Abstract
The phenolic compounds play an important role in production of quality grapes and wines. The current investigation focused on optimization of an extraction method for targeted analysis of 33 phenolic compounds in grapes by liquid chromatography tandem mass spectrometry (LC–MS/MS). The optimized method was successfully used for phenolic profiling of two wine grape varieties, Sauvignon blanc (white) and Shiraz (red) originated from Pune and Nasik regions of Maharashtra State, India. The optimized sample preparation procedure involved liquid–liquid extraction with acidified methanol by vortexing for 2 min followed by analysis on LC–MS/MS. The limit of quantification of the targeted compounds was in the range of 29 to 411 µg/L. The results indicated that skin of both varieties contained the highest amount of flavonols (69.47 ± 14.74 mg/kg in Sauvignon blanc and 129.47 ± 10.05 mg/kg in Shiraz) compared to pulp. The highest amounts of flavan-3-ols were present in grape seed collected from the Pune region (2016.84 ± 14.73 mg/kg in Sauvignon blanc and 1945.06 ± 32.69 mg/kg in Shiraz). The concentration of stilbenes was the highest in grape skin (0.13 ± 0.52 to 5.78 ± 5.45 mg/kg) compared to seed and pulp of both varities. Hydroxybenzoic acid (vanillin), hydroxycinnamic acid (p-coumaric acid) and anthocyanins (oenin, malvidin, cyanidin and kuromanin) were found only in Shiraz variety. The results of antioxidant activity (FRAP and DPPH assay) indicated the highest scavenging activity in seed (978.64 ± 56.23 to1133.38 ± 143.65 µMol TE/g DW FRAP and 594.93 ± 37.94 to 631.94 ± 56.45 µMol TE/g DW in DPPH). The phenolic contents in Sauvignon blanc and Shiraz grapes between Pune and Nasik regions did not have any significant difference.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03802-w) contains supplementary material, which is available to authorized users.
Keywords: Grape, Sauvignon blanc, Shiraz, Phenolic profile, LC–MS/MS, Antioxidant activity
Introduction
Phenolic compounds are phytochemicals that play a crucial role in the nutritional and sensory properties of fruits and vegetables. These are the secondary metabolites with diverse chemical structures and functions. In plants, the phenolic compounds do not participate directly in reproduction, fertility and growth, but play a protective role against various forms of environ-mental stress (Naczk and Shahidi 2004). Phenolic compounds can be classified into two major groups: non-flavonoids viz. hydroxybenzoic acids, hydroxycinnamic acids and stilbenes, and flavonoids viz. anthocyanins, flavan-3-ols and flavonols (Rodriguez et al. 2006). The reported literature suggests involvement of phenolic compounds in many positive health benefits such as protective effects against cancer and cardiovascular diseases (Arts and Hollman 2005). Such effects are attributed to their free radical scavenging activity or anti-oxidant property (Jayaprakasha et al. 2003; Rockenbach et al. 2011).
Phenolic compounds are abundant in both red and white wine varieties of grapes. Grape is an important component of human diet because of its nutritional value and various health benefits. The phenolic compounds are present in the skin, pulp and seed of grape berries and these compounds are responsible for colour, taste, mouth feel and oxidation status of wine (Juana et al. 2010). Phenolic compounds play important roles in defining the quality of grapes and wines. The non-flavonoid compounds are mostly present in pulp and the flavonoids are located in the skins, seeds, and stems (Monagas et al. 2005). Besides, phenolic compounds reported in grape seed, skin and stem extracts (Jayaprakasha et al. 2003) are also known to have antimicrobial property. Anthocyanins are directly responsible for red colour in grapes (Rolle and Guidoni 2007). Cultivation period, variety, environmental and climatic conditions, plant health, soil type, geographical region, and maturity stage are the factors that influence the concentration of phenolic compounds in grapes (Sellappan and Akoh 2002). Several regional climate models have been proposed in order to forecast the overall effects of individual or combined variables related to climate change on phenolic profile (Mira de Orduña 2010). Hence, investigation of phenolic profile of grape is important to assess the potential of grapes for various functional food applications.
The techniques of HPLC–UV (Tarola et al. 2007; Rodriguez et al. 2006, Dejan et al. 2010) and LC–MS/MS (Pang et al. 2016; Jaitz et al. 2010) have been commonly used for analysis of phenolic compounds. However, identification of compounds by HPLC–UV is challenging since many compounds show similar UV–Vis spectra. To overcome this problem, laborious sample preparation may be required, principally for pre-concentration of the desired compounds and removal of unwanted ones (e.g. sugars). On the other hand, LC–MS/MS offers the unique advantages of selectivity and sensitivity in detection which is based on compounds specific - multiple reaction monitoring transitions. Hence, liquid chromatography-tandem mass spectrometry (LC–MS/MS) technique has become a popular tool for the targeted identification of phenolic compounds. Previous researchers have reported a large number of phenolic compounds in grape berries and wines based on the analysis by LC–MS/MS (Jaitz et al. 2010). In the current study, a total of 33 phenolic compounds belonging to different classes were identified by LC–MS/MS in two varieties of grapes, namely Sauvignon blanc (white grape) and Shiraz (red grape) from Pune and Nasik regions of India. The goal was to find out the geographical and varietal influence on the profile of these phenolic compounds. Additionally, the phenolic composition of different anatomical parts of grape berries, viz. pulp, skin, seeds and whole fruit was comparatively evaluated along with measurement of their antioxidant activities.
Materials and methods
Chemicals
Certified reference srtandards (75–99% purity) of all 33 targeted phenolic compounds viz; malvidin, oenin, cyanidin, kaempferol-3-O- glucoside, quercetin, quercitrin hydrate, quercetin-3-O-glucoside, rutin hydrate, kaempferol, 3-hydroxyflavone, 7,4-dihydroxy flavone, (−)-epicatechin, (+)-catechin hydrate, procyanidin B1, B2 and C1, (−)-catechin gallate, (−)-epicatechin gallate, epigallocatechin, (−)-epigallocatechin gallate, caftaric acid, chlorogenic acid, p-coumaric acid, caffeic acid, vanillic acid, syringic acid, vanillin, ellagic acid, picetannol, resveratrol, pterostilbene and quinidine were purchased from Merck, Bangalore, India. For the antioxidant assays, chemicals including (±)-6-Hydroxy-2,5,7,8-tetramethyl-chromane-2-carboxylic acid (Trolox), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,4,6-Tris (2-pyridyl)-s-triazine (TPTZ), and solvents including gradient grade methanol (MeOH), acetonitrile (ACN), formic acid (FA) and hydrochloric acid were purchased from Merck India, Bangalore. The HPLC grade water (> 18 MΩcm) was prepared by a water purification system (Sartorius, Germany).
