Abstract
Background and Aims
No prospective studies substantiate 15 eos/hpf as an appropriate endpoint for treatment of eosinophilic esophagitis (EoE). We aimed to determine a histologic cutpoint that identifies successful treatment of EoE by assessing symptomatic and endoscopic improvement.
Methods
We performed a prospective cohort study of 62 consecutive adult patients undergoing outpatient esophagogastroduodenoscopy at the University of North Carolina from 2009 through 2014. At diagnosis of EoE and after 8 weeks of standard treatment, symptom and endoscopic responses were measured using a visual analogue scale and an endoscopic severity score (ESS), and eosinophil counts were assessed. Receiver operator curves and logistic regression models evaluated the histologic threshold that best predicted symptomatic and endoscopic response. For symptoms, analysis was limited to patients without baseline esophageal dilation.
Results
The mean eosinophil count at diagnosis was 124 eos/hpf, falling to 35 eos/hpf after treatment. The mean visual analogue scale decreased from 3.4 at baseline to 1.7 after treatment, and the mean ESS decreased from 3 to 1.6. Twenty-nine patients had symptom responses (47%) and 34 had endoscopic responses (55)%. Post-treatment eosinophil count thresholds of 8, 15, and 5 eos/hpf best predicted symptom, endoscopic and combined responses, respectively. On logistic regression, decreasing eosinophil count was significantly associated with the probability of symptomatic (P=.01) and endoscopic response (P<.001).
Conclusions
In a prospective study of patients with EoE, we found that a cutpoint of <15 eos/hpf identifies most patients with symptom and endoscopic improvements, providing support for the current diagnostic threshold. A lower threshold (<5 eos/hpf) identifies most patients with a combination of symptom and endoscopic responses; this cutpoint might be used in situations that require a stringent histologic.
Keywords: cut-off value, predicted outcome, corticosteroids, dietary therapy
Introduction
Eosinophilic esophagitis (EoE) is a chronic, immune/antigen mediated disorder characterized histologically by esophageal inflammation with intraepithelial eosinophils and clinically by symptoms of esophageal dysfunction.1 The current criteria for diagnosis require the presence of at least 15 eosinophils per high-power field (eos/hpf) in esophageal biopsies and the exclusion of alternative etiologies of esophageal eosinophilia, in the correct clinical context.2–4 Since its original description 2 decades ago, EoE emerged as an important and increasingly recognized etiology of gastrointestinal morbidity.5
Treatment options for EoE include medications, dietary elimination, and esophageal dilation.1 However, what constitutes a response to these treatment modalities remains unclear. Possible outcome measures include symptoms, quality of life, endoscopic findings, biomarkers, and histologic findings.6 From a clinical standpoint, the goal is to decrease or eliminate symptoms, improve or normalize the endoscopic appearance, and decrease or resolve esophageal eosinophilia.1 However, no guidelines set firm response thresholds for any outcome measure.3,4 Most studies set histology as the primary outcome measure, as this may be assessed objectively, but used a range of values (from 0 eos/hpf to <15 eos/hpf).4,6,7 There are only a limited number of studies assessing the utility of different histologic endpoints for response in treated EoE patients.8 Though 15 eos/hpf provides conceptual symmetry with the diagnostic threshold, no prospective data substantiate that this threshold constitutes an appropriate treatment endpoint. Furthermore, it remains unknown which degrees of esophageal eosinophilia represent the most clinically relevant outcome measure. The degree of concordance between symptomatic, endoscopic, and histologic outcomes also remains unclear.9–11 This knowledge gap impedes patient care as well as the synthesis of the medical literature.12,13
The aim of this study was to determine an optimal histologic cutpoint after EoE treatment that maximizes symptomatic and endoscopic improvement.
