 | 156.98 | 12.57 | 51.9 | 50.9 | 1.02 | 13.2 | 5.7 |
| 5 | 0 | 1 | 0 | 288.2 | 303.74 | −15.51 | 96.5 | 99.64 | −3.12 | 0 | 0 |
| 6 | 1/6 | 2/3 | 1/6 | 226.5 | 208.07 | 18.5 | 72.1 | 67.44 | 4.7 | 13.3 | 9.7 |
| 7 | 0 | 0 | 1 | 5.9 | 10.22 | −4.26 | 1.3 | 2.16 | −0.83 | 0 | 0 |
| 8 | 0 | 1 | 0 | 298.0 | 303.74 | −5.72 | 98.5 | 99.64 | −1.12 | 0 | 0 |
| 9 | 1 | 0 | 0 | 21.0 | 23.24 | −2.18 | 3.5 | 3.89 | −0.38 | 0 | 0 |
| 10 | 2/3 | 1/6 | 1/6 | 69.3 | 67.82 | 1.5 | 19.5 | 19.56 | 0.023 | 10.1 | 5.7 |
| 11 | 0 | 0 | 1 | 6.1 | 10.22 | −4.06 | 1.6 | 2.16 | −0.54 | 0 | 0 |
| 12 | 1/2 | 0 | 1/2 | 14.8 | 16.73 | −1.87 | 2.3 | 3.03 | −0.66 | 12.9 | 4.2 |
| 13 | 1/3 | 1/3 | 1/3 | 110.7 | 112.4 | −1.61 | 35.7 | 35.23 | 0.52 | 5.5 | 5.7 |
non-randomized sequence.
2.4. Total antioxidant activity
Both, the FRAP and ABTS radical scavenging methods were used because only one of them would not offer enough information for all of the antioxidant functional groups in the extracted compounds (Lai et al., 2018). The methods were simple and robust, and the assays were performed quickly, providing precise results (Prior et al., 2005).
2.4.1. FRAP assay
The reagent was freshly prepared for use by adding 2.5 mL of 10 mM 2,4,6-tripyridyl -s-triazine in 40 mM HCl and 2.5 mL of 20 mM FeCl3.6H2O to 25 mL of 0.3 M of an acetate buffer (pH = 3.6) solution. At the beginning, 80 μL of each EO mixture was placed in a 10 mL volumetric flask and 5 mL of a FRAP reagent was added. After being filled with distilled water, the volumetric flask was shaken and allow to stand for 30 min at 37 °C in a RY-DHG-9030A electric heat laboratory oven. No phase separation was observed since the sample completely reacted with FRAP reagent (Seo et al., 2015). The calibration curve was assembled based on TROLOX pure standard in the range 10−4 – 10−3 mg L−1. The spectrophotometric measurements at the wavelength of 593 nm were performed using a Thermo Scientific GENESYS 10S UV-Vis Spectrophotometer.
2.4.2. ABTS assay
Antioxidant activity was determined by means of the ABTS method (Baananou et al., 2013). For the radical formation, 3.3 mg of sodium persulfate was placed together with 19.4 mg of ABTS•+ in a dark bottle. 5 mL of distilled water were added. The mixture was left to stand at room temperature for 16 hours. After that, the solution was diluted in a 1:10 ratio with ethanol in order to get an absorbance value near to 0.8730 (Ai) at 754 nm against ethanol blank (Thermo Scientific GENESYS 10S UV-Vis Spectrophotometer). Then, 40 μL of each EO mixture, (obtained according to the SL-MD), was added, shaken, and, after 7 minutes, the final absorbance (Af) was determined. The antioxidant activity of the EOs was expressed using the following equation:
| (1) |
2.5. Simple lattice mixture design to determine antioxidant response
A Simple Lattice Mixture Design was applied to find out the interactive effects of antioxidant activity from the EOs extracted from A. graveolens, T. vulgaris and C. sativum. The response variable was determined by FRAP and ABTS antioxidant activity assays. Mixture design experiments were conceived and analyzed by means of Design-Expert 10 software (Trial key: 2182-0638-8336-EVAL). The entire 13 mixtures are shown in Table 1.
