Abstract
The endocannabinoid (eCB) system has been implicated in a variety of physiological functions due to abundant expression of its receptors and endogenous ligands in the central nervous system. Substantial progress has been made in understanding how the eCB system influences the brain norepinephrine (NE) system, an important neurochemical target in the continued development of new therapies for stress-induced psychiatric disorders. We, and others, have characterized the neuroanatomical, biochemical and pharmacological effects of cannabinoid receptor modulation on brain noradrenergic circuitry and defined how molecular elements of the eCB system are positioned to directly impact the locus coeruleus (LC)-prefrontal cortex pathway, a neural circuit well recognized for contributing to symptoms of hyperarousal, a key pathophysiological feature of stress-related disorders. We also described molecular and electrophysiological properties of LC noradrenergic neurons and NE release in the medial prefrontal cortex under conditions of cannabinoid type 1 receptor deletion. Finally, we identified how stress influences cannabinoid modulation of the coeruleo-cortical pathway. A number of significant findings emerged from these studies that will be summarized in the present review and have important implications for clinical studies targeting the eCB system in the treatment of stress-induced psychiatric disorders.
Keywords: Psychiatric disorder, Corticotropin-releasing factor, Noradrenergic, Cannabinoid type 1 receptor, Sex difference, Posttraumatic stress disorder
List of abbreviations
- μm
micrometer
- 2-AG
2-arachidonylglycerol
- AA
arachidonic acid
- ACTH
adrenocorticotropic hormone
- AEA
N-arachidonylethanolamide/anandamide
- ANOVA
analyses of variance
- AR
adrenoceptor
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- Ca2+
calcium
- CB1r
cannabinoid type 1 receptor
- CNA
central nucleus of the amygdala
- cort
cortisol
- COX-2
cyclooxygenase-2
- CRF
corticotropin releasing factor
- CRFr
corticotropin releasing factor receptor
- CRFr1
corticotropin releasing factor receptor type 1
- DGL
diacylglycerol lipase
- eCB
endocannabinoid
- ELISA
enzyme-linked immunosorbent assay
- EMT
endocannabinoid membrane transporter
- FAAH
fatty acid amide hydrolase
- g
gram
- GABA
gamma-aminobutyric acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HPA
hypothalamic-pituitary adrenal (axis)
- KCl
potassium chloride
- KO
knockout
- LC
locus coeruleus
- MGL
monoacylglycerol lipase
- min
minute
- mPFC
medial prefrontal cortex
- mRNA
messenger ribonucleic acid
- NAPE-PLD
n-acyl-phosphatidylethanolamine-hydrolyzing phospholipase-D
- NE
norepinephrine
- nM
nanomolar
- PHAL
phaseolus vulgaris-leucoagglutinin
- PFC
prefrontal cortex
- PGi
nucleus paragigantocellularis
- PrH
nucleus prepositus hypoglossi
- PTSD
posttraumatic stress disorder
- PVN
paraventricular nucleus
- sec
second
- SEM
standard error of the mean
- TH
tyrosine hydroxylase
- THC
Δ-9 tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid receptor type 1
- WT
wild type
1. Introduction
Chronic stress exposure is associated with the onset and severity of numerous psychiatric diseases including substance abuse, mood and anxiety disorders (McEwen, 2008; Carvalho and Van Bockstaele, 2012). However, not all individuals exposed to the same stress will develop these impairments and a challenge of modern medicine is to distinguish the neurobiological substrates underlying differences between vulnerable and resilient individuals. Thus, identifying cellular mechanisms that confer stress resilience and vulnerability is critically important to develop modalities to increase resistance and prevent stress-induced psychiatric disease. Sex, childhood trauma and genetics are also important factors that influence vulnerability. While most studies have used exclusively male subjects, females are more susceptible to many stress initiated neuropsychiatric disorders (Kendler et al., 1995; Marcus et al., 2005). Women are about twice as likely as men to suffer from anxiety disorders, such as panic disorder or from posttraumatic stress disorder (PTSD) and have higher rates of depression (Marcus et al., 2005). Delineating sex-dependent interactions between neurochemical mediators of stress resilience and components of the locus coeruleus (LC)-norepinephrine (NE) system is critical to our continued understanding of cellular mechanisms underlying effective coping to stress. An approach towards elucidating the link between stress and psychiatric disease is to investigate mechanisms by which neurobiological substrates of the stress response interface with neurotransmitter systems that have been implicated in these disorders.
Modulation of noradrenergic neurotransmission is an important mechanism for adapting to stress. While moderate levels of NE release may enhance working memory and prefrontal cortex function via high affinity α2 adrenoceptors, high levels of NE impair prefrontal cortical function via low affinity α1 and perhaps β1 adrenoceptors (Sara, 2009). The locus coeruleus (LC) provides the sole source of NE in the mammalian forebrain thus mediating a variety of brain functions and behaviors such as arousal, memory acquisition, attention, vigilance, and responses to stress (Aston-Jones et al., 1996; Valentino and Van Bockstaele, 2008; Sara, 2009). It is well recognized that the central noradrenergic system, and the LC in particular, plays a key role in the pathophysiology of stress-related disorders.
The LC-NE system is a major stress response system that is regulated by the pro-stress neuropeptide, corticotropin-releasing factor (CRF) as well as the endocannabinoid (eCB) system, an anti-stress modulator. The eCB system has profound effects on mood and behavior, in part through their modulation of the stress-integrative LC-NE system. Cannabinoid type 1 receptor (CB1r) agonists are capable of increasing noradrenergic activity and anxiety-like behaviors; however, they can also decrease stress-induced anxiety (Wyrofsky et al., 2015). This review will summarize our current understanding of how the eCB system modulates LC-NE activity and stress-related afferents to the LC. Specifically, we will review the neuroanatomical, biochemical and pharmacological effects of cannabinoid receptor modulation on brain noradrenergic circuitry as well as newer neuroanatomical findings demonstrating the cellular localization of CB1r with respect to CRF afferents in the LC. We will also describe findings from recent electrophysiological studies examining eCB modulation of CRF afferents in the LC and finally, adaptations in eCB signaling in the LC in a resident-intruder model of social stress that reveals that chronic stress differentially alters eCB system protein expression levels in the LC across sexes. Taken together, the findings illustrate how targeting CB1r specifically in the LC-NE circuit, a pathway dysregulated following chronic stress, could restore homeostasis in noradrenergic activity that could contribute therapeutic relief for stress-induced anxiety disorders, notably post-traumatic stress disorder. We begin with a brief overview of the eCB system and stress signaling processes in the mammalian central nervous system, then explore how the eCB system interfaces specifically with the LC-NE system in modulating the stress response.
2. The eCB system: an “anti-stress” neuromediator
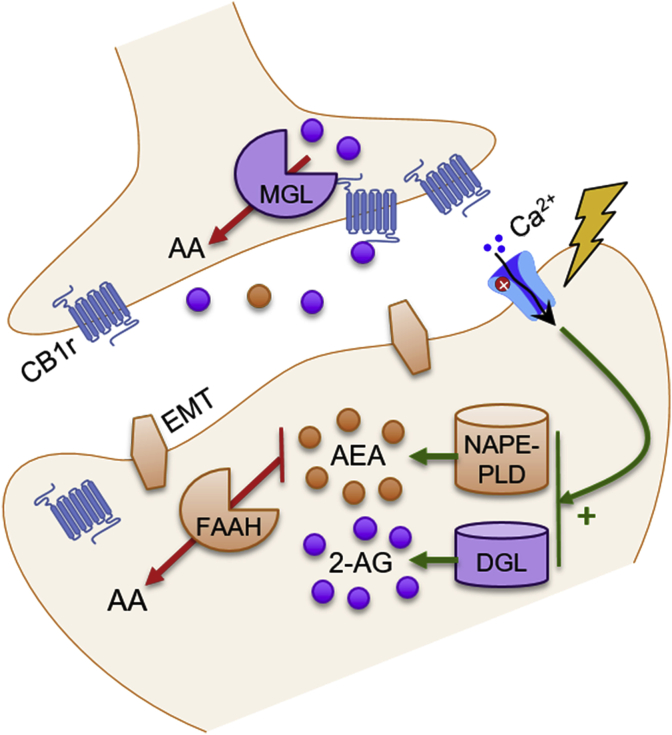
Cannabis sativa, or marijuana, has been used throughout human history as a stress-reducing agent, as well as a drug to reduce anxiety, pain, muscle spasms, and seizures (Zuardi, 2006). Modern research has confirmed that several compounds found in cannabis, namely Δ9-tetrahydrocannabinol (THC) and cannabidiol, are successful at reducing stress and providing anxiolytic behavioral effects (Green et al., 2003; Bergamaschi et al., 2011). Though there are many endogenous cannabinoids, the two most prevalent are N-arachidonoylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG) (Di Marzo et al., 2004). Traditionally, the eCB system is thought to signal in a retrograde fashion (Castillo et al., 2012). Postsynaptic depolarization-induced increases in intracellular calcium (Ca2+) and activation of phospholipase C cause diacylglycerol lipase (DGL) and N-acyl-phosphatidyl-ethanolamine-hydrolyzing phospholipase-D (NAPE-PLD) to synthesize 2-AG and AEA, respectively (Castillo et al., 2012). 2-AG and AEA then cross the synapse, where they bind to presynaptic Gi-coupled CB1r, thereby inhibiting subsequent neurotransmitter release. 2-AG and AEA are then degraded by presynaptic monoacylglycerol lipase (MGL) and postsynaptic fatty acid amide hydrolase (FAAH), respectively (Fig. 1; Okamoto et al., 2007; Di Marzo, 2011; Castillo et al., 2012). Postsynaptic cyclo-oxygenase 2 (COX-2) can terminate eCB signaling by turning 2-AG and AEA into prostaglandins (Hermanson et al., 2013). Within the LC, neuroanatomical evidence supports differential localization of eCB metabolizing enzymes both pre- and post-synaptically. MGL has been shown to be localized in presynaptic axon terminals synapsing with LC-NE neurons and FAAH has been localized in postsynaptic LC-NE somatodendritic processes (Van Bockstaele et al., 2017). Evidence also suggests that eCB signaling can occur in autocrine and non-retrograde fashions, via binding to postsynaptic CB1r and transient receptor potential vanilloid receptor type 1 (TRPV1) receptors (Ross, 2003; Bacci et al., 2004).
Fig. 1.
The endocannabinoid system.
