Short abstract
Alzheimer’s disease (AD) ranks sixth on the Centers for Disease Control and Prevention Top 10 Leading Causes of Death list for 2016, and the Alzheimer’s Association attributes 60% to 80% of dementia cases as AD related. AD pathology hallmarks include accumulation of senile plaques and neurofibrillary tangles; however, evidence supports that soluble amyloid beta (Aβ), rather than insoluble plaques, may instigate synaptic failure. Soluble Aβ accumulation results in depression of long-term potentiation leading to cognitive deficits commonly characterized in AD. The mechanisms through which Aβ incites cognitive decline have been extensively explored, with a growing body of evidence pointing to modulation of the glutamatergic system. The period of glutamatergic hypoactivation observed alongside long-term potentiation depression and cognitive deficits in later disease stages may be the consequence of a preceding period of increased glutamatergic activity. This review will explore the Aβ-related changes to the tripartite glutamate synapse resulting in altered cell signaling throughout disease progression, ultimately culminating in oxidative stress, synaptic dysfunction, and neuronal loss.
Keywords: α7 nicotinic acetylcholine receptors (α7nAChRs), amyloid oligomers, synaptic dysfunction, excitotoxicity, mild cognitive impairment, N-methyl-D-aspartic acid (NMDA)
Introduction
The glutamatergic synapse, referred to as the tripartite synapse, involves three elements: presynaptic neurons, postsynaptic neurons, and astrocytes. There are several different synaptic components expressed on both neurons and astrocytes in the glutamate synapse, as summarized in Table 1. These components include two types of glutamate receptors that exist on these synaptic elements: metabotropic and ionotropic. Metabotropic glutamate receptors (mGluRs) are present on both presynaptic and postsynaptic neurons (Revett et al., 2013) and involve three different groups of receptors: Group I (mGluR1 and mGluR5), Group II (mGluR 2 and 3), and Group III (mGluR4, mGluR6, mGluR7, and mGluR8; Petralia et al., 1996; Rudy et al., 2015). Group I mGluRs are Gq-coupled and are expressed postsynaptically, depolarizing the postsynaptic neuron upon stimulation (Petralia et al., 1996; Ferraguti and Shigemoto, 2006; Revett et al., 2013). Groups II and III mGluRs are Gi/o-coupled inhibitory autoreceptors and are expressed both pre- and postsynaptically (Petralia et al., 1996; Rudy et al., 2015), leading to inhibition of presynaptic glutamate release or postsynaptic excitation (Ambrosini et al., 1995; Ferraguti and Shigemoto, 2006).
Table 1.
Brief Overview of Glutamatergic Synapse Components: Localization and Function.
| Glutamate synapse components | Localization | Function | References |
|---|---|---|---|
| VGluT | Presynaptic neuron | Packaging glutamate into vesicles | Takamori et al., 2000; Fremeau et al., 2001, 2004 |
| Glutaminase | Presynaptic neuron | Synthesizes glutamate from glutamine | Revett et al., 2013 |
| α7nAChR | Both neurons and glia | Soluble Aβ binding (in low concentrations) triggers Ca2+-dependent release of glutamate from the presynaptic neuron, as well as stimulation of the postsynaptic neuron | Wang et al., 2000b; Gahring et al., 2004; Magdesian et al., 2005; Puzzo et al., 2008; Mura et al., 2012;Hascup and Hascup, 2016 |
| mGluR Group II/III | Pre- and postsynaptic neuron | Gi/o-coupled receptor, inhibition of presynaptic release of glutamate or inhibition of postsynaptic response to stimulation | Ambrosini et al., 1995; Petralia et al., 1996; Ferraguti and Shigemoto, 2006; Rudy et al., 2015 |
| mGluR Group I | Postsynaptic neuron | Gq-coupled receptor, depolarizes neuron upon binding of glutamate and results in Ca2+ release from intracellular stores | Petralia et al., 1996; Ferraguti and Shigemoto, 2006; Revett et al., 2013 |
| AMPA | Pre- and postsynaptic neuron | Presynaptically promotes the formation of synapses. Postsynaptically depolarizes the neuron upon glutamate binding | Wisden and Seeburg, 1993; Isaac et al., 2007; Rudy et al., 2015 |
| NMDA | Postsynaptic neuron | Contains a magnesium block that is removed upon depolarization of postsynaptic membrane, allowing for Ca2+ influx into the neuron | Calabresi et al., 1992; Wisden and Seeburg, 1993; Pandis et al., 2006; Parsons et al., 2007; Rudy et al., 2015 |
| GLT-1 (EAAT1)/GLAST (EAAT2) | Astrocytes | Clearance of glutamate from the synapse by uptake into astrocytes | Lehre et al., 1995; Revett et al., 2013 |
| GS | Astrocytes | Conversion of glutamate to glutamine so that it may be transported back to the presynaptic neuron | Norenberg and Martinez-Hernandez, 1979; Parsons et al., 2007; Revett et al., 2013 |
Note. Outline of glutamate neuronal and astrocytic components and their functions in glutamatergic neurotransmission.
α7nAChR = alpha-7 nicotinic acetylcholine receptor; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; EAAT = excitatory amino acid transporter; GLAST = glutamate aspartate transporter; GLT-1 = glutamate transporter-1; GS = glutamine synthetase; mGluR = metabotropic glutamate receptor; NMDA = N-methyl-D-aspartic acid; VGluT = vesicular glutamate transporter.
The ionotropic glutamate receptors are expressed both pre- and postsynaptically (Wisden and Seeburg, 1993; Rudy et al., 2015) and include α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), N-methyl-D-aspartic acid receptors (NMDAR), and kainate receptors. AMPARs function presynaptically to promote synapse and spine formation (Isaac et al., 2007). On the postsynaptic side, all three ionotropic receptors are expressed (Wisden and Seeburg, 1993; Rudy et al., 2015). AMPARs are bound by glutamate and lead to membrane depolarization, while NMDARs have a magnesium block which is released upon membrane depolarization. This results from either high-frequency stimulation inducing Na+ influx through AMPARs or disinhibition of GABAergic synapses (Parsons et al., 2007), which then opens the NMDAR ion channel (Calabresi et al., 1992; Pandis et al., 2006; Parsons et al., 2007). In fact, AMPARs and NMDARs are colocalized on the postsynaptic membrane because of the cooperation required between the two receptors in response to membrane depolarization (Takumi et al., 1999; Antal et al., 2008). The opening of NMDARs results in Ca2+ influx into the postsynaptic neuron and, along with Group I mGluRs, increases intracellular Ca2+ (Chen et al., 2002; Hardingham et al., 2002; Hardingham and Bading, 2003; Verkhratsky and Kirchhoff, 2007; Yamin, 2009; Zhang et al., 2016). NMDA interacts with glutamate such that the NR2 subunit of the NMDA receptor binds glutamate, with NR2A and NR2B subtypes mediating excitotoxicity in cultured cortical neurons (von Engelhardt et al., 2007). The NR2 subunit is expressed both synaptically (NR2A) and extrasynaptically (NR2B; Rudy et al., 2015). Extrasynaptic NMDARs (E-NMDARS) are activated only by high concentrations of glutamate, unlike synaptic NMDARs (S-NMDARS) that are located closer to the synaptic cleft and are activated by presynaptic glutamate release (Groc et al., 2009; Newpher and Ehlers, 2009; Rudy et al., 2015). Both E-NMDAR stimulation and NMDA/mGluR-mediated Ca2+ influx constitute important factors in soluble amyloid beta (Aβ)-mediated neurotoxicity as explored further in this review.
Glutamate is synthesized from glutamine by glutaminase in the presynaptic neuron (Revett et al., 2013), and then transported to the synaptic terminals where vesicular glutamate transporter-1/2 (VGluT-1/2) packages glutamate into vesicles, which release glutamate upon neuronal depolarization (Takamori et al., 2000; Fremeau et al., 2001, 2004). Glutamate clearance from the synapse is carried out through high-efficiency excitatory amino acid transporters (EAATs) located primarily on astrocytes. Glutamate transporter-1 (GLT-1) and glutamate aspartate transporter (GLAST) allow for uptake of glutamate into astrocytes (Lehre et al., 1995), where glutamate is then converted into glutamine by glutamine synthetase (GS) and transported back to the presynaptic neuron (Norenberg and Martinez-Hernandez, 1979). This is referred to as the glutamate/glutamine cycle (Revett et al., 2013), a critical regulation point for the glutamatergic system to terminate receptor signaling while preventing excess accumulation of synaptic glutamate potentially leading to excitoxicity (Olney et al., 1997). As well, dysregulation of glucose metabolism can potentially impact glutamate synthesis in the glutamate/glutamine cycle (Knight et al., 2014), underlining the importance of this regulatory cycle for learning and memory (Valladolid-Acebes et al., 2012; Hascup et al., 2019a).
Alterations in Glutamatergic Signaling Throughout Alzheimer’s Disease Progression
The Relationship Between Aβ and Glutamate
Alzheimer’s disease (AD) is characterized by excitotoxic levels of extracellular glutamate alongside accumulation of soluble Aβ and hyperphosphorylated tau protein leading to neuronal cell death (Hiruma et al., 2003; Kopeikina et al., 2012). Evidence shows that soluble Aβ accumulation results in synaptic failure, potentially through modifications to the glutamatergic system (Walsh et al., 2002; Wang et al., 2002; Barghorn et al., 2005; Cleary et al., 2005; Selkoe, 2008; Ferreira and Klein, 2011; Wilcox et al., 2011). Early stages of the disease show an increased excitability of pyramidal neurons (Grutzendler et al., 2007; Šišková et al., 2014; Hascup and Hascup, 2015) and an upregulation of glutamatergic presynaptic boutons as observed in mild cognitive impairment (MCI; Bell et al., 2007) and in mouse models of AD (Hascup et al., 2019a). Several studies have noted hyperactivity in the hippocampus of MCI patients (Bell et al., 2007; Miller et al., 2008; O’Brien et al., 2010; Huijbers et al., 2015) with familial history of AD (Okonkwo et al., 2014) and elevated Aβ deposition (Huijbers et al., 2015) also contributing to excitability. In fact, hyperactivity at baseline, as detected by functional magnetic resonance imaging, is associated with increased severity of cognitive decline (Miller et al., 2008; O’Brien et al., 2010; Huijbers et al., 2015).
Along with increased excitability, morphological changes in dendritic structure of hippocampal pyramidal neurons have been noted (Grutzendler et al., 2007; Šišková et al., 2014), with specific responsiveness to amyloid plaques (Masliah et al., 1993; Ovsepian et al., 2018). Whole-cell patch clamp recordings of CA1 pyramidal neurons in AβPP/PS1ΔE9 (APP/PS1; RRID:MMRRC_034832-JAX) mice show reductions in length, branching, and surface area of dendrites while also displaying a localized hyperactivity effect and increased synaptic integration that is attributed to the changes in dendritic structure (Šišková et al., 2014). Similar morphological changes appear in the PSAPP double transgenic mouse model (Tg2576 RRID: IMSR_TAC:1349; PSEN1 (M146L): RRID: IMSR_JAX:033255) and postmortem AD brain samples (Grutzendler et al., 2007).
Later stages of AD show markedly decreased glutamatergic activity in stark juxtaposition to earlier hyperactivity. The APP/PS1 AD mouse model shows increased stimulus-evoked glutamate release at younger ages, which then steadily decreases with age and Aβ accumulation (Minkeviciene et al., 2008; Hascup and Hascup, 2015; Hascup et al., 2019b). Busche et al. (2012) also note increased glutamatergic hyperactivity in young APPswe/PS1G384A double transgenic mice (APPswe: RRID: MGI: 3665286; PS1G384A: RRID: MGI: 4819108), with hyperactive neurons preferentially surrounding amyloid plaques. Such findings are observed in AD patients, with a decrease in presynaptic glutamatergic boutons and glutamate signaling noted in later disease stages (Bell et al., 2007; Miller et al., 2008; O’Brien et al., 2010). This could result in chronically elevated glutamate levels eventually leading to inhibition of axonal transport and neurodegeneration through Aβ accumulation and increased Ca2+ intracellular concentrations (Hiruma et al., 2003; Stutzmann, 2005).
