Abstract
Background:
Hepatitis C virus (HCV) infection causes many extrahepatic malignancies; whether it increases gastric cancer risk and the risk reverses after anti-HCV therapy remain elusive.
Method:
A nationwide population-based cohort study of Taiwan National Health Insurance Research Database (TNHIRD) was conducted. In parallel, the risk factors and HCV-core-protein expressions were surveyed in gastric cancer patients from a tertiary care center.
Results:
From 2003 to 2012, of 11,712,928 patients, three 1:4:4, propensity-score-matched TNHIRD cohorts including HCV-treated (7545 patients with interferon-based therapy ⩾ 6 months), HCV-untreated (n = 30,180), and HCV-uninfected cohorts (n = 30,180) were enrolled. The cumulative incidences of gastric cancer [HCV-treated: 0.452%; 95% confidence interval (CI): 0.149–1.136%; HCV-untreated: 0.472%; 95% CI: 0.274–0.776%; HCV-uninfected: 0.146%; 95% CI 0.071–0.280%] were lowest in HCV-uninfected cohort (p = 0.0028), but indifferent between treated and untreated cohorts. HCV infection [hazards ratio (HR): 2.364; 95% CI: 1.337–4.181], male sex (HR: 1.823; 95% CI: 1.09–3.05) and age ⩾ 49 years (HR: 3.066; 95% CI: 1.56–6.026) were associated with incident gastric cancers. Among 887 (males: 68.4%; mean age: 66.5 ± 12.9 years, 2008–2018) hospitalized gastric cancer patients, HCV Ab-positive rate was 7.8%. None of the investigated factors exhibited different rates between HCV Ab-positive and Ab-negative patients. No HCV-core-positive cells were demonstrated in gastric cancer tissues.
Conclusions:
HCV infection, male sex and old age were risk factors for gastric cancer development. HCV-associated gastric cancer risk might be neither reversed by interferon-based therapy, nor associated with in situ HCV-core-related carcinogenesis.
Keywords: age, gastric cancer, HCV, sex
Introduction
Hepatitis C virus (HCV) is a human pathogen responsible for acute and chronic liver disease that infects an estimated 150 million individuals worldwide.1 In addition to hepatic complications such as steatosis, cirrhosis and hepatocellular carcinoma (HCC), HCV causes extrahepatic complications including mixed cryoglobulinemia,2 dyslipidemia, diabetes, obesity, cardiovascular events1 and neurological manifestations.3 More-over, several population-based, large case-control or cohort studies had demonstrated the associations between HCV infection and extrahepatic malignancies including lymphoid,4 head and heck,5 pancreas, lung, renal, rectal,6 esophageal, prostate, thyroid,7 breast, kidney and colon cancers.6 Since gastric cancer is the fourth most common incident cancer, the second most common cause of cancer death8 and most prevalent in East Asia,9 whether HCV infection accelerates the risk of gastric cancer thus draws our attention. Although one study based in the USA showed gastric cancer was not more frequent among HCV-infected patients compared with the general population,6 several evidences have directed the potential connections between HCV infection and gastric cancer. For example, a study using gene-set enrichment and signaling-pathway-impact analyses showed HCV infection is involved in gastric acid secretion;10 gastric-cancer-derived FU97 cells exhibited a much higher susceptibility to cell-culture-adapted HCV/JFH-2 infection than observed in Huh7 cells;11 among patients with HCC, the presence of HCV antibody (Ab) was positively correlated with the frequency of gastric cancer;12 HCV-ribonucleic acid (RNA) has been frequently detected in gastric mucosa,13,14 and a case report even showed spontaneous elimination of serum HCV-RNA after total gastrectomy for early gastric cancer in a patient with chronic hepatitis C (CHC).15 In addition, some infectious agents, including Helicobacter pylori (HP)16 and human papilloma virus,17 are involved in the development of gastric cancer, while the role of HCV infection in gastric cancer remains elusive.
Accordingly, we conducted a nationwide population-based cohort study to investigate the association of HCV infection with the development of gastric cancer in Taiwan, an Asian country endemic for HCV infection.18 The impact of HCV infection on the risk of gastric cancer was investigated by comparing the cumulative incidence of gastric cancers and gastric-cancer-associated mortalities among the HCV-infected subjects with and without anti-HCV therapy, and the participants without HCV infection. In parallel, the risk factors and the gastric HCV-core-protein expressions were surveyed in patients with gastric cancer in a tertiary referral center.
Methods
Taiwan National Health Insurance Research Database (TNHIRD) samples and measurements
This population-based retrospective cohort study used nation-level data, including the National Health Insurance (NHI) administrative database, the Cancer Registry Database, and the Death Registry Database of Taiwan. This mandatory, single-payer NHI program provides comprehensive coverage that includes ambulatory care, hospital services, laboratory tests, and prescription drugs. More than 99% of the population is enrolled in the program and about 92% of healthcare organizations are contracted with the NHI Administration. Given that Taiwan is a hyperendemic area for hepatitis B virus (HBV) infection, which is highly oncogenic, causes many hepatic complications19,20 and prominently biases the phenotype of HCV infection,21 those who were diagnosed with HBV infection in the observation period (2003–2012), or with any cancer or mortality occurring prior to 6 months after completing anti-HCV treatment (the baseline), which is the time to ensure therapeutic response,22 were excluded.