This schematic illustrates the basic components of the endocannbinoid (eCB) system. Postsynaptic depolarization and influx of calcium (Ca2+) stimulates eCB synthesis. N-acyl-phosphatidylethanolamine-hydrolyzing phospholipase-D (NAPE-PLD) is the main enzyme responsible for synthesizing anandamide (AEA), while diacylglycerol lipase (DGL) synthesizes 2-arachidonylglycerol (2-AG). These eCBs can then cross through the membrane, either via passive diffusion or with the help of endocannabinoid membrane transporters (EMTs), and travel across the synapse, where they retrogradely activate presynaptic cannabinoid type 1 receptors (CB1r). Presynaptic monoacylglycerol (MGL) then metabolizes 2-AG, and fatty acid amide hydrolase (FAAH) breaks down AEA into arachidonic acid (AA).
During development, the eCB system plays a critical role in neural growth and connectivity. CB1r and eCBs are found abundantly in the white matter of perinatal rodent and human brains, but not in adult brains (Viveros M-P et al., 2011). Additionally, maximal levels of CB1r and eCBs have been observed in adolescent rats, decreasing during adulthood (Rodriguez de Fonseca et al., 1993; Belue et al., 1995; Harkany et al., 2007). In adults, CB1r is highly expressed in brain regions associated with cognitive functioning, motor control, and emotional processing (Glass et al., 1997). It is well established that the eCB system plays a role in the regulation of mood and the stress response, and its dysregulation results in the development of stress-induced psychiatric disorders (Parolaro et al., 2010; Wyrofsky et al., 2015). AEA levels decrease to initiate an acute stress response, while 2-AG levels are subsequently increased to help terminate the stress response and enable adaptation to chronic stress (Morena et al., 2016). Additionally, CB1r levels are decreased in most brain regions following chronic stress (Morena et al., 2016). CB1r-knock out (KO) mice have hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, and show an increase in passive behaviors in the forced swim test and an increase in immobility time in the tail suspension test compared to wild type (WT) mice, suggesting heightened depressive-like phenotype (Uriguen et al., 2004; Aso et al., 2008; Cota, 2008; Steiner et al., 2008). Conversely, administration of CB1r agonists and FAAH inhibitors produce antidepressant-like effects in the forced swim and tail suspension tests (Gobbi et al., 2005; Bambico et al., 2007). In the clinic, a CB1r antagonist, rimonabant, originally intended as a weight-loss drug, was withdrawn from the market due to significant psychological side effects, including depression and anxiety (Nissen et al., 2008). These studies suggest a protective role of eCB signaling against the development of psychiatric disorders, which may result from the interplay between the eCB system and stress circuitry.
3. The mammalian stress response
The ability to recognize and deal with a threat ensures survival in dangerous situations or environments. When facing a physiological or psychological stressor, parallel engagement of the HPA axis and brainstem noradrenergic circuits underlying the cognitive limb of the stress response contributes to adaptive responses necessary to confront a perceived threat (Valentino and Van Bockstaele, 2008). CRF, a neurohormone that is a critical orchestrator of the stress response, coordinates both limbs of the stress response, eliciting widespread autonomic and behavioral responses (Vale et al., 1981; Valentino and Van Bockstaele, 2008).
3.1. The HPA axis activation: endocrine limb of the stress response
Any perceived threat to homeostasis causes activation of the HPA axis via excitatory and inhibitory inputs to the paraventricular nucleus of the hypothalamus (PVN) from the amygdala, prefrontal cortex (PFC), LC, dorsal raphe nucleus, and other limbic and brainstem regions (Herman et al., 2005; Ulrich-Lai and Herman, 2009). The PVN contains a large population of CRF-producing neurons, which release CRF upon activation (Swanson and Sawchenko, 1980). CRF then travels to the anterior pituitary, causing adrenocorticotropic hormone (ACTH) to be released into the blood stream (Herman et al., 2005). When ACTH reaches the adrenal cortex, it stimulates the production and release of adrenal steroids, such as glucocorticoids, which helps prepare the body to respond to the stressor by reallocating energy resources (Herman and Cullinan, 1997; Herman et al., 2005).
3.2. LC-NE system: cognitive limb of the stress response
CRF released from the amygdala and PVN targets the LC (Valentino and Van Bockstaele, 2008; Valentino et al., 2010). The LC, a dense region of noradrenergic neurons located off of the fourth ventricle in the brainstem, innervates many regions of the neuraxis and provides the sole source of NE to the medial prefrontal cortex (mPFC) (Sara, 2009). CRF fibers have been shown to strongly innervate the peri-LC, a region where dendritic processes extend from the core of the LC (Shipley et al., 1996). Tract tracing and immuno-electron microscopy studies have revealed topographic CRF innervation of the LC, with limbic regions such as the amygdala and bed nucleus of the stria terminalis (BNST) targeting the peri-LC, and autonomic-related brain areas such as the PVN, Barrington's nucleus, nucleus paragigantocellularis (PGi), and nucleus prepositus hypoglossi (PrH) targeting the LC core (Van Bockstaele et al., 2001). LC-NE neurons express CRF receptor 1 (CRFr1), and the release of CRF leads to increases in the firing of these neurons (Curtis et al., 1996; Reyes et al., 2008). This shifts LC activity to a high tonic state, which is adaptive during acute stressor exposure as it promotes behavioral flexibility and environmental scanning (Valentino and Van Bockstaele, 2008; Curtis et al., 2012). Chronic CRF release and stimulation of LC-NE neurons increases anxiety and depressive-like behaviors (Valentino and Van Bockstaele, 2008). Indeed, studies have found that CRF released from amygdalar afferents in the LC increases the tonic firing of LC neurons and causes stress-induced anxiety and aversive behaviors (McCall et al., 2015).
LC projections to both the mPFC and the amygdala play important and distinct roles in aversion; the LC-amygdala microcircuit is critical in promoting aversive learning, while the LC-mPFC microcircuit is critical for extinguishing aversive responses (Uematsu et al., 2017). NE release in the amygdala modulates aversive memory formation in Pavlovian fear conditioning models (Díaz-Mataix et al., 2017; Schiff et al., 2017). This effect of NE is mediated by β-AR activation in both the central nucleus of the amygdala (CeA) and the basolateral amygdala (BLA) (Campese et al., 2017; McCall et al., 2017). Similarly, overactivation of the LC-BLA pathway via β-AR activation plays a critical role in pain-induced anxiety and enhances aversive learning (Llorca-Torralba et al., 2019). In addition to its role in extinguishing aversive memories, NE dysregulation in the mPFC can precipitate the development of psychiatric disorders (Mueller et al., 2008; Mueller and Cahill, 2010).
3.3. Dysregulation following chronic stress
While activation of the HPA axis and the cognitive limb of the stress response are beneficial during exposure to acute stress, continued exposure to chronic stress is maladaptive. Long-term exposure to stress is known to contribute to the pathophysiology of psychological disorders, including depression, anxiety, and drug abuse (Kendler et al., 1995, 1999; Breese et al., 2005). Rats exposed to repeated social defeat develop long lasting hyper-activation of stress signaling and HPA axis activity (Wood et al., 2010; Wood and Bhatnagar, 2015). Chronic glucocorticoid release can lead to maladaptive metabolic, cardiovascular, and neurological states, and HPA hyperactivity is common in individuals suffering from PTSD and major depression (McEwen, 2008). Aberrant monoaminergic signaling also plays a significant role in the development of psychiatric disorders, and many traditional antidepressants target noradrenergic and serotonergic neurotransmission (Carvalho et al., 2010; Valentino et al., 2010). Dysregulation of NE release in cortical and limbic brain regions contributes to the debilitating symptoms of depression and anxiety (Johnston et al., 1999; Carvalho and Van Bockstaele, 2012). In addition to NE, the LC also releases galanin in the ventral tegmental area following stress-induced hyperactivity, and antidepressants have been shown to reduce galanin mRNA in the LC (Rovin et al., 2012). Therefore, regulation of the stress response by the eCB system is of paramount importance, in order to protect against the development of these stress-induced psychiatric disorders.
4. eCB regulation of stress circuitry
4.1. eCB regulation of the HPA axis
In order to help protect against the damage that excessive glucocorticoids can cause, negative feedback mechanisms exist to dampen HPA axis activity after an adequate stress response has occurred. A short feedback loop exists, where stress causes the release of glucocorticoids into circulation via stimulation of the HPA axis (Hill and McEwen, 2009). Increases in hypothalamic eCB levels caused by glucocorticoid circulation cause subsequent suppression of glutamatergic signaling onto the CRF-releasing neurons in the PVN (Patel et al., 2004; Hill and McEwen, 2009; Hill and Tasker, 2012). Similar to their effect in the PVN, circulating glucocorticoids stimulate DGL in the mPFC to increase 2-AG synthesis. 2-AG then binds to presynaptic CB1r localized to GABAergic interneurons, causing disinhibition of excitatory projection neurons to GABAergic neurons within the BNST. This time-delayed feedback leads to an increase in inhibitory output from the BNST to the PVN, ultimately causing a dampening of PVN activity and decreased CRF efflux (Hill et al., 2011). In contrast to the mPFC and PVN, where eCBs appear to be produced on demand, tonic AEA signaling in the basolateral amygdala (BLA) serves as a gatekeeper of limbic activity by causing basal inhibition of the HPA axis (Hill and McEwen, 2009). When an acute stressor occurs, increased FAAH activity degrades AEA, causing disinhibition of BLA neurons and a subsequent increase in HPA axis activity via increased amygdalar output to the PVN (Hill and McEwen, 2009; Hill et al., 2010). While acute stressors alter AEA levels in the amygdala, 2-AG signaling is upregulated following chronic stress, suggesting another mechanism for the eCB system to dampen HPA axis hyperactivity via inhibition of amygdalar output (Patel et al., 2009).
4.2. eCB regulation of LC-NE activity
CB1r mRNA and protein expression have been localized within the LC (Herkenham et al., 1991; Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993; Derbenev et al., 2004). Additionally, electron microscopy studies have shown that CB1r are found on both glutamatergic and GABAergic presynaptic axon terminals that synapse with NE-producing LC neurons as well as post-synaptically in somatodendritic processes of LC cells (Scavone et al., 2010). The presence of CB1r on LC-NE neurons is functional, as indicated by electrophysiological studies showing that CB1r agonists and FAAH inhibitors increase the basal firing rate of LC-NE cells, c-Fos expression of LC neurons, and NE efflux in the mPFC (Gobbi et al., 2005; Oropeza et al., 2005; Mendiguren and Pineda, 2006; Muntoni et al., 2006; Page et al., 2008). The eCB system also regulates NE microcircuits in LC target regions. Footshock is known to enhance hyperarousal and inhibitory avoidance in rodents due to the LC-BLA microcircuit, and FAAH inhibitors administered in the BLA decreases the startle response and other anxiety-like behaviors (Aisenberg et al., 2017; Sabban et al., 2018). Additionally, β1-AR have been found to play a role in the impaired memory consolidation that occurs following post-training injections of a CB1r agonist directly into the CeA (Zarrindast MR et al., 2012). Within the mPFC, CB1r activation has been shown to inhibit NE release, and it has inversely been suggested that increased NE levels might downregulate CB1r (Richter et al., 2012).