Studies attempting to characterize disease cell signaling and pathology in human AD typically use postmortem tissue due to logistical difficulties in obtaining tissues and data from patients in earlier disease stages. Such limitations hinder study of earlier cell signaling changes, although there are some methods currently used to examine glutamate in live patients. Of note, studies using proton magnetic resonance spectroscopy (1H MRS) and glutamate chemical exchange saturation transfer (GluCEST) show decreased overall glutamate concentration in APP/PS1 mice and AD patients (Rupsingh et al., 2011; Haris et al., 2013). The hippocampus was specifically noted as having a large decrease in glutamate concentration, and as previously discussed, the hippocampus is especially vulnerable to Aβ accumulation. The CA1 region is particularly susceptible to disease-related neuronal loss (West et al., 1994; Hof et al., 2003) and shows decreased stimulus-evoked glutamate with age in APP/PS1 mice (Hascup et al., 2016). This supports that the decrease in total concentration observed may be a consequence of neuronal loss resulting from excitotoxicity, as opposed to decreased glutamate concentration in the hippocampus.
These findings display a flexibility in glutamatergic characterization such that it is specific to the disease stage. The paradoxical nature of glutamate signaling in AD petitions for a deeper look into the individual impact on glutamatergic synaptic components throughout disease progression. Modifications in expression of synaptic elements could lead to alterations in cell signaling, allowing for excitotoxic conditions to grow and building the foundation for cognitive decline.
Neuronal Glutamate Synaptic Component Changes in AD
Individual changes in glutamatergic synaptic components underlie the altered glutamate release observed throughout disease progression. As mentioned previously, earlier stages are marked with elevated glutamate release that eventually increases glutamate concentrations in and around the synapse. Evidence supports that this starts presynaptically, with Aβ and VGluT1 colocalizing on glutamatergic synaptic boutons and preferentially accumulating in these terminals (Sokolow et al., 2012). This coincides with elevated expression of VGluT1 in mouse models of AD, supporting increased vesicle trafficking of glutamate (Hascup et al., 2019a). Several studies have also shown a downregulation of VGluT1 expression in AD (Kashani et al., 2008; Canas et al., 2014; Rodriguez-Perdigon et al., 2016), but only VGluT2 downregulation in MCI subjects (Kashani et al., 2008), supporting that VGluT1 levels are not downregulated until later stages of AD. This expression pattern matches that of glutamate release such that initially more glutamate is packaged into vesicles for release, contributing to an increase in extracellular glutamate levels into a toxic range.
Postsynaptically, the impact of Aβ accumulation on AMPA/NMDA receptors has been well documented. Bath application of Aβ reduces both the amplitude and frequency of AMPA postsynaptic currents in CA1 pyramidal neurons (Parameshwaran et al., 2007). Neuronal cell cultures taken from the cortex and hippocampus of Tg2576 transgenic AD mice show decreased expression of the GluR1 AMPA subunit as Aβ concentrations increased (Almedia et al., 2005). A decrease in GluR1 and GluR2/3 expression has also been observed in postmortem AD entorhinal cortex (Yasuda et al., 1995), although GluR2/3 is expressed presynaptically as well. In the hippocampus, ionotropic glutamate receptor AMPA subunit-4 expression is downregulated in sporadic AD (Jacob et al., 2007), along with decreased AMPA binding in the CA1 (Dewar et al., 1991). It is arguable that the decrease in AMPA binding may be due to decreased GluR1 surface expression caused by decreased expression of synaptic calcium-calmodulin II (CaMKII) as seen in APP/PS1 mice (Gu et al., 2009). The decreased release of stimulus-evoked glutamate mentioned earlier could also account for this decrease in binding (Hascup and Hascup, 2015). In cell culture, GluA1 expression is upregulated in APP knockout corticohippocampal neurons supporting that Aβ directly impacts expression levels of AMPA subunits (Martinsson et al., 2019). Interestingly, GluR2 has been shown to be upregulated in incipient AD patients (Williams et al., 2009), supporting that AMPA expression may follow the same cycle of early upregulation and then subsequent downregulation with AD progression. Early upregulation is likely a response to increased presynaptic glutamate stimulation, but overtime, chronic excessive stimulation leads to desensitization and internalization of the AMPA receptor (Esposito et al., 2013).
NMDA receptors are a cornerstone in the relationship between Aβ accumulation and glutamate toxicity. Aβ42 preferentially binds to glutamatergic neurons expressing NR1 or NR2B NMDA subunits compared with other subunits (Lacor et al., 2007). Aβ42 can stimulate glutamate release, potentially through α7 nicotinic acetylcholine receptors (α7nAChR; discussed later), to activate E-NMDARs (Talantova et al., 2013). This can result in long-term potentiation (LTP) inhibition (Li et al., 2011; Kervern et al., 2012) and contribute to synaptic spine loss (Talantova et al., 2013). This Aβ-mediated increase in glutamate concentration leads to endocytosis and decreased surface expression of NR1 and NR2B (Snyder et al., 2005). Downregulated NMDA subunit expression in the hippocampus has been observed in several postmortem studies in human AD subjects (Hynd et al., 2004a, 2004b; Jacob et al., 2007).
NMDARs are believed to mediate soluble Aβ-induced cell death such that persistent activation of NMDA from excessive glutamate release leads to selective neuronal death from chronic excitotoxicity (Butterfield and Pocernich, 2003). E-NMDARs allow for Ca2+ entry into the cell (Zhang et al., 2016), which causes increased dendritic calcium-induced calcium release from ryanodine receptors, thus increasing intracellular Ca2+ concentrations (Goussakov et al., 2010). In fact, MCI patients show increased expression of ryanodine receptor 2 (Bruno et al., 2012), which would allow for further NMDA-mediated increases in intracellular Ca2+ concentrations. Overtime, this can eventually result in toxic Ca2+ levels leading to depolarization of the mitochondrial membrane, free radical production, and cell death. (Hardingham et al., 2002; Hardingham and Bading, 2003; Bezprozvanny and Mattson, 2008; Zhang et al., 2016). Along with this, E-NMDAR-mediated Ca2+ influx activates cAMP-regulatory element binding protein (CREB) shut off pathways (Hardingham et al., 2002). CREB plays a central role in long-term memory (Yin and Tully, 1996; Barco et al., 2003; Tully et al., 2003), and downregulation results in memory impairment (Xiong et al., 2013; Zhang et al., 2014). Thus, increased intracellular Ca2+ levels resulting from E-NMDAR stimulation contributes to both cognitive decline and neuronal loss in AD pathology.
Furthermore, soluble Aβ acts through NMDAR to activate nicotinamide adenine dinucleotide phosphate oxidase leading to induction of reactive oxygen species (ROS; Kishida and Klann, 2006) and release of arachidonic acid (Shelat et al., 2008). The influx of Ca2+ from NMDAR activation is required for ROS formation (De Felice et al., 2007). Although NMDA production of ROS is a necessary element for LTP (Kishida and Klann, 2006), Aβ stimulates an excessive ROS induction from NMDARs, leading to oxidative damage and synaptic failure (De Felice et al., 2007; Shelat et al., 2008). In fact, elevated ROS levels contribute to impairment of LTP (Serrano and Klann, 2004) and spatial learning (Nicolle et al., 2001) with age. NMDA activation also increases nitric oxide (NO) synthesis (Garthwaite et al., 1989) in a Ca2+ (Law et al., 2001) and postsynaptic density-95 dependent manner (Sattler et al., 1999). The 3xTg-AD (RRID: MMRRC_034830-JAX) mouse model shows significant increases in NMDA-mediated NO concentration peaks in the CA1 at earlier disease stages, which then substantially decreased with age (Dias et al., 2016), an effect that could underlie the changes in glutamate cell signaling seen throughout disease progression. Furthermore, inducible nitric oxide synthase (iNOS) mediates Aβ-induced LTP inhibition (Wang et al., 2004), while soluble Aβ works through both NMDA and NOS to increase oxidative stress (Parks et al., 2001). However, NO can in turn inhibit NMDAR action (Manzoni et al., 1992), which could play a role in decreased NMDAR activity in later stages of AD. Even with increasing neurotoxicity, NOS+ neurons in the hippocampus are relatively spared (Hyman et al., 1992), supporting a complicated relationship between NO and glutamate such that NO provides some neuroprotection while also contributing to the consequences of excitotoxicity.
Studies performed in primary cortical neuron cultures have shown seemingly opposing results on NMDA modulation of APP processing. NMDA (50 µM) treatment showed both induction of Kunitz protease inhibitory domain (KPI)-APP neuronal expression promoting production of Aβ42 (Lesné et al., 2005), and increases in α-carboxyterminal fragment levels, supporting enhancement of nonamyloidogenic α-secretase cleavage (Hoey et al., 2009). Interestingly, the increase in α-carboxyterminal fragment levels was not observed in isolated E-NMDARs, supporting that this is solely an S-NMDAR effect (Hoey et al., 2009). Furthermore, Lesné et al. (2005) used a longer NMDA incubation period (24 hr) to create an excitotoxic environment, as would be observed with chronic E-NMDAR stimulation. Bordji et al. (2010) addressed these conflicting findings by isolating either S-NMDARs or E-NMDARs in primary cortical neuron culture. While S-NMDARs were shown to have no impact on KPI-APP neuronal expression, E-NMDARs increased KPI-APP expression at the 12- and 24-hr time points. This E-NMDAR-mediated increase in KPI-APP expression was also found to be calcium-calmodulin dependent, supporting induction through NMDA-mediated Ca2+ entry into the neuron. Along with these findings, NMDA antagonists are capable of blocking Aβ42 uptake into hippocampal neurons (Bi et al., 2002) where Aβ can reduce axonal transport through an NMDA/glycogen synthase kinase-3β dependent mechanism (Decker et al., 2010). This further emphasizes the critical roles of E-NMDARs in AD, impacting both APP processing and Aβ42 internalization, and thereby establishing E-NMDARs as a central mediator to the neurotoxic effects of Aβ.
Autoradiography studies of postmortem brain tissue shows decreased binding to mGluRs and decreased mGluR/neuronal density ratio (Dewar et al., 1991; Albasanz et al., 2005). This effect was associated with a decrease in mGluR1 expression, which continuously declines with AD progression (Albasanz et al., 2005). Interestingly, another study found mGluR2, which is primarily expressed presynaptically, to be upregulated in AD (Lee et al., 2004). mGluR2 protects against excitotoxicity by inhibiting presynaptic glutamate release (Buisson and Choi, 1995; Imre, 2007; Hascup et al., 2010; Quintero et al., 2011; Hascup et al., 2019b) and has been shown to directly activate extracellular signal-regulated kinase (ERK) in a phosphoinositide 3-kinase (P13K)-dependent manner (Ferraguti et al., 1999). ERK impacts CREB phosphorylation and promotes cell survival (Lu and Xu, 2006; Lee et al., 2009) but also phosphorylates tau which can contribute to aberrant hyperphosphorylation resulting in neurofibrillary tangles (Lee et al., 2009). These findings support that while this mechanism may initially be neuroprotective (Bond et al., 2000), over time, it may contribute to disease pathology.
Astrocytic Glutamate Component Changes in AD
Astrocytes play a key role both in the tripartite glutamatergic synapse and in AD pathogenesis (Rudy et al., 2015). Reactive astrocytes are known to associate with senile plaques in AD mouse models and human tissue (Verkhratsky et al., 2010; Rodríguez-Arellano et al., 2016), leading to astrogliosis in the hippocampus and cortex characterized by increased expression of glial fibrillary acidic protein (GFAP; Nagele et al., 2004; Olabarria et al., 2010; Hascup et al., 2019a). This response is triggered by damaged neuronal signals, referred to as damaged-associated molecular patterns, and Aβ plaque deposition (Verkhratsky et al., 2010) leading to increased release of proinflammatory factors such as interleukin-1β (IL-1β) and tumor necrosis factor-α (Morales et al., 2014). Continuing induction of astrocytic response leads to chronic inflammation that results in cell damage (Streit et al., 2004).
