Abstract
Polyaniline/ Sawdust /Poly Ethylene Glycol/ (PANi/SD/PEG) composite synthesized chemically is used as an adsorbent to remove hexavalent chromium from water. Adsorption experiments have been done in batch and continuous (column) mode. Some parameter such as pH, contact time, PANi/SD/PEG dose, isotherms in batch mode and pH, column bed depth and fluid flow rate in column mode were investigated. Result shows that PANi/SD/PEG has a good performance to remove hexavalent chromium ion from aqueous media. By presence of PEG, prepared composite has been homogenized and further absorption has been occurred. The best adsorption occurs under pH 2 and optimum contact time for removal of hexavalent chromium ion in batch experiment was about 30 min. Adsorption of Cr (VI) by PANi/SD/PEG fitted well in Langmuir isotherms. Maximum adsorption of hexavalent chromium was calculated 3.2 (mg/g). In column experiments, pH and column bed depth were found to be more prominent than fluid flow rate. Though, about 22% of Cr (VI) can be recovered using 0.1 M NaOH in the batch system, the recovered Cr (VI) in column system was less than 7.9%.
Keywords: Chromium, Adsorption, Polyaniline, Composite, Wood sawdust
Introduction
Growing industrial development and population explosion in the world resulted in an increasing amount of different pollutants daily discharged into environment. Some pollutant, such as heavy metals, even at low concentrations, are harmful to human health. Chromium is not naturally found in water; however, but it enters to environment as chromate and dichromate through industrial pollution caused by effluents from metal plating, tanning, dye making, and stereotyping workshops and by oil varnish and ink [1]. Chromium ions can have various valence states: Cr(II), Cr(III), Cr(V), and Cr(VI), with Cr(III) and Cr(VI) being the most stable [2]. Chromium can cause special hazards because it accumulates in most organs. It seems that plants do not need chromium and excessive amounts of it are toxic to them [3]. Chromate dissolved in water can disrupt wastewater treatment processes. Dichromate, which is a strong oxidizing agent and hence a carcinogen, can be absorbed into the skin. If this happens, it will damage enzymes and biological systems [4]. According to world health organization the maximum allowable concentration of Cr (VI) is 0.05 mg/L for drink water. Various methods have been developed to remove chromium from aqueous solutions such as chemical precipitation. Ion exchange, coagulation, reverse osmosis and adsorption [5–17]. Each of these methods has its own advantages and disadvantages [18]. All these methods are somehow efficient and effective in removing heavy metals, but chemical precipitation method hase been employed more extensively because this method is easier and less expensive. However, chemical precipitation method produce sludge and are not very effective at low chromium concentration. Many researchers have shown interest in the adsorption method of removing heavy metals at low concentrations because it does not produce sludge and can remove metallic ions having low concentrations [19]. One of the disadvantages of the adsorption method is its high cost. Therefore, low-cost materials have been utilized as adsorbents of heavy metals [20–23]. Recently, new adsorbents have been used for chromium removal purposes such as nano-particle resin lewatit [24], lewatit fo36 nano ion exchange resin [25], nano carbon-onions [26], waste tire [27], modified carbon foam [28], biogenic iron based nanoparticles [29], reticulated chitosan [30], surface modified composite nanofibers [31], natural biomaterials [32], iron activated Carbon produced from coconut shell [33], Bagasse Ashes [34], activated Carbon produced from rice busk [35], distillery sludge [36], polymers such as polypyrrole [37–40] and polyaniline [41]. Polyaniline (PANi) is one of the most important conductive polymers. Polyaniline is synthesized very simple and inexpensive in the form of powder and film and used in many applications including supercapacitors [42], biosensors [43], electrochemical sensors [44], electrochemical energy storage [45, 46], biomedical application [47], photocatalytic application [48], and microwave absorption [49]. Polyaniline has shown good potential for removing heavy metals from water and wastewater due to its high content of amine group [50]. The various kinds of polyaniline have been tested for the removal of some heavy metals [51, 52]. Reduction of hexavalent chromium from water has also been studied using conductive polymer films [53–55]. The solvent type in which the polyaniline is synthesized and the types of oxidizing agent in the polymerization stage have shown an important role in increasing the removal capacity of polyaniline [56, 57]. The new researches show that synthesis of polyaniline attended by some other polymers increase polyaniline capacity for heavy metals removal. For example Removal of heavy metals from aqueous solution using polyaniline and polyaniline/ferricyanide [58], polyaniline/graphene oxide [59], polyaniline/silica gel [60], polyaniline/polyvinyl alcohol [61, 62], polyaniline/polystyrene [63, 64], polyaniline–poly ethylene glycol [65] have been reported and all have mentioned a significant improvement of removal efficiency. The chemically synthesized polyaniline naturally exists in powder shape and is unable to be used in column system. The conducting polymers such as Polyaniline can be easily coated on the surface of some solids such as cloth, glass, paper and sawdust [66–68]. The coating of polyaniline on the stable solids was reported a good methods for solving the mentioned problem [69–72]. Among these, sawdust has been used a lot to coating polyaniline [73–75].
There is evidence that synthesis of polyaniline attended by some other polymers such as poly ethylene glycol (PEG) exerts a marked effect on the surface and morphology properties of the synthesized polymers [65]. It, therefore, appears that entering some polymers such as PEG into the structure of polyaniline could produce effect on capacity of chromium uptake by PANi due to a change in molar mass of the polymer and its electrochemical properties [65]. At the other hand by coating polyaniline/poly ethylene glycol on wood saw dust, prepared composite was able to use in both bath and column system and recycling the polymer after treatment become simple In this study, the composite of polyaniline with poly ethylene glycol and wood sawdust (PANi/SD/PEG) was chemically synthesized and tested as an adsorbent for removal of toxic hexavalent chromium from aqueous solutions in batch and column modes. Then the effect of some parameters such as contact time of PANi/SD/PEG and solution, pH of chromium solution, PANi/SD/PEG dose, fluid flow rate, column bed depth and adsorption isotherms were tested.
Materials and methods
Materials and instrumentation
Sawdust (30–50 mesh size), Sulfuric acid with a degree of purity between 95 to 97%, Ammonium persulfate (APS), potassium dichromate, aniline monomer and poly ethylene glycol (Mw = 35,000) were all provided from Merck company (Germany).
The pH meter (CH 9101-Herisau, Metrohm, Switzerland), Fourier transform infrared (FT-IR) spectrometer (VERTEX 70, Brucker, Germany) digital scale (BP 211 D, Sartorius,Germany), Scanning electron microscope (LEO 440i, USA), magnetic mixer (MK 20, Helmer, Germany), and a glass column (1 cm*50 cm) were used for experiments. UV-Visible Spectrophotometer (Cary 300, Varian, USA) is used for measuring Hexavalent chromium concentration. Cr (VI) concentrations were determined by employing a spectrophotometer at pH values higher than 12 (adjusted by using 0.2 M NaOH) and at λmax = 375. To draw tables and curves, Excel and Tecplot software packages were used.
Chemical synthesis
To prepare PANi/SD/PEG, first 1 g of APS as an initiator of oxidative polymerization was dissolved in 100 mL of 1 M H2SO4 in the presence 0.2 g PEG, then 1 g of sawdust was added to solution and after 30 min, 1 mL aniline monomer was added to stirred solution. The polymerization was occurred for 3 h. After polymerization, PANi/SD/PEG was washed with distilled water used after it dried [65].
