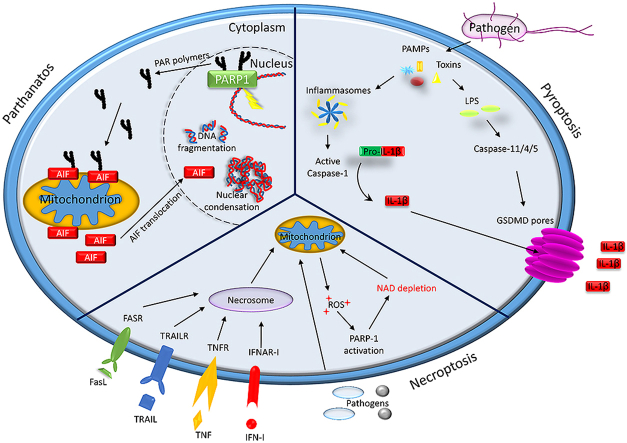
Abstract
Macrophages are highly plastic cells of the innate immune system. Macrophages play central roles in immunity against microbes and contribute to a wide array of pathologies. The processes of macrophage activation and their functions have attracted considerable attention from life scientists. Although macrophages are highly resistant to many toxic stimuli, including oxidative stress, macrophage death has been reported in certain diseases, such as viral infections, tuberculosis, atherosclerotic plaque development, inflammation, and sepsis. While most studies on macrophage death focused on apoptosis, a significant body of data indicates that programmed necrotic cell death forms may be equally important modes of macrophage death. Three such regulated necrotic cell death modalities in macrophages contribute to different pathologies, including necroptosis, pyroptosis, and parthanatos. Various reactive oxygen and nitrogen species, such as superoxide, hydrogen peroxide, and peroxynitrite have been shown to act as triggers, mediators, or modulators in regulated necrotic cell death pathways. Here we discuss recent advances in necroptosis, pyroptosis, and parthanatos, with a strong focus on the role of redox homeostasis in the regulation of these events.
Keywords: Cell death, Macrophage, Regulated necrosis, Pyroptosis, Necroptosis, Parthanatos, Redox, Pathogens, Myeloid cells
Graphical abstract
1. Introduction
Macrophages (MΦs) are phagocytic cells of the innate immune system that play central roles in tissue homeostasis and response to pathogenic stimuli. Circulating monocytes can enter the tissues and differentiate into MΦs in response to CSF1 and GM-CSF. On the other hand, tissue resident MΦs (e.g. Kupffer cells in the liver and brain microglia) have been shown to arise from yolk sac-derived (and not bone marrow-derived) primitive MΦs and settle in organs prenatally [1,2] or may even transdifferentiate from smooth muscle cells, as demonstrated in the wall of atherosclerotic arteries [3].
In tissues, MΦs sample the environment and respond to the pathogen- or damage-associated molecular patterns (PAMPs and DAMPs, respectively), toxins, cytokines, and chemokines. Depending on the composition of the sampled environment, MΦs differentiate into a diverse set of phenotypes, which are usually characterized by two extremes of the MΦ differentiation spectrum, M1 (inflammatory) and M2 (resolutory). However, MΦs are characterized by high plasticity and their phenotype can easily change in response to alterations of the microenvironment.
Classically activated (M1-like) MΦs produce large amounts of reactive oxygen and nitrogen species (ROS and RNS, respectively), mostly via activation of NADPH Oxidase 2 (NOX2) and inducible nitric oxide synthase (iNOS). These reactive species are effective tools to combat pathogens but also contribute to organ damage in inflamed tissues. MΦs also succumb to this ROS and RNS-filled environment and undergo cell death. While research on programmed cell death initially focused on apoptosis, in recent decades several novel forms of programmed necrotic cell death (necroptosis, pyroptosis, parthanatos, oxytosis, ferroptosis, NETosis) have attracted increased attention [4]. Many of these modalities are relevant to MΦs. Here, we will discuss the mechanisms and pathological consequences of programmed necrotic cell death of MΦs focusing on pyroptosis, necroptosis, and parthanatos.
2. Pyroptosis
The term pyroptosis (from Greek “pyro,“- fire or fever, and “ptosis” – falling) was initially proposed by Cookson and Brennan [5] for a novel form of inflammatory programmed cell death. The first observations of pyroptosis were made with the invasive pathogenic bacteria Shigella and Salmonella, which triggered lytic cell death by activating caspase-1 in MΦs via the secreted effector proteins, SipB and IpaB, respectively [6,7]. Initially, this process was incorrectly classified as apoptosis, but was subsequently recognized as a form of programmed cell death different from apoptosis.
Pyroptosis is now recognized as its own entity of programmed cell death. Initially, pyroptosis was described as being dependent on caspase-1 and gasdermin D (GSDMD), discriminating it on the mechanistic level from other forms of cell death, such as apoptosis, which depends on caspase-8, and necroptosis, which depends on RIP3 and mixed lineage kinase domain-like (MLKL) [8]. The prototypical form of pyroptosis is triggered by activation of proinflammatory caspases (caspase-1,-4,-5 in man and caspase-1 and -11 in mice). The terminal cell lysis is then mediated by cleavage of GSDMD by one of these caspases [9,10]. The active form of GSDMD, which consists of an N-terminal domain, can assemble to form pores in the cell membrane [11,12]. These pores lead to collapse of the membrane, potentially initiating death of the cell, but at the same time leading to the release of inflammatory mediators, including IL-1β and IL-18 [9,10,13]. Compared to apoptosis, where the cell content of the dying cell is sealed in apoptotic bodies, this form of cell death is inflammatory and the highly inflammatory cytokines of the IL-1 family and cellular danger signals (damage-associated molecular pattern - DAMP) are released. Besides GSDMD, other members of the gasdermin family participate in pyroptotic cell death. GSDME (DFNA5) cleavage by caspase-3 can induce pyroptosis in certain cancer cells upon chemotherapy [14], a process termed non-canonical pyroptosis. GSDMB is highly expressed in septic shock and might contribute to GSDMD processing [15].
Recently, it was shown that caspase-8 also cleaves GSDMD, leading to caspase-8-mediated GSDMD-dependent cell death in response to extrinsic triggers of apoptosis [16]. This form of pyroptosis is induced by inhibition of pro-survival signals, mainly by pharmaceutical or bacterial targeting of TAK1 kinase [[17], [18], [19]]. These examples illustrate that different forms of pyroptotic cell death exist and that these are interconnected with apoptotic and necroptotic pathways.
