Abstract
A 58-year-old man with previous myocardial infarction presented to our hospital with fever, cough, and dyspnea. PCR testing with nasopharyngeal swabs confirmed influenza virus infection, and enhanced computed tomography and transthoracic echocardiography revealed bilateral ground-glass opacities and consolidation, deep venous thrombosis, acute pulmonary artery embolism, and acute arterial embolism that appeared to originate from thrombus in the left ventricle. Combination of a neuraminidase inhibitor, antibiotics, an anticoagulant, and anti-platelet agent improved these complications; however, amputation of the patient's right foot was required. Because influenza can cause vascular events, physicians should pay attention to this complication in patients with influenza-associated pneumonia.
Keywords: Influenza, Pneumonia, Acute arterial embolism, Deep venous thrombosis, Gangrene
Abbreviations
- APTE
Acute pulmonary thromboembolism
- CT
Computed tomography
- DVT
Deep vein thrombosis
- HD
Hospital day
- LV
Left ventricle
- TTE
Transthoracic echocardiogram
1. Introduction
Influenza is a common infection that causes epidemics every winter season. Major complications of influenza include pneumonia, encephalopathy, and myocarditis. However, patients with influenza can also develop arterial and venous vascular events. We recently experienced a patient with influenza-associated pneumonia complicated with deep venous thrombosis, acute pulmonary artery embolism, and acute arterial embolism that presumably originated from the left ventricle (LV). To our knowledge, there have been only two reports [1,2] of acute arterial embolism complicating influenza-associated pneumonia.
2. Case presentation
A 58-year-old man presented to Saitama Cardiovascular and Respiratory Center with dyspnea, fever, and cough for 2 weeks. His mother had suffered from influenza several days before his initial symptoms. He had a history of inferior LV wall myocardial infarction and hypertension in 2013 but had stopped regular follow-up. He had not been vaccinated for influenza or pneumococcus. He had smoked from 20 years of age but did not drink alcohol. He had never been exposed to dust and had no problematic family history including that of thrombosis.
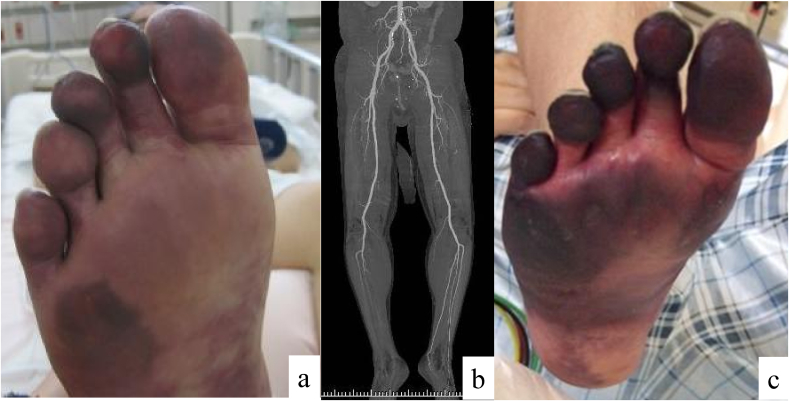
On presentation, his vital signs included a body temperature of 37.7 °C, heart rate of 129/min, blood pressure of 115/66 mmHg, and respiratory rate of 38/min. His height was 174 cm and body weight was 66 kg. Chest auscultation revealed a gallop and fine crackles in the bilateral lung fields. Lower extremity pulses in the dorsal artery of his right foot were absent, and the toes of his right foot were blue (Fig. 1a). Neurological findings were within normal limits except for the disappearance of sensory and motor signs in the right foot (paralysis).
Fig. 1.
Appearance of the right foot. The patient's toes were pale on admission (a), and enhanced computed tomography showed contrast delay in the lower extremities (b). This condition progressed to gangrene by hospital day 18 (c).