Removal of chromium
For Batch experiments PANi/SD/PEG composite added to 50 mL of hexavalent chromium solution and mixed by using a magnetic stirrer with speed of 300 rpm. After mixing time, the solutions were filtered and filtrate was used to measure chromium concentration. After removal experiments the efficiency of removal has been calculated from Eq. (1),
| 1 |
Where, C0 is the initial concentration of chromium (mg/L) and Ce is the final concentration of chromium (mg/L) after removal experiment. Some parameter such as pH (2–7), contact time (2–90 min), PANi/SD/PEG dose (1–40 g/L) and isotherms were investigated in batch mode.
A 1 cm diameter glass column was used for column adsorption experiments. After passing Cr (VI) through the column, treated solution samples were collected and used to measure hexavalent chromium concentration. The pH (2,5,7), column bed depth (10, 20 cm) and fluid rate flow(2,5 ml/min) in column mode were investigated.
Results and discussion
Batch experiments
Removal of Cr (VI) using prepared polymers
According the results (Fig. 1) the PANi/SD/PEG composite has a good performance on the removal of Cr (VI) from aqueous media As shown using PANi/SD/PEG composite, removal percentage was about six times higher compared to employing only sawdust. Coating of PANi/PEG; on saw dust has a noticeable effect on the adsorptive properties of saw dust. SEM micrographs of SD, PANi/SD and PANi/SD/PEG composite are shown in Fig. 2. SEM micrographs of SD, PANi/SD and PANi/SD/PEG composite are shown in Fig. 2. In the PANi/SD, distribution of PANi particles is scattered and needle-shaped (0.01 μ diameter, 3 μ length), but in the PANi/SD/PEG, the polymer coating is uniformly and have sharp scale-like surfaces. With the presence of PEG, prepared composite has been homogenized and further absorption has been occurred.
Fig. 1.
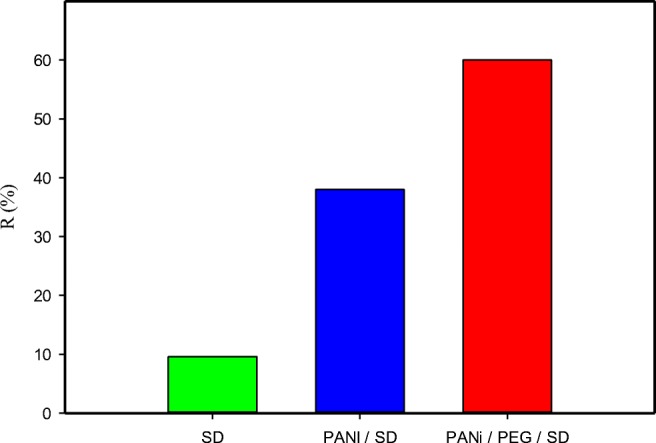
Removal percentage of Cr(VI) (sorbent dose 10 g/L; initial Cr(VI) 50 ppm; pH 5 agitation time 30 min) using SD, PANi/SD and PANi/SD/PEG
Fig. 2.
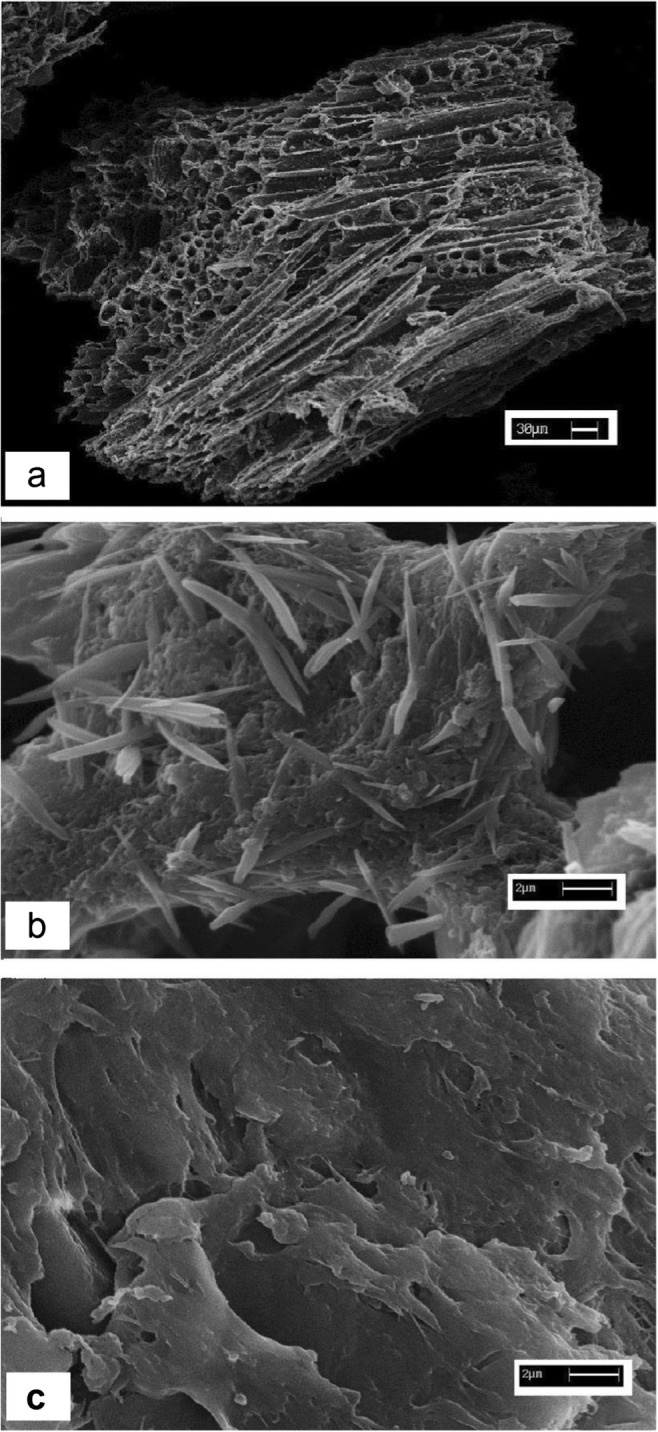
SEM micrograph of: a: saw dust (×150), b: Polyaniline/ saw dust (×5000), c: Polyaniline/ saw dust/poly ethylene glycol (×5000)
In previous research, it was stated that sawdust adsorbed chromium [75]. The mechanism of chromium removal by the polyaniline and sawdust composite was also said to be a combination of adsorption and reduction [75]. PANi/SD/PEG composite was able to desirably remove chromium from aqueous environments. This happened due to the presence of nitrogen in prepared composite. Nitrogen compounds can attract metals because they have free electrons in their last orbit. Nitrogen in amine groups is able to attract metals due to the presence of electrons in its S2P3 orbit. In acidic environments also nitrogen is protonated and electrostatic attraction takes place.