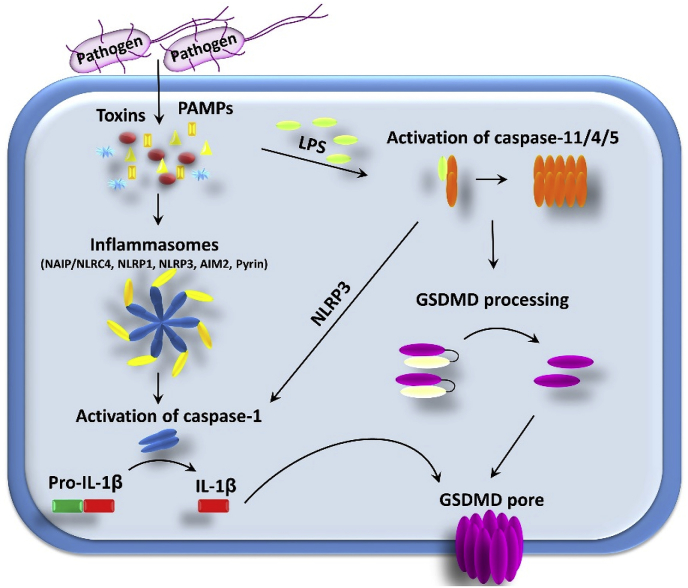
The main function of the classical pyroptosis pathway is thought to be defense against infection [12]. Pathogens and pathogen-derived toxins are sensed by cytosolic pattern-recognition receptors (PRRs). Pyrin domain (PYD)-containing members of the Nod-like receptor (NLR) family and AIM2 are the most thoroughly studied members of the cytosolic PRRs. Upon activation, PYD-containing NLR proteins and AIM2 form a multimeric high-molecular weight complex in the cell, containing the adaptor protein apoptosis associated speck-like protein containing a CARD (ASC). These complexes, named “inflammasomes” by Jürg Tschopp, are scaffolds for the activation of pro-caspase-1, which is induced by proximity induced auto-processing [20]. ASC consists of a PYD and a CARD domain. The PYD domain can form homo-oligomeric interactions with the PYD from NLR or AIM2 and the CARD forms homo-oligomeric interactions with the CARD of caspase-1. In addition to the recruitment of effector cells by the released cytokines, one effector mechanism induced by the pyroptotic program is the expulsion of invaded bacterial pathogens from the affected cell in small membrane-enclosed structures. These pore-induced intracellular traps (PIT) can induce efferocytosis of the engulfed material by macrophages and neutrophils [21].
Currently, five PRRs are described as sensor proteins that induce pyroptosis (see Table 1). PRRs, including AIM2, NAIP/NLRC4 oligomer, NLRP3, Pyrin (TRIM20), and NLRP1, sense a variety of structurally different PAMPs. AIM2 reacts to double-stranded DNA in the cytosol [22], NAIP/NLRC4 senses bacterial type III secretion apparatus proteins and flagellin [23], NLRP3 is activated by different kinds of membrane damage [24], Pyrin is activated by bacterial modification of host proteins [25], and NLRP1 senses anthrax lethal toxin and Toxoplasma [26]. All of these pathways induce the activation of caspase-1, which ultimately processes GSDMD (Fig. 1). An alternative pathway to activate pyroptosis is triggered by activation of caspase-11/4/5. This non-canonical inflammasome is activated by cytosolic lipopolysaccharide (LPS) [27,28]. Caspase-11 in mice and caspase-4 and -5 in man bind to LPS, leading to activation of these caspases [29]. Although caspase-11/4/5 can cleave and activate GSDM in human myeloid cells to induce pyroptosis, IL-1β production upon cytosolic LPS sensing by this pathway depends on NLRP3 activation [30]. GSDMD cleavage is needed to induce activation of NLRP3 [9]. The mechanism of NLRP3 activation is poorly understood; however, GSDMD and GSDME both target mitochondrial membranes and stimulate the release of ROS, which can trigger NLRP3 activation [31,32].
Table 1.
Pyroptosis inducers (see text for details and references).
| Inducer | PRR | Pathway |
|---|---|---|
| cytosolic dsDNA | AIM2 | Canonical pyroptosis |
| bacterial type III SA, flagellin | NAIP/NLRC4 | Canonical pyroptosis |
| membrane damage | NLRP3 | Canonical pyroptosis |
| Bacterial modification of GTPases | Pyrin | Canonical pyroptosis |
| Anthrax lethal toxin, Toxoplasma ssp. | NLRP1 | Canonical pyroptosis |
| Cytosolic LPS | Caspase4,5/11 | Canonical pyroptosis |
| Chemotherapy drugs | GSDME | Non-canonical, caspase-3, GSDME |
| Targeting of TAK1 | Caspase-8-mediated GSDMD cleavage |
Fig. 1.
Schematic representation of the pyroptosis pathways in a mammalian cell. See text for details.
Release of the potent inflammatory cytokine, IL-1β, is important in controlling infection. However, IL-1β has detrimental effects in sepsis, a life-threatening organ dysfunction caused by an overwhelming cytokine response towards bacterial pathogens. Neutrophils are emerging as important players in this condition and targeting the modulation of pyroptosis in neutrophils might be a viable treatment option [33].
