Abstract
Particulate matter (PM) as the carcinogenic air pollutants can lead to aggravated health outcomes. Epidemiological studies demonstrated that PM can be engaged in different diseases such as cardiovascular, respiratory and cancer. The in vitro secretion of proinflammatory cytokines by human peripheral blood mononuclear cells (PBMCs) has been used to assess the effects of PM with an aerodynamic diameter < 10 μm (PM10). This study compared the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1-beta (IL1-β) secretions of PBMCs exposed to PM10 of dust storm and inversion. We collected PM10 samples during the spring and autumn seasons in two locations. Isolated PBMCs were exposed separately to 50, 150, and 300 μg/ml of different type of PM10 for 4 and 24 h. The mean concentrations of TNF-α for the PM of dust storm and inversion were 6305.61 ± 2421 and 6651.74 ± 2820, respectively. Also the mean concentrations of IL1-β for the PM of dust storm and inversion were 556.86 ± 162 and 656.35 ± 196, respectively. Furthermore, these values for the production of IL-6 were 12,655 ± 5661 and 16,685 ± 8069, respectively. Although no significant difference was observed between the PM of dust storm and that of inversion with regard to PBMCs, the results showed a significant increase in the proinflammatory cytokine secretion of both PMs compared with the controls. Moreover, TNF-α, IL1-β, and IL-6 secreted in cells exposed to PM10 of dust storm were about 10 times more than the controls, these values for cells exposed to PM10 of inversion were around 10, 12, and 14 times more than the controls, respectively. It can be concluded that the PM10 of both dust storm and inversion can play a significant role in proinflammatory cytokine secretion due to its harmful effect on human health.
Graphical abstract.
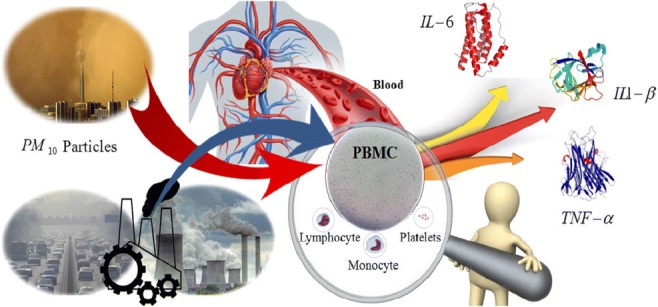
This picture shows the Proinflammatory cytokine producing potential of PM10 with two sources (dust storm and urban air pollution) in exposure with human PBMCs in vitro.
Keywords: Proinflammatory cytokine, Inversion, Dust storm, Tehran, PBMCs, In vitro
Introduction
Air pollution in megacities is one of the main environmental threats to public health in today’s modern life [1, 2]. Air pollution consists of gas-phase and particle-phase pollutants. The latter often refer to airborne PM which can originate from multiple natural and man-made sources [3]. Dust storm events are natural sources of PM which can move and disperse huge concentrations of particulate matter in long distances [4]. A broad range of diseases has been attributed to PM including chronic and acute health effects such as skin diseases [5], myocardial infarction, cardiovascular [6–8] and respiratory diseases, asthma, chronic obstructive pulmonary disease, lung cancer [9–12] as well as arteriosclerosis and premature morbidity and mortality [6, 13–16]. Although the developing paths causing cardiovascular and respiratory diseases as well as cancer and death are not thoroughly clear, numerous studies have proved several mechanisms of PM-induced harmful effects on cells such as DNA oxidative damage and genotoxicity [17–19], mutagenicity, and stimulation of proinflammatory cytokines and cytotoxicity [20–26].
The formation of reactive oxygen species (ROS) including hydroxyl radicals (OH°), superoxide anion (O2−), hydrogen peroxide (HOOH), and oxygen radicals results in oxidative stress. As a complex process, PM-induced ROS leads to oxidative potential (OP), increased functions of mitochondria, and stimulated inflammatory mediators, macrophages, dendritic cells, and lymphocytes [27, 28].
Inflammation is regulated by some mediators like multifunctional cytokines including IL1-β, IL-6, and TNF released by some tissue and inflammatory cells [29]. However, proinflammatory responses have been investigated in cultured human monocytes (THP-1) with exposure to PM10 and PM2.5 samples in vitro [30], murine macrophages (J774.1 cells), and PBMCs in exposure to ultrafine particles (UFPs) generated by combustion processes [31], particle pollution in Rio de Janeiro, Brazil, in exposure to human lung cells (BEAS-2B) [32]. In addition, proinflammatory cytokines have been studied with regard to airborne PM from different sites in Aachen, Germany, exposed to human alveolar epithelial cells (A549), in THP-1 cells as an in vitro model for evaluating monocyte/macrophage lineage cell responses exposed to PM in Los Angeles [33], in MLE-12 (mouse lung epithelial) cells, and RAW 264.7 (mouse monocyte/macrophage) cells [34].
Dust storm events during recent years have had negative effects on air quality and human health due to their prolonged atmospheric stay and carrying PM10 in an extended area [13, 35]. The Middle East is located in an arid area of dust belt with annual rainfall under 200–250 mm [36]. Iran is one of the Middle East countries which face several dust storms yearly. Residents of central and western parts of Iran where populated cities like Tehran (with a population of around 9 million people) are located are exposed to high PM concentrations of both dust storms and urban air pollution [37, 38]. Tehran is the 19th largest city in the world with about 4 million daily roaming vehicles consisting of 90% light-duty passenger cars and motorcycles and 10% other vehicles which accounts for 70% of PM urban pollution, while the power and industry sectors are responsible for 20% and 8% of PM urban pollution, respectively [39]. Also, since Tehran is located in a valley and due to the presence of medium to high mountain ranges (1000–3800 m) in the north, northwest, south, and southeast, the outflow of pollutants by humid winds is prevented. Tehran’s inhabitants usually experience high concentrations of PM especially during autumn and winter due to trapped air pollution and thermal inversion [38, 40]. This situation is aggravated by occasional dust storms originating from neighboring countries such as Iraq and Syria [37].
Although inflammatory responses to PM10 and its constituents on the alveolar macrophages and lung epithelial cells (A549 cell line) have been well reported [29], few studies have clarified the health effects of the PM of inversion and dust storm on PBMCs in vitro [41–43]. The current research is part of a comprehensive study which was done on the different biological and chemical aspects of PM10 originating from dust storm and non-dust storm sources in Tehran [44, 45]. The present study aims at evaluating the inflammatory effect of PM10 caused by the two air pollution sources of dust storm and inversion on human PBMCs. We examine the concentration changes of proinflammatory cytokines including TNF-α, IL-6, and IL1-β as biomarkers of active immune cells in PBMCs in vitro.
