Abstract
Effect of postharvest applications of sodium benzoate on physico-chemical properties and enzymatic activities of pear fruit cv. Patharnakh during cold storage were examined. Uniform and healthy fruits of pear cv. Patharnakh were treated with sodium benzoate (0.0, 1.0, 2.0 and 3.0%) and thereafter stored at low temperature conditions (0–1 °C and 90–95% RH) for 70 days. Evaluation of physico-chemical parameters and enzymatic activities were made at 0, 20, 40, 60 and 70 days of storage. Sodium benzoate treatments effectively retained higher fruit firmness, sensory quality, total phenolics content and alongside suppressed the degradation of total soluble solids, ascorbic acid content and titratable acidity. Sodium benzoate treated fruits also exhibited higher efficacy in maintaining less enzymatic activity of cellulase, pectin methyl esterase and polyohenol oxidase. It was inferred that the postharvest treatments of sodium benzoate proved to be an effective means of improving quality and postharvest life of ‘Patharnakh’ pear fruit.
Keywords: Sodium benzoate, Fruit quality, Pear, Cold storage, Enzymatic activity
Introduction
Subtropical pears, [Pyrus pyrifolia (Burm.) Nak.] are successfully cultivated in the plains of north-western India where winter period is short and summers are very hot. Amongst several recommended varieties grown in this region, ‘Patharnakh’ is most popular due to its high yield potential, low chill requirement, adaptation to various biotic and abiotic stresses. Fruits harvested in July when temperature is high and not ideal for ambient storage which causes high postharvest losses. The high water loss, more transpiration and mechanical damage reduced the quality of produce and lower its market value (Jawandha et al. 2014). Various attempts have been made to increase the postharvest life of pear fruit under low temperature storage for a longer period to enhance its marketing.
Sodium benzoate is a generally-recognized-as-safe (GRAS) preservative for miscellaneous and general-purpose usage (Code of Federal Regulations, title 21, section 184.1733). Its antibacterial properties in solution are due to the undissociated benzoic acid molecule. Only those organic acids that are lipophilic, such as benzoic acid, have antimicrobial activity that involves interference with the permeability of the microbial cell membrane (Doores 1993). Much research evidence shows that sodium benzoate has ability to delay senescence & ripening, reduction in respiration rate, prolongs shelf-life, reduced physiological disorders and also preserves the quality parameters of fruits (Wang and Baker 1979). Sodium benzoate is the sodium salt of benzoic acid and works well in acidic media to inhibit yeasts, molds & bacterial growth and also used as fungistatic preservative. Sodium benzoate has also been used to inhibit postharvest changes in fruits and vegetables. Sodium Benzoate can also maintain the good quality of fruit juices during preservation (Ayub et al. 2010) and found to control both growth and alfatoxin production by aspergillus parasitic (El-Gazzar et al. 1987). Postharvest application of sodium benzoate of 500 ppm effectively lowered weight loss percentage and increased shelf life of grapes (Venkatram et al. 2015). Best results were obtained by HPMC-BW coating containing 2.0% sodium benzoate for control of alternaria black spot in cherry tomatoes (Fagundes et al. 2015). The effect of sodium benzoate on the growth and survival of some yeast strains, food poisoning and spoilage organisms has been widely reported. Sodium benzoate inhibits the ethylene production interfering essentially at the conversion of aminocyclopropane-1 carboxylic acid (ACC) to ethylene, thereby increasing the shelf life of tomato (Srividya et al. 2014).
Therefore, considering the positive effects of sodium benzoate on fresh horticultural commodities, the present research was conducted to investigate the effects of postharvest treatments of sodium benzoate on physico-chemical and enzymatic changes in pear cv. Patharnakh during low temperature storage.
Materials and methods
Experimental procedure
For the experiment, fruits of pear cv. Patharnakh were harvested from the Fruit Research Farm, Department of Fruit Science, Punjab Agricultural University, Ludhiana (India) in the year 2017. The harvested fruits were sorted on the basis of uniformity in size, colour, and absence of visible injury and then divided into 4 lots. Fruits of first three lots were dipped for 5 mintues in solution of sodium benzoate at different concentrations (1, 2 and 3%). Control fruits were dipped in water only. The experiment comprised of four treatments with four replications in each treatment. One kg of fruit from every replication of each treatment was packed in corrugated fibreboard (CFB) boxes (5% perforation) with paper lining and kept at 0–1 °C and 90–95% RH for 70 days. Before packing in CFB boxes fruits of pear were air dried in shade. For study of storage behaviour, fruit samples were analyzed after 0, 20, 40, 60 and 70 days of storage for various physico-chemical characteristics.