Glutamate clearance from the synapse is hindered in AD through decreased uptake into astrocytes. This is supported by decreased expression of both GLT-1 and GLAST in the hippocampus (Cassano et al., 2012) that appears prior to plaque deposition (Schallier et al., 2011). In later stages, GLT-1 expression is markedly decreased around plaques (Hefendehl et al., 2016). Expression of GLT-1 may be directly impacted by Aβ through lipid peroxidation and 4-hydroxynonenal modification as a result of oxidative stress (Butterfield et al., 2002). This change in GLT-1 expression contributed to spatial learning deficits in earlier stages (6 months old) but did not cause a significant deviation in cognitive performance in 9-month-old APP/PS1 mice lacking one GLT-1 allele (Mookherjee et al., 2011). This effect is also observed with decreased expression of EAAT-2 (the human GLT-1 equivalent) in postmortem AD cortex (Scott et al., 2011) as well as EAAT-1 (GLAST) reduction in the hippocampus (Jacob et al., 2007). Again, this reduction was specifically noted in the vicinity of senile plaques (Jacob et al., 2007; Hefendehl et al., 2016), supporting elevated basal glutamate surrounding plaques in both mouse and human models. Interestingly, several disease-specific splice variants of EAAT2 exist that reduce the glutamate transport capacity of EAAT2 in AD postmortem tissue (Scott et al., 2011). This may offer an additional avenue for excitoxicity, whereby glutamate transporters are both downregulated and have decreased functional glutamate clearance.
GS action upon glutamate is a critical component in preventing excitotoxicity (Verkhratsky and Kirchhoff, 2007; Rudy et al., 2015) but shows altered expression in AD (Robinson, 2001; Boyd-Kimball et al., 2005; Olabarria et al., 2011; Palmieri et al., 2017; Huang et al., 2016). In 3xTg-AD mice, GS+ astrocyte distribution mirrored that of GFAP+ distribution in the DG and CA1, but by 12 months of age, GS+ astrocyte cell counts decreased, and by 18 months of age, downregulation of GS expression in the hippocampus was observed (Olabarria et al., 2011). Human tissue taken from patients with advanced AD also shows a decrease in GS expression. Interestingly, less GS staining was observed clustering around plaques, unlike GFAP+ astrocytes (Robinson, 2001). This is possibly due to the level of neuronal loss experienced during advanced stages of AD (DeKosky and Scheff, 1990; Selkoe, 2002). GS is particularly vulnerable to oxidative modification (Boyd-Kimball et al., 2005) that makes it a potential target of Aβ-induced oxidative damage (Huang et al., 2016). Along with this, GS inhibition in activated microglia results in stronger induction of inflammatory markers which increases neuronal toxicity (Palmieri et al., 2017).
The evidence presented for both neuronal and astrocytic components of the tripartite glutamate synapse involvement in AD progression support that Aβ can impact the glutamatergic system through various mechanisms. Changes in expression of each of the synaptic components lead to chronically elevated extracellular glutamate that becomes excitotoxic and underlies AD pathology.
Aβ–α7nAChR Interactions
α7nAChRs are expressed on both neurons and glia, with hippocampal immunostaining showing the highest density of α7nAChR-expressing neurons in the neuropil and α7nAChR-expressing glia in the distal regions of the stratum radiatum (Gahring et al., 2004). It has been well established in the literature that application of nicotine elicits glutamate release both in vitro and in vivo (Marchi et al., 2002; Lambe et al., 2003; Konradsson-Geuken et al., 2009). In vivo microelectrode array recording studies have shown nicotine-induced glutamate release in the prefrontal cortex of freely moving rats, that is attenuated with application of α-bungarotoxin, an α7nAChR antagonist (Konradsson-Geuken et al., 2009). Soluble Aβ40 and Aβ42 interact with nAChRs at these synapses (Wang et al., 2000a, 2000b) impacting glutamate release. Aβ interacts with both α7 and α4β2 nAChRs; however, α4β2nAChR receptor binding requires a significantly higher concentration than α7nAChR (Wang et al., 2000b). Regarding differences between Aβ40 and Aβ42, Aβ40 was found to bind with less affinity and decreased potency compared with Aβ42 (Wang et al., 2000a; Dineley et al., 2002a). More specifically, Aβ42 binds with femtomolar affinity to the nicotine/acetylcholine (ACh)-binding pocket of α7nAChR (Wang et al., 2000b; Magdesian et al., 2005) and can elicit different responses dependent on Aβ preparation and concentration used (Parri et al., 2011; Hascup and Hascup, 2016). High concentrations of Aβ (nM-µM) noncompetitively block hippocampal α7nAChRs in in vitro hippocampal cell culture and slices (Liu et al., 2001; Mura et al., 2012). Alternatively, low concentrations of Aβ (fM-pM) have been shown to potentiate glutamate release (Puzzo et al., 2008; Mura et al., 2012; Hascup and Hascup, 2016) with some effects on aspartate and GABA release also observed in vivo (Mura et al., 2012). In fact, Aβ-stimulated α7nAChR glutamate release from neurons and astrocytes can result in rising extracellular glutamate levels that can chronically activate E-NMDARs, thereby contributing to excitotoxicity (Rudy et al., 2015).
Lower concentrations of Aβ42 have been shown to enhance LTP and spatial memory performance in C57BL/6 mice (RRID: IMSR_JAX:000664; Puzzo et al., 2008), while higher concentrations result in LTP deficits (Chen et al., 2006, 2010; Gu and Yakel, 2011). The LTP-enhancing aspect of Aβ42 is not mediated by AMPA/NMDA receptors, as Aβ42 perfusion of hippocampal slices does not impact AMPA/NMDA receptor currents or amplitude frequency and distribution (Puzzo et al., 2008). This concentration-dependent action of Aβ supports a transformation from excitation to inhibition of α7nAChRs as AD progresses, with Aβ levels rising from picomolar to nanomolar levels (Näslund et al., 1994; Auld et al., 1998; Puzzo and Arancio, 2012). It should be noted that presynaptic α7nAChRs are known to interact with neighboring NMDARs such that the chronic inactivation of α7nAChR, resulting from desensitization by Aβ, could result in enhanced NMDAR function (Lin et al., 2010). However, this increased presynaptic glutamate release may not evoke LTP enhancement as postsynaptic AMPA/NMDA receptors become desensitized and downregulated in the excitotoxic state associated with AD (Yasuda et al., 1995; Selkoe, 2002; Hynd et al., 2004a, 2004b; Parameshwaran et al., 2007).
Evidence of Aβ42 concentration-dependent α7nAChR modulation is further characterized through microelectrode array recordings in the hippocampus. Hascup and Hascup (2016) showed glutamatergic stimulation with local application of Aβ42 in hippocampal recordings from anesthetized C57BL/6 mice, an effect that was blocked with co-application of α-bungarotoxin supporting involvement of α7nAChR. Interestingly, different responses to Aβ42 application were observed depending on the hippocampal subfield, with the CA1 and dentate gyrus responding most to lower concentrations of Aβ42 compared with the CA3. These findings mirror current knowledge of AD disease progression, with the CA1 showing the earliest increases in glutamate release in the APP/PS1 transgenic AD mouse model (Hascup and Hascup, 2015) and the earliest site of plaque deposition for the hippocampus in human AD patients (Thal et al., 2000; Shie et al., 2003).
α7nAChRs open upon nicotine binding, allowing a Ca2+ influx into the presynaptic neuron (Gray et al., 1996). Similarly, binding of soluble Aβ to the α7nAChR triggers an influx of Ca2+ into the presynaptic neuron (Dineley et al., 2001, 2002a; Dougherty et al., 2003) and has been shown to activate the ERK/mitogen-activated protein kinases pathway (Dineley et al., 2001; Bell et al., 2004; Abbott et al., 2008; Young et al., 2009) in a P13K-dependent manner (Bell et al., 2004) which in turn impacts the phosphorylation of CREB (Dineley et al., 2001). Intraperitoneal injection of α7nAChR selective agonist A-582941 resulted in increased ERK 1/2 and CREB phosphorylation that improved behavioral performance on delayed matching to sample titration, inhibitory avoidance, and social recognition (Bitner et al., 2007). However, in the Tg2576 mouse model, CREB was found to be upregulated at 13 months of age and then downregulated by 20 months of age (Dineley et al., 2001). This supports that CREB activation mirrors the Aβ-α7nAChR interaction such that CREB expression is enhanced in the early stages of AD when there are lower concentrations of Aβ, and then decreases with disease progression corresponding to Aβ accumulation.
The potentiation of glutamate release from Aβ activation of α7nAChR contributes to excitotoxicity while also resulting in rapid desensitization of the α7nAChR (Dineley et al., 2002b). Aβ42 and α7nAChR have been shown to form a complex that becomes internalized in the neuron and leads to cell lysis and plaque deposition (D’Andrea et al., 2001; Nagele et al., 2002; Deutsch et al., 2014; Godyń et al., 2016). In fact, Aβ accumulates at a faster rate in neuroblastoma cells transfected with α7nAChR (Nagele et al., 2002). The Aβ42-α7nAChR complex internalization leads to decreased surface expression of α7nAChR (Nagele et al., 2002), coinciding with upregulation of α7nAChR seen throughout AD progression (Hellström-Lindahl et al., 1999; Dineley et al., 2001, 2002b; D’Andrea and Nagele, 2006) supporting an α7nAChR compensatory mechanism for decreased surface expression. Interestingly, this chronic desensitization of α7nAChR has been shown to increase NMDAR surface expression (Lin et al., 2010), suggesting a complex compensatory response to accumulation of Aβ42.
Learning and Memory Consequences of Altered Glutamatergic Signaling
Glutamatergic dysfunction due to deregulation of synaptic components has phenotypic consequences in the form of decreased learning and memory performance. Presynaptically, reductions in VGluT1 and VGluT2 correlated with decline in cognitive status and disease duration in AD patients (Kashani et al., 2008). Cognitive ability is negatively correlated with glutamate presynaptic bouton density in MCI patients such that the increase in bouton density leads to decreased cognitive ability (Bell et al., 2007). Similarly, elevated hippocampal glutamate release negatively correlated with cognitive performance prior to cognitive decline in APP/PS1 mice (Hascup and Hascup, 2015). This is in stark contrast to cognitively normal patients that have a positive correlation (Bell et al., 2007), supporting a threshold in which increased glutamate signaling switches from stimulating to hindering cognition.
NMDA and mGluRs are both known to play vital roles in induction of LTP and long-term depression (LTD; Parameshwaran et al., 2007; Shankar et al., 2008). Soluble Aβ inhibits S-NMDARs that are required for LTP conduction (Collingridge and Bliss, 1995; Parsons et al., 2007). Treatment with (2R)-amino-5-phosphonovaleric acid (D-AP5), a selective NMDA antagonist, impairs LTP in vivo supporting that the involvement of NMDARs in LTP (Davis et al., 1992). However, in GLT-1 knockout mice, the LTP impairment observed was reversed with application of D-AP5, supporting that both blockade and overactivation of NMDARs can interfere with LTP (Katagiri et al., 2001). This effect may be due to the involvement of E-NMDARs, whereby inhibition of E-NMDARs prevents Aβ-mediated LTP impairments (Li et al., 2011; Rammes et al., 2011). Memantine, an FDA-approved noncompetitive NR2 antagonist (Parsons et al., 1998) for treating AD has shown efficacy in improving Morris water maze performance (Barnes et al., 1996; Van Dam et al., 2005; Banerjee et al., 2006) and blocked Aβ inhibition of LTP in 3xTg-AD hippocampal slices (Parsons et al., 2009; Martinez-Coria et al., 2010). Thus, supporting that noncompetitive inhibition of NR2+ NMDARs provides a counterbalance to both inhibition of S-NMDARS and overactivation of E-NMDARS, explored further in the next section.mGluRs are involved in both the Aβ-induced suppression of LTP and potentiation of LTD (Wang et al., 2004; Shankar et al., 2008; Rammes et al., 2011). Aβ-mediated LTP impairments can be blocked by inhibition of mGluR5 (Wang et al., 2004; Rammes et al., 2011). As NMDARs and mGluRs are mechanistically coupled, chronic stimulation of either can lead to synaptic failure as seen in AD (Rammes et al., 2011; Kervern et al., 2012). This failure is potentially due to Ca2+ dysregulation and dephosphorylation of CREB as both E-NMDARs and mGluR5 elevate intracellular Ca2+ (Chen et al., 2002; Hardingham et al., 2002; Hardingham and Bading, 2003; Verkhratsky and Kirchhoff, 2007; Yamin, 2009; Zhang et al., 2016). In addition, mGluR1 has been shown to be involved in synaptic plasticity and LTP in the CA1 (Neyman and Manahan-Vaughan, 2008; Rudy et al., 2015). Of note, agonism of mGluR1 and mGluR5 with 3-hydroxyphenylglycine potentiated NMDAR-induced neurotoxicity, juxtaposing the neuroprotective effects observed with mGluR2 agonism (Buisson and Choi, 1995; Tyszkiewicz and Yan, 2005) and further supporting a deleterious relationship between NMDAR and mGluRs upon LTP and excitotoxicity. Furthermore, mGluRs are required for soluble Aβ-mediated LTD enhancement (Shankar et al., 2008; Palop and Mucke, 2010; Um et al., 2013). Treatment with a similar Group I mGluR agonist, 3,5-dihydroxyphenylglycine, results in decreased AMPAR surface expression during LTD (Um et al., 2013), supporting another mechanism by which soluble Aβ impacts learning and memory signal conductance.