Methods
Study design, patients, and measures
We analyzed data collected during a prospective cohort study at the University of North Carolina at Chapel Hill from 2009–2014 that enrolled consecutive adult patients undergoing outpatient esophagogastroduodenoscopy (EGD).14,15 The University of North Carolina institutional review board approved this study. Incident EoE cases were diagnosed per consensus guidelines. Cases had symptoms of esophageal dysfunction and at least 15 eosinophils per high-power field (hpf area = 0.24mm2) on esophageal biopsy after an 8 week high-dose proton-pump inhibitor trial to exclude PPI-responsive esophageal eosinophilia; competing causes of eosinophilia were excluded.2–4
After incident diagnosis, treatment of cases was at the discretion of their primary gastroenterologist with either a topical corticosteroid (tCS; budesonide viscous slurry dosed at 1 mg twice daily16,17 or fluticasone 880 mcg twice daily18–20) or dietary elimination therapy (six food elimination diets or targeted elimination diets).21–23 Patients were treated for 8 weeks and then reassessed with EGD. During the baseline and post-treatment endoscopies, a total of 5 research protocol biopsies obtained from the distal, mid, and proximal esophagus (two biopsies at 3, one biopsy at 8, and 2 biopsies 13cm, respectively, above the gastroesophageal junction) determined eosinophil counts. Quantification of eosinophils for the maximum number per high-power field (eos/hpf) utilized our previously validated protocol.24
At baseline and follow-up, a visual analogue scale (VAS) recorded dysphagia severity, which we previously showed to represent a reliable and responsive measure.25 For the VAS, patients placed a mark on a 10 cm line to answer the question, “How bad, on average, has your swallowing difficulty been over the past 30 days?” The VAS was anchored at 0 with “no trouble swallowing” and at 10 with “unable to even swallow saliva”. The mark was measured in mm to provide a 0–100 VAS score, with higher scores indicating more severe dysphagia.
Endoscopic findings of EoE were prospectively recorded at baseline and post-treatment. As data gathering pre-dated the EoE endoscopic reference score system (EREFS)26, we utilized a simplified endoscopic severity score (ESS) where each EREFS finding (exudates, rings, edema, furrows, stricture) was rated as absent or present. Scores ranged from 0–5, with higher scores representing a larger number of findings.
We also recorded patient demographics and co-morbidities using standardized data collection tools.
Statistical analysis
We examined the characteristics of the sample to determine the distribution of the variables and to assess any impact of missing data or extreme values. For continuous variables, the mean, standard deviation, and the shape of the distribution were determined. Frequencies were tabulated for categorical variables. Non-parametric testing did not alter the interpretation of the data. Bivariable analysis was described the entire patient cohort. To explore the relationship between baseline and follow-up VAS, ESS, and eosinophil counts, paired t-tests compared continuous variables (VAS and ESS) and McNemar’s chi-squared test compared categorical variables (endoscopic findings).
Because we aimed to evaluate histologic cutpoints optimizing symptomatic and endoscopic responses to treatment, we assessed whether esophageal dilation explained discordance between post-treatment histologic and symptom response. As baseline dilation did impact results, analyses of symptom and combined symptomatic and endoscopic responses were restricted to patients who not undergoing baseline esophageal dilation. Assessment of post-treatment endoscopic response alone included dilated and non-dilated patients. We empirically defined symptomatic and endoscopic responses as a ≥50% decrease in the VAS or ESS, respectively.
We used logistic regression to estimate the probability of symptomatic, endoscopic and combined symptomatic and endoscopic response after treatment. Models were constructed using post-treatment eosinophil count or percentage change in the eosinophil count as a continuous variable. Adjustment for confounding was limited given the available sample size and the number of outcomes. When appropriate, models were adjusted for baseline VAS and/or ESS measurement. These baseline covariates were included to help control for regression to the mean. Additionally, baseline scores negatively correlated with change, as low baseline scores generally improved more than higher scores.27
We constructed receiver operator curves (ROC) to assess the impact of a range of cutoff values for both post-treatment eosinophil counts and percentage change in eosinophil counts on VAS and ESS scores. To determine the optimal histologic threshold that best predicted symptomatic, endoscopic, and combined responses, we found the maximum sensitivity and specificity and the associated probability defining response from each ROC curve. We also performed a sensitivity analysis to determine if a 100% change in the VAS, ESS, or both (i.e. complete response as measured by no symptoms and/or normalization of the esophagus) would change our conclusions. Methods for sensitivity analyses related to endoscopic phenotypes and histology by esophageal level are in the Supplemental Materials. All data analysis was performed using Stata 14.1 (StataCorp, College Station, TX).