Physico- chemical analysis
Fruit firmness and weight loss
Firmness of fruits was measured with stand mounted penetrometer (Model FT-327, Italy). With the help of peeler, about one centimetre of peel was removed from the shoulders of fruit. The fruit firmness from peeled off area was recorded and expressed in terms of lbf. Weight loss was calculated by subtracting final weight from initial weight and expressed in percentage (%) with reference to the initial weight.
Sensory quality, spoilage, total sugars and ascorbic acid
Sensory evaluation of treated fruits was done by a panel of 5 members. Panelists evaluated the fruits on basis of freshness colour, aroma, texture, taste and overall acceptance according to 9-point scale described by Amerine et al. (1965). Spoilage percentage was measured as a proportion of rotton fruits weight relative to total weight of fruits within each replication. Total sugars were deliberated by Lane and Eynon’s titration method as given by Ranganna (2000) and expressed in percentage. The ascorbic acid was estimated by using 2, 6-dichlorphenol-indophenol dye method as described Ranganna (2000) and expressed in percentage.
Total soluble solids, TA and total phenolics content
Total soluble solids were determined by digital refractometer (ATAGO, PAL-1, Japan) and expressed in °Brix. TA was recorded as per AOAC (2005) and expressed in percent of malic acid. For determining total phenolics content, extraction was done by taking 1 g tissue homogenized with 10 ml of methanol after constant shaking for 1 h at room temperature. After filtering the solution whole process was repeated for residue. Finally the extracts were combined and diluted to 100 ml with methanol. Then, total phenolics content was estimated by using Folin–Ciocateu method as described by Singh et al. (2002) and results were expressed as mg/100 g fresh weight basis.
Pectin methylesterase, cellulase and PPO activities
Enzyme extraction of PME and cellulase: For the purpose of extracting PME and cellulase enzyme, blended frozen fruit pulp with NaCl solution (0.15 m) measuring 60–100 ml quantity, is then passed through a cheese cloth and centrifuge at 2000 rpm for 30 min at 4 °C. Enzyme source taken was supernatant (Mahadevan and Sridhar 1998).
PME assay
After the hydrolysis of pectin by the enzymes, the activity of PME was estimated by measuring the increase in acidity. In 50 ml beaker, take 20 ml of 1.0% pectin solution and adjusting the pH to 7.0 and then addition of 10 ml enzyme solution with immediate adjustment of pH to 7.0 by addition of 1 N NaOH, which was considered as zero time. Then for 15 min, the beaker placed in water bath and pH again checked and adjusted to 7.0 after every 15 min interval by usage of 0.02 N NaOH, alongwith the stirring of content and noting down amount of 0.02 N NaOH used (Mahadevan and Sridhar 1998).
Cellulase assay
The percent reduction in the substrate viscosity is used to estimate the cellulase activity during fruit storage at various intervals. For assay, in the viscometer, pipette out 4.0 ml of carboxy methyl cellulose (CMC) solution, 1.0 ml of sodium acetate acetic acid buffer (pH 5.2) and 2.0 ml of enzyme source (extract). Content mixing was done through drawing air rapidly by suction through large arm of viscometer, thereafter suction was applied to viscometer through the small arm and the efflux time of the mixture was determined. It was considered as zero time. Determination of efflux time of mixture at various intervals was done and cellulase activity expressed as per cent reduction in substrate’s viscosity (Mahadevan and Sridhar 1998). PPO activity was estimated as method reported by Serradell et al. (2000) and expressed in units mg−1 FW.
Statistical analysis
The experiment was set up as a completely randomized design and the data analysis was performed using the Statistical Analysis Software System SAS version 9.3 (SAS Institute, Inc, 1992; Cary, NC, USA). The analysis of variance (ANOVA) was done using PROC GLM. Mean comparisons were performed using LSD test at p <0.05. Results were expressed as mean ± standard deviation. Further, data was subjected to Pearson correlation analysis to assess the nature and extent of relationship between them.