Additionally, there is tonic eCB production in the LC, as sole application of a CB1r antagonist is capable of decreasing LC-NE activity (Muntoni et al., 2006; Carvalho and Van Bockstaele, 2012). Interestingly, the LC appears to be under biphasic regulation of the eCB system, and both too much and too little CB1r activity can increase LC-NE firing. For example, other studies have found that systemic administration of rimonabant, a CB1r antagonist, increases NE levels in the mPFC and PVN (Tzavara et al., 2001, 2003), and low levels of THC can cause a decrease in NE release from synaptosomes (Poddar and Dewey, 1980). Additionally, various doses of CB1r agonists and antagonists can produce opposite effects, further highlighting the biphasic nature of the eCB system within the LC (Carvalho et al., 2010).
Studies have found that CB1r in the PFC and hippocampus have differential sensitivity to CB1r agonists, depending on their neuronal subpopulations – CB1r on glutamatergic terminals have a higher sensitivity to CB1r agonists compared to CB1r on GABAergic terminals (Ohno-Shosaku et al., 2002; Aparisi Rey et al., 2012). Tonic eCB activity has also been shown to differentially affect various populations of CB1r, with basal tonic CB1r activation having a stronger effect on GABAergic terminals while glutamatergic CB1r are activated during phasic eCB release (Marsicano et al., 2003; Slanina and Schweitzer, 2005; Katona and Freund, 2008; Roberto et al., 2010; Aparisi Rey et al., 2012). It is possible that this might be the case within the LC, as CB1r have been localized to both glutamatergic and GABAergic terminals in the LC (Scavone et al., 2010). It could therefore be hypothesized that within the LC, tonic eCB release primarily regulates GABAergic synapses. Following postsynaptic depolarization, phasic release of eCBs help gate further glutamatergic excitation of LC neurons. However, when large levels of CB1r agonists are present, GABAergic CB1r also become activated. This hypothesis might account for the biphasic nature of CB1r activation within the LC. Taken together, it appears that the eCB system serves to modulate the LC-NE system to maintain an optimal level of activity.
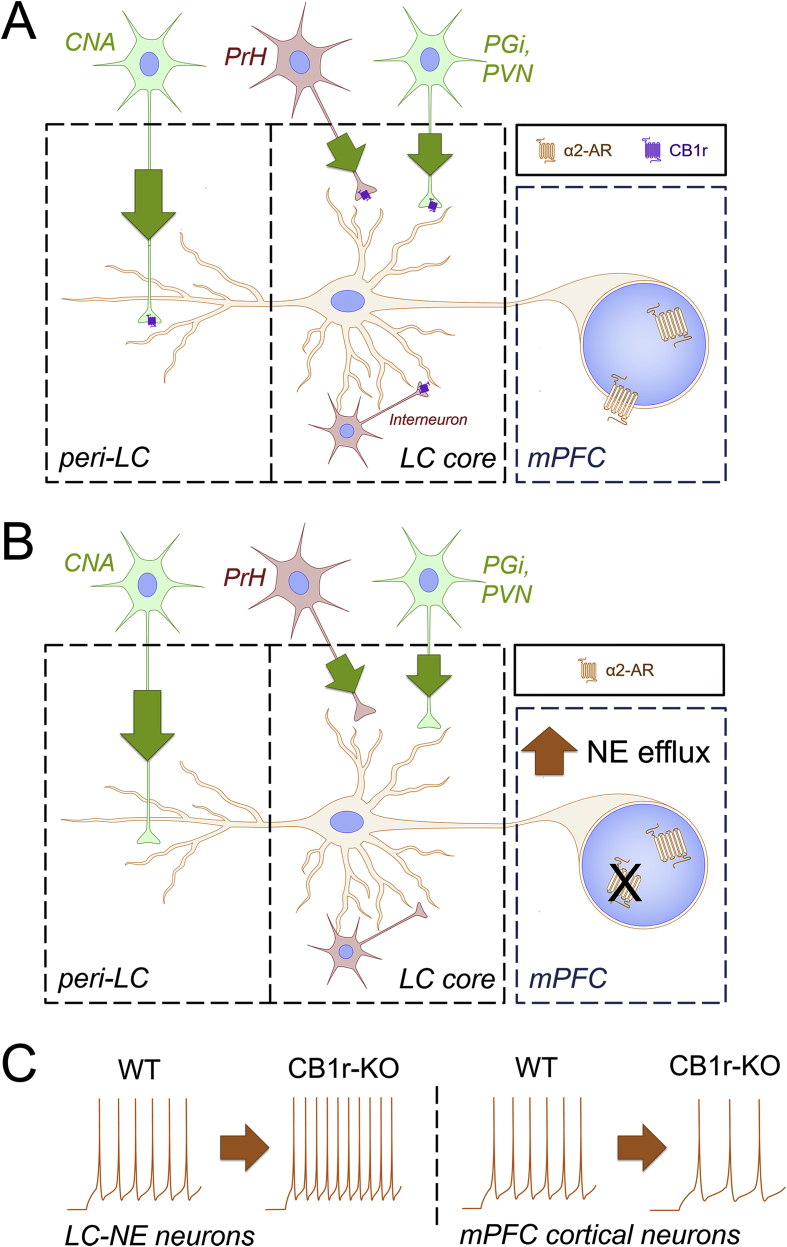
In support of this, our laboratory has recently demonstrated a reduction in basal mPFC neuronal excitability in CB1r-KO mice, caused by desensitization of the normally excitatory mPFC α2-adrenoceptors (ARs) (Reyes et al., 2017). This indicates that without a functioning eCB system, aberrant LC-NE activity is observed, where CB1r-KO mice have increased LC-NE activity, resulting in mPFC α2-AR desensitization, and subsequent decreased mPFC output (Fig. 2). This finding also supports the hypothesis that tonic eCB signaling primarily affects GABAergic CB1r within the LC. Additionally, when LC-NE neurons are excited via potassium chloride (KCl) bath application, CB1r agonist pre-treatment is capable of attenuating the KCl-induced increases in LC-NE firing (Mendiguren and Pineda, 2007). These data suggest that the eCB system might function to prevent over-activation of LC-NE neurons.
Fig. 2.
Schematic depicting alterations to LC-mPFC microcircuit in male CB1r-KO mice.A. A depiction of the LC-medial prefrontal cortex (mPFC) microcircuit in male wild type (WT) mice. The nucleus paragigantocellularis (PGi) and paraventricular nucleus of the hypothalamus (PVN) provide excitatory/glutamatergic (green neurons) input and the nucleus prepositus hypoglossi (PrH) provides inhibitory/GABAergic (red neurons) to the locus coeruleus (LC) core, while the amygdala (CNA) provides excitatory/glutamatergic input to the peri-LC. These afferents can also release corticotropin-releasing factor (CRF), as indicated by the green arrows. Additionally, GABAergic interneurons are present in the LC. Cannabinoid type 1 receptors (CB1r) have been localized to excitatory and inhibitory synapses in the core and peri-LC, providing a mechanism for eCB modulation of limbic and autonomic projections. B. A depiction of the LC-mPFC microcircuit in male CB1r-knock out (KO) mice. CB1r localization in the LC suggests that CB1r deletion could result in dysregulation of neurotransmitter and CRF release in the LC. Indeed, male CB1r-KO mice have a significant increase in NE levels in the mPFC compared to WT, resulting in the desensitization and decreased expression of α2-adrenoceptors. C.In vitro whole-cell patch clamp recordings reveal that male CB1r-KO mice have increased LC-NE excitability compared to WT, which corresponds with the increase in mPFC NE levels. Additionally, male CB1r-KO mice have a decrease in mPFC cortical neuron excitability, likely resulting from desensitization of excitatory mPFC α2-adrenoceptors. Traces are examples of neuronal excitability, as would be measured by counting the number of action potentials that occur during application of increasing current pulses. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
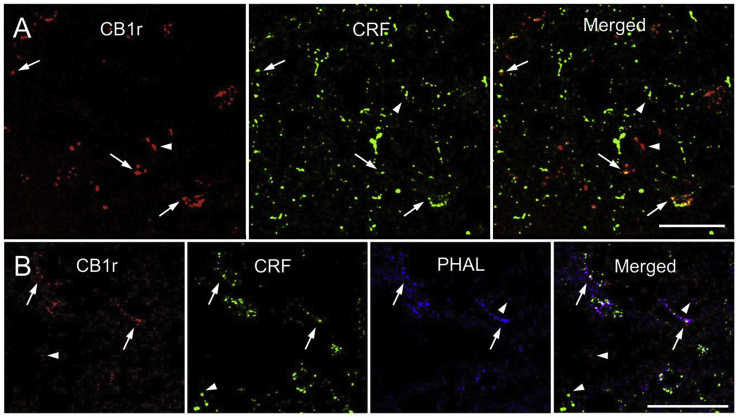
Since CRF release in the LC increases LC-NE excitability and activity, it is tempting to speculate that the eCB system can serve to attenuate these CRF-induced increases as well (Curtis et al., 1996). Indeed, recent immunofluorescence studies from our laboratory have demonstrated that CB1r are co-localized with CRF in the rat LC (Fig. 3A) (Wyrofsky et al., 2017). Additionally, using Phaseolus Vulgaris Leucoagglutinin (PHAL) as an anterograde tracer injected into the amygdala, CB1r are directly positioned to regulate CRF release from amygdalar limbic afferents (Fig. 3B) (Wyrofsky et al., 2017). Immunoelectron quantification has confirmed that CB1r are localized both pre- and post-synaptically with respect to CRF in both the core and peri-LC and in both excitatory and inhibitory synapses (Wyrofsky et al., 2017). Specific quantification values can be found in our previous manuscript (Wyrofsky et al., 2017). These findings provide an anatomical substrate for direct eCB modulation of CRF. Because aberrant NE release in the mPFC and amygdala contribute to the development of stress-induced psychiatric disorders, eCB modulation of CRF release in LC microcircuits during stress could play a role in the anxiolytic effects of CB1r agonists (see functional implications).
Fig. 3.
Co-localization of CB1r and CRF-amygdalar afferents in the LC. A.