It is important to encourage that, although not all possible combinations were made with replicates and not all possible mixtures or combinations were represented in the study, precisely the advantage of this type of design is that the data can be processed statistically with a minimum number of points (Cornell, 2011).
The following polynomial equation of function Xi was fitted for each factor evaluated at each experimental point, where Y is the variable response and β1, β2, β3, β12, β13, and β23 are constant regression coefficients (Eq. 2):
| (2) |
The information was analyzed using a general linear model. Values of antioxidant properties were explained by the three-dimensional response surface analysis of the independent variable (Xi) and dependent variable (Y) representing the measured antioxidant activity.
2.6. Selection and validation of SL-MD model
The statistical attributes of the models were considered in selection. The adjusted and predicted R2 coefficient values were determined. The weight for each experimental run was obtained, and the appropriateness of the model was calculated by analysis of variance (ANOVA) (Nazir et al., 2017; Pompeu et al., 2009).
Finally, the ‘synergistic effect’ was calculated as the percentage of increase shown in relation to the algebraic sum of the proportion, taking into consideration the individual contribution of each pure oil.
3. Results and discussions
3.1. Antioxidant activity
The highest antioxidant activity was observed using pure EOs, for T. vulgaris (Table 1). Pure EO of A. graveolens presented much lower activity, while C. sativum showed the lowest antioxidant activity. This is in accordance with other studies of these EOs. Al-Asmari et al. (2017) also found T. vulgaris rich in pinene, thymol and caryophyllene, to be a strong antioxidant. A. graveolens, as previously described, has free radical suppression activity which could be attributed to its major component 4-chloro-4,4-dimethyl-3-(1-imidazolyl)-valerophenone (Nagella et al., 2012), whereas the same attributes found in C. sativum could be attributed to the action of (E)-2-Decenal and linalool (Chahal et al., 2018). Lower antioxidant activity is typically seen in EOs rich in monoterpenes (pinenes) (Miller et al., 1993) and C. sativum is no exception (Prachayasittikul et al., 2018).
All the experimental mixtures obtained had higher antioxidant activity than A. graveolens and C. sativum alone. The addition of T. vulgaris to the mixtures resulted in higher antioxidant activity as well. It is obvious that antioxidant activity depends on the T. vulgaris content in any given mixture. If the proportional contribution of the individual EOs to the mixture is compared according to the actual antioxidant activity shown, a synergistic effect is evidenced (see Table 1). Obtained results are in accordance with those found by other authors in mixture design studies for optimum antioxidant activity of mixtures of essential oils (Baj et al., 2018), and avocado byproducts (Calderón-Oliver et al., 2016).
3.2. SL-MD and antioxidant response
Response Surface Methodology is a compilation of statistical and mathematical techniques that are established on the fit of polynomial equation to the experimental data (Mansouri et al., 2018). It describes well the behavior of data set aiming to make statistical previsions. It is more favorable than the traditionally used single parameter optimization since it reduces time, space and raw material usage (Anderson and Whitcomb, 2016a).
The SL-MD allowed for the finding of a combination of the three EOs studied that guarantees a synergic potentiation of the antioxidant activity. This could have a practical use in the food industry for food preservation and conservation. Using mixtures of different essential oils, in addition to potentiating antioxidant activity, guarantees other important organoleptic properties for consumers, for example increased smell and taste.
Using a Simplex Lattice Mixture Design, a linear model was obtained for both methods with an R2 value of nearly 0.99. The expected R2 was in agreement with the adjusted R2; the difference was lower than 0.2 (Anderson and Whitcomb, 2016b). With respect to the ANOVA (Table 2), the lack of fit (F value) was not significant. Additionally, a non-significant lack of fit indicated that the calculated model was suitable for an experimental run. The model was deemed satisfactory because it generated results higher than four (Crespo et al., 2017).
Table 2.