Results and discussions
Weight loss and fruit firmness
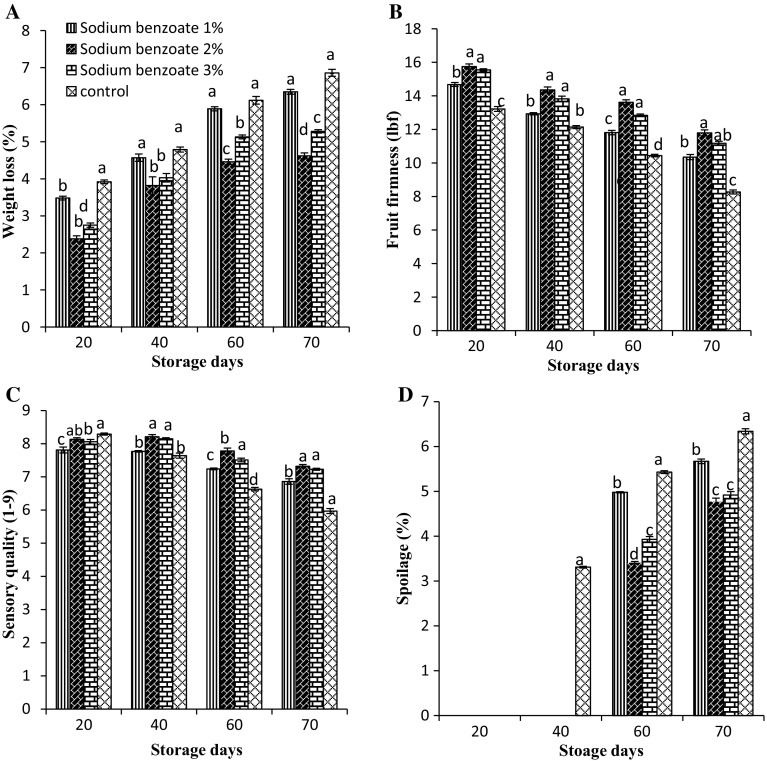
In Fig. 1a shows the weight loss of sodium benzoate treated fruits stored at 0–1 °C for 70 days. Weight loss of fruits significantly increased from 0 to 70 days of storage irrespective of the applied treatments. However, sodium benzoate treated fruits showed less weight loss as compared to untreated fruits. Maximum weight loss of fruits was recorded in untreated fruits and minimum was observed in sodium benzoate 2.0% treated fruits. From the 20th day of storage to end of studies, the weight loss of fruits increased from 2.38 to 4.62% in sodium benzoate (2.0%) treatment and 3.92–6.86% in control fruits. Weight loss of fruits is mainly due transpiration, respiration and other metabolic activities. For instance results are also confirmed with earlier reports where loss in weight of fruits significantly decreased with sodium benzoate treatments and found to be most effective (Kaur et al. 2014). ‘Balanagar’ custard apple fruits treated with sodium benzoate recorded least physiological weight loss of fruits during cold storage (Venkatram and Bhagwan 2013). In pear, fruit firmness is considered as important physical parameter used to assess the texture, storage life and progression in fruit softening. Membrane permeability increased due to cellullar disintrgration and causes softening of fruits. A decline in fruit firmness was recorded during storage of pear fruits in all the treatments. Fruits treated with sodium benzoate exhibited higher fruit firmness as compared to control. At 70 days of storage, the mean fruit firmness of sodium benzoate 2% treated fruits was 20.6% higher than control fruits (Fig. 1b). These results are in line with the findings of Silvia et al. (2011) who reported that sodium benzoate coating maintained higher fruit firmness of ‘Clemenules’ mandarin up to 30 days of storage.
Fig. 1.