Materials and methods
PM10 sampling and dust storm trajectory
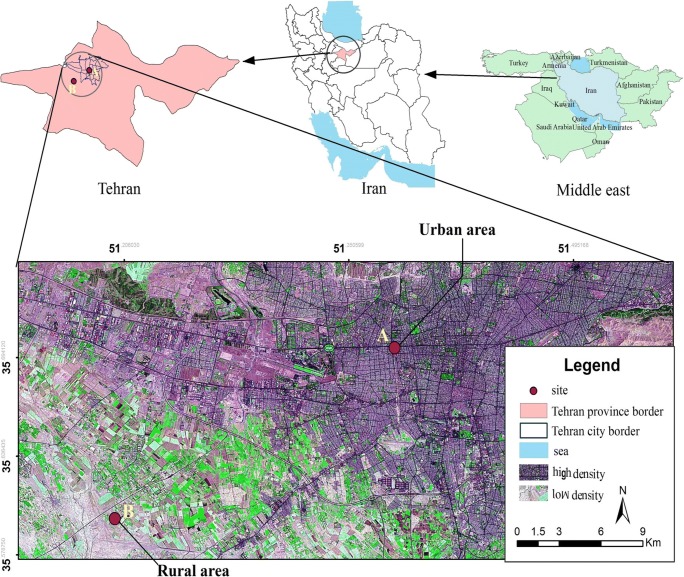
Tehran, the capital and most populated city of Iran, is located in a relatively limited area of the Alborz mountains in UTM (35° 34–35° 50′ N and 51° 08–51° 37′ E). The city is expanded in a total area of 730 km2 with around 9 million dwellers frequently facing metropolitan air pollution and dust storm events [46]. PM10 samples were collected simultaneously at two stations. Figure 1 presents the sampling sites and their geographical locations. Station (A) (35°42′ 71 ˝ N, 51°23′ 19 ˝ E), located in the central part of Tehran, is characterized by busy and high motor-vehicle traffic density. Also the second station (B) (35°38′ 10 ˝ N, 51°12′ 77 ˝ E) situated in a rural area in the southwestern part of the city is not exposed to urban air pollution due to the direction of dominant west winds.
Fig. 1.
Map of the sampling sites location (A: urban area and B: rural area)
To prevent any possible changes in the air flow and PM concentration based on the United States Environmental Protection Agency (USEPA) regulation, both samplers were set at the height of nearly 10 m above the ground level so that there were no natural and man-made barriers [47]. PM10 was sampled using two calibrated and standard size selective inlet high-volume samplers (Grasebey-Anderson, USA) at a flow rate of l.3–1.7 m3 min−1. The instruments were programmed to operate for 24 h. Normally, dust storm and inversion events take place in spring and autumn in Tehran. Hence, the samples were collected on fiberglass filters (G653, 8″ × 10″ Fiberglass Filters, Whatman, USA) from 21 April to 7 June and from 24 September to 15 November 2016. Before sampling, the filters were heated for 2 h at 550 °C to remove any organic pollution and kept in a desiccator. Before and after sampling, they were weighted in an environmentally controlled room (20–30 °C and 30–40% relative humidity) with a precise microbalance (Mettler-Toledo Inc. ±10 mg) to determine the mass of collected PM. All the sampling processes included blank filters along with field filters for quality assurance and quality control.
Meteorological data were received from the nearest (Mehrabad Airport and Shahriar, out of the 25 permanent stations) meteorological stations of Tehran Air Quality Control Company (TAQCC). Differentiation of inversion from dust storm was conducted by TAQCC daily reports and Hoffmann’s criteria based on the wind speed, visibility, and PM10 concentration [48]. In this study, we selected one day as dust storm and four days as inversion for extracting PM10 samples based on Hoffmann’s criteria and TAQCC reports (Table 1).
Table 1.
PM concentration and meteorological characteristics of selected dusty and inversion days
| Condition | Dusty Day | Inversion Days | |||
|---|---|---|---|---|---|
| Date | June 6, 2016 | November 12, 2016 | November 13, 2016 | November 14, 2016 | November 15, 2016 |
| Temperature (°C) | 28.25 | 14.12 | 13.87 | 16 | 15.37 |
| Pressure (hPa) | 881.82 | 888.22 | 889.20 | 886.31 | 884.65 |
| Dominant wind direction | Northwest | North | Northwest | West | Northwest |
| Average wind speed (m/s) | 5.12 | 0.50 | 1.37 | 2.12 | 1.5 |
| Horizontal Visibility (m) | 4200 | 4370 | 4500 | 4870 | 4060 |
| Precipitation (mm/day) | 0 | 0 | 0 | 0 | 0 |
| PM10 concentration (μg/m3) | 348.40 | 236.60 | 201.19 | 211.32 | 233.05 |
PM10 preparation
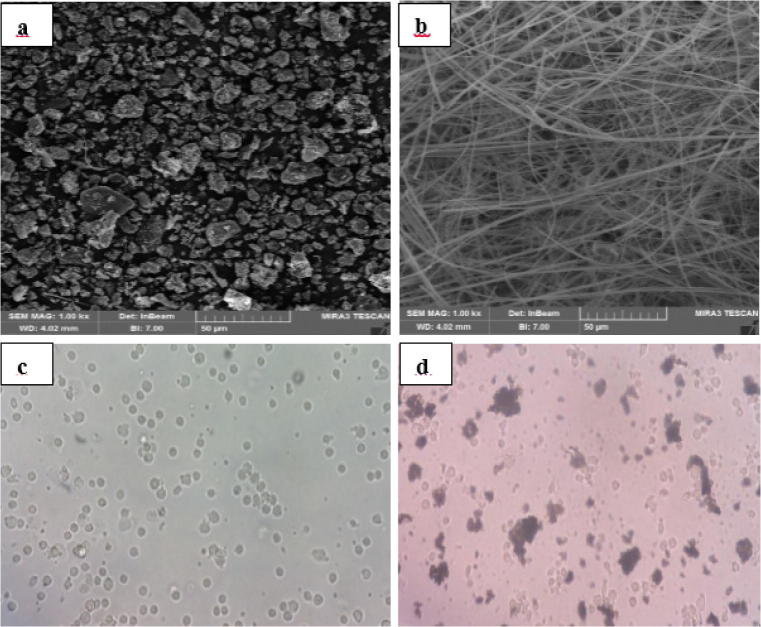
The filters were put in a clean tube before dry sweep sonication for 45 min to improve PM detaching (Elma, model D-78224 Singen/Htw, Germany). Then, the surfaces of the fiberglass filters were mechanically brushed using a very fine brush to avoid scratching them [49, 50]. The extracted mass of PM10 was placed in endotoxin-free microtubes in a − 18 °C freezer to be used for the biological experiments. Although fiberglass filters have toxic effects on cells, trivial amounts of fibers in PM concentrations used for cellular experiments do not have significant biological effects on the cells [50, 51]. Moreover, we investigated the extracted PM mass as well as blank filters using scanning electron microscope (SEM) (MIRA3-TESCAN, Czech Republic) to ensure that no fibers exist in the extracted samples (Fig. 2a and b).
Fig. 2.