3. Redox control of pyroptosis
As eluded to above, the pyroptosome triggering inflammasomes can be activated by microbial substances. In the case of the NLRP3 inflammasome, there is good evidence that its activity is also controlled by the redox state of the cell (for an excellent overview the reader is referred to Ref. [34]). The presence of ROS, produced by the MΦs upon microbial insult, was shown to contribute to NLRP3 activation by the redox sensor thioredoxin-interacting protein (TXNIP) (reviewed in Ref. [35]). However, the contribution of TXNIP to NLRP3 activation is controversial, as TXNIP knockout mice were reported to have no defects in IL-1β production [36]. NADPH oxidase, the enzyme that produces ROS for an oxidative burst in MΦs, is also not needed for this activation, as both NADPH oxidase knock out mice and chronic granulomatosis patients show normal IL-1β production [[37], [38], [39]]. In contrast, several reports suggest a role for mitochondrial-derived ROS (mitoROS) [40]. In a model of Shiga toxin and LPS-induced cell activation, mitoROS plays a critical role in IL-1β release and pyroptosis, mediated by both NLRP3 and GSDMD [31]. The master transcriptional regulator of redox homeostasis nuclear factor E2-related factor 2 (Nrf2) further contributes to NLRP3 activation and IL-1β secretion is inhibited by Nrf2 silencing [41,42]. The functional mechanism remains elusive, but is likely to be indirect, as no contribution of Nrf2 to inflammasome complex formation has yet been identified. The redox status can directly affect the activity of initiator caspases. Caspase-1 can be regulated by superoxide via reversible oxidation and glutathionylation of redox-sensitive cysteine residues. Accordingly, depletion of superoxide dismutase 1 (SOD1) leads to an oxygen-dependent reduction of caspase-1 activation [43]. For caspase-11, extracellular ROS can induce its expression and activation, which involves JNK activation [44].
Our knowledge about the fine-tuning of the final steps of pyroptosis is still very fragmentary, but redox status seems to contribute here as well. ROS have recently been shown to oxidize GSDM, which enhances GSDM cleavage by caspase-1 [45].
4. Necroptosis
Caspases are the executioner proteins of both apoptosis and pyroptosis. In contrast, necroptosis is a caspase-independent necrotic cell death program regulated by receptor-interacting protein (RIP) kinases. Necroptosis was initially discovered when cells, stimulated with FasL, tumor necrosis factor (TNF), or a TNF ligand, were additionally treated with the pan-caspase inhibitor, Z-VAD-FMK. Necrosis is an unprogrammed cell death and occurs due to an irreversible injury to the cell. In contrast, necroptosis is programmed and regulated by receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3) [46]. Most of the current knowledge about necroptosis is primarily derived from investigating tumor necrosis factor (TNF) signaling. Engagement of TNF with its cognizant receptor results in the formation of complex I at the cell membrane. Complex I is composed of tumor necrosis factor receptor (TNFR)-associated death domain (TRADD), Fas-associated death domain (FADD), RIPK1, TNFR-associated factors (TRAF), and cellular inhibitor of apoptosis protein 1 (cIAP1) and cIAP2. TRAF proteins ubiquinate and stabilize RIP1 at the plasma membrane, leading to the activation of nuclear factor kappa B (NFκB) and cell survival [47]. Activation of necroptosis is initiated by the deubiquitination of RIPK1 by cylindromatosis protein (CYLD), which dislodges RIPK1 from complex I and forms a complex II with FADD, TRADD, and caspase-8. Active caspase-8 can cleave RIPK1 and RIPK3; however, inhibition of caspase-8 facilitates the interaction of RIPK1 and RIPK3 through their RIP homotypic interaction motives (RHIM) [48]. The RIP complex induces the phosphorylation of the pseudokinase mixed-lineage kinase domain-like protein (MLKL) [49,50]. Phosphorylation of MLKL exposes the amino acid-terminal 4-helical bundle domain, which forms a pore in the cell membrane by interacting with negatively charged phospholipids. Pore formation in the cell membrane ultimately leads to cell death [51]. The pathway leading to necroptosis has been largely deciphered through the discovery of small molecule inhibitors that block RIPK1 (necrostatin-1) [52], RIPK3 (GSK′872) [53], and MLKL (necrosulfonamide) [49].
4.1. Necroptosis in MΦs
Necroptosis in MΦs was first reported in the second mitochondria-derived activator of caspase (SMAC) mimetics mediated inhibition of cellular inhibitor of apoptosis proteins (cIAPs) and the expression of the caspase inhibitor XIAP in TNF-α stimulated bone marrow-derived MΦs (BMDMs) [54]. Consistently, inhibition of proteasomes in MΦs, using PS-341, suppresses the degradation of cIAPs, and, thus, attenuates necroptosis [55]. More recent reports suggest that the expression of pro-inflammatory cytokines is elevated, while RIPK1-dependent cell death is reduced, during the differentiation of MΦs and RIPK3-caspase-8 is important in the differentiation of MΦs. Resistance to cell death in differentiated MΦs is mediated by the p38/MK2 pathway [56].
Necroptosis plays a crucial role in pathophysiology. Unlike apoptosis, which is immunologically quiescent, necroptosis results in the release of cytoplasmic contents, which can activate MΦs during infection and other sterile inflammatory conditions. Atherogenic ligands stimulate the expression of the necroptotic genes, RIPK3 and MLKL. Thus, necroptosis occurs in MΦs associated with human atherosclerotic plaques, which becomes the driver of necrotic core formation in atherosclerosis [57]. Plant sterols, such as sitosterol, promote atherosclerosis by inducing necroptosis in MΦs [58]. Heme released during hemolysis is also known to induce necroptosis through ROS and TNF production due to the activation of TLR [59].
On the other hand, bacterial pathogens target MΦs and mitigate host defense mechanisms by eliminating MΦs via necroptosis. Excess TNF induces RIPK1-RIPK3-dependent mitochondrial ROS in Mycobacterium tuberculosis infected MΦs. The authors propose that induction of necroptosis is through the modulation of cyclophilin D that regulates mitochondrial membrane permeability pore formation and ceramide synthesis [60]. A similar phenomenon has also been observed in ischemia-associated oxidative damage wherein p53 associates with cyclophilin D and opens the mitochondrial permeability transition pore resulting in necrotic cell death [61]. Mycobacterium tuberculosis also secretes tuberculosis necrotizing toxin (TNT), a nicotinamide adenine dinucleotide (NAD+) glycohydrolase that induces necroptosis in infected MΦs. Interestingly, depletion of NAD+ is sufficient to induce necroptosis in MΦs [62]. Loss of NAD+ may also result from PARP activation, which suggests that necroptosis and parthanatos, which is reviewed in the next section of the paper, may be linked. Interestingly in other cellular models, administration of NAD+ decreases oxidative stress induced by H2O2 and protects cells from necrosis [63].