Confocal fluorescence micrographs showing that cannabinoid type 1 receptor (CB1r, red) and corticotropin-releasing factor (CRF, green) are co-localized in the locus coeruleus (LC). CB1r was detected using a rhodamine isothiocyanate-conjugated secondary antibody and CRF was detected using an Alexafluor 647-conjugated secondary antibody (pseudocolored in green). Co-localization of CB1r and CRF (yellow) is shown in a merged image. Arrows highlight points of CB1r and CRF co-localization, while arrowheads point to singly labeled points of CB1r and CRF. B. Confocal fluorescence micrographs showing that CB1r (red), CRF (green), and phaseolus vulgaris-leucoagglutinin (PHAL, blue) are co-localized in the LC. CB1r was detected using a rhodamine isothiocyanate-conjugated secondary antibody, CRF was detected using an Alexafluor 647-conjugated secondary antibody (pseudocolored in green), and PHAL was detected using fluorescein isothiocyanate-conjugated secondary antibody (pseudocolored in blue). Triple co-localization (white) can be observed, and is depicted by arrows. Arrowheads highlight singly labeled points of CB1r, CRF, and PHAL. This figure represents data previously published in (Wyrofsky et al., 2017). Scale bars = 25 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4.3. Dysregulation of eCB system in psychiatric disorders
Chronic stress is known to precipitate psychiatric disorders, and CB1r-KO mice show increased susceptibility to stress-induced anxiety and depressive-like phenotypes (Martin et al., 2002; Wyrofsky et al., 2015). HPA axis hyperactivity, a common trait in depressed patients, is also observed in CB1r-KO mice (Uriguen et al., 2004). Compared to WT mice, CB1r-KO mice spend significantly less time in the open arms of the elevated plus maze and more time around the border in the open field test, demonstrating an increase in anxiety behaviors (Parolaro et al., 2010). When exposed to chronic stress, CB1r-KO mice develop anhedonia faster than WT mice, suggesting that lack of CB1r signaling leads to increased vulnerability to stress-induced psychiatric disorders (Martin et al., 2002). In the foot-shock model of PTSD, impaired traumatic memory extinction is associated with increased hippocampal and PFC CB1r levels, and mice with increased CB1r expression in the mPFC and CB1r-KO mice both exhibit more persistent fear behaviors during the extinction sessions when compared to WT mice (Marsicano et al., 2002; Korem and Akirav, 2014). These opposing findings suggest that CB1r dysregulation affects fear behaviors and memory extinction. Additionally, rodents exposed to chronic stress have increased 2-AG levels and decreased CB1r expression and function throughout the brain (Patel et al., 2005, 2009; Rademacher and Hillard, 2007; Hill et al., 2008; Rossi et al., 2010; Wamsteeker et al., 2010; Wang et al., 2010; Hu et al., 2011; Lee and Hill, 2013; Morena et al., 2016). Importantly, this effect appears to be mediated by corticosterone, as the effect of stress on CB1r expression and function is blocked by glucocorticoid receptor antagonists (Hill et al., 2008; Wamsteeker et al., 2010; Hong et al., 2011; Bowles et al., 2012; Morena et al., 2016).
Dysregulation of the eCB system is also observed clinically (Ashton and Moore, 2011). Patients suffering from major depression have decreased serum AEA and 2-AG levels that corresponds with the length of the depressive episodes (Gobbi et al., 2005; Hill and Gorzalka, 2005; Miller et al., 2005; Serra and Fratta, 2007). Interestingly, depressed suicide victims have an increase in AEA and 2-AG levels specifically within the dorsal PFC (Kofalvi and Fritzsche, 2008; Tzavara and Witkin, 2008). Furthermore, increased sensitization of CB1r in the PFC contributes to the pathophysiology of suicide in depressed individuals (Tzavara and Witkin, 2008). These preclinical and clinical studies show the importance of a functioning eCB system in protecting against the development of stress-induced psychiatric disorders.
5. Sex differences in stress-induced psychiatric disorders
There is a known bias in susceptibility to psychiatric disorders between the sexes. Males are more prone to drug abuse, while females are about twice as likely to develop stress-induced disorders such as depression and anxiety (Kendler et al., 1995; Marcus et al., 2005, Bangasser et al., 2016). As stress and eCB system dysregulation are known to play a role in the development of stress-induced psychiatric disorders, it is important to consider how both systems are affected across the sexes in order to fully understand what might be contributing to the differences in prevalence of depression and anxiety between males and females.
5.1. Sex differences in stress circuitry
Stress affects both sexes differently; females are more sensitive to low levels of CRF due to both augmented CRF receptor (CRFr) signaling and diminished CRFr internalization after exposure to stress when compared to males (Bangasser et al., 2010). In males, CRF binding to its receptor shows biased signaling towards the recruitment of β-arrestin and receptor internalization, but in females, CRF binding causes a biased response for Gs signaling (Valentino et al., 2013). Additionally, following stressors, female rats have increased dendritic extension into the peri-LC, the region surrounding the LC nucleus where a majority of limbic CRF afferents terminate (Bangasser et al., 2010; Valentino et al., 2013). Basal sex differences exist in noradrenergic neurons, with females having an increase in the expression of genes associated with the development of major depressive disorder compared to males (Mulvey et al., 2018). Females have heightened HPA axis activity, coupled with slower negative feedback of the HPA axis (Handa et al., 1994; Handa and Weiser, 2014). All of these discoveries lead to the generalized conclusion that females have heightened stress signaling compared to males (Bangasser et al., 2016).
5.2. Sex differences in eCB signaling
Differences across sexes are also observed in the eCB system, both anatomically and behaviorally. Females have a greater sensitivity to cannabinoid abuse, dependence, withdrawal, and relapse (Craft et al., 2013). Females also have decreased CB1r density in certain brain regions, including the amygdala and cingulate areas 1 and 3 (Castelli et al., 2014). Additionally, in human depressed patients, while both sexes show a rise in serum AEA, only females show a decrease in 2-AG (Reich et al., 2009). Therefore, it is important to look at both males and females when designing experiments investigating psychiatric disorders, since sex differences exist in both the stress and eCB systems.
5.3. Sex differences in LC-NE neurons of CB1r-KO mice
Our laboratory has recently found that CB1r deletion differentially affects male and female LC neurons (Wyrofsky et al., 2018). Using in vitro slice electrophysiology, Western blotting, and ELISA analysis, we discovered that CB1r-KO caused a significant increase in noradrenergic indices in male mice compared to WT: male KO mice had an increase in LC-NE excitability, input resistance, tyrosine hydroxylase (TH) expression within the LC, and NE levels in the mPFC (Fig. 3). These noradrenergic indices were not altered following CB1r deletion in female mice. Western blot analyses of LC tissue from male and female CB1r/CB2r-KO mice also highlighted several sex differences. Male CB1r/CB2r-KO mice showed a significant increase in CRF expression and in NET expression compared to male WT mice, while female CB1r/CB2r-KO mice showed a significant increase in α2-AR expression compared to female WT mice, and these adaptations might play a role in the resulting dysregulation of LC-NE activity that occurs in male but not female CB1r-KO mice. Additionally, we tested LC-NE activity in response to CRF under conditions of CB1r deletion. While 300 nM CRF was capable of increasing LC-NE excitability in WT brain slices from both male and female mice, LC-NE neurons from CB1r-KO mice were not affected by 300 nM CRF bath application. This could be attributed to cellular adaptations observed in the CB1r-KO mice, such as increased α2-AR signaling in female KO mice, saturation of CRFr1 in male KO mice resulting from their increased endogenous CRF levels, or alterations to CRFr1 trafficking or synthesis. Chronic stress is known to alter CRFr1 trafficking in the LC. Therefore, it is possible that increased CRF levels in the LC could lead to CRFr1 desensitization, especially in males (Bangasser et al., 2010; Valentino et al., 2013). This shows the importance of the eCB system in maintaining normal LC-NE excitability and responsiveness to stress signaling.
6. Social stress alters eCB system in the LC: novel data
6.1. Social defeat stress as a model of chronic stress
One of the most well - established animal models for social stress is the resident-intruder paradigm, in which a smaller intruder rat is exposed to a more aggressive resident rat. Rats exposed to repeated social defeat by the larger resident rat develop increased depressive- and anxiety-like phenotypes, long-lasting hyperactivation of stress signaling and the HPA axis, and increased inflammatory processes in vulnerable subpopulations (Wood et al., 2010; Chen et al., 2015; Wood and Bhatnagar, 2015; Pearson-Leary et al., 2017). Additionally, an individual's ability to cope with stressors bears importance on the development of psychiatric disorders. Active coping strategies center around behavioral responses in an attempt to minimize harm and reduce stress, and often lead to resilience from the anxiogenic effects of the social stressor (Veenema et al., 2003; Wood and Bhatnagar, 2015). Passive coping strategies involve feelings of helplessness and immobility, which are associated with an increased susceptibility to depression and anxiety disorders (Billings and Moos, 1984; Wood and Bhatnagar, 2015). These two coping strategies can be observed in the resident-intruder model by separating rats based on their average latency to assume a defeated posture. Rats belonging to the short latency group exhibit HPA axis dysregulation and depressive-like behaviors, while those in the long latency group show decreased efficacy of CRF and appear to be more resilient to the development of depressive-like behaviors (Wood et al., 2010; Wood and Bhatnagar, 2015).
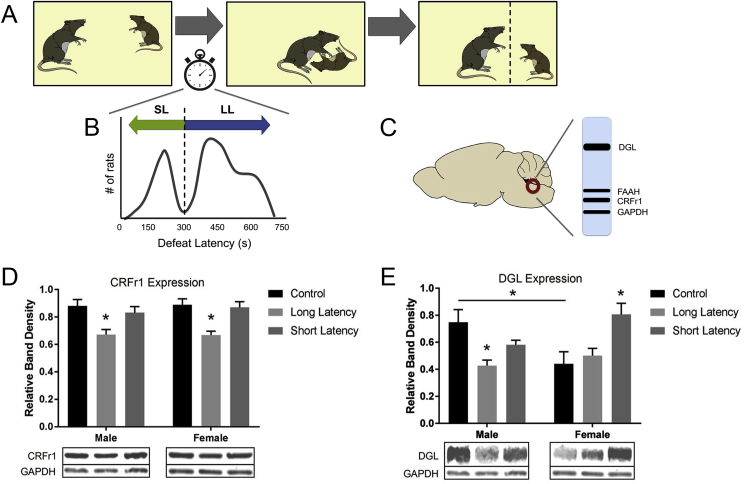
The LC is sensitive to social stressors (Chaijale et al., 2013), and the resident-intruder paradigm robustly increases sympathetic activation acutely compared to non-social stress paradigms (Sgoifo et al., 1999). Previous studies investigating the effect of social stress on the LC in male rats have found a decrease in LC-NE activity following repeated stress, compared to an increase in activity after an acute stressor (Chaijale et al., 2013). Additionally, differences in neurochemical adaptations in the LC occur across behavioral phenotypes, with an increase in opioid signaling in long latency rats compared to short latency and control groups, suggesting one mechanism for combating chronic stress in the LC (Chaijale et al., 2013; Reyes et al., 2015). Recently, we have begun to investigate how social stress might affect eCB levels within the LC, and whether differences in long and short latency groups exist. We used the resident-intruder paradigm as a model for social stress (Miczek, 1979; Wood et al., 2010), and performed Western blot analyses to examine the effects of social stress on the eCB levels within the LC (Fig. 4A–C). See the supplemental materials for a more detailed description of the methods performed.