The HCV-treated cohort included subjects who had an HCV-RNA test and received ribavirin and pegylated interferon (Peg-IFN) in 2003–2012. Their first HCV test was assumed to be the index date of diagnosis. The baseline for the HCV-treated cohort was the date of 6 months after completing the combination therapy. Untreated HCV-infected patients were those who had HCV test (HCV Ab or HCV-RNA test; their first HCV test being the index date), were diagnosed with hepatitis C [International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes: 070.41, 070.44, 070.51, 070.54, 070.70, 070.71, V02.62], were prescribed hepatoprotective agents (silymarin, liver hydrolysate, choline bitartrate, or ursodeoxycholic acid), but did not receive any anti-HCV therapy (ribavirin or Peg-IFN). HCV-uninfected individuals had no HCV diagnosis or HCV tests, and received no hepatoprotective agents or anti-HCV therapy. The HCV-treated cohort was matched with untreated HCV-infected patients (HCV-untreated cohort) and with HCV-uninfected individuals (HCV-uninfected cohort) through a propensity-score-matching method indicating the probability of receiving the combination therapy, which was estimated by using a logistic model. The covariates in the model included sex (male, female), age (20–39, 40–49, 50–59, ⩾60), NHI registration location (city, township, rural area), the Charlson Comorbidity Index (CCI) score (0, 1, ⩾2),23 and year of the index date (2003–2006, 2007–2009, 2010–2012).This method was used to assure that the HCV-treated cohort and the selected counterparts were comparable in observed characteristics. The baselines for the HCV-untreated and HCV-uninfected cohorts were assigned according to the period from the index date to the baseline of their matched counterparts of the HCV-treated cohort. The index date of the HCV-uninfected individuals was the date of one of their physician visits randomly selected from their claims database. The matching process for the three cohorts is shown in Supplementary Figure 1.
Outcomes were defined as the development of gastric cancers [ICD-9-CM code: 151; International Classification of Diseases for Oncology (ICD-O-3): C16]. Types of cancers and dates of diagnosis were retrieved from the Cancer Registry Database. Participants were followed until the date of the event, death, or the end of follow up (31 December 2013), whichever came first. Dates of death were adopted from the Death Registry database. For the HCV-treated group, only the gastric cancer or mortality occurring 6 months after the complement of anti-HCV therapy were recorded.
10-year risk factor survey of gastric cancer in a tertiary referral center
The patients older than 18 years with a diagnosis of gastric cancer were consecutively recruited at a Taiwan tertiary referral center between July 2008 and June 2018. The baseline demographic data including sex, age, body mass index (BMI), as well as habit of smoking, alcohol and betel-nut chewing, HP infection, HCV Ab positivity, and hepatitis B surface antigen (HBsAg) positivity were recorded and analyzed by chart review. The diagnosis of HP infection was based upon the results of histology or rapid urease tests of the gastric wall, or the results of urea breath tests.24
Immunohistochemical staining of gastric cancers
The immunohistochemical (IHC) staining of the gastric cancers from patients with CHC and with past HCV infection were performed using paraffinized samples in the tissue bank of the tertiary referral center with cases of gastric cancers between July 2008 and June 2018. CHC was defined as the presence of documented HCV Abs and detectable HCV-RNA for >24 weeks. Past HCV infection was defined as positive HCV Ab but negative HCV-RNA. The IHC staining for HCV-core protein in gastric tissues was performed according to the manufacturer’s protocol. The liver tissue samples from the HCV-core transgenic mice served as positive control.25 In brief, the cells were permeabilized with 0.1% Triton-100 (Sigma-Aldrich corp., St. Louis, MO, USA); incubated with the anti-HCV core Ab (mouse monoclonal antibody, Virostat, Westbrook, ME, USA); washed, and then incubated with a secondary Ab (rabbit anti-mouse Ab) (Vector Laboratories, Burlingame, CA, USA). The intensity of protein expression was determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/). The pathology of all IHC staining was reviewed by gastrointestinal pathologists at participating sites who were blinded to study participation and cancer prediction.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science (SPSS package version 21, SPSS Inc., Chicago, IL, USA) or Statistical Analysis System (SAS version 9.4, SAS Institute Inc., Cary, NC, USA) software. Continuous variables were analyzed using a Student’s t test, and categorical variables were analyzed using a chi-square test or Fisher’s exact test, as appropriate. Nonparametric tests were applied where indicated. Kaplan–Meier or univariate Cox regression analyses were used to assess the relationship among baseline variables and the development of gastric cancers. Multivariate Cox regression models were used to assess the relationship between various dependent and independent variables by adjusting for all the independent variables with a p value < 0.1 in the univariate analyses. Binary logistic regression analysis was performed to access the variables that accounted for emergence of events. Cumulative incidences of outcomes were estimated and compared by using the modified Kaplan–Meier method and the Gray method, with death being a competing risk event.26 Subdistribution hazards models for competing risks,27 an extension of Cox proportional-hazards models, taking competing mortality into consideration, were used to estimate adjusted hazards ratio of developing gastric cancer, adjusting for age, sex, NHI registration location, the CCI score, year of the index date, and comorbid liver cirrhosis, chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), diabetes mellitus (DM), hypertension, dyslipidemia, cardiovascular events [including percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), myocardial infarction (MI), heart failure, cardiogenic shock, and peripheral vascular disease], stroke, HP infection, and HP therapy. Statistical significance was defined at the 5% level based on two-tailed tests of the null hypothesis.
Informed consent
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB No: 104-7005B). The need for consent was waived because the national-level data used in this study were de-identified by encrypting personal identification information.