Theories on the Paradoxical Nature of Glutamate in AD
The two-stage model theory was first set forth by Olney et al. (1997) describing two stages of NMDAR-mediated toxicity in AD pathology. The first stage, referred to as NRHyper, describes persistent activation of NMDA receptors. Chronic NMDAR stimulation then leads to the second disease stage, referred to as NRHypo, in which the NMDARs become hypoactive as a result of chronic overstimulation leading to inhibition of LTP conductance and cognitive decline. These disease stages are driven by soluble Aβ accumulation. During the NRHyper phase, Aβ potentiates glutamate release and increases NMDA activation and sensitivity to glutamate. This leads to partial membrane depolarization allowing tonic activation of NMDA receptors (Koh et al., 1990; Gray and Patel, 1995; Danysz and Parsons, 2012). This overstimulation subsequently results in an NRHypo state in which glutamatergic activation is depressed and synaptic components are downregulated. NMDARs become hypofunctional in normal brain aging; however, this is experienced to an extreme in AD possibly due to the increase hyperactivation state experienced in AD that precedes hypoactivation. NMDAR hypoactivation applies to NMDARs on GABAergic neurons as well, causing disinhibition of the glutamatergic system and resulting in increased extracellular glutamate concentrations and neuronal loss. This is expanded upon by the findings of Huijber et al. (2015) and the trait versus state hypothesis set forth by Aizenstein and Klunk (2015), describing two models of hippocampal activity such that some individuals have a trait of high levels of hippocampal activation prior to MCI and some experience hyperactivation as a state with MCI onset. Both models subsequently result in cognitive decline and support a period of hippocampal hyperactivation occurring before hypoactivation.
Similarly, Parsons et al. (2007) proposed the signal-to-noise ratio hypothesis. This hypothesis notes that in AD pathology, NMDAR is stimulated by glutamate for longer periods of time due to a hyperactive glutamatergic system (Buisson et al., 1992; Mitani et al., 1992). As such, this leads to tonic activation of NMDAR that generates increasing amounts of noise. This noise raises the threshold by which neuronal stimulation must pass to generate a stimulus-evoked signal. With disease progression, the increased noise eventually drowns out the signal, leading to learning and memory deficits seen in AD. This effect has been observed in our lab, with APP/PS1 mice showing a decline in stimulus-evoked glutamate and an increase in basal glutamate levels with disease progression, impacting spatial memory performance (Hascup and Hascup, 2015). This hypothesis is further elucidated with the proposed action of memantine, the noncompetitive NR2 antagonist, which shows fast unblocking kinetics dependent upon membrane potential. This characteristic allows memantine to quickly unblock the NMDAR during strong depolarization of the postsynaptic membrane and remain bound during tonic stimulation. In fact, recovery from memantine blockade is shown to be even faster in the presence of higher synaptic glutamate concentrations (Clements et al., 1992). Memantine benefits both S-NMDARs and E-NMDARs in LTP such that LTP conductance is not hindered at the synapse but chronic stimulation of E-NMDARs is blocked, ultimately helping to alleviate excitotoxic effects. This is seen with memantine improvements in LTP induction and Morris water maze performance as discussed earlier (Barnes et al., 1996; Van Dam et al., 2005; Banerjee et al., 2006; Parsons et al., 2009; Martinez-Coria et al., 2010). Of note, memantine has also showed efficacy in reducing Aβ production brought on by E-NMDAR prolonged activation (Bordji et al., 2010), as well as reducing the levels of both soluble Aβ and insoluble Aβ plaques in 3xTg-AD mice (Martinez-Coria et al., 2010) and blocking soluble Aβ-induced oxidative stress (De Felice et al., 2007). As such, memantine shows modulation of Aβ-and-glutamate-induced neurotoxicity giving support to the idea that noise created by extracellular glutamate overpowers stimulus-evoked glutamate signals in AD.
In clinical trials, memantine was shown to reduce cognitive deficits in mild to severe AD patients (Winblad and Poritis, 1999; Reisberg et al., 2003; Peskind et al., 2006). A meta-analysis of memantine trials also revealed efficacy in treating patients with moderate to severe AD (Winblad et al., 2007). However, the weak procognitive effects observed in AD patients treated with memantine (McShane et al., 2006; Schneider et al., 2011) may be due to the disease stage when treatment was initiated. If memantine treatment is initiated too late in disease progression, neuronal loss may be too severe to yield procognitive effects. As such, post hoc analysis of nine clinical trials of memantine show delay of clinical worsening in moderate to severe AD patients (Hellweg et al., 2012). Danysz and Parsons (2012) argue that starting treatment in the prodromal stage of AD would allow for memantine-induced neuroprotection, which could yield more promising clinical results and further delay cognitive decline.
In addition, NMDARs are not the only components that modulate the signal-to-noise ratio. mGluRs are multifaceted due to their G-protein and NMDAR coupling and as such are also a vital mediator in LTP. Riedel (1996) has argued that mGluRs are responsible for the initial setup of the signal-to-noise ratio to allow for efficient plasticity. Pretraining injection of mGluR agonists leads to nonspecific activation of Group I mGluRs, generating an increase in noise and preventing memory formation. Groups II and III mGluRs then act as mediators to reduce noise and allow for signal conductance and memory formation. This initial organization of the signal-to-noise ratio is impacted at the mGluR level in AD pathology, as mentioned earlier with mGluR1 and mGluR5 potentiating NMDA-induced neurotoxicity and then experiencing a downregulation in expression in later disease stages (Buisson and Choi, 1995; Albasanz et al., 2005), following a similar hyper- to hypoactivation trend. This initial noise generation overpowers the inhibitory modulation of mGluR2, despite its upregulation in early AD (Buisson and Choi, 1995). However, our laboratory recently demonstrated that prodromal treatment with the mGluR Group II agonist LY379268 does not offer long-term procognitive benefits in APP/PS1 mice (Hascup et al., 2019b).
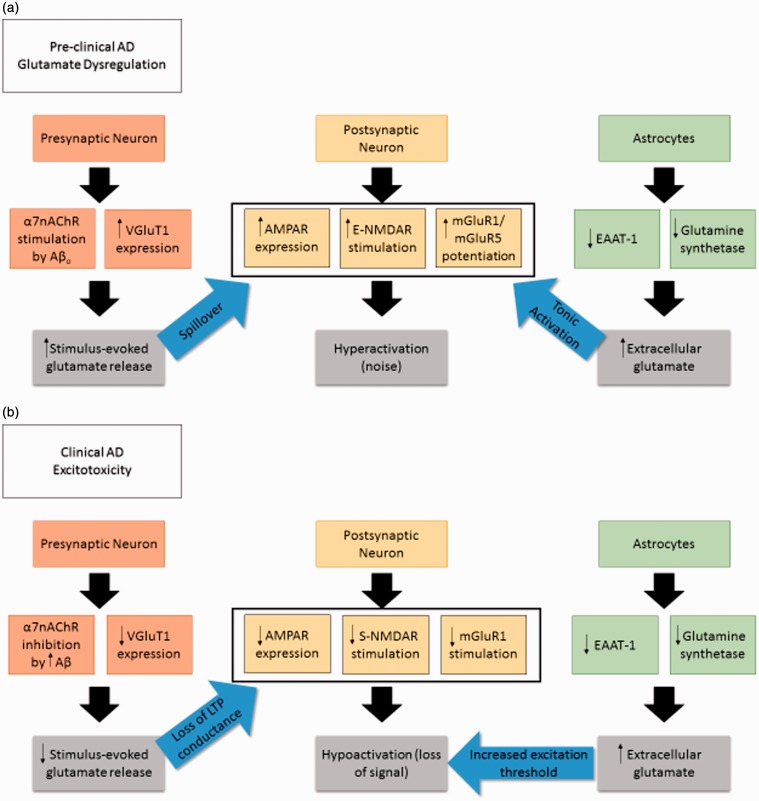
Furthermore, our laboratory and others have reported a relationship between Aβ and glutamate mediated by α7nAChR stimulation that contributes to increases in glutamate release (Puzzo et al., 2008; Mura et al., 2012; Hascup and Hascup, 2016), generating a large amount of signal. This relationship changes as AD progresses and Aβ increases in concentration and plaque formation such that we see an opposite impact through α7nAChR in which glutamate release is inhibited and thus contributes to overall hypoactivation (Liu et al., 2001; Mura et al., 2012). Interestingly, α7nAChR desensitization leads to increased NMDAR surface expression and enhanced function (Lin et al., 2010), and both pathways modulate Aβ toxicity such that α7nAChR and E-NMDARs lead to Aβ42 internalization (D’Andrea et al., 2001; Bi et al., 2002; Nagele et al., 2002; Deutsch et al., 2014; Godyń et al., 2016), and E-NMDARs lead to increased amyloidogenic processing (Bordji et al., 2010). In addition, nicotine-mediated enhancement of LTP is both NMDA and mGluR5 dependent (Welsby et al., 2006), and because Aβ42 binds to the same pocket as nicotine, it follows that mGluR5 may potentiate both NMDA and α7nAChR-induced excitotoxicity. Other pathways previously discussed in this review also result in toxic Ca2+ intracellular levels and modulation in CREB expression that result in neuronal cell loss. This interaction between multiple glutamatergic pathways emphasizes the breadth of Aβ modulation of the glutamatergic system to generate neurotoxicity as outlined in Figure 1.
Figure 1.
Changes in glutamatergic synapse component expression and signaling with AD progression. (a) Preclinical AD upregulation of several neuronal components contributing to hyperactivation and building the foundation for excitotoxicity. (b) Clinical AD is characterized by hypoactivation of the glutamatergic system, possibly a consequence of the earlier preclinical stage. This results in cognitive deficits due to signal-to-noise ratio imbalance.
AD = Alzheimer’s disease; α7nAChR = alpha-7 nicotinic acetylcholine receptor; Aβo = amyloid beta oligomer; VGluT1 = vesicular glutamate transporter 1; AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; E-NMDAR = extrasynaptic N-methyl-D-aspartic acid receptor; mGluR = metabotropic glutamate receptor; S-NMDAR = synaptic N-methyl-D-aspartic acid receptor; EAAT = excitatory amino acid transporter.
Conclusion
Taken together, the presented evidence supports a signal-to-noise ratio imbalance occurring in AD pathology. Aβ modulates several parts of the tripartite glutamatergic synapse that culminates in an excitotoxic environment. The excitation threshold at which glutamate signals must surpass for learning and memory increases while also undergoing an overall dampening of the glutamatergic system. Loss of signal detection due to persistently elevated synaptic and extrasynaptic glutamate levels leads to the hallmark symptoms of cognitive deficits and eventual neuronal loss in AD disease progression.
Summary
Glutamatergic transmission displays stark changes throughout AD progression. Earlier stages are characterized by upregulation of synaptic components contributing to increased glutamate signaling. Later stages exhibit hypoactivation possibly due to cell damage and neuronal loss, subsequently resulting in cognitive decline.
Author Contributions
All authors were involved with the article preparation and editing.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG057767 and R01AG061937, the Illinois Department of Public Health under Award Numbers 63282003D and 83282002F, the Center for Alzheimer’s Disease and Related Disorders at Southern Illinois University School of Medicine, the Kenneth Stark Endowment, the Fraternal Order of Eagles, and the American Diabetes Association under Award Number 1-19-IBS-126 and does not necessarily represent the official views of the funding agencies.