Fig. 4.
LC Western blot analysis of rats exposed to social stress.
Unpublished data. A. The resident-intruder paradigm used as a model for social stress was based on the model established originally by Miczek (Miczek, 1979), and has been previously described (Wood et al., 2010). B. Intruder rats were separated into short or long latency groups based on the average time it took for them to reach subordination: short latency <300 s, long latency >300 s, as described in previous publications (Wood et al., 2010; Wood and Bhatnagar, 2015). C. Western blot analysis was performed on LC micropunches from each group as previously described (Wyrofsky et al., 2018). For Western blot analysis, data represents the following number of animals per group: N = 6 male control rats, N = 7 male long latency rats, N = 5 male short latency rats, N = 6 female control rats, N = 5 female long latency rats, N = 5 female short latency rats. Differences in protein expression levels were tested using two-way ANOVAs (sex vs. phenotype) followed by post-hoc Tukey's multiple comparison adjustments. Statistics were performed using GraphPad Prism 7.03 (GraphPad Software, San Diego, CA, USA). Results are presented as mean±standard error of the mean (SEM). Bands shown are representative of one sample obtained from one animal per group. Relative band density is determined by normalizing each sample to loading control glyceraldehyde 3-phosphate dehydrogenase (GAPDH). D. Western blot analysis for CRFr1 expression in protein extracts from the locus coeruleus (LC) revealed a significant decrease in levels in male and female long latency groups compared to control (p < 0.05), and no change between short latency groups and control. Relative protein expression for CRFr1 across sex and phenotype was as follows: male controls – 0.880 ± 0.047, male long latency – 0.684 ± 0.042, male short latency – 0.817 ± 0.052, female control – 0.888 ± 0.036, female long latency – 0.678 ± 0.018, female short latency 0.870 ± 0.042. E. Western blot analysis for DGL expression in protein extracts from the LC showed that DGL expression is decreased in male long latency rats compared to control (p < 0.05), while DGL expression is increased in female short latency rats compared to control (p < 0.05). Additionally, female control rats showed significantly less DGL expression compared to males (p < 0.05). Relative protein expression for DGL across sex and phenotype was as follows: male controls – 0.748 ± 0.094, male long latency – 0.457 ± 0.046, male short latency – 0.581 ± 0.033, female control – 0.489 ± 0.092, female long latency – 0.514 ± 0.063, female short latency 0.807 ± 0.082. Asterisks indicate a significant difference between groups as determined by two-way ANOVA/mixed-effects regression model (*p < 0.05).
6.2. Effect of social stress on CRFr1 expression in the LC
CRF exerts its effects on LC-NE neurons via CRFr1, which are expressed throughout the LC and are very prominent in the peri-LC region (Valentino and Van Bockstaele, 2005; Reyes et al., 2008). Western blot analysis revealed that, in addition to altering the eCB system, changes in CRFr1 receptor expression were identified across phenotypes (Fig. 4D). CRFr1 levels in LC tissue from both long latency males and females were significantly decreased compared to male and female controls, and no significant difference between short latency and control groups was detected. Decreased CRFr1 expression in the LC of long latency male rats was previously identified (Chaijale et al., 2013). A similar trend was found in females, confirming that the resilient long latency rats have decreased responsiveness to CRF following chronic social stress across both sexes, suggesting one mechanism by which long latency rats are protecting themselves from social stress and a resulting increase in CRF levels.
6.3. Effect of social stress on eCB protein expression in the LC
Western blot analysis of LC tissue from various social defeat groups across sexes revealed differential expression of DGL levels (Fig. 4E). Analysis of control rats showed that under basal conditions, there is a sex difference in DGL levels in the LC: females had significantly lower expression compared to males. Regarding change in expression across phenotypes, males had a significant reduction in DGL expression in the long latency group compared to control, while no significant change between control and short latency groups was observed. In females, no change between control and long latency groups was found; however, there was a significant increase in short latency females compared to control. When examining FAAH (data not shown), no significant changes in expression levels were detected between sexes or phenotypes.
DGL is one of the main proteins responsible for the synthesis of eCB 2-AG (Castillo et al., 2012). A decrease in DGL expression would suggest a decrease in 2-AG synthesis while, conversely, an increase in DGL expression would suggest an increase in 2-AG levels. Based on the data above, short latency rats have more 2-AG in the LC compared to long latency, suggesting there is increased eCB signaling in the short latency groups. In regards to the basal sex difference observed in DGL levels in control rats, greater expression was observed in males compared to females. This might suggest that the eCB system is primed and ready to combat stress in males, and that females have less tonic 2-AG regulation of LC-NE excitability. Indeed, Krebs-Craft et al. (2010) have discovered that males have higher levels of AEA and 2-AG in the amygdala compared to females (Krebs-Kraft et al., 2010; Craft et al., 2013), confirming region specific sex differences within the eCB system.
One hallmark characteristic of the short latency group is an inability to attenuate HPA axis hyperactivity, while the rats in the long latency group have both decreased HPA axis activity as well as a decreased efficacy of CRF in PVN (Wood et al., 2010). Studies have found that elevated corticosterone levels are responsible for increasing 2-AG (Morena et al., 2016), thus HPA axis hyperactivity in short latency rats could be responsible for the heightened DGL expression. Since short latency rats have heightened stress signaling, increased DGL and 2-AG levels could be an attempt to counteract increased CRF levels and aberrant LC-NE activity. Conversely, long latency rats have developed adaptations in brain regions such as the PVN to keep HPA axis and stress levels in balance (Wood et al., 2010); therefore, an increase in eCB synthesis within the LC is not necessary to maintain normal LC-NE activity. Another potential explanation might be that the increase in DGL levels in short latency rats causes an increase in 2-AG levels large enough to activate the less-sensitive CB1r localized to GABAergic terminals, leading to decreased inhibition of LC-NE activity.
7. Functional implications
7.1. Working model of eCB regulation of LC-NE activity
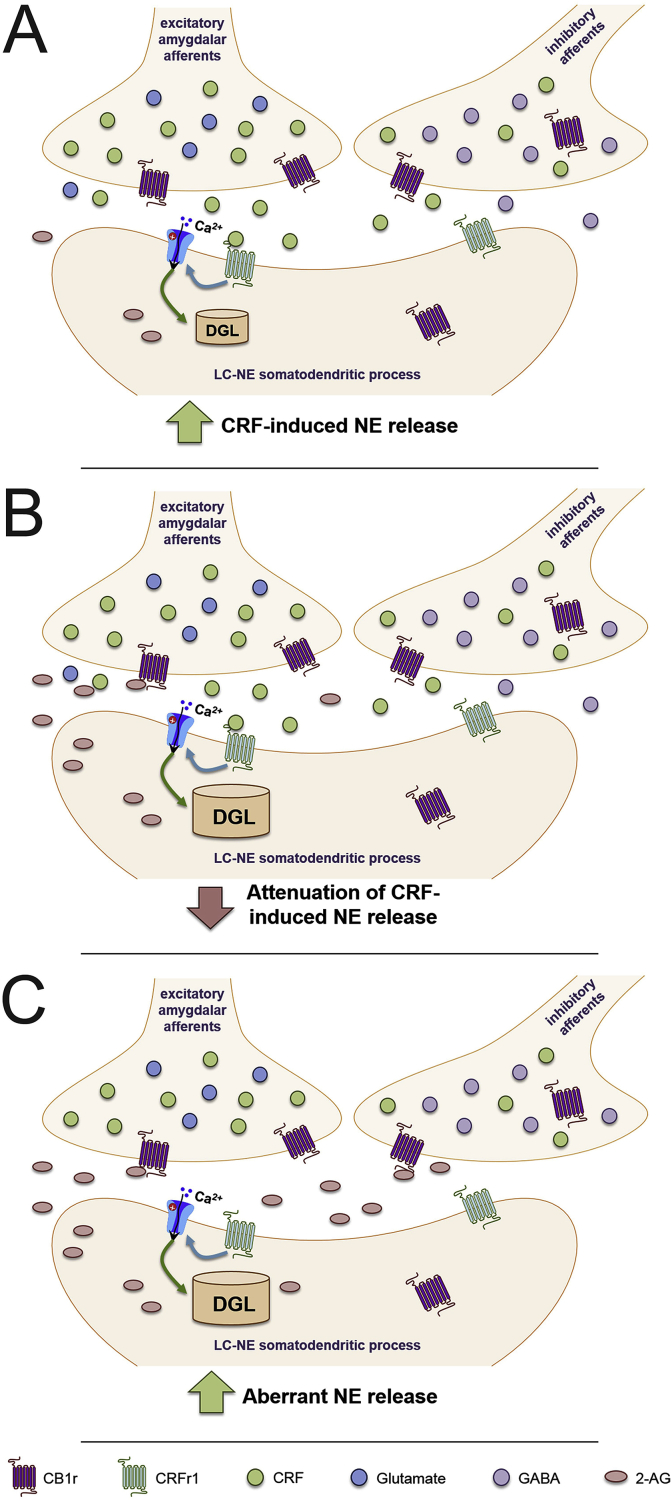
Based on our collective data, we propose the following model for eCB regulation of LC-NE neuronal activity. Initially, acute stress exposure causes release of CRF into the LC, from a variety of brain regions, but predominantly from amygdalar afferents (Fig. 5A). CRF then binds to CRFr1 on LC-NE somatodendritic processes, causing postsynaptic depolarization and an increase in LC-NE activity and NE release in the mPFC. This depolarization and influx in intracellular calcium levels stimulates an increase in DGL activity, causing the synthesis and release of the eCB 2-AG (Fig. 5B). 2-AG retrogradely crosses the synaptic cleft to bind to presynaptic CB1r, which have been localized to amygdalar-CRF afferents, where its activation leads to a decrease in CRF release and subsequent attenuation of LC-NE activity. However, under conditions of chronic stress in vulnerable subpopulations, such as short latency females in our social stress model, we have found an increase in DGL expression. This could present a problem when stressors are no longer present, as the increased DGL levels could lead to an increase in 2-AG synthesis, even when a stressor is not present. While eCBs are capable of attenuating LC-NE excitability, overexpression of eCBs have been shown to also increase basal LC-NE activity. Therefore, hyperactivity of the eCB system following chronic stress might lead to activation of CB1r at other neighboring synapses in the LC, such as inhibitory interneurons, where CB1r have also been localized (Fig. 5C). Additionally, if GABAergic CB1r are indeed less sensitive to phasic CB1r agonism than glutamatergic CB1r within the LC, the large increase in eCB levels during chronic stress could be further inhibiting GABAergic neurotransmission within the LC. This disinhibition onto LC dendrites would cause an increase in LC-NE activity, and aberrant NE release in the mPFC could contribute to anxiety and depressive-like behaviors. As females are known to be more susceptible to stress-induced psychiatric disorders, it would be intriguing to hypothesize that a significant increase in LC DGL expression following chronic stress in vulnerable female populations might be contributing to the disparity in prevalence of PTSD and depression. It should be noted that this proposed model is based on current literature and our novel data presented above – direct measurement of eCB levels or MGL were not directly measured in this study, and future projects should investigate this.