Apparatus
The apparatus used included a mixer of 1 L capacity (Bajaj India Pvt Ltd., Mumbai, India), precision balance (GR-202, A&D Company, India), homogenizer (Heidolph 900, Heidolph Instrument GmbH & KG, Germany), vortex mixer (Geni2T, Imperials biomedical, Mumbai, India), high speed refrigerated centrifuge (Kubota, Japan), microcentrifuge (Microfuge, Pico kendro D-37,520, Germany), incubated shaker (JEIO TECH, Korea), water bath (JEIO TECH, Korea) and a UV–Vis spectrophotometer (model no. 1700, Shimadzu Corporation, Japan).
Preparation of standard solutions
Accurately weighed 10 mg (± 0.1 mg) certified reference standard of each analyte was dissolved in 10 mL methanol. These were stored in dark vials in a refrigerator at - 20 °C. An intermediate stock of standard mixture (10 µg/mL) was prepared by mixing appropriate quantities of individual stock solutions followed by dilution in methanol. A working standard mixture of 1 µg/mL was prepared from the above stock by serial dilution in methanol. The calibration standards within the range of 0.05–1.00 µg/mL were prepared by serial dilution of the working standard in methanol–water with 0.1% formic acid (1:1, v/v).
Sample collection
Sauvignon blanc and Shiraz grapes (2 kg each) were collected from the vineyards located in Pune and Nasik region of Maharashtra state, India and stored at − 20 °C until analysis. Skin, seeds and pulp were separated, homogenized separately and then stored at − 20 °C for further study.
Sample size
Based on the previous reports, 10 (± 0.1) g and 5 (± 0.1) g of the homogenates from skin, pulp and whole berry of S. blanc and Shiraz grapes respectively were evaluated. For the analysis of seeds, 2 g homogenate of both varieties was chosen as the optimal sample size.
Optimization of extraction protocol for polyphenols
Selection of extraction solvent
Two different solvents with different compositions were evaluated for the extraction of polyphenols (Benmeziane et al. 2014). The solvents used in the experiment were: 100% acetonitrile, 100% methanol, 100% methanol with 1% formic acid, acetonitrile: methanol (50:50, v/v), acetonitrile: methanol (50:50, v/v) with 1% formic acid and acetonitrile: methanol (75:25, v/v). The grape homogenates (10 g for S. blanc and 5 g for Shiraz) were taken in 50 mL polypropylene tubes, to which 20 mL extraction solvent was added, vortexed for 2 min and centrifuged at 5000 rpm for 5 min. The organic extract (2 mL) was taken in a 2 mL tube and centrifuged at 10,000 rpm for 5 min after which the supernatant was separated and injected into LC–MS/MS for analysis. The performance of the extraction solvents was compared based on the number of phenolic compounds detected, relative response of the compounds in mass detector and % RSD values (n = 3).
Solvent ratio optimization
The optimized solvent combination was then used under different sample: solvent ratios, viz., 1:1, 1:2 and 1:3 (w/v) for S. blancand 1:1, 1:2, 1:5 and 1:10 (w/v) for Shiraz. The optimum sample: solvent ratio was selected based on the same criteria mentioned in the selection of extraction solvent.
Extraction time optimization
The optimized parameters of extraction solvent and sample:solvent ratio were used to evaluate the following various durations of extraction (in min): 2 min (vortexing) and the following durations of shaking 30, 60, 120, 180, 240, 300, 360 and 1440 min at 25 °C.
Optimized extraction procedure
For S. blanc variety, 10 (± 0.1) g of whole berry, skin and pulp homogenate was taken in separate 50 mL polypropylene tubes and was extracted with 20 mL of methanol with 1% formic acid. In case of Shiraz variety, 5 g each of homogenized whole grape berry, skin and pulp was taken in a 120 mL wide mouth plastic bottle and to it, 50 mL methanol with 1% formic acid was added and wrapped with aluminium foil. Each mixture was vortexed for 2 min and centrifuged at 5000 rpm for 5 min at room temperature. The organic phase (2 mL) was taken in an eppendorf tube and again centrifuged at 10,000 rpm for 5 min at room temperature. For LC–MS/MS analysis, the supernatant (0.5 mL) was transferred to a dark colour vial and diluted with 0.5 mL HPLC grade water before injection. For seeds, 2.0 (± 0.1) g of homogenized sample of both varieties were taken in 50 mL polypropylene tubes (wrapped in aluminium foil) and 12 mL methanol/water (50/50, v/v) containing 1% formic acid was added to it. Samples were prepared for further analysis in the same way as described above.
HPLC–MS/MS analysis
A high performance liquid chromatography (Waters 2695 separation module, Waters Corp., USA) hyphenated to a triple quadrupole (Quattro Premier, Waters Corp., Milford, MA, USA) mass spectrometer equipped with electrospray ionization (ESI) probe was used for polyphenol analysis. The extract (20 µL) was injected through an autosampler and chromatographic separation was performed on an Atlantis® T3 (100 × 3.0 mm, 5 µm) column, with a flow rate of 0.350 mL/min. The mobile phase was composed of methanol: water (10: 90, v/v) with 0.5% formic acid (Solvent A) and methanol: water (90:10, v/v) with 0.5% formic acid (Solvent B). The gradient flow of 0–0.5 min/10%B, 3.0 min/60%B, 7.0–13.0 min/98%B, 14.0–20.0 min/10% was applied for a total run time of 20 min. The column temperature was maintained at 25 °C. The ESI analysis was performed in positive polarity using multiple reaction monitoring (MRM) mode with a dwell time of 0.02 s. The other LC–MS/MS parameters included capillary voltage of 3.0 kV, source temperature 120 °C, desolvation temperature 500 °C, desolvation gas flow 900 L/h and cone gas 50 L/h.
The LOQs were determined based on the sensitivity wherein the quantifier MRM transition was evaluated at S/N of ≥ 10. The analyte dependent mass spectrometric parameters and retention time of each analyte are given in Table 1.
Table 1.