Analysis of variance (ANOVA) for linear model.
| FRAP | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | |
|---|---|---|---|---|---|---|
| Model Linear | 15561.76 | 2 | 7780.88 | 2100.33 | <0.0001 | significant |
| Residual | 37.05 | 10 | 3.7 | |||
| Lack of Fit | 34.96 | 7 | 4.99 | 7.18 | 0,0664 | not significant |
| Pure Error | 2.09 | 3 | 0.7 | |||
| Cor Total |
15598.8 |
12 |
||||
|
ABTS |
Sum of Squares |
df |
Mean Square |
F Value |
p-value Prob > F |
|
| Model Linear | 1.38E+05 | 2 | 68752.6 | 794.55 | <0.0001 | significant |
| Residual | 865.3 | 10 | 86.53 | |||
| Lack of Fit | 816.69 | 7 | 116.67 | 7.2 | 0,0667 | not significant |
| Pure Error | 48.6 | 3 | 16.2 | |||
| Cor Total | 1,38E+05 | 12 |
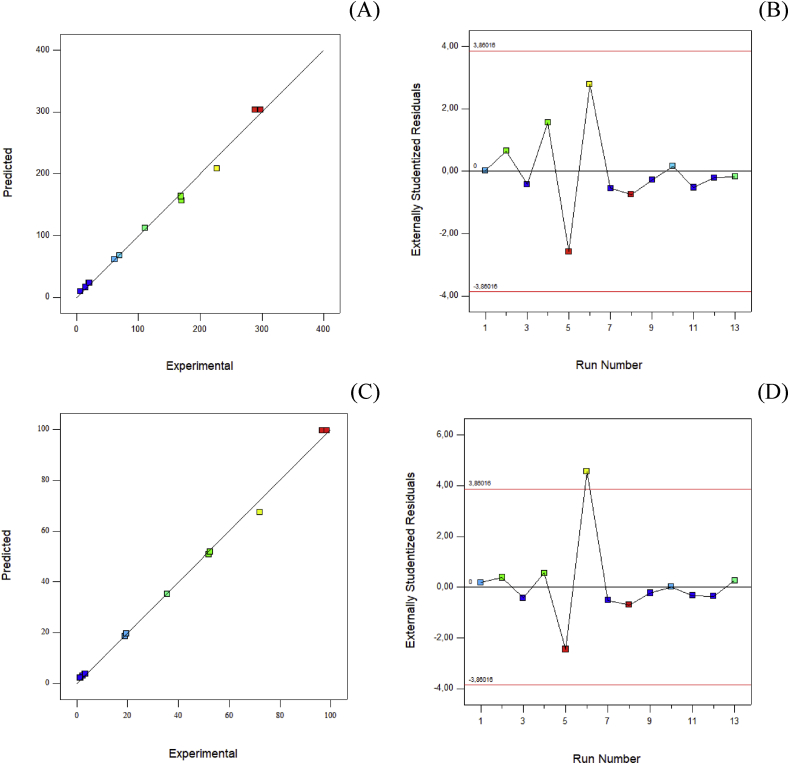
The antioxidant response values expected for the linear model and the real ones obtained from the experiment are shown in Fig. 1. The scatter plot confirms the accuracy of the model in reflecting the entire range of experiments conducted.
Fig. 1.
Relationship between experimental and predicted values and residues vs. run number (A and B, FRAP method; C and D, ABTS method).
It is important to note that the linear equation found may be used to predict the response for several levels of each factor considered. A coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients. The regression model for the experiment is shown in Eq. 3 and Eq. 4:
| (3) |
| (4) |
X1, X2, X3 – single components in mixtures.
The regression analysis results showed that T. vulgaris had greater weight than the rest of the EOs, where C. sativum < A. graveolens < T. vulgaris. In order to establish the validity of the regression, a residue analysis was carried out. A normal distribution was achieved (Fig. 1 B and D).
In the prepared mixtures where the T. vulgaris was in 66.7% proportion, the activity was higher, regardless of the second ingredient in the mixture (exp. 6). However, the obtained activity was always higher for the mixtures than for the pure T. vulgaris essential oil. This could be indicative of the potentiated properties of the mixtures compared to the those of the essential oils individually (see Table 1). The following mixture proportions showed high values of antioxidant activity and also greater synergism: 66.7% T. vulgaris, 16.7% C. sativum and 16.7% A. graveolens.