Fig. 5.
Working model for eCB modulation of LC following chronic stress.
Cannabinoid type 1 receptor (CB1r) has been localized to excitatory and inhibitory corticotropin-releasing factor (CRF) afferents in the locus coeruleus (LC), and anterograde tract tracing has found CB1r specifically on excitatory amygdalar-CRF afferents, the main source of CRF to the LC. A. Following a stressor, CRF is released in the LC, where it binds to corticotropin-releasing factor receptor type 1 (CRFr1). This causes postsynaptic depolarization of LC-norepinephrine (NE) neurons, leading to an increase in activity and NE efflux in the medial prefrontal cortex (mPFC). B. CRF-induced depolarization and influx in intracellular calcium (Ca2+) stimulates diacylglycerol lipase (DGL) to synthesize and release 2-arachidonlyglycerol (2-AG) into the synaptic cleft. 2-AG then crosses the synapse and binds to CB1r. This inhibits the continued release of CRF, attenuating the CRF-induced increases in LC-NE activity and NE efflux, and helping to diminish the stress response. C. Chronic stress, especially in vulnerable female subpopulations, results in high DGL expression. Increased DGL would suggest greater production of 2-AG, which could bind to CB1r on neighboring synapses, causing inhibition of GABAergic interneurons and non-CRF releasing afferents. This dysregulation synaptic activity and disinhibition could further excite LC-NE neurons can cause aberrant NE release in the mPFC, and could contribute to the increased propensity for females to develop stress-induced psychiatric disorders.
7.2. Implications for eCB-targeted therapies
As the experiments and literature discussed in this review suggest, the eCB system represents an attractive target for the treatment of stress-induced psychiatric disorders. Selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and other current antidepressants target monoamines, which are dysregulated during depression and anxiety (Valentino et al., 2010; Carvalho and Van Bockstaele, 2012); however, the eCB system targets both monoamines and the HPA axis, which is also dysregulated during stress-induced psychiatric disorders, making it a more inclusive therapeutic (Wyrofsky et al., 2015). Repeated re-consolidation of fear memories coupled with an inability to extinguish aversive memories due to hyperactivation of the limbic circuity and decreased cognitive flexibility contributes heavily to the pathophysiology of PTSD and other anxiety disorders (Lehner et al., 2009; Jovanovic and Ressler, 2010). Both the amygdala and mPFC are targeted by LC-NE afferents, and pharmacological manipulation of noradrenergic neurotransmission has provided symptomatic relief for individuals suffering from PTSD (Taylor et al., 2008; Byers et al., 2010). Recent evidence suggests that the eCB and noradrenergic systems play a role together in stress-related memory consolidation (Campolongo et al., 2009; Hill and McEwen, 2010), and eCB signaling also modulates the noradrenergic-mPFC microcircuit where memory extinction occurs (Cathel et al., 2014; McLaughlin et al., 2014; Bedse et al., 2015; Wyrofsky et al., 2015). CB1r activation-induced impairment of context-dependent fear memory acquisition is dependent on α2-AR activation in the BLA {Nasehi et al. (2016). Therefore, targeting eCB-noradrenergic interactions could help improve PTSD and anxiety-like behaviors associated with chronic stress.
Indeed, several compounds targeting the eCB system are being investigated in clinical trials for the treatment of anxiety, depression, and PTSD (Wyrofsky et al., 2015). Also, many individuals suffering from PTSD and other psychiatric disorders self-medicate with marijuana, with a strong correlation between use and the severity of PTSD symptoms (Cougle et al., 2011; Potter et al., 2011; Passie et al., 2012; Bonn-Miller et al., 2014; Wyrofsky et al., 2015). One study has found that nabilone, a synthetic cannabinoid, results in improved sleep quality and diminished traumatic flashbacks in PTSD treatment-resistant patients, and can improve chronic pain, nightmares, and insomnia associated with PTSD (Fraser, 2009; Cameron et al., 2014). Additional studies discovered THC treatment to effectively reduce nightmares and increase sleep quality in PTSD patients (Shalev et al., 2013; Yarnell, 2015). Specifically, THC causes a decrease in PTSD-associated behaviors and hyperarousal symptoms (Roitman et al., 2014), further implicating involvement and dysregulation of eCB signaling in the LC-NE system. Based on the preclinical and clinical data described in this review, other compounds that increase eCB signaling, such as FAAH inhibitors or allosteric CB1r modulators, should be investigated as novel therapeutics for PTSD and stress-induced psychiatric disorders.
Recent data have revealed important sex differences in the eCB system within the LC. Electrophysiology results suggest that CB1r deletion has less of an effect in female rodents compared to males, as male CB1r-KO mice showed a significant increase in LC-NE excitability compared to WT, but no change was observed in females (Wyrofsky et al., 2018). Coupled with the new data presented here, showing a significant reduction in basal DGL levels in female rats compared to males, indeed it seems that females have less tonic eCB signaling than males. Therefore, removal of eCB signaling might have less profound of an effect on female LC-NE activity, and eCB-targeting therapeutics might affect males and females differently. This is in line with analyses performed on the adverse effects of CB1r antagonist rimonabant, which suggested that the odds ratio for developing anxiety and depression following treatment was the largest in males aged 35–38 (Pi-Sunyer et al., 2006; Nissen et al., 2008).
The therapeutic benefits of cannabis have long been known (Zuardi, 2006). In 1996, California became the first state in the United States to legalize medical marijuana (Freisthler and Gruenewald, 2014). As of now, 28 states and the District of Colombia have legalized medical cannabis use (Sarvet et al., 2018); however, there are challenges to its therapeutic development. Marijuana is still classified as a Schedule I drug under the federal Controlled Substance Act (CSA) (Mead, 2017). In order to perform research on cannabinoids, investigators need to obtain a Schedule I research registration/license, which can be an arduous process (Mead, 2017). Additionally, as a Schedule I drug, the federal government currently does not accept medicinal use of marijuana (Mead, 2017).
Future studies should continue to discern the reciprocal interaction between NE and the eCB system during stress. Electron microscopy can be used to determine basal sex differences in CB1r expression and trafficking following exposure to chronic stress. Additionally, microdialysis should be used to observe actual changes in eCB levels within the LC, amygdala, and mPFC in different models of stress, as differences in FAAH and DGL expression only suggest changes to AEA and 2-AG. Using conditional knockout mice to delete CB1r selectively from NE-producing neurons and assessing them in models of PTSD and anxiety would help further elucidate the role of eCB-NE interactions in psychiatric disorders. Continuing to investigate the role of the eCB system in LC-mPFC and -amygdala microcircuit regulation is very important. The field should continue to research the effectiveness of eCB-targeting compounds at treating stress-induced psychiatric disorders across sexes, in hopes of finding better therapeutic interventions for those suffering from anxiety, depression, and PTSD.
Acknowledgements
This project was supported by the National Institutes of Health grants DA020129 to E.J. Van Bockstaele, P30 DA013429 to L.G. Kirby, and MH093981 to S. Bhatnagar.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100176.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aisenberg N., Serova L., Sabban E.L., Akirav I. The effects of enhancing endocannabinoid signaling and blocking corticotrophin releasing factor receptor in the amygdala and hippocampus on the consolidation of a stressful event. Eur. Neuropsychopharmacol. 2017;27(9):913–927. doi: 10.1016/j.euroneuro.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Aparisi Ray A., Purrio M., Viveros M.-P., Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton C., Moore P. Endocannaginoid system dysfunction in mood and related disorders. Acta Psychiatr. Scand. 2011;124:250–265. doi: 10.1111/j.1600-0447.2011.01687.x. [DOI] [PubMed] [Google Scholar]
- Aso E., Ozaita A., Valdizan E.M., Ledent C., Pazos A., Maldonado R., Valverde O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Rajkowski J., Kubiak P., Valentino R.J., Shipley M.T. Role of the locus coeruleus in emotional activation. Prog. Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Bacci A., Huguenard J.R., Prince D.A. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bambico F.R., Katz N., Debonnel G., Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotoninergic neurons through the medial prefrontal cortex. J. Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I., Ischiropoulos H., Van Bockstaele E.J., Valentino R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry. 2010;15:877–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wiersielis K.R., Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641(Pt B):177–188. doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G., Romano A., Tempesta B., Lavecchia M.A., Pace L., Bellomo A., Duranti A., Di Bonaventura M.V.M., Cifani C., Cassano T., Gaetani S. Inhibition of anandamide hydrolysis enhances noradrenergic and GABAergic transmission in the prefrontal cortex and basolateral amygdala of rats subjected to acute swim stress. J. Neurosci. Res. 2015;93:777–787. doi: 10.1002/jnr.23539. [DOI] [PubMed] [Google Scholar]
- Belue R.C., Howlett A.C., Westlake T.M., Hutchings D.E. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol. Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Bergamaschi M.M., Queiroz R.H., Chagas M.H., de Oliveira D.C., De Martinis B.S., Kapczinski F., Quevedo J., Roesler R., Schroder N., Nardi A.E., Martin-Santos R., Hallak J.E., Zuardi A.W., Crippa J.A. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings A., Moos R.H. Coping, stress, and social resources among adults with unipolar depression. J. Personal. Soc. Psychol. 1984;46:877–891. doi: 10.1037//0022-3514.46.4.877. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller M.O., Babson K., Vandrey R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136:162–165. doi: 10.1016/j.drugalcdep.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles N.P., Hill M.N., Bhagat S.M., Karatsoreos I.N., Hillard C.J., McEwen B.S. Chronic, noninvasive glucocorticoid administration suppresses limbic endocannabinoid signaling in mice. Neuroscience. 2012;204:83–89. doi: 10.1016/j.neuroscience.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese G.R., Overstreet D.H., Knapp D.J. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacology. 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers M., Allison K., Wendel C., Lee J. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J. Clin. Psychopharmacol. 2010;30:225–229. doi: 10.1097/JCP.0b013e3181dac52f. [DOI] [PubMed] [Google Scholar]
- Cameron C., Watson D., Robinson J. Use of a synthetic cannbinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J. Clin. Psychopharmacol. 2014;5:559–564. doi: 10.1097/JCP.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese V.D., Soroeta J.M., Vazey E.M., Aston-Jones G., LeDoux J.E., Sears R.M. Noradrenergic regulation of central amygdala in aversive Pavlovian-to-instrumental transfer. eNeuro. 2017;4(5) doi: 10.1523/ENEURO.0224-17.2017. 0024-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P., Roozendaal B., Trezza V., Hauer D., Schelling G., McGaugh J.L., Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Nation. Academy. Sci. USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.F., Mackie K., Van Bockstaele E.J. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur. J. Pharmacol. 2010;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.F., Van Bockstaele E.J. Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2012;38:59–67. doi: 10.1016/j.pnpbp.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli M., Fadda P., Casu A., Spano M., Casti A., Fratta W., Fattore L. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr. Pharmaceut. Des. 2014;20 doi: 10.2174/13816128113199990430. [DOI] [PubMed] [Google Scholar]