References
- Abbott J. J., Howlett D. R., Francis P. T., Williams R. J. (2008). Aβ1-42 modulation of Akt phosphorylation via α7 nAChR and NMDA receptors. Neurobiol Aging, 29(7), 992–1001. doi:10.1016/j.neurobiolaging.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Aizenstein H. J., Klunk W. E. (2015). Where is hippocampal activity in the cascade of Alzheimer’s disease biomarkers? Brain, 138(4), 831–833. doi:10.1093/brain/awv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasanz J. L., Dalfó E., Ferrer I., Martín M. (2005). Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol Dis, 20(3), 685–693. doi:10.1016/j.nbd.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Almeida, C. G., Tampellini, D., Takahashi, R. H., Greengard, P., Lin, M. T., Snyder, E. M., Gouras, G. K. (2005). Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis, 20(2), 187–198. doi:10.1016/j.nbd.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Ambrosini A., Bresciani L., Fracchia S., Brunello N., Racagni G. (1995). Metabotropic glutamate receptors negatively coupled to adenylate cyclase inhibit N-methyl-D-aspartate receptor activity and prevent neurotoxicity in mesencephalic neurons in vitro. Mol Pharmacol, 47(5), 1057–1064. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7746273 [PubMed] [Google Scholar]
- Antal M., Fukazawa Y., Eördögh M., Muszil D., Molnár E., Itakura M., Takahashi M., Shigemoto R. (2008). Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci, 28(39), 9692–9701. doi:10.1523/JNEUROSCI.1551-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld D. S., Kar S., Quirion R. (1998). β-Amyloid peptides as direct cholinergic neuromodulators: A missing link? Trends Neurosci, 21(1), 43–49. doi:10.1016/S0166-2236(97)01144-2 [DOI] [PubMed] [Google Scholar]
- Banerjee P. K., Billings Luhrs L., LeFerla F. M. (2006). P1-001. Alzheimers Dement, 2(3), S94. doi:10.1016/j.jalz.2006.05.376 [Google Scholar]
- Barco A., Pittenger C., Kandel E. R. (2003). CREB, memory enhancement and the treatment of memory disorders: Promises, pitfalls and prospects. Expert Opin Ther Targets, 7(1), 101–114. doi:10.1517/14728222.7.1.101 [DOI] [PubMed] [Google Scholar]
- Barghorn S., Nimmrich V., Striebinger A., Krantz C., Keller P., Janson B., Bahr M., Schmidt M., Bitner R. S., Harlan J., Barlow E., Ebert U., Hillen H. (2005). Globular amyloid beta-peptide1-42 oligomer – A homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem, 95(3), 834–847. doi:10.1111/j.1471-4159.2005.03407.x [DOI] [PubMed] [Google Scholar]
- Barnes C. A., Danysz W., Parsons C. G. (1996). Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci, 8(3), 565–571. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8963448 [DOI] [PubMed] [Google Scholar]
- Bell K. A., O’Riordan K. J., Sweatt J. D., Dineley K. T. (2004). MAPK recruitment by beta-amyloid in organotypic hippocampal slice cultures depends on physical state and exposure time. J Neurochem, 91(2), 349–361. doi:10.1111/j.1471-4159.2004.02722.x [DOI] [PubMed] [Google Scholar]
- Bell K. F. S., Bennett D. A., Cuello A. C. (2007). Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci, 27(40), 10810–10817. doi:10.1523/JNEUROSCI.3269-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Mattson M. P. (2008). Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci, 31(9), 454–463. doi:10.1016/J.TINS.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, X., Gall, C. M., Zhou, J., Lynch, G. (2002). Uptake and Pathogenic Effects of Amyloid Beta 1-42 Are Enhanced by Integrin Antagonists and Blocked by NMDA Receptor Antagonists. Neuroscience, 112(4), 827–840. Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/12088742 [DOI] [PubMed] [Google Scholar]
- Bitner R. S., et al. (2007). Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci, 27(39), 10578–10587. doi:10.1523/JNEUROSCI.2444-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A., Jones N. M., Hicks C. A., Whiffin G. M., Ward M. A., O’Neill M. F., Kingston A. E., Monn J. A., Ornstein P. L., Schoepp D. D., Lodge D., O’Neill M. J. (2000). Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: Investigations into possible mechanism of action in vivo. J Pharmacol Exp Ther, 294(3), 800–809. [PubMed] [Google Scholar]
- Bordji K., Becerril-Ortega J., Nicole O., Buisson A. (2010). Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ß production. J Neurosci, 30(47), 15927–15942. doi:10.1523/JNEUROSCI.3021-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Kimball D., Sultana R., Fai Poon H., Lynn B. C., Casamenti F., Pepeu G., Klein J. B., Butterfield D. A. (2005). Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid β-peptide (1–42) into rat brain: Implications for Alzheimer’s disease. Neuroscience, 132(2), 313–324. doi:10.1016/j.neuroscience.2004.12.022 [DOI] [PubMed] [Google Scholar]
- Bruno A. M., Huang J. Y., Bennett D. A., Marr R. A., Hastings M. L., Stutzmann G. E. (2012). Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging, 33(5), 1001.e1–1001.e6. doi:10.1016/J.NEUROBIOLAGING.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson A., Callebert J., Mathieu E., Plotkine M., Boulu R. G. (1992). Striatal protection induced by lesioning the substantia nigra of rats subjected to focal ischemia. J Neurochem, 59(3), 1153–1157. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1353789 [DOI] [PubMed] [Google Scholar]
- Buisson A., Choi D. W. (1995). The inhibitory mGluR agonist, S-4-carboxy-3-hydroxy-phenylglycine selectively attenuates NMDA neurotoxicity and oxygen-glucose deprivation-induced neuronal death. Neuropharmacology, 34(8), 1081–1087. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8532157 [DOI] [PubMed] [Google Scholar]
- Busche M. A., Chen X., Henning H. A., Reichwald J., Staufenbiel M., Sakmann B., Konnerth A. (2012). Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A, 109(22), 8740–8745. doi:10.1073/pnas.1206171109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D. A., Castegna A., Lauderback C. M., Drake J. (2002). Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging, 23(5), 655–664. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12392766 [DOI] [PubMed] [Google Scholar]
- Butterfield D. A., Pocernich C. B. (2003). The glutamatergic system and Alzheimer’s disease. CNS Drugs, 17(9), 641–652. doi:10.2165/00023210-200317090-00004 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Pisani A., Mercuri N. B., Bernardi G. (1992). Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci, 4(10), 929–935. doi:10.1111/j.1460-9568.1992.tb00119.x [DOI] [PubMed] [Google Scholar]
- Canas, P. M., Simoes, A. P., Rodrigues, R. J., Cunha, R. A. (2014). Predominant loss of glutamatergic terminal markers in a β-amyloid peptide model of Alzheimer’s disease. Neuropharmacology, 76(A), 51–56. doi:10.1016/j.neuropharm.2013.08.026 [DOI] [PubMed] [Google Scholar]
- Cassano T., Serviddio G., Gaetani S., Romano A., Dipasquale P., Cianci S., Bellanti F., Laconca L., Romano A. D., Padalino I., LaFerla F. M., Nicoletti F., Cuomo V., Vendemiale G. (2012). Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging, 33(6), 1121.e1–1121.e12. doi:10.1016/J.NEUROBIOLAGING.2011.09.021 [DOI] [PubMed] [Google Scholar]
- Chen L., Wang H., Zhang Z., Li Z., He D., Sokabe M., Chen L. (2010). DMXB (GTS-21) ameliorates the cognitive deficits in beta amyloid(25-35) injected mice through preventing the dysfunction of alpha7 nicotinic receptor. J Neurosci Res, 88(8), 1784–1794. doi:10.1002/jnr.22345 [DOI] [PubMed] [Google Scholar]
- Chen L., Yamada K., Nabeshima T., Sokabe M. (2006). α7 Nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in β-amyloid infused rats. Neuropharmacology, 50(2), 254–268. doi:10.1016/j.neuropharm.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Chen Q.-S., Wei W.-Z., Shimahara T., Xie C.-W. (2002). Alzheimer amyloid β-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem, 77(3), 354–371. doi:10.1006/nlme.2001.4034 [DOI] [PubMed] [Google Scholar]
- Cleary J. P., Walsh D. M., Hofmeister J. J., Shankar G. M., Kuskowski M. A., Selkoe D. J., Ashe K. H. (2005). Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci, 8(1), 79–84. doi:10.1038/nn1372 [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. (1992). The time course of glutamate in the synaptic cleft. Science (New York, N.Y.), 258(5087), 1498–1501. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1359647 [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Bliss T. V. (1995). Memories of NMDA receptors and LTP. Trend Neurosci, 18(2), 54–56. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7537406 [PubMed] [Google Scholar]
- D’Andrea M., Nagele R. (2006). Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimers disease pyramidal neurons. Curr Pharm Des, 12(6), 677–684. doi:10.2174/138161206775474224 [DOI] [PubMed] [Google Scholar]
- D’Andrea M. R., Nagele R. G., Wang H. Y., Peterson P. A., Lee D. H. (2001). Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology, 38(2), 120–134. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11207825 [DOI] [PubMed] [Google Scholar]
- Danysz W., Parsons C. G. (2012). Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine – Searching for the connections. Br J Pharmacol, 167(2), 324–352. doi:10.1111/j.1476-5381.2012.02057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Butcher S. P., Morris R. G. (1992). The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci, 12(1), 21–34. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1345945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F. G., Velasco P. T., Lambert M. P., Viola K., Fernandez S. J., Ferreira S. T., Klein W. L. (2007). Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem, 282(15), 11590–11601. doi:10.1074/jbc.M607483200 [DOI] [PubMed] [Google Scholar]
- Decker H., Lo K. Y., Unger S. M., Ferreira S. T., Silverman M. A. (2010). Amyloid-β peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci, 30(27), 9166–9171. doi:10.1523/jneurosci.1074-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S. T., Scheff S. W. (1990). Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol, 27(5), 457–464. doi:10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- Deutsch S. I., Burket J. A., Benson A. D. (2014). Targeting the α7 nicotinic acetylcholine receptor to prevent progressive dementia and improve cognition in adults with Down’s syndrome. Prog Neuropsychopharmacol Biol Psychiatry, 54, 131–139. doi:10.1016/J.PNPBP.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Dewar D., Chalmers D. T., Graham D. I., McCulloch J. (1991). Glutamate metabotropic and AMPA binding sites are reduced in Alzheimer’s disease: An autoradiographic study of the hippocampus. Brain Res, 553(1), 58–64. doi:10.1016/0006-8993(91)90230-S [DOI] [PubMed] [Google Scholar]
- Dias C., Lourenço C. F., Ferreiro E., Barbosa R. M., Laranjinha J., Ledo A. (2016). Age-dependent changes in the glutamate-nitric oxide pathway in the hippocampus of the triple transgenic model of Alzheimer’s disease: Implications for neurometabolic regulation. Neurobiol Aging, 46, 84–95. doi:10.1016/J.NEUROBIOLAGING.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Dineley K. T., Bell K. A., Bui D., Sweatt J. D. (2002. a). β- Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem, 277(28), 25056–25061. doi:10.1074/jbc.M200066200 [DOI] [PubMed] [Google Scholar]
- Dineley K. T., Westerman M., Bui D., Bell K., Ashe K. H., Sweatt J. D. (2001). Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci, 21(12), 4125–4133. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11404397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley K. T., Xia X., Bui D., Sweatt J. D., Zheng H. (2002. b). Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem, 277(25), 22768–22780. doi:10.1074/jbc.M200164200 [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Wu J., Nichols R. A. (2003). Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci, 23(17), 6740–6747. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12890766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito Z., Belli L., Toniolo S., Sancesario G., Bianconi C., Martorana A. (2013). Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: Are we on the right track? CNS Neurosci Ther, 19(8), 549–555. doi:10.1111/cns.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F., Baldani-Guerra B., Corsi M., Nakanishi S., Corti C. (1999). Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci, 11(6), 2073–2082. doi:10.1046/j.1460-9568.1999.00626.x [DOI] [PubMed] [Google Scholar]