Variation in a weight loss, b fruit firmness, c sensory quality and d spoilage in pear fruits during low temperature storage (0–1 °C, 90–95% RH) in relation to postharvest treatments with different concentration of sodium benzoate. Vertical bars represent ± standard error of mean of 4 replicates
Sensory quality and spoilage
Fruit quality is a major factor from consumer’s perspective which includes sensory evaluation as well as visual criteria. Patharnakh pear fruit consists of abundance of sclereid cells due to which it has gritty texture. Sensory quality of fruits improved during storage period due to increase in sugar content and reduction in acid concentration. The sensory quality increased from 0 to 40 days of cold storage in Patharnakh fruits treated with different concentrations of sodium benzoate. However, in control fruits (8.29) sensory quality increased only up to 20 days of storage which decreased rapidly with progression in storage period (Fig. 1c). Conversely, the rate of reduction in sensory quality of sodium benzoate treated fruits was steady with respect to storage time. At the end of studies, the sensory scores of sodium benzoate treated fruits were above the moderately acceptability level. However, control fruits were moderately acceptable on 60th days of storage. In the similar studies were found in strawberries, where sodium benzoate were found effective in maining organoleptic properties (Khan et al. 2014). In cucumber fruit, sodium benzoate maintained the sensory quality up to 10 days of storage (Zhang et al., 2004). On 20th day of storage no spoilage was observed in any concentration of sodium benzoate, however, untreated fruits exhibited 3.31% spoilage. Spoilage percentage continuously increased during storage. At 70th day untreated fruits showed 6.34 percent spoilage while spoilage in sodium benzoate 2% treated fruit was 4.77% and it was 32.91% lower than untreated fruits (Fig. 1d). Sodium salts are GRAS compounds which are widely used for controlling postharvest decay of horticultural crops. Fagundes et al. (2015) reported that coating containing sodium benzoate was found to be most effective in controlling disease incidence in cherry tomatoes. The treatment of sodium benzoate gives long term protection and prevents from decay incidence in grapefruit (Abdel-Kader et al. 2011).
TSS, total sugars content, TA and ascorbic acid content
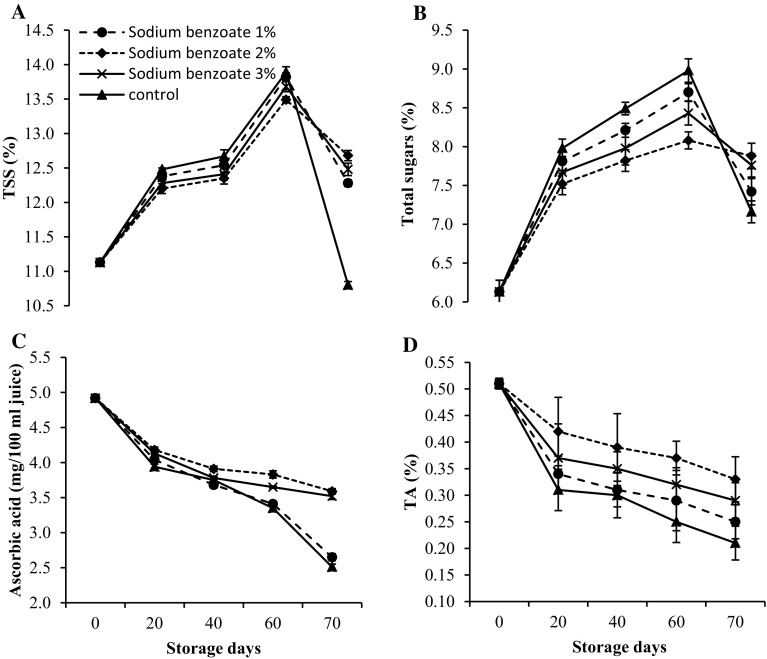
A gradual progression in TSS and total sugars was recorded in all the treatments till 60 days of storage followed by a decline (Fig. 2a, b). In conversely, ascorbic acid and TA content significantly declined in pear fruits during storage irrespective to the treatments. During storage untreated fruits exhibited significantly higher mean TSS and total sugars content than sodium benzoate treatments. However on 70th day, sodium benzoate 2.0% treatment recorded significantly higher TSS and total sugars over other treatments. At the end of studies, mean TSS and total sugars were significantly lower (0.78 and 3.92%) in sodium benzoate treated fruits than control. Postharvest applications of sodium benzoate significantly maintained higher level of ascorbic acid and TA (3.59 and 0.33%), as compared to untreated fruits (Fig. 2c, d). The decline in TSS and total sugars after 60 days of storage is due to utilization of carbohydrates in other metabolic activities (Champa et al. 2014). Lower TSS and total sugars in sodium benzoate treated fruits may be due to reduction of ripening process. Hydrolysis of starch, water loss from fruit surface and other polysaccharides insoluble form to soluble form of sugar leads to increase in TSS and sugars content. The consumption of organic acids in respiratory process resulted in reduction in acidity level of fruit (Maftoonazad et al. 2008). In storage, oxygen influenced the activities of phenoloxidase and ascorbic acid oxidase which primarily regulates ascorbic acid content (Zhou et al. 2008). Khan et al. (2014) reported that the use of sodium benzoate as a preservative in strawberry maintained overall quality of fruits during storage. Lower value of total sugars and higher ascorbic acid content were also observed in sodium benzoate treated mango (Farzana and Baloch 2014). The reduction in ascorbic acid content with storage are confirmed by Talasila et al. (2012).