Other bacterial pathogens are also known to produce toxins that induce necroptosis in MΦs. For instance, pathogens such as Serratia marcescens, Listeria monocytogenes, Staphylococcus aureus, Streptococcus pneumoniae, and uropathogenic Escherichia coli (UPEC) produce pore-forming toxins that trigger necroptosis and disrupt the cell membrane, damage mitochondria, decrease ATP, and increase ROS-generation [64,65]. These findings demonstrate that the necroptotic death of MΦs is a major cause of lung pathology in pneumonia. A strain of Streptococcus pneumoniae (TIGR4) is able to invade the heart and cause cardiac damage. Authors have demonstrated that the damage is critically associated with the necroptotic death of MΦs induced by pneumolysin, the toxin produced by the bacteria [66]. Similarly, Yersinia outer protein J (YopJ) of Yersinia pestis induces necroptotic death in MΦs, thus allowing the lymphatic spread of the pathogen [67].
Although most studies describe TNFα as the primary factor regulating necroptosis, TNFα-independent necroptosis upon the activation of TLRs in MΦs has also been reported [68]. Our findings demonstrate that Salmonella enterica ssp. enterica ser. Typhimurium (S. Typhimurium) promotion of necroptotic death in MΦs is dependent on type I interferon (IFN–I) signaling. IFN-induced inflammatory pathology is predominantly due to necroptosis. Mice lacking the cognate receptor for IFN-I (IFNAR) showed reduced bacterial burden and pathology associated with the infection [69]. IFN–I drives necroptosis through IFN-stimulated gene factor 3 (ISGF3) signaling, which leads to persistent expression of STAT1, STAT2, and IRF9. Strikingly, MLKL is one of the interferon-stimulated genes (ISGs) [70]. Constitutive interferon signaling, such as in autoimmune diseases, primes MΦs to undergo necroptosis by maintaining adequate levels of MLKL [71].
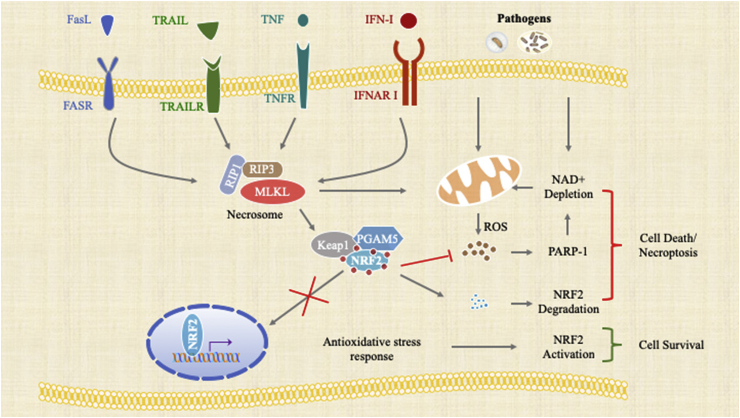
Oxidative stress is a common feature in macrophage necroptosis induced by pathogens or under sterile inflammatory conditions. Bacterial toxin-stimulated necroptosis is prevented using Coenzyme Q10 in combination with a RIPK1 inhibitor. Antioxidants have also been shown to ameliorate heme-induced necroptosis. Moreover, heme oxygenase 1 (HO-1) reduces oxidative stress and, thus, provides cytoprotection [59]. The transcription factor, Nrf2, transcriptionally regulates HO-1 and other cytoprotective genes by binding to cis-acting antioxidant responsive elements (ARE). Thus, the Nrf2-regulated antioxidative response inhibits heme-induced cell death [72]. More recently, we have provided evidence that IFN-I-regulated RIPK3 activation sequesters Nrf2 in the cytoplasm by activating PGAM5 during S. Typhimurium infection. Importantly, pharmacological activation of Nrf2, using the synthetic triterpenoid compound, CDDO (2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid), was able to prevent necroptosis [73]. Upon TNFα-induced necroptosis, PGAM5 is recruited to the RIPK1/RIPK3 complex on the outer mitochondrial membrane, where it triggers Drp1-mediated mitochondrial fragmentation, which is considered an obligatory step in necroptosis [74]. Intriguingly, RIPK3 has been shown to regulate mitochondrial metabolism by targeting the pyruvate dehydrogenase complex [75]. Mitochondrial oxidative stress associated cell death also coincides with another form of cell death known as ferroptosis, which is caused by the accumulation of lipid-based ROS. Expression of glutathione peroxidase, which repairs oxidized lipid species, is also transcriptionally driven by Nrf2. Hence, Nrf2 is known to play a critical role in mitigating ferroptosis [76]. Inhibition of PARP has also been shown to reduce ROS generation and protect mitochondria [77,82]. Consistently, we and others have reported that during Mycobacterium tuberculosis [62] and S. Typhimurium induced necroptosis in MΦs, NAD+ is depleted [78] and PARP-1 is activated [69]. Taken together, this evidence suggests that oxidative stress plays a significant part in the execution of necroptosis (Fig. 2).
Fig. 2.
Schematic diagram representing the convergence of necroptotic signaling and oxidative stress.
5. Parthanatos
Parthanatos is a relatively new addition to the growing list of established cell death forms. The term parthanatos was coined to reflect the dependence of this cell death pathway on the formation of poly (ADP-ribose) (PAR), while the second part of the name refers to Thanatos, the personification of death in Greek mythology [79]. The PAR polymer is synthesized by some poly (ADP-ribose) polymerase (PARP) enzyme family members (PARP1, PARP2 and tankyrases) and parthanatos is triggered by the DNA breakage-induced activation of the founding member of this enzyme family, PARP1. Poly (ADP-ribosyl)ation (PARylation) of proteins, including PARP1 itself (auto-PARylation), near the DNA damage site facilitates the recruitment of DNA repair effector proteins and, thus, contributes to DNA repair. While PARylation is primarily a survival mechanism, in cells experiencing excessive DNA damage, high PARylation activity can cause regulated necrotic cell death. PARP1-mediated cell suicide was first described by Nathan Berger [80] and was thought to result from the depletion of the enzyme's substrate, NAD+, and, consequently, ATP in the cells. The signaling pathway for PARP1-mediated cell death, however, proved to be more complex than a metabolic collapse. Virág et al. demonstrated that PARP1-mediated cell death displays the features of necrosis[[81], [82]]. Inhibition of PARP1 (e.g. by PARP inhibitors or inactivation of PARP1 gene) diverts cells to the “default” apoptotic route [81,82]. Key features of parthanatos include: a) its independence from caspases [81]; b) mitochondrial membrane depolarization and secondary ROS production [82]; c) dependence on calcium signaling [83]; d) independence from the cytoprotective effect of Bcl-2 [84]; e) synergism between PARG and PARP1 in cell death regulation [85].