- Ferraguti F., Shigemoto R. (2006). Metabotropic glutamate receptors. Cell Tissue Res, 326(2), 483–504. doi:10.1007/s00441-006-0266-5 [DOI] [PubMed] [Google Scholar]
- Ferreira S. T., Klein W. L. (2011). The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem, 96(4), 529–543. doi:10.1016/j.nlm.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau R. T., Kam K., Qureshi T., Johnson J., Copenhagen D. R., Storm-Mathisen J., Chaudhry F. A., Nicoll R. A., Edwards R. H. (2004). Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science, 304(5678), 1815–1819. doi:10.1126/science.1097468 [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Troyer M. D., Pahner I., Nygaard G. O., Tran C. H., Reimer R. J., Bellocchio E. E., Fortin D., Storm-Mathisen J., Edwards R. H. (2001). The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron, 31(2), 247–260. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11502256 [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Persiyanov K., Dunn D., Weiss R., Meyer E. L., Rogers S. W. (2004). Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J Comp Neurol, 468(3), 334–346. doi:10.1002/cne.10943 [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M. J., Moncada S. (1989). NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol, 172(4–5), 413–416. doi:10.1016/0922-4106(89)90023-0 [DOI] [PubMed] [Google Scholar]
- Godyń J., Jończyk J., Panek D., Malawska B. (2016). Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep, 68(1), 127–138. doi:10.1016/J.PHAREP.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Goussakov I., Miller M. B., Stutzmann G. E. (2010). NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci, 30(36), 12128–12137. doi:10.1523/JNEUROSCI.2474-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. W., Patel A. J. (1995). Neurodegeneration mediated by glutamate and beta-amyloid peptide: A comparison and possible interaction. Brain Res, 691(1–2), 169–179. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8590049 [DOI] [PubMed] [Google Scholar]
- Gray R., Rajan A. S., Radcliffe K. A., Yakehiro M., Dani J. A. (1996). Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature, 383(6602), 713–716. doi:10.1038/383713a0 [DOI] [PubMed] [Google Scholar]
- Groc L., Bard L., Choquet D. (2009). Surface trafficking of N-methyl-d-aspartate receptors: Physiological and pathological perspectives. Neuroscience, 158(1), 4–18. doi:10.1016/j.neuroscience.2008.05.029 [DOI] [PubMed] [Google Scholar]
- Grutzendler J., Helmin K., Tsai J., Gan W. B. (2007). Various dendritic abnormalities are associated with fibrillar amyloid deposits in Alzheimer’s disease. Ann N Y Acad Sci, 1097(1), 30–39. doi:10.1196/annals.1379.003 [DOI] [PubMed] [Google Scholar]
- Gu Z., Liu W., Yan Z. (2009). Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem, 284(16), 10639–10649. doi:10.1074/jbc.M806508200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Yakel J. L. (2011). Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron, 71(1), 155–165. doi:10.1016/j.neuron.2011.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G. E., Bading H. (2003). The Yin and Yang of NMDA receptor signalling. Trend Neurosci, 26(2), 81–89. doi:10.1016/S0166-2236(02)00040-1 [DOI] [PubMed] [Google Scholar]
- Hardingham G. E., Fukunaga Y., Bading H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci, 5(5), 405–414. doi:10.1038/nn835 [DOI] [PubMed] [Google Scholar]
- Haris M., Nath K., Cai K., Singh A., Crescenzi R., Kogan F., Verma G., Reddy S., Hariharan H., Melhem E. R., Reddy R. (2013). Imaging of glutamate neurotransmitter alterations in Alzheimer’s disease. NMR Biomed, 26: 386–391. doi:10.1002/nbm.2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup E. R., Broderick S. O., Russell M. K., Fang Y., Bartke A., Boger H. A., Hascup K. N. (2019. a). Diet‐induced insulin resistance elevates hippocampal glutamate as well as VGLUT1 and GFAP expression in AβPP/PS1 mice. J Neurochem, 148, 219–237. doi:10.1111/jnc.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup E. R., Hascup K. N., Stephens M., Pomerleau F., Huettl P., Gratton A., Gerhardt G. A. (2010). Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem, 115(6), 1608–1620. doi:10.1111/j.1471-4159.2010.07066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup K. N., Britz J., Findley C. A., Tischkau S., Hascup E. R. (2019. b). LY379268 does not have long-term procognitive effects nor attenuate glutamatergic signaling in AβPP/PS1 mice. J Alzheimers Dis, 68(3), 1193–1209. doi:10.3233/JAD-181231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup K. N., Broderick S. O., Hascup E. R. (2016). Can Alzheimer’s-Related Cognitive Decline Be Delayed Through Prodromal Treatment? Evidence from a mouse model of Alzheimer’s disease. Alzheimers Dement, 12(7), P1017. doi:10.1016/j.jalz.2016.06.2097 [Google Scholar]
- Hascup K. N., Hascup E. R. (2015). Altered neurotransmission prior to cognitive decline in AβPP/PS1 mice, a model of Alzheimer’s disease. J Alzheimers Dis, 44, 771–776. doi:10.3233/JAD-142160 [DOI] [PubMed] [Google Scholar]
- Hascup K. N., Hascup E. R. (2016). Soluble amyloid-β42 stimulates glutamate release through activation of the α7 nicotinic acetylcholine receptor. J Alzheimers Dis, 53, 337–347. doi:10.3233/JAD-160041 [DOI] [PubMed] [Google Scholar]
- Hefendehl J. K., LeDue J., Ko R. W. Y., Mahler J., Murphy T. H., MacVicar B. A. (2016). Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat Commun, 7(1), 13441. doi:10.1038/ncomms13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström-Lindahl E., Mousavi M., Zhang X., Ravid R., Nordberg A. (1999). Regional distribution of nicotinic receptor subunit mRNAs in human brain: Comparison between Alzheimer and normal brain. Brain Res, 66(1–2), 94–103. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10095081 [DOI] [PubMed] [Google Scholar]
- Hellweg R., Wirth Y., Janetzky W., Hartmann S. (2012). Efficacy of memantine in delaying clinical worsening in Alzheimer’s disease (AD): Responder analyses of nine clinical trials with patients with moderate to severe AD. Int J Geriatr Psychiatry, 27(6), 651–656. doi:10.1002/gps.2766 [DOI] [PubMed] [Google Scholar]
- Hiruma H., Katakura T., Takahashi S., Ichikawa T., Kawakami T. (2003). Glutamate and amyloid beta-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J Neurosci, 23(26), 8967–8977. doi:10.1523/JNEUROSCI.23-26-08967.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey S. E., Williams R. J., Perkinton M. S. (2009). Synaptic NMDA receptor activation stimulates α-secretase amyloid precursor protein processing and inhibits amyloid-β production. J Neurosci, 29(14), 4442–4460. doi:10.1523/JNEUROSCI.6017-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof P. R., Bussière T., Gold G., Kövari E., Giannakopoulos P., Bouras C., Perl D. P., Morrison J. H. (2003). Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropathol Exp Neurol, 62(1), 55–67. doi:10.1093/jnen/62.1.55 [DOI] [PubMed] [Google Scholar]
- Huang, W., Zhang X., Chen, W. (2016). Role of oxidative stress in Alzheimer’s disease. Biomed Rep, 4(5), 519–522. doi:10.3892/br.2016.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W., Mormino E. C., Schultz A. P., Wigman S., Ward A. M., Larvie M., Amariglio R. E., Marshall G. A., Rentz D. M., Johnson K. A., Sperling R. A. (2015). Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain, 138(4), 1023–1035. doi:10.1093/brain/awv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. T., Marzloff K., Wenniger J. J., Dawson T. M., Bredt D. S., Snyder S. H. (1992). Relative sparing of nitric oxide synthase-containing neurons in the hippocampal formation in Alzheimer’s disease. Ann Neurol, 32(6), 818–820. doi:10.1002/ana.410320618 [DOI] [PubMed] [Google Scholar]
- Hynd M. R., Scott H. L., Dodd P. R. (2004. a). Differential expression of N-methyl-D-aspartate receptor NR2 isoforms in Alzheimer’s disease. J Neurochem, 90(4), 913–919. doi:10.1111/j.1471-4159.2004.02548.x [DOI] [PubMed] [Google Scholar]
- Hynd M. R., Scott H. L., Dodd P. R. (2004. b). Selective loss of NMDA receptor NR1 subunit isoforms in Alzheimer’s disease. J Neurochem, 89(1), 240–247. doi:10.1111/j.1471-4159.2003.02330.x [DOI] [PubMed] [Google Scholar]
- Imre G. (2007). The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Reviews, 13(4), 444–464 doi:10.1111/j.1527-3458.2007.00024.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. T. R., Ashby M. C., McBain C. J. (2007). The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron, 54(6), 859–871. doi:10.1016/J.NEURON.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Jacob C. P., Koutsilieri E., Bartl J., Neuen-Jacob E., Arzberger T., Zander N., Roggendorf W., Riederer P., Grünblatt E. (2007). Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis, 11(1), 97–116. doi:10.3233/JAD-2007-11113 [DOI] [PubMed] [Google Scholar]
- Kashani A., Lepicard È., Poirel O., Videau C., David J. P., Fallet-Bianco C., Simon A., Delacourte A., Giros B., Epelbaum J., Betancur C., El Mestikawy S. (2008). Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging, 29, 1619–1630. doi:10.1016/j.neurobiolaging.2007.04.010 [DOI] [PubMed] [Google Scholar]
- Katagiri H., Tanaka K., Manabe T. (2001). Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eu rJ Neurosci, 14(3), 547–553. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11553304 [DOI] [PubMed] [Google Scholar]
- Kervern M., Angeli A., Nicole O., Léveillé F., Parent B., Villette V., Buisson A., Dutar P. (2012). Selective impairment of some forms of synaptic plasticity by oligomeric amyloid-β peptide in the mouse hippocampus: Implication of extrasynaptic NMDA receptors. J Alzheimers Dis, 32(1), 183–196. doi:10.3233/JAD-2012-120394 [DOI] [PubMed] [Google Scholar]
- Kishida K. T., Klann E. (2006). Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal, 9(2), 233–244. doi:10.1089/ars.2007.9.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E. M., Martins I. V. A., Gümüsgöz S., Allan S. M., Lawrence C. B. (2014). High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging, 35(8), 1821–1832. doi:10.1016/J.NEUROBIOLAGING.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. (1990). Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res, 533(2), 315–320. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2289145 [DOI] [PubMed] [Google Scholar]
- Konradsson-Geuken A., Gash C. R., Alexander K., Pomerleau F., Huettl P., Gerhardt G. A., Bruno J. P. (2009). Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse, 63(12), 1069–1082. doi:10.1002/syn.20693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina K. J., Hyman B. T., Spires-Jones T. L. (2012). Soluble forms of tau are toxic in Alzheimer’s disease. Transl Neurosci, 3(3), 223–233. doi:10.2478/s13380-012-0032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor P. N., Buniel M. C., Furlow P. W., Sanz Clemente A., Velasco P. T., Wood M., Viola K. L., Klein W. L. (2007). Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci, 27(4), 796–807. doi:10.1523/JNEUROSCI.3501-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe E. K., Picciotto M. R., Aghajanian G. K. (2003). Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology, 28(2), 216–225. doi:10.1038/sj.npp.1300032 [DOI] [PubMed] [Google Scholar]
- Law A., Gauthier S., Quirion R. (2001). Say NO to Alzheimer’s disease: The putative links between nitric oxide and dementia of the Alzheimer’s type. Brain Res Rev, 35(1), 73–96. doi:10.1016/S0165-0173(00)00051-5 [DOI] [PubMed] [Google Scholar]
- Lee H., Ogawa O., Zhu X., O’Neill M. J., Petersen R. B., Castellani R. J., Ghanbari H., Perry G., Smith M. A. (2004). Aberrant expression of metabotropic glutamate receptor 2 in the vulnerable neurons of Alzheimer’s disease. Acta Neuropathol, 107(4), 365–371. doi:10.1007/s00401-004-0820-8 [DOI] [PubMed] [Google Scholar]