Fig. 2.
Variation in a TSS, b total sugars, c ascorbic acid content and d TA in pear fruits during low temperature storage (0–1 °C, 90–95% RH) in relation to postharvest treatment with different concentrations of sodium benzoate. Vertical bars represent ± standard error of mean of 4 replicates
Total phenolics content and PPO activity
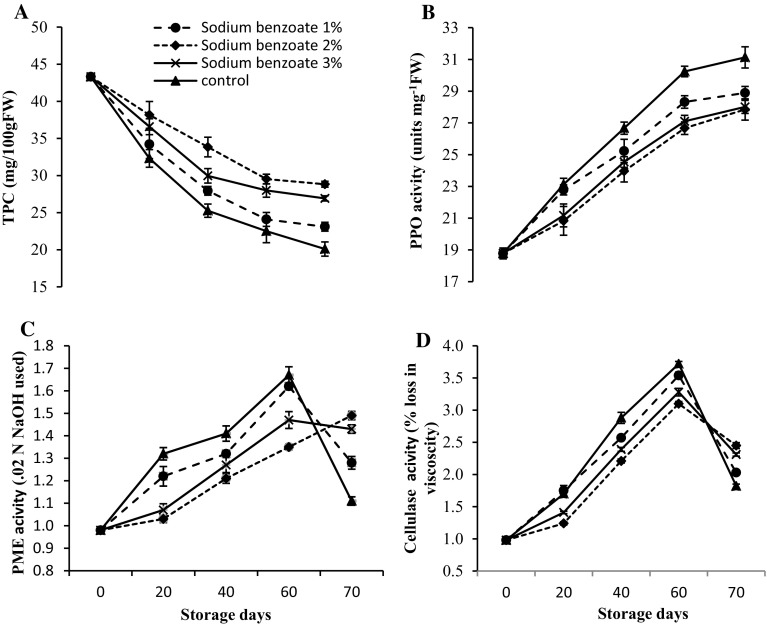
Phenolics content a substrate of PPO was closely linked with tissue browning. Polyphenols are important antioxidants which protects biological systems against oxygen radicals (Bendini et al. 2007). As storage time progressed, pear fruits exhibited a linear reduction in total phenolices content. However, decrease in total phenolics content was less prominent in sodium benzoate treated fruits. At the end of storage, sodium benzoate 2% treated fruits exhibited 23.16% higher total phenolics content as compared to control (Fig. 3a, b). The accumulation of total phenolics content with ethylene climacteric may be attributed to the role of ethylene in phenolic metabolism (Blankenship and Richard-unrath 1988). Positive effects of sodium benzoate on total phenolics content might contribute to lower rate of spoilage percentage of sodium benzoate treated pear fruits over the control. PPO enzyme plays an important role in deteriorating the quality of pear fruits and causes enzymatic browning. PPO activity progressed with advancement of storage period. The mean minimum PPO activity was recorded in sodium benzoate 2% treated fruits and mean maximum PPO activity was recorded in control fruits. At the end of storage, control fruits recorded 6.47% higher PPO activity than sodium benzoate 2% treated fruits. The amount of O-quinones depends mainly on amount of best substrates (5-caffeic derivative and catehins) and on polyphenol oxidase activity. PPO comes in a direct contact with substrates. The degradation of phenolics compound in pear could be result of direct oxidation by polyphenol oxidases and oxidation, as described in as grapes (Cheynier et al. 1988; Cheynier and Ricardo da Siliva 1991).
