Abstract
Background:
Cladribine tablets 3.5 mg/kg cumulative over 2 years (CT3.5) had significant clinical/imaging effects in patients with clinically isolated syndrome (CIS; ORACLE-MS) or relapsing-remitting MS (RRMS; CLARITY and CLARITY Extension). This analysis compared the effect of cladribine tablets on the dynamics of immune cell reduction and reconstitution in ORACLE-MS, CLARITY, and CLARITY Extension during the first year of treatment (i.e. the first course of CT1.75) in patients randomized to CT3.5.
Methods:
Lymphocyte subtypes were analyzed using multiparameter flow cytometry. Changes in cell counts and relative proportions of lymphocytes were evaluated at weeks 5, 13, 24, and 48.
Results:
Across studies, consistent and comparable selective kinetics of immune cell populations occurred following the first treatment year with CT. A rapid reduction in CD16+/CD56+ cells (week 5 nadir), a more marked reduction in CD19+ B cells (week 13 nadir), and a less-pronounced effect on CD4+ (week 13 nadir) and CD8+ T cells (week 24 nadir) was shown. There was little effect on neutrophils or monocytes. Lymphocyte recovery began after treatment with CT3.5. Regarding relative proportions of naïve and memory T-cell subtypes in ORACLE-MS, the proportion of naïve-like naturally occurring T-regulatory cells (nTregs) decreased, and the proportion of memory-like nTregs increased, relative to total CD4+ T cells.
Conclusions:
CT3.5 has comparable effects on the immune systems of patients with CIS or RRMS. The pronounced reduction and recovery dynamics of CD19+ B cells and relative changes in the proportion of some immune cell subtypes may underlie the clinical effects of CT3.5.
Keywords: cladribine tablets, CLARITY, CLARITY Extension, lymphocytes, multiple sclerosis, ORACLE-MS, subsets
Introduction
Advances over the last few decades have demonstrated the autoimmune nature of multiple sclerosis (MS) and the role of immune cell subsets in disease development and progression.1 Much of what is known about the involvement of the immune system in MS is derived from studies in patients with moderately advanced relapsing–remitting multiple sclerosis (RRMS). In contrast, less is known about the role of the immune system in patients with clinically isolated syndrome (CIS), the earliest clinical MS phenotype. While it is conceivable that there are no or only minor biological differences between the initial and subsequent clinical MS events, it is relevant to study the involvement of immune cell types in CIS in order to understand their contribution in conversion from CIS to RRMS.2 Variation in clinical response may potentially indicate differences in immune cell populations in CIS and RRMS. In patients with CIS, early treatment with disease-modifying therapies (DMTs) has been shown to delay the time of conversion to clinically definite MS and to slow the progression of disability.3 In patients with RRMS, earlier treatment, as well as a longer duration of treatment, has been associated with delayed conversion to secondary progressive MS.4 It should be noted that the terms CIS and first clinical demyelinating event (used in some publications) are typically used interchangeably and describe the same presentation. Therefore, these terms are considered equivalent herein.
In RRMS, the involvement of immune cell subtypes has been studied extensively, including the role of B and T lymphocytes and subtypes.5 In patients with CIS, the role of immune cell subtypes has been investigated to a lesser degree, although decreases in CD4+ T lymphocytes and CD19+ B lymphocytes have been associated with disease worsening.6 Assessment of the speed and nature of lymphocyte reduction and reconstitution may help to further shed light on the role of specific immune cell subpopulations on disease activity (magnetic resonance imaging [MRI] activity, relapses, or disability worsening). Immune reconstitution therapy (IRT) is characterized by transient effects on lymphocyte counts or lymphocyte subtype populations. This is followed by a period of recovering cell numbers with reconstitution of immune function and durable efficacy beyond the initial immunosuppression.7 An IRT that selectively targets B and T cells, without significant impact on innate immunity or induction of secondary autoimmunity through impaired immune homeostasis, would be of considerable interest for the treatment of MS.7,8
Cladribine tablets 10 mg (MAVENCLAD) were recently approved for the treatment of active RRMS in Europe and other countries and regions. The approved indication in Europe is a cumulative dose of cladribine tablets 3.5 mg/kg (CT3.5) over 2 years for the treatment of adult patients with highly active relapsing MS as defined by clinical or imaging features. In patients with active RRMS, the CLARITY study showed that 2 years’ treatment, given as a first short course of CT1.75 at the beginning of the first year (Year 1) followed by a second short course of CT1.75 at the beginning of the second year (Year 2) with a cumulative dose of CT3.5, was effective on clinical and neurological outcomes.9–11 Durable clinical and imaging benefits were observed in the 2-year CLARITY Extension study, in the group of patients who received no additional active treatment following 2 years of treatment in CLARITY.12,13 Cladribine tablets are not approved for patients with CIS. In the 2-year ORACLE-MS study, treatment with CT3.5 significantly delayed time to conversion to clinically definite MS in patients presenting with CIS.14 Subgroup analyses in patients retrospectively classified according to whether or not they met the 2010 McDonald criteria for MS also demonstrated efficacy, consistent with the main study findings.15
The effects of CT3.5 in CLARITY have been assessed for several immune cell types, including mature CD19+ B cells, as well as several naïve and memory CD4+ and CD8+ T-lymphocyte subtypes and CD16+/56+ natural killer (NK) cells. That analysis found that CT treatment produced marked reductions in B cells from baseline (70–90%), and more modest reductions in T cells (up to 45%) and NK cells (47%).16 Naïve T cells and memory T cells displayed similar reduction and recovery kinetics, albeit with a marginally more modest effect on the memory T cells (CD45RA-) than the naïve T cells (CD45RA+).16 Furthermore, a recent study with parenteral cladribine has suggested that the large reductions in B cells was characterized by marked reductions in memory B cells.17
Investigating the effects of CT treatment in ORACLE-MS provides an opportunity to analyze the dynamics of reduction and reconstitution of immune cell subtypes in patients with CIS and to compare those dynamics with an RRMS population. The objective of the present analysis is to compare reduction and reconstitution of CD19+ B cells, memory and naïve CD4+ and CD8+ T cells, NK cells, neutrophils, and monocytes after the first of the two annual treatment courses of CT in patients with CIS versus patients with established MS receiving placebo or a first course of CT3.5 as part of one of the three clinical trials (CLARITY, CLARITY Extension, and ORACLE-MS). In addition, the analysis assessed an extended surface marker panel of T-lymphocyte subtypes in ORACLE-MS using fluorescence-activated cell sorting (FACS). This panel includes central and effector memory CD4+ cells, Th1-type T-helper cells, and naïve and memory naturally occurring regulatory T cells (nTregs), which have not previously been assessed in patients with CIS treated with cladribine tablets.
