Abstract
Diabetes Mellitus (DM) is one of the major metabolic disorders and its severity leads to death. Enhancement in hyperglycaemic conditions of DM gives rise to endothelial impairment in small and large blood vessels contributing towards microvascular and macrovascular complications respectively. The pathogenesis of diabetic complications is associated with interruption of various signal transduction pathways due to epigenetic modifications such as aberrant histone modifications, DNA methylation and expression of miRNAs along with the long non-coding RNAs (lncRNAs). Amongst these epigenetic alterations, modulated expressions of miRNAs confer to apoptosis and endothelial dysfunction of organs that gives rise to vascular complications. In this review, we principally focussed on physiological role of miR29 family in DM and have discussed crosstalk between miR29 family and numerous genes involved in signal transduction pathways of Diabetic vascular complications. Incidences of diabetic retinopathy exploiting the role of miR29 in regulation of EMT process, differential expression patterns of miR29 and promising therapeutic role of miR29 have been discussed. We have summarised the therapeutic role of miR29 in podocyte impairment and how miR29 regulates the expressions of profibrotic, inflammatory and ECM encoding genes in renal fibrosis under diabetic conditions. We have also highlighted impact of miR29 expression patterns in cardiac angiopathy, cardiomyocyte’s apoptosis and cardiac fibrosis. Additionally, we have also presented the contradictory actions of miR29 family in amelioration as well as in enhancement of diabetic complications.
Keywords: Diabetes mellitus, Epigenetics, miRNA29, Diabetic vascular complications, Diabetic retinopathy, Diabetic nephropathy, Diabetic cardiomyopathy
Introduction
Among several kinds of RNAs, some RNAs are involved in regulation of the gene expressions. These regulatory RNAs are recently discovered as microRNAs [1, 2]. miRNAs exhibit an unique set of small, non-coding RNAs consisting of 18–24 nucleotides in order to negatively govern the expression of genes causing degradation of target sequence of mRNA or through inhibition of translation [3, 4]. Single miRNA can control the expressions of several genes as it has the ability to bind to its target sequence of mRNA as imperfect or perfect complement. Hence, a group of miRNAs may regulate about 30% of gene expressions in a cell. This implies to the pivotal role played by miRNAs in maintenance of various physiological functions [5]. These miRNAs are highly preserved from plants to humans encoded by their respective genes [6]. Since many miRNAs have not been clearly understood and their existence is still in dubious state, recent findings have contributed important insights into physiological role of miR29 family. The miR29 family especially in human involves hsa-miR29a, hsa-miR29b-1, and hsa-miR29b-2 and hsa-miR-29 c. Out of these, miR29b-1 and miR29b-2 have similar sequences and together were called as ‘miR29b’. Mature miR29b sequences were found to be conserved in mouse, rat and human [7].
Pathogenesis of diabetes and its complications involves many genetic factors and biochemical pathways. It affects cellular transcriptions of target organs and biochemical pathways resulting into abnormal gene expression of pro-apoptotic, pro-fibrotic, pro-inflammatory and growth promoting genes. Epigenetics have also shown to play a vital role in gene interactions [8]. The major molecular events involved in epigenetic phenomenon are histone modifications, DNA methylation and interfering RNAs. A study have reported that interruption in signalling pathways (inflammation, apoptosis and stress due to increased oxidation, etc.) occur as a result of abnormal histone modifications, DNA methylation and modulated expressions of miRNAs and lncRNAs (long non-coding RNAs) in T2DM [9]. As reported earlier, miRNAs have demonstrated their crucial role in enhancing diabetes-related chronic complications [8]. miRNAs control the expressions of their target genes involved in signal transduction pathways mediating insulin secretion in insulin-receptive tissues. These are also involved in regulation of vascular homeostasis and repression of diabetes-mediated end-stage organ impairment [10]. Earlier reports have shown that lncRNAs (long non-coding RNAs) and miRNAs maintained the gene expression and functional control of inflammatory cytokines and growth factors involved in diabetic complications. Altered expression of such miRNAs in hyperglycaemic conditions causes apoptosis and endothelial dysfunction of organs that further lead to vascular complications [8].
In this review, we have focussed on physiological role of miR29 family and construed a crosstalk between miR29 family and genes involved in signal transduction mechanisms of diabetic vascular complications.
Genomic constitution of miR29 family
The mature form of miR29 was found to be preserved among mice, rats and humans possessing similar seed sequences (AGCACC) [11]. The miR29 family consists of hsa-miR29a, hsa-miR29b and hsa-miR29c [12]. The miR29b have also been divided into two subtypes: miR29b-1 and miR29b-2 and the difference between these two subtypes is based on their genomic locus and possessing similar mature forms of miR29b [13]. A single nucleotide present outside the target sequence was used to distinguish between miR29a, miR29c and miR29b [14]. The precursor gene for miR29b-1 and miR29a was found to be positioned on chromosome 7q32.3 in human. Although precursor for miR29b and miR29 was found to be in cis position on chromosome 1q32.2 [7]. The transcription sequence of primary transcripts of miR29b1, miR29b2, miR29a together with miR29c was reported to be present in upstream direction. Exon of miR29b/c primary transcript encodes miR29b-2 and miR29c while miR29b-1 and miR29a are transcribed from the last intron of primary transcript EU1543532 [7]. The two miR29 expressing gene encoding clusters are located 1 kb apart from each other. However, both the genomic clusters transcribed simultaneously as primary transcripts. A study has demonstrated a novel splice variant which have suggested that precursors of miR29a and miR29b-1 can also be synthesized from the end exon of another primary transcript (GU321462). Therefore, both exonic as well as intronic coding will ultimately lead to synthesis of mature miR29a and miR29b-1, but alternative splicing can affect the maintenance of expression patterns of such miRNAs [7].
MicroRNA29 family: A biomarker of diabetes mellitus
Hyperinsulinemia and hyperglycemia conditions have shown increased expression of miR29 in the region of adipose tissue. It has been also reported that when hyperglycemic stress was given to 3 T3-L1 adipocytes, miR29 expression level was elevated [15]. Kong et al. have also reported the higher expression of miR29 family in patients susceptible to T2DM. Differential expressions of miR29 was also observed in T1DM patients worldwide. It was found that miR29 directly involved in the maintenance of β-cell apoptosis and their networks of regulation [16]. Analyses of islets of T1DM mice model demonstrate that the expression of miRNA leads to detrimental effects in promotion of β-cell destruction. The elevation in miR29 level was observed in insulin targeted tissues that created resistance towards insulin in these mice [17]. Another experiment which was carried out in Diabetic Goto-Kakizaki rats, miR29a along with miR29b showed elevated expression patterns in liver and white adipose tissue (WAT). Such elevated expressions of miR29a and miR29b was thought to be induced due to decrease in caveolin protein (CAV2), which is associated with lipid raft. The expression of CAV2 occurs in response to hyperinsulinemia as an intermediate of insulin signal transduction pathway i.e. phosphatidylinositol 3-kinase regulatory subunit-α (PIK3R1) and Insulin-induced gene 1 (INSIG) [18]. miR29 have also targeted Mct1 (Monocarboxylate Transporter 1) to maintain insulin expression [19]. Since last 10 years, it has been known that regulation of insulin signaling cascade involved miR29 family via negative feedback mechanism. However, an absolute mechanism is not yet known. An experiment carried out by Kurtz et al. in diabetic mice exhibited that transcription factor, FOXA2 was targeted by miR29. FOXA-2 have shown to induce maintenance of HMGSC2, ABHD5 and PPARGC1A [20]. It has been also reported in one experiment that rise in miR29 expression in obese and pregnant sheep resulted in increased expression levels of miR29 in offspring lambs specifically in the region of liver tissue [21]. Some studies have reported exalted expressions of miR29b in T2DM patients [22] while some studies have shown exalted expression levels of miR29a in T2DM patients [22–25]. Such studies have proposed that miR29 family could be a propitious target in therapy of diabetes mellitus.