- Lee H., Zhu X., Casadesus G., Pallàs M., Camins A., O’Neill M. J., Nakanishi S., Perry G., Smith M. A. (2009). The effect of mGluR2 activation on signal transduction pathways and neuronal cell survival. Brain Res, 1249, 244–250. doi:10.1016/J.BRAINRES.2008.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre K. P., Levy L. M., Ottersen O. P., Storm-Mathisen J., Danbolt N. C. (1995). Differential expression of two glial glutamate transporters in the rat brain: Quantitative and immunocytochemical observations. J Neurosci, 15(3), 1835–1853. doi:10.1523/JNEUROSCI.15-03-01835.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S., Ali C., Gabriel C., Croci N., Mackenzie E. T., Glabe C. G., Plotkine M., Marchand-Verrecchia C., Vivien D., Buisson A. (2005). NMDA receptor activation inhibits-secretase and promotes neuronal amyloid-production. J Neurosci, 25(41), 9367–9377. doi:10.1523/JNEUROSCI.0849-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Jin M., Koeglsperger T., Shepardson N. E., Shankar G. M., Selkoe D. J. (2011). Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci, 31(18), 6627–6638. doi:10.1523/JNEUROSCI.0203-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Vicini S., Hsu F.-C., Doshi S., Takano H., Coulter D. A., Lynch D. R. (2010). Axonal α7 nicotinic ACh receptors modulate presynaptic NMDA receptor expression and structural plasticity of glutamatergic presynaptic boutons. Proc Natl Acad Sci U S A., 107(38), 16661–16666. doi:10.1073/pnas.1007397107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.-S., Kawai H., Berg D. K. (2001). β-Amyloid peptide blocks the response of 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci, 98(8), 4734–4739. doi:10.1073/pnas.081553598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Xu S. (2006). ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life, 58(11), 621–631. doi:10.1080/15216540600957438 [DOI] [PubMed] [Google Scholar]
- Magdesian M. H., Nery A. A., Martins A. H. B., Juliano M. A., Juliano L., Ulrich H., Ferreira S. T. (2005). Peptide blockers of the inhibition of neuronal nicotinic acetylcholine receptors by amyloid β. J Biol Chem, 280(35), 31085–31090. doi:10.1074/jbc.M502406200 [DOI] [PubMed] [Google Scholar]
- Manzoni O., Prezeau L., Marin P., Deshager S., Bockaert J., Fagni L. (1992). Nitric oxide-induced blockade of NMDA receptors. Neuron, 8(4), 653–662. doi:10.1016/0896-6273(92)90087-T [DOI] [PubMed] [Google Scholar]
- Marchi M., Risso F., Viola C., Cavazzani P., Raiteri M. (2002). Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem, 80(6), 1071–1078. doi:10.1046/j.0022-3042.2002.00805.x [DOI] [PubMed] [Google Scholar]
- Martinez-Coria H., Green K. N., Billings L. M., Kitazawa M., Albrecht M., Rammes G., Parsons C. G., Gupta S., Banerjee P., LaFerla F. M. (2010). Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. Am J Pathol, 176(2), 870–880. doi:10.2353/AJPATH.2010.090452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson I., Capetillo-Zarate E., Faideau M., Willén K., Esteras N., Frykman S., Tjernberg L. O., Gouras G. K. (2019). APP depletion alters selective pre- and post-synaptic proteins. Mol Cell Neurosci, 95, 86–95. doi:10.1016/j.mcn.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., Alford M., DeTeresa R., Terry R. (1993). An antibody against phosphorylated neurofilaments identifies a subset of damaged association axons in Alzheimer’s disease. Am J Pathol, 142(3), 871–882. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8456946 [PMC free article] [PubMed] [Google Scholar]
- McShane R., Areosa Sastre A., Minakaran N. (2006). Memantine for dementia. Cochrane Database Syst Rev, 2(2), CD003154. doi:10.1002/14651858.CD003154.pub5 [DOI] [PubMed] [Google Scholar]
- Miller S. L., Fenstermacher E., Bates J., Blacker D., Sperling R. A., Dickerson B. C. (2008). Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry, 79(6), 630–635. doi:10.1136/jnnp.2007.124149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkeviciene R., Ihalainen J., Malm T., Matilainen O., Keksa-Goldsteine V., Goldsteins G., Iivonen H., Leguit N., Glennon J., Koistinaho J., Banerjee P., Tanila H. (2008). Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. J Neurochem, 105(3), 584–594. doi:10.1111/j.1471-4159.2007.05147.x [DOI] [PubMed] [Google Scholar]
- Mitani A., Andou Y., Kataoka K. (1992). Selective vulnerability of hippocampal CA1 neurons cannot be explained in terms of an increase in glutamate concentration during ischemia in the gerbil: Brain microdialysis study. Neuroscience, 48(2), 307–313. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1351267 [DOI] [PubMed] [Google Scholar]
- Mookherjee P., Green P. S., Watson G. S., Marques M. A., Tanaka K., Meeker K. D., Meabon, James S., Li N., Zhu P., Olson V. G., Cook D. G. (2011). GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J Alzheimers Dis, 26(3), 447–455. doi:10.3233/JAD-2011-110503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I., Guzmán-Martínez L., Cerda-Troncoso C., Farías G. A., Maccioni R. B. (2014). Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci, 8, 112. doi:10.3389/fncel.2014.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura E., Zappettini S., Preda S., Biundo F., Lanni C., Grilli M., Cavallero A., Olivero G., Salamone A., Marchi M. (2012). Dual effect of beta-amyloid on α7 and α4β2 nicotinic receptors controlling the release of glutamate, aspartate and GABA in rat hippocampus. PLoS One, 7(1), e29661. doi:10.1371/journal.pone.0029661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele R. G., D’Andrea M. R., Anderson W. J., Wang H.-Y. (2002). Intracellular accumulation of beta-amyloid(1-42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience, 110(2), 199–211. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11958863 [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Wegiel J., Venkataraman V., Imaki H., Wang K.-C., Wegiel J. (2004). Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging, 25(5), 663–674. doi:10.1016/j.neurobiolaging.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Näslund J., Schierhorn A., Hellman U., Lannfelt L., Roses A. D., Tjernberg L. O., Silberring J., Gandy S. E., Winblad B., Greengard P. (1994). Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci U S A., 91(18), 8378–8382. doi:10.1073/PNAS.91.18.8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher T. M., Ehlers M. D. (2009). Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol, 19(5), 218–227. doi:10.1016/j.tcb.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Neyman S., Manahan-Vaughan D. (2008). Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci, 27(6), 1345–1352. doi:10.1111/j.1460-9568.2008.06109.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle M. M., Gonzalez J., Sugaya K., Baskerville K. A., Bryan D., Lund K., Gallagher M., McKinney M. (2001). Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience, 107(3), 415–431. doi:10.1016/S0306-4522(01)00374-8 [DOI] [PubMed] [Google Scholar]
- Norenberg M. D., Martinez-Hernandez A. (1979). Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res, 161(2), 303–310. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/31966 [DOI] [PubMed] [Google Scholar]
- O’Brien J. L., O’Keefe K. M., LaViolette P. S., DeLuca A. N., Blacker D., Dickerson B. C., Sperling R. A. (2010). Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology, 74(24), 1969–1976. doi:10.1212/WNL.0b013e3181e3966e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo O. C., Oh J. M., Koscik R., Jonaitis E., Cleary C. A., Dowling N. M., Bendlin B. B., Larue A., Hermann B. P., Barnhart T. E., Murali D., Rowley H. A., Carlsson C. M., Gallagher C. L., Asthana S., Sager M. A., Christian B. T., Johnson S. C. (2014). Amyloid burden, neuronal function, and cognitive decline in middle-aged adults at risk for Alzheimer’s disease. J Int Neuropsychol Soc, 20(4), 422–433. doi:10.1017/S1355617714000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabarria M., Noristani H. N., Verkhratsky A., Rodríguez J. J. (2010). Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia, 58(7), 831–838. doi:10.1002/glia.20967 [DOI] [PubMed] [Google Scholar]
- Olabarria M., Noristani H. N., Verkhratsky A., Rodríguez J. J. (2011). Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: Mechanism for deficient glutamatergic transmission? Mol Neurodegener, 6, 55. doi:10.1186/1750-1326-6-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney J. W., Wozniak D. F., Farber N. B. (1997). Excitotoxic neurodegeneration in Alzheimer disease. New hypothesis and new therapeutic strategies. Arch Neurol, 54(10), 1234–1240. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9341569 [DOI] [PubMed] [Google Scholar]
- Ovsepian S. V, OLeary V. B., Zaborszky L., Ntziachristos V., Oliver Dolly J. (2018). Amyloid plaques of Alzheimer’s disease as hotspots of glutamatergic activity. Neuroscientist, 0, 1–2. doi:10.1177/1073858418791128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri E. M., Menga A., Lebrun A., Hooper D. C., Butterfield D. A., Mazzone M., Castegna A. (2017). Blockade of glutamine synthetase enhances inflammatory response in microglial cells. Antioxid Redox Signal, 26(8), 351–363. doi:10.1089/ars.2016.6715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J. J., Mucke L. (2010). Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. NatNeurosci, 13(7), 812–818. doi:10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandis C., Sotiriou E., Kouvaras E., Asprodini E., Papatheodoropoulos C., Angelatou F. (2006). Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience, 140(1), 163–175. doi:10.1016/J.NEUROSCIENCE.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Parameshwaran K., Sims C., Kanju P., Vaithianathan T., Shonesy B. C., Dhanasekaran M., Bahr B. A., Suppiramaniam V. (2007). Amyloid β-peptide Aβ1–42 but not Aβ1–40 attenuates synaptic AMPA receptor function. Synapse, 61(6), 367–374. doi:10.1002/syn.20386 [DOI] [PubMed] [Google Scholar]
- Parks J. K., Smith T. S., Trimmer P. A., Bennett J. P., Parker W. D. (2001). Neurotoxic Aβ peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem, 76(4), 1050–1056. doi:10.1046/j.1471-4159.2001.00112.x [DOI] [PubMed] [Google Scholar]
- Parri H. R., Hernandez C. M., Dineley K. T. (2011). Research update: Alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer’s disease. Biochem Pharmacol, 82, 931–942. doi:10.1016/j.bcp.2011.06.039 [DOI] [PubMed] [Google Scholar]
- Parsons C. G., Albrecht M., Rammes G. (2009). Memantine reverses ß-amyloid oligomers-induced deficits in long term potentiation (LTP) in murine hippocampal slices. Alzheimers Dement, 5(4), P176. doi:10.1016/j.jalz.2009.04.020 [Google Scholar]
- Parsons C. G., Danysz W., Quack G. (1998). Glutamate in CNS disorders as a target for drug development: An update. Drug News Perspect, 11(9), 523–569. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15616669 [DOI] [PubMed] [Google Scholar]
- Parsons C. G., Stöffler A., Danysz W. (2007). Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system – Too little activation is bad, too much is even worse. Neuropharmacology, 53(6), 699–723. doi:10.1016/j.neuropharm.2007.07.013 [DOI] [PubMed] [Google Scholar]
- Peskind E. R., Potkin S. G., Pomara N., Ott B. R., Graham S. M., Olin J. T., McDonald S. (2006). Memantine Treatment in Mild to Moderate Alzheimer Disease: A 24-Week Randomized, Controlled Trial. Am J Geriatr Psychiatry, 14(8), 704–715. doi:10.1097/01.JGP.0000224350.82719.83 [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wang Y.-X., Niedzielski A. S., Wenthold R. J. (1996). The metabotropic glutamate receptors, MGLUR2 and MGLUR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience, 71(4), 949–976. doi:10.1016/0306-4522(95)00533-1 [DOI] [PubMed] [Google Scholar]
- Puzzo D., Arancio O. (2012). Amyloid-β peptide: Dr. Jekyll or Mr. Hyde? J Alzheimers Dis, 33(S1), S111–S120. doi:10.3233/JAD-2012-129033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D., Privitera L., Leznik E., Fa M., Staniszewski A., Palmeri A., Arancio O. (2008). Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci, 28(53), 14537–14545. doi:10.1523/JNEUROSCI.2692-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero J. E., Pomerleau F., Huettl P., Johnson K. W., Offord J., Gerhardt G. A. (2011). Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: Basic characterization and drug pharmacology. Brain Res, 1401, 1–9. doi:10.1016/j.brainres.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G., Hasenjäger A., Sroka-Saidi K., Deussing J. M., Parsons C. G. (2011). Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology, 60(6), 982–990. doi:10.1016/j.neuropharm.2011.01.051 [DOI] [PubMed] [Google Scholar]