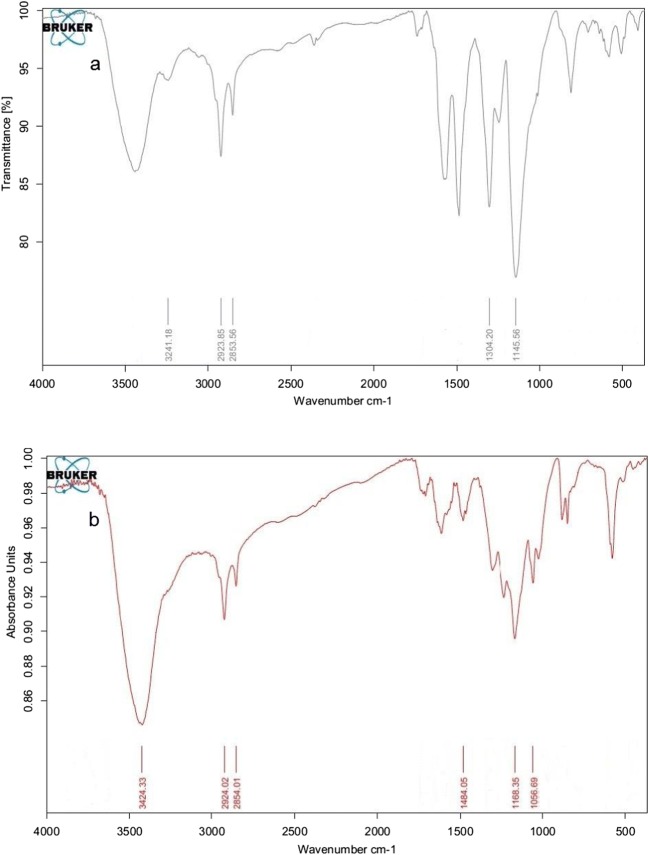
For PANi/SD Fig. 3a 1578 peak represents a carbon-carbon double bond in the quinoid ring, 1145 and 2923 peaks are respectively related to carbon-nitrogen and carbon-hydrogen bonds. For PANi/SD/PEG Fig. 3b 1458 peak represents the double carbon-carbon bond of benzene ring. 1168 and 2924 peaks are respectively related to carbon-nitrogen and carbon-hydrogen bonds. As seen in Fig. 3 the PEG changed FTIR spectrum of prepared composite. For PANi/PSD/PEG the peaks moved slightly when the PEG has been added which is due to the penetration of PEG to the polymer structure and the interaction effects on the energy of synthesized composite bonds.
Fig. 3.
FTIR spectra of: a PANi/SD and (b) PANi/SD/PEG
Effect of pH
Numerous studies have shown that the initial pH value of a solution is the most important parameter in removing heavy metals by adsorbents. This parameter influences both the metal dissolved in the solution and the adsorption sites. Chromium ions have different forms at various pH values. Cr+6 appear as Cr2O72 at pH values below 4, mostly as Cr2O72− and HCrO4− at pH values 4–8, and mainly as CrO42− at pH values above 8. In this research, the effect of pH on adsorption of Cr (VI) by PANi /PEG/SD was tested to find the best pH for chromium removal. As shown in Fig. 4, The best pH values for chromium removal by this adsorbent were acidic pH values, especially below 2. At this pH value, the nitrogen atoms in this adsorbent are protonated and attract chromate and dichromate anions.
Fig. 4.
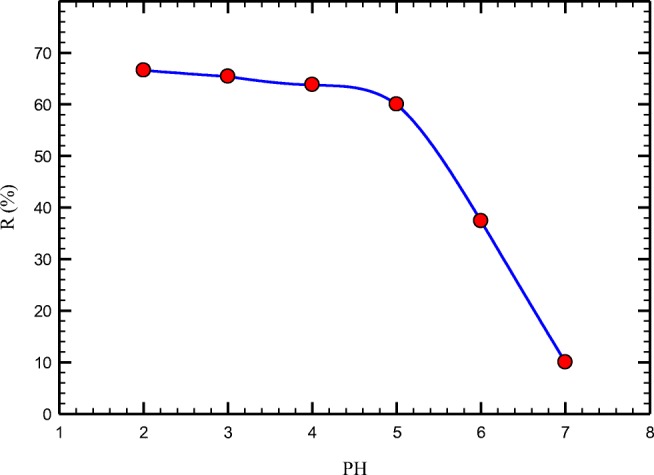
Effect of pH on hexavalent chromium removal (PANi/SD/PEG dose 10 g/L; initial Cr (VI) 50 ppm; agitation time 30 min)
Effect of contact time
Equilibrium time is another important parameter in adsorption process. Usually, with increase of agitation time removal efficiency increases until equilibrium is obtained. In this study, 0.5 g of PANi/PEG/SD was exposed to r(VI) solution for different equilibration times (2–90 min). As results show (Fig. 5), majority of Cr (VI) removal occurred within 30 min. With increase of the exposure time after 30 min, increase of removal efficiency is insignificant.
Fig. 5.
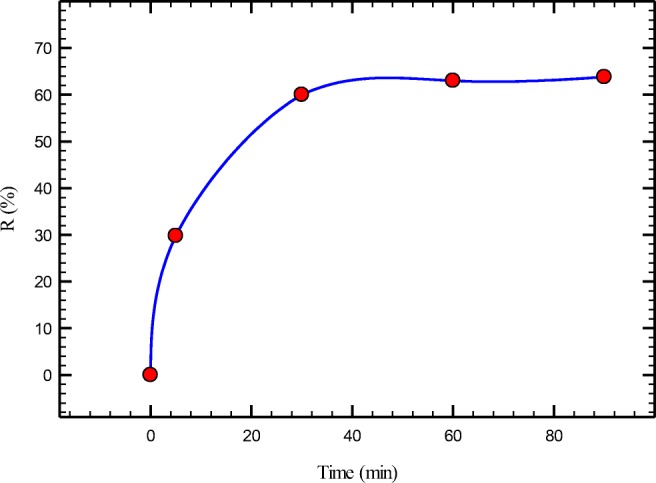
Effect of contact time on chromium removal (PANI/PEG/SD dose 10 g/L; initial Cr(VI) 50 ppm; pH 5)
Effect of PANi/SD/PEG dose
Effect of different dosage of PANi/SD/PEG on the removal of Cr (VI) was investigated. For this purpose 0.25–4 g of PANi/SD/PEG) were mixed to 50 mL of Cr (VI) solution. The results show that (Fig. 6) the Cr (VI) removal efficiency is increased by PANi/SD/PEG dose from 0 to 4 g. Using 40 g/L PANi/SD/PEG, about 98% chromium removal occur.
Fig. 6.