Optimized LC–MS/MS parameters for studied phenolic compounds
| Sr. No | Name of compound | RT (min) | Adduct | Quantitative transition | Cone (V) | CE | Confirmatory transition | CE | LOQ (µg/L) | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Quinidine | 4.56 | [M + H]+ | 325 > 307 | 20 | 20 | 325 > 184 325 > 253 | 30 | 411 | 0.98 |
| 2. | Caftaric acid | 4.62 | [M + H]+ | 313 > 165 | 20 | 15 | 313 > 135 | 25 | 147 | 0.99 |
| 3. | Epigallocatechin | 4.65 | [M + H]+ | 307 > 139 | 25 | 15 | 307 > 151 | 12 | 161 | 0.99 |
| 4. | (+)-Catechin hydrate | 4.78 | [M + H]+ | 291 > 165 | 38 | 17 | 291 > 139 291 > 123 | 22 | 173 | 0.98 |
| 5. | Procyanid in B1 | 4.78 | [M + H]+ | 579 > 457 | 20 | 20 | 579 > 427 579 > 305 | 25 | 59 | 0.99 |
| 6. | Procyanid in B2 | 4.78 | [M + H]+ | 579 > 457 | 20 | 20 | 579 > 427 579 > 305 | 25 | 81 | 0.98 |
| 7. | Procyanidin C1 | 4.91 | [M + H]+ | 867 > 849 | 25 | 10 | 867 > 715 867 > 593 | 25 | 69 | 0.99 |
| 8. | (−)-Epigallocatechin- gallate | 5.00 | [M + H]+ | 459 > 139 | 25 | 25 | 459 > 151 459 > 289 | 20 | 154 | 0.98 |
| 9. | (−)-Epicatechin gallate | 5.00 | [M + H]+ | 443 > 123 | 51 | 19 | 443 > 273 | 12 | 174 | 0.99 |
| 10. | Chlorogenic acid | 5.04 | [M + H]+ | 355 > 163 | 25 | 30 | 355 > 148 | 30 | 143 | 0.99 |
| 11. | Cyanidin | 5.04 | [M]+ | 287 > 137 | 20 | 35 | 287 > 109 | 40 | 51 | 0.99 |
| 12. | Kuromanin | 5.04 | [M]+ | 448 > 287 | 25 | 18 | 448 > 137 | 40 | 45 | 0.99 |
| 13. | (−)-Epicatechin | 5.22 | [M + H]+ | 291 > 165 | 38 | 17 | 291 > 139 291 > 123 | 22 | 31 | 0.99 |
| 14. | Malvidin | 5.29 | [M]+ | 331 > 315 | 25 | 26 | 331 > 299 | 30 | 30 | 0.99 |
| 15. | Oenin | 5.29 | [M + H]+ | 493 > 331 | 45 | 29 | 493 > 315 | 50, 55 | 40 | 0.99 |
| 493 > 287 | ||||||||||
| 16. | Vanillic acid | 5.42 | [M + H]+ | 169 > 125 | 25 | 14 | 169 > 93 | 21 | 190 | 0.99 |
| 17. | Caffeic acid | 5.43 | [M + H]+ | 181 > 135 | 16 | 15 | 181 > 165 | 27 | 150 | 0.99 |
| 18. | Syringic acid | 5.51 | [M + H]+ | 199 > 155 | 20 | 14 | 199 > 123 199 > 77 | 18, 38 | 150 | 0.99 |
| 19. | (−)-Catechin gallate | 5.52 | [M + H]+ | 442.99 > 139 | 25 | 15 | 443 > 122.9 | 20 | 40 | 0.99 |
| 20. | Vanillin | 5.71 | [M + H]+ | 152.1 > 125 | 20 | 10 | 152 > 93 | 20 | 168 | 0.99 |
| 21. | Piceatanol | 5.93 | [M + H]+ | 244.07 > 135 | 20 | 20 | 244 > 121 244 > 123 | 25 | 83 | 0.99 |
| 22. | Resveratrol | 6.28 | [M + H]+ | 229 > 135 | 29 | 22 | 229 > 107 229 > 119 | 30, 55 | 41 | 0.99 |
| 23. | Rutin hydrate | 6.38 | [M + H]+ | 611 > 303 | 25 | 20 | 611 > 465 611 > 85 | 29, 59 | 29 | 0.99 |
| 24. | Quercetin-3-O-glucoside | 6.45 | [M + H]+ | 464 > 303 | 20 | 15 | 464 > 153 | 30 | 171 | 0.99 |
| 25. | Quercitrin hydrate | 6.93 | [M + H]+ | 449 > 303 | 25 | 15 | 449 > 129 449 > 85 | 22, 35 | 54 | 0.99 |
| 26. | Ellagic acid | 6.94 | [M + H]+ | 303 > 257 | 30 | 40 | 303 > 229 303 > 201 | 45, 50 | 150 | 0.99 |
| 27. | Kaempferol-3-O-glucoside | 7.01 | [M + H]+ | 448.1 > 287 | 25 | 20 | 448.1 > 87 | 30 | 29 | 0.99 |
| 28. | p-Coumaricacid | 7.86 | [M + H]+ | 163 > 147 | 25 | 16 | 163 > 119 163 > 91 | 25, 35 | 78 | 0.99 |
| 29. | Quercetin | 7.89 | [M + H]+ | 303 > 153 | 55 | 45 | 303 > 137 303 > 69 | 45, 80 | 30 | 0.99 |
| 30. | 7,4 dihydroxy flavone | 8.03 | [M + H]+ | 255 > 118 | 40 | 35 | 255 > 137 | 30 | 47 | 0.99 |
| 31. | Kaempferol | 8.7 | [M + H]+ | 287 > 153 | 20 | 30 | 287 > 165 | 30 | 143 | 0.99 |
| 32. | Pterostilbene | 10.14 | [M + H]+ | 257 > 225 | 20 | 10 | 225 > 133 225 > 105 | 25 | 76 | 0.99 |
| 33. | 3-hydroxyflavone | 10.53 | [M + H]+ | 239 > 105 | 40 | 25 | 239 > 121 | 30 | 37 | 0.99 |
RT retention time, CV cone voltage, CE collision energy, LOQ limit of quantification, R2 correlation coefficient
Evaluation of antioxidant activity
The antioxidant activity of both the studied grape varieties was evaluated for Pune and Nasik regions. A homogenate of 5 g of whole grape berry, skin, pulp and seed was taken initially. Sauvignon blanc samples were extracted with 25 mL of 80% aqueous methanol (Bonilla et al. 2003), while Shiraz samples were extracted with 0.01% hydrochloric acid in 80% aqueous methanol with overnight shaking on an environmental shaker. All extracts were centrifuged at 12,000 rpm for 15 min at 4 °C and stored at − 20 °C till further analysis.The residues were re-extracted (twice, 3 h each) using 10 mL solvent under similar conditions for both varieties. The organic layer was separated and stored at − 20 °C till further analysis.
DPPH radical scavenging assay
The protocol described by Arnous et al. 2001 was used for the determination of free radical scavenging activity. The extract (100 μL) was mixed with 4000 μL of DPPH solution and incubated in dark for 30 min followed by measurement of absorbance at 515 nm. The suppression of absorbance of DPPH radical by sample antioxidants was compared with that of Trolox standard. The standard calibration curve was obtained using trolox in the concentration range of 300–700 µM (r2 = 0.99). The scavenging activity of DPPH radical in the sample was expressed as µMol trolox equivalent/g dry weight (TE).
Determination of reducing power (FRAP assay)
Total antioxidant activity was measured by Ferric Reducing Antioxidant Power (FRAP) assay (Benzie and Strain 1996). Each reaction mixture containing 100 μL of sample extract was added to 3000 μL of FRAP reagent [Acetate buffer (pH 3.6, 300 mM), 10 mM of TPTZ in 40 mM HCL and 20 mM iron(III) chloride in 10:1:1 proportion] and incubated in a water bath at 37 °C for 30 min. Absorbance was measured at 593 nm. The standard calibration curve was obtained from Trolox in the concentration range of 300–700 µM (r2 = 0.99). The sample results were expressed as µMol Trolox equivalent/g dry weight (TE).