Fig. 3.
Variation in a TPC, b PPO, c PME activity and d cellulase activity in pear fruits during low temperature storage (0–1 °C, 90–95% RH) in relation to postharvest treatment with different concentrations of sodium benzoate. Vertical bars represent ± standard error of mean of 4 replicates
PME and cellulase activities
The rapid decrease in fruit firmness might be attributed to rapid progression in PME activity, depolymerization of cell wall pectins and also associated with changes in composition of cell wall pectin and turgor pressure. Pectic substances, cellulose, and hemicellulose are the major cell wall polysaccharides, some of which are depolymerized during ripening leading to fruit softening. Pectin degradation in fruits is occurred initially by the action of PME. It catalyses the hydrolysis of methyl-ester groups from galacturonosyl residues and plays important role in determining the extent to which demethylated polygalacturonase are accessible to degradation by polygalacturonases (Barnavon et al. 2001). As compared to untreated fruits postharvest treatments of sodium benzoate at different concentrations significantly lowered PME activity. The PME and cellulase activities of pear fruit significantly increased slowly up to 60 days of storage and afterwards all the treatments exhibited a sharp decline in enzyme activities. However, sodium benzoate treated fruits illustrated lower PME and cellulase activities (7.29% and 11.06%) in contrast to control fruits (Fig. 3c, d). The decline in enzyme activities was prominent in control fruits as compared to sodium benzoate treated samples. On 70th day, fruits treated with sodium benzoate had higher activity of PME and cellulase as compared to control fruits. The higher activity of PME and cellulase in sodium benzoate treated fruits might be due to higher level of substrate for enzyme activity which was already decomposed in control fruits. Therefore, the positive affect of sodium benzoate on suppression of cell wall degrading enzymes might accounts firmer fruits in treated group as compared to untreated group. The activity of PME and cellulase activity exhibited significant interaction between sodium benzoate and storage interval. These results are lined with the previous study in apple (Mahajan 1994), ber (Jawandha et al. 2012), mango (Ali et al. 2004), grapes (Champa et al. 2014) who reported decrease in PME activity during storage at later stages.
Correlation analysis
The correlation analysis (Table 1) in our studies showed that fruit firmness is negatively correlated with, weight loss (r = − .930, p ≤ 0.01) enzymatic activities of pectin methyl esterase (r = − .403, p ≤ 0.01) and cellulase (r = − .362, p ≤ 0.01). The chemical treated fruits showed higher amounts of total sugars in ripe fruits which may be due to a higher activity of fruit softening enzymes in those fruit, leading to higher cell wall disassembly providing more sources of carbon for sugar synthesis (Fabi et al. 2007). The analysis further demonstrated a negative correlation of total phenolics content with spoilage (r = − .757, p ≤ 0.01) and PPO activity (r = − .772, p ≤ 0.01). This coincidence of high PPO and phenolics enabled the fruits to become more susceptible to enzymatic browning and increases spoilage percentage. As the storage progressed, total phenolics content decreased, while PPO activity increased in the fruit. This reverse change between PPO activity and total phenolics content in pear fruit during storage can be attributed to an increase of the catalytic efficiency of enzyme during ripening. Accordingly it can be stated that as fruit matures, in contrast to PPO activity, there was a significant decrease in amount of total phenolics content.
Table 1.