The central mediator of this cell death pathway is the DNA damage response protein PARP1. PARP1 activation is considered a hallmark of oxidative stress. As a DNA nick sensor enzyme, PARP1 binds to broken DNA resulting in its activation. Activated PARP1 cleaves NAD+ into ADP-ribose and nicotinamide and attaches ADP-ribose to acceptor proteins near the damage site. The enzyme can add further ADP-ribose units to the protein proximal moiety to generate an (ADP-ribose)n polymer known as poly (ADP-ribose) (PAR). PAR polymers are degraded by poly (ADP-ribose) glycohydrolase (PARG) and ADP-ribosylhydrolase-3 (ARH3) enzymes [86].
5.1. The canonical route of parthanatos
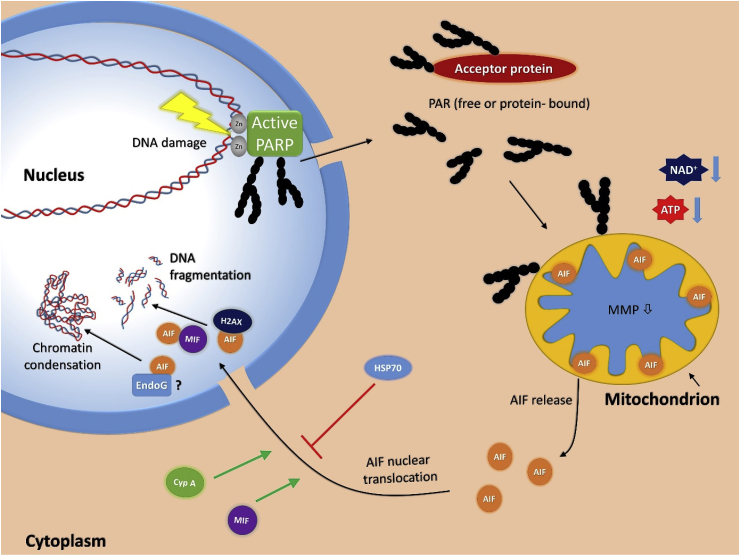
The fact that PARP1-mediated cell death is now recognized as a stand-alone cell death entity is mainly due to discoveries made in the lab of Valina and Ted Dawson at Johns Hopkins University. Their lab showed that cerebral ischemic injury [87], N-methyl-d-aspartate (NMDA) excitotoxicity [87], 1-metil-4-fenil-1,2,3,6-tetrahidropiridin (MPTP)-induced Parkinsonism, and neurodegenerative diseases [88,89] are mediated by PARP1 activation. While characterizing the molecular events leading to and following PARP1 activation in excitotoxicity, they identified apoptosis-inducing factor (AIF) as the downstream mediator of cell death [90]. Their proposed model relies on the following key events of parthanatos: stimulation of the NMDA receptor in neurons, glutamate triggering of calcium signaling, and calcium-dependent activation of the neuronal isoform of nitric oxide synthase (nNOS). Neuronal NOS produces nitric oxide, which combines with superoxide anion radical to form peroxynitrite (ONOO−). Peroxynitrite is highly reactive and causes, among other macromolecular damages, DNA strand breaks [91]. Once DNA breaks are formed, the pathway may show an overlap with other cell death models in which DNA damage is triggered by direct DNA damaging stimuli (e.g. treatment with exogenous peroxynitrite, hydrogen peroxide, or DNA alkylating or crosslinking agents). DNA breaks are recognized by PARP1 and the enzyme becomes activated. Active PARP1 synthesizes the PAR polymer from NAD+ to mark the site of DNA damage. PARG and ARH3 enzymes cleave the polymer off of PARylated proteins and the polymers (whether protein-bound or “naked” polymers is not known) leave the nucleus and translocate to the mitochondria (Fig. 3). PAR triggers the release of AIF from the mitochondrial intermembrane space and AIF begins its journey towards the nucleus [79]. The mechanism of AIF release is not fully understood. PARP1-mediated NAD+ depletion may trigger mitochondrial membrane depolarization, facilitating AIF release. Alternatively, the polymer may directly interact with the C-terminus of membrane-bound AIF. As an alternative to the consumption of NAD+, suppression of glycolysis may result from PAR binding to and inhibition of hexokinase, a key regulatory control point of glycolysis. Either way, AIF leaves the mitochondria and interacts with macrophage migration inhibitory factor (MIF) in the cytoplasm. Nuclear translocation of MIF is followed by MIF-mediated DNA fragmentation, as a result of the newly discovered nuclease activity of this multifunctional protein [92].
Fig. 3.
Parthanatos. Nuclear mitochondrial crosstalk in parthanatos is triggered by DNA damaging stimuli activating PARP1. PAR synthesized in response to DNA breaks travels to the mitochondria and induces liberation of AIF. In turn, AIF interacts with MIF and the latter degrades DNA.
5.2. Parthanatos in MΦs
Zingarelli et al. [93] was the first to demonstrate that PARP1-mediated cell death is an important cell death modality in activated MΦs. [Of note, at the time of their investigation PARP1 was the only known PARylating enzyme and was referred to as poly (ADP-ribose) synthase; PARS in this study.] They showed that exposure of murine peritoneal MΦs or J774 macrophage cells to high concentrations (10 μg/ml) of LPS triggered a rapid burst of superoxide production and a slower upregulation of iNOS. As a result, the ideal conditions were created for ONOO− formation, which triggered the following sequence of events: DNA breakage - PARP1 activation – NAD+/ATP depletion – cell death pathway. Follow up in vivo studies showed that inhibition of this pathway led to reduced organ damage and improved survival in endotoxemic or septic animals [91,94,95].
TLR ligands, such as LPS, also induce production of inflammatory cytokines, with TNFα considered to be the master cytokine regulator of inflammation [96]. MΦs are not only the primary source of TNFα, but also important targets of TNFα. MΦs express both TNFα receptors (TNFR1 and TNFR2) and respond to TNFα stimulation [96]. TNFα –induced cell death has been shown to be mediated by PARP1 in Actinomycin D-pretreated L929 and ME-180 human cervical carcinoma cells, but whether or not MΦs react similarly to the toxic effect of this cytokine remains to be seen [97].