SEM of extracted PM10 (a) and blank fiberglass filter (b); microscopic images of isolated PBMCs (c) and PM exposed PBMCs (d)
Isolation of PBMCs
This study was approved by the Ethics Committee of Tehran University of Medical Sciences (ethic code: IR-TUMS-SPH-REC-1395-869). Blood donors for this study consisted of nonsmoking, healthy, young adults who lived in rural areas. They volunteered to participate in the study after the research objectives were explained to them. Five men in the range of 21–25 years old (average age of 23.4 ± 1.6) participated in this research. All participants were requested to answer some questions about probable confounders such as their background diseases which might have affected the level of biomarkers in blood. In this study, inclusion criteria were normal healthy male individuals living in rural area for more than 6 months (and at least one-week permanent residence). Exclusion criteria were being a smoker or an addict, passive smokers, having any acute or chronic diseases, taking any medicine even supplements, professional athletes, having intense physical performance before blood sampling, depression, occupations in the environment with any kind of dust, smoke, or combustion products (such as farmers, bakers, and construction workers) and people with special nutrition habits (like being a vegetarian). All participants signed a written informed consent form. A 20 ml whole blood sample was obtained from each participants.
Heparinized blood samples of five volunteers were immediately processed within 2 h of sampling. To ensure the participants’ health, routine tests were performed on them. Parts of their blood was sent to the laboratory for routine tests such as CBC diff, total cholesterol, triglycerides, fasting blood sugar, CRP (C-reactive protein), and ESR (erythrocyte sedimentation rate). PBMCs were isolated from fresh whole blood by density centrifugation on Ficoll-Hypaque gradient with Histopaque-1077 (Sigma-Aldrich). Having lower density, mononuclear cells and platelets gather on top of the Ficoll-Hypaque layer whereas red blood cells (RBC) and granulocytes accumulate at the bottom of the Ficoll-Hypaque layer due to their higher density. The isolation method began by diluting and mixing whole blood using 40 ml Ca2+/Mg2+-free PBS (Biosera, France). Then, 30 ml Ficoll-Hypaque solution (Biosera, France) was added to the diluted blood. The tube was centrifuged for 22 min at 2000 rpm with no acceleration and no brake. The observed buffy coat was collected precisely and washed by lysis buffer and isolation buffer. The isolated solution was centrifuged at 400 g, for 14 min, with an acceleration of 6, and brake of 4. Next, PBMCs were suspended in 1 ml complete RPMI-1640 culture medium (Gibco BRL, San Diego, CA) (Fig. 2c) [52]. The living-cells portion was measured by trypan blue solution. The viability of the cells was over 98%.
Cell toxicity and MTT assay
Cytotoxicity assay was conducted on the extracted and pooled particles of dust storm and inversion to determine the toxicity of PM particles showing their maximum concentration used in the biological experiments. In this study, the tetrazolium-based colorimetric assay (MTT test), a well-known standardized method, was used to measure cell viability, proliferation, and/or toxicity of dust storm and inversion PMs. The amount of living cells is proportional to the amount of formazan and is therefore proportional to color intensity. In each well of a 96-well plate, 200,000 cells were seeded in 100 mL RPMI-1640 culture medium and kept in an incubator at 37 °C with 5% (v/v) CO2. Then, the prepared cells were exposed to low, medium, and high concentrations (50, 100, 200, 300, 400, 500, and 700 μg/mL) of PM in duplicate. After 24 h incubation, 0.5 mg/mL MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide, Sigma, USA) was added to each well. The spectrophotometric measurement of the formazan crystal was achieved by prolonging the incubation period to 4 h. The plates were then centrifuged at 800 g for 5 min. The supernatant was elutriated by removing the untransformed MTT, and 150 μL dimethylsulfoxide (DMSO) (Sigma, USA) was added to each well. By shaking the plate, the purple crystals of formazan were solved and the absorbance of the solution was measured using microplate spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA) at 570 nm. The results were expressed as OD (optical density) after blank (cell and medium only) subtraction. The cell viability of less than 80% attributed to each PM concentration was considered as a toxic concentration for PBMCs.
In vitro PM treatment
Isolated PBMCs of each blood donor were separately treated with pooled PMs of both dust and inversion. PM concentrations were treated in the RPMI-1640 culture medium which was seeded by 1 × 106 cell/ml PBMCs a day before and incubated at 37 °C with 5% (v/v) CO2 in a 95% humidified atmosphere (Fig. 2d). Before treating, the PM stock was thoroughly vortexed (2 min) to obtain a homogeneous dose at three concentrations of 50, 150, and 300 μg/ml before being added to each well for exposure times of 4 and 24 h. Our preliminary experiments suggested 4 and 24 h as suitable exposure times for studying the release of TNF-α, IL1-β, and IL-6. Untreated cells in each cell culture plate were taken as controls. Moreover, the control and treatment wells were considered in duplicate. At the end of each exposure time, the cell culture medium was collected and centrifuged at 250 g for 10 min to remove the particles and cell debris. Thereafter, the final supernatants (in duplicate for each cytokine) were stored at −80 °C for future cytokine assays. Finally, protein levels of TNF-α, IL-6, and IL1-β were determined by enzyme-linked immunosorbent assay (ELISA).
Enzyme-linked immunosorbent assay (ELISA)
Proinflammatory secretion of TNF-α, IL1-β, and IL-6 was measured in the supernatants of PBMCs exposed to non-lethal concentrations of PM10 (50, 150, and 300 μg/ml) using sandwich type ELISA commercial kits [(DTA00C), (DLB50), and (D6050) Quantikine ELISA Development System, R&D Systems, USA)], according to the manufacturer’s directions. The minimum detectable dose (MDD) of human TNF-α ranged from 0.5 to 5.5 pg/ml. Also, MDD for IL-1β and IL-6 was typically less than 1 and 0.70 pg/ml, respectively. Non-exposed cells were used as controls. ELISA determinations of TNF-α, IL1-β, and IL-6 were run in duplicate. The plates were read at 450 nm, with the correction wavelength set at 540 nm or 570 nm using a plate reader (Bio-Tek Instruments, Winooski, VT, USA). Finally, cytokine concentrations were computed against a standard curve provided with the kits.
Statistical analyses
Data analysis was accomplished by means of the statistical software package SPSS (version 19.0). Differences were considered to be statistically significant at Pvalue < 0.05. Also, Prism 6.0 for windows (GraphPad Software, San Diego, CA) was used for drawing the graphs reported as means ± standard error (SE). The results were expressed as mean values and standard deviations. For the specified exposure times (4 and 24 h), data from control wells and PMs of dust storm- and inversion-exposed cells were compared to those of non-exposed cells. The statistical analyses of cytokine responses for different concentrations of the PMs of dust storm and inversion were performed by the non-parametric Friedman Test. Also, Wilcoxon signed-rank test was used to compare the differences between dust storm and inversion PM groups in terms of the amount of cytokines released.
Results
PM10 concentration and dust storm trajectory
The extracted PM mass from the filters ranged from 186 to 1438 μg for the rural site and from 259 to 1309 μg for the urban site. Dust storm and inversion days in Tehran were observed on 6 June 2016 and on 12–15 November 2016, respectively. Also, the World Meteorological Organization (WMO) protocol consists of four categories for comparing different kinds of dust storms in terms of visibility: dust-in-suspension, blowing dust, dust storm, and severe dust storm. Blowing dust (visibility of less than 10 km) and dust-in-suspension (visibility of 1 to 10 km) matched Tehran’s dust storm conditions at the time of observation [53, 54]. In this research, the dusty day is within dust-in-suspension and blowing dust categories. Inversion days lasted for four days from 12 to 15 of November 2016. Based on the daily report announcements of TAQCC, these days were categorized as thermal inversion which was a dangerous condition for vulnerable groups of people in Tehran. The average daily concentration of PM10 was 220.54 μg/m3 during the inversion days with the highest concentration of 236.60 μg/m3 (Table 1). According to these data, the daily mean concentrations of PM10 on the dust storm day and the inversion days were about 7 times and 4.4 times higher than the WHO (World Health Organization) air quality guideline value (50 μg/m3), respectively. Therefore, Tehran province residents face a high concentration of PM10 originating from more than 3 million roaming vehicles [38].