Methods
ORACLE-MS, CLARITY, and CLARITY Extension were undertaken in compliance with the Declaration of Helsinki and standards of Good Clinical Practice according to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Independent ethics committees approved the studies and all patients gave written informed consent before screening.
ORACLE-MS
The phase III ORACLE-MS study (ClinicalTrials.gov identifier: NCT00725985) has been described previously. Briefly, patients with CIS (n = 617) were randomized (1:1:1) to 96 weeks (2 years) of double-blind treatment with placebo, a cumulative dose of CT3.5 or CT 5.25 mg/kg bodyweight (CT5.25).14 In the first year of the study, patients randomized to the CT3.5 treatment arm received two short (4 or 5 days) weekly treatments. The two weekly treatments were repeated in the second year of the study. Therefore, patients received a total of 1.75 mg/kg of cladribine tablets in the first year (year 1). The first weekly treatment was at the beginning of the first month of the double-blind period, and the second weekly treatment was at the start of the second month (this is consistent with the approved dosing regimen in the Summary of Product Characteristics).18 The ORACLE-MS safety analysis set included all randomized patients who received at least one dose of study medication and had at least one safety assessment during the initial treatment period.
CLARITY and CLARITY Extension
In the CLARITY study (ClinicalTrials.gov identifier: NCT00213135), patients with RRMS (n = 1326) were randomized (1:1:1) to receive either placebo or a cumulative dose of CT3.5 or CT5.25 over 2 years. Patients who completed CLARITY were eligible to enter the CLARITY Extension study (ClinicalTrials.gov identifier: NCT00641537; n = 806), in which patients on placebo during the CLARITY study were assigned CT3.5 for a further 2 years. Patients on CT during the CLARITY study were randomized to CT3.5 or placebo for the same duration. These studies have been described previously, including primary safety and efficacy outcomes.9,10,12,13,19,20 In each of these studies, the dosing schedule was similar to that used in ORACLE-MS.
Lymphocytes and myeloid cells
Counts of lymphocytes, neutrophils, and monocytes were assessed centrally in all randomized patients in ORACLE-MS, CLARITY, and CLARITY Extension. These analyses are of data collected during the first 48 weeks of each study (i.e. patients had received only the first year of the 2-year treatment course). Samples were collected at baseline and weeks 2, 5, 9, 13, 16, 24, 36, 44, and 48 in ORACLE-MS, and at weeks 5, 9, 13, 16, 20, 24, 36, 40, and 48 in CLARITY and CLARITY Extension.
Lymphocyte surface marker (LSM) analyses were restricted to patients randomized to treatment with placebo or CT3.5 who had signed an informed consent form specifically for the LSM analyses. Certain patients and time periods were excluded from the analyses due to study-site-related requirements, including the need for specific informed consent. Data gathered after week 48 in each study (to include the second year of the 2-year treatment course) are not included in the present analyses.
LSM analyses
The LSM set comprised all randomized patients who provided informed consent, received at least one dose of study drug (cladribine tablets or placebo) and who had at least one LSM assessment between baseline and the end of Year 1 (i.e. the Week 48 visit). Analyses were performed centrally, using the as-treated principle.
In the ORACLE-MS study an extended panel of LSM was assessed via FACS. The markers included in the analysis of each of the three studies, and the extended panel of markers analyzed in ORACLE-MS only are listed in Supplementary Table 1. Analysis of LSM was carried out at day 1 (baseline) and weeks 5, 13, 24, and 48. For each LSM, the median cell count, change from baseline, and the percentage change was summarized descriptively at each time point. The cell count for each marker was also assessed at absolute lymphocyte count (ALC) nadir. This was defined for each patient as the lowest post-baseline value relative to their baseline ALC value up to the end of year 1. The change from baseline at ALC nadir (absolute cell numbers, change, and percentage change) was summarized descriptively.
In addition to assessing changes in counts of lymphocyte subtypes, proportions of CD4+ and CD8+ T cells were calculated relative to ALC at each time point. Median proportions of CD4+ T-cell subtypes were measured relative to total CD4+ T-cell counts. Exceptions to this were naïve- and memory-like nTregs, which were measured relative to total nTreg counts.
Further detail on methods used for immunophenotyping is provided in the Supplementary Material. International reference ranges for lymphocyte subpopulations where available and as reported by the central laboratories are provided in Supplementary Table 2A and B.
Statistical analyses
All analyses of the year 1 LSM data should be considered as exploratory. Year 2 analyses were not conducted. The results are presented with no formal statistical testing since the clinical studies were not powered to detect changes between each timepoint for each lymphocyte subset. Continuous variables were summarized using descriptive statistics, including number of patients, number of patients with nonmissing values, mean, standard deviation, median, 25th percentile–75th percentile (Q1–Q3), minimum, and maximum. Qualitative variables were summarized by counts and percentages. Unless otherwise stated, the calculation of proportions was based on the number of patients in the analysis set of interest.
Results
Patients
In ORACLE-MS, a total of 88 patients were included in the LSM analysis set: 47 received placebo and 41 received CT3.5. In the LSM set, the mean age was 31.7 years and 71.6% of patients were female; in the overall ORACLE-MS population, the mean age was 31.9 years and 65% were female.14 The total lymphocyte count in the LSM analysis set (see below) was similar to the overall ORACLE-MS population (data not shown). The LSM analyses of CLARITY and CLARITY Extension included a total of 281 patients. In the CLARITY LSM set, 93 patients received placebo and 97 received CT3.5 (for a total of 190 patients); in the CLARITY Extension LSM set, 136 patients received CT3.5 (these patients had previously been treated with placebo during CLARITY). A total of 45 patients are common to the CLARITY and CLARITY Extension LSM sets (received placebo in CLARITY and CT3.5 in CLARITY Extension). The mean age in the CLARITY and CLARITY Extension LSM sets was 38.6 and 39.7 years, respectively, and 64.7% and 61.8% of patients were female. For the overall CLARITY population the mean age was 38.6 years and 67.7% of patients were female and at baseline of CLARITY Extension, the mean age was 41.1 years with 65.9% female patients.