Relationship between some selected quality parameters of pear fruits treated with different concentrations of sodium benzoate during of cold storage
| Variables compared | Relationship |
|---|---|
| Pearson correlation coefficient (r) | |
| Firmness versus WL | − .930** |
| Firmness versus PME | − .403** |
| Firmness versus cellulase | − .362** |
| TPC versus PPO | − .772** |
| TPC versus spoilage | − .757** |
WL Weight loss, PME pectin methylesterase, TPC total phenolics content, PPO polyphenol oxidase
**Significant at p < 0.01
Conclusion
It can be concluded from the study that postharvest treatment of 2% sodium benzoate were found most effective in maintaining physico-chemical attributes and enzymatic activities of pear fruits under low temperature storage. It also significantly reduced the postharvest spoilage and enhanced the storage of life of fruits.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Kader M, El-Mougy N, Lashin S. Evaluation of grapefruit coating with chemical preservative as control measure against postharvest decay. Phytopathologia. 2011;59:25–38. [Google Scholar]
- Ali ZM, Chin LH, Lazan H. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Sci. 2004;167:317–327. doi: 10.1016/j.plantsci.2004.03.030. [DOI] [Google Scholar]
- Amerine MA, Pangborn RM, Roessler EB. Principles of sensory evaluation of food. Food science and technology monographs. New York: Elsevier; 1965. pp. 338–339. [Google Scholar]
- AOAC . Official method of analysis of AOAC International. 18. Rockville: AOAC; 2005. [Google Scholar]
- Ayub M, Ullah J, Muhammad A, Zeb A. Evaluation of strawberry juice preserved with chemical preservatives at refrigeration temperature. Int J Nutr Metab. 2010;2:27–32. [Google Scholar]
- Barnavon L, Doco T, Terrier N, Ageorges A, Romieu C, Pellerina P. Involvement of pectin methyl-esterase during the ripening of grape berries: partial cDNA isolation, transcript expression and changes in the degree of methyl-esterification of cell wall pectins. Photochem. 2001;58:693–701. doi: 10.1016/S0031-9422(01)00274-6. [DOI] [PubMed] [Google Scholar]
- Bendini A, Cerretani L, Carrasco-Pancorbo A, Gomez-Caravaca AM, Segura-Carretero A, Fernandez-Gutierrez A, Lercker G. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship SM, Richard Unrath C. PAL and ethylene content during maturation of red and golden delicious apples. Phytochem. 1988;27:1001–1002. doi: 10.1016/0031-9422(88)80260-7. [DOI] [Google Scholar]
- Champa WHA, Gill MIS, Mahajan BVC, Arora NK. Postharvest treatment of polyamines maintains quality and extends shelf-life of table grapes (Vitis vinifera L.) cv. flame seedless. Postharvest Biol Technol. 2014;91:57–63. doi: 10.1016/j.postharvbio.2013.12.014. [DOI] [Google Scholar]
- Cheynier V, Ricardo da Silva JM. Oxidation of grape procyanidins in model solutions containing trans-caffeoyltartaric acid and polyphenol oxidase. J Agric Food Chem. 1991;39:1047–1049. doi: 10.1021/jf00006a008. [DOI] [Google Scholar]
- Cheynier V, Basire N, Rigaud J. Mechanism of trans-caffeoyltartaric acid and catechin oxidation in model solutions containing grape polyphenoloxidase. J Agric Food Chem. 1988;37:1069–1071. doi: 10.1021/jf00088a055. [DOI] [Google Scholar]
- Doores S. Organic acids, antimicrobials in foods. 2. New York: Marcel Dekker; 1993. pp. 95–136. [Google Scholar]
- EL-gazzar FE, Rusul G, Marth EH. Growth and aflatoxin production by Aspergillus parasiticus NRRL 2999 in the presence of lactic acid and at different initial pH values. J Food Protect. 1987;50:940–944. doi: 10.4315/0362-028X-50.11.940. [DOI] [PubMed] [Google Scholar]
- Fabi JP, Cordenunsi BR, Mattos Barreto GPD, Mercadante AZ, Lajolo FM, Nascimento JROD. Papaya fruit ripening: response to ethylene and 1-methylcyclopropene (1-MCP) J Agric Food Chem. 2007;55:6118–6123. doi: 10.1021/jf070903c. [DOI] [PubMed] [Google Scholar]