Vascular complications of diabetes mellitus
Vascular complications of DM are of two types: Macrovascular and microvascular complications. Microvascular complications of DM are basically small blood vessels' diseases including diabetic nephropathy, diabetic retinopathy and diabetic neuropathy which cause end-stage renal disorder (ESRD), blindness and severe neuropathic pain conditions respectively. While the macrovascular complications are the intricacies of large blood vessels i.e. impairment in arterial walls of peripheral and coronary artery systems but such macrovascular complications majorly arise due to kidney disease in diabetes. These includes cerebrovascular, cardiovascular and peripheral vascular complications [26, 27]. In hyperglycaemic conditions, alteration in microvasculature influences the capillary basement membrane involving arterioles of retina, glomeruli, myocardium, muscle and skin. It builds up microangiopathy and macrovascular complications which arise due to complexities and structural aberrations like hypertrophy, inappropriate blood flow and loss of physiological function [28].
Role of microRNA29 in microvascular complications
MicroRNA29 family targets in diabetic retinopathy
Diabetic retinopathy (DR) is a critical factor for blindness in the world. Amongst the world’s population, about 1/3rd of diabetic patients undergo DR and it further enhances the risk factors involved in macrovascular complications such as coronary heart disease, stroke, etc. [29] Approximately, 11% of deviation in retinopathy risk is characterised by duration of disease and level of HbA1c [30]. Clinical indications of DR consist of retinal haemorrhage, recurrent bleeding by neovascularisation and retinal detachment leading to blindness [29]. There are several miRNAs expressed in the tissues of retina but expression of miR376c, miR136 [31], miR31, miR184, miR150, miR10a, miR-10b, miR19a-3p, miR-338, miR-144, miR-219-2-3p, 146a [32], miR-10a and miR-199a-3p [33] were found to be significantly downregulated in retinopathy conditions. The contribution of miRNAs in DR pathogenesis was also illustrated by microarray expression profiling techniques where expression of miR96, miR182, miR211, miR183, miR135b, miR124, miR135, miR190b, miR592, miR29c, and miR363 were found to be upregulation while miR10a, miR10b, miR199a-3p, miR338, miR144and miR219–2-3p were downregulated [34]. Additionally, miR125b has been found to be involved in migration, proliferation and apoptosis of retinal cells. miR125b was also reported to be involved in neovascularization processes in DR and demonstrated upregulated or downregulated expression in retinopathy conditions [35]. The role of miR29 family in various signal transduction pathways involved in pathogenesis of DR has been discussed in Fig. 1.
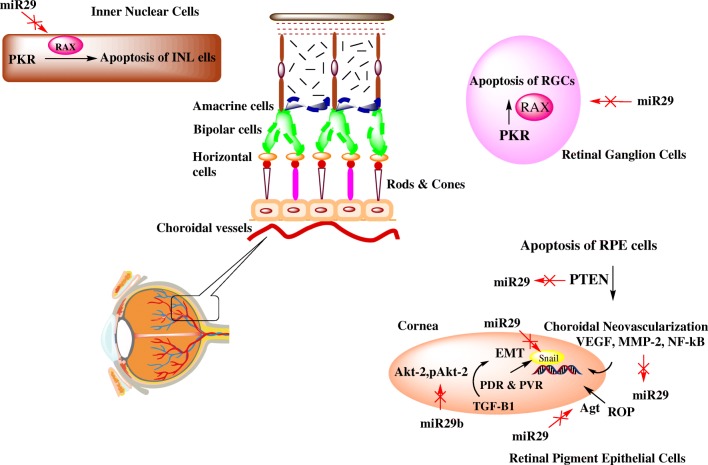
Fig. 1.
The schematic demonstrates the crosstalk between miR29 family and several signal transduction pathways involved in pathogenesis of diabetic retinopathy. In inner nuclear cells and retinal ganglion cells, miR29 shows its protective action via targeting PKR pathway and inhibition of RAX to prevent cells’ apoptosis while in retinal pigment epithelial cells, apoptosis is prevented via inhibition of PTEN pathway. Choroidal Neovascularisation occurs in RPE cells via suppression of miR29 and upregulation of VEGF, MMP-2 and NF-κβ. miR29 prevents ROP via targeting Ang II. In addition to this, miR29 targets transcription factor, Snail as well as Akt-2 and pAkt-2 involved in progression of EMT. However, TGF-β1 downregulates miR29 and promotes EMT. PKR, Protein kinase RNA-activated pathway; RAX, Retinal homeobox protein; PTEN, Phosphatase and tensin homolog; RPE, Retinal pigment epithelial cells; VEGF, Vascular endothelial growth factor; MMP-2, Matrix metalloproteinase-2; NF-κβ, Nuclear factor-κβ; ROP, Retinopathy of prematurity; Ang II, Angiotensin II; Akt-2 and pAKt-2, serine/threonine specific protein; EMT, Epithelial-mesenchymal transition; TGF-β1, Transforming growth factor-β1
Regulation of EMT process by miR29
It was established that retinal pigment epithelial (RPE) cells were involved in the progression of fibrosis in retina. It has been also reported that RPE to mesenchymal cell transformation can be termed as EMT (Epithelial-to-mesenchymal transition) involved in proliferative diabetic retinopathy (PDR) and proliferative vitreo retinopathy(PVR) [36, 37]. The mechanism of EMT is mainly activated by release of specific cytokines especially TGF-β1 (Transforming Growth Factor) which has been reported to be associated with several fibrotic conditions [38]. Previous study also reported that miR29a/b/c level is downregulated in response to upregulation of TGF-β1 [39]. This imbalance of miR29a/b/c level further promoted EMT process and hence, downregulation of miR29 family could be a possible therapeutic cause for disorders related to fibrosis in retina [37]. Moreover, it was also found that Snail is the principle transcription factor involved in the process of EMT and was found to be targeted by miR29b [40]. It was also been observed that miR29b could acts as an essential regulator of EMT processing in ARPE-19 cells (Human Retinal Pigment Epithelial Cell Line). The upregulation of miR29b has stimulated resistance for EMT in ARPE-19 cells. This study has also proved that Akt-2 and p-Akt-2 involved in EMP process could be a potential targets of miR29b [36]. Additionally, expression of hsa-miR29a was found to be remarkably high in fibrosis and angiogenesis in the vitreous of eyes suffering from PDR [41].
miR29 involvement in apoptosis of retinal cells
The involvement of miR29 in apoptosis was established in the study conducted on RPE cells grown under HG. The upregulation of miR29 expression was found in RPE cells grown under HG which may be linked to enhanced apoptosis because downregulation of miR29 have shown to be responsible for protection against apoptosis in RPE cells via targeting PTEN (Phosphatase and tensin homolog) pathway and decreased production of caspase-9 in RPE cells [42]. A study conducted in STZ-induced diabetic rats showed that RAX (Pro-apoptotic RNA-dependant protein kinase (PKR)-associated protein X) was responsible for apoptosis and miR29b were located in the retinal ganglion cells (RGCs) and in the region of inner nuclear layer (INL). It was observed that regulation of RAX activity is indirectly controlled by miR29b. This signifies that miR29b has a sheltering effect against apoptosis in RGCs and INL cells through regulation of RAX-mediated PKR pathway [43]. Moreover, a correlation between transcription factor Sp1 (Specific protein1) and miR29b was established where Sp1 which is a potential target for miR29b binds to the promotor sequence of miR29b and prevents its expression [44]. However, another study have opposed these results where it was found that miR29b inhibited Sp1 transcription and elevated its own expression [45]. It was observed that under hyperglycaemic condition, Sp1 expression was highly increased in rat retinal muller cells (rMC-1) which was directly targeted by miR29b at its promoter region [44]. This observation was found to be associated with myocardial infarction-associated transcript (MIAT) [46]. MIAT is a transcription factor activated by NF-κβ that have shown its expression majorly in brain tissue and heart. But the abrupt expression pattern of MIAT was found to be responsible for cell proliferation, migration and apoptosis in diabetic conditions [47]. It has been already reported that under hyperglycaemia, cell apoptosis gets elevated. However, it was also observed that suppression of MIAT suppression can reverse the cell apoptosis and regulate the expression of miR29b as well as maintain high level of Sp1. The suggested mechanism has showed defensive role of miR29 by promoting cell survival and prevention of cellular apoptosis via regulation of miR29b/Sp1 network [48].