Results
Baseline characteristics
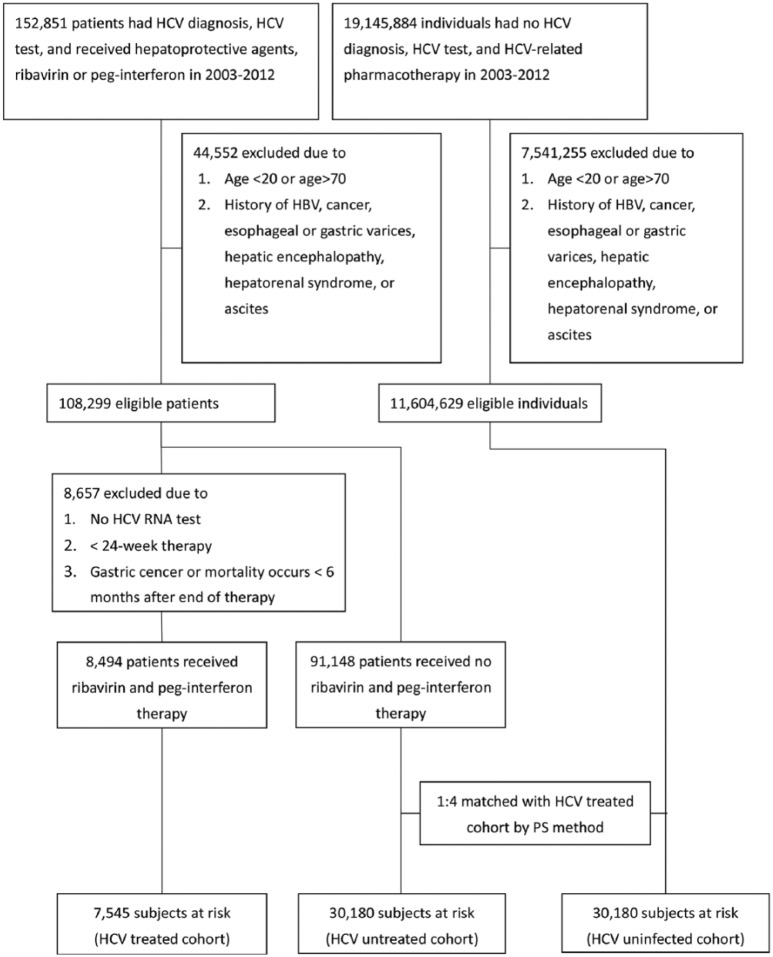
From 1 January 2003 to 31 December 2012, a total of 11,712,928 patients without HBV infection and any malignancies had been eligible in the current study (Figure 1). After matching with aforementioned baseline factors, 7545 patients with HCV infection and had ever received Peg-IFN with ribavirin for at least 6 months (HCV-treated cohort), 30,180 patients with HCV infection but had never been treated (HCV-untreated cohort), and 30,180 patients without HCV infection (HCV-uninfected cohort) had been enrolled. The three cohorts were matched in the propensity scores, did not differ in baseline demographic factors, residency, CCI and index year, although baseline comorbidities were not similar. Compared with HCV-untreated cohorts, the HCV-treated cohort had higher rates of cirrhosis, HP infection and HP therapy, but lower rates of baseline ESRD, DM, hypertension, dyslipidemia, cardiovascular events, and stroke. Compared with the HCV-uninfected cohort, the HCV-treated cohort had higher rates of cirrhosis, COPD, ESRD, hypertension, HP infection and HP therapy, but lower rates of dyslipidemia and stroke (Table 1). To lineate the HCV-associated complications, we compared the baseline factors between the HCV-infected cohort (which was a combination of the HCV-treated and HCV-untreated cohorts) and the HCV-uninfected cohort. The HCV-infected cohort had higher rates of all baseline comorbidities except dyslipidemia, than the HCV-uninfected cohort (Supplementary Table 1).
Figure 1.
Flow chart of TNHIRD study participant selection.
HBV, hepatitis B virus; HCV, hepatitis C virus; Peg-IFN, pegylated interferon; PS, propensity score; RNA, ribonucleic acid; TNHIRD, Taiwan National Health Insurance Research Database.
Table 1.
Baseline characteristics of the three HCV cohorts of TNHIRD.
| (1) |
(2) |
(3) |
p values |
|||
|---|---|---|---|---|---|---|
| HCV-treated | HCV-untreated | HCV-uninfected | (1)–(2) | (1)–(3) | (2)–(3) | |
| n | 7545 | 30,180 | 30,180 | |||
| Gender, n (%) | ||||||
| Male | 4023 (53.32) | 16,092 (53.32) | 16,092 (53.32) | 1 | 1 | 1 |
| Female | 3522 (46.68) | 14,088 (46.68) | 14,088 (46.68) | |||
| Age (mean ± SD), years | 49.68 ± 10.76 | 49.73 ± 11.25 | 49.12 ± 48.98 | |||
| Age range, years, n (%) | ||||||
| 20–39 | 1433 (18.99) | 5714 (18.93) | 5732 (18.99) | 0.9993 | 1 | 0.9973 |
| 40–49 | 2107 (27.93) | 8446 (27.99) | 8428 (27.93) | |||
| 50–59 | 2655 (35.19) | 10,620 (35.19) | 10,620 (35.19) | |||
| ⩾60 | 1350 (17.89) | 5400 (17.89) | 5400 (17.89) | |||
| Area, n (%) | ||||||
| City | 1798 (23.83) | 7174 (23.77) | 7192 (23.83) | 0.9933 | 1 | 0.9833 |
| Township | 2334 (30.93) | 9350 (30.98) | 9336 (30.93) | |||
| Rural area | 3413 (45.24) | 13,656 (45.25) | 13,652 (45.24) | |||
| CCI score, n (%) | ||||||
| 0 | 3815 (50.56) | 15,260 (50.56) | 15,260 (50.56) | 0.9995 | 1 | 0.9987 |
| 1 | 2610 (34.59) | 10,444 (34.61) | 10,440 (34.59) | |||
| ⩾2 | 1120 (14.84) | 4476 (14.83) | 4480 (14.84) | |||
| Index year, n (%) | ||||||
| 2003–2006 | 3683 (48.81) | 14,736 (48.83) | 14,732 (48.81) | 0.9989 | 1 | 0.9973 |
| 2007–2009 | 2464 (32.66) | 9859 (32.67) | 9856 (32.66) | |||
| 2010–2012 | 1398 (18.53) | 5585 (18.51) | 5592 (18.53) | |||
| Baseline factor, n (%) | ||||||
| Liver cirrhosis | 669 (8.87) | 1427 (4.73) | 13 (0.04) | <0.0001 | <0.0001 | <0.0001 |