MTT assay
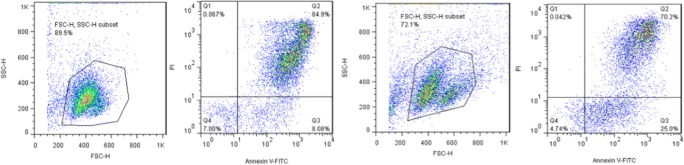
Table 2 shows the results of the MTT assay for the PM10 of both dust storm and inversion days. These results indicated that the necrosis and apoptosis of PBMCs have increased by both types of particles in comparison to the control. Moreover, the viability of PBMCs decreased more by inversion particles than by dust storm particles. In other words, particulate matters inhibit cell proliferation. The percentages of viable cells exposed to the PMs (with concentrations of 50–700 μg/mL) of dust storm and inversion were 96.65–7.00 and 93.06–4.74, respectively. Flow cytometry analysis was used to monitor the viability of PBMCs exposed to dust storm and inversion PM10 for the concentration of 700 (μg/ml) (Fig. 3).
Table 2.
Viability percentage of PBMCs exposed to the PM10 of dust storm and inversion (50–700 μg/ml) in MTT assay
| PM concentration (μg/ml) |
Percent of Cell viability | |||
|---|---|---|---|---|
| Dust storm PM10 | Inversion PM10 | |||
| Mean | SD | Mean | SD | |
| Untreated cells | 98.00 | 2.78 | 98.00 | 2.78 |
| 50 | 96.65 | 3.36 | 93.06 | 3.48 |
| 100 | 94.12 | 4.49 | 91.03 | 4.21 |
| 150 | 92.56 | 3.38 | 90.14 | 3.89 |
| 200 | 90.85 | 4.65 | 88.09 | 5.55 |
| 250 | 86.77 | 4.41 | 82.33 | 4.49 |
| 300 | 84.00 | 5.43 | 80.11 | 5.08 |
| 400 | 63.58 | 4.89 | 57.61 | 3.84 |
| 500 | 23.51 | 3.87 | 20.01 | 2.86 |
| 700 | 7.00 | 1.43 | 4.74 | 1.17 |
Fig. 3.
Flow cytometry analysis in the PBMCs exposed to PM10 of dust storm (a) and inversion (b) [concentration of 700 (μg/ml)] (Note: Q1, Q2, Q3, and Q4 stand for cell necrosis, late apoptosis, early apoptosis, and viable cells)
In the current study, the maximum concentration of 300 μg/ml for PM was selected as the uppermost nonlethal exposure concentration for PBMCs. Particle contents produce reactive oxygen species (ROS) in cells which is responsible for decreased mitochondrial functionality, cell damage, and death. Thus, a significant positive correlation with cytotoxicity is provided [55]. In the current study, it was observed that the reduction of cell viability was more in the PM of inversion than that of dust storm in all concentrations. This finding is in agreement with the results of Gualtieri et al. (2010, 2012) who reported the correlation, which was more in winter than in summer, between the airborne PM of Milan (Italy) and cytotoxic and genotoxic effects. In addition, in the study of Roig et al. (2013) in Catalonia (Spain), the PM of traffic-impacted areas was more cytotoxic and genotoxic [55–57].
The proinflammatory response of PBMCs to PM10
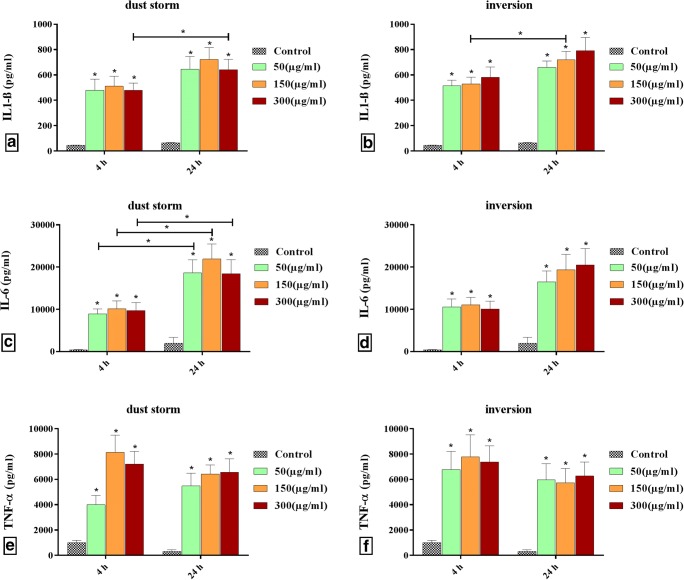
Dose-dependency responses: Fig. 4a and b shows the dose-related effects of the PM10 of dust storm and inversion on IL1-ß production in human PBMCs. There was a statistically significant dose-dependent increase in IL1-ß production of PM10 for both dust storm and inversion. However, the responses related to 300 μg/ml PM10 of dust storm in both exposure times decreased. Also, the responses to PM10 of inversion were larger than those of dust storm. There were significant differences between the control and IL1-ß production in PM10 of dust storm and inversion in both exposure times. The production of IL-6 increased significantly in the PM10 of both dust storm and inversion by increasing dose whereas for a longer exposure time, the PM10 of dust storm played a more important role in the production of IL-6 (Fig. 4c and d). The PM10 of dust storm was more influential on the production of TNF-α cytokine. A significant increase in the production of TNF-α for the PM10 of dust storm and inversion compared to the control can be seen in Fig. 4e and f. According to Fig. 4, the production of TNF-α and IL1-ß is more dose-dependent than that of IL-6. However, it is clear that the production of all cytokines was increased several times through the stimulation of PBMCs by PM10.
Fig. 4.
Dose and time-dependency of cytokines in exposure times of PBMCs in duplicate a: IL1-β produced by PM10 of dust storm; b: IL1-β produced by PM10 of inversion; c: IL-6 produced by PM10 of dust storm; d: IL-6 produced by PM10 of inversion; e: TNF-α produced by PM10 of dust storm; f: TNF-α produced by PM10 of inversion. [* = statistically significant differences (P < 0.05)], (asterisks above the bars indicate statistically significant differences from the control)
Time-dependency responses: Time-related effects of the PM10 of dust storm and inversion on IL1-ß production in human PBMCs are shown in Fig. 4a and b. There was a time-dependent increase in IL1-ß production for the PM10 of both dust storm and inversion, but the responses in the exposure time of 4 h were more modest than those of 24 h. In comparing 4 h and 24 h exposure times, significant differences in the secretion of IL1-ß were observed in PM concentrations of 150 and 300 μg/ml for inversion and dust storm, respectively. Figure 4c and d demonstrates that the increase in the secretion of IL-6 in the PM of dust storm at the exposure time of 4 h compared to that of 24 h was significant at all concentrations, while this increase was not statistically significant in the PM of inversion. Also, as can be observed in Fig. 4e and f, exposure time had a reverse effect on cytokine release in both PM types. This means that by increasing the exposure time, TNF-α production was decreased independently of PM dose.