miR29s in retinal vascularization
Choroidal neovascularization (CNV) is the principal cause of diabetic retinopathy which involves vascular degeneration and pathological myopia. Overexpression of MMP-2 and VEGF via retinal pigment epithelial (RPE) cells is the critical step of angiogenesis in CNV of retina [49]. Previous studies have also reported that NF-кβ have contributed during initial stages of angiogenesis and causes downregulation of miR29s. NF-кβ have also regulated MMP-2 transcription through its direct activation or by downregulation of miR29s which could lead to the invasion of basement membrane as well as ECM. This signifies that miR29s could be the promising therapeutic targets to cure CNV [50]. Earlier reports have proved that Renin-angiotensin-aldosterone system (RAAS) plays an important role in the pathological condition of DR and causes imbalance between Ang-I and Ang-II. Moreover, in case of retinopathy of prematurity (ROP), Ang-II interacts with growth factors such as vascular endothelial growth factors (VEGF), which could further induce retinal angiogenesis leading to retinal neovascularization. Retinal neovascularization is a predominant factor involved in ROP. miR29a could suppress this retinal neovascularisation condition by downregulation of AGT and could prevent the development of ROP [51]. In addition to this, upregulation of miR29a could diminish vascular density of retina of rat models and endothelial cell nuclei reduction [29].
MicroRNA29 family targets in diabetic nephropathy
Diabetic Nephropathy (DN) is another microvascular complication involved in both type 1 and type 2 diabetes mellitus. The prevalence of DN have been rising with approximately 9% of the total world population affected by DN [52]. Development of DN further gives rise to end-stage renal disease (ESRD) which has become a predominant cause of death [52, 53]. The patients suffering from DN are at major risk to develop macrovascular complications. The key features involved in renal failure are mesangial hypertrophy and ECM proteins aggregation in renal tubules and glomerulus. Other prognosticators of DN pathogenesis are due to the loss of integrity and apoptosis of podocytes. The growth factors, oxidative stress, advanced glycation end products (AGEs), metabolic memory and release of inflammatory cytokines have been reported as principle mediators involved in development of DN and their activation gave rise to epigenetic modifications [53]. Several experiments have shown aberrant expressions of miRNAs in DN. Role of miRNAs in kidney impairment conditions has emerged from reports of renal phenotypes found in mice with podocyte specific removal of Dicer that involved glomerulosclerosis, tubulointerstitial fibrosis, proteinuria and destruction of podocyte foot processes [54]. miR29 family plays an imperative role in chronic conditions of kidney diseases. Various targets of miR29 family in signal transduction pathways involved in pathogenesis of DN are discussed in following sections as well as in Fig. 2.
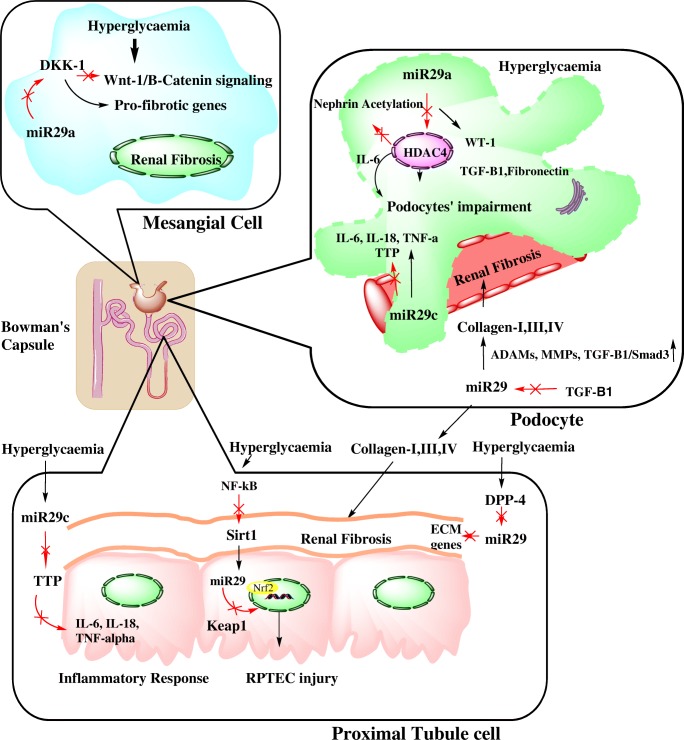
Fig. 2.
The schematic portrays crosstalk between miR29 family and various signal transduction pathways involved in pathogenesis of Diabetic Nephropathy. In mesangial cells, miR29a prevents activation of pro-fibrotic genes via targeting Wnt/β-Catenin signaling pathway and shows defensive role in renal fibrosis while in podocytes, in incidences of hyperglycaemia, downregulation of miR29a occurs which further rises HDAC4 expression ultimately resulting into podocytes’ inflammation followed by their impairment along with promotion of renal fibrosis via activation of TGF-β1 and Fibronectin. In contrast to this, miR29c causes activation of inflammatory cytokines and Rho-Kinase while suppression of TTP (anti-inflammatory protein) conferring to podocytes’ as well as proximal tubular cells’ inflammation. In proximal tubular cells, NF-κ β followed by Sirt1 causes downregulation of miR29 causing deregulation of Keap1/Nrf2 pathway subsequently causing RPTEC injury while renal fibrosis occurs due to three major factors i.e. TGF-β1, Ang II and DPP4 contributing to overexpression of ECM genes and downregulation of miR29a and miR29b. HDAC4, Histone deacetylase 4; TGF-β1, Transforming growth factor-β1; TTP, Tristetraprolin; NF-κβ, Nuclear factor-κβ; Keap1, Kelch-like ECH-associated protein 1; Nrf2, Nuclear factor erythroid 2; RPTEC, Renal proximal tubular epithelial cells; Ang II, Angiotensin II; DPP4, Dipeptidyl peptidase 4; ECM, Extracellular matrix
miR29 family and histone deacetylase circuitry in podocyte dysfunction
Podocytes dysfunction is a hallmark in pathophysiology of DN. Impairment in kidney tissue under hyperglycaemic conditions becomes a major incidence of glomerulopathy and contributes to proteinuria and other kidney diseases such as renal fibrosis [55]. Suppression of miR29 has already been reported in renal fibrosis induced by TGF-β1 [56]. HDACs (Histone Deacetylases) involved in deacetylation of histone and non-histone proteins were also found to be involved in homeostasis of glucose [57]. Amongst them, class II HDACs consist of HDAC4 (Histone Deacetylase 4) which interferes with stability of nephrin and responsible for development of podocyte’s impairment [58]. Nephrin is a slit diaphragm protein present in renal tissue, is a tyrosine kinase that have shown to maintain proper functioning of podocytes and filtration capacity of glomerular basement membrane (GBM). Loss of podocyte’s integrity as well as activity of nephrin has shown to cause decreased filtration capacity of glomerular filtration membrane. It results into saturation of several high molecular weight proteins in urine leading to proteinuria [59, 60]. In an earlier report, it was found that upregulation of HDAC4 was also observed in diabetic condition which have shown to inhibit miR29a signal transduction and promoted podocyte’s dysfunction. Moreover, overexpression of miR29b has shown to alleviate HDAC4 expression that re-established nephrin acetylation process and prevented podocyte’s dysfunction. Upregulation of miR29a signalling have also promoted expression of podocyte-specific marker WT-1 which have shown to take part in the maintenance of podocyte’s integrity and viability of tubular cells. In addition to this, miR29a mitigated glomerulopathy and inflammation in diabetic kidneys of mice. This signifies that miR29a could be an essential therapeutic tool in podocytes’ impairment [58]. Another class of histone deacetylase, Sirt1 have demonstrated to regulate the inflammatory responses in DN under hyperglycaemic conditions [61]. In euglycemic conditions, Sirt1 have caused suppression of NF-κβ by controlling its silencing of transcription [62]. In hyperglycaemic conditions, deacetylation activity of Sirt1 was altered by NF-κβ which have shown to be associated with maintenance of inflammatory responses in tissues [63]. In addition to this, it was found that