ALCs
At baseline, the median ALC in the ORACLE-MS safety analysis set and the LSM analysis sets were similar. In the ORACLE-MS safety analysis set, the median baseline ALC was 1.92 × 109/l (Q1–Q3 1.64–2.34) in patients treated with CT3.5 (n = 206), and 1.89 × 109/l, Q1–Q3 1.63–2.35) in placebo recipients (n = 206). In the LSM analysis set, the median baseline ALC was 1.76 × 109/l (Q1–Q3 1.48–2.10) in the placebo group, and 1.76 × 109/l, Q1–Q3 1.58–2.22) in the CT3.5 group.
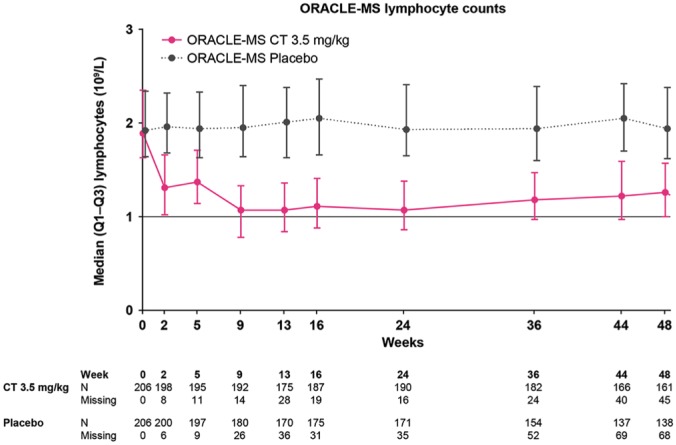
In patients treated with CT3.5, median ALC showed a rapid decline from baseline to week 2, which was maintained until week 5. A further decrease was observed after week 5, following the second treatment week; this decrease was followed by gradual recovery (Figure 1). In patients treated with CT3.5, median time to lowest concentration point (nadir) was 106.0 days (Q1–Q3 64.0–168.0), median ALC at nadir was 0.78 × 109/l (Q1–Q3 0.62–0.99). For CLARITY and CLARITY Extension, longitudinal data on ALC have been published elsewhere.21 Patients who were randomized to placebo in CLARITY were assigned to CT3.5 in CLARITY Extension; patients who were randomized to CT3.5 in CLARITY were re-randomized to CT3.5 or placebo in CLARITY Extension.
Figure 1.
Median absolute lymphocyte counts over time in patients treated with cladribine tablets 3.5 mg/kg or placebo in the first year of the ORACLE-MS study.
Lower and upper error bars indicate first and third quartile values. The lower limit of normal for lymphocyte counts is indicated by a horizontal line.
Cell types analyzed in CLARITY, CLARITY Extension and ORACLE-MS
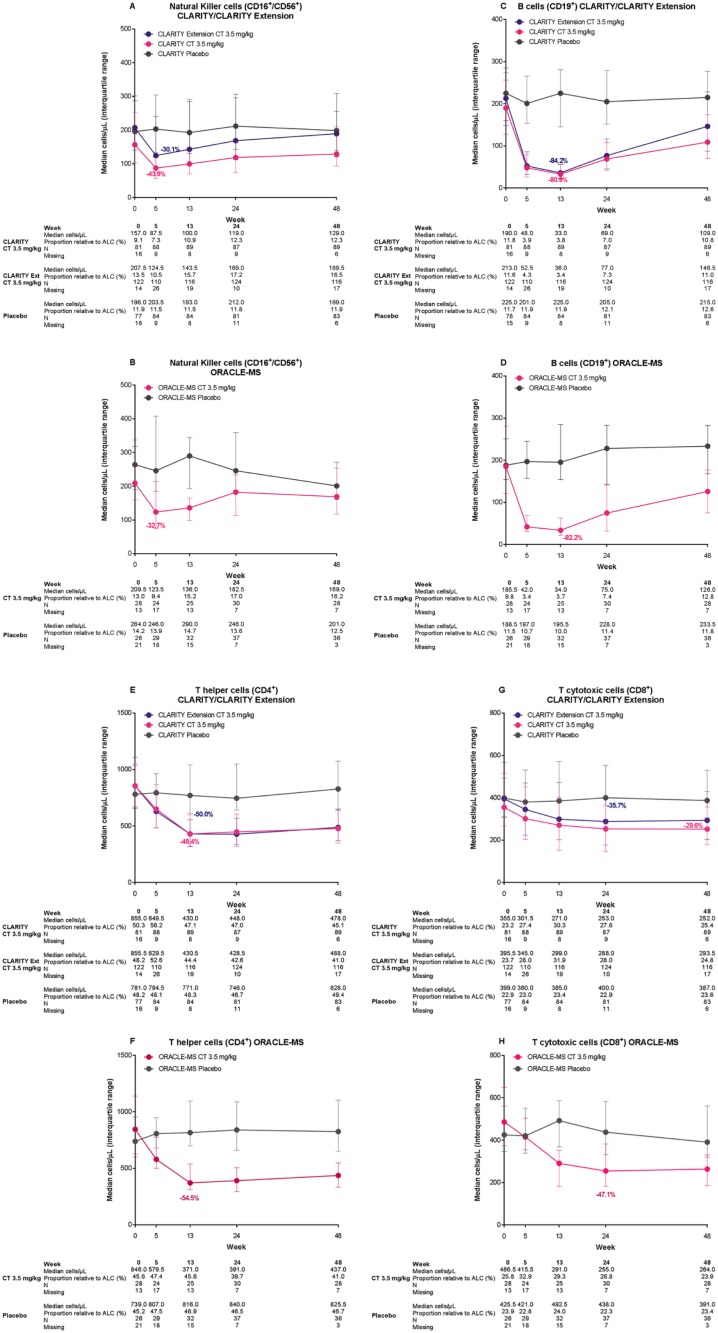
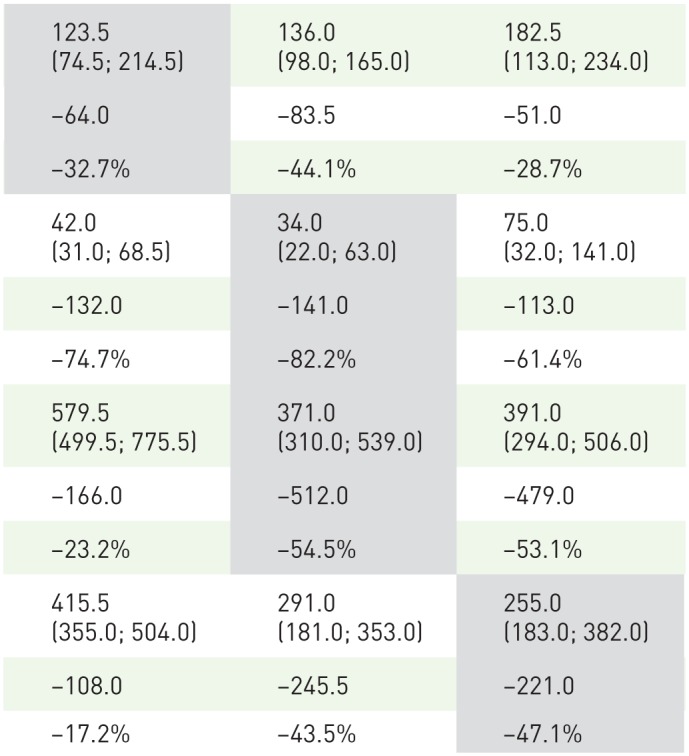
Natural killer cells (CD16+/CD56+). In each study, median counts of CD16+/CD56+ cells decreased rapidly from baseline to reach nadir at week 5. These represented changes from baseline of −43.9%, −30.1%, and −32.7% in CLARITY, CLARITY Extension, and ORACLE-MS, respectively (Table 1 and Figure 2A and B). Recovery of CD16+/CD56+ was rapid in each study, and well advanced at week 24. The interquartile range (IQR) values for the placebo and CT3.5 groups in each study overlapped for the majority of the treatment period timepoints and approached baseline counts at week 48.
Table 1.
Median (interquartile range) counts of NK cells (CD16+/56+), B cells (CD19+), T-helper cells (CD4+), and T-cytotoxic cells (CD8+) in patients treated with cladribine tablets 3.5 mg/kg in the ORACLE-MS study.
| Lymphocyte subset | Week 0 | Week 5 | Week 13 | Week 24 | Week 48 | ALC nadir |
|---|---|---|---|---|---|---|
| Absolute lymphocyte counts |
|
|||||
| NK cells (CD16 + /56 + ) a | ||||||
| Change versus baseline | ||||||
| Percentage change versus baseline | ||||||
| B cells (CD19 + ) a | ||||||
| Change versus baseline | ||||||
| Percentage change versus baseline | ||||||
| T-helper cells (CD4 + ) a | ||||||
| Change versus baseline | ||||||
| Percentage change versus baseline | ||||||