- Reisberg B., Doody R., Stöffler A., Schmitt F., Ferris S., Möbius H. J. (2003). Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med, 348(14), 1333–1341. doi:10.1056/NEJMoa013128 [DOI] [PubMed] [Google Scholar]
- Revett T. J., Baker G. B., Jhamandas J., Kar S. (2013). Glutamate system, amyloid ß peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci, 38(1), 6–23. doi:10.1503/jpn.110190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. (1996). Function of metabotropic glutamate receptors in learning and memory. Trend Neurosci, 19(6), 219–223. doi:10.1016/0166-2236(96)20012-8 [DOI] [PubMed] [Google Scholar]
- Robinson S. R. (2001). Changes in the cellular distribution of glutamine synthetase in Alzheimer’s disease. J Neurosci Res, 66, 972–980. doi:10.1002/jnr.10057 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arellano J. J., Parpura V., Zorec R., Verkhratsky A. (2016). Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience, 323, 170–182. doi:10.1016/j.neuroscience.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Perdigon M., Tordera R. M., Gil-Bea F. J., Gerenu G., Ramirez M. J., Solas M. (2016). Down-regulation of glutamatergic terminals (VGLUT1) driven by Aβ in Alzheimer’s disease. Hippocampus, 26(10), 1303–1312. doi:10.1002/hipo.22607 [DOI] [PubMed] [Google Scholar]
- Rudy C. C., Hunsberger H. C., Weitzner D. S., Reed M. N. (2015). The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging Dis, 6(2), 131–148. doi:10.14336/AD.2014.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupsingh R., Borrie M., Smith M., Wells J. L., Bartha R. (2011). Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging, 32, 802–810. doi:10.1016/j.neurobiolaging.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Sattler R., Xiong Z., Lu W. Y., Hafner M., MacDonald J. F., Tymianski M. (1999). Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science (New York, N.Y.), 284(5421), 1845–1848. doi:10.1126/SCIENCE.284.5421.1845 [DOI] [PubMed] [Google Scholar]
- Schallier A., Smolders I., Van Dam D., Loyens E., De Deyn P. P., Michotte A., Massie A. (2011). Region- and age-specific changes in glutamate transport in the AβPP23 mouse model for Alzheimer’s disease. J Alzheimers Dis, 24(2), 287–300. doi:10.3233/JAD-2011-101005 [DOI] [PubMed] [Google Scholar]
- Schneider L. S., Dagerman K. S., Higgins J. P. T., McShane R. (2011). Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol, 68(8), 991. doi:10.1001/archneurol.2011.69 [DOI] [PubMed] [Google Scholar]
- Scott H. A., Gebhardt F. M., Mitrovic A. D., Vandenberg R. J., Dodd P. R. (2011). Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging, 32(3), 553.e1–553.e11. doi:10.1016/j.neurobiolaging.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. (2002). Alzheimer’s disease is a synaptic failure. Science, 298(5594), 789–791. doi:10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. (2008). Soluble oligomers of the amyloid β-protein: Impair synaptic plasticity and behavior. Behav Brain Res, 192(1), 106–113. doi:10.1007/978-3-540-76330-7_8 [DOI] [PMC free article] [PubMed]
- Serrano F., Klann E. (2004). Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev, 3(4), 431–443. doi:10.1016/J.ARR.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008). Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med, 14(8), 837–842. doi:10.1038/nm1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat P. B., Chalimoniuk M., Wang J.-H., Strosznajder J. B., Lee J. C., Sun A. Y., Simonyi A., Sun G. Y. (2008). Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem, 106(1), 45–55. doi:10.1111/j.1471-4159.2008.05347.x [DOI] [PubMed] [Google Scholar]
- Shie F.-S., LeBoeuf R. C., Jin L.-W., LeBoeur R. C. (2003). Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. Neuroreport, 14(1), 123–129. doi:10.1097/01.wnr.0000051151.87269.7d [DOI] [PubMed] [Google Scholar]
- Šišková Z., Justus D., Kaneko H., Friedrichs D., Henneberg N., Beutel T., Pitsch J., Schoch S., Remy S. (2014). Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron, 84(5), 1023–1033. doi:10.1016/J.NEURON.2014.10.024 [DOI] [PubMed] [Google Scholar]
- Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., Greengard P. (2005). Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci, 8(8), 1051–1058. doi:10.1038/nn1503 [DOI] [PubMed] [Google Scholar]
- Sokolow S., Luu S. H., Nandy K., Miller C. A., Vinters H. V., Poon W. W., Gylys K. H. (2012). Preferential accumulation of amyloid-beta in presynaptic glutamatergic terminals (VGluT1 and VGluT2) in Alzheimer’s disease cortex. Neurobiol Dis, 45(1), 381–387. doi:10.1016/j.nbd.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Mrak R. E., Griffin W. S. T. (2004). Microglia and neuroinflammation: A pathological perspective . J Neuroinflammation, 1(1), 14. doi:10.1186/1742-2094-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann G. E. (2005). Calcium dysregulation, IP3 signaling, and Alzheimer’s disease. Neuroscientist, 11(2), 110–115. doi:10.1177/1073858404270899 [DOI] [PubMed] [Google Scholar]
- Takamori S., Rhee J. S., Rosenmund C., Jahn R. (2000). Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature, 407(6801), 189–194. doi:10.1038/35025070 [DOI] [PubMed] [Google Scholar]
- Takumi Y., Ramírez-León V., Laake P., Rinvik E., Ottersen O. P. (1999). Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci, 2(7), 618–624. doi:10.1038/10172 [DOI] [PubMed] [Google Scholar]
- Talantova M., et al. (2013. ). Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A., 110(27), E2518–E2527. doi:10.1073/pnas.1306832110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D. R., Rüb U., Schultz C., Sassin I., Ghebremedhin E., Del Tredici K., Braak E., Braak H. (2000). Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol, 59(8), 733–748. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10952063; https://academic.oup.com/jnen/article-abstract/59/8/733/2610065 [DOI] [PubMed] [Google Scholar]
- Tully T., Bourtchouladze R., Scott R., Tallman J. (2003). Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov, 2(4), 267–277. doi:10.1038/nrd1061 [DOI] [PubMed] [Google Scholar]
- Tyszkiewicz J. P., Yan Z. (2005). β-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons. J Neurophysioly, 93(6), 3102–3111. doi:10.1152/jn.00939.2004 [DOI] [PubMed] [Google Scholar]
- Um J. W., Kaufman A. C., Kostylev M., Heiss J. K., Stagi M., Takahashi H., Kerrisk M. E., Vortmeyer A., Wisniewski T., Koleske A. J., Gunther E. C., Nygaard H. B., Strittmatter S. M. (2013). Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron, 79(5), 887–902. doi:10.1016/J.NEURON.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladolid-Acebes I., Merino B., Principato A., Fole A., Barbas C., Lorenzo M. P., García A., Del Olmo N., Ruiz-Gayo M., Cano V. (2012). High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab, 302(4), E396–E402. doi:10.1152/ajpendo.00343.2011 [DOI] [PubMed] [Google Scholar]
- Van Dam D., Abramowski D., Staufenbiel M., De Deyn P. P. (2005). Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology, 180(1), 177–190. doi:10.1007/s00213-004-2132-z [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Kirchhoff F. (2007). Glutamate-mediated neuronal-glial transmission. J Anat, 210(6), 651–660. doi:10.1111/j.1469-7580.2007.00734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky, A., Olabarria, M., Noristani, H. N., Yeh, C., Rodriguez, J. J. (2010). Astrocytes in Alzheimer’s Disease. Neurotherapeutics, 7(4), 399–412. doi:10.1016/j.nurt.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J., Coserea I., Pawlak V., Fuchs E. C., Köhr G., Seeburg P. H., Monyer H. (2007). Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology, 53(1), 10–17. doi:10.1016/J.NEUROPHARM.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002). Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature, 416(6880), 535–539. doi:10.1038/416535a [DOI] [PubMed] [Google Scholar]
- Wang H.-W., Pasternak J. F., Kuo H., Ristic H., Lambert M. P., Chromy B., Viola K.L., Klein W.L., Trommer B. L. (2002). Soluble oligomers of β amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res, 924(2), 133–140. doi:10.1016/S0006-8993(01)03058-X [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Lee D. H., D’Andrea M. R., Peterson P. A., Shank R. P., Reitz A. B. (2000. a). Beta-amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology . J Biol Chem, 275(8), 5626–5632. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10681545 [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Lee D. H. S., Davis C. B., Shank R. P. (2000. b). Amyloid peptide Aβ1-42 binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. J Neurochem, 75(3), 1155–1161. doi:10.1046/j.1471-4159.2000.0751155.x [DOI] [PubMed] [Google Scholar]
- Wang Q., Walsh D. M., Rowan M. J., Selkoe D. J., Anwyl R. (2004). Block of long-term potentiation by naturally secreted and synthetic amyloid-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci, 24(13), 3370–3378. doi:10.1523/JNEUROSCI.1633-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby P., Rowan M., Anwyl R. (2006). Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci, 24(11), 3109–3118. doi:10.1111/j.1460-9568.2006.05187.x [DOI] [PubMed] [Google Scholar]
- West M. J., Coleman P. D., Flood D. G, Troncoso J. C. (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet, 344(8925), 769–772. doi:10.1016/S0140-6736(94)92338-8 [DOI] [PubMed] [Google Scholar]
- Wilcox K. C., Lacor P. N., Pitt J., Klein W. L. (2011). Aβ oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol Neurobiol, 31(6), 939–948. doi:10.1007/s10571-011-9691-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C., Mehrian Shai R., Wu Y., Hsu Y.-H., Sitzer T., Spann B., McCleary C., Mo Y., Miller C. A. (2009). Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer’s disease. PloS One, 4(3), e4936. doi:10.1371/journal.pone.0004936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B., Jones R. W., Wirth Y., Stöffler A., Möbius H. J. (2007). Memantine in moderate to severe Alzheimer’s disease: A meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord, 24(1), 20–27. doi:10.1159/000102568 [DOI] [PubMed] [Google Scholar]
- Winblad B., Poritis N. (1999). Memantine in severe dementia: Results of the9M-best study (benefit and efficacy in severly demented patients during treatment with memantine). Int J Geriatr Psychiatry, 14(2), 135–146. doi:10.1002/(SICI)1099-1166(199902)14:2<135::AID-GPS906>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. (1993). Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol, 3(3), 291–298. doi:10.1016/0959-4388(93)90120-N [DOI] [PubMed] [Google Scholar]
- Xiong W.-X., Zhou G.-X., Wang B., Xue Z.-G., Wang L., Sun H.-C., Ge S.-J. (2013). Impaired spatial learning and memory after sevoflurane–nitrous oxide anesthesia in aged rats is associated with down-regulated cAMP/CREB signaling. PLoS One, 8(11), e79408. doi:10.1371/journal.pone.0079408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin G. (2009). NMDA receptor-dependent signaling pathways that underlie amyloid β-protein disruption of LTP in the hippocampus. J Neurosci Res, 87(8), 1729–1736. doi:10.1002/jnr.21998 [DOI] [PubMed] [Google Scholar]
- Yasuda R. P., Ikonomovic M. D., Sheffield R., Rubin R. T., Wolfe B. B., Armstrong D. M. (1995). Reduction of AMPA-selective glutamate receptor subunits in the entorhinal cortex of patients with Alzheimer’s disease pathology: A biochemical study. Brain Res, 678(1–2). doi:10.1016/0006-8993(95)00178-S [DOI] [PubMed] [Google Scholar]
- Yin J. C., Tully T. (1996). CREB and the formation of long-term memory. Curr Opin Neurobiol, 6(2), 264–268. doi:10.1016/S0959-4388(96)80082-1 [DOI] [PubMed] [Google Scholar]
- Young K. F., Pasternak S. H., Rylett R. J. (2009). Oligomeric aggregates of amyloid β peptide 1–42 activate ERK/MAPK in SH-SY5Y cells via the α7 nicotinic receptor. Neurochem Int, 55(8), 796–801. doi:10.1016/j.neuint.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Zhang L., Jin C., Lu X., Yang J., Wu S., Liu Q., Chen R., Bai C., Zhang D., Zheng L., Du Y., Cai Y. (2014). Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology, 323, 95–108. doi:10.1016/J.TOX.2014.06.011 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li P., Feng J., Wu M. (2016). Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol Sci, 37(7), 1039–1047. doi:10.1007/s10072-016-2546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]