NF-κβ signaling decreases the expression levels of miR29 [64]. It has also been reported that Sp1 is the transcription factor for NF-κβ signalling [65] and could be a potential target of miR29b. Therefore, miR29b causes suppression of renal inflammation via inhibition of NF-κβ/Sp1 signalling [66]. The transcription factor Nrf2 (NF-E2-related factor2) was known as the main regulator of anti-cell press genes and it has been observed that its loss causes rise in oxidative stress as shown in the experiment conducted on streptozotocin-induced diabetic mice [67]. The regulator of Nrf-2 function is Keap1 (Kelch-like ECH-associated protein) that have regulated Nrf-2 expression level through ubiquitination followed by degradation [68]. The reduction in Sirt1 deacetylase activity was also observed in hyperglycaemic condition which have contributed in downregulation of miR29, followed by suppression of Keap1/Nrf2 signaling. Nrf-2 loss followed by Keap1 upregulation was also observed through bioinformatic analysis. It was found that miR29 binds to 3’-UTR of mRNA of Keap1 and controls its expression. All these findings signify that DN have involved Sirt1/NF-κβ/miR-29/Keap1/Nrf2 signalling pathways and miR29 could be one of the regulator of these signalling pathways [69].
Role of miR29 family in regulation of profibrotic genes, encoding of ECM proteins and inflammatory genes expression in renal injury
The miR29 targets various pathways involved in expressions of profibrotic, ECM proteins encoding and inflammatory genes that have shown to enhance DN conditions. A higher expression of miR29 has been reported in VSMCs (vascular smooth muscle cells) and fibroblasts. It was studied that miR29 family causes suppression of 16 ECM proteins encoding genes [70]. Schaper et al. has reported that TGF-β1 stimulated fibrosis via activation of Smads which are effector molecules of downstream pathways [71]. Wang et al. reported that in hyperglycaemic conditions, TGF-β1 reduces miR29 family expressions in podocytes as well as tubular epithelial cells. Simultaneously, rise in expression of collagen I, III and IV was observed during renal fibrosis [39]. Recent reports have shown reduction in miR29b expression in a rat model of hypertensive renal disease [72]. A study have shown that Smad3 negatively regulates miR29b expression by physical interaction with its promoter site in response to TGF-β1 in DN [56]. It was concluded that regulation of miR29 was processed via canonical TGF-β1/Smad3pathway [73]. It was earlier reported that TGF-β1-mediated renal fibrosis was induced by interaction of Sp1 and Smad3 [74] and inhibition of this interaction could be the another defensive mechanism shown by miR29b [66]. miR29 family is involved in regulation of fibrotic genes expression such as collagens and matrix metalloproteinases (MMPs) [75]. Among them, ADAMs (disintegrin metalloproteases) has maintained the cellular and cellular processes. Therefore, they are involved in regulation of fibrotic and inflammatory genes in nephropathy [76]. It was reported that ADAM 10, 12, 17 and 19 were found to be involved in regulation of TGF-β1/Smad3 pathway. It was also shown that miR29 has complementary binding sites to 3’-UTR of ADAM12 and regulation of expression of ADAM12 and ADAM19 governed by miR29.These interactions demonstrated a molecular crosstalk between miR29 family, ADAMs and TGF-β1/Smad3 pathway [77]. Fabbri et al. has reported that miR29 also targets the enzymes called ‘DNA methyltransferases’ and promotes their methylation activities. The expression of genes involved in synthesis of ECM is suppressed and fibrosis is ameliorated due to hypermethylation [78]. DPP-4 (Dipeptidyl peptidase-4) which is widely expressed in kidneys was found to be involved in remodelling of ECM as well as in cell survival [79]. Activation of DPP-4 causes degradation of integrin β1 via phosphorylation that stimulates upregulation of TGF-β1 and activates VEGF (Vascular Endothelial Growth Factor). In hyperglycaemic conditions, DPP-4 expression was observed on the surface of tubular epithelial cells and endothelial cells [80]. As reported by Cushing et al., miR29 was involved in maintenance of genes expression that encode for ECM proteins such as collagens, laminins and integrins [81]. In correlation with this, normal glucose conditions had showed that miR29 suppresses the expression of DPP-4 expression while in hyperglycaemic conditions, miR29 activity was lost and the level of DPP-4 expression was elevated. It has been also observed that DPP-4 was found to be involved in fibroblast activation pathway and miR29 was acted as a regulator of DPP-4 expression via suppression of fibroblast activation pathway. Glomerulosclerosis is a predominant feature involved in pathogenesis of DN which occurs due to enormous alterations in kidney mesangium [82]. Wnt//β-catenin signalling pathway was also found to be involved in renal fibrosis in in-vitro and in-vivo studies [83]. It was reported that DKK1 (Dickkopf-1) acts as a Wnt antagonist in mesangial cells under hyperglycaemic conditions [84]. However, knockdown of miR29a have caused downregulation of Wnt/β-catenin signalling pathway while upregulation of DKK1 resulted into increased expression of pro-fibrotic genes and development of glomerulosclerosis. Moreover, imbalance between DKK1 and Wnt/β-catenin signalling contributed to fibrotic matrix synthesis and apoptosis of kidney cells under hyperglycaemic conditions. miR29a was found to act as a regulator of Wnt/β-catenin signalling and DKK1 expression to conserve the mesangial cells from fibrosis and apoptosis. The experimental results have revealed that miR29a could provide a therapy for glomerulosclerosis by acting as a regulator of Wnt/β-catenin signalling pathway and can also act as an anti-fibrotic factor [82].
Role of miR29c in enhancement of DN conditions
Among the miR29 family, miR29c had showed distinct expression patterns in urinary sediment, plasma and renal tissues of DN patients and have become a biomarker for enhancement of DN [85]. Clinical data have shown upregulation of inflammatory cytokines such as IL-6, IL-18, TNF-α while downregulation of anti-inflammatory protein TTP in diabetic patients suffering from proteinuria [86]. Tristetraprolin (TTP) is an anti-inflammatory protein which causes suppression of IL-6, IL-18 and TNF-α expressions [87]. However, miR29c overexpression was also observed along with the upregulation of inflammatory cytokines promoting inflammation while downregulation of TTP was observed under hyperglycaemic conditions. This infers that in hyperglycaemic conditions, miR29c promotes the inflammatory response by inhibiting TTP expression causing further development of DN [88]. In addition to this, it has also been observed that miR29c expression got elevated in db/db mice that may have activated Rho kinase by targeting Sprouty homolog 1, thus favouring fibronectin assembly and podocytes’ apoptosis [89].