| Cytotoxic T cells (CD8 + ) a | ||||||
| Change versus baseline | ||||||
| Percentage change versus baseline | ||||||
ALC, absolute lymphocyte count; NR, not reported.
Values are cells/µl.
Shaded cells indicate the time points at which the largest changes from week 0 occurred. Change versus baseline and percentage change versus baseline are median values; calculations are described in the Supplementary Material.
Figure 2.
Median counts over time in patients treated with cladribine tablets 3.5 mg/kg or placebo for natural killer cells (CD16+/CD56)+ in (A) CLARITY and CLARITY Extension and (B) ORACLE-MS; for B cells (CD19+) in (C) CLARITY and CLARITY Extension and (D) ORACLE-MS; for T-helper cells (CD4+) in (E) CLARITY and CLARITY Extension and (F) ORACLE-MS; and for T-cytotoxic cells (CD8+) (G) in CLARITY and CLARITY Extension and (H) ORACLE-MS.
Lower and upper error bars indicate first and third quartile values. The value shown on the figure indicates the percentage change from baseline at nadir in patients treated with cladribine tablets 3.5 mg/kg. CLARITY Extension patients are those who received placebo in CLARITY.
B cells (CD19+)
Changes in median CD19+ B-cell counts in patients treated with CT3.5 in CLARITY, CLARITY Extension, and ORACLE-MS were relatively rapid (approximately 70% reduction from baseline at week 5 in each study, Table 1 and Figure 2C and D) and reached nadir at week 13 in each study, with a decline of 81–84% from baseline. Median CD19+ B-cell counts recovered towards baseline after week 13, showing reductions of approximately 60% and 30% at week 24 and 48, respectively. Further subsets of B lymphocytes were not analyzed in the CLARITY, CLARITY Extension, or ORACLE-MS studies.
T-helper cells (CD4+)
The pattern of response to treatment with CT3.5 was similar across the three studies. Median counts of CD4+ T cells showed a slow decrease from baseline to nadir, followed by stabilization and a relatively minor recovery towards baseline: IQRs for the CT3.5 and placebo groups did not overlap in any study at weeks 13 and 24, but did so at week 48. In CLARITY and in ORACLE-MS, nadir was at week 13 (representing a change of approximately −50% from baseline; Table 1 and Figure 2E and F). In CLARITY Extension, nadir was at week 24 (Figure 2E).
Cytotoxic T cells (CD8+)
The response of CD8+ T cells showed some similarities to CD4+ T cells: a slow decline from baseline was followed by stabilization with little sign of recovery until week 48. However, there was a notable difference compared with CD4+ T cells, with the extent of reduction being smaller for CD8+ T cells; the IQRs for placebo and CT3.5 groups overlapped at the majority of timepoints. In CLARITY, the lowest numbers of CD8+ T cells was at week 48 (representing a change from baseline of approximately −30%). In CLARITY Extension and ORACLE-MS, the nadir for CD8+ T cells was at week 24 (representing changes of −36% to −48%, Table 1 and Figure 2G and H).