Statistical analysis
The data was evaluated statistically using the Student’s t test for unequal variances, ANOVA- single factor and correlation analysis in SAS software, version 9.3.
Results and discussion
Extraction solvent
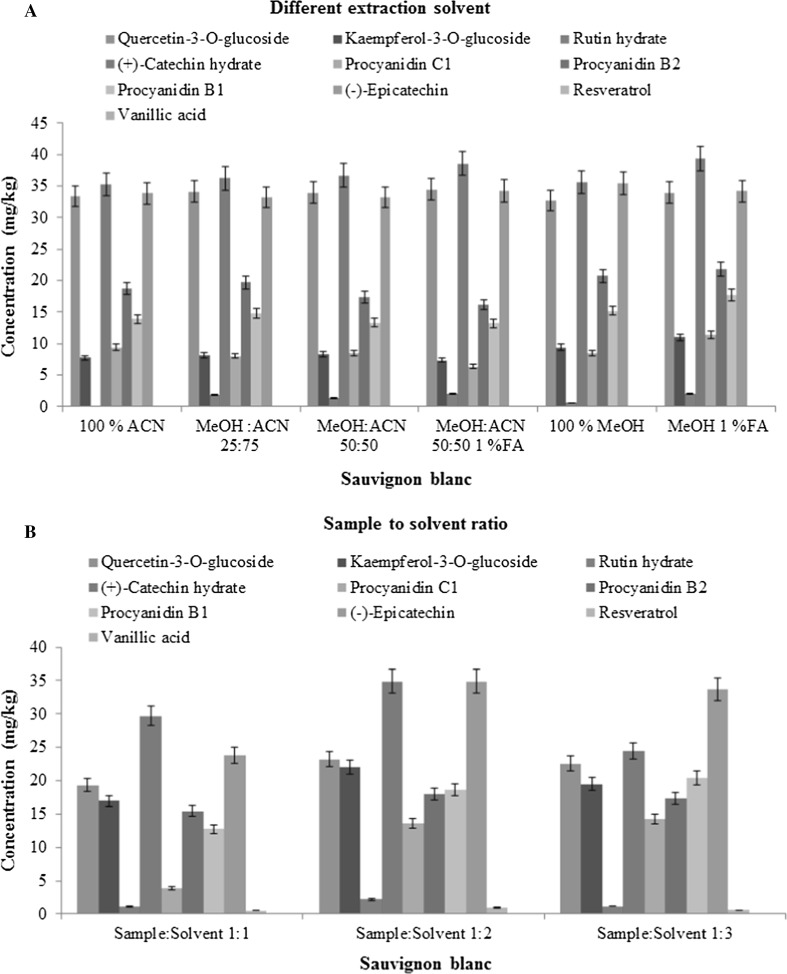
Phenolic compounds are polar in nature and hence a polar solvent is expected to provide higher extraction efficiency (Wissam et al. 2012). Previous literature has recommended use of methanol and water for effective extraction of polyphenols (Roobha et al. 2011; Juana et al. 2010, Zan-Min Jin et al. 2009). However, we avoided water in the extraction solvent considering the fact that the grape matrix inherently contains more than 70% moisture. When acetonitrile was used for extraction, the recovery of the phenolics was comparatively less. Addition of methanol in a proportion of 25% and 50% increased the extraction efficiency of certain compounds including kaempferol-3-O-glucoside (from 5.6 to 6.9%), (+)-catechin hydrate (from 2.5 to 3.8%) and resveratrol (from 34.3 to 41.8%) with satisfactory repeatability (RSD within 7.20 to 9.45%). An addition of 1% formic acid to methanol: acetonitrile (50:50, v/v) further improved the extraction efficiency of quercetin-3-O-glucoside by 3.2%, (−)-epicatechin by 1.2% and (+)-catechin hydrate by 8.5% in Sauvignon blanc. Similarly, in Shiraz, the extraction efficiency of rutin hydrate, (+)-catechin hydrate and procyanidin B1 increased by 53.4, 29.8 and 6.9%, respectively. However, when 100% methanol was used as the extraction solvent, the extraction efficiency of these compounds was significantly improved from 4.7 to 43.9% in S. blanc and 8.2 to 85.5% in Shiraz. An addition of 1% formic acid to methanol resulted in a further increase in the extraction efficiency of the studied compounds from 1.7 to 85.3%. The addition of acid to solvent enhanced the recovery of phenolic compounds probably due to the following two reasons; 1. the acidic condition led to denaturation of the cell membrane, increasing interactions between the solvent and the target compounds; 2. the free hydrogen ions (H+) led to stabilization of the flavylium cation in the structure of anthocyanins (Blackhall et al. 2018). Also, most of the previous studies have preferred the use of weak organic acids such as acetic acid or formic acid instead of any strong acid (such as HCl) since anthocyanins tend to degrade in presence of a strong acid (Salamon et al. 2015).
Statistical analysis revealed significant difference (p-value is < 0.05 and F > Fcritical) in the performance of various solvent combinations. Superior recoveries were recorded when 1% formic acid in methanol was used for extraction. For example, the concentrations of epicatechin with 1% formic acid in methanol was much higher as compared to acetonitrile (by 23%), MeOH:ACN 50:50 1%FA (by 18%), MeOH:ACN 50:50 (by 28%), MeOH:ACN 25:75 (by 19%) and methanol (by 18%). A similar trend was observed for other identified phenols. Thus, 1% formic acid in methanol was finally selected as the extraction solvent.
Sample to solvent ratio
In case of Sauvignon blanc, the extraction efficiency of phenolic compounds was improved when the sample: solvent ratio was set at 1:2 (Benmeziane et al. 2014). A further increase in sample: solvent ratio either decreased the extraction efficiency (by around 3 to 45.5%) for certain compounds (e.g. quercetin-3-O-glucoside, epicatechin, rutin hydrate or there was no significant improvement found for most of the compounds. Hence a ratio of 1:2 (sample: solvent) ratio was selected as optimum for the S. blanc variety. For Shiraz, the extraction efficiency was improved (from 0.50 to 81.76%) when the sample: solvent ratio was increased from 1:5 to 1:10 (e.g., oenin − 2.7%, quercetin-3-O-glucoside − 32.6%, (+)-catechin hydrates - 50.2% and resveratrol − 43.8% without affecting the extraction efficiency of the rest of the compounds. This is in accordance with Li et al. 2010. A further increase in sample: solvent ratio (> 1:10) affected the LOQ of certain compounds like kaempferol-3-O-glucoside due to dilution effect. Hence, 1:10 sample to solvent ratio was selected as optimum for Shiraz variety.