Role of microRNA29 in macrovascular complications
MicroRNA29 family targets in diabetes-induced cardiovascular diseases
Diabetes-induced cardiomyopathy was firstly discovered in year 1972. Pathogenesis of cardiomyopathy-mediated by diabetes comprised of imbalance between cardiac ionic currents, cardiomyocyte’s apoptosis, aberrant energy usage, cardiac neuropathy and interstitial fibrosis. Another feature of diabetes-induced cardiomyopathy includes resistance of myocardium generated towards insulin which further promoted decrease in GLUT4 (Glucose transporter 4) expression at the level of plasma membrane [90]. Current studies have reported that expressions of lncRNAs and their respective physiological functions get modified on incidence of cardiovascular diseases such as cardiac hypertrophy, CAD (Coronary artery diseases), MI etc [91]. Several reports have also shown that dysregulation of miRNAs were involved in cardiac hypertrophy, heart failure, cardiac arrhythmias and atherosclerosis [90–92]. It has been further studied that miR29 family plays several roles in such incidences like controlling apoptosis of cardiomyocytes and formation of aortic aneurysm [93], regulation of cardiac fibrosis via [94] and maintenance of atrial fibrillation [93]. The involvement of miR29 with various signal transduction pathways occurring in pathogenesis of diabetic cardiomyopathy has been discussed below.
miR29/IGF-1/MCL-1 interrelationship and analogy between miR29 and IL-6 in cardiomyopathy
Among several miRNAs involved in cardiac functions, it had showed abnormal expression patterns of miR29 in myocardial infarction and were involved in cardiac fibrosis [95]. This signifies that miR29 plays a central part in diabetes-mediated cardiomyopathy. One of the key features of cardiomyopathy is diabetes-induced angiopathy [96] where inadequate recovery of angiogenesis process may lead to death due to cardiomyopathy [97]. In a study, it was found that IGF-1 (Insulin Growth Factor-1) is a potential target for miR29 [98] which is an essential growth factor involved in the angiogenesis process [99]. Enhancement in pro-angiogenic processes such as cell proliferation and migration occurred due to downregulation of miR29 in diabetes-induced angiopathy. It was suggested that IGF-1 could be a directly involved in regulation process of angiogenesis in diabetic cardiomyopathy mediated via miR29 [98]. It is well-known that oestrogen plays a vital role in protection of cardiovascular functions [100]. miR29 has been known as major regulator of physiology of heart which can maintain the apoptosis of cardiomyocytes, cardiac fibrosis and hypertrophy [101]. A recent report have suggested that miR29 significantly reduces collagen accumulation and ventricular compliance [102]. In an experiment of ovariectomy, downregulation of miR29 was observed as it acts as one of the biomarkers of apoptosis in diabetic hearts of rats. Downregulation in miR29 expression pattern contributed to rise in IGF-1 and BCL-2 expressions and both are responsible for apoptosis of cardiomyocytes and cardiac fibrosis in cardiomyopathy. It had indicated that miR29 downregulation in ovariectomised diabetic rats enhanced cardiomyopathic conditions [103]. miR29 family was also found to be involved in myeloid cell leukaemia-1 (MCL-1) gene suppression which is a member of BCL-2 family of pro-survival genes and due to MCL-1 gene suppression, β-cell death of pancreas occurs. Therefore, miR29 family and MCL-1 gene repression play a pivotal role in pancreatic impairment resulting into T1DM [104]. It has been reported that prevention of mTORC1 signal transduction pathway contributed for increased expression of miR29 and downregulation of MCL-1 gene in cardiomyocytes under hyperglycaemic conditions. However, inhibition of miR29 gene expression was occurred by insulin. This correlation had showed the lack of insulin under hyperglycaemic conditions due to inhibition of mTORC1 signal transduction pathway. It leads to deregulation of miR29-MCL-1 circuitry resulting into damage of myocardium in DM [105]. Another major cause involved in pathogenesis of cardiomyopathy is the occurrence of interstitial fibrosis of heart due to diabetes [106]. Interleukin-6 (IL-6) is one of the inflammatory cytokines which plays a crucial role in interstitial fibrosis, deposition of collagen and formation of fibroblasts and gives rise to cardiovascular diseases [107]. In an experiment, it was observed that upon administration of IL-6, collagen deposition was triggered via enhancement of TGF-β1 expression and suppression of miR29 expression while its deletion has alleviated collagen expression in streptozotocin-mediated diabetic cardiomyopathy. This signifies that IL-6 might be involved in cardiac fibrosis enhancement via targeting TGFβ1/miR29 loop and IL-6 deletion could protect against cardiac fibrosis in diabetic cardiomyopathy [108]. The expression of miR29 affecting cardiac dysfunctions is summarized in Table 1.
Table 1.
This table depicts the role of miR29 in modulating cell signalling molecules affecting cardiac dysfunction. MCL-1, Myeloid leukaemia cell-1; IGF-1, Interstitial growth factor-1; BCL-2, B cell lymphoma 2; IL-6, Interleukin-6; TGF-β1, Transforming growth factor-β1
| Hyperglycaemia | Cardiac dysfunction | |
|---|---|---|
| Expression of miR29 | Target | |
| Upregulation of miR29 | Decreased mTORC1 signalling and MCL-1 | Prevention of mTORC1 signal transduction pathway contributed for increased expression of miR29 and downregulation of MCL-1 gene in cardiomyocytes [105]. |
| Downregulation of miR29 | Increased IGF-1 | IGF-1 is a direct factor involved in regulation process of angiogenesis in diabetic cardiomyopathy mediated via miR29 [98]. |
| Increased IGF-1 and BCL-2 | miR29 downregulation expression pattern contributed to rise in IGF-1 and BCL-2 causing apoptosis of cardiomyocytes and cardiac fibrosis in cardiomyopathy [103]. | |
| Increased TGF-β and IL-6 | IL-6 is involved in cardiac fibrosis enhancement via targeting TGFβ1/miR29 loop [108]. | |
Conclusion
In current scenario, diabetes is a leading cause of compromised lifestyle due to debilitating diabetic complications. Several signal transduction pathways profoundly get influenced in pathogenesis of diabetic complications. Epigenetic phenomenon is an imperative biological mechanism played a vital role in enhancement of diabetic severity. miRNAs are one of the key factors of epigenetics that were found to be engaged in the pathogenesis of diabetic complications. In this review, we have summarised the performance of miR29 family in vascular complexities of diabetes. miR29 family consists of miR29a, miR29b and miR29c which have shown to target multiple genes involved in prognosis of vascular diabetic complications. A pivotal role of miR29 in regulation of EMT process under hyperglycaemic conditions via targeting multiple signal transduction pathways involved in the process, differential expression patterns of miR29 in apoptotic conditions of retinal cells playing both protective and apoptosis promoting actions. It has shown promising therapeutic role in retinal vascularisation. In episodes of diabetic nephropathy, we have summarised how miR29 can acts as a therapeutic tool in podocyte’s impairment via targeting histone deacetylase circuitry. It was observed that miR29 regulates the expressions of profibrotic, inflammatory and ECM encoding genes in renal fibrosis under diabetic conditions. In occurrence of diabetic cardiomyopathy, miR29 suppression under hyperglycaemic conditions causes development of angiopathy and contradictory actions played by miR29 was found in the development and prevention of apoptosis of cells and cardiac fibrosis. From these studies, we inferred that role of miR29 in diabetic complications is quite uncertain as in some pathological conditions. It was also found to be involved in amelioration of diabetic severity while in few incidences it was implicated in endorsement of the same. By virtue of such contradictory actions showed by miR29 family, it could be considered as the potential pharmacological target in diabetic complications. However, the exact mechanism should be further studied and validated in a more elaborative way to have a firm approach towards therapeutic aid of miR29 family.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aishwarya P. Dasare and Piyush Gondaliya contributed equally to this work.