PM10 of dust storm versus PM10 of inversion
As was mentioned above, PBMCs were exposed to different doses of PM10 of dust storm and inversion for 4 and 24 h. Tables 3 and 4 show descriptive and test statistics of proinflammatory cytokines in two exposure times with all concentrations of PM10. The inflammatory responses to PM10 of dust storm were not statistically significant compared to those of the inversion. However, it can be seen that except TNF-α and IL-6 in exposure time of 24 h, all mean concentrations of proinflammatory cytokines in inversion group were slightly higher than those of dust storm group. Considering all exposure times and PM concentrations together, all cytokine secretions except TNF-α were higher in the inversion group than the dust storm group.
Table 3.
Descriptive statistics of proinflammatory cytokines in all exposure times and all concentrations of PM10
| Cytokine | PM10 Type | Exposure time (hour) | Mean (Pg/ml) | Std. Deviation (Pg/ml) | Minimum (Pg/ml) | Maximum (Pg/ml) |
|---|---|---|---|---|---|---|
| TNF-α | Dust storm | 4 | 7526.9115 | 2523.19912 | 3801.34 | 12,430.90 |
| IL-6 | Dust storm | 4 | 9616.2183 | 3418.61905 | 5660.33 | 16,179.75 |
| IL1-ß | Dust storm | 4 | 490.1885 | 156.30834 | 295.13 | 799.61 |
| TNF-α | Dust storm | 24 | 6160.8471 | 1992.58299 | 3727.20 | 9241.48 |
| IL-6 | Dust storm | 24 | 19,687.1562 | 7062.51486 | 7335.60 | 29,439.60 |
| IL1-ß | Dust storm | 24 | 670.3960 | 192.69945 | 382.61 | 1035.60 |
| TNF-α | Dust storm | All | 6843.8793 | 2339.41401 | 3727.20 | 12,430.90 |
| IL-6 | Dust storm | All | 14,651.6873 | 7480.09645 | 5660.33 | 29,439.60 |
| IL1-ß | Dust storm | All | 580.2923 | 195.24307 | 295.13 | 1035.60 |
| TNF-α | Inversion | 4 | 7310.0585 | 3116.43783 | 3624.32 | 12,865.11 |
| IL-6 | Inversion | 4 | 10,571.2228 | 3781.52195 | 5502.01 | 16,621.25 |
| IL1-ß | Inversion | 4 | 542.1740 | 130.52705 | 288.17 | 785.24 |
| TNF-α | Inversion | 24 | 5993.4367 | 2415.70382 | 3683.40 | 10,374.52 |
| IL-6 | Inversion | 24 | 18,809.0470 | 7219.65384 | 7197.92 | 29,192.45 |
| IL1-ß | Inversion | 24 | 723.6740 | 169.15735 | 492.11 | 1170.60 |
| TNF-α | Inversion | All | 6651.7476 | 2820.31139 | 3624.32 | 12,865.11 |
| IL-6 | Inversion | All | 14,690.1349 | 7043.92484 | 5502.01 | 29,192.45 |
| IL1-ß | Inversion | All | 632.9240 | 174.80907 | 288.17 | 1170.60 |
Table 4.
Test statistics of proinflammatory cytokines in all exposure times and all concentrations of PM10
| TNF-α | IL-6 | IL1-ß | TNF-α | IL-6 | IL1-ß | TNF-α | IL-6 | IL1-ß | |
|---|---|---|---|---|---|---|---|---|---|
| Exposure time (h) | 4 | 4 | 4 | 24 | 24 | 24 | All | All | All |
| Z | −.738 | −1.250 | −1.817 | −.682 | −1.193 | −.966 | −.936 | −.257 | −1.656 |
| Asymp. Sig. (2-tailed) | .460 | .211 | .069 | .496 | .233 | .334 | .349 | .797 | .098 |
Discussion
The rapid growth of technology and industry, besides human manipulation of nature, have resulted in a massive increase of air pollution in the world. Based on recent epidemiological studies, air pollution has become a global issue due to its widespread and different effects on human health [58]. These health effects are altered temporally and spatially depending on the origin of PMs from urban or rural areas which affects their size and characteristics. About 40% of the particle mass in urban areas is attributable to fossil fuels, while particle masses in rural areas originate from the earth’s crust [59]. However, the specific emission sources of PM as a likely contributor to determining health risks have not been fully addressed. To the best of our knowledge, this is the first study that shows the comparative proinflammatory effects of two types of airborne particulate matter (PM10) of dust storms and urban air pollution events on human PBMCs in Iran. We now provide some new insights into the inflammatory cytokine produced by human blood cells in exposure to PM with different sources. In the current study, the proinflammatory cytokines (TNF-α, IL-6, and IL1-β) produced by the Tehran airborne particulate matter samples of rural and urban areas during two seasons (spring and autumn 2016) were investigated on human peripheral blood mononuclear cells (PBMCs) and measured by ELISA.
In a study conducted by Steenhof et al. (2011) on murine macrophage cell line in vitro, different concentrations of proinflammatory cytokines were released in response to the PM samples of different sites and sizes. Thus, the evidence to date proposes that toxicological responses differ between urban and rural PM sources [60, 61]. Similarly, Rezaei et al. (2018) demonstrated that different chemical constituents of PM from two conditions lead to differences in ROS levels which in turn induce different oxidative stresses [45]. This disparity in oxidative stresses is related to distinct chemical characteristics of PM samples from two origins [45]. It will result in different modifications of proinflammatory cytokine secretion. Moreover, the analyses of metals, ions, and PAHs resemble different characteristics of PMs, inflammatory responses, and the generation of cellular oxidative stress (ROS) influenced by chemical components especially metals [62].
The rural sample of coarse PM caused a significant increase in TNF-α concentration. This phenomenon has been well illustrated in the study of Dergham et al. (2012) in rural particles compared to particles with urban and industrial origins in BEAS-2B cells [63]. In agreement with our study, TNF-α cytokine secretion decreased with increasing exposure time. This decrease did not have a regular trend and was not statistically significant in both types of particles [63].
The study of Yang et al. (2018) on human bronchial epithelial (BEAS-2B) cell lines as an in vitro model demonstrated that high concentrations of PAHs and elemental Ni were strongly associated with high apoptosis rates and high expression of IL-1β. Furthermore, the ROS level was altered by Fe element whereas Fe and Cr elements induced DNA damage [58]. Another research revealed that urban PM extract-exposed BEAS-2B cell line containing PAHs, volatile organic compounds (VOCs), and transition metals by producing ROS can participate in lipid peroxidation, DNA mutations, protein oxidative damage, upregulated gene expression, and production and release of inflammatory cytokines and chemokines [64].