In case of seed of both Shiraz and Sauvignon blanc, a sample size of 2 g was selected with 12 mL methanol: water (50/50, v/v) containing formic acid (0.1/10, v/v) for extraction, and this provided stability to the phenolic compounds (Dejan et al. 2010). In all cases, the extent of homogenization was quite satisfactory with relative standard deviation (RSD) < 5% for each compound when analysed in triplicates. The extraction efficiency of the target compounds with regards to different solvents and sample to solvent ratio and are presented in Fig. 1a, b (for Sauvignon blanc) and Supplementary Fig. 1. A and Fig. 1. B (for Shiraz).
Fig. 1.
a Comparative evaluation of extraction different extraction solvents in terms of concentration of phenolic compounds in S. blanc grape. b Comparative evaluation of different sample to solvent ratios in terms of concentration of phenolic compounds in S. blanc grape
Statistically, a significant difference was recorded among various combinations of sample:solvent ratios as demonstrated by ANOVA analysis with the p-value < 0.05 and F > Fcrit. For example, in Shiraz, a higher concentration of malvidin was recorded in 1:10 sample:solvent ratio as compared to 1:1 (by 55%), 1:2 (by 49%), and 1:5 (by 1.4%). Similar values and pattern were observed for the other identified phenols and also in S. blanc grapes.
Optimization of extraction time
Extraction time played a vital role in providing satisfactory recoveries. For both S. blanc and Shiraz, vortexing for 2 min provided the highest recovery. Shaking was not effective. Shaking for even longer durations (30 to 1440 min) did not help much in improving the recoveries of the compounds (non-significant results, p-value > 0.05). This was in accordance with the observations of Hismath et al. 2011 and Uma et al. 2010. Since the extraction efficiency was better with higher throughput, a 2 min of vortexing was selected as the optimal extraction time.
Phenolic profiling of Sauvignon blanc and Shiraz varieties
Phenolic profiling of S. blanc and Shiraz from two different geographical locations, viz. Pune and Nasik was carried out in whole grape and different anatomical parts viz. skin, seed and pulp. The Total Ion Chromatogram (TIC) and Extracted Ion Chromatogram (XIC) of the phenolic standards mixture (1 mg/kg) in LC–MS/MS is given in Fig. 2. The results for S. blanc and Shiraz are presented in Table 2 and Table 3 respectively. The results for individual phenolic groups are discussed below.
Fig. 2.

TIC and XIC of phenolic standards (1 mg/kg) in LCMS/MS
Table 2.
Content of phenolic compounds (mg/kg) in anatomical parts of S. blanc grape from different growing region
| Analyte | Whole berry (Mean ± RSD) | Skin (Mean ± RSD) | Pulp (Mean ± RSD) | Seed (Mean ± RSD) | ||||
|---|---|---|---|---|---|---|---|---|
| Pune | Nasik | Pune | Nasik | Pune | Nasik | Pune | Nasik | |
| Flavonols | ||||||||
| Quercetin-3-O- glucoside | 39.33 ± 8.80 | 24.32 ± 12.38 | 41.37 ± 13.70 | 23.74 ± 0.61 | 4.58 ± 1.03 | 2.02 ± 0.69 | 2.55 ± 0.06 | 4.14 ± 0.17 |
| Kaempferol-3-O- glucoside | 10.76 ± 3.73 | 8.37 ± 2.18 | 22.45 ± 1.01 | 3.91 ± 3.78 | 2.77 ± 3.61 | 0.64 ± 0.89 | ND | ND |
| Rutin hydrate | 1.35 ± 0.10 | 2.50 ± 1.75 | 5.65 ± 0.03 | 2.71 ± 1.48 | 0.39 ± 0.36 | 0.46 ± 1.05 | ND | ND |
| SUM | 51.44 ± 12.63 | 35.19 ± 25.24 | 69.47 ± 14.74 | 30.36 ± 22.51 | 7.74 ± 31.02 | 3.12 ± 3.96 | 2.55 ± 0.06 | 4.14 ± 0.17 |
| Flavanol | ||||||||
| (+)-Catechin hydrate | 30.81 ± 2.46 | 32.63 ± 0.90 | 4.70 ± 0.44 | 4.65 ± 5.62 | ND | ND | 729.66 ± 3.59 | 163.23 ± 7.57 |
| (−)-Epicatechin | 28.93 ± 0.71 | 17.17 ± 1.81 | 1.08 ± 0.03 | 1.00 ± 0.15 | ND | ND | 502.08 ± 5.70 | 150.32 ± 5.50 |
| Procyanidin B1 | 20.67 ± 4.81 | 18.29 ± 0.60 | 1.37 ± 0.90 | 1.39 ± 1.28 | ND | 0.26 ± 2.65 | 380.09 ± 1.14 | 123.56 ± 5.48 |
| Procyanidin B2 | 14.07 ± 8.80 | 21.15 ± 1.66 | 2.08 ± 5.02 | 2.01 ± 4.71 | ND | 0.37 ± 1.86 | 286.53 ± 1.13 | 115.88 ± 3.26 |
| Procyanidin C1 | 5.67 ± 0.57 | 12.49 ± 3.14 | ND | 3.34 ± 2.71 | ND | ND | 118.48 ± 3.17 | 27.15 ± 1.44 |
| SUM | 100.15 ± 17.35 | 101.73 ± 8.11 | 9.23 ± 5.58 | 12.39 ± 14.47 | ND | 0.63 ± 4.51 | 2016.84 ± 14.73 | 580.14 ± 23.25 |
| Stilbene | ||||||||
| Resveratrol | 1.23 ± 3.13 | 1.52 ± 8.69 | 3.17 ± 4.19 | 2.40 ± 2.62 | ND | 0.13 ± 0.52 | 0.62 ± 0.89 | 0.20 ± 2.76 |
| Pterostilbene | 0.17 ± 0.52 | 0.15 ± 3.74 | 0.38 ± 3.40 | 0.16 ± 1.03 | ND | ND | ND | ND |
| SUM | 1.40 ± 3.65 | 1.67 ± 12.43 | 3.55 ± 7.59 | 2.56 ± 3.65 | ND | 0.13 ± 0.52 | 0.62 ± 0.89 | 0.20 ± 2.76 |
| Total | 152.99 ± 33.66 | 138.59 ± 45.78 | 82.25 ± 27.91 | 45.31 ± 23.99 | 7.74 ± 5.00 | 3.88 ± 7.63 | 2019.39 ± 15.68 | 584.34 ± 26.18 |
RSD relative standard deviation, ND not detected
Table 3.