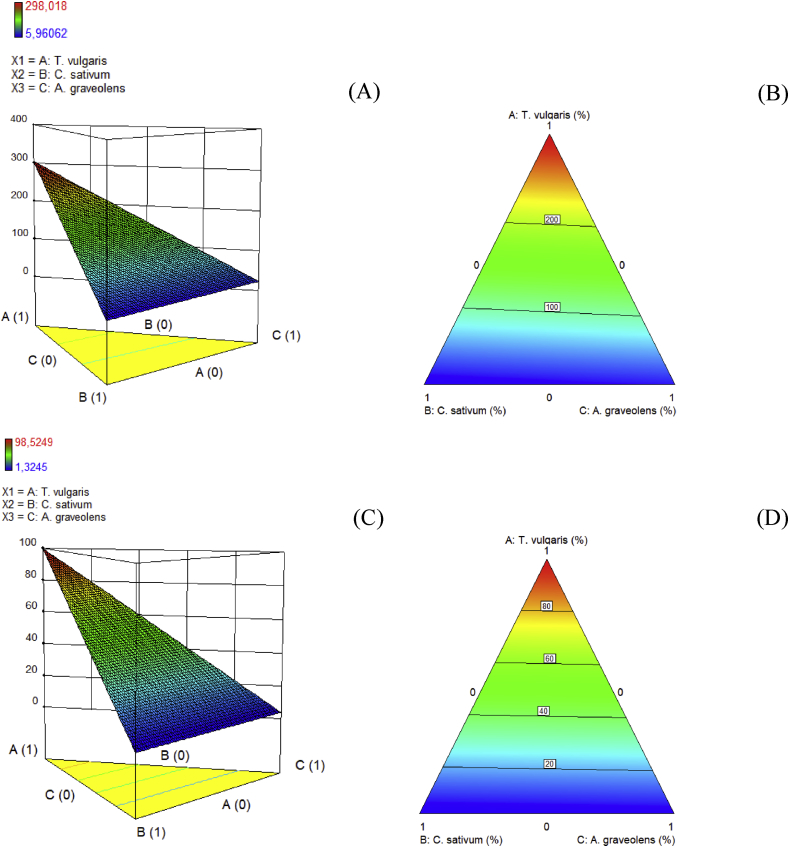
By using the SL-MD, every mixture was analyzed, and in each case, a higher antioxidant property was obtained when T. vulgaris was added (Fig. 2). An optimization procedure was conducted in order to find the mixture having the highest antioxidant activity.
Fig. 2.
Antioxidant responses of the mixture of A. graveolens, T. vulgaris and C. sativum (A and B, FRAP method; C and D, ABTS method).
Previous studies have used a mixture design in order to achieve the best antioxidant mixture. Higher synergistic results have recently been published by Calderón-Oliver et al. (2016); the authors found synergistic effects in the range: 16.5–45.8%. A study on the application of a mixture design for optimal antioxidant activity of EO mixtures has recently been published, which analyzed Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. (Baj et al., 2018). The percentages of synergy found were in the range of 6.6–57.2%. Additional effects of EO mixtures have been reported, including the synergistic antibacterial effects of EO mixtures using Origanum compactum L., O. majorana and Thymus serpyllum L. (Ouedrhiri et al., 2016).