Results
Patient characteristics and overall treatment responses
A total of 62 adult patients newly diagnosed with EoE met inclusion criteria for this study. The mean age was 38 years, and cases were predominately white (94%) and male (55%) (Table 1). The mean peak eosinophil count at diagnosis was 123.7 ± 120.5, and this decreased to 34.6 ± 69.5 after treatment (Table 2). The baseline mean VAS score was 3.4, which decreased to 1.7 after treatment (p < 0.001). The mean ESS decreased from 3.0 to 1.6 (p < 0.001). For all patients, symptomatic response occurred in 29 (47%), endoscopic response in 34 (55%) and both symptomatic and endoscopic response in 16 (26%).
Table 1.
Baseline characteristics (n = 62)
| Demographics | |
| Age (mean years ± SD) | 38 ± 12 |
| Male (n, %) | 34 (55) |
| White (n, %) | 58 (94) |
| Any atopic disease (n, %) | 50 (86) |
| Asthma | 23 (37) |
| Rhinitis/sinusitis | 48 (77) |
| Dermatitis | 7 (11) |
| Food allergies | 12 (29) |
| Symptoms (n, %) | |
| Dysphagia | 61 (98) |
| Heartburn | 7 (11) |
| Abdominal pain | 7 (11) |
| Treatment type (n, %) | |
| Topical steroids | 59 (95) |
| Oral viscous budesonide | 51 (86) |
| Mean dose (mcg ± SD) | 2140 ± 700 |
| Fluticasone inhaler | 8 (14) |
| Mean dose (mcg ± SD) | 1790 ± 85 |
| Dietary elimination | 3 (5) |
| Targeted | 2 (67) |
| Six food elimination diet | 1 (33) |
Table 2.
Symptom, endoscopic and histologic findings before and after treatment
| Baseline (n = 62) | Follow-up (n = 62) | p | |
|---|---|---|---|
| Dysphagia severity (mean VAS ± SD) | 3.4 ± 3.0 | 1.7 ± 2.0 | < 0.001 |
| Mean % change in VAS | −26.7 ± 104.0 | ||
| 50% improvement in VAS (n, %) | 29 (47) | ||
| Endoscopic severity (mean ESS ± SD) | 3.0 ± 1.3 | 1.6 ± 1.3 | < 0.001 |
| Mean % change in ESS | −38.1 ± 51.9 | ||
| 50% improvement in ESS (n, %) | 34 (55) | ||
| Endoscopic findings (n, %) | |||
| Normal | 2 (3) | 11 (18) | 0.007 |
| Rings | 54 (87) | 39 (67) | < 0.001 |
| Stricture | 15 (24) | 11 (17) | 0.21 |
| Narrowing | 20 (32) | 18 (29) | 0.53 |
| Furrows | 54 (87) | 23 (27) | < 0.001 |
| Crêpe-paper mucosa | 5 (8) | 1 (2) | 0.05 |
| White plaques/exudates | 29 (47) | 12 (19) | < 0.001 |
| Edema/decreased vascularity | 31 (50) | 13 (21) | < 0.001 |
| Dilation performed | 18 (29) | 17 (27) | 0.48 |
| Eosinophil count (mean eos/hpf ± SD) | 123.7 ± 120.5 | 34.6 ± 69.5 | < 0.001 |
| Histologic responses (n, %) | |||
| < 15 eos/hpf | 44 (71) | ||
| < 5 eos/hpf | 35 (57) | ||
| < 1 eos/hpf | 27 (44) | ||
| 50% decrease | 50 (81) | ||
| 90% decrease | 41 (66) |
Abbreviations: VAS = visual analogue scale; ESS = endoscopic severity score; eos/hpf = eosinophils per high-power field; SD = standard deviation
Considering several histologic outcome values, 44 (71%) patients had post-treatment eosinophil counts <15 eos/hpf, while only 27 (44%) had 0 eos/hpf. In 50 (81%) patients, eosinophil counts decreased from baseline by ≥50%, while 41 (66%) had a decrease in counts of ≥90%.