| COPD | 846 (11.21) | 3422 (11.34) | 2851 (9.45) | 0.7575 | <0.0001 | <0.0001 |
| ESRD | 41 (0.54) | 635 (2.1) | 80 (0.27) | <0.0001 | 0.0001 | <0.0001 |
| DM | 1336 (17.71) | 6539 (21.67) | 5451 (18.06) | <0.0001 | 0.4733 | <0.0001 |
| Hypertension | 2155 (28.56) | 10,285 (34.08) | 7984 (26.45) | <0.0001 | 0.0002 | <0.0001 |
| Dyslipidemia | 913 (12.1) | 5964 (19.76) | 5354 (17.74) | <0.0001 | <0.0001 | <0.0001 |
| Cardiovascular events* | 175 (2.32) | 1218 (4.04) | 746 (2.47) | <0.0001 | 0.4429 | <0.0001 |
| Stroke | 225 (2.98) | 1410 (4.67) | 1460 (4.84) | <0.0001 | <0.0001 | 0.3389 |
| HP infection | 182 (2.41) | 544 (1.8) | 480 (1.59) | 0.0009 | <0.0001 | 0.047 |
| HP therapy | 55 (0.73) | 140 (0.46) | 116 (0.38) | 0.0053 | 0.0002 | 0.1496 |
Cardiovascular events including percutaneous coronary intervention, coronary artery bypass graft, myocardial infarction, heart failure, cardiogenic shock, and peripheral vascular disease.
CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; DM, diabetes; ESRD, end-stage renal disease; HCV, hepatitis C virus; HP, Helicobacter pylori; SD, standard deviation; TNHIRD, Taiwan National Health Insurance Research Database.
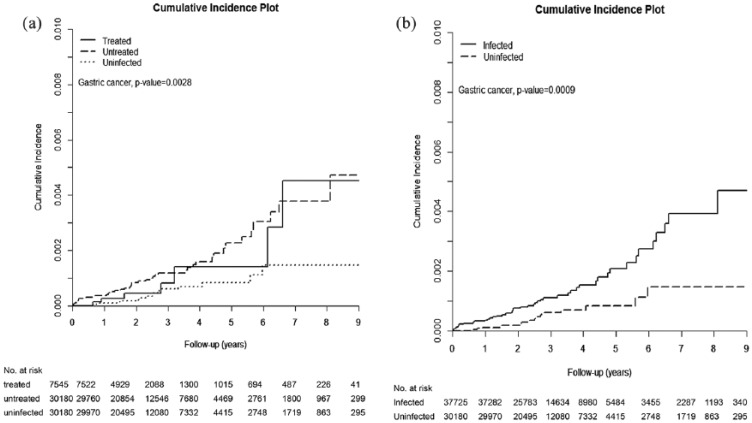
Cumulative incidences of gastric cancers and gastric cancer-associated mortality
The HCV-treated, untreated, and uninfected cohorts were followed up until death for a duration (mean ± standard deviation) of 2.899 ± 1.804 years, 3.165 ± 1.863 years, and 3.124 ± 1.844 years, respectively, with the longest observation of 9 years. The incidences of gastric cancer were lowest in the HCV-uninfected cohort, as gastric cancer occurred cumulatively in 0.452% [95% confidence interval (CI): 0.149–1.136%], 0.472% (95% CI: 0.274–0.776%), and 0.146% (95% CI: 0.071–0.280%) of the HCV-treated, -untreated, and -uninfected cohorts, respectively [Table 2, Figure 2(a)]. However, antiviral treatment was not associated with an attenuated risk of gastric cancer, as the cumulative incidence of gastric cancer was indifferent between the treated and untreated cohorts (p = 0.4446). We thus pooled the data of the HCV-treated and HCV-untreated cohorts to form an HCV-infected cohort and compared the data between the HCV-infected and HCV-uninfected cohorts. The HCV-uninfected cohort had lower risk of incident gastric cancer than the HCV-infected cohort [Table 3, Figure 2(b)]. Likewise, the cumulative incidence of gastric-cancer-associated mortality was significantly lowest in the HCV-uninfected cohort (0.007%; 95% CI: 0.001–0.024%) as compared with the HCV-treated (0.177 %; 95% CI: 0.018–0.953%) and HCV-untreated cohorts (0.16%; 95% CI: 0.073–0.325%; p = 0.0056; Table 4).
Table 2.
Comparison of cumulative incidence of gastric cancers among three HCV cohorts.
| Gastric cancer | HCV-treated | HCV-untreated | HCV-uninfected | p value |
|---|---|---|---|---|
| n | 7545 | 30,180 | 30,180 | |
| Follow up (years), mean ± SD | 2.899 ± 1.804 | 3.165 ± 1.863 | 3.124 ± 1.844 | |
| Event number, n (%) | 7 (0.09) | 44 (0.15) | 16 (0.05) | |
| Competing mortality, n (%) | 127 (1.68) | 1830 (6.06) | 635 (2.10) | |
| Cumulative incidence, % (95% CI) | 0.452 (0.149–1.13) | 0.472 (0.274–0.776) | 0.146 (0.071–0.280) | 0.0028 |
Comparison of cumulative incidence of gastric cancers among HCV-treated, HCV-untreated and HCV-uninfected cohorts.