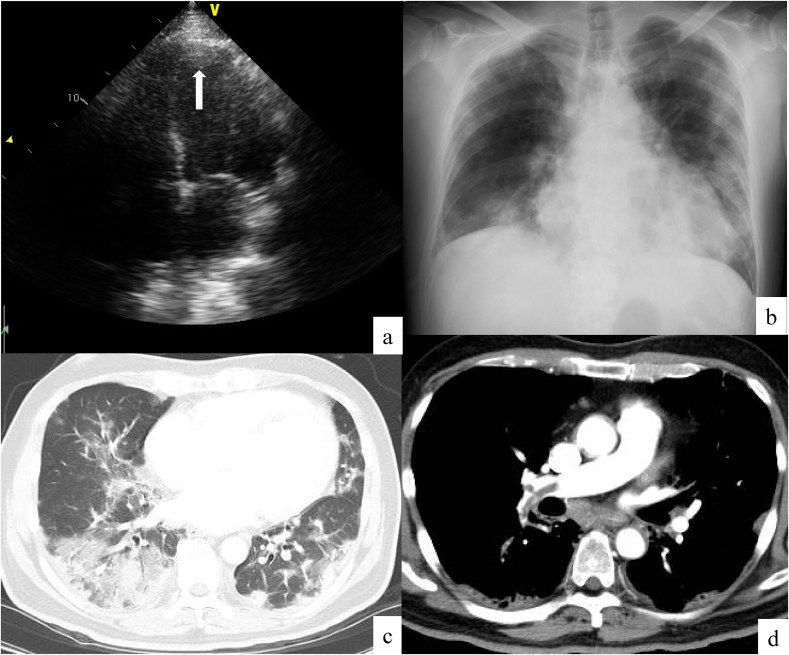
A 12-lead electrocardiogram showed wide QRS waves, ST elevation in the chest leads, and a significant Q wave in leads II, III, and aVF. Transthoracic echocardiogram (TTE) showed impaired LV motion, especially in the inferior LV wall and apex, with a reduced LV ejection fraction of 30–35%. A low-echoic nodule of 12 mm in size was detected in the LV apex (Fig. 2a). The caliber of the inferior vena cava was enlarged. Arterial blood gas analysis under 5 L/min of O2 showed a pH of 7.53, PaCO2 of 26.4 Torr, PaO2 of 99.9 Torr, HCO3− of 21.5 mmol/L, and lactate of 2.24 mmol/L. Blood test results included white blood cells of 25,400/mm3 (neutrophils, 23,400/mm3; lymphocytes, 1400/mm3; eosinophils, 200/mm3; monocytes, 300/mm3, and basophils, 100/mm3), hemoglobin of 12.0 g/dL, and platelets of 32.8 × 104/mm3. Serum tests showed a total protein of 6.6 g/dL, albumin of 2.2 g/dL, urea nitrogen of 19 mg/dL, creatinine of 1.29 mg/dL, AST of 170 IU/L, ALT of 87 IU/L, lactate dehydrogenase of 1040 IU/L, creatine kinase of 10,337 IU/L, creatine kinase MB of 28 IU/L, troponin-T of 1.34 ng/mL, C-reactive protein of 18.1 mg/dL, KL-6 of 1881 U/mL, and BNP of 797.8 pg/mL. Antinuclear antibody was negative. Coagulation test showed an activated partial thromboplastin time of 27.1 s, prothrombin time of 14.0 s, fibrinogen of 349 mg/dL, fibrin degradation product of 118.5 μg/mL, and D-dimer of 59.8 μg/mL. Protein C and protein S values were within normal range, and lupus anticoagulant was negative. Rapid diagnostic Mycoplasma pneumoniae and influenza antigen tests were both negative, as were urinary antigen tests for Streptococcus pneumoniae and Legionella sp. Our patient could not expectorate sputum, and a blood culture test was negative. Chest X-ray showed bilateral consolidations and cardiomegaly (Fig. 2b). Chest computed tomography showed consolidations and ground-glass opacities distributed in the bilateral lung fields (Fig. 2c). There was no pleural effusion or lymphadenopathy. Enhanced computed tomography (CT) detected filling defects in the pulmonary arteries (Fig. 2d), and a vascular echogram of the lower extremities showed low-echoic thrombus in the right femoral vein, great saphenous vein, bilateral popliteal vein, and the cnemial veins (posterior tibial vein, peroneal vein, and soleus muscle vein). Enhanced CT also showed contrast delay in the lower extremities (Fig. 1b), especially distal to the right ankle joint.
Fig. 2.
Echocardiographic and chest and imaging on admission. Thrombus was detected in the left ventricle by transthoracic echocardiography (arrow) (a). Chest X-ray on admission showed bilateral consolidations and cardiomegaly (b). Chest computed tomography (CT) showed bilateral consolidations and ground-glass opacities (c). Enhanced CT showed filling defects in the pulmonary arteries (d).