Symptomatic and endoscopic responses by histologic response and baseline dilation
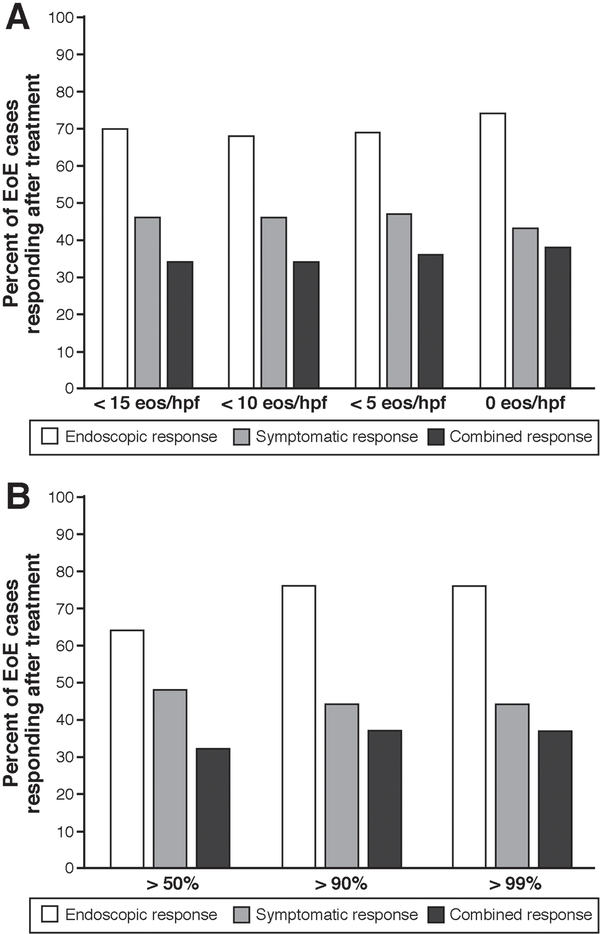
Symptomatic, endoscopic, and combined treatment responses were assessed for all patients after their initial treatment course. Among patients achieving <15 eos/hpf, 70% had an endoscopic response, 46% had a symptomatic response, and 34% had both a symptomatic and endoscopic response (Figure 1A). Among patients with 0 eos/hpf post-treatment, 74% had an endoscopic response, 43% had symptomatic response, and 38% had both. Results were similar using a percent change in the post-treatment eosinophil count: a decrease of 50% from the baseline eosinophil count was associated with an endoscopic and a symptom response rate of 64% and 46%, and both a symptomatic and endoscopic response in 33%. With a 90% decrease from the baseline eosinophil count, 76% had an endoscopic response, 44% had symptomatic response, and 38% had both a symptomatic and endoscopic response (Figure 1B; Supplementary figures A, B, C).
Figure 1.
(A) Endoscopic, symptomatic, and combined response depending on post-treatment eosinophil count thresholds. (B) Endoscopic, symptomatic, and combined response depending on percent change in post-treatment eosinophil counts.
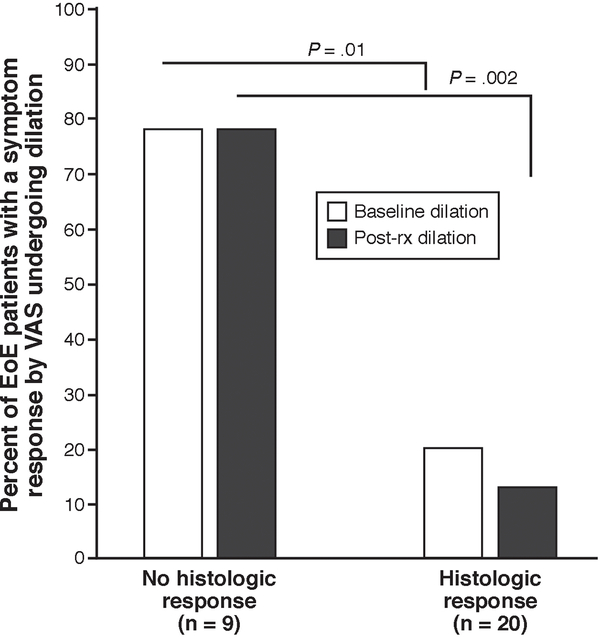
Because we hypothesized that esophageal dilation produces symptom response despite the tissue effect of anti-inflammatory therapy, we explored the relationship between post-treatment eosinophil counts and dilation among symptomatic responders. When examining the entire patient cohort, 18 patients did not have a histologic response (as defined by a post-treatment eosinophil count of < 15 eos/hpf). There were 9 of 18 (50%) histologic non-responders who reported a symptom response. Of these 9 non-responders with a symptom response, 7 (78%) were dilated at baseline. In contrast, just 4 of 20 (20%) of those with both a histologic and symptomatic response had baseline dilation (p = 0.01). Esophageal dilation likely explains why many histologic non-responders reported symptom responses (Figure 2). We thus restricted further statistical analysis of symptoms, as well as combined symptom and endoscopic responses, to patients who did not undergo esophageal dilation during the endoscopy when EoE was diagnosed.