CI, confidence interval; HCV, hepatitis C virus; SD, standard deviation.
Figure 2.
Cumulative incidence of gastric cancers among three TNHIRD cohorts, including HCV-treated, HCV-untreated and HCV-uninfected cohorts.
Cumulative incidence of gastric cancers among the three TNHIRD cohorts, including HCV-treated, HCV-untreated and HCV-uninfected cohorts (a) and between two TNHIRD cohorts including HCV-infected (pooled data of HCV-treated and HCV-untreated cohorts) and HCV-uninfected cohorts (b).
HCV, hepatitis C virus; TNHIRD, Taiwan National Health Insurance Research Database.
Table 3.
Comparison of the cumulative incidence of gastric cancer between the HCV-infected and HCV-uninfected cohorts.
| Gastric cancer | HCV-infected | HCV-uninfected | p value |
|---|---|---|---|
| Number | 37,725 | 30,180 | |
| Mean years’ follow up ± SD | 3.11 ± 1.85 | 3.12 ± 1.84 | |
| Event number, n (%) | 51 (0.14) | 16 (0.05) | |
| Competing mortality, n (%) | 1957 (5.19) | 635 (2.10) | |
| Cumulative incidence, % (95% CI) | 0.470 (0.289–0.736) | 0.146 (0.071–0.280) | 0.0009 |
The HCV-infected cohort comprises the HCV-treated and HCV-untreated cohorts.
CI, confidence interval; HCV, hepatitis C virus; SD, standard deviation.
Table 4.
Comparison of the cumulative incidence of gastric-cancer-associated mortality among three HCV cohorts.
| Gastric cancer | HCV-treated | HCV-untreated | HCV-uninfected | p value |
|---|---|---|---|---|
| Number | 7545 | 30,180 | 30,180 | |
| Mean years’ follow up ± SD | 2.901 ± 1.804 | 3.167 ± 1.864 | 3.124 ± 1.843 | |
| Event number, n (%) | 1 (0.01) | 15 (0.05) | 2 (0.01) | |
| Competing mortality, n (%) | 127 (1.68) | 1837 (6.09) | 636 (2.11) | |
| Cumulative incidence, % (95% CI) | 0.177 (0.018–0.953) | 0.16 (0.073–0.325) | 0.007 (0.001–0.024) | 0.0056 |
Comparison of cumulative incidence of gastric-cancer-associated mortality among HCV-treated, HCV-untreated, and HCV-uninfected cohorts.
CI, confidence interval; HCV, hepatitis C virus; SD, standard deviation.
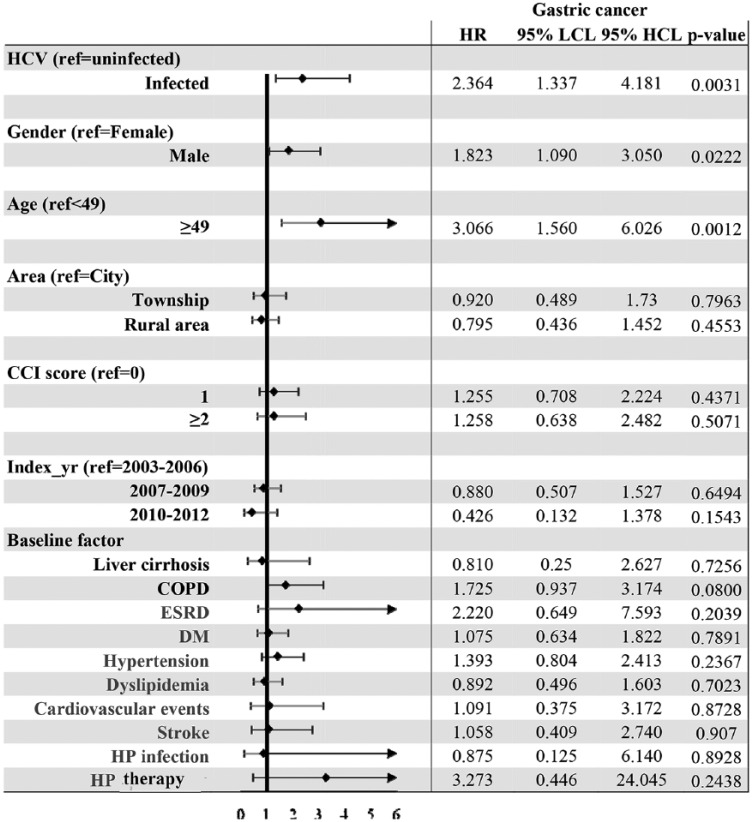
Factors associated with the cumulative incidences of gastric cancer
Subdistribution hazards models showed HCV infection [hazards ratio (HR): 2.364; 95% CI: 1.337–4.181], male sex (HR: 1.823; 95% CI: 1.09–3.05) and age ⩾ 49 years (HR: 3.066; 95% CI: 1.56–6.026) were independent factors associated with the development of gastric cancers (Figure 3). Since HP infection plays a significant role in gastric cancer,28 we undertook several subanalyses to focus on HP status: (a) one multivariate analysis with HP infection survey [HCV-treated, HCV-untreated and HCV-uninfected cohorts, i.e. three cohorts; HCV infection and HCV-uninfected cohorts, i.e. two cohorts; Supplementary Figure 2(a) and (b)] and the other with HP therapy survey [three and two cohorts; Supplementary Figure 3(a) and (b)]; (b) multivariate analyses of three cohorts with various HP-infection-status surveys (no HP infection, and HP infection with and without therapy; Supplementary Figure 4). However, none of the analyses showed any connection between HP infection and the development of gastric cancer.