We suspected the patient of having pneumonia, especially viral pneumonia, complicated with deep vein thrombosis (DVT), acute pulmonary thromboembolism (APTE), acute arterial embolism, and acute myocardial infarction. Coronary arteriography showed a stenosis of the left anterior descending artery, and we performed percutaneous coronary intervention with stenting. To treat the pneumonia, we started ampicillin/sulbactam, minocycline, and peramivir. Methylprednisolone was also started at 1 g daily for 3 days because we could not rule out diffuse alveolar damage by CT findings. On hospital day (HD) 2, PCR testing using nasopharyngeal swabs was performed that was positive for influenza A virus. Prednisolone 40 mg daily was started from HD 4 and was tapered to 20 mg daily, 10 mg daily, 5 mg daily, and stopped every 3 days. We also started heparin, urokinase, and apixaban for DVT and APTE. TTE performed on HD 15 and enhanced CT performed on HD 19 showed regression of the DVT. We also started aspirin and atorvastatin for acute arterial embolism. TTE performed on HD 8 also detected the LV thrombus, but it could not be found on HD 18. His respiratory condition improved, and oxygen treatment was stopped; however, the toes of his right foot progressed to gangrene (Fig. 1c), and he was transferred to the Department of Orthopedics at another hospital for amputation of the foot.
3. Discussion
Influenza-associated pneumonia is classified into primary viral pneumonia, mixed viral and bacterial pneumonia, and secondary bacterial pneumonia [3]. Although sputum or bronchial aspirates could not be tested, other pathogens including viruses and bacteria were not found, and we diagnosed our patient as having primary viral pneumonia.
The D-dimer value on presentation was elevated, and enhanced CT detected DVT and APTE. A high incidence of DVT has been reported in patients with influenza, and we also reported the incidence of APTE in hospitalized patients with influenza-associated pneumonia to be 1% [4]. DVT regressed by HD 18 due to effective treatment with apixaban. In addition, there have been reports of high rates of arterio-vascular complications in influenza: the incidence ratio of an admission for acute myocardial infarction, which our patient developed, during the risk interval as compared with the control interval was 6.05 (95% confidence interval, 3.86 to 9.50) [5]. However, there have been only 2 reports of acute arterial embolism complicating influenza-associated pneumonia [1,2], and we have not experienced such cases [4]. Bunce et al. reported a 50-year-old woman with acute infra-renal aortic embolism who underwent surgical de-embolization, bilateral aortoiliac stenting, and left above-knee amputation [1]. Hüzmeli et al. reported a 28-year-old man with acute infra-renal aortic embolism who received enoxaparin treatment but developed acute kidney injury requiring hemodialysis [2]. The patient died of Acinetobacter spp. bacteremia.
Influenza has been reported as a risk factor for thrombosis. Interactions among influenza, platelets, and endothelial cells have been known to explain vascular events accompanying influenza [6]. An in vitro study reported that monocytes and endothelial cells that were incubated with influenza were able to activate coagulation via endothelial dysfunction and elevated tissue factor levels [7,8]. Influenza virus A subtype H3N2 infects endothelial cells and induces their apoptosis, which promotes adhesion of platelets to the cells [9]. In addition to the endothelium, the influenza virus may also directly affect platelets. An H3N2 virus added directly to platelets was found to induce clumping of both human and rabbit platelets [10]. Cytokines can also induce endothelial cell retraction, exposing the pro-atherogenic extracellular matrix. In our patient, thrombus was detected by TTE in the LV apex, and we suspected this thrombus to be the cause of the acute arterial embolism. A detailed mechanism for the development of thrombus in the LV is unclear, but our patient had a history of myocardial infarction 6 years before and suffered an acute myocardial infarction at this admission. Impaired LV wall motion may allow the formation of thrombus in the LV. We did not perform surgery to remove the emboli because of the unclear duration from onset and the disappearance of sensory and motor signs in the right foot. Enhanced CT indicated an embolism peripheral to the ankle joint, and although we administered heparin and urokinase, unfortunately, amputation could not be prevented.
The rapid influenza diagnostic test is a useful diagnostic tool that facilitates testing for influenza infection, but its low sensitivity is problematic. PCR using nasopharyngeal swabs was positive for influenza, whereas the rapid influenza diagnostic test was negative. Although PCR is expensive and technically demanding, its high sensitivity was useful in diagnosing our patient. CT findings were compatible with those of primary influenza viral pneumonia [11,12], and PCR is desirable in such cases for confirmation.
We administered peramivir and corticosteroid on admission. Although the efficacy of these agents on influenza-associated pneumonia is controversial, favorable results have been reported in severely ill patients [13]. We have also experienced patients with influenza-associated pneumonia effectively treated with corticosteroids. To avoid the development of infection in the patient's gangrenous foot, systemic corticosteroid therapy was ceased after 2 weeks.