Figure 2.
Frequency of baseline esophageal dilation in symptom responders (defined by a 50% decrease in the VAS), as stratified by histologic response (defined as <15 eos/hpf).
Assessment of histologic response thresholds
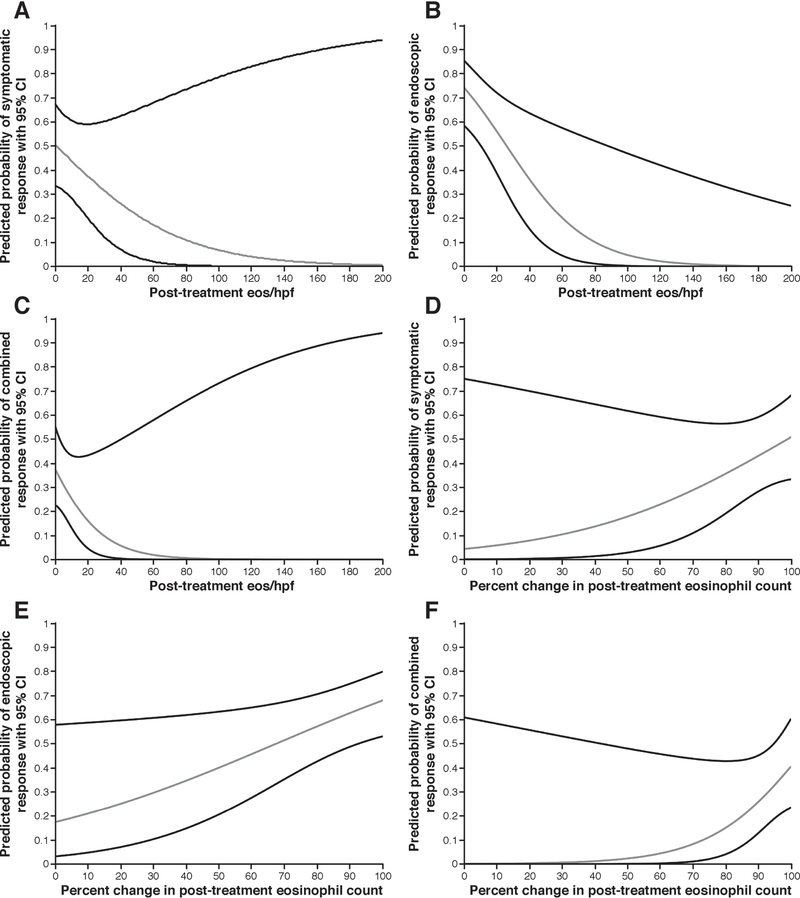
For every post-treatment decrease of 10 eos/hpf, the probability of symptomatic response increased by approximately 6% (p = 0.01) (Figure 3A), endoscopic response by approximately 7% (p < 0.001) (Figure 3B) and both symptomatic and endoscopic response by approximately 5% (p < 0.001) (Figure 3C). Percentage change in the post-treatment eosinophil counts produced similar results. For every 10% decrease in eosinophil count, symptom response increased by approximately 7% (p = 0.04) (Figure 3D), endoscopic response by 6% (p < 0.001) (Figure 3E), and combined symptomatic and endoscopic responses by 10% (p = 0.01) (Figure 3F). As post-treatment eosinophil counts clustered toward lower values, the limited number of patients with high post-treatment counts inflated variance and the width of associated predicted 95% CI’s.
Figure 3.
(A) Predicted probability of symptom response per post-treatment eosinophil count. (B) Predicted probability of endoscopic treatment response per post-treatment eosinophil count. (C) Predicted probability of combined treatment response per post-treatment eosinophil count. (D) Predicted probability of symptom response per percent change in post-treatment eosinophil count. (E) Predicted probability of endoscopic treatment response per percent change in post-treatment eosinophil count. (F) Predicted probability of combined treatment response per percent change in post-treatment eosinophil count.