It is worthwhile to mention that PM10, on the whole, consists of coarse, fine, and UFPs. Finer fractions of PM are inhaled and can reach to peripheral blood stream and the brain by penetrating the blood–air barrier of the lungs [65, 66]. Moreover, in recent years, in vitro PM exposures have been used in many human studies. Although we applied dry mechanical extraction of particulate matters from the surface of filters to prevent any manipulation and change in the physical and chemical characteristics of PMs, it should be mentioned that the simulation of the real concentration of PM, to which human blood cells are exposed, is actually challenging. By the way, by using this method, in vitro exposure of isolated human organs (such as blood cells) to PM can provide useful data about the mechanism of PM health effects. In vitro models besides animal models and epidemiological studies can provide essential information for fully understanding the consequences of exposure to air pollution and definite carcinogenic effects of particulate matters.
Limitations
Like other studies, this study has some limitations that are inevitable. Since the particle sampling depended on the location, season of the year, and the weather conditions, the determination of and planning for the number of days with dust storms and inversion was not under the control of the researchers. By the way, we aimed to find the proinflammatory effects of two types of PM10 on human PBMCs in dust storm and inversion conditions. Although due to the limitations of in vitro studies, the findings of such researches may be specific to certain conditions, complementary in vivo and ex vivo researches should be expanded to better define the underlying mechanisms. Additional epidemiological and toxicological studies are recommended to continue and complete the other aspects of the current study. The other limitation of these kinds of studies is to simulate the real concentration and conditions of human blood cells exposed to particulate matters although we tried to minimize the environmental confounders as much as possible.
Conclusion
The urban and rural airborne PM samples collected in spring and autumn induced different proinflammatory profiles in the in vitro model. We supposed that PM10 with dust storm and inversion sources can affect the expression of proinflammatory cytokines. We could not find any study in Iran comparing the effects of PM10 with different origins on human PBMCs in vitro. In conclusion, we demonstrated that exposure to the PM10 of both inversion and dust storm significantly increased proinflammatory cytokines in PBMCs without showing clear differences between PM10 origins, although the results showed a slight cytokine increase in PBMCs exposed to the PM of inversion. Although the exposure conditions of the current research may not have been exactly the same as real human exposure to PM, it is obvious that using in vitro models as valuable basic studies in a highly controlled condition will produce valuable information without discomforting the subjects participating in experiments in a cheaper and faster way compared with other researches on health effects of PM. These findings besides other complementary investigations lead to acquiring relevant knowledge about the dangerous effects of PM in cellular scale so that proper management and control measures can be adopted to reduce the harmful effects of PM on human health.
Acknowledgments
This work was part of the Ph.D thesis of Zahra Atafar (first author), a student of the Tehran University of Medical Sciences (TUMS). This study was financially and technically supported by the Institute for Environmental Research (IER) (grant number: 95-03-46-32835) of Tehran University of Medical Sciences. The authors are also grateful to the manager and staff of the Immunology, Asthma and Allergy Research Institute (IAARI) of Tehran University of Medical Sciences for their generous cooperation and technical support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Osseiran N, Chriscaden K. Air pollution levels rising in many of the world’s poorest cities. 2016. https://www.who.int/news-room/detail/12-05-2016-air-pollution-levels-rising-in-many-of-the-world-s-poorestcities.
- 2.Ghorani-Azam A, Riahi-Zanjani B, Balali-Mood M. Effects of air pollution on human health and practical measures for prevention in Iran. J Res Med Sci. 2016;21:65. [DOI] [PMC free article] [PubMed]
- 3.Kim K-H, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Khaniabadi YO, Daryanoosh SM, Amrane A, Polosa R, Hopke PK, Goudarzi G, Mohammadi MJ, Sicard P, Armin H. Impact of middle eastern dust storms on human health. Atmos Pollution Res. 2017;8(4):606–613. [Google Scholar]
- 5.Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Abdolahnejad A, Jafari N, Mohammadi A, Miri M, Hajizadeh Y, Nikoonahad A. Cardiovascular, respiratory, and total mortality ascribed to PM10 and PM2. 5 exposure in Isfahan, Iran. J Educ Health Promot. 2017;6. [DOI] [PMC free article] [PubMed]
- 7.Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016;8(1):E8. doi: 10.3978/j.issn.2072-1439.2015.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf K, Schneider A, Breitner S, Meisinger C, Heier M, Cyrys J, Kuch B, von Scheidt W, Peters A, KORA Study Group Associations between short-term exposure to particulate matter and ultrafine particles and myocardial infarction in Augsburg, Germany. Int J Hyg Environ Health. 2015;218(6):535–542. doi: 10.1016/j.ijheh.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Khaefi M, Geravandi S, Hassani G, Yari AR, Soltani F, Dobaradaran S, Moogahi S, Mohammadi MJ, Mahboubi M, Alavi N, Farhadi M, Khaniabadi YO. Association of particulate matter impact on prevalence of chronic obstructive pulmonary disease in Ahvaz, Southwest Iran during 2009–2013. Aerosol Air Qual Res. 2017;17(1):230–237. [Google Scholar]
- 10.Ghozikali MG, Ansarin K, Naddafi K, Nodehi RN, Yaghmaeian K, Hassanvand MS, Yunesian M. Prevalence of asthma and associated factors among male late adolescents in Tabriz, Iran. Environ Sci Pollut Res. 2018;25(3):2184–2193. doi: 10.1007/s11356-017-0553-6. [DOI] [PubMed] [Google Scholar]
- 11.Gharibvand L, Shavlik D, Ghamsary M, Beeson WL, Soret S, Knutsen R, Knutsen SF. The association between ambient fine particulate air pollution and lung cancer incidence: results from the AHSMOG-2 study. Environ Health Perspect. 2016;125(3):378–384. doi: 10.1289/EHP124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharibvand L, Beeson WL, Shavlik D, Knutsen R, Ghamsary M, Soret S, et al. The association between ambient fine particulate matter and incident adenocarcinoma subtype of lung cancer. Environ Health. 2017;16(1):71. doi: 10.1186/s12940-017-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crooks JL, Cascio WE, Percy MS, Reyes J, Neas LM, Hilborn ED. The association between dust storms and daily non-accidental mortality in the United States, 1993–2005. Environ Health Perspect. 2016;124(11):1735–1743. doi: 10.1289/EHP216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa AF, Hoek G, Brunekreef B, de Leon ACP. Air pollution and deaths among elderly residents of Sao Paulo, Brazil: an analysis of mortality displacement. Environ Health Perspect. 2017;125(3):349–354. doi: 10.1289/EHP98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 16.Daryanoosh M, Goudarzi G, Rashidi R, Keishams F, Hopke PK, Mohammadi MJ, et al. Risk of morbidity attributed to ambient PM10 in the western cities of Iran. Toxin Rev. 2017:1–6.