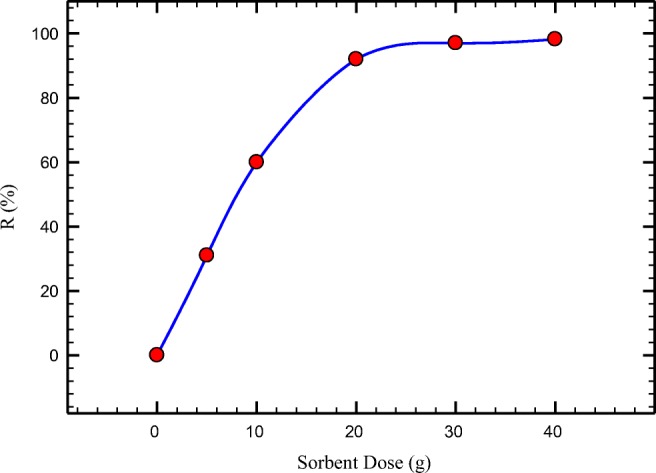
Effect of PANi/SD/PEG dose on chromium removal (initial Cr(VI) 50 ppm; pH 5, agitation time 30 min)
Adsorption isotherms
In order to model the adsorption mechanism, adsorption isotherms were studied at room temperature. Different isotherms such as Langmuir, Freundlich and BET which all express surface adsorption process. In this study the Langmuir and Freundlich equations were tested to find the most suitable isotherm model. The Freundlich and Langmuir equations are defined as Eqs. 2 and 3, respectively.
| 2 |
| 3 |
Where, x is the amount adsorbed (mg), m is weight of adsorbent (gr), Ce is the final concentration of chromium in solution (mg/L), X is the amount adsorbed by sorbent (mg/g) and Xm is the maximum amount adsorbed by adsorbent (mg/g. The plots of log (x/m) versus log (Ce) and 1/X versus 1/Ce are used to find the constants of isotherms [65].
Different amounts of PANi/SD/PEG (0.25–4 g) were mixed to 50 mL of 50 ppm Cr (VI) solution. As shown in Figs. 7 and 8, adsorption of Cr (VI) by PANi/SD/PEG can be fitted well in Langmuir isotherms. For suitable adsorbents, constant n in Freundlich isotherm is more than 2. The n constant was calculated about 4 for PANi/SD/PEG. For PANi/SD/PEG composite maximum adsorption of hexavalent chromium was calculated 3.2 (mg/g).
Fig. 7.
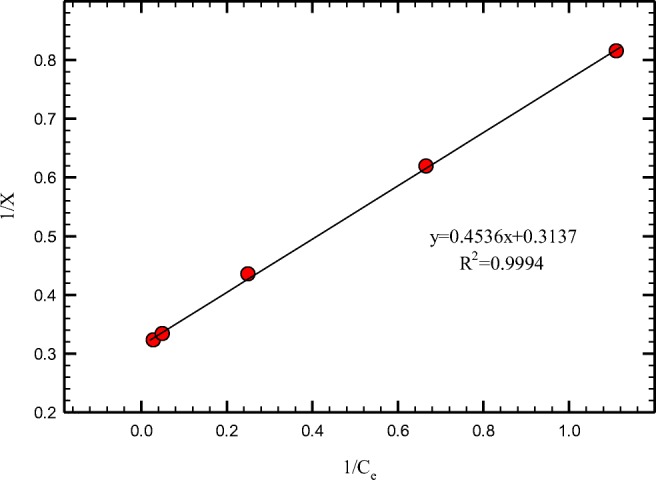
Adsorption isotherm of chromium using Langmuir isotherms
Fig. 8.
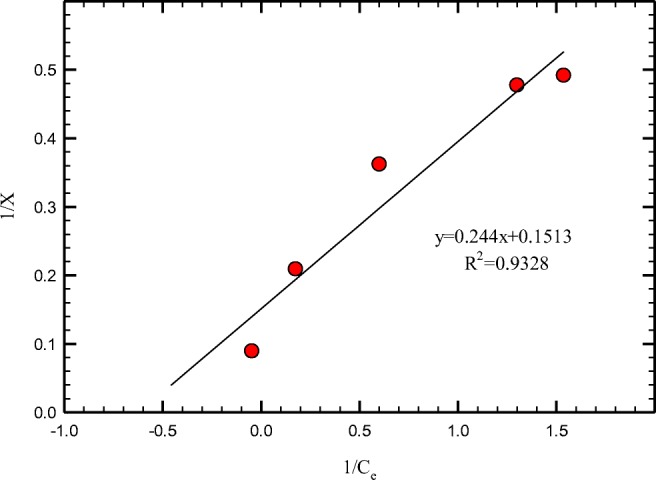
Adsorption isotherm of chromium using Freundlich isotherms
Desorption and recovery of adsorbed chromium
In this study, 50 mL Cr(VI) 50 ppm was treated with 0.5 g PANi/PEG/SD for 30 min with pH 5. Chromium containing PANi/PEG/SD was transferred to 50 mL of 0.2 M NaOH and stirred for 60 min. During desorption, 22.33% of chromium was released into solution. The reused PANi/PEG/SD was washed with deionized water to obtain neutral pH and was used again for adsorption experiments after it dried. Result shows that the used adsorbent can be reused for Cr (VI) efficient removal with 8% loss of its capacity even after its partial regeneration.
In Table 1, the performance of the PANi/SD/PEG is compared with some adsorbents. According to table, PANi/SD/PEG has a acceptable performance to remove chromium from aqueous solutions.
Table 1.
Comparison of different absorbent performance in chromium removal
| Adsorbent | Contact time (min.) |
Optimum pH | Maximum adsorpyion of chromium mg/g | Reference |
|---|---|---|---|---|
| Poly aniline poly ethylene glycol coated on saw dust | 30 | 2 | 3.2 | This research |
| Polyaniline-Poly ethylen glycol composite | 30 | 4 | 68.97 | Riahi [65] |
| Polyaniline synthesized on jute fiber | 50 | 3 | 62.9 | Kumar [67] |
| Polypyrrole coated on sawdust | 45 | 1–11 | 3.4 | Ansari [75] |
| Saw dust | 30 | 2 | 1.9 | Ansari [75] |
| Benetonite | 10 | 2 | .57 | Khan [39] |
| Activated Carbon produced from coconut shell | 30 | 6 | 2.18 | Babel [33] |
| Bagasse Ashes | 150 | 1 | 260 | Gupta [34] |
| Activated Carbon produced from rice busk | 45 | 2 | 456 | Srivastava [35] |
| Distillery sludge | 90 | 3 | 5.7 | Selvarg [36] |
Column experiments
Since most water and wastewater treatment facilities employ adsorption columns, performance of sawdust covered polyaniline and polyethylene glycol composite must also be studied in adsorption columns. If polyaniline and polyethylene glycol composite powder is used, a substantial pressure drop happens in the system.
Removal of Cr (VI) from solution in column systems
In this study, the column (as described before) was packed with 1.7 g of adsorbent (PANi/SD/PEG, SD). The 500 mL of 50 ppm Cr (VI) solution with 5 mL/min flow rate was passed through the column. According to Fig. 9, for PANi/SD/PEG the breakthrough curve is very extensive. The breakthrough (Ce = 2.5 mg/L) and exhaustion(Ce = 47.5 mg/L) occur in 10 mL and 45 mL, respectively for SD but this event occurs in 40 and 400 mL for PANi/SD/PEG. According to results, about 80 mL of 50 ppm Cr (VI) solution can be treated by 1.7 g PANi/SD/PEG in column system.
Fig. 9.