- Fagundes C, Palou L, Monteiro AR, Perez-Gago MB. Hydroxypropyl methylcellulose-bee wax edible coating formulated with antifungal food additives to reduce Alternaria black spot and maintain post harvest quality of cold-stored cherry tomatoes. Sci Hort. 2015;193:249–257. doi: 10.1016/j.scienta.2015.07.027. [DOI] [Google Scholar]
- Farzana B, Baloch MK. Postharvest quality and shelf life of mango (Mangifera indica L.) fruit as affected by various coatings. J Food Proc Preserv. 2014;38:499–507. doi: 10.1111/j.1745-4549.2012.00800.x. [DOI] [Google Scholar]
- Jawandha SK, Gupta N, Randhawa S. Effect of postharvest treatments on enzyme activity and quality of cold stored ber fruit. Not Sci Biol. 2012;4:86–89. doi: 10.15835/nsb448181. [DOI] [Google Scholar]
- Jawandha SK, Gill PPS, Annu V, Kaur N. Effect of coatings on storage quality of pear. Int J Sci Nat. 2014;5:703–706. [Google Scholar]
- Kaur S, Jawandha SK, Singh H. Response of baramasi lemon to various post-harvest treatments. Int J Agric Environ Biotechnol. 2014;7:895–902. doi: 10.5958/2230-732X.2014.01402.8. [DOI] [Google Scholar]
- Khan A, Shamrez B, Litaf U, Zeb A, Rehman Z, Naz R, Khan SH, Shah AS. Effect of sucrose solution and chemical preservatives on overall quality of strawberry fruit. J Food Process Technol. 2014;6:1–6. [Google Scholar]
- Maftoonazad N, Ramaswamy HS, Marcotte M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int J Food Sci Technol. 2008;43:951–957. doi: 10.1111/j.1365-2621.2006.01444.x. [DOI] [Google Scholar]
- Mahadevan A, Sridhar R. Methods in physiological plant pathology. Madras: Sivagami Publisher; 1998. [Google Scholar]
- Mahajan BVC. Biochemical and enzymatic changes in apple during cold storage. J Food Sci Technol. 1994;31:142–144. [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetables. 2. New Delhi: Tata McGraw-Hill publishing Company Limited; 2000. [Google Scholar]
- Serradell MDLA, Rozenfeld PA, Martínez GA, Civello PM, Chaves AR, Añón MC. Polyphenoloxidase activity from strawberry fruit (Fragaria × ananassa. Duch. cv. Selva): characterization and partial purification. J Sci Food Agric. 2000;80:1421–1427. doi: 10.1002/1097-0010(200007)80:9<1421::AID-JSFA649>3.0.CO;2-K. [DOI] [Google Scholar]
- Silvia A, Lluis PA, Río ADM, Perez-Gago ME. Performance of hydroxypropyl methylcellulose (HPMC)-lipid edible coatings with antifungal food additives during cold storage of ‘Clemenules’ mandarins. Food Sci Technol. 2011;44:2342–2348. [Google Scholar]
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extract using in vitro models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Srividya S, Reddy SS, Sudhavani P, Ramanjaneya V. Effect of post harvest chemicals on fruit physiology and shelf life of tomato under ambient conditions. Int J Agric Food Sci Technol. 2014;5:99–104. [Google Scholar]
- Talasila U, Vechalapu RR, Shaik KB. Storage stability of cashew apple juice-use of chemical preservative. J Food Technol. 2012;10:117–123. [Google Scholar]
- Venkatram A, Bhagwan A. Storage life improvement of custard apple (Annona squamosa L.) ‘Balanagar’ fruits by post harvest application of antioxidants. J Appl Hortic. 2013;15:1–5. [Google Scholar]
- Venkatram A, Bhagwan A, Thirupathi J, Kumar S. Effect of antioxidants and modified atmospheric packaging on physic-chemical characteristics of Balanagar custard apple (Annona squamosa L.) fruits. Environ Ecol. 2015;33:1513–1518. [Google Scholar]
- Wang CY, Baker JE. Vase life of cut flowers treated with rhizobittoxine analogs, sodium benzoate and isopentenyl adenosine. Hortic Sci. 1979;14:59–60. [Google Scholar]
- Zhang M, Xiao G, Lou G, Peng J, Salokhe VM. Effect of coating treatments on the extension of the shelf-life of minimally processed cucumber. Int Agrophys. 2004;18:97–102. [Google Scholar]
- Zhou R, Mo Y, Li Y, Zhao Y, Zhang G, Hu Y. Quality and internal characteristics of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biol Technol. 2008;49:171–179. doi: 10.1016/j.postharvbio.2007.12.004. [DOI] [Google Scholar]