Figure 3.
Forest plot of factors associated with incident gastric cancers in the TNHIRD cohorts.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes; ESRD, end-stage renal disease; HCL, higher confidence limit; HCV, hepatitis C virus; HP, Helicobacter pylori; HR, hazards ratio; LCL, lower confidence limit; TNHIRD, Taiwan National Health Insurance Research Database.
Risk factor survey in hospitalized gastric cancer cases
Among the 887 hospitalized gastric cancer patients (mean age: 66.5 ± 12.9 years), the males (n = 605, 68.4%) accounted for the majority. The rates of HCV Ab-positive, HBsAg-positive and HP-positive patients, and those with habits of smoking, alcohol and betel-nut chewing of the enrolled patients were 7.8%, 14.9%, 23.4%, 33.9%, 30.7% and 7.2%, respectively. None of the rates of the investigated factors were different between the HCV Ab-positive and HCV Ab-negative patients (Supplementary Table 2).
Among the investigated factors, male sex was correlated with age [Pearson correlation coefficient (PCC): 0.09, p = 0.007], habits of smoking (PCC: 0.406, p < 0.001), alcohol (PCC: 0.337, p < 0.01), and betel-nut chewing (PCC: 0.141; p < 0.001). The male patients were older (67.26 ± 12.1 versus 64.74 ± 14.1, p = 0.011), had higher smoking-habit rates (47.4% versus 6.0%, p < 0.001), alcohol intake (38.2% versus 5.7%, p < 0.001) and betel-nut chewing (9.6% versus 1.8%, p < 0.001) than the female patients. The HCV Ab positivity was not associated with any of the investigated factors.
IHC studies
No HCV-core-positive cells could be demonstrated in the gastric cancer tissue, for both CHC and past HCV-infected patients (Supplementary Figure 5).
Discussion
The key results of the current study are: (a) the incidence of gastric cancer was lowest in the HCV-uninfected cohort and indifferent between the HCV-treated and HCV-untreated cohorts; (b) HCV infection, male sex and age ⩾ 49 years were factors associated with the development of gastric cancers; (c) no HCV-core-positive cells could be demonstrated in the gastric cancer tissue.
The higher rate of baseline cirrhosis in the HCV-treated versus the HCV-untreated cohorts of TNHIRD coincided with the fact that only patients with fibrosis score ⩾ 1 were reimbursed for anti-HCV therapy,29 and the other different variables between these two cohorts highlight that the patients with comorbidities ineligible for the IFN-based therapy had been excluded for therapy. The differences in the baseline variables between the HCV-infected and HCV-uninfected cohorts were consistent with the fact that HCV infection elicits many cardiometabolic events.1 Besides, the higher rate of HP infection in the HCV-infected cohort versus the HCV-uninfected cohort echoed the strong association between HP and CHC, albeit the mechanism being uncertain.30 Thus, the baseline comparisons of the three cohorts confirmed the reliability of the TNHIRD data.
Of note, consistent with the fact that the HCV-uninfected cohort had the lowest cumulative incidences of gastric cancer among the three cohorts, HCV infection was a positive factor for the development of gastric cancer. By contrast, although HP infection has been established as a risk factor for gastric cancer28 and the TNHIRD study confirmed the high prevalence of HP infection in the HCV-infected cohort as mentioned above, all the associated analyses excluded HP infection being the risk factor for gastric cancer among the three cohorts. Given that the HCV-infected cohort had a higher HP therapy rate than the HCV-uninfected cohort, it is likely that the HP-associated gastric carcinogenesis had been blunted by the effective anti-HP therapy. On the other hand, the hospital-based study had comprehensively surveyed the potential risk factors for gastric cancer. Compared with the general population in Taiwan, the prevalence rates of HP and HBV infections, and habits of alcohol and betel chewing were either similar or lower in the patients with gastric cancer, regardless of sex effects.31–34 Thus, the hospital-based study did not support the aforementioned factors to be strong risk factors for gastric cancer. However, both the HCV Ab-positive (7.8%) and smoking rates (34.3%) of the hospitalized gastric cancer patients were higher than that of the general population (2% of HCV viremia35 and 2.7% of estimated HCV Ab positivity,36 and 14.5% of smoking rate37) in Taiwan. Both HCV infection and smoking are thus potential risk factors for gastric cancers based on the hospital study. Moreover, HCV infection was not associated with smoking, and none of the investigated factors exhibited different rates between the HCV Ab-positive and HCV Ab-negative patients, the impact of HCV infection on the development of gastric cancer might thus be independent. Since we did not show any HCV-core-positive cells in the gastric cancer tissue, no evidence of HCV replication in stomach tissue could thus be provided in the current study. Certainly, this cannot preclude the possibility of the carcinogenic role of direct invasion of HCV in the stomach, since the HCV protein expression in the host cells are hardly visible,38 despite HCV-RNA being frequently detected in gastric mucosa.13,14 However, compared with local effect,3 systemic parameters such as endocrine effects or a heightened immune reaction subsequent to HCV infection3 are probably more likely the culprits of accelerated risk of gastric cancer in patients with HCV infection. In addition, given that the cumulative incidence of gastric cancer between the HCV-treated and untreated cohorts were indifferent, the HCV-associated risk in gastric cancer development seems to not be attenuated by viral clearance once the process of gastric carcinogenesis has been initiated. Although most HCV infections are currently curable with potent, direct-acting antiviral agents,39 special caution is still warranted for the incident gastric cancer in patients with HCV infection who have achieved a sustained virological response following anti-HCV therapy.