Content of phenolic compounds (mg/kg) in anatomical parts of Shiraz grape from Pune and Nasik regions
| Analyte | Whole berry (Mean ± RSD) | Skin (Mean ± RSD) | Pulp (Mean ± RSD) | Seed (Mean ± RSD) | ||||
|---|---|---|---|---|---|---|---|---|
| Pune | Nasik | Pune | Nasik | Pune | Nasik | Pune | Nasik | |
| Flavonols | ||||||||
| Quercetin-3-O- glucoside | 25.06 ± 8.45 | 4.48 ± 7.63 | 45.16 ± 1.23 | 40.35 ± 6.40 | 5.07 ± 0.42 | 2.02 ± 0.86 | 1.96 ± 0.40 | 1.80 ± 3.35 |
| Kaempferol-3-O- glucoside | 9.76 ± 0.21 | 1.20 ± 0.14 | 16.17 ± 4.58 | 25.78 ± 2.59 | 2.97 ± 1.17 | 1.78 ± 0.32 | ND | ND |
| Quercetin | 1.33 ± 0.16 | 0.68 ± 0.37 | 5.31 ± 0.38 | 2.04 ± 0.02 | 0.98 ± 0.10 | 0.63 ± 0.15 | ND | ND |
| Rutin hydrate | 26.76 ± 7.17 | 19.41 ± 5.00 | 45.04 ± 2.21 | 61.29 ± 1.04 | 34.15 ± 0.03 | 40.31 ± 0.16 | ND | ND |
| SUM | 62.92 ± 15.99 | 25.79 ± 13.14 | 111.68 ± 8.4 | 129.47 ± 10.05 | 42.06 ± 1.72 | 44.77 ± 1.59 | 1.96 ± 0.40 | 1.80 ± 3.35 |
| Flavanol | ||||||||
| (+)-Catechin hydrate | 61.37 ± 0.87 | 15.77 ± 0.01 | 0.89 ± 0.16 | ND | ND | ND | 470.17 ± 3.70 | 193.68 ± 13.71 |
| (−)-Epicatechin | 72.08 ± 13.68 | 35.40 ± 0.92 | ND | ND | 0.73 ± 0.04 | 1.53 ± 0.41 | 610.62 ± 13.68 | 457.20 ± 7.50 |
| Procyanidin B1 | 68.19 ± 11.18 | 46.46 ± 12.60 | 1.45 ± 0.18 | 1.07 ± 0.12 | ND | 1.45 ± 0.19 | 407.94 ± 3.09 | 346.64 ± 0.20 |
| Procyanidin B2 | 51.69 ± 9.40 | 34.44 ± 2.73 | 1.64 ± 0.25 | 1.44 ± 0.12 | 0.76 ± 0.34 | 1.69 ± 0.31 | 345.87 ± 1.78 | 273.05 ± 0.31 |
| Procyanidin C1 | 43.76 ± 4.71 | 30.13 ± 6.50 | 14.12 ± 7.04 | 28.77 ± 13.86 | ND | 2.27 ± 0.33 | 110.46 ± 10.44 | 168.08 ± 4.32 |
| SUM | 297.09 ± 39.84 | 162.2 ± 22.76 | 18.10 ± 7.63 | 31.28 ± 14.10 | 1.49 ± 0.38 | 6.94 ± 1.24 | 1945.06 ± 32.69 | 1438.65 ± 26.04 |
| Stilbene | ||||||||
| Resveratrol | 1.22 ± 0.44 | 0.46 ± 0.48 | 5.45 ± 2.43 | 1.32 ± 0.76 | ND | 1.70 ± 0.37 | 0.72 ± 0.13 | 0.82 ± 0.17 |
| Pterostilbene | 0.16 ± 3.92 | ND | 0.33 ± 3.02 | 0.11 ± 4.58 | ND | ND | ND | ND |
| SUM | 1.38 ± 4.36 | 0.46 ± 0.48 | 5.78 ± 5.45 | 1.43 ± 5.34 | ND | 1.70 ± 0.37 | 0.72 ± 0.13 | 0.82 ± 0.17 |
| Hydroxybenzoic acid | ||||||||
| Vanillin | ND | ND | ND | ND | ND | ND | 0.39 ± 2.56 | 0.18 ± 3.68 |
| SUM | ND | ND | ND | ND | ND | ND | 0.39 ± 2.56 | 0.18 ± 3.68 |
| Hydroxycinnamic acid | ||||||||
| p-Coumaric acid | ND | 0.76 ± 0.23 | ND | 1.65 ± 0.35 | ND | ND | ND | ND |
| SUM | ND | 0.76 ± 0.23 | ND | 1.65 ± 0.35 | ND | ND | ND | ND |
| Anthocyanin | ||||||||
| Oenin | 107.14 ± 6.99 | 214.11 ± 0.43 | 237.05 ± 1.08 | 453.97 ± 2.99 | 31.61 ± 0.27 | 94.26 ± 1.25 | 2.11 ± 5.53 | 10.20 ± 0.21 |
| Cyanidin | 0.82 ± 1.22 | 0.63 ± 1.59 | 3.74 ± 2.69 | 1.46 ± 3.01 | 0.14 ± 2.74 | 0.20 ± 2.96 | 0.09 ± 3.81 | 0.25 ± 0.28 |
| Malvidin | 72.60 ± 0.08 | 152.72 ± 0.02 | 150.78 ± 0.06 | 274.51 ± 0.21 | 22.71 ± 0.09 | 74.37 ± 0.02 | 2.01 ± 0.04 | 9.17 ± 0.02 |
| Kuromanin | 1.02 ± 1.71 | 0.49 ± 3.70 | 3.17 ± 2.79 | 1.44 ± 1.39 | 0.13 ± 6.85 | 0.13 ± 0.05 | ND | ND |
| SUM | 181.58 ± 10.00 | 367.95 ± 5.74 | 394.74 ± 6.62 | 731.38 ± 7.6 | 54.59 ± 9.95 | 168.96 ± 4.28 | 4.21 ± 9.38 | 19.62 ± 0.51 |
| Total | 542.97 ± 70.19 | 557.16 ± 42.35 | 530.30 ± 28.1 | 895.21 ± 37.44 | 98.14 ± 12.05 | 222.37 ± 7.38 | 1952.35 ± 45.16 | 1461.07 ± 34.38 |
RSD relative standard deviation, ND not detected
Flavonols
In Sauvignon blanc, three flavonols, namely quercetin-3-O-glucoside (2.02 ± 0.69 to 41.37 ± 13.70 mg/kg), kaempferol-3-O-glucoside (0.64 ± 0.89 to 22.45 ± 1.01 mg/kg) and rutin hydrate (0.39 ± 0.36 to 5.65 ± 0.03 mg/kg) were detected in various anatomical parts.
In Shiraz, the identified flavonols included quercetin-3-O-glucoside (1.80 ± 3.35 to 45.16 ± 1.23 mg/kg), kaempferol-3-O-glucoside (1.20 ± 0.14 to 25.78 ± 2.59 mg/kg), rutin hydrate (19.41 ± 5.00 to 61.29 ± 1.04 mg/kg) and quercetin (0.63 ± 0.15 to 5.31 ± 0.38 mg/kg). The levels of flavonols were maximum in skin and negligible or non-detectable in seed. This result is in agreement with literature (Zan-Min Jin et al. 2009). The total flavonol content was also higher in the skin of both varieties, i.e. 69.47 ± 14.74 (in Sauvignon blanc) and 129.47 ± 10.05 mg/kg (in Shiraz).