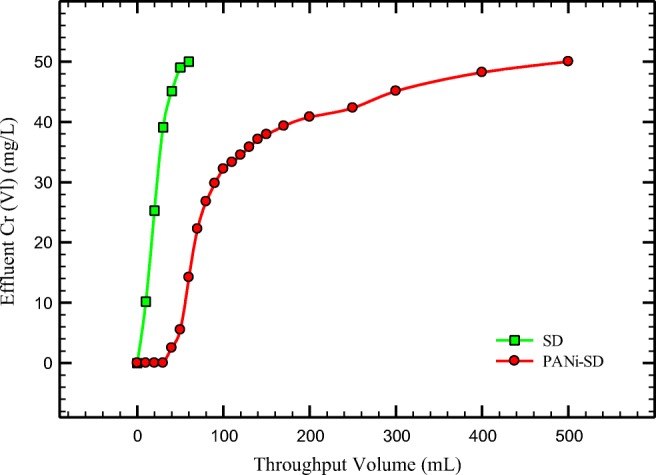
Breakthrough curve obtained for SD and PANi/SD/PEG (sorbent weight 1.7 g; initial Cr (VI) 50 ppm; Flow rate 5 mL/min; pH = 5)
Effect of influent pH
As our result shows (Fig. 10), adsorption of Cr (VI) ion increased as pH of solutions decreased. The most breakthrough and exhaustion point occurred at pH = 2. In this condition, breakthrough and exhaustion were calculated 75 mL and 1700 mL, respectively. The pH of solution has an important effect on Cr (VI) removal by PANi/SD/PEG in column systems.
Fig. 10.
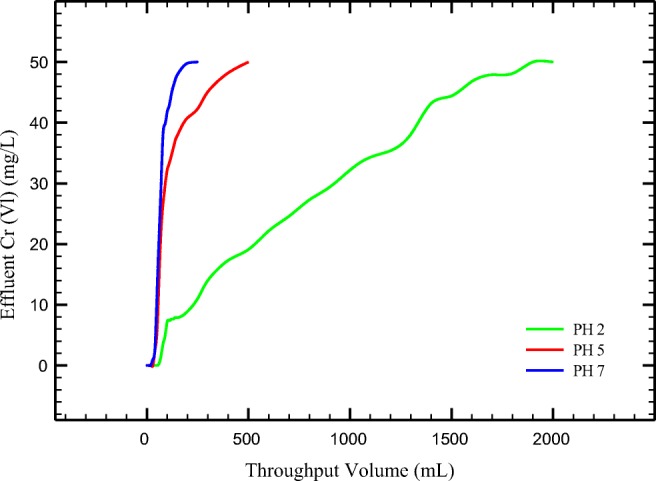
Breakthrough curve obtained for PANi/SD/PEG at various pH (sorbent weight 1.7 g; initial Cr (VI) 50 ppm; Flow rate 5 mL/min)
Effect of column bed depth
To study the effect of bed depth, experiments were done with column filled with 1.7 (about 10 cm) and 3.4 g (about 20 cm) PANi/SD/PEG, respectively. Then 50 ppm Cr (VI) solution was passed through the column with flow rate of 5 mL/min. As results show (Fig. 11) the breakthrough and exhaustion were calculated 40 and 225 mL for 10 cm PANi/SD/PEG bed depth, respectively. This event occurred in 110, 1600 mL for 20 cm bed depth, respectively. The bed depth has a significant effect on adsorption of Cr (VI) using PANi/SD/PEG in column systems.
Fig. 11.
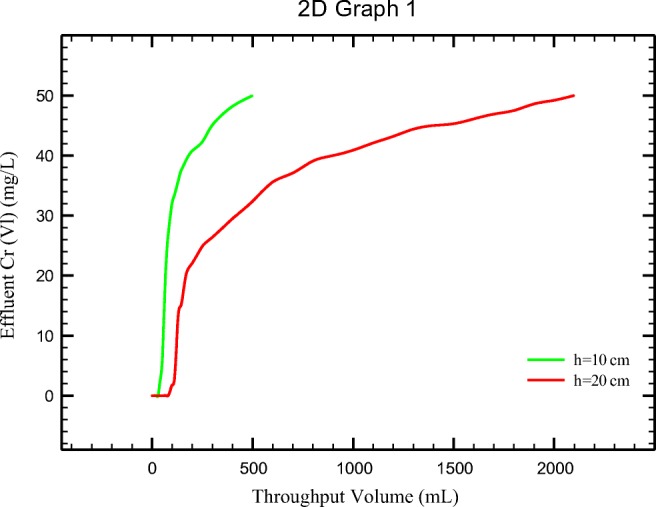
Breakthrough curve obtained for PANi/SD/PEG at various bed depths (initial Cr (VI) 50 ppm; Flow rate 5 mL/min; pH = 5)
Effect of fluid flow rate
According to Fig. 12, adsorption of Cr (VI) ion increased as flow rate decreased. The breakthrough and exhaustion occurred in 40 mL and 400 mL, respectively for flow rate of 5 mL/min but this occurred in 45and 650 mL for flow rate of 2 mL/min.
Fig. 12.
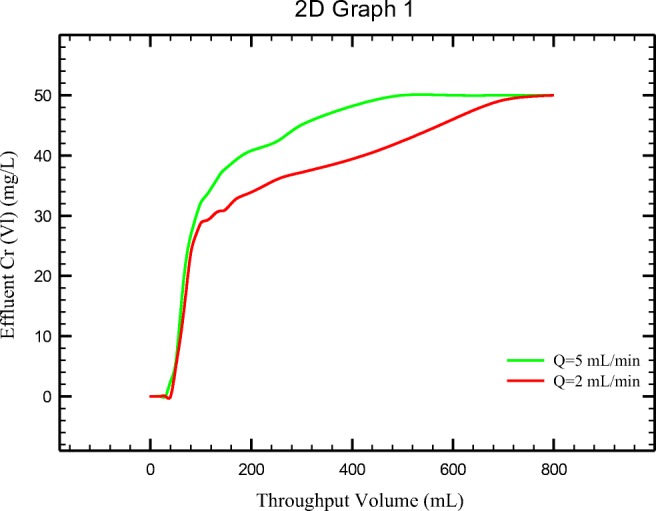
Breakthrough curve obtained for PANi/SD/PEG at various influent flow rate (sorbent weight 1.7 g; initial Cr (VI) 50 ppm; pH = 5)
Desorption and recoveru
Two hundred and fifty ml of chromium (at 50 mg/l) with the flow rate of 5 ml/min was passed through the column and the column was washed with 250 ml of 0.2 M NaOH with the flow rate of 2 ml/min. Results of these experiments show that recovery from the column was 7.9%, which is a low recovery percentage.
Conclusion
This study has been conducted to evaluate the performance of polyaniline/ wood sawdust /poly ethylene glycol/ (PANI/SD/PEG) composite on hexavalent chromium from aqueous solution. The experiment conducted in batch and column mode. In batch experiments the best adsorption occurs at pH 2 and 30 min contact time. Removal of chromium by using PANi/SD/PEG composite was highly in accordance with Langmuir’s isotherm. For PANi/SD/PEG composite the maximum adsorption of hexavalant chromium was calculated 3.2 (mg/g). In column experiments, fluid pH and column bed depth were found to be more prominent than fluid flow rate. During desorption experiment, 22.33, 7.9% of chromium was released into solution in batch and column experiment respectively. The presence of PEG at the synthesis stage had effect on prepared composite. With the presence of PEG, prepared composite has been homogenized and further absorption has been occurred. Chromium removal percentage for PANi/SD/PEG was about six times higher compared to employing only sawdust. According to this study, PANi/SD/PEG composite has a good performance on the removal of Cr (VI) from aqueous media. The extension of this paper according previous works [76–90] affords engineers a good option for experimental investigations.