The area under the ROC curves (AUC) for symptomatic, endoscopic, and combined symptomatic and endoscopic responses, utilizing post-treatment eosinophil counts as cutpoints, were 0.67, 0.85 and 0.83 respectively (Supplementary figure D). When considering all patients including those dilated at baseline, the AUC for symptomatic response decreased to 0.56. When utilizing a 50% change in eosinophil counts the cutpoint, the AUC for symptomatic, endoscopic and both symptomatic and endoscopic responses were 0.61, 0.74 and 0.80, respectively (Supplementary figure E). The ROC AUC for a 90% change in the eosinophil count was 0.60, 0.86 and 0.81 for symptom, endoscopic and combined responses (Supplementary figure F).
The post-treatment eosinophil count best predicting a symptomatic response was 8 eos/hpf (Figure 3). This corresponded to 63% of patients classified corrected, a sensitivity of 61% and a specificity of 64%. The post-treatment eosinophil count best predicting an endoscopic response was 15 eos/hpf. This corresponded to 76% of patients being correctly classified and a sensitivity and specificity of 79% and 71%, respectively. Last, the post-treatment eosinophil count found best predicting a combined response was 5 eos/hpf. Here, 79% of patients were correctly classified, and the sensitivity was 88% and specificity 76%.
We analyzed the impact of utilizing a 100% improvement in the VAS and ESS as the definition of outcome responses. A 100% improvement in the VAS score produced an AUC of 0.59, which was similar to the utilizing a 50% definition. A 100% improvement in the ESS resulted in an AUC of 0.66, which was less discriminative than the 50% definition. Only one patient achieved a 100% improvement in his/her VAS and ESS, which precluded a model examining this combined outcome.
Results from the sensitivity analyses related to endoscopic phenotypes and histology by esophageal level are presented in the Supplemental Materials and Supplemental Table 1.
Discussion
Few data describe the association of symptomatic, endoscopic, and histologic outcomes in EoE, and at this time, no consensus exists on the definition of an optimal post-treatment histologic cutpoint. Complicating this, studies have also documented discordance in these outcomes.28,29,30 Our paper further suggests a nebulous relationship between EoE-related symptoms and histologic activity. The variable histologic endpoints selected in clinical trials of EoE treatment add to this confusion. However, concordant symptomatic and endoscopic response was common in studies reaching a post-treatment eosinophil count of < 15 eos/hpf (despite started histologic outcome).18,20,31–34 Only one trial achieved <1eos/hpf without improvement in symptoms or endoscopy.19 In contrast, trials failing to lower eosinophil counts to <15 had inconsistent symptomatic and endoscopic outcomes.16,29
In our study, the post-treatment eosinophil counts best predicting symptom, endoscopic, and combined responses were 8, 15, and 5 eos/hpf. Making the count thresholds more restrictive did not result in substantial gains in symptomatic or endoscopic response. If a histologic response outcome were used as a measure of treatment efficacy, we favor using the absolute eosinophil count over the percentage change. The ongoing presence of eosinophils, which would occur in patients with high baseline counts treated to an endpoint of 50 or 90% reduction, may produce ongoing risk for fibrotic remodeling of the esophagus.33,35 Considering the sum of our results, a threshold of <15 eos/hpf appears a reasonable endpoint, particularly in clinical settings. However, our analysis demonstrates that the rate of endoscopic and symptomatic response increases with decreasing eosinophil counts. While pushing the response threshold lower than <15 eos/hpf may not result in large gains of response, settings exist (e.g. clinical trials) where a stringent histologic threshold is desired. In these cases, we found a threshold of <5 eos/hpf maximized a combined symptomatic and endoscopic response. The feasibility of achieving these response thresholds must be considered. In our data, 71% of patients obtained a post-treatment eosinophil count of <15 eos/hpf, while only 44% achieved <1 eos/hpf. This suggests that a sizable percentage of EoE patients would require intensified (or alternative) therapy to reach a more stringent threshold: this subjects patients to additional risks of treatment with marginal improvement in their disease process. We also determined the post-treatment eosinophil counts best predicting the resolution of phenotypic features of EoE. The resolution of fibrostenotic features required near normalization of biopsies, while resolution of inflammatory findings occurred with a less stringent reduction in eosinophil counts. Our models also better predicted symptomatic, endoscopic, and combined responses in patients with involvement of all esophageal segments (proximal, middle, and distal) at baseline, though this was most pronounced for symptom improvement.