Contributor Information
Akshay Srivastava, Email: akshay.srivastava@niperahm.ac.in.
Kiran Kalia, Email: director@niperahm.ac.in.
References
- 1.Liang M, Liu Y, Mladinov D, Cowley AW, Trivedi H, Fang Y, et al. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Ren Physiol. 2009;297(3):F553–F5F8. doi: 10.1152/ajprenal.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu X, Han Y, Wu Y, Zhu X, Lu X, Mao F, Wang X, He X, Zhao Y, Zhao Y. Prognostic role of microRNA-21 in various carcinomas: a systematic review and meta-analysis. Eur J Clin Investig. 2011;41(11):1245–1253. doi: 10.1111/j.1365-2362.2011.02535.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C. MicroRNA-145 in vascular smooth muscle cell biology: a new therapeutic target for vascular disease. Cell Cycle. 2009;8(21):3469–3473. doi: 10.4161/cc.8.21.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: A review. J Biochem Mol Toxicol. 2012;26(2):79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Cheng Y, Liu X, Yang J. MicroRNA-145 in vascular smooth muscle cell biology and vascular disease. Am Heart Assoc. 2009. [DOI] [PMC free article] [PubMed]
- 6.Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70(18):7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44(4):237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khullar M, Cheema BS, Raut SK. Emerging evidence of epigenetic modifications in vascular complication of diabetes. Front Endocrinol. 2017;8:237. doi: 10.3389/fendo.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for MicroRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev. 2017;38(2):145–168. doi: 10.1210/er.2016-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slusarz A, Pulakat L. The two faces of miR-29. J Cardiovasc Med (Hagerstown) 2015;16(7):480–490. doi: 10.2459/JCM.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Zhang G, Wu J-H, Jiang C-P. Diverse roles of miR-29 in cancer. Oncol Rep. 2014;31(4):1509–1516. doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- 13.Fiserova B, Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. The miR-29 family in hematological malignancies. Biomed Papers. 2015;159(2):184–191. doi: 10.5507/bp.2014.037. [DOI] [PubMed] [Google Scholar]
- 14.Kollinerova S, Vassanelli S, Modriansky M. The role of miR-29 family members in malignant hematopoiesis. Biomed Papers. 2014;158(4):489–501. doi: 10.5507/bp.2014.029. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Valverde SL, Taft RJ, Mattick JS. MicroRNAs in β-cell biology, insulin resistance, diabetes and its complications. Diabetes. 2011;60(7):1825–1831. doi: 10.2337/db11-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48(1):61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhao E, Keller MP, Rabaglia ME, Oler AT, Stapleton DS, Schueler KL, Neto EC, Moon JY, Wang P, Wang IM, Lum PY, Ivanovska I, Cleary M, Greenawalt D, Tsang J, Choi YJ, Kleinhanz R, Shang J, Zhou YP, Howard AD, Zhang BB, Kendziorski C, Thornberry NA, Yandell BS, Schadt EE, Attie AD. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome. 2009;20(8):476–485. doi: 10.1007/s00335-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey AK, Verma G, Vig S, Srivastava S, Srivastava AK, Datta M. miR-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on PEPCK gene expression in HepG2 cells. Mol Cell Endocrinol. 2011;332(1–2):125–133. doi: 10.1016/j.mce.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic β-cell-specific silencing of monocarboxylate transporter 1 (Mct1) Mol Cell Biol. 2011;31(15):3182–3194. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz CL, Peck BC, Fannin EE, Beysen C, Miao J, Landstreet SR, et al. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes. 2014:DB_131015. [DOI] [PMC free article] [PubMed]
- 21.Nicholas LM, Rattanatray L, MacLaughlin SM, Ozanne SE, Kleemann DO, Walker SK, et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 2013;27(9):3786–3796. doi: 10.1096/fj.13-227918. [DOI] [PubMed] [Google Scholar]
- 22.C Melnik B. The pathogenic role of persistent milk signaling in mTORC1-and milk-microRNA-driven type 2 diabetes mellitus. Curr Diabetes Rev. 2015;11(1):46–62. doi: 10.2174/1573399811666150114100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagge A, Clausen TR, Larsen S, Ladefoged M, Rosenstierne MW, Larsen L, Vang O, Nielsen JH, Dalgaard LT. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem Biophys Res Commun. 2012;426(2):266–272. doi: 10.1016/j.bbrc.2012.08.082. [DOI] [PubMed] [Google Scholar]
- 24.Yang W-M, Jeong H-J, Park S-Y, Lee W. Induction of miR-29a by saturated fatty acids impairs insulin signaling and glucose uptake through translational repression of IRS-1 in myocytes. FEBS Lett. 2014;588(13):2170–2176. doi: 10.1016/j.febslet.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Gu P, Shi W, Li J, Hao Q, Cao X, et al. MicroRNA-29a induces insulin resistance by targeting PPARδ in skeletal muscle cells. Int J Mol Med. 2016;37(4):931–938. doi: 10.3892/ijmm.2016.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes JM, Fotheringham AK. Vascular complications in diabetes: old messages, new thoughts. Diabetologia. 2017:1–10. [DOI] [PubMed]
- 27.Ighodaro O, Adeosun A. Vascular complications in diabetes mellitus. Kidney. 4:16.
- 28.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–551. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L-Q, Cui H, Wang L, Fang X, Su S. Role of microRNA-29a in the development of diabetic retinopathy by targeting AGT gene in a rat model. Exp Mol Pathol. 2017;102(2):296–302. doi: 10.1016/j.yexmp.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Tom L, Davoudi S, Sobrin L. Genetic epidemiology of diabetic retinopathy. Ann Eye Sci. 2017;2(8).