3.3. Correlation between FRAP and ABTS assays
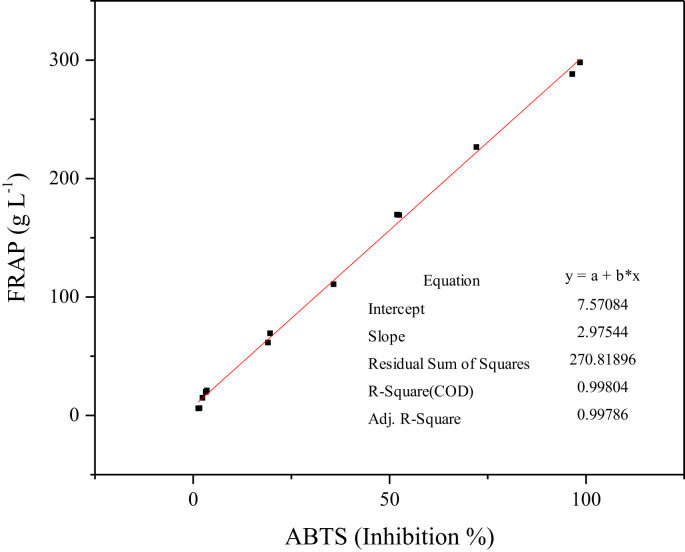
Both of these spectrophotometric analytical methods are based on different chemical mechanisms. The measurements were taken in order to evaluate the antioxidant biological activity of the EO mixture, and were achieved by employing a linear regression model (Fig. 3).
Fig. 3.
Relationship between FRAP and ABTS assays.
4. Conclusions
The spectrophotometric measurements based on different chemical mechanisms allowed evaluation of the antioxidant activity of the EO mixtures.
The highest antioxidant activity was observed for pure T. vulgaris with intermediate activity for A. graveolens, and the lowest antioxidant activity for C. sativum.
The mixture proportions of 66.7% T. vulgaris, 16.7% C. sativum and 16.7% A. graveolens showed higher values of antioxidant activity. A mild synergistic relationship between EOs with regard to their antioxidant activity was predicted by a linear regression model using SL-MD and was observed at various proportions.
Declarations
Author contribution statement
Yasiel Arteaga Crespo, Luis Ramón Bravo Sánchez: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yudel García Quintana, Andrea Silvana Tapuy Cabrera: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Abdel Bermúdez del Sol, Doris Magaly Guzmán Mayancha: Performed the experiments; Wrote the paper.
Funding statement
This work was supported by Universidad Estatal Amazónica. Ecuador.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thanks to the Universidad Estatal Amazónica for their financial support.
References
- Al-Asmari A.K., Athar M.T., Al-Faraidy A.A., Almuhaiza M.S. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed. 2017;7:147–150. [Google Scholar]
- Amerah A.M., Ouwehand A.C. Essential Oils in Food Preservation, Flavor and Safety. Elsevier; 2016. Use of essential oils in poultry production; pp. 101–110. [Google Scholar]
- Anderson M.J., Whitcomb P.J. Productivity press; 2016. DOE Simplified: Practical Tools for Effective Experimentation. [Google Scholar]
- Anderson M.J., Whitcomb P.J. Productivity press; 2016. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments. [Google Scholar]
- Aruoma O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baananou S., Bouftira I., Mahmoud A., Boukef K., Marongiu B., Boughattas N.A. Antiulcerogenic and antibacterial activities of Apium graveolens essential oil and extract. Nat. Prod. Res. 2013;27:1075–1083. doi: 10.1080/14786419.2012.717284. [DOI] [PubMed] [Google Scholar]
- Baj T., Baryluk A., Sieniawska E. Application of mixture design for optimum antioxidant activity of mixtures of essential oils from Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. Ind. Crops Prod. 2018;115:52–61. [Google Scholar]
- Bajpai V.K., Baek K.H. Biological efficacy and application of essential oils in foods-A review. Essent. Oil-Bear. Plants. 2016;19:1–19. [Google Scholar]
- Bajpai V.K., Baek K.H., Kang S.C. Control of Salmonella in foods by using essential oils: a review. Food Res. Int. 2012;45:722–734. [Google Scholar]
- Bajpai V.K., Sharma A., Baek K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32:582–590. [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Burdock G.A., Carabin I.G. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem. Toxicol. 2009;47:22–34. doi: 10.1016/j.fct.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Calderón-Oliver M., Escalona-Buendía H.B., Medina-Campos O.N., Pedraza-Chaverri J., Pedroza-Islas R., Ponce-Alquicira E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;65:46–52. [Google Scholar]
- Cornell J.A. John Wiley & Sons; 2011. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data. [Google Scholar]
- Crespo Y.A., Naranjo R.A., Quitana Y.G., Sanchez C.G., Sanchez E.M.S. Optimisation and characterisation of bio-oil produced by Acacia mangium Willd wood pyrolysis. Wood Sci. Technol. 2017;51:1155–1171. [Google Scholar]