As demonstrated by the actual rather than predicted outcome responses, improvements in outcomes at higher post-treatment eosinophil counts occur. However, as a limited number of patients exhibited high post-treatment eosinophil counts, cautious conclusions should be drawn for responses at these levels.
This study has several limitations. We relied on non-validated measures of symptom and endoscopic response. This was required, as no validated measures existed when the study was designed and initiated. However, the VAS provided an objective measure of dysphagia severity and we demonstrated that this is a simple and accurate measure that is treatment responsive.25 Similarly, the ESS can be thought of as closely related to the EREFS but with the categories collapsed. We do acknowledge that validated symptom and endoscopic assessments for evaluating EoE have now been published,9–11,36 and these should be applied in future prospective studies. Additionally, we utilized a 30-day recall period for the VAS. This period is relatively long and may have produced inaccuracies not seen with a shorter recall period. Furthermore, as we limited our final analyses to non-dilated patients, the sample size analyzing symptomatic and combined symptomatic and endoscopic outcomes was smaller than the entire cohort. This may have limited power to detect meaningful changes but not the validity of the findings. Outcomes were also assessed after an initial 8-week treatment course. Therefore, we are unable to comment on what histologic threshold might decrease long-term complications such as strictures of the esophagus, or intermittent outcomes such as food bolus impactions. Last, most patients received tCS, so the results may not be fully applicable to dietary elimination.
This study also has multiple strengths. This is one of the largest prospective cohorts with post-treatment data. Additionally, because these results were found outside of a clinical trial, we believe they represent “real-world” response rates that could be typical of clinical practice, giving them broad applicability. The analyses linking specific histologic treatment outcomes to symptomatic and endoscopic responses are also relatively unique in the EoE literature, particularly when considering prospectively collected data. We utilized a VAS for symptomatic outcomes, which we previously documented to represent a reliable and responsive measure.25 This allowed us to capture patient symptom experience along a continuum, rather than relying on physician impression of symptom improvement. In addition, endoscopic outcomes were captured prospectively with an ESS that is conceptually similar to the EREFS scoring system.10 We also excluded patients with baseline esophageal dilation to obviate confounding. Lastly, the post-treatment eosinophil cutpoints were similar to that which we previously reported in a retrospective study, which strengthens the validity of our current conclusions.7
In conclusion, we identified post-treatment eosinophil counts that best predicted symptom, endoscopic, and combined responses of 8, 15, and 5 eos/hpf, respectively. After exploring potential histologic outcome thresholds, we favor an eosinophil cut-point of <15 eos/hpf as this optimizes the probability of symptomatic and endoscopic improvement while also being readily attainable in most patients. It also provides conceptual symmetry, mirroring the current diagnostic threshold of ≥ 15 eos/hpf, which has been supported by empiric data.37 Though difficult to obtain in routine clinical practice, a threshold of 5 eos/hpf maximizes symptomatic and endoscopic responses. This cutpoint may be more appropriate in settings like clinical trials if a stringent histologic threshold is desired. These cutpoints provide a starting point for future investigations where the merits of this response threshold in additional prospective cohorts of EoE can be assessed.
Supplementary Material
Acknowledgments
Grant Support: This research was supported, in part, by NIH awards T32DK007634 (CCR, WAW, CCC), K24DK100548 (NJS), K23DK090073 (ESD), and P30DK034987
Disclosures: Dr. Dellon has received research funding from Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, an educational grant from Banner, and is a consultant for Adare, Alvio, Banner, Enumeral, GSK, Receptos/Celegene, Regeneron, and Shire. None of the other authors have any relevant conflicts of interest to disclose.