Compliance with ethical standards
Conflict of interest
There is not any conflict of interest in this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papaevangelou VA, Gikas GD, Tsihrintzis VA. Chromium removal from waste water using HSF and VF pilot-scale constructed wetlands: Overall performance, and fate and distribution of this element within the wetland environment. Chemosphere. 2017;168:716–730. doi: 10.1016/j.chemosphere.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton EM, Young SD, Bailey EH, Watts MLJ. Chromium speciation in foodstuffs: A review. Food Chem. 2018;250:105–112. doi: 10.1016/j.foodchem.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Shahid M, Shamshad S, Rafiq M, Khalid S, Rashid MI. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 4.Jobby R, Jha P, Kumar Yadav A, Desai N. Biosorption and biotransformation of hexavalent chromium [Cr (VI)]: A comprehensive review. Chemosphere. 2018;207:255–266. doi: 10.1016/j.chemosphere.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Deng H, Chen C, Yang Y, Xu H. Biosorption of malachite green from aqueous solutions by Pleurotus ostreatus using Taguchi method. J Environ Health Sci Eng. 2014;12:1–10. doi: 10.1186/2052-336X-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashrafi SD, Kamani H, Arezomand HS, Yousefi N, Mahvi AH. Optimization and modeling of process variables for adsorption of Basic Blue 41 on NaOH-modified rice husk using response surface methodology. Desal Wat Treat. 2016;57:14051–14059. [Google Scholar]
- 7.Lyu H, Tang J, Huang Y, Gai L, Zeng EY, Liber K, Gong Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem Eng J. 2017;322:516–524. [Google Scholar]
- 8.Huang D, Wang G, Shi Z, Li Z, Kang F, Liu F. Removal of hexavalent chromium in natural groundwater using activated carbon and cast iron combined system. J Clean Prod. 2017;165:667–676. [Google Scholar]
- 9.Lv X, Xu J, Jiang G, Xu X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere. 2011;85:1204–1209. doi: 10.1016/j.chemosphere.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Chen Z, Chen D, Xiong W. Removal of hexavalent chromium from contaminated waters by ultrasound-assisted aqueous solution ball milling. J Environ Sci. 2017;52:276–283. doi: 10.1016/j.jes.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Mamais D, Noutsopoulos C, Kavallari I, Nyktari E, Kaldis A, Panousi E, Nikitopoulos G, Antoniou K, Nasioka M. Biological groundwater treatment for chromium removal at low hexavalent chromium concentrations. Chemosph. 2016;152:238–244. doi: 10.1016/j.chemosphere.2016.02.124. [DOI] [PubMed] [Google Scholar]
- 12.Gaikwad MS, Balomajumder C. Simultaneous electrosorptive removal of chromium(VI) and fluoride ions by capacitive deionization (CDI): Multicomponent isotherm modeling and kinetic study. Sep Purif Technol. 2017;186:272–281. [Google Scholar]
- 13.Fu R, Yang Y, Xu Z, Zhang X, Guo X, Bi D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI) Chemosphere. 2015;138:726–734. doi: 10.1016/j.chemosphere.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini SS, Nazif A, Shahmirzadi MAA, Ortiz I. Fabrication, tuning and optimization of poly (acrilonitryle) nanofiltration membranes for effective nickel and chromium removal from electroplating wastewater. Sep Purif Technol. 2017;187:46–59. [Google Scholar]
- 15.Geng B, Jin Z, Li T, Qi X. Kinetics of, hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosph. 2009;75:825–830. doi: 10.1016/j.chemosphere.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Asiabi H, Yamini Y, Shamsayei M. Highly selective and efficient removal of arsenic (V), chromium (VI) and selenium (VI) oxyanions by layered double hydroxide intercalated with zwitterionic glycine. J Hazard Mater. 2017;339:239–247. doi: 10.1016/j.jhazmat.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, He Y, Lan Y, Mao J, Chen S. Influence of complex reagents on removal of chromium(VI) by zero-valent iron. Chemosphere. 2008;72:870–874. doi: 10.1016/j.chemosphere.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadian M, Yosefi N, Toolabi A, Khanjani N, Rahimi-Keshari S, Fatehizadeh A. Adsorption of direct yellow 9 and acid orange 7 from aqueous solutions by modified pumice. Asia J Chemis. 2012;24:3094. [Google Scholar]
- 19.Pourfadakari S, Yousefi N, Mahvi A. Removal of Reactive Red 198 from aqueous solution by combined method multi-walled carbon nanotubes and zero-valent iron: Equilibrium, kinetics, and thermodynamic. Chin J Chem Eng. 2016;24:1448–1455. [Google Scholar]
- 20.Kalhor MM, Rafati AA, Rafati L, Rafati AA. Synthesis, characterization and adsorption studies of amino functionalized silica nano hollow sphere as an efficient adsorbent for removal of imidacloprid pesticide. J Mol Liq. 2018;266:453–459. [Google Scholar]
- 21.Kamranifar M, Khodadadi M, Samiei V, Dehdashti B, Noori Sepehr M, Rafati L, Nasseh N. Comparison the removal of reactive red 195 dye using powder and ash of barberry stem as a low cost adsorbent from aqueous solutions: Isotherm and kinetic study. J Mol Liq. 2018;255:572–577. [Google Scholar]
- 22.Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH. Removal of ibuprofen from aqueous solution by functionalized strong nano-clay composite adsorbent: kinetic and equilibrium isotherm studies. Int J Environ Sci Technol. 2018;15:513–524. [Google Scholar]
- 23.Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH. Modeling of adsorption kinetic and equilibrium isotherms of naproxen onto functionalized nano-clay composite adsorbent. J Mol Liq. 2016;224:832–841. [Google Scholar]
- 24.Rafati L, Nabizadeh R, Mahvi AH, Dehghani MH. Removal of phosphate from aqueous solutions by iron nano-particle resin Lewatit (FO36) Kore J Chemic Eng. 2012;29:473–477. [Google Scholar]
- 25.Rafati L, Mahvi AH, Asgari AR, Hosseini SS. Removal of chromium (VI) from aqueous solutions using lewatit fo36 nano ion exchange resin, inter. J Environ Sci Technol. 2010;7:147–156. [Google Scholar]
- 26.Sakulthaew C, Chokejaroenrat C, Poapolathep A, Satapanajaru T, Poapolathep S. Hexavalent chromium adsorption from aqueous solution using carbon nano-onions (CNOs) Chemosphere. 2017;184:1168–1174. doi: 10.1016/j.chemosphere.2017.06.094. [DOI] [PubMed] [Google Scholar]
- 27.Bhatti IA, A N, Iqbal N, Zahid M, Iqbal M. Chromium adsorption using waste tire and conditions optimization by response surface methodology. J Environ Chemic Eng. 2017;5:2740–2751. [Google Scholar]