Neutrophils and monocytes
In ORACLE-MS, median neutrophil counts showed a short-duration drop at week 2, but remained above the lower limit of normal (2.03 × 109 cells/l) and recovered quickly to baseline levels (Supplementary Figure 1A). Median monocyte counts showed no substantial reductions and remained close to baseline levels at all time points during ORACLE-MS (Supplementary Figure 1B). In CLARITY and CLARITY Extension, median neutrophil counts also remained within the normal range at all times up to and including week 48 (not shown). Monocyte counts for the CT3.5 and placebo groups showed comparable median (0.4 × 109/l) and Q1; Q3 values (0.3; 0.5 × 109/l) during CLARITY and CLARITY Extension, although there were differences in minimum and maximum values (data not shown).
Extended analysis of T-lymphocyte subtypes in ORACLE-MS
Overall, reduction and recovery dynamics for CD4+ T-lymphocyte subtype counts in ORACLE-MS were broadly similar to those seen in total CD4+ T cells.
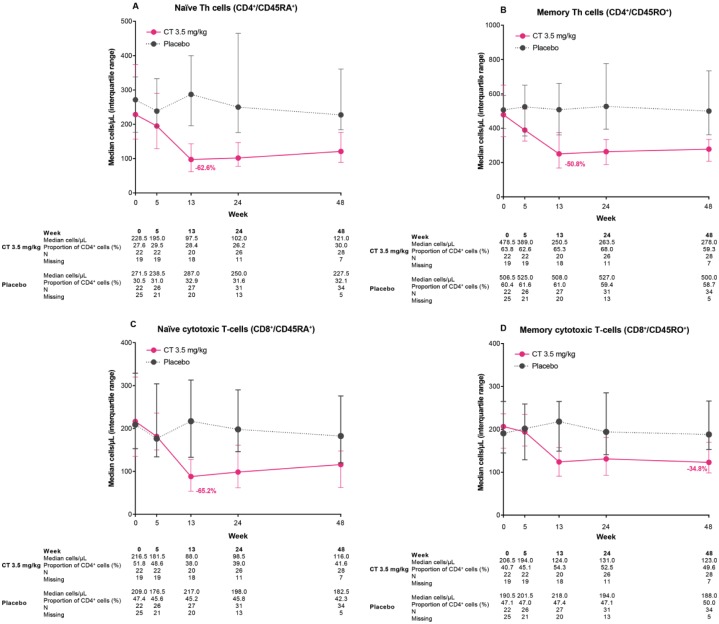
Naïve (CD4+CD45RA+) and memory (CD4+CD45RO+) T-helper cells
Nadir was at week 13 for CD4+CD45RA+ and CD4+CD45RO+ cells (Figure 3A and B), with naïve T-helper cells being slightly more affected than memory T helper (changes from week 0 of −62.6% and −50.8%, respectively).
Figure 3.
Median counts over time for T-cell subtypes: (A) naïve T-helper cells (CD4+CD45RA+); (B) memory T-helper cells (CD4+CD45RO+); (C) naïve cytotoxic T cells (CD8+CD45RA+); and (D) memory cytotoxic T cells (CD8+CD45RO+) in the ORACLE-MS study.
Lower and upper error bars indicate first and third quartile values. The value shown on the figure indicates the percentage change from baseline at nadir in patients treated with cladribine tablets 3.5 mg/kg.
Naïve (CD8+CD45RA+) and memory (CD8+CD45RO+) cytotoxic T cells
Median counts of CD8+CD45RA+ and CD8+CD45RO+ cells (Figure 3C and D, respectively) reached nadir at week 13 and week 48, respectively, with naïve cytotoxic T cells showing a greater reduction than memory cytotoxic T cells (changes from baseline of −65.2% and −34.8%, respectively). Naïve cytotoxic T-cell recovery was minimal, and memory cytotoxic T cells did not recover by week 48.
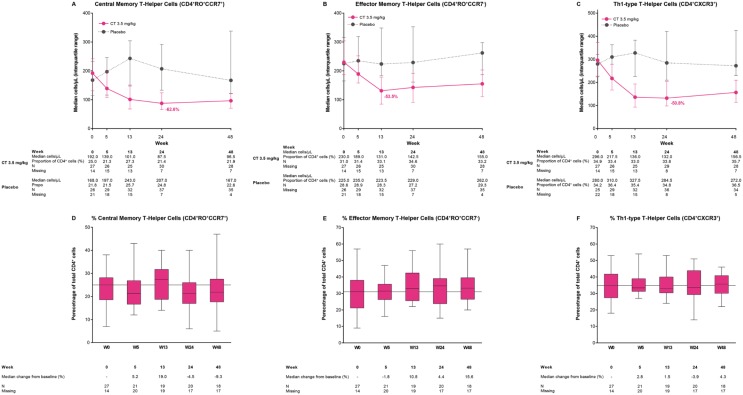
Central (CD4+RO+CCR7+) and effector (CD4+RO+CCR7−) memory T cells
Median counts of CD4+RO+CCR7+ and CD4+RO+CCR7− cells reached nadir at week 24 (–62.6% change from week 0) and at week 13 (–53.5% change from baseline), respectively, and showed little sign of recovery by week 48 (Figure 4A and B). There were minor perturbations of the proportion of each subgroup from the starting median proportions (Figure 4D and E). The proportion of CD4+RO+CCR7+ cells measured as a percentage of total CD4+ T cells was 25.0% at week 0. This proportion tended to decrease during the study, falling by 5.2% at week 5 and 9.3% at week 48. The proportion of CD4+RO+CCR7− cells as a percentage of total CD4+ T cells at week 0 was 31.0%. In contrast to CD4+RO+CCR7+ cells, the proportion of CD4+RO+CCR7− cells relative to total CD4+ T cells increased during the study, by 10.8% at week 13, and by 15.6% at week 48.
Figure 4.