Flavanols (Flavan-3-ols)
Five flavanols were identified in the seeds of both varieties, which included (+)-catechin hydrate, (−)-epicatechin, procyanidin B1, procyanidin B2 and procyanidin C1. Their concentrations are presented in Tables 2 and 3. The concentration of (−)-epicatechin in seeds of S. blanc (150.32 ± 5.50 mg/kg) was significantly different (p-value < 0.05) from Shiraz (457.20 ± 7.50 mg/kg) in Nasik region. However, a study conducted by Rodriguez et al. 2006 in Spain reported similar concentrations of epicatechin in the seeds of S. blanc (130 ± 68 mg/kg) and Shiraz (130 ± 34 mg/kg) grapes. This observation indicates influence of geographic locations on the concentration of polephenols across various grape varieties.
The levels of flavanols were negligible or non-detectable in pulp. The results are in agreement with literature where flavan-3-ols have been mentioned as the richest class of phenolics in grape with major accumulation in seeds (Teixeira et al. 2013).
Stilbenes
The stilbenes included resveratrol and pterostilbene. As expected, the concentration of these compounds was the highest in grape skin (Tables 2 and Table 3) since their biosynthesis occurs mainly in the skin at the mature stage and contents also vary across varieties (Gatto et al. 2008).
Hydroxybenzoic acid
In this group, only vanillin was detected in the seeds of Shiraz variety with values being 0.39 ± 2.56 mg/kg in Pune and 0.18 ± 3.68 mg/kg in Nashik.
Hydroxycinnamic acid
In this group, only p-coumaric acid was detected in whole grape berries (0.76 ± 0.23 mg/kg) and skin (1.65 ± 0.35 mg/kg) of Shiraz variety from Nasik region. Previous research also supported that hydroxycinnamates commonly accumulate in skin and flesh of white and red vinifera and non-vinifera varieties (Teixeira et al. 2013).
Anthocyanins
Four compounds of anthocyanin group were detected in Shiraz variety, viz. oenin, cyanidin,malvidin and kuromanin in the range of 2.11 to 453.97, 0.09 to 3.74, 2.01 to 274.51, and 0.13 to 3.17 mg/kg, respectively. As also observed in previous studies, their levels were found to be the highest in the skin (Braidot et al. 2008). As expected, no anthocyanin was detected in S. blanc grapes as it is a white variety (Heredia et al. 1998).The overall trend observed in our study for the concentrations of individual and total phenols in both grape varieties as well as locations followed the trend: seed > skin > pulp. Similar pattern was also reported by Bonilla et al. 2003 in muscadine grapes.
The content of individual and total phenols in the whole berry of Shiraz and S. blanc grapes in Pune and Nasik demonstrated no significant difference (p-value two tail > 0.05 and t Stat < t Critical two-tail) when compared statistically. Similar observation was noted for other anatomical parts such as skin, pulp and seed in both regions. This may be due to very similar climatic conditions in both regions.
Antioxidant activity analysis
FRAP assay
In Sauvignon blanc, the highest reducing power was measured in seed: 1133.38 ± 143.65 µMol TE/g DW and 922.89 ± 1.01 µMol TE/g DW in Nasik and Pune regions, respectively (Fig. 3). Skin and pulp showed 98.63–99.45% less reducing power compared to seed. This is in accordance with Guo et al. 2003 who also reported a higher FRAP value in seed as compared to skin and pulp in a study conducted in the grape varieties of China region.
Fig. 3.
Comparison of DPPH and FRAP antioxidant properties across Pune and Nasik regions in Shiraz and S. blanc grapes
In Shiraz, the highest reducing power was measured in seeds: 978.64 ± 56.23 µMol TE/g DW and 959.58 ± 84.45 µMol TE/g DW in Pune and Nasik regions, respectively. In this variety also, skin and pulp showed 83.25–95.32% less reducing power. The berries from Nasik (215.66 ± 7.95 µMol TE/g DW) showed more reducing power than Pune region (194.51 ± 25.87 45 µMol TE/g DW) (Fig. 3).
DPPH assay
The DPPH assay is usually considered more accurate because in this assay, the radical compound is more stable and does not have to be generated as it happens in other radical scavenging assays such as ABTS (Sanchez-Moreno 2002). The highest scavenging activity was observed in seed with nominal activity in skin and pulp, which is in agreement with the observations of Butkhupl et al. 2010 and Bonilla et al. 2003. In Sauvignon blanc, the scavenging activity of seed in both locations was similar, 630.6 ± 19.31 and 631.94 ± 56.45 µMol TE/g DW in Pune and Nasik regions, respectively. The whole berry also showed nearly the same activity, measured as 46.58 ± 1.00 µMol TE/g DW and 42.10 ± 0.59 µMol TE/g DW for Pune and Nasik regions, respectively (Fig. 3).
In case of Shiraz, the seeds of Pune region (594.93 ± 37.94 µMol TE/g DW) showed a better scavenging activity than Nasik (478.07 ± 49.48 µMol TE/g DW). However, grape berries of both regions had approximately the same activity, i.e. 114.72 ± 11.65 µMol TE/g DW and 108.27 ± 11 µMol TE/g DW in Pune and Nasik, respectively (Fig. 3).
Statistical analysis also proved that there is no significant difference in DPPH and FRAP values across Pune and Nasik regions (p-values > 0.05). A positive correlation (0.7 to 0.96) was also found between DPPH, FRAP and phenolic contents which depicts that the antioxidant activities are proportional to the phenolic contents.