- 28.Lee C, Lee S, Park JA, Park C, Lee SJ, Kim S, An B, Yun S, Lee S, Choi J. Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere. 2017;166:203–211. doi: 10.1016/j.chemosphere.2016.09.093. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Z, Zhang H, Xu Y, Yuan M, Jing X, Huang J, Li Q, Sun D. Ultra-efficient removal of chromium from aqueous medium by biogenic iron based nanoparticles. Sep Purif Technol. 2017;174:466–473. [Google Scholar]
- 30.Dima JB, Cynthia S, Zaritzky NE. Hexavalent chromium removal in contaminated water using reticulated chitosan micro/nanoparticles from seafood processing wastes. Chemosph. 2015;141:100–111. doi: 10.1016/j.chemosphere.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed A, Nasser WS, Osman TA, Toprak MS, Muhammed M, Uheida A. Removal of chromium (VI) from aqueous solutions using surface modified composite nanofibers. J Colloid Interface Sci. 2017;505:682–691. doi: 10.1016/j.jcis.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 32.Park D, Lim S, Yun Y, Park JM. Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosph. 2007;70:298–305. doi: 10.1016/j.chemosphere.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Babel S, Kurniawan TA. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemos. 2004;54:951–967. doi: 10.1016/j.chemosphere.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Gupta VK, Morhan D, Sharma S, Park KT. Removal of chromium from electroplating industry wastewater using bagasse fly ash-a sugar industry waste materials. Environmentalist. 1999;19:129–136. [Google Scholar]
- 35.Srivastava K, Balasubramanian N, Ramakhrisna TV. Studies on chromium removal by rice busk carbon. Ind J Environ Healt. 1998;30:376–387. [Google Scholar]
- 36.Selvarg K, Chandramohan V, PattebhiI S. Removal of hexavalent chromium using distillery sludge. Bioresour Technol. 1997;89:207–211. doi: 10.1016/s0960-8524(03)00062-2. [DOI] [PubMed] [Google Scholar]
- 37.Amalraj A, Selvi MK, Rajeswari A, Christy EJS. A. Pius, Efficient removal of toxic hexavalent chromium from aqueous solution using threonine doped polypyrrole nanocomposite. J Wat Proc Engin. 2016;13:88–99. [Google Scholar]
- 38.Bhaumik M, Setshedi K, Maity A, Onyango MS. Chromium(VI) removal from water using fixed bed column of polypyrrole/Fe3O4 nanocomposite. Sep Purif Technol. 2013;110:11–19. [Google Scholar]
- 39.Baig U, Rao RAK, Khan AA, Sanagi MM, Gonda MA. Removal of carcinogenic hexavalent chromium from aqueous solutions using newly synthesized and characterized polypyrrole–titanium(IV)phosphate nanocomposite. Chem Eng J. 2015;280:494–504. [Google Scholar]
- 40.Ballav N, Maity A, Mishra SB. High efficient removal of chromium(VI) using glycine doped polypyrrole adsorbent from aqueous solution. Chem Eng J. 2012;198–199:536–546. [Google Scholar]
- 41.Bhaumik M, Maity A, Srinivasu VV, Onyango MS. Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem Eng J. 2012;181–182:323–333. [Google Scholar]
- 42.Shabani-Nooshabadi M, Zahedi F. Electrochemical reduced graphene oxide-polyaniline as effective nanocomposite film for high-performance supercapacitor applications. Electrochimi Act. 2017;245:575–586. [Google Scholar]
- 43.Jasim A, Ullah MW, Shi Z, Lin X, Yang G. Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr Polym. 2017;163:62–69. doi: 10.1016/j.carbpol.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 44.Farias EAO, Santos MC, Dionísio NA, Quelemes PV, Leite JRSA, Eaton P, Silva DA, Eiras C. Layer-by-Layer films based on biopolymers extracted from red seaweeds and polyaniline for applications in electrochemical sensors of chromium VI. Mater Sci Eng B. 2015;200:9–21. [Google Scholar]
- 45.Patil BH, Jang K, Lee S, Kim JH, Yoon CS, Kim J, Kim DH, Ahn H. Periodically ordered inverse opal TiO2/polyaniline core/shell design for electrochemical energy storage applications. J Alloys Compd. 2017;694:111–118. [Google Scholar]
- 46.Liu M, He S, Fan W, Miao Y, Liu T. Filter paper-derived carbon fiber/polyaniline composite paper for high energy storage applications. Compos Sci Technol. 2014;101:152–158. [Google Scholar]
- 47.Stejskal J, Hajná M, Kašpárková V, Humpolíček P, Zhigunov A, Trchová M. Purification of a conducting polymer, polyaniline, for biomedical applications. Synth Met. 2014;195:286–293. [Google Scholar]
- 48.X H, Zhang J, Chen Y, Lu H, Zhuang J, Li J. Synthesis of polyaniline-modified MnO2 composite nanorods and their. Mater Lett. 2014;117:21–23. [Google Scholar]
- 49.Yang C, Li H, Xiong D, Cao Z. Hollow polyaniline/Fe3O4 microsphere composites: Preparation, Characterization and application in microwave absorption. React Funct Polym. 2009;69:137–144. [Google Scholar]
- 50.Samani MR, Borghei SM, Olad A, Chaichi MJ. Adsorption of chromium from aqueous solution using polyaniline. Wat Wastewat. 2011;379:2–9. doi: 10.1016/j.jhazmat.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 51.Samani MR, Borghei SM, Olad A, Chaichi MJ. Removal of Chromium from Aqueous Solution Using Two Kinds of Polyaniline. J Env Stud. 2010;55:25. doi: 10.1016/j.jhazmat.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 52.Eisazadeh H, Samani MR. Chromium removal from Chromium-plating industry waste water using conductive polymers. IranPolym J. 2006;19:137–141. [Google Scholar]
- 53.Farrell ST, Breslin CB. Reduction of Cr (VI) at a polyaniline film: influence of film thickness and oxidation state. Environ Sci Technol. 2004;38:4671–4676. doi: 10.1021/es0498585. [DOI] [PubMed] [Google Scholar]
- 54.Ruotolo LAM, Gubulin JC. Chromium (VI) reduction using conducting polymer films. React Funct Polym. 2005;62:141–151. [Google Scholar]
- 55.Olad A, Nabavi R. Application of polyaniline for the reduction of toxic Cr(VI) in water. J Hazard Mater. 2007;147:845–851. doi: 10.1016/j.jhazmat.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 56.Samani MR, Borghei SM. Removal of Chromium from Aqueous Solution using Synthesized Polyaniline in Acetonitrile. World Acad Sci Eng Technol. 2012;68:1282–1285. [Google Scholar]
- 57.Samani MR, Borghei SM, Olad A, Chaichi MJ. Influence of Polyaniline Synthesis Conditions on its Capability for Removal and Recovery of Chromium from Aqueous Solution. Iran J Chem Chemic Eng (IJCCE) 2011;30:97–100. [Google Scholar]
- 58.Rafiqi FA, Majid K. Removal of copper from aqueous solution using polyaniline and polyaniline/ferricyanide composite. J Env Chem Eng. 2015;3:2492–2501. [Google Scholar]
- 59.Liu Y, Chen L, Li Y, Wang P, Dong Y. Synthesis of magnetic polyaniline/graphene oxide composites and their application in the efficient removal of Cu(II) from aqueous solutions. J Env Chem Eng. 2016;4:825–834. [Google Scholar]