In addition to HCV infection, male sex and age ⩾ 49 years were risk factors for gastric cancer development based on the THNIRD study. The associations between gastric cancer and male sex or old age has been consistently reported40,41 and strengthen the reliability of our results. Moreover, the hospital-based study confirmed that patients with gastric cancer were male dominant (68.4%) and old (mean age: 66.5 years). Estrogens may protect against the development of gastric cancer42 and decrease the risk of gastric cancer in females.43 On the other hand, environmental or occupational exposures may play a role in the male-dominant trend of gastric cancer, as men are more likely to smoke tobacco products.44 The results of our hospital-based study also confirm the high correlation of male sex with the habits of smoking, alcohol and betel-nut chewing.
There are several limitations recognized in the current study. First, given that the TNHIRD did not contain direct laboratory results, we could not clarify the correlation of sustained virological response (SVR) to anti-HCV therapy with gastric cancer. However, we are confident of the antiviral efficacy in the HCV-treated cohort since IFN-based therapy for HCV infection generally achieves an SVR rate ranged from 70% to 90% in Taiwan,22,45 where a favorable genetic variation in interleukin-28B is prevalent.46,47 Second, there were likely some undiagnosed HCV-infected patients in the HCV-uninfected cohort (who never received HCV tests). However, since the reimbursement of anti-HCV therapy in Taiwan is nationwide, and only 2.0–2.7%35,36 of the Taiwanese were supposed to be HCV positive, the rate of undiagnosed HCV infection of the HCV-uninfected cohort should be negligible and might not bias our results. Third, information of important risk factors of gastric cancers including smoking and alcohol consumption were lacking in the TNHIRD, and the impact of these risk factors on gastric cancer cannot be assessed, although our hospital-based data provided some clues in linking smoking and gastric cancer. Fourth, variations in physician visits among different individuals of the HCV-uninfected cohort were not considered, and whether different underlying disease affects the statistical results remains elusive. Fifth, the precise mechanism of the increased risk of gastric cancer in the patients with HCV infection was undetermined. Future prospective studies in other independent cohorts with identifiable SVR following anti-HCV therapy, comprehensive surveys of risk factors, considered stratification of patient subpopulation, and sophisticated molecular or animal investigations are required to elucidate the fundamental mechanisms underlying the findings described here.
Taken together, HCV infection, male sex and old age were positive factors for the development of gastric cancer. However, the HCV-associated risk for gastric cancer development might be neither reversed by IFN-based anti-HCV therapy, nor associated with in situ HCV-core-related carcinogenesis.
Supplemental Material
Supplemental material, Supplementary_figure_1_matching_process_20190416__3_ for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Figure_2 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Figure_3 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Figure_4 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Figure_5 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_Tables_1_and_2_042819 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Footnotes
Funding: This study was supported by grants from the Chang Gung Medical Research Program (CIRPD1D0032, CMRPG3F0473, CRRPG3F0013 and CMRPG3I0411) and the National Science Council, Taiwan (106-2314-B-182-041-MY2). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Ming-Ling Chang  https://orcid.org/0000-0003-4902-4401
https://orcid.org/0000-0003-4902-4401
Contributor Information
Chun-Wei Chen, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Jur-Shan Cheng, Clinical Informatics and Medical Statistics Research Center, Chang Gung University, Taoyuan, Taiwan; Department of Emergency Medicine, Chang Gung Memorial Hospital, Keelung, Taiwan.
Tai-Di Chen, Department of Anatomic Pathology, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Puo-Hsien Le, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Hsin-Ping Ku, Clinical Informatics and Medical Statistics Research Center, Chang Gung University, Taoyuan, Taiwan.
Ming-Ling Chang, Liver Research Center, Division of Hepatology, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, No 5, Fu Hsing Street, Kuei Shan, Taoyuan, 33305, Taiwan.
References
- 1. Chang ML. Metabolic alterations and hepatitis C: from bench to bedside. World J Gastroenterol 2016; 22: 1461–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramos-Casals M, Stone JH, Cid MC, et al. The cryoglobulinaemias. Lancet 2012; 379: 348–360. [DOI] [PubMed] [Google Scholar]
- 3. Negro F, Forton D, Craxì A, et al. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 2015; 149: 1345–1360. [DOI] [PubMed] [Google Scholar]
- 4. Su TH, Liu CJ, Tseng TC, et al. Hepatitis C viral infection increases the risk of lymphoid-neoplasms: a population-based cohort study. Hepatology 2016; 63: 721–730. [DOI] [PubMed] [Google Scholar]
- 5. Mahale P, Sturgis EM, Tweardy DJ, et al. Association between hepatitis C virus and head and neck cancers. J Natl Cancer Inst 2016; 108: djw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allison RD, Tong X, Moorman AC, et al. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J Hepatol 2015; 63: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis 2012; 206: 469–477. [DOI] [PubMed] [Google Scholar]
- 8. Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010; 19: 1893–1907. [DOI] [PubMed] [Google Scholar]
- 9. Forman D, Burley V. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 2006; 20: 633–649. [DOI] [PubMed] [Google Scholar]
- 10. Wu ZY, Li JR, Huang MH, et al. Internal driving factors leading to extrahepatic manifestation of the hepatitis C virus infection. Int J Mol Med 2017; 40: 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiokawa M, Fukuhara T, Ono C, et al. Novel permissive cell lines for complete propagation of hepatitis C virus. J Virol 2014; 88: 5578–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kai K, Miyoshi A, Kitahara K, et al. Analysis of extrahepatic multiple primary malignancies in patients with hepatocellular carcinoma according to viral infection status. Int J Hepatol 2012; 2012: 495950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tursi A, Brandimante G, Chiarelli F, et al. Detection of HCV RNA in gastric mucosa-associated lymphoid tissue by in situ hybridization: evidence of a new extrahepatic localization of HCV with increased risk of gastric malt lymphoma. Am J Gastroenterol 2002; 97: 1802–1806. [DOI] [PubMed] [Google Scholar]