Cytotoxicity of exogenous DNA damaging agents, such as H2O2, has also been investigated in MΦs. MΦs are often exposed to reactive oxygen species and are quite resistant to H2O2, mainly due to the constitutively active PI3K-Akt pathway [98]. Nonetheless, high concentrations of H2O2 cause PARP1-mediated necrosis-like cell death characterized by plasma membrane permeabilization and lack of caspase activation [99]. Interestingly, even though this cell death is PARP1 dependent, no sign of AIF translocation could be observed. Thus, this type of cell death showed some key signs of parthanatos (PARP1-dependence, necrotic phenotype, energetic collapse), while lacking other key features (e.g. AIF translocation). Similar non-canonical parthanatos could also be observed in other models of cell death [[100], [101], [102]] raising the question as to which cell death events should be considered essential for the definition of parthanatos. Based on the above controversy, we propose a less restrictive definition that defines parthanatos simply as PARP1-dependent regulated necrosis.
6. Outlook
Several open questions remain regarding the execution of necrotic MΦ cell death. The pathways leading to various forms of cell death are thought to be distinct. However, some evidence suggests that the necrotic cell death modalities discussed in this review could be interlinked. Studies have demonstrated that IAPs prevent RIPK3-dependent necroptosis and IL-1 activation [103,104]. Consistently, loss of XIAP triggers RIPK3- and caspase-8-driven IL-1β activation and cell death [105]. Activated MLKL also triggers the NLRP3 inflammasome in a cell intrinsic manner [106]. Staphylococcus toxin-induced necroptosis also promotes MLKL-NLRP3-mediated inflammation [65]. On the other hand, RIPK3 has also been shown to activate the inflammasome independent of MLKL [107]. These studies provide vital evidence of cross-talk between necroptosis and pyroptosis. Interestingly, a recent report demonstrated that the MLKL inhibitor, necrosulfonamide (NSA), which has been frequently used to prevent necroptosis in cells of human origin, also inhibits Gasdermin D, the pyroptosis-executioner [108]. Prevention of cell death using NSA has been interpreted as necroptosis, but it is possible that the drug also inhibited pyroptosis. Therefore, the existing data using NSA to inhibit necroptosis need to be revisited to understand if necroptosis and pyroptosis coexist. The simultaneous existence of multiple forms of necrotic cell death has not been scrutinized. For instance, S. Typhimurium infection in macrophages induces caspase-1/11 activation and IL-1β secretion. Additionally, we have shown that the pathogen induces necroptosis and also a differential PARP-1 cleavage and activation [69]. Similarly, Rhodococcus equi induced necrosis is also associated with PARP-1 activation [109]. PARP-1 activation leads to depletion of NAD+, which is sufficient to induce necroptosis during M. tuberculosis infection in MΦs. These findings provoke us to ask if pyroptosis, necroptosis, and parthanatos occur in different subsets of MΦs and how does the mode of cell death switch from one form to the other, depending on the functional state of the cell? Moreover, it is increasingly evident that mitochondrial metabolism, as well as glycolytic regulation, is a major contributor to regulated necrotic cell death modalities in MΦs. Compelling factors that might link various forms of cell death are reactive oxygen and nitrogen species, which sensitize macrophages to necrosis. However, this aspect of cell death has not yet been investigated in detail. For necroptosis, the modus operandi of RIP signaling in causing oxidative stress in addition to MLKL dependent membrane damage requires further investigation.
For pyroptosis, a key question is if cell death related to IL-1 release can be uncoupled from IL-1 release in the cells. The notion that the initiation of events of programmed cell death pathways can be revoked and cells rescued from different forms of death is emerging [110]. The endosomal sorting complexes required for transport (ESCRT) machinery can repair damaged cell membranes and inhibit cell death [111], suggesting that this might allow for non-cell death mediated release of IL-1 cytokines.
As for parthanatos, a detailed molecular characterization of this death pathway in MΦs, as well as proof for its in vivo relevance, is missing. Parthanatotic cell death is likely to contribute to inflammation via the release of DAMPs, due to its programmed necrotic phenotype. The release of the prototypical DAMP protein, HMGB1, is induced by PARP1 activation [112]. In LPS-treated MΦs, HMGB1 is PARylated, leading to acetylation, which is essential for HMGB1 release [113]. In MΦs, the interplay between PARP1 and DAMPs is a two-way communication. For example, in brain injury, microglial activation is mediated in part by the release of alarmins from damaged cells. Microglial signaling induced by the alarmin S100B is mediated by PARP1 [114]. Thus, the hypothesis that parthanatos is an inflammatory cell death pathway is plausible. However, the role of parthanatos in various forms of inflammation requires further investigation.
Understanding the regulation of MΦ cell death modalities may open new avenues for therapeutic interventions in a wide range of inflammations ranging from microbial infections and atherosclerosis to toxic liver injuries. Exploiting our expanding knowledge of MΦ cell death pathways, the molecular switches diverting cells from one route to another, and the molecular determinants of MΦ sensitivity to cytotoxic stimuli may provide new opportunities for potential clinical treatments of various inflammatory diseases, atherosclerosis, microbial infections, toxic organ damage, and even cancer.
Acknowledgements
LV is funded by the National Research Development and Innovation Office (Hungary) grants GINOP-2.3.2-15-2016-00048-STAYALIVE, GINOP-2.3.2-15-2016-00020 TUMORDNS”, OTKA K112336, and K132193. CH was supported by the National Research Development and Innovation Office (Hungary) grant OTKA PD 116845 and by a Bolyai postdoctoral fellowship ÚNKP-18-4 (Bolyai+) fellowship. TAK acknowledges support by the German Research Foundation (Germany), grant KU 1945/4-1. Work in the laboratory of NR is supported by funds from the Department of State Development, South Australia and the University of South Australia. The authors are grateful for the careful English language editing by Dr. Karen Uray.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101239.
Contributor Information
Nirmal Robinson, Email: nirmal.robinson@unisa.edu.au.