- 60.Karthik R, Meenakshi S. Removal of hexavalent chromium ions using polyaniline/silica gel composite. J Wat Proc Eng. 2014;1:37–45. [Google Scholar]
- 61.Roghani M, Nakhli SA, Aghajani M, Rostami MH, Borghei SM. Adsorption and oxidation study on arsenite removal from aqueous solutions by polyaniline/polyvinyl alcohol composite. J W Proc Eng. 2016;14:101–107. [Google Scholar]
- 62.Samani MR, Ebrahimbabaie P, Molamahmood HV. Hexavalent chromium removal by using synthesis of polyaniline and polyvinyl alcohol. Water Sci Technol. 2016;74:2305–2313. doi: 10.2166/wst.2016.412. [DOI] [PubMed] [Google Scholar]
- 63.Gupta RK, Singh RA, Dubey SS. Removal of mercury ions from aqueous solutions by composite fo polyaniline with polystyrene. Sep Purif Technol. 2004;38:225–232. [Google Scholar]
- 64.Davodi B, Jahangiri M. Determination of optimum conditions for removal of As (III) and As(V) by polyaniline/polystyrene nanocomposite. Synth Met. 2014;194:97–101. [Google Scholar]
- 65.Samani MR, Borghei SM, Olad A, Chaichi MJ. Removal of chromium from aqueous solution using polyaniline–poly ethylene glycol composite. J Hazard Mater. 2010;184:248–254. doi: 10.1016/j.jhazmat.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Qiua B, Xu C, Sun D, Wangb Q, Gua H, Brandon XZ, Weeksd L, Hoppera J, Hoa TC, Guoa Z, Wei S. Polyaniline coating with various substrates for hexavalent chromium removal. Appl Surf Sci. 2015;334:7–14. [Google Scholar]
- 67.Kumar PA, Chakraborty S, Ray M. Removal and recovery of chromium from wastewater using short chain polyaniline synthesized on jute fiber. Chem Eng J. 2008;141:130–140. [Google Scholar]
- 68.Harijan DKL, Chandra V. Polyaniline functionalized Graphene sheets for treatment of toxic hexavalent chromium. J Env Chem Eng. 2016;4:3006–3012. [Google Scholar]
- 69.Qomia MH, Eisazadeh H, Hosseinid M, Namaghia HA. Manganese removal from aqueous media using polyaniline nanocomposite coated on wood sawdust. Synth Met. 2014;194:153–159. [Google Scholar]
- 70.Eisazadeh A, Eisazadeh H, Kassim KA. Removal of Pb (II) using polyaniline composites and iron oxide coated natural sand and clay from aqueous solution. Synth Met. 2013;171:56–61. [Google Scholar]
- 71.Ren J, Huang X, Wang N, Lu K, Zhang X, Li W, Liu D. Preparation of polyaniline-coated polyacrylonitrile fiber mats and their application to Cr(VI) removal. Synth Met. 2016;222:255–266. [Google Scholar]
- 72.Karthik R, Meenakshi S. Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem Eng J. 2015;263:168–177. [Google Scholar]
- 73.Ansari R. Application of polyaniline and its composites for adsorption /recovery of chromium (VI) from aqueous solutions. Act Chem Solv. 2006;53:88–94. [Google Scholar]
- 74.Ansari R, Raofie F. Removal of lead ion from aqueous solutions using sawdust coated by polyaniline. E J Chem. 2006;3:49–59. [Google Scholar]
- 75.Ansari R, Raofie F. Removal of mercuric ion from aqueous solutions using sawdust coated by polyaniline. E J Chem. 2006;3:35–43. [Google Scholar]
- 76.Esfe MH, Saedodin S, Bahiraei M, Toghraie D, Mahian O, Wongwises S. Thermal conductivity modeling of MgO/EG nanofluids using experimental data and artificial neural network. J Therm Anal Calorim. 2014;118:287–294. [Google Scholar]
- 77.Zarringhalam M, Karimipour A, Toghraie D. Experimental study of the effect of solid volume fraction and Reynolds number on heat transfer coefficient and pressure drop of CuO–Water nanofluid. Exp Thermal Fluid Sci. 2016;76:342–351. [Google Scholar]
- 78.Esfe MH, Akbari M, Semiromi DT, Karimiopour A, Afrand M. Effect of nanofluid variable properties on mixed convection flow and heat transfer in an inclined two-sided lid-driven cavity with sinusoidal heating on sidewalls. Heat Transfer Res. 2014;45:409–432. [Google Scholar]
- 79.Afrand M, Toghraie D, Ruhani B. Effects of temperature and nanoparticles concentration on rheological behavior of Fe3O4–Ag/EG hybrid nanofluid: an experimental study. Exp Thermal Fluid Sci. 2016;77:2016. [Google Scholar]
- 80.Hemmat Esfe M, Yan WM, Afrand M, Sarraf M, Toghraie D, Dahari M. Estimation of thermal conductivity of Al2 O3 /Water (40%)–ethylene-glycol (60%) by artificial neural network and correlation using experimental data. Int Commun Heat Mass Transf. 2016;74:125–128. [Google Scholar]
- 81.Toghraie D, Chaharsoghi VA, Afrand M. Measurement of thermal conductivity of ZnO–TiO2/EG hybrid nanofluid. J Therm Anal Calorim. 2016:1–9. 10.1007/s10973-016-5436-4.
- 82.Toghraie D, Alempour SMB, Afrand M. Experimental determination of viscosity of Water based magnetite nanofluid for application in heating and cooling systems. J Magn Magn Mater. 2016;417:243–248. [Google Scholar]
- 83.Hemmat EM, Saedodin S, Wongwises S, Toghraie D. An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/Water nanofluids. Therm Anal. 10.1007/s10973-014-4328-8.
- 84.Hemmat Esfe M, Afrand M, Gharehkhani S, Rostamiand H, Toghraie D, Dahari M. An experimental study on viscosity of alumina-engine oil: Effects of temperature and nanoparticles concentration. Int Commun Heat Mass Transf. 2016;76:202–208. [Google Scholar]
- 85.Hemmat Esfe M, Afrand M, Yan WM, Yarmand H, Toghraie D, Dahari M. Effects of temperature and concentration on rheological behavior of MWCNTs/ SiO2 (20–80)-SAE40 hybrid nano-lubricant. Int Commun Heat Mass Transf. 2016;76:133–138. [Google Scholar]
- 86.Hemmat Esfe M, Ahangar H, Rejvani M, Toghraie D, Hajmohammad MH. Designing an artificial neural network to predict dynamic viscosity of aqueous nanofluid of TiO2 using experimental data. Int Commun Heat Mass Transf. 2016;75:192–196. [Google Scholar]
- 87.Afrand M, Toghraie D, Sina N. Experimental study on thermal conductivity of Water-based Fe3O4 nanofluid: Development of a new correlation and modeled by artificial neural network. Int Commun Heat Mass Transf. 2016;75:262–269. [Google Scholar]
- 88.Esfe MH, Afrand M, Rostamian SH, Toghraie D. Examination of rheological behavior of MWCNTs/ZnO-SAE40 hybrid nano-lubricants under various temperatures and solid volume fractions. Exp Thermal Fluid Sci. 2017;80:384–390. [Google Scholar]
- 89.Esfe MH, Rostamian H, Toghraie D, Yan WM. Using artificial neural network to predict thermal conductivity of ethylene glycol with alumina nanoparticle. J Therm Anal Calorim. 2016;126(2):643–648. [Google Scholar]
- 90.Zadkhast M, Toghraie D, Karimipour A. Developing a new correlation to estimate the thermal conductivity of MWCNT-CuO/water hybrid nanofluid via an experimental investigation. J Therm Anal Calorim. 2017;129:859–867. [Google Scholar]