- Castillo P.E., Younts T.J., Chavez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathel A.M., Reyes B.A., Wang Q., Palma J., Mackie K., Van Bockstaele E.J., Kirby L.G. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur. J. Neurosci. 2014;40:2014. doi: 10.1111/ejn.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale N., Curtis A., Wood S., Zhang X.-Y., Bhatnagar S., Reyes B.A., Van Bockstaele E.J., Valentino R.J. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology. 2013;38:1833. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.J., Kelly G., Sengupta A., Heydendael W., Nicholas B., Beltrami S., Luz S., Peixoto L., Abel T., Bhatnagar S. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience. 2015;305:36–48. doi: 10.1016/j.neuroscience.2015.07.045. [DOI] [PubMed] [Google Scholar]
- Cota D. The role of the endocannabinoid system in the regulation of the hypothalamic-pituitary-adrenal axis activity. J. Neuroendocrinol. 2008;20:35–38. doi: 10.1111/j.1365-2826.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- Cougle J.R., Bonn-Miller M.O., Vujanovic A.A., Zvolensky M.J., Hawkins K.A. Posttraumatic stress disorder and cannabins use in a nationally representative sample. Psychol. Addict. Behav. 2011;25:554–558. doi: 10.1037/a0023076. [DOI] [PubMed] [Google Scholar]
- Craft R.M., Marusich J.A., Wiley J.L. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A., Lechner S.M., Pavcovich L.A., Valentino R.J. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J. Pharmacol. Exp. Ther. 1996;281:163–172. [PubMed] [Google Scholar]
- Curtis A., Leiser S.C., Snyder K., Valentino R.J. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology. 2012;62:1737–1745. doi: 10.1016/j.neuropharm.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbenev A.V., Stuart T.C., Smith B.N. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J. Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat. Neurosci. 2011;14:9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Bifulco M., De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L., Piper W.T., Schiff H.C., Roberts C.H., Campese V.D., Sears R.M., LeDoux J.E. Characterizatino of the amplificatory effect of norepinephrine in the acquisition of Pavlovian threat associations. Learn. Mem. 2017;24:432–435. doi: 10.1101/lm.044412.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser G.A. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS Neurosci. Ther. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freisthler B., Gruenewald P.J. Examining the relationship between the physical availability of medical marijuana and marijuana use across fifty California cities. Drug Alcohol Depend. 2014;143:244–250. doi: 10.1016/j.drugalcdep.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M., Dragunow M., Faull R.L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gobbi G., Bambico F.R., Mangieri R., Bortolato M., Campolongo P., Solinas M., Cassano M., Morgese M.G., Debonnel G., Duranti A., Tontini A., Tarzia G., Mor M., Trezza V., Goldberg S.R., Cuomo V., Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Nation. Academy. Sci. USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B., Kavanagh D., Young R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Handa R., Burgess L., Kerr J., O'Keefe J. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa R., Weiser M. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T., Guzman M., Galve-Roperh I., Berghuis P., Devi L.A., Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalmo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Ostrander M.M., Mueller N.K., Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hermanson D.J., Hartley N.D., Gamble-George J., Brown N., Shonesy B.C., Kingsley P.J., Colbran R.J., Reese J., Marnett L.J., Patel S. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat. Rev. Neurosci. 2013;16:1291–1298. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Carrier E., Ho W., Shi L., Patel S., Gorzalka B.B., Hillard C.J. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Gorzalka B.B. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression. Behav. Pharmacol. 2005;16:333–352. doi: 10.1097/00008877-200509000-00006. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McEwen B.S. Endocannabinoids: the silent partner of glucocorticoids in the synapse. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:4579–4580. doi: 10.1073/pnas.0901519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McEwen B.S. Involvement of the endocannabinoid system in the neurobehavioral effects of stress and glucocorticoids. Progress in Neuropsychopharmacology & Biological Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Pan B., Fitzgerald M.L., Roberts C.J., Lee T.T.-Y., Karatsoreos I.N., Mackie K., Viau V., Pickel V.M., McEwen B.S., Liu Q-s, Gorzalka B.B., Hillard C.J. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S., Campolongo P., Tasker J.G., Wotjak C.T., Bains J.S. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Tasker J.G. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Zheng G., Wu X., Snider N.T., Owyang C., Wiley J.W. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140:627–637. doi: 10.1053/j.gastro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Zhang M., Czeh B., Zhang W., Flugge G. Chronic restraing stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Res. Bull. 2011;85:374–379. doi: 10.1016/j.brainresbull.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Johnston T.G., Kelly C.B., Stevenson M.R., COoper S.J. Plasma norepinephrine and prediciton of outcome in major depressive disorder. Biol. Psychiatry. 1999;46:1253–1258. doi: 10.1016/s0006-3223(99)00134-1. [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Ressler K.J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I., Freund T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L., Prescott C. Casual relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Kessler R.C., Walkers E.E., MacLean C., Neale M.C., Heath A.C., Eaves L.J. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am. J. Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kofalvi A., Fritzsche M. Springer Science + Business Media, LLC; Coimbra, Portugal: 2008. The Endocannabinoid System Is a Major Player in Schizophrenia. [Google Scholar]
- Korem N., Akirav I. Cannabinoids prevent the effects of a footshock followed by situational reminders on emotional processing. Neuropsychopharmacology. 2014;39(12):2709–2722. doi: 10.1038/npp.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Kraft D., Hill M.N., Hillard C.J., McCarthy M. Sex differences in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc. Nation. Academy. Sci USA. 2010;107:20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.T.Y., Hill M.N. Age of stress sxposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience. 2013;249:106–114. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Lehner M., Wilslowska-Stanek A., Plaznik A. Extinction of emotional response as a novel approach of pharmacotherapy of anxiety disorders. Psychiatr. Pol. 2009;43:639–653. [PubMed] [Google Scholar]
- Llorca-Torralba M., Suarez-Pereira I., Bravo L., Camarena-Delgado C., Garcia-Partida J.A., Mico J.A., Berrocoso E. Chemogenetic silencing of the locus coeruleus-basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol. Psychiatry. 2019 doi: 10.1016/j.biopsych.2019.02.018. (in press) [DOI] [PubMed] [Google Scholar]
- Mailleux P., Vanderhaeghen J.-J. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Marcus S.M., Young E.A., Kerber K.B., Kornstein S., Farabaugh A.H., Mitchell J., Wisniewski S.R., Balasubramani G.K., Trivedi M.H., Rush A.J. Gender differences in depression: findings from the STAR*D study. J. Affect. Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S.C., Cascio M.G., Gutierrez S.O., van der Stelt M., Lopez-Rodriguez M.L., Casanova E., Schutz G., Zieglgansberger W., Di Marzo V., Behl C., Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Hermann H., Tang J., Hofmann C., Zieglgansberger W., Di Marzo V., Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;481:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M., Ledent C., Parmentier M., Maldonado R., Valverde O. Involvement of CB1 cannabinoid receptors in emotional behavior. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Matsuda L.A., Bonner T.I., Lolait S.J. Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- McCall J.G., Al-Hasani R., Siuda E.R., Hong D.Y., Norris A.J., Ford C.P., Bruchas M.R. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall J.G., Siuda E.R., Bhatti D.L., Lawson L.A., McElligott Z.A., Stuber G.D., Bruchas M.R. Locus coeruleus to basolateral amygdala noradreneric projections promote anxiety-like behavior. eLife. 2017;6 doi: 10.7554/eLife.18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R.J., Hill M.N., Gorzalka B.B. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Behav. Review. 2014;42:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Mendiguren A., Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur. J. Pharmacol. 2006;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mendiguren A., Pineda J. CB1 cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–625. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Miczek K. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miller G., Hill M.N., Ho W., Hillard C.J. Society for Neuroscience Washington, DC. 2005. Bidirectional alterations in serum endocannabinoids in minor and major depression. [Google Scholar]
- Morena M., Patel S., Bains J.S., Hill M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D., Cahill S.P. Noradrenergic modulation of extinctino learning and exposure therapy. Behav. Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Mueller D., Porter J.T., Quirk G.J. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci. 2008;28(2):369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey B., Bhatti D.L., Gyawali S., Lake A.M., Kriaucionis S., Ford C.P., Bruchas M.R., Heintz N., Dougherty J.D. Molecular and functional sex differences in noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 2018;23(8):2225–2235. doi: 10.1016/j.celrep.2018.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni A.L., Pillolla G., Melis M., Perra S., Gessa G.L., Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur. J. Pharmacol. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Nasehi M., Zamanparvar M., Ebrahimi-Ghiri M., Zarrindast M.-R. Modulation of cannabinoid siglnaling by amygdala a2-adrenergic system in fear conditioning. Behav. Brain. Res. 2016;300:114–122. doi: 10.1016/j.bbr.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Nicholls S.J., Wolski K., Rodes-Cabau J., Cannon C.P., Deanfield J.E., Despres J.P., Kastelein J.J., Steinhubl S.R., Kapadia S., Yasin M., Ruzyllo W., Gaudin C., Job B., Hu B., Bhatt D.L., Lincoff A.M., Tuzcu E.M. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. J. Am. Med. Assoc. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Tsubokawa H., Mizushima I., Yoneda N., Zimmer A., Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Wang J., Morishita J., Ueda N. Biosynthetic pathways of the endocannabinoid anandamide. Chem. Biodivers. 2007;4:1842–1857. doi: 10.1002/cbdv.200790155. [DOI] [PubMed] [Google Scholar]