Median counts for T-cell subtypes (A) central memory cells (CD4+RO+CCR7+), (B) effector memory cells (CD4+RO+CCR7−), and (C) Th1-type helper cells (CD4+CXCR3+) and proportions of lymphocyte subtypes relative to CD4+ cells for (D) central memory cells (CD4+RO+CCR7+), (E) effector memory cells (CD4+RO+CCR7−), and (F) Th1=type helper cells (CD4+CXCR3+) in the ORACLE-MS study.
In (A)–(C), lower and upper error bars indicate first and third quartile values. Values shown in (A)–(C) indicate the percentage change from baseline at nadir in patients treated with cladribine tablets 3.5 mg/kg. In (D)–(F), the horizontal line indicate median values (data expressed as percentage of total CD4+ cell counts), lower and upper box edges indicate Q1 and Q3 values, and whisker extremities indicate maximum and minimum values. Values shown in (D)–(F) indicate percentage change from week 0 in the proportion of cell types relative to total CD4+ cells in patients treated with cladribine tablets 3.5 mg/kg.
Th1-type helper T cells (CD4+CXCR3+)
In CD4+CXCR3+ T cells, nadir was reached at week 24 (–50.8% change from baseline) and followed by a slow return towards baseline (Figure 4C). The proportion of CD4+CXCR3+ cells as a percentage of total CD4+ T cells was 34.9% at baseline, and showed only small changes over time (Figure 4F).
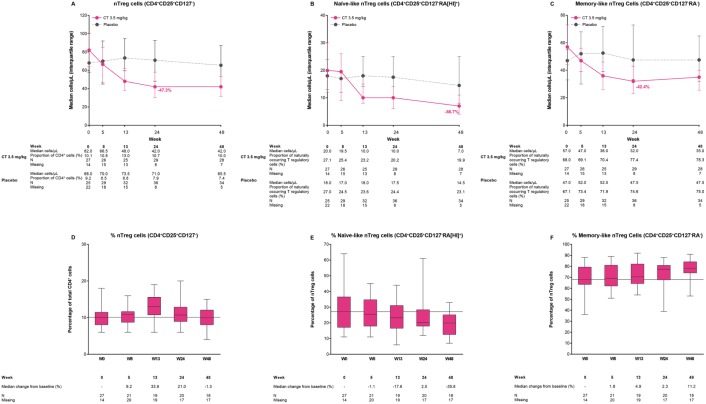
Naturally occurring T-regulatory cells (nTreg; CD4+CD25+CD127−)
Median CD4+CD25+CD127− cell counts reached nadir at week 24 (–47.3% change from baseline), and remained close to this level until week 48 (Figure 5A). The proportion of CD4+CD35+CD127− cells as a percentage of CD4+ T cells (10.1% at week 0) increased by 9.2% and 33.9% at weeks 5 and 13, respectively, and then returned towards baseline at week 48 (Figure 5D).
Figure 5.
Median counts over time for T-cell subtypes: (A) naturally occurring T-regulatory (nTreg) cells (CD4+CD25+CD127−); (B) naïve-like nTreg cells (CD4+CD25+CD127−RA(HI)+); and (C) memory-like nTreg cells (CD4+CD25+CD127−RA−); proportions of lymphocyte subtypes relative to CD4+ cells for (D) naturally occurring T-regulatory (nTreg) cells (CD4+CD25+CD127−); and relative to (E) nTreg cells for naïve-like nTreg cells (CD4+CD25+CD127−RA(HI)+); and (F) memory-like nTreg cells (CD4+CD25+CD127−RA−) in the ORACLE-MS study.
In (A)–(C), the lower and upper error bars indicate first and third quartile values. In (D)–(F), the horizontal line indicates median values, lower and upper box edges indicate Q1 and Q3 values (data in (D) expressed as a percentage of total CD4+ cell counts; data in (E) and (F) expressed as percentage of nTreg cell counts). Values in (D) indicate percentage changes from week 0 in the proportion of cells relative to total CD4+ cells. Values shown in (E) and (F) indicate percentage change from week 0 in the proportion of cell types relative to total nTregs. There was an increase in memory-like nTreg cells towards the end of sampling.
Naïve-like (CD4+CD25+CD127−RA[HI]+) and memory-like (CD4+CD25+CD127−RA−) nTreg cells
Median CD4+CD25+CD127−RA(HI)+ cell counts declined throughout the duration of the study period, and reached their lowest concentration at week 48 (–66.7% change from baseline; Figure 5B). Median CD4+CD25+CD127−RA− cell counts reached nadir at week 24 (–42.4% change from baseline), then increased slightly by week 48 (Figure 5C). The proportion of CD4+CD25+CD127−RA(HI)+ cells as a percentage of total nTreg cells measured at week 0 was 27.0%. This decreased over time, with a reduction of 35.8% from week 0 to week 48 (Figure 5E). In contrast, the proportion of memory-like CD4+CD25+CD127−RA−cells as a percentage of total nTreg cells at week 0 was 68.0%. This proportion increased during the study from week 0 to 11.2% at week 48 (Figure 5F).
Discussion
Our findings show that following the first treatment year for patients randomized to CT3.5 (the first of 2 years of treatment), the effects of CT3.5 on B cells, T cells, NK cells, and innate immune system cells in patients with CIS are comparable with the effects observed in patients with established RRMS. This immune cell analysis comprised data from only the first year of a total CT3.5 treatment. It is important to note that CT1.75 is not an indicated dose, and results from analysis of 1-year data should not be interpreted as any indication of a 1-year clinical benefit.
Across three studies, we observed a large reduction in B cells (approximately 80% at nadir, Figure 2C and D), a moderate reduction in T cells (CD4+ approximately 50%, Figure 2E and F; and CD8+ approximately 40% at nadir, Figure 2G and H), and smaller reductions in NK cells (between 30% and 44% at nadir; Figure 2A and B). Innate immune system cells were largely unaffected by treatment with CT3.5 (Supplementary Figure 1): neutrophil counts remained within the lower limit of normal and had recovered by week 5. Monocyte counts were largely unaffected.