- 31.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2011;52(7):4402–4409. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 32.Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res. 2014;43:92–107. doi: 10.1016/j.preteyeres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Wu J-h, Gao Y, A-j R, Zhao S-h, Zhong M, Y-j P, et al. Altered microRNA expression profiles in retinas with diabetic retinopathy. Ophthalmic Res. 2012;47(4):195–201. doi: 10.1159/000331992. [DOI] [PubMed] [Google Scholar]
- 34.Gong Q, Su G. Roles of microRNAs and Long noncoding RNAs in the progression of diabetic retinopathy. Biosci Rep. 2017;37:BSR20171157. doi: 10.1042/BSR20171157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Q, Jn X, Liu Y, Li Y, Su G. Differentially expressed MicroRNAs in the development of early diabetic retinopathy. J Diabetes Res. 2017;2017:1–10. doi: 10.1155/2017/4727942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Li H, Liu X, Xu D, Wang F. MicroRNA-29b regulates TGF-β1-mediated epithelial–mesenchymal transition of retinal pigment epithelial cells by targeting AKT2. Exp Cell Res. 2016;345(2):115–124. doi: 10.1016/j.yexcr.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Ye S, Xiao W, Luo L, Liu Y. Differentially expressed microRNAs in TGFβ2-induced epithelial-mesenchymal transition in retinal pigment epithelium cells. Int J Mol Med. 2014;33(5):1195–1200. doi: 10.3892/ijmm.2014.1688. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Zhang N, Crombruggen K, Hu G, Hong S, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy. 2012;67(10):1193–1202. doi: 10.1111/j.1398-9995.2012.02880.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K, Gregorevic P, Cooper ME, Kantharidis P. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23(2):252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Wang H, Wang F, Gu Q, Xu X. Snail involves in the transforming growth factor β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS One. 2011;6(8):e23322. doi: 10.1371/journal.pone.0023322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirota K, Keino H, Inoue M, Ishida H, Hirakata A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):335–342. doi: 10.1007/s00417-014-2692-5. [DOI] [PubMed] [Google Scholar]
- 42.Lin X, Zhou X, Liu D, Yun L, Zhang L, Chen X, Chai Q, Li L. MicroRNA-29 regulates high-glucose-induced apoptosis in human retinal pigment epithelial cells through PTEN. In Vitro Cell Dev Biol Anim. 2016;52(4):419–426. doi: 10.1007/s11626-015-9990-z. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Zhang X, Wang T. MicroRNA-486 down-regulates p53 expression in the diabetic retinopathy. Int J Clin Exp Pathol. 2016;9(5):5034–5044. [Google Scholar]
- 44.Jia L-F, Huang Y-P, Zheng Y-F, Ming-Yue L, Wei S-B, Meng Z, et al. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN–AKT signaling pathway by targeting Sp1. Oral Oncol. 2014;50(11):1062–1071. doi: 10.1016/j.oraloncology.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Luo H, Li N, Duan X. Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway, part ii: an in vivo investigation. Invest Ophthalmol Vis Sci. 2015;56(10):6019–6028. doi: 10.1167/iovs.15-16558. [DOI] [PubMed] [Google Scholar]
- 46.Papait R, Kunderfranco P, Stirparo GG, Latronico MV, Condorelli G. Long noncoding RNA: a new player of heart failure? J Cardiovasc Transl Res. 2013;6(6):876–883. doi: 10.1007/s12265-013-9488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, Wong D. Long non-coding RNA-mediated regulation of glucose homeostasis and diabetes. Am J cardiovasc Dis. 2016;6(2):17–25. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Chen M, Chen J, Lin S, Cai D, Chen C, Chen Z. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Biosci Rep. 2017;37(2):BSR20170036. doi: 10.1042/BSR20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilcock DM, Morgan D, Gordon MN, Taylor TL, Ridnour LA, Wink DA, Colton CA. Activation of matrix metalloproteinases following anti-Aβ immunotherapy; implications for microhemorrhage occurrence. J Neuroinflammation. 2011;8(1):115. doi: 10.1186/1742-2094-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai J, Yin G, Lin B, Wang X, Liu X, Chen X, Yan D, Shan G, Qu J, Wu S. Roles of NFκB-miR-29s-MMP-2 circuitry in experimental choroidal neovascularization. J Neuroinflammation. 2014;11(1):88. doi: 10.1186/1742-2094-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X-K, Ouyang L-J, Yin Z-Q, Xia Y-Y, Chen X-R, Shi H, et al. Effects of microRNA-29a on retinopathy of prematurity by targeting AGT in a mouse model. Am J Transl Res. 2017;9(2):791. [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diab Rep. 2016;16(3):35. doi: 10.1007/s11892-016-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato M, Natarajan R. MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci. 2015;1353(1):72–88. doi: 10.1111/nyas.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M, Natarajan R. Diabetic nephropathy [mdash] emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuen DA, Stead BE, Zhang Y, White KE, Kabir MG, Thai K, Advani SL, Connelly KA, Takano T, Zhu L, Cox AJ, Kelly DJ, Gibson IW, Takahashi T, Harris RC, Advani A. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol. 2012;23(11):1810–1823. doi: 10.1681/ASN.2011121170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin W, Chung AC, Huang XR, Meng X-M, Hui DS, Yu C-M, et al. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22(8):1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud P-D, Ruth TY, Alvarez JG, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145(4):607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin C-L, Lee P-H, Hsu Y-C, Lei C-C, Ko J-Y, Chuang P-C, Huang YT, Wang SY, Wu SL, Chen YS, Chiang WC, Reiser J, Wang FS. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol. 2014;25(8):1698–1709. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George B, Verma R, Soofi AA, Garg P, Zhang J, Park T-J, Giardino L, Ryzhova L, Johnstone DB, Wong H, Nihalani D, Salant DJ, Hanks SK, Curran T, Rastaldi MP, Holzman LB. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest. 2012;122(2):674–692. doi: 10.1172/JCI60070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia Y, Zheng Z, Wang Y, Zhou Q, Cai W, Jia W, Yang L, Dong M, Zhu X, Su L, Hu D. SIRT1 is a regulator in high glucose-induced inflammatory response in RAW264. 7 cells. PLoS One. 2015;10(3):e0120849. doi: 10.1371/journal.pone.0120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ceolotto G, De Kreutzenberg SV, Cattelan A, Fabricio AS, Squarcina E, Gion M, et al. Sirtuin 1 stabilization by HuR represses TNF-α-and glucose-induced E-selectin release and endothelial cell adhesiveness in vitro: relevance to human metabolic syndrome. Clin Sci. 2014;127(7):449–461. doi: 10.1042/CS20130439. [DOI] [PubMed] [Google Scholar]
- 63.Hah Y-S, Cheon Y-H, Lim HS, Cho HY, Park B-H, Ka S-O, Lee YR, Jeong DW, Kim HO, Han MK, Lee SI. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS One. 2014;9(2):e87733. doi: 10.1371/journal.pone.0087733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110(5):1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N, Cui J, Duan X, Chen H, Fan F. Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway in human Tenon's fibroblasts. Invest Ophthalmol Vis Sci. 2012;53(3):1670–1678. doi: 10.1167/iovs.11-8670. [DOI] [PubMed] [Google Scholar]
- 66.Chen H-Y, Zhong X, Huang XR, Meng X-M, You Y, Chung AC, et al. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther. 2014;22(4):842–853. doi: 10.1038/mt.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Si Y, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13(11):1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 68.Vriend J, Reiter RJ. The Keap1-Nrf2-antioxidant response element pathway: a review of its regulation by melatonin and the proteasome. Mol Cell Endocrinol. 2015;401:213–220. doi: 10.1016/j.mce.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 69.Zhou L, D-y X, W-g S, Shen L, G-y L, Yin X, et al. High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J Transl Med. 2015;13(1):352. doi: 10.1186/s12967-015-0710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, et al. Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol Med. 2016:e201506031. [DOI] [PMC free article] [PubMed]