- 17.Ghanbarian M, Nicknam MH, Mesdaghinia A, Yunesian M, Hassanvand MS, Soleimanifar N, et al. Investigation and comparison of in vitro genotoxic potency of PM 10 collected in rural and urban sites at Tehran in different metrological conditions and different seasons. Biol Trace Elem Res. 2018:1–10. [DOI] [PubMed]
- 18.Srivastava A, Yadav S, Pandey AK, Dwivedi UN, Parmar D. Ultrafine particles of diesel exhaust induces cytochrome P450 1A1 mediated oxidative stress and DNA damage in cultured blood and lung cells. Def Life Sci J. 2016;1.
- 19.Marcoccia M, Ronci L, De Matthaeis E, Setini A, Perrino C, Canepari S. In-vivo assesment of the genotoxic and oxidative stress effects of particulate matter on Echinogammarus veneris. Chemosphere. 2017;173:124–134. doi: 10.1016/j.chemosphere.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Velali E, Papachristou E, Pantazaki A, Choli-Papadopoulou T, Argyrou N, Tsourouktsoglou T, et al. Cytotoxicity and genotoxicity induced in vitro by solvent-extractable organic matter of size-segregated urban particulate matter. Environmental pollution. 2016;218(Supplement C):1350–62. 10.1016/j.envpol.2016.09.001. [DOI] [PubMed]
- 21.MohseniBandpi A, Eslami A, Shahsavani A, Khodagholi F, Alinejad A. Physicochemical characterization of ambient PM2. 5 in Tehran air and its potential cytotoxicity in human lung epithelial cells (A549). Sci Total Environ. 2017;593:182–90. [DOI] [PubMed]
- 22.Øvrevik J, Refsnes M, Låg M, Holme J, Schwarze P. Activation of proinflammatory responses in cells of the airway mucosa by particulate matter: oxidant-and non-oxidant-mediated triggering mechanisms. Biomolecules. 2015;5(3):1399–1440. doi: 10.3390/biom5031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez Y, Carranza C, Iñiguez M, Torres M, Quintana R, Osornio A, Gardner C, Sarkar S, Schwander S. Effect of inhaled air pollution particulate matter in alveolar macrophages on local pro-inflammatory cytokine and peripheral interferon γ production in response to mycobacterium tuberculosis. Lancet Glob Health. 2018;6:S29. [Google Scholar]
- 24.Ma Q-Y, Huang D-Y, Zhang H-J, Wang S, Chen X-F. Exposure to particulate matter 2.5 (PM2. 5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int Immunopharmacol. 2017;50:139–145. doi: 10.1016/j.intimp.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Landkocz Y, Ledoux F, André V, Cazier F, Genevray P, Dewaele D, Martin PJ, Lepers C, Verdin A, Courcot L, Boushina S, Sichel F, Gualtieri M, Shirali P, Courcot D, Billet S. Fine and ultrafine atmospheric particulate matter at a multi-influenced urban site: physicochemical characterization, mutagenicity and cytotoxicity. Environ Pollut. 2017;221:130–140. doi: 10.1016/j.envpol.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 26.Luo M, Bao Z, Xu F, Wang X, Li F, Li W, et al. Unrepaired DNA damage in macrophages causes elevation of particulate matter-induced airway inflammatory response. Aging (Albany NY) 2018;10(4):549. doi: 10.18632/aging.101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellack B, Quass U, Nickel C, Wick G, Schins RP, Kuhlbusch TA. Oxidative potential of particulate matter at a German motorway. Environ Sci: Processes Impacts. 2015;17(4):868–876. doi: 10.1039/c4em00605d. [DOI] [PubMed] [Google Scholar]
- 28.Calas A, Uzu G, Martins JM, Voisin D, Spadini L, Lacroix T, et al. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci Rep. 2017;7(1):11617. doi: 10.1038/s41598-017-11979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Møller P, Danielsen PH, Karottki DG, Jantzen K, Roursgaard M, Klingberg H, Jensen DM, Christophersen DV, Hemmingsen JG, Cao Y, Loft S. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res/Rev Mutat Res. 2014;762:133–166. doi: 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Manzano-León N, Serrano-Lomelin J, Sánchez BN, Quintana-Belmares R, Vega E, Vázquez-López I, Rojas-Bracho L, López-Villegas MT, Vadillo-Ortega F, de Vizcaya-Ruiz A, Perez IR, O'Neill MS, Osornio-Vargas AR. TNF α and IL-6 responses to particulate matter in vitro: variation according to PM size, season, and polycyclic aromatic hydrocarbon and soil content. Environ Health Perspect. 2015;124(4):406–412. doi: 10.1289/ehp.1409287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Falco G, Terlizzi M, Sirignano M, Commodo M, D’Anna A, Aquino RP et al. Human peripheral bloodmononuclear cells (PBMCs) from smokers release higher levels of IL-1-like cytokines after exposure to combustion-generated ultrafine particles. Sci Rep. 2017;7:43016. 10.1038/srep43016. [DOI] [PMC free article] [PubMed]
- 32.Rodríguez-Cotto RI, Ortiz-Martínez MG, Rivera-Ramírez E, Mateus VL, Amaral BS, Jiménez-Vélez BD, et al. Particle pollution in Rio de Janeiro, Brazil: Increase and decrease of pro-inflammatory cytokines IL-6 and IL-8 in human lung cells. Environ Pollut. 2014;194(Supplement C):112–120. doi: 10.1016/j.envpol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu W, Robin M, Kiros B, Scott F, Feifei L, Ilona J, et al. Inflammatory response of monocytes to ambient particles varies by highway proximity. Am J Respir Cell Mol Biol. 2014;51(6):802–809. doi: 10.1165/rcmb.2013-0265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musah S, DeJarnett N, Hoyle GW. Tumor necrosis factor-α mediates interactions between macrophages and epithelial cells underlying proinflammatory gene expression induced by particulate matter. Toxicology. 2012;299(2–3):125–132. doi: 10.1016/j.tox.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Najafi MS, Khoshakhllagh F, Zamanzadeh SM, Shirazi MH, Samadi M, Hajikhani S. Characteristics of TSP loads during the Middle East springtime dust storm (MESDS) in Western Iran. Arab J Geosci. 2014;7(12):5367–5381. [Google Scholar]
- 36.Ashrafi K, Shafiepour-Motlagh M, Aslemand A, Ghader S. Dust storm simulation over Iran using HYSPLIT. J Environ Health Sci Eng. 2014;12(1):9. doi: 10.1186/2052-336X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Givehchi R, Arhami M, Tajrishy M. Contribution of the middle eastern dust source areas to PM 10 levels in urban receptors: case study of Tehran, Iran. Atmos Environ. 2013;75:287–295. [Google Scholar]
- 38.Naddafi K, Hassanvand MS, Yunesian M, Momeniha F, Nabizadeh R, Faridi S, Gholampour A. Health impact assessment of air pollution in megacity of Tehran, Iran. Iran J Environ Health Sci Eng. 2012;9(1):28. doi: 10.1186/1735-2746-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taghvaee S, Sowlat MH, Mousavi A, Hassanvand MS, Yunesian M, Naddafi K, et al. Source apportionment of ambient PM 2.5 in two locations in Central Tehran using the positive matrix factorization (PMF) model. Sci Total Environ. 2018;628:672–686. doi: 10.1016/j.scitotenv.2018.02.096. [DOI] [PubMed] [Google Scholar]