4. Conclusion
We reported a case of primary influenza virus pneumonia complicated by severe vascular events. Influenza is a common infection, and millions of people are infected every year in Japan. Careful attention to its complications is needed, and acute arterial embolism should also be considered because it can potentially result in a functional handicap.
Conflicts of interest
All authors have no conflict of interest to declare.
Funding
A part of this study was supported by a Research Fund from Saitama Cardiovascular and Respiratory Center under Grant (No. 16ES).
Acknowledgement
We thank our colleagues in the Departments of Respiratory Medicine, Cardiology, and Nursing at Saitama Cardiovascular and Respiratory Center.
Contributor Information
Takashi Ishiguro, Email: ishiguro.takashi@pref.saitama.lg.jp.
Keisuke Matsuo, Email: matsuo.keisuke@pref.saitama.lg.jp.
Shinya Fujii, Email: fujii.shinya@pref.saitama.lg.jp.
Noboru Takayanagi, Email: takayanagi.noboru@pref.saitama.lg.jp.
References
- 1.Bunce P.E., High S.M., Nadjafi M., Stanley K., Liles W.C., Christian M.D. Pandemic H1N1 influenza infection and vascular thrombosis. Clin. Infect. Dis. 2011;52:e14–e17. doi: 10.1093/cid/ciq125. [DOI] [PubMed] [Google Scholar]
- 2.Hüzmeli C., Saglam M., Arıkan A., Doner B., Akıncı G., Candan F. Infrarenal aorta thrombosis associated with H1N1 influenza A virus infection. Case Rep. Infect. Dis. 2016;2016:9567495. doi: 10.1155/2016/9567495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louria D.B., Blumenfeld H.L., Ellis J.T., Kilbourne E.D., Rogers D.E. Studies on influenza in the pandemic of 1957-1958. II. Pulmonary complications of influenza. J. Clin. Investig. 1959;3:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishiguro T., Kagiyama N., Uozumi R., Odashima K., Takaku Y., Kurashima K., Morita S., Takayanagi N. Clinical characteristics of influenza-associated pneumonia of adults: clinical features and factors contributing to severity and mortality. Yale J. Biol. Med. 2017;90(2):165–181. [PMC free article] [PubMed] [Google Scholar]
- 5.Kwong J.C., Schwartz K.L., Campitelli M.A., Chung H., Crowcroft N.S., Karnauchow T., Katz K., Ko D.T., McGeer A.J., McNally D., Richardson D.C., Rosella L.C., Simor A., Smieja M., Zahariadis G., Gubbay J.B. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong S.M., Darwish I., Lee W.L. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence. 2013;4:537–542. doi: 10.4161/viru.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visseren F.L., Bouwman J.J., Bouter K.P., Diepersloot R.J., de Groot P.H., Erkelens D.W. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb. Haemostasis. 2000;84:319–324. [PubMed] [Google Scholar]
- 8.Keller T.T., van der Sluijs K.F., de Kruif M.D., Gerdes V.E., Meijers J.C., Florquin S., van der Poll T., van Gorp E.C., Brandjes D.P., Buller H.R., Levi M. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ. Res. 2006;99:1261–1269. doi: 10.1161/01.RES.0000250834.29108.1a. [DOI] [PubMed] [Google Scholar]
- 9.Bombeli T., Schwartz B.R., Harlan J.M. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999;93:3831–3838. [PubMed] [Google Scholar]
- 10.Terada H., Baldini M., Ebbe S., Madoff M.A. Interaction of influenza virus with blood platelets. Blood. 1966;28:213–228. [PubMed] [Google Scholar]
- 11.Ishiguro T., Takayanagi N., Kanauchi T., Uozumi R., Kawate E., Takaku Y., Kagiyama N., Shimizu Y., Hoshi T., Morita S., Sugita Y. Clinical and radiographic comparison of influenza virus-associated pneumonia among three viral subtypes. Intern. Med. 2016;55(7):731–737. doi: 10.2169/internalmedicine.55.5227. [DOI] [PubMed] [Google Scholar]
- 12.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18–39. doi: 10.1148/radiol.11092149. [DOI] [PubMed] [Google Scholar]
- 13.Quispe-Laime A.M., Bracco J.D., Campagne C.G., Barberio P.A., Campagne C.G., Rolfo V.E., Umberger R., Meduri G.U. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36(1):33–41. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]