- 71.Ajila V, Shetty H, Babu S, Shetty V, Hegde S. Human papilloma virus associated squamous cell carcinoma of the head and neck. J Sex Transm Dis. 2015;2015:1–5. doi: 10.1155/2015/791024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Taylor NE, Lu L, Cowley AW, Ferreri NR, Yeo NC, et al. Renal medullary MicroRNAs in dahl salt-sensitive rats. Hypertension. 2010;55(4):974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou L, Wang L, Lu L, Jiang P, Sun H, Wang H. Inhibition of miR-29 by TGF-beta-Smad3 signaling through dual mechanisms promotes transdifferentiation of mouse myoblasts into myofibroblasts. PLoS One. 2012;7(3):e33766. doi: 10.1371/journal.pone.0033766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poncelet A-C, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-β1-induced α2 (I) collagen expression in human glomerular mesangial cells. J Biol Chem. 2001;276(10):6983–6992. doi: 10.1074/jbc.M006442200. [DOI] [PubMed] [Google Scholar]
- 75.He Y, Huang C, Lin X, Li J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie. 2013;95(7):1355–1359. doi: 10.1016/j.biochi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Shiomi T, Lemaître V, D'armiento J, Okada Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60(7):477–496. doi: 10.1111/j.1440-1827.2010.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramdas V, McBride M, Denby L, Baker AH. Canonical transforming growth factor-β signaling regulates disintegrin metalloprotease expression in experimental renal fibrosis via miR-29. Am J Pathol. 2013;183(6):1885–1896. doi: 10.1016/j.ajpath.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci. 2007;104(40):15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother. 2009;58(11):1723–1747. doi: 10.1007/s00262-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi S, Koya D, Kanasaki K. Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogenesis Tissue Repair. 2016;9(1):1. doi: 10.1186/s13069-016-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu Y-C, Chang P-J, Ho C, Huang Y-T, Shih Y-H, Wang C-J, et al. Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/β-catenin signaling. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed]
- 83.Lin C-L, Wang J-Y, Huang Y-T, Kuo Y-H, Surendran K, Wang F-S. Wnt/β-catenin signaling modulates survival of high glucose–stressed mesangial cells. J Am Soc Nephrol. 2006;17(10):2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 84.Lin C-L, Wang J-Y, Ko J-Y, Huang Y-T, Kuo Y-H, Wang F-S. Dickkopf-1 promotes hyperglycemia–induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol. 2010;21(1):124–135. doi: 10.1681/ASN.2008101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chien H-Y, Chen C-Y, Chiu Y-H, Lin Y-C, Li W-C. Differential microRNA profiles predict diabetic nephropathy progression in Taiwan. Int J Med Sci. 2016;13(6):457–465. doi: 10.7150/ijms.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu F, Guo J, Zhang Q, Liu D, Wen L, Yang Y, Yang L, Liu Z. The expression of Tristetraprolin and its relationship with urinary proteins in patients with diabetic nephropathy. PLoS One. 2015;10(10):e0141471. doi: 10.1371/journal.pone.0141471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patial S, Blackshear PJ. Tristetraprolin as a therapeutic target in inflammatory disease. Trends Pharmacol Sci. 2016;37(10):811–821. doi: 10.1016/j.tips.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo J, Li J, Zhao J, Yang S, Wang L, Cheng G, et al. MiRNA-29c regulates the expression of inflammatory cytokines in diabetic nephropathy by targeting tristetraprolin. Sci Rep. 2017;7. [DOI] [PMC free article] [PubMed]
- 89.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286(13):11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Figueira M, Monnerat-Cahli G, Medei E, Carvalho A, Morales M, Lamas M, et al. MicroRNAs: potential therapeutic targets in diabetic complications of the cardiovascular and renal systems. Acta Physiol. 2014;211(3):491–500. doi: 10.1111/apha.12316. [DOI] [PubMed] [Google Scholar]
- 91.Papageorgiou N, Tslamandris S, Giolis A, Tousoulis D. MicroRNAs in cardiovascular disease: perspectives and reality. Cardiol Rev. 2016;24(3):110–118. doi: 10.1097/CRD.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 92.Samanta S, Balasubramanian S, Rajasingh S, Patel U, Dhanasekaran A, Dawn B, Rajasingh J. MicroRNA: A new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc Med. 2016;26(5):407–419. doi: 10.1016/j.tcm.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heid J, Cencioni C, Ripa R, Baumgart M, Atlante S, Milano G, Scopece A, Kuenne C, Guenther S, Azzimato V, Farsetti A, Rossi G, Braun T, Pompilio G, Martelli F, Zeiher AM, Cellerino A, Gaetano C, Spallotta F. Age-dependent increase of oxidative stress regulates microRNA-29 family preserving cardiac health. Sci Rep. 2017;7(1):16839. doi: 10.1038/s41598-017-16829-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abonnenc M, Nabeebaccus AA, Mayr U, Barallobre-Barreiro J, Dong X, Cuello F, Sur S, Drozdov I, Langley SR, Lu R, Stathopoulou K, Didangelos A, Yin X, Zimmermann WH, Shah AM, Zampetaki A, Mayr M. Extracellular matrix secretion by cardiac FibroblastsNovelty and significance. Circ Res. 2013;113(10):1138–1147. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- 95.Deng Z, He Y, Yang X, Shi H, Shi A, Lu L, et al. MicroRNA-29: A crucial player in fibrotic disease. Mol Diagn Ther. 2017:1–10. [DOI] [PubMed]
- 96.Kuo Y-R, Chien C-M, Kuo M-J, Wang F-S, Huang E-Y, Wang C-J. Endothelin-1 expression associated with lipid peroxidation and nuclear factor-κB activation in type 2 diabetes mellitus patients with Angiopathy and limb amputation. Plast Reconstr Surg. 2016;137(1):187e–195e. doi: 10.1097/PRS.0000000000001886. [DOI] [PubMed] [Google Scholar]
- 97.Gu J, Wang S, Tan Y, Cai L, editors. Inhibition of P53 Prevents Diabetic Cardiomyopathy by Attenuating the Early-Stage Apoptosis and Improving Late-Stage Senescence and Defects in Glycolysis and Angiogenesis. Diabetes; 2017: AMER Diabetes Assoc 1701 N Beauregard ST, Alexandria, VA 22311–1717 USA.
- 98.Li Z, Jiang R, Yue Q, Peng H. MicroRNA-29 regulates myocardial microvascular endothelial cells proliferation and migration in association with IGF1 in type 2 diabetes. Biochem Biophys Res Commun. 2017;487(1):15–21. doi: 10.1016/j.bbrc.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 99.van Beijnum JR, Pieters W, Nowak-Sliwinska P, Griffioen AW. Insulin-like growth factor axis targeting in cancer and tumour angiogenesis–the missing link. Biol Rev. 2017;92(3):1755–1768. doi: 10.1111/brv.12306. [DOI] [PubMed] [Google Scholar]
- 100.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33. doi: 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roncarati R, Anselmi CV, Losi MA, Papa L, Cavarretta E, Martins PDC, et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2014;63(9):920–927. doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 102.Fernandes T, Baraúna VG, Negrão CE, Phillips MI, Oliveira EM. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Phys Heart Circ Phys. 2015;309(4):H543–HH52. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Habibi P, Alihemmati A, Nasirzadeh M, Yousefi H, Habibi M, Ahmadiasl N. Involvement of microRNA-133 and-29 in cardiac disturbances in diabetic ovariectomized rats. Iran J Basic Med Sci. 2016;19(11):1177–1185. [PMC free article] [PubMed] [Google Scholar]
- 104.Roggli E, Gattesco S, Caille D, Briet C, Boitard C, Meda P, Regazzi R. Changes in microRNA expression contribute to pancreatic β-cell dysfunction in prediabetic NOD mice. Diabetes. 2012;61(7):1742–1751. doi: 10.2337/db11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnold N, Koppula PR, Gul R, Luck C, Pulakat L. Regulation of cardiac expression of the diabetic marker microRNA miR-29. PLoS One. 2014;9(7):e103284. doi: 10.1371/journal.pone.0103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goyal B, Mehta A. Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfuntion. Hum Exp Toxicol. 2013;32(6):571–590. doi: 10.1177/0960327112450885. [DOI] [PubMed] [Google Scholar]
- 107.González GE, Rhaleb N-E, D’ambrosio MA, Nakagawa P, Liu Y, Leung P, et al. Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J Hypertens. 2015;33(1):144–152. doi: 10.1097/HJH.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Wang J-H, Zhang Y-Y, Wang Y-Z, Wang J, Zhao Y, Jin XX, Xue GL, Li PH, Sun YL, Huang QH, Song XT, Zhang ZR, Gao X, Yang BF, du ZM, Pan ZW. Deletion of interleukin-6 alleviated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy of mice through affecting TGFβ1 and miR-29 pathways. Sci Rep. 2016;6:23010. doi: 10.1038/srep23010. [DOI] [PMC free article] [PubMed] [Google Scholar]