- 40.Faridi S, Shamsipour M, Krzyzanowski M, Künzli N, Amini H, Azimi F, et al. Long-term trends and health impact of PM 2.5 and O 3 in Tehran, Iran, 2006–2015. Environ Int. 2018;114:37–49. doi: 10.1016/j.envint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 41.He M, Ichinose T, Kobayashi M, Arashidani K, Yoshida S, Nishikawa M, Takano H, Sun G, Shibamoto T. Differences in allergic inflammatory responses between urban PM2. 5 and fine particle derived from desert-dust in murine lungs. Toxicol Appl Pharmacol. 2016;297:41–55. doi: 10.1016/j.taap.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, Baumgartner J, Zhang Y, Schauer JJ. Source apportionment of Beijing air pollution during a severe winter haze event and associated pro-inflammatory responses in lung epithelial cells. Atmos Environ. 2016;126:28–35. [Google Scholar]
- 43.Ortiz-Martínez MG, Rodríguez-Cotto RI, Ortiz-Rivera MA, Pluguez-Turull CW, Jiménez-Vélez BD. Linking endotoxins, African dust PM10 and asthma in an urban and rural environment of Puerto Rico. Mediat Inflamm. 2015;2015:1–14. doi: 10.1155/2015/784212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naddafi K, Atafar Z, Faraji M, Ghanbarian M, Rezaei S, Ghozikali MG, et al. Health effects of airborne particulate matters (PM10) during dust storm and non-dust storm conditions in Tehran. J Air Pollut Health. 2017;1(4):259–268. [Google Scholar]
- 45.Rezaei S, Naddafi K, Hassanvand MS, Nabizadeh R, Yunesian M, Ghanbarian M, et al. Physiochemical characteristics and oxidative potential of ambient air particulate matter (PM 10) during dust and non-dust storm events: a case study in Tehran, Iran. Journal of Environmental Health Science and Engineering. 2018;16(2):147. [DOI] [PMC free article] [PubMed]
- 46.Sarkhosh M, Shamsipour A, Yaghmaeian K, Nabizadeh R, Naddafi K, Mohseni SM. Dispersion modeling and health risk assessment of VOCs emissions from municipal solid waste transfer station in Tehran, Iran. J Environ Health Sci Eng. 2017;15(1):4. doi: 10.1186/s40201-017-0268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.U.S. Environmental Protection Agency Monitoring and Quality Assurance Group Emissions M, and Analysis, Division Office of Air Quality Planning and Standards Research Triangle Park, NC 27711. Particulate Matter (PM2.5) Speciation Guidance. 1999.
- 48.Hoffmann C, Funk R, Sommer M, Li Y. Temporal variations in PM 10 and particle size distribution during Asian dust storms in Inner Mongolia. Atmos Environ. 2008;42(36):8422–8431. [Google Scholar]
- 49.Alfaro-Moreno E, Martínez L, García-Cuellar C, Bonner JC, Murray JC, Rosas I, Rosales SPL, Osornio-Vargas AR. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ Health Perspect. 2002;110(7):715–720. doi: 10.1289/ehp.02110715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osornio-Vargas ÁR, Bonner JC, Alfaro-Moreno E, Martínez L, García-Cuellar C, Rosales SP-d-L, et al. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 2003;111(10):1289. doi: 10.1289/ehp.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alfaro-Moreno E, Torres V, Miranda J, Martínez L, García-Cuellar C, Nawrot TS, et al. Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban particulate matter collected with a modified method of PM sampling. Environ Res. 2009;109(5):528–535. doi: 10.1016/j.envres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Lan K, Verma SC, Murakami M, Bajaj B, Robertson ES. Isolation of human peripheral blood mononuclear cells (PBMCs) Curr Protocols Microbiol. 2017;6(1):A. 4C. 1–A. 4C. 9. doi: 10.1002/9780471729259.mca04cs6. [DOI] [PubMed] [Google Scholar]
- 53.Cuevas, E. Establishing a WMO Sand and Dust Storm Warning Advisory and Assessment System Regional Node for West Asia: Current Capabilities and Needs, WMO-No. 1121, Chair, Publications Board World Meteorological Organization (WMO). 10.13140/RG.2.2.20089.57447.
- 54.Shao Y, Dong CH. A review on East Asian dust storm climate, modelling and monitoring. Glob Planet Chang. 2006;52(1):1–22. [Google Scholar]
- 55.Roig N, Sierra J, Rovira J, Schuhmacher M, Domingo JL, Nadal M. In vitro tests to assess toxic effects of airborne PM10 samples. Correlation with metals and chlorinated dioxins and furans. Sci Total Environ. 2013;443:791–797. doi: 10.1016/j.scitotenv.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Gualtieri M, Longhin E, Mattioli M, Mantecca P, Tinaglia V, Mangano E, Proverbio MC, Bestetti G, Camatini M, Battaglia C. Gene expression profiling of A549 cells exposed to Milan PM2. 5. Toxicol Lett. 2012;209(2):136–145. doi: 10.1016/j.toxlet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 57.Gualtieri M, Øvrevik J, Holme JA, Perrone MG, Bolzacchini E, Schwarze PE, Camatini M. Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol in Vitro. 2010;24(1):29–39. doi: 10.1016/j.tiv.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Yang L, Liu G, Lin Z, Wang Y, He H, Liu T, et al. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ Toxicol. 2016;31(8):923–936. doi: 10.1002/tox.22102. [DOI] [PubMed] [Google Scholar]
- 59.Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15(1):1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- 60.Mirowsky J, Hickey C, Horton L, Blaustein M, Galdanes K, Peltier RE, Chillrud S, Chen LC, Ross J, Nadas A, Lippmann M, Gordon T. The effect of particle size, location and season on the toxicity of urban and rural particulate matter. Inhal Toxicol. 2013;25(13):747–757. doi: 10.3109/08958378.2013.846443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steenhof M, Gosens I, Strak M, Godri KJ, Hoek G, Cassee FR, et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential–the RAPTES project. Part Fibre Toxicol. 2011;8(1):1–15. doi: 10.1186/1743-8977-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perrone MG, Zhou J, Malandrino M, Sangiorgi G, Rizzi C, Ferrero L, et al. PM chemical composition and oxidative potential of the soluble fraction of particles at two sites in the urban area of Milan, Northern Italy. Atmos Environ. 2016;128:104–113. [Google Scholar]
- 63.Dergham M, Lepers C, Verdin A, Billet S, Cazier F, Courcot D, Shirali P, Garçon G. Prooxidant and proinflammatory potency of air pollution particulate matter (PM2. 5–0.3) produced in rural, urban, or industrial surroundings in human bronchial epithelial cells (BEAS-2B) Chem Res Toxicol. 2012;25(4):904–919. doi: 10.1021/tx200529v. [DOI] [PubMed] [Google Scholar]
- 64.Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;10(9):3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sah D, Verma PK, Kandikonda MK, Lakhani A. Pollution characteristics, human health risk through multiple exposure pathways, and source apportionment of heavy metals in PM10 at indo-Gangetic site. Urban Climate. 2019;27:149–162. [Google Scholar]