Abbreviations
- AUC
Area under the curve
- EGD
esophagogastroduodenoscopy
- EoE
eosinophilic esophagitis
- eos/hpf
eosinophils per high-power field
- EREFS
EoE endoscopic reference score system
- ESS
endoscopic severity score
- EEsAI
EoE symptom activity index
- Hpf
high-power field
- ROC
receiver operator curve
- tCS
topical corticosteroid
- VAS
visual analogue scale
References
- 1.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147(6):1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuta GT, Liacouras C a, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–1363. [DOI] [PubMed] [Google Scholar]
- 3.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. [DOI] [PubMed] [Google Scholar]
- 4.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–92. [DOI] [PubMed] [Google Scholar]
- 5.Safroneeva E, Coslovsky M, Kuehni CE, et al. Eosinophilic oesophagitis: Relationship of quality of life with clinical, endoscopic and histological activity. Aliment Pharmacol Ther. 2015;42(8):1000–1010. [DOI] [PubMed] [Google Scholar]
- 6.Hirano I Editorial: Should patients with suspected eosinophilic esophagitis undergo a therapeutic trial of proton pump inhibition? Am J Gastroenterol. 2013;108(3):373–375. [DOI] [PubMed] [Google Scholar]
- 7.Wolf WA, Cotton CC, Green DJ, et al. Evaluation of Histologic Cutpoints for Treatment Response in Eosinophilic Esophagitis. J Gastroenterol Hepatol Res. 2015;4(10):1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano I Therapeutic End Points in Eosinophilic Esophagitis: Is Elimination of Esophageal Eosinophils Enough? Clin Gastroenterol Hepatol. 2012;10(7):750–752. [DOI] [PubMed] [Google Scholar]
- 9.Schoepfer AM, Straumann A, Panczak R, et al. Development and Validation of a Symptom-Based Activity Index for Adults With Eosinophilic Esophagitis. Gastroenterology. 2014;147(6):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Irani A-M, Hill MR, Hirano I. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38(6):634–642. [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102(10):2300–2313. [DOI] [PubMed] [Google Scholar]
- 13.Sperry SLW, Shaheen NJ, Dellon ES. Toward uniformity in the diagnosis of eosinophilic esophagitis (EoE): the effect of guidelines on variability of diagnostic criteria for EoE. Am J Gastroenterol. 2011;106(5):824–32; quiz 833. [DOI] [PubMed] [Google Scholar]
- 14.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis From Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2009;7(12):1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellon ES, Chen X, Miller CR, et al. Diagnostic utility of major basic protein, eotaxin-3, and leukotriene enzyme staining in eosinophilic esophagitis. Am J Gastroenterol. 2012;107:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellon ES, Sheikh A, Speck O, et al. Viscous Topical is More Effective than Nebulized Steroid Therapy for Patients with Eosinophilic Esophagitis. Gastroenterology. 2012;143(2):321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellon ES K Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139(2):418–429.e1 [DOI] [PubMed] [Google Scholar]
- 18.Konikoff MR, Noel RJ, Blanchard C, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Fluticasone Propionate for Pediatric Eosinophilic Esophagitis. Gastroenterology. 2006;131(5):1381–1391. [DOI] [PubMed] [Google Scholar]
- 19.Alexander JA, Jung KW, Arora AS, et al. Swallowed Fluticasone Improves Histologic but Not Symptomatic Response of Adults With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–749. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13(1):66–76. [DOI] [PubMed] [Google Scholar]
- 21.Wolf WA, Jerath MR, Sperry SLW, et al. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(8):1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonsalves N, Yang GY, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; Food reintroduction identifies causative factors. Gastroenterology. 2012;142(7):1451–1459.e1. [DOI] [PubMed] [Google Scholar]
- 23.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130(2). [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Fritchie KJ, Rubinas TC, et al. Inter- and Intraobserver Reliability and Validation of a New Method for Determination of Eosinophil Counts in Patients with Esophageal Eosinophilia. Dig Dis Sci. 2011;55(7):1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed C, Wolf W, Cotton C, Dellon E. A visual analogue scale and a Likert scale are simple and responsive tools for assessing dysphagia in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol. 2016;14(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2009;48(2):152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debrosse CW, Franciosi JP, King EC, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011;128(1):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults with Eosinophilic Esophagitis. Gastroenterology. 2016;150(3):581–590e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–1604. [DOI] [PubMed] [Google Scholar]
- 32.Wolf WA, Cotton CC, Green DJ, et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin Gastroenterol Hepatol. 2015;13(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dellon ES, Kim HP, Sperry SLW, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dellon ES, Katzka DA, Collins MH, et al. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared with Placebo in Patients with Eosinophilic Esophagitis. Gastroenterology. 2016;In press,. [DOI] [PubMed] [Google Scholar]
- 35.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–1236. [DOI] [PubMed] [Google Scholar]
- 36.Franciosi JP, Hommel K, DeBrosse CW, et al. Development of a validated patient-reported symptom metric for pediatric Eosinophilic Esophagitis: qualitative methods. BMC Gastroenterol. 2011;11(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dellon S, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol. 2015;28(3):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.