Thomas A. Kufer, Email: thomas.kufer@uni-hohenheim.de.
László Virág, Email: lvirag@med.unideb.hu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gomez Perdiguero E. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginhoux F. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankman L.S. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015;21(6):628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanden Berghe T. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014;15(2):135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 5.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 6.Hersh D. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. U. S. A. 1999;96(5):2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilbi H. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 1998;273(49):32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 8.Murphy J.M., Silke J. Ars Moriendi; the art of dying well - new insights into the molecular pathways of necroptotic cell death. EMBO Rep. 2014;15(2):155–164. doi: 10.1002/embr.201337970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayagaki N. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 10.Shi J. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 11.Sborgi L. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017;17(3):151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W.T. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J. Mol. Cell Biol. 2018:1–13. doi: 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram A.M., Booty L.M., Bryant C.E. Chopping GSDMD: caspase-8 has joined the team of pyroptosis-mediating caspases. EMBO J. 2019;38(10) doi: 10.15252/embj.2019102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarhan J. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. U. S. A. 2018;115(46):E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orning P. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen K.W. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38(10) doi: 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen I. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 2016;213(10):2113–2128. doi: 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lugrin J., Martinon F. The AIM2 inflammasome: sensor of pathogens and cellular perturbations. Immunol. Rev. 2018;281(1):99–114. doi: 10.1111/imr.12618. [DOI] [PubMed] [Google Scholar]
- 23.Vance R.E. The NAIP/NLRC4 inflammasomes. Curr. Opin. Immunol. 2015;32:84–89. doi: 10.1016/j.coi.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M., Kanneganti T.D. Nlrp3: an immune sensor of cellular stress and infection. Int. J. Biochem. Cell Biol. 2010;42(6):792–795. doi: 10.1016/j.biocel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilig R., Broz P. Function and mechanism of the pyrin inflammasome. Eur. J. Immunol. 2018;48(2):230–238. doi: 10.1002/eji.201746947. [DOI] [PubMed] [Google Scholar]
- 26.Chavarria-Smith J., Vance R.E. The NLRP1 inflammasomes. Immunol. Rev. 2015;265(1):22–34. doi: 10.1111/imr.12283. [DOI] [PubMed] [Google Scholar]
- 27.Kayagaki N. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 28.Hagar J.A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 30.Baker P.J. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 2015;45(10):2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 31.Platnich J.M. Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep. 2018;25(6):1525–1536 e7. doi: 10.1016/j.celrep.2018.09.071. [DOI] [PubMed] [Google Scholar]
- 32.Rogers C. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019;10(1):1689. doi: 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L., Sun B. Neutrophil pyroptosis: new perspectives on sepsis. Cell. Mol. Life Sci. 2019;76(11):2031–2042. doi: 10.1007/s00018-019-03060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubartelli A. Redox control of NLRP3 inflammasome activation in health and disease. J. Leukoc. Biol. 2012;92(5):951–958. doi: 10.1189/jlb.0512265. [DOI] [PubMed] [Google Scholar]
- 35.Martinon F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010;40(3):616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 36.Masters S.L. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 2010;11(10):897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Bruggen R. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood. 2010;115(26):5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 38.van de Veerdonk F.L. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. U. S. A. 2010;107(7):3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meissner F. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116(9):1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heid M.E. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 2013;191(10):5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao C. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J. Biol. Chem. 2014;289(24):17020–17029. doi: 10.1074/jbc.M114.563114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jhang J.J. Monosodium urate crystals trigger Nrf2- and heme oxygenase-1-dependent inflammation in THP-1 cells. Cell. Mol. Immunol. 2015;12(4):424–434. doi: 10.1038/cmi.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meissner F., Molawi K., Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 2008;9(8):866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 44.Lupfer C.R. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014;10(9) doi: 10.1371/journal.ppat.1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019 doi: 10.1093/jmcb/mjz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenabeele P. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 47.Welz P.S. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477(7364):330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 48.Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25(6):347–353. doi: 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Sun L. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy J.M. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Degterev A. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 53.Mandal P. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell. 2014;56(4):481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McComb S. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 2012;19(11):1791–1801. doi: 10.1038/cdd.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y. Proteasome inhibitor PS-341 limits macrophage necroptosis by promoting cIAPs-mediated inhibition of RIP1 and RIP3 activation. Biochem. Biophys. Res. Commun. 2016;477(4):761–767. doi: 10.1016/j.bbrc.2016.06.132. [DOI] [PubMed] [Google Scholar]
- 56.Rijal D. Differentiated macrophages acquire a pro-inflammatory and cell death-resistant phenotype due to increasing XIAP and p38-mediated inhibition of RipK1. J. Biol. Chem. 2018;293(30):11913–11927. doi: 10.1074/jbc.RA118.003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karunakaran D. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016;2(7) doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bao L. Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J. Biol. Chem. 2006;281(44):33635–33649. doi: 10.1074/jbc.M606339200. [DOI] [PubMed] [Google Scholar]