The dynamics of lymphocyte reduction demonstrated that CD16+/56+ NK cells were the most rapid to reach nadir, doing so by week 5. This may, of course, be attributable to requiring less time to reach a modest nadir, as discussed above, with other subsets requiring more time to reach deeper nadirs. Both CD19+ B cells and CD4+ T-helper cells reached nadir at week 13, with a particularly profound reduction of the CD19+ B cells (changes from baseline: −82.2%). CD8+ cytotoxic T cells were the slowest to reach nadir.
The dynamics of reconstitution were similar to the observed patterns for reduction, with recovery towards baseline levels by week 24 for CD16+/56+ NK cells and by week 48 for CD19+ B cells (Table 1). Given the small reduction for the CD16+/CD56+ NK cells, a relatively quick recovery may have been expected. More noteworthy is the speed of recovery towards baseline for the CD19+ B cells given their large reduction from baseline. The CD4+ and CD8+ T cells had a more gradual recovery (minimal recovery at week 48; Figure 2). This is perhaps unsurprising, given the slow rate of reduction of cell numbers leading to nadir. Retreatment guidelines based on lymphocyte counts were applied during the conduct of ORACLE-MS and are included in the approved prescribing information for cladribine tablets.
The effect of the first year of treatment with CT3.5 on proportions of lymphocyte subtypes (Figures 4D–F and 5D–F) was of interest in this study because homeostasis and function of immune cells is dependent upon their relative ratios. Since the majority of autoreactive T cells in MS appear to be memory CD4+ T cells,22 with a large proportion derived from CCR7+ central memory cells,23 the effects of CT3.5 on these cell populations was of high interest. In ORACLE-MS, counts of both memory and effector CD4+ T lymphocytes decreased after treatment with CT3.5 (63% and 54%, respectively, at nadir) and this was accompanied by changes in the ratio of these subtypes (Figure 4D and E). Interestingly, CD4+ central memory and effector memory T cells displayed quite different reconstitution dynamics, with the former remaining lower at 48 weeks than at baseline but with effector memory T cells being ~16% higher at week 48 than at baseline.
The effect of treatment on subtypes and ratios of nTregs was also studied as these cells have a strong suppressive effect on T cells and myeloid cells.24,25 At week 48, nTreg counts had decreased from baseline by nearly half. Naïve-like nTregs had a larger reduction than memory-like nTregs (Figure 5A–C). Considered together with the effects on CD4+ and cytotoxic CD8+ T cells (Figure 3A and B), naïve-like nTregs may be more sensitive to cladribine than memory-like nTregs. The larger decrease in naïve-like nTregs was accompanied by a reduction in their proportion at week 48 compared with week 0 (~36%; Figure 5E). In contrast, the proportion of memory-like nTregs increased at week 48 compared with week 0 (~11%; Figure 5F). It remains to be determined whether these findings are confined to the peripheral immune compartment or affects both peripheral cells and immune cells resident in the central nervous system (CNS). Further work is ongoing to characterize the effects of treatment with CT3.5 on FOX P3+ nTregs, and also nTreg to T-effector cell ratios, which may be important immunological changes in MS and therefore may lead to a better understanding of the effects of DMTs.26,27
A key differentiator of CT3.5 is the discontinuous nature of peripheral lymphocyte reductions. In ORACLE-MS, ALC and CD19+ B-cell counts were recovering towards baseline levels at week 48. Findings from longer-term studies (using data from CLARITY and CLARITY Extension) demonstrate that ALC and CD19+ B-cell counts recover and stabilize.28 It should be noted that other B-cell subtypes were not analyzed, so it is not possible to comment on their reduction and repopulation dynamics. In contrast, reductions in T-cell subsets were modest in size but had a more gradual recovery up to week 48 of treatment. The first year of treatment with CT3.5 did not appear to be associated with an above-baseline rise in B or T cells following the initial reduction phase by week 48. Immune dysfunction associated with homeostatic proliferation after treatment with lymphocyte-depleting therapies (e.g. alemtuzumab and rabbit antithymocyte globulin) has been shown in transplant and MS patients. In this context, the skewing of repopulating lymphocytes towards a more memory-type phenotype has been described, but a deeper understanding of the underlying biology is still warranted.29–32 Homeostatic proliferation from the peripheral pool may predispose to autoimmunity.31
There has been considerable debate about the relative importance of B cells and B-cell subsets in the pathogenesis and treatment of RRMS.16,17 There are studies indicating an important pathological role for memory B cells in RRMS and, more recently, in primary progressive MS.17,33,34 However, reduction of B cells alone may be insufficient to explain efficacy in RRMS, as rituximab treatment significantly decreases counts of both B and T cells and attenuates proinflammatory responses, suggesting that reduction of both cell types may be important.35,36 A recent publication by Jelcic et al. suggested that memory, notably unswitched memory, B cells directly promote autoproliferation and activation of ‘brain-homing’ CD4+ T cells.37 The autoproliferating CD4+ T cells have a strong affinity for some antigens expressed by the brain or by B cells.37 If corroborated, these findings may provide further rationale for drugs that target B cells, as they demonstrate a complex and closely intertwined relationship between B cells and T cells in MS pathogenesis.
Alemtuzumab markedly decreases B-cell and CD4+ and CD8+ lymphocyte counts and has some effects on NK cells. The magnitude of effect of alemtuzumab and cladribine were compared by Baker et al. and found to be comparable with respect to B-cell reduction; T-cell reduction with cladribine was more modest than was shown for alemtuzumab.16 Fingolimod treatment reduces total B-cell counts but increases the relative proportion of naïve B cells,38 with similar effects (an increase in transitional B cells) reported for patients treated with interferon-β.39 Furthermore, the potentially complex role of B cells in RRMS is highlighted by studies with atacicept, a fusion protein with potent B-cell suppressive effects. In patients with RRMS, atacicept reduced B-cell counts but increased relapse activity compared with placebo.40
In the present analysis, following treatment with CT3.5, B cells showed fast (approximately 70% reduction at week 5) and large reductions (>80% reduction at the week 13 nadir) compared with T cells (approximately 50% reduction at week 5). The rapid recovery of B cells should be considered in the context of the durable and relatively rapid clinical effect of CT3.5 (in CLARITY, T1 Gd+ MRI activity was undetectable at week 24 in patients treated with CT3.5).11 One question is how CT3.5 produces durable clinical effect over several years, after two short treatment periods in Year 1 and Year 2. In addition, how the changed proportion of memory-like Tregs may contribute to this long-term efficacy observed in studies. How this may affect B cells, T-effector cells and other T-cell subtypes is uncertain and will be key areas for future research. A general limitation of immunophenotyping studies after treatment with lymphocyte-depleting therapies is the fact that only blood has been analyzed in most MS trials, and the level of depletion and order of repopulation in primary and secondary lymphoid organs or cerebrospinal fluid is still unknown. Fully characterizing the reconstitution of the adaptive immune system after treatment with CT3.5 may require further functional and qualitative analysis of study of lymphocytes subsets.