Conclusion
The optimized LC–MS/MS method correctly identified all the target phenolic compounds in whole berry as well as its anatomical parts. The skin of both varieties contained the highest amount of flavonols and stilbenes compared to pulp and seed. Hydroxybenzoic acid, hydroxycinnamic acid and anthocyanins were predominant in Shiraz grapes, and their contents and antioxidant activities in Pune and Nasik regions did not have any significant difference. The same was also true for S. blanc grapes in both locations. The highest free radical scavenging activity was recorded in seeds followed by skin and pulp. We can also conclude that the concentration and distribution of phenolic compounds in berries vary across its anatomical parts, although the results may be largely similar across geographical locations of similar weather conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the funding supports from Indian Council for Agricultural Research (ICAR), New Delhi, India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arnous A, Makris DP, Kefalas P. Effect of principle polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem. 2001;49:5736–5742. doi: 10.1021/jf010827s. [DOI] [PubMed] [Google Scholar]
- Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies1-4. Am J ClinNutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Benmeziane F, Djamai R, Cadot Y, Seridi R. Optimization of extraction parameters of phenolic compounds from Algerian fresh table grapes (Vitisvinifera) Food Res Int. 2014;21:1025–1029. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Blackhall ML, Berry R, Davies NW, Walls JT. Optimized extraction of anthocyanins from Reid Fruits’ Prunusavium ‘Lapins’ cherries. Food Chem. 2018;256:280–285. doi: 10.1016/j.foodchem.2018.02.137. [DOI] [PubMed] [Google Scholar]
- Bonilla EP, Akoh CC, Sellapan S, Krewer G. Phenolics content an antioxidant capacity of Muscadine grapes. J Agric Food Chem. 2003;51:5497–5503. doi: 10.1021/jf030113c. [DOI] [PubMed] [Google Scholar]
- Braidot E, Zancani M, Petrussa E, Peresson C, Bertolini A, Patio S, Macrì F. Transport and accumulation of flavonoids in grapevine (Vitisvinifera L.) Plant Signal Behav. 2008;3:626–632. doi: 10.4161/psb.3.9.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkhupl L, Chowtivannakul S, Gaensakoo R, Prathepha P, Samappito S. Study of the phenolic composition of Shiraz red grape cultivar (Vitisvinfera L.) Cultivated in North-eastern Thailand and its antioxidant and antimicrobial activity. S Afr J Enol. 2010;31:89–98. [Google Scholar]
- Dejan G, Vele T, Milovan V, Ljubodrag V, Vlatka V, Slobodan M. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J Serb Chem Soc. 2010;75:1641–1652. doi: 10.2298/JSC100519131G. [DOI] [Google Scholar]
- Gatto P, Vrhovsek U, Muth J, Segala C, Romualdi C, Fontana P, Pruefer D, Stefanini M, Moser C, Mattivi F, Velasco R. Ripening and genotype control stilbene accumulation in healthy grapes. J Agric Food Chem. 2008;56:11773–11785. doi: 10.1021/jf8017707. [DOI] [PubMed] [Google Scholar]
- Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res. 2003;23:1719–1726. doi: 10.1016/j.nutres.2003.08.005. [DOI] [Google Scholar]
- Heredia FJ, Francia-Aricha EM, Rivas-Gonzalo JC, Vicario IM, Santos-Buelga C. Chromatic characterization of anthocyanin’s from red grapes - I. pH effect. Food Chem. 1998;63:491–498. doi: 10.1016/S0308-8146(98)00051-X. [DOI] [Google Scholar]
- Hismath I, Want Aida WM, Ho CW. Optimization of extraction conditions for phenolic compounds from neem (Azadirachtaindica) leaves. Int Food Res J. 2011;18:931–939. [Google Scholar]
- Jaitz L, Siegl K, Eder R, Rak G, Abranko L, Koellensperger G, Hann S. LC-MS/MS analysis of phenols for classification of red wine according to geographic origin, grape variety and vintage. Food Chem. 2010;122:366–372. doi: 10.1016/j.foodchem.2010.02.053. [DOI] [Google Scholar]
- Jayaprakasha GK, Selvi T, Sakariah KK. Antibacterial and antioxidant activities of grape (Vitisvnifera) seed extracts. Food Res Int. 2003;36:117–122. doi: 10.1016/S0963-9969(02)00116-3. [DOI] [Google Scholar]
- Jin Zan-Min, He Jian-Jun, Bi He-Qiong, Cui Xiang-Yun, Duan Chang-Qing. Phenolic compound profiles in berry skins from nine red wine grape cultivars in northwest china. Molecules. 2009;14:4922–4935. doi: 10.3390/molecules14124922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juana M, Francisco P, Pilar Z. Antioxidant activity and phenolic composition of organic and conventional grapes and wines. J Food Compos Anal. 2010;23:569–574. doi: 10.1016/j.jfca.2010.05.001. [DOI] [Google Scholar]
- Li Zheng, Pan Qiuhong, Cui Xiangyun, Duan Changqing. Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci Bio technol. 2010;19:1047–1053. [Google Scholar]
- Mira de Orduña R. Climate change associated effects on grape and wine quality and production. Food Res Int. 2010;43:1844–1855. doi: 10.1016/j.foodres.2010.05.001. [DOI] [Google Scholar]
- Monagas M, Bartolome B, Gomez-Cordoves C. Updated knowledge about the presence of phenolic compounds in wine. Crit Rev Food Sci Nutr. 2005;45:85–118. doi: 10.1080/10408690490911710. [DOI] [PubMed] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- Pang B, Zhu Y, Lu L, Gu F, Chen H. The applications and features of liquid chromatography-mass spectrometry in the analysis of traditional chinese medicine. Evid Based Complement Altern Med. 2016;2016:3837270. doi: 10.1155/2016/3837270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenbach II, Gonzaga LV, Rizelio VM, Goncalves A, Genovese MI, Fett R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitisvinifera and Vitislabrusca) pomace from Brazilian winemaking. Food Res Int. 2011;44:897–901. doi: 10.1016/j.foodres.2011.01.049. [DOI] [Google Scholar]
- Rodriguez MR, Romero Peces R, Chacon Vozmediano JL, MartinezGascuena J, Garcia Romero E. Phenolic compounds in skins and seeds of ten grape Vitisvinifera varieties grown in a warm climate. J Food Compos Anal. 2006;19:687–693. doi: 10.1016/j.jfca.2005.05.003. [DOI] [Google Scholar]
- Rolle L, Guidoni S. Color and anthocyanin evaluation of red winegrapes CIE L*, a*, b* parameters. J Int Sci Vigne Vin. 2007;41:193–201. [Google Scholar]
- Roobha JJ, Saravanakumar M, Aravindhan KM, Devi PS. The effect of light, temperature, pH on stability of anthocyanin pigments in Musa acuminata bract. Res Plant Biol. 2011;1:05–12. [Google Scholar]
- Salamon I, Mariychuk R, Grulova D. Optimal extraction of pure anthocyanins from fruits of Sambucusnigra. Acta Hortic. 2015;1061:73–78. doi: 10.17660/ActaHortic.2015.1061.6. [DOI] [Google Scholar]
- Sanchez-Moreno C. Review: methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002;8:121–137. doi: 10.1177/1082013202008003770. [DOI] [Google Scholar]
- Sellappan S, Akoh CC. Flavonoids and antioxidant capacity of Georgia-Grown vidalia onions. J Agric Food Chem. 2002;50:5338–5342. doi: 10.1021/jf020333a. [DOI] [PubMed] [Google Scholar]
- Tarola AM, Milano F, Giannetti V. Simultaneous determination of phenolic compounds in red wines by HPLC-UV. Anal Lett. 2007;40:2433–2445. doi: 10.1080/00032710701577666. [DOI] [Google Scholar]
- Teixeira A, Eiras-Dias J, Castellarin SD, Gerós H. Berry phenolics of grapevine under challenging environments. Int J Mol Sci. 2013;14:18711–18739. doi: 10.3390/ijms140918711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uma DB, Ho CW, Wan Aida WM. Optimization of extraction parameters of total phenolic compounds from henna (Lawsoniainermis) Leaves. Sains Malays. 2010;39:119–128. [Google Scholar]
- Wissam Z, Ghada B, Wassim A, Warid K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int J Pharm Pharm Sci. 2012;4:675–682. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.