- Oropeza V.C., Page M.E., Van Bockstaele E.J. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Page M.E., Oropeza V.C., Van Bockstaele E.J. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci. Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolaro D., Realini N., Vigano D., Guidali C., Rubino T. The endocannabinoid system and psychiatric disorders. Exp. Neurol. 2010;224:3–14. doi: 10.1016/j.expneurol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Passie T., Emrich H.M., Karst M., Brandt S.D., Halpern J.H. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug Test. Anal. 2012;4:649–659. doi: 10.1002/dta.1377. [DOI] [PubMed] [Google Scholar]
- Patel S., Kingsley P.J., Mackie K., Marnett L.J., Winder D.G. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhanves short-term endocannabinoid signalling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Roelke C.T., Rademacher D.J., Cullinan W.E., Hillard C.J. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S., Roelke C.T., Rademacher D.J., Hillard C.J. Inhibition of restraint stress-induced neural and behavioral activation by endogenous cannabinoid signaling. Eur. J. Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pearson-Leary J., Eacret D., Chen R., Takano H., Nicholas B., Bhatnagar S. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl. Psychiatry. 2017;7(6) doi: 10.1038/tp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer F., Aronne L., Heshmati H., Devin J., Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight of obese patients: RIO-North America: a randomized controlled trial. J. Am. Med. Assoc. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Poddar M.K., Dewey W.L. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J. Pharmacol. Exp. Ther. 1980;214:63–67. [PubMed] [Google Scholar]
- Potter C.M., Vujanovic A.A., Marshall-Berenz E.C., Bernstein A., Bonn-Miller M.O. Posttraumatic stress and marijuana use coping motives: the mediating role of distress tolerance. J. Anxiety Disord. 2011;25:437–443. doi: 10.1016/j.janxdis.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher D.J., Hillard C.J. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2007;31:633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C., Taylor M., McCarthy M. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B.A., Valentino R.J., Van Bockstaele E.J. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B.A., Zitnik G., Foster C., Van Bockstaele E.J., Valentino R.J. Social stress engages neurochemically-distinct afferents to the rat locus coeruleus depending on coping strategy. eNeuro. 2015;2:1–12. doi: 10.1523/ENEURO.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B.A.S., Carvalho A.F., Szot P., Kalamarides D.J., Wang Q., Kirby L.G., Van Bockstaele E.J. Cortical adrenoceptor expression, function and adaptation under conditions of cannabinoid receptor deletion. Exp. Neurol. 2017;292:179–192. doi: 10.1016/j.expneurol.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H., Teixeira F.M., Ferreira S.G., Kittel A., Köfalzi A., Sperlágh B. Presynaptic α2-adrenoceptors control the inhibitory action of presynaptic CB1 cannabinoid receptors on prefrontocortical norepinephrine release in the rat. Neuropharmacology. 2012;63(5):784–797. doi: 10.1016/j.neuropharm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Roberto M., Cruz M., Bajo M., Siggins G.R., Parsons L.H., Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F., Ramos J.A., Bonnin A., Fernandez-Ruiz J.J. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Roitman P., Mechoulam R., Cooper-Kazaz R., Shalev A. Preliminary, open-label, pilot study of add-on oral d9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin. Drug Investig. 2014;34:587–591. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- Ross R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., De Chiara V., Musella A., Sacchetti L., Cantarella C., Castelli M., Cavasinni F., Motta C., Studer V., Bernardi G., Cravatt B.F., Maccarrone M., Usiello A., Centonze D. Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acif amide hydrolase inhibition. Mol. Pharmacol. 2010;78:260–268. doi: 10.1124/mol.110.064196. [DOI] [PubMed] [Google Scholar]
- Rovin M.L., Ross-Williams K.A., Alisch R.S., Ritchie J.C., Weinshenker D., West C.H., Weiss J.M. Influence of chronic administration of antidepressant drugs on mRNA for galanin, galanin receptors, and tyrosine hydroxyls in catecholaminergic and serotonergic cell-body regions in rat brain. Neuropeptides. 2012;46(2):81–91. doi: 10.1016/j.npep.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban E.L., Serova L.I., Newman E., Aisenberg N., Akirav I. Changes in gene expression in the locus coeruleus-amygdala circuitry in inhibitory avoidance PTSD model. Cell. Mol. Neurobiol. 2018;38(1):273–280. doi: 10.1007/s10571-017-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sarvet A.L., Wall M.M., Fink D.S., Greene E., Le A., Boustead A.E., Pacula R.L., Keyes K.M., Cerda M., Galea S., Hasin D.S. Medical marijuana laws and adolescent marijuana use in the United States: a systematic review and meta-analysis. Addiction. 2018;113(6):1003–1016. doi: 10.1111/add.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone J.L., Mackie K., Van Bockstaele E.J. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff H.C., Johansen J.P., Hou M., Bush D.E.A., Smith E.K., Klein J.E., LeDoux J.E., Sears R.M. β-adrenergic receptors regulate the acquisition and consolidation phases of aversive memory formation through distinct, temporally regulated signaling pathways. Neuropsychopharmacology. 2017;42:895–903. doi: 10.1038/npp.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra G., Fratta W. A possible role for the endocannabinoid system in the neurobiology of depression. Clin. Pract. Epidemiol. Ment. Health. 2007;3:25. doi: 10.1186/1745-0179-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A., Koolhaas J., Musso E., De Boer S. Different sympathovagal modulation of heart rate during social and nonsocial stress episodes in wild-type rats. Physiol. Behav. 1999;67:733–738. doi: 10.1016/s0031-9384(99)00134-1. [DOI] [PubMed] [Google Scholar]
- Shalev A., Roitman P., Mechoulam R. Israel Society for Biological Psychiatry abstract book Hagoshrim; Israel: 2013. Pilot Open Label Trial on Oral Absorbable Delta9-THC for Chronic PTSD. 2013. [Google Scholar]
- Shipley M.T., Fu L., Ennis M., Liu W.L., Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J. Comp. Neurol. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Slanina K.A., Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippotampus. Neuropharmacology. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Steiner M.A., Wanisch K., Monory K., Marsicano G., Borroni E., Bachli H., Holsboer F., Lutz B., Wotjak C.T. Impaired cannabinoid receptor type 1 signaling interferes with stress-coping behavior in mice. Pharmacogenomics J. 2008;8:196–208. doi: 10.1038/sj.tpj.6500466. [DOI] [PubMed] [Google Scholar]
- Swanson L.W., Sawchenko P.E. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Taylor H., Freeman M., Cates M. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am. J. Health Syst. Pharm. 2008;65:716–722. doi: 10.2146/ajhp070124. [DOI] [PubMed] [Google Scholar]
- Tzavara E.T., Davis R.J., Perry K.W., Li X., Salhoff C., Bymaster F.P., Witkin J., Nomikos G. The CB1 receptor antagonist ST141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex; implications for therapeutic actions. Br. J. Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara E.T., Perry K.W., Rodriguez D.E., Bymaster F.P., Nomikos G. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur. J. Pharmacol. 2001;426:R3–R4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- Tzavara E.T., Witkin J. The cannabinoid controversy: cannabinoid agonists and antagonists as potential novel therapies for mood disorders. In: A K., editor. Cannabinoids and the Brain. Springer Science; Coimbra, Portugal: 2008. pp. 529–558. [Google Scholar]
- Uematsu A., Tan B.Z., Ycu E.A., Cuevas J.S., Koivumaa J., Junyent F., Kremer E.J., Witten I.B., Deisseroth K., Johansen J.P. Modular organizatino of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017;20:1602–1611. doi: 10.1038/nn.4642. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriguen L., Perez-Rial S., Ledent C., Palomo R., Manzanares J. vol. 46. 2004. pp. 966–973. (Impaired Action of Anxiolytic Drugs in Mice Deficient in Cannabinoid CB1 Receptors Neuropharmacology). [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C.L., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of cortico-tropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino R.J., Bangasser D.A., Van Bockstaele E.J. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol. Pharmacol. 2013;83:737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., Lucki I., Van Bockstaele E.J. Corticotropin-releasing factor in the dorsal raphe nucleus: linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., Van Bockstaele E.J. Functional interactions between stress neuromediators and the locus coeruleus-norepinephrine system. Tech. Behav. Neural Sci. 2005;15:465–486. [Google Scholar]
- Valentino R.J., Van Bockstaele E.J. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele E.J., Bajic D., Proudfit H., Valentino R.J. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol. Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele E.J., Mackie K., Reyes B.A. In: Cellular Sites for Interactions between the Endocannabionid Metabolic Enzyme Monoacylglycerol Lipase and Norepinephrine in the Rat Frontal Cortex. Neuroscience S. f., editor. 2017. Washington, DC. [Google Scholar]
- Veenema A., Meijer O., De Kloet E., Koolhaas J. Genetic selection for coping style predicts stressor susceptiblity. J. Neuroendocrinol. 2003;15:256–267. doi: 10.1046/j.1365-2826.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- Viveros M.-P., Marco E.M., Lopez-Gallardo M., Garcia-Segura L.M., Wagner E.J. Framework for sex differences in adolescent neurobiology: a focus on cannabinoids. Neurosci. Behav. rev. 2011;35:1740–1751. doi: 10.1016/j.neubiorev.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsteeker J.I., Kuzmiski J.B., Bains J.S. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2010;30:11188–11196. doi: 10.1523/JNEUROSCI.1046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sun D., Pan B., Roberts C.J., Sun X., Hillard C.J., Liu Q.S. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropharmacology. 2010;35:2249–2261. doi: 10.1038/npp.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol. Stress. 2015;1:164–173. doi: 10.1016/j.ynstr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Walker H., Valentino R.J., Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Neuroendocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrofsky R.R., McGonigle P., Van Bockstaele E.J. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin. Drug Discov. 2015;10:17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrofsky R.R., Reyes B.A.S., Van Bockstaele E.J. Co-localization of the cannabinoid type 1 receptor with corticotropin-releasing factor-containing afferents in the noradrenergic nucleus locus coeruleus: implications for the cognitive limb of the stress response. Brain Struct. Funct. 2017:1–17. doi: 10.1007/s00429-017-1381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrofsky R.R., Yu D., Kirby L.G., Van Bockstaele E.J. Effect of cannabinoid type 1 receptor deletion on locus coeruleus-norepinephrine neurons and corticotropin releasing factor-mediated responses. Eur. J. Neurosci. 2018;48(5):2118–2138. doi: 10.1111/ejn.14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell S. The use of medical marijuana for posttraumatic stress disorder: a review of the current literature. The Primary Care Companion for CNS Disorders. 2015;17 doi: 10.4088/PCC.15r01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast M.R., Ghiasvand M., Rezayof A., Ahmadi S. The amnesic effect of intra0central amygdala administration of a cannabinoid CB1 receptor agonist, WIN 55, 212-2, is mediated by a beta-1 noradrenergic system in rat. Neuroscience. 2012;212:77–85. doi: 10.1016/j.neuroscience.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Zuardi A.W. History of cannabis as a medicine: a review. Rev. Bras. Psiquiatr. 2006;28:153–157. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.