- 59.Fortes G.B. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood. 2012;119(10):2368–2375. doi: 10.1182/blood-2011-08-375303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roca F.J., Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153(3):521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaseva A.V. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149(7):1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pajuelo D. NAD(+) depletion triggers macrophage necroptosis, a cell death pathway exploited by Mycobacterium tuberculosis. Cell Rep. 2018;24(2):429–440. doi: 10.1016/j.celrep.2018.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Y. Exogenous NAD(+) decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci. Rep. 2016;6:26322. doi: 10.1038/srep26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez-Juarbe N. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 2015;11(12) doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitur K. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11(4) doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilley R.P. Infiltrated macrophages die of pneumolysin-mediated necroptosis following pneumococcal myocardial invasion. Infect. Immun. 2016;84(5):1457–1469. doi: 10.1128/IAI.00007-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arifuzzaman M. Necroptosis of infiltrated macrophages drives Yersinia pestis dispersal within buboes. JCI Insight. 2018;3(18) doi: 10.1172/jci.insight.122188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He S. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. U. S. A. 2011;108(50):20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson N. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat. Immunol. 2012;13(10):954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knuth A.K. Interferons transcriptionally up-regulate MLKL expression in cancer cells. Neoplasia. 2019;21(1):74–81. doi: 10.1016/j.neo.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarhan J. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019;26(2):332–347. doi: 10.1038/s41418-018-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallorini M. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials. 2015;56:114–128. doi: 10.1016/j.biomaterials.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 73.Hos N.J. Type I interferon enhances necroptosis of Salmonella Typhimurium-infected macrophages by impairing antioxidative stress responses. J. Cell Biol. 2017;216(12):4107–4121. doi: 10.1083/jcb.201701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1–2):228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 75.Yang Z. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 2018;20(2):186–197. doi: 10.1038/s41556-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 76.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hocsak E. PARP inhibition protects mitochondria and reduces ROS production via PARP-1-ATF4-MKP-1-MAPK retrograde pathway. Free Radic. Biol. Med. 2017;108:770–784. doi: 10.1016/j.freeradbiomed.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 78.Ganesan R. Salmonella Typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrabi S.A., Dawson T.M., Dawson V.L. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann. N. Y. Acad. Sci. 2008;1147:233–241. doi: 10.1196/annals.1427.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berger N.A. Poly(ADP-ribose) polymerase mediates the suicide response to massive DNA damage: studies in normal and DNA-repair defective cells. Princess Takamatsu Symp. 1983;13:219–226. [PubMed] [Google Scholar]
- 81.Virag L. Peroxynitrite-induced thymocyte apoptosis: the role of caspases and poly (ADP-ribose) synthetase (PARS) activation. Immunology. 1998;94(3):345–355. doi: 10.1046/j.1365-2567.1998.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Virag L., Salzman A.L., Szabo C. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J. Immunol. 1998;161(7):3753–3759. [PubMed] [Google Scholar]
- 83.Virag L. Requirement of intracellular calcium mobilization for peroxynitrite-induced poly(ADP-ribose) synthetase activation and cytotoxicity. Mol. Pharmacol. 1999;56(4):824–833. [PubMed] [Google Scholar]
- 84.Virag L., Szabo C. BCL-2 protects peroxynitrite-treated thymocytes from poly(ADP-ribose) synthase (PARS)-independent apoptotic but not from PARS-mediated necrotic cell death. Free Radic. Biol. Med. 2000;29(8):704–713. doi: 10.1016/s0891-5849(00)00359-2. [DOI] [PubMed] [Google Scholar]
- 85.Erdelyi K. Dual role of poly(ADP-ribose) glycohydrolase in the regulation of cell death in oxidatively stressed A549 cells. FASEB J. 2009;23(10):3553–3563. doi: 10.1096/fj.09-133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crawford K. Specificity of reversible ADP-ribosylation and regulation of cellular processes. Crit. Rev. Biochem. Mol. Biol. 2018;53(1):64–82. doi: 10.1080/10409238.2017.1394265. [DOI] [PubMed] [Google Scholar]
- 87.Eliasson M.J. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997;3(10):1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 88.Kam T.I. Poly(ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson's disease. Science. 2018;362(6414) doi: 10.1126/science.aat8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan J., Dawson T.M., Dawson V.L. Cell death mechanisms of neurodegeneration. Adv Neurobiol. 2017;15:403–425. doi: 10.1007/978-3-319-57193-5_16. [DOI] [PubMed] [Google Scholar]
- 90.Yu S.W. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 91.Virag L. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol. Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science. 2016;354(6308) doi: 10.1126/science.aad6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zingarelli B. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J. Immunol. 1996;156(1):350–358. [PubMed] [Google Scholar]
- 94.Erdelyi K. Pathophysiologic role of oxidative stress-induced poly(ADP-ribose) polymerase-1 activation: focus on cell death and transcriptional regulation. Cell. Mol. Life Sci. 2005;62(7–8):751–759. doi: 10.1007/s00018-004-4506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Virag L., Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002;54(3):375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 96.Parameswaran N., Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010;20(2):87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agarwal S., Drysdale B.E., Shin H.S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J. Immunol. 1988;140(12):4187–4192. [PubMed] [Google Scholar]
- 98.Liu H. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J. Exp. Med. 2001;194(2):113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Regdon Z. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic. Biol. Med. 2019;131:184–196. doi: 10.1016/j.freeradbiomed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 100.Jang K.H. AIF-independent parthanatos in the pathogenesis of dry age-related macular degeneration. Cell Death Dis. 2017;8(1):e2526. doi: 10.1038/cddis.2016.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang F. PARP-1 hyperactivation and reciprocal elevations in intracellular Ca2+ during ROS-induced nonapoptotic cell death. Toxicol. Sci. 2014;140(1):118–134. doi: 10.1093/toxsci/kfu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Douglas D.L., Baines C.P. PARP1-mediated necrosis is dependent on parallel JNK and Ca(2)(+)/calpain pathways. J. Cell Sci. 2014;127(Pt 19):4134–4145. doi: 10.1242/jcs.128009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vince J.E. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36(2):215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 104.Yabal M. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7(6):1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 105.Lawlor K.E. XIAP loss triggers RIPK3- and caspase-8-driven IL-1beta activation and cell death as a consequence of TLR-myd88-induced ciap1-TRAF2 degradation. Cell Rep. 2017;20(3):668–682. doi: 10.1016/j.celrep.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 106.Conos S.A. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. U. S. A. 2017;114(6):E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lawlor K.E. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rathkey J.K. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3(26) doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luhrmann A. Necrotic death of Rhodococcus equi-infected macrophages is regulated by virulence-associated plasmids. Infect. Immun. 2004;72(2):853–862. doi: 10.1128/IAI.72.2.853-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gong Y.N. To the edge of cell death and back. FEBS J. 2019;286(3):430–440. doi: 10.1111/febs.14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruhl S. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362(6417):956–960. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- 112.Ditsworth D., Zong W.X., Thompson C.B. Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J. Biol. Chem. 2007;282(24):17845–17854. doi: 10.1074/jbc.M701465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z. PARP-1 mediates LPS-induced HMGB1 release by macrophages through regulation of HMGB1 acetylation. J. Immunol. 2014;193(12):6114–6123. doi: 10.4049/jimmunol.1400359. [DOI] [PubMed] [Google Scholar]
- 114.Xu J. Microglial activation induced by the alarmin S100B is regulated by poly(ADP-ribose) polymerase-1. Glia. 2016;64(11):1869–1878. doi: 10.1002/glia.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.