- Chahal K., Singh R., Kumar A., Bhardwaj U. Chemical composition and biological activity of Coriandrum sativum L.: a review. Indian J. Nat. Prod. Resour. (IJNPR)[Formerly Natural Product Radiance (NPR)] 2018;8:193–203. [Google Scholar]
- Elgndi M.A., Filip S., Pavlić B., Vladić J., Stanojković T., Žižak Ž., Zeković Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja Montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluids. 2017;128:128–137. [Google Scholar]
- Firenzuoli F., Jaitak V., Horvath G., Bassolé I.H.N., Setzer W.N., Gori L. Essential oils: new perspectives in human health and wellness. J. Evid Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/467363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatou G.P.P., Viljoen A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010;87:1–7. [Google Scholar]
- Lai P., Rao H., Gao Y. Chemical composition, cytotoxic, antimicrobial and antioxidant activities of essential oil from Anthriscus caucalis M. Bieb grown in China. Record Nat. Prod. 2018;12:290–294. [Google Scholar]
- Lee S.-J., Umano K., Shibamoto T., Lee K.-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–137. [Google Scholar]
- Mancini E., Senatore F., Del Monte D., De Martino L., Grulova D., Scognamiglio M., Snoussi M., De Feo V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules. 2015;20:12016–12028. doi: 10.3390/molecules200712016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri N., Aoun L., Dalichaouche N., Hadri D. Yields, chemical composition, and antimicrobial activity of two Algerian essential oils against 40 avian multidrug-resistant Escherichia coli strains. Vet. World. 2018;11(11):1539–1550. doi: 10.14202/vetworld.2018.1539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.J., Rice-Evans C., Davies M.J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Nagella P., Ahmad A., Kim S.J., Chung I.M. Chemical composition, antioxidant activity and larvicidal effects of essential oil from leaves of Apium graveolens. Immunopharmacol. Immunotoxicol. 2012;34:205–209. doi: 10.3109/08923973.2011.592534. [DOI] [PubMed] [Google Scholar]
- Nazir S., Wani I.A., Masoodi F.A. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J. Adv. Res. 2017;8:235–244. doi: 10.1016/j.jare.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedrhiri W., Balouiri M., Bouhdid S., Moja S., Chahdi F.O., Taleb M., Greche H. Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: optimization of their antibacterial effect. Ind. Crops Prod. 2016;89:1–9. [Google Scholar]
- Pompeu D.R., Silva E.M., Rogez H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009;100:6076–6082. doi: 10.1016/j.biortech.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Prachayasittikul V., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Coriander (Coriandrum sativum): a promising functional food toward the well-being. Food Res. Int. 2018;105:305–323. doi: 10.1016/j.foodres.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Rożek E., Nurzyńska-Wierdak R., Sałata A., Gumiela P. The chemical composition of the essential oil of leaf celery (Apium graveolens L. var. secalinum alef.) under the plants’irrigation and harvesting method. Acta Sci. Pol-Hortoru. 2016;15 [Google Scholar]
- Seo S.-M., Jung C.-S., Kang J., Lee H.-R., Kim S.-W., Hyun J., Park I.-K. Larvicidal and acetylcholinesterase inhibitory activities of Apiaceae plant essential oils and their constituents against Aedes albopictus and formulation development. J. Agric. Food Chem. 2015;63:9977–9986. doi: 10.1021/acs.jafc.5b03586. [DOI] [PubMed] [Google Scholar]
- Valgimigli L., Valgimigli M., Gaiani S., Pedulli G.F., Bolondi L. Measurement of oxidative stress in human liver by EPR spin-probe technique. Free Radic. Res. 2000;33:167–178. doi: 10.1080/10715760000300721. [DOI] [PubMed] [Google Scholar]