- 14. De Vita S, De Re V, Sansonno D, et al. Gastric mucosa as an additional extrahepatic localization of hepatitis C virus: viral detection in gastric low-grade lymphoma associated with autoimmune disease and in chronic gastritis. Hepatology 2000; 31: 182–189. [DOI] [PubMed] [Google Scholar]
- 15. Yoshikawa M, Morimoto Y, Shiroi A, et al. Spontaneous elimination of serum HCV-RNA after total gastrectomy for early gastric cancer in a patient with chronic hepatitis C. Am J Gastroenterol 2001; 96: 922–923. [DOI] [PubMed] [Google Scholar]
- 16. De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13: 607–615. [DOI] [PubMed] [Google Scholar]
- 17. Zeng ZM, Luo FF, Zou LX, et al. Human apillomavirus as a potential risk factor for gastric cancer: a meta-analysis of 1,917 cases. Onco Targets Ther 2016; 9: 7105–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang SW, Cheng ML, Shiao MS, et al. Recovery of lipid metabolic alterations in hepatitis C patients after viral clearance: incomplete restoration with accelerated ω-oxidation. J Clin Lipidol 2018; 12: 756–766. [DOI] [PubMed] [Google Scholar]
- 19. Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol 2019, 16: 57–73. [DOI] [PubMed] [Google Scholar]
- 20. Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol 2014; 61: 1407–1417. [DOI] [PubMed] [Google Scholar]
- 21. Chang ML, Lin YJ, Chang CJ, et al. Occult and overt HBV co-infections independently predict postoperative prognosis in HCV-associated hepatocellular carcinoma. PLoS One 2013; 8: e64891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu CH, Liu CJ, Lin CL, et al. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C virus genotype 1 infection: a multicenter, randomized controlled trial. Clin Infect Dis 2008; 47: 1260–1269. [DOI] [PubMed] [Google Scholar]
- 23. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J ClinEpidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 24. Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - recent developments in diagnosis. World J Gastroenterol 2014; 20: 9299–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang ML, Yeh HC, Tsou YK, et al. HCV core-induced nonobese hepatic steatosis is associated with hypoadiponectinemia and is ameliorated by adiponectin administration. Obesity (Silver Spring) 2012; 20: 1474–1480. [DOI] [PubMed] [Google Scholar]
- 26. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1998; 16: 1141–1154. [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 28. Conteduca V, Sansonno D, Lauletta G, et al. H. pylori infection and gastric cancer: state of the art (review). Int J Oncol 2013; 42: 5–18. [DOI] [PubMed] [Google Scholar]
- 29. Website for Ministry of Health and Welfare, Taiwan, https://www.nhi.gov.tw/Content_List.aspx?n=A4EFF6CD1C4891CA&topn=3FC7D09599D25979.
- 30. Wang J, Li WT, Zheng YX, et al. The association between helicobacter pylori infection and chronic hepatitis C: a meta-analysis and trial sequential analysis. Gastroenterol Res Pract 2016; 2016: 8780695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu YC, Yeh CC, Chen RY, et al. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ 2018; 6: e4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin JT, Wang JT, Wang TH, et al. Helicobacter pylori infection in a randomly selected population, healthy volunteers, and patients with gastric ulcer and gastric adenocarcinoma. A seroprevalence study in Taiwan. Scand J Gastroenterol 1993; 28: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 33. Website for Ministry of Health and Welfare, Taiwan, http://www.mohw.gov.tw/dl-13429–288bc79a-e335–4e5c-9657-f63628dbd05f.html.
- 34. Website for Ministry of Health and Welfare, Taiwan, http://www.mohw.gov.tw/dl-13430-ec859f62-a27c-444e-b452–00d417f55fa5.html.
- 35. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2: 161–176. [DOI] [PubMed] [Google Scholar]
- 36. Cheng YL, Wang YC, Lan KH, et al. Anti-hepatitis C virus seropositivity is not associated with metabolic syndrome irrespective of age, gender and fibrosis. Ann Hepatol 2015; 14: 181–189. [PubMed] [Google Scholar]
- 37. http://www.mohw.gov.tw/dl-13428-be854fc8-ddc3–49d5-b16d-a36a7309990c.html.
- 38. Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol 2014; 61: S3–S13. [DOI] [PubMed] [Google Scholar]
- 39. Vermehren J, Park JS, Jacobson I, et al. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J Hepatol 2018; 69: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 40. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 41. Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute, 2011, p.19. [Google Scholar]
- 42. Sheh A, Ge Z, Parry NMA, et al. 17β-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in helicobacter pylori–infected INS-GAS male mice. Cancer Prev Res 2011; 4: 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Derakhshan MH, Liptrot S, Paul J, et al. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut 2009; 58: 16–23. [DOI] [PubMed] [Google Scholar]
- 44. Freedman N, Derakhshan M, Abnet C, et al. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women. Eur J Cancer 2010; 46: 2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu ML, Dai CY, Huang JF, et al. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut 2007; 56: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461: 399–401. [DOI] [PubMed] [Google Scholar]
- 47. Yu ML, Huang CF, Huang JF, et al. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology 2011; 53: 7–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_figure_1_matching_process_20190416__3_ for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Figure_2 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Figure_3 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Figure_4 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Figure_5 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Tables_1_and_2_042819 for The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: a joint study of hospital-based cases and nationwide population-based cohorts by Chun-Wei Chen, Jur-Shan Cheng, Tai-Di Chen, Puo-Hsien Le, Hsin-Ping Ku and Ming-Ling Chang in Therapeutic Advances in Gastroenterology