The low level of opportunistic infections in the clinical development program is evidence of immune system competence following CT3.5 treatment. Lymphocyte recovery began soon after treatment, which may, in part, account for the low frequency of severe lymphopenia associated with CT3.5.13 Data from CLARITY and CLARITY Extension also show that no cases of Common Terminology Criteria for Adverse Events (CTCAE) Grade 4 lymphocyte counts were present at the end of any treatment year in patients treated with CT3.5 according to treatment guidelines.41 Consistent with this, neutrophil recovery in ORACLE-MS was also rapid, with a low frequency of severe neutropenia (Supplementary Figure 2) and no cases of CTCAE Grade 4 (serious) neutropenia at any time.
This study was limited by its exploratory nature. No statistical testing for multiplicity was carried out and the outcomes reported here should be considered as suggestive, highlighting future areas for research. Furthermore, measurements of lymphocyte counts were made using peripheral blood only. Cladribine is highly distributed in tissue shortly after administration, and the possible effects of treatment on lymphocytes within tissues such as the CNS have not been determined. In addition, assessment of B-lymphocyte subsets, particularly memory subsets, was not included in these analyses.
Conclusion
In patients with CIS and RRMS, treatment with cladribine tablets in the first year led to substantial but transient reductions in CD19+ B cells and smaller but more long-lasting reductions in T-lymphocyte subtypes followed by gradual recovery. The relative selectivity of CT3.5 for lymphocytes was demonstrated by the lack of effect on neutrophils and monocytes, and a moderate and transient effect on NK cells. In the extended analysis of patients from the ORACLE-MS study, there were alterations in the proportions of some CD4+ T-cell subtypes, relative to the overall CD4+ T-cell population, with small decreases in the proportions of central memory T cells. In contrast, memory-like Treg cells appeared to increase as a proportion of the T-lymphocyte pool by week 48. Furthermore, nTreg cells increased as a proportion of CD4+ cells, before returning to baseline levels by week 48. However, the changes in nTregs were underpinned by decreasing proportions of naïve-like nTreg cells in parallel with increased proportions of memory-like nTreg cells.
It must be noted that these lymphocyte population dynamics are not yet known following the second year of treatment, as approved, and contributions of such changes to a clinical therapeutic effect are currently unknown. This immune cell analysis comprised data from only the first year of a total CT3.5 treatment. It is important to note that CT1.75 is not an indicated dose, and results from analysis of 1-year data should not be interpreted as any indication of a 1-year clinical benefit.
These findings provide further insights into how CT3.5 may selectively target and reduce T and B lymphocytes with transient effects on NK cells and minimal innate immune system impact, as part of a unique approach to IRT in MS.
Supplemental Material
Supplemental material, Supplementary_Materials for Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers by Olaf Stuve, Per Soelberg Soerensen, Thomas Leist, Gavin Giovannoni, Yann Hyvert, Doris Damian, Fernando Dangond and Ursula Boschert in Therapeutic Advances in Neurological Disorders
Footnotes
Funding: This study was sponsored by EMD Serono, Inc., a business of Merck KGaA, Darmstadt, Germany (in the United States), and Merck Serono SA, Geneva, an affiliate of Merck KGaA Darmstadt, Germany (rest of the world).
Medical writing assistance was provided by Phil Jones and Ash Dunne of inScience Communications, Springer Healthcare, Chester, UK, and supported by Merck KGaA, Darmstadt, Germany.
Conflict of interest statement: OS serves on the editorial boards of the Multiple Sclerosis Journal, and Therapeutic Advances in Neurological Disorders. He has served on data monitoring committees for Genentech-Roche, Pfizer, and TG Therapeutics without monetary compensation. He has advised EMD Serono, Celgene, Genzyme, and Serono. He currently receives grant support from Sanofi Genzyme. He received travel support from Shire
PSS has served on advisory boards for Biogen, Merck KGaA, Novartis, Teva, MedDay Pharmaceuticals, and GSK; on steering committees or independent data monitoring boards in trials sponsored by Merck KGaA, Teva, GSK, and Novartis; has received speaker honoraria from Biogen Idec, Merck KGaA, Teva, Sanofi-Aventis, Genzyme, and Novartis. His department has received research support from Biogen, Merck KGaA, Teva, Novartis, Roche, and Genzyme.
TL has received consultancy fees or clinical research grants from Acorda, Bayer, Biogen, Daiichi, EMD Serono, Novartis, ONO, Pfizer, and Teva Neuroscience.
GG has received speaker honoraria and consulting fees from Abbvie, Actelion, Atara Bio, Almirall, Bayer Schering Pharma, Biogen Idec, FivePrime, GlaxoSmithKline, GW Pharma, Merck & Co., Merck KGaA, Pfizer Inc, Protein Discovery Laboratories, Teva Pharmaceutical Industries Ltd, Sanofi-Genzyme, UCB, Vertex Pharmaceuticals, Ironwood, and Novartis; and has received research support unrelated to this study from Biogen Idec, Merck & Co, Novartis, and Ironwood.
YH, DD, FD, and UB are employees of EMD Serono Research & Development Institute Inc., a business of Merck KGaA, Darmstadt, Germany.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Olaf Stuve  https://orcid.org/0000-0002-0469-6872
https://orcid.org/0000-0002-0469-6872
Contributor Information
Olaf Stuve, Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center at Dallas, 6000 Harry Hines Boulevard, Dallas, TX 75390-8813, USA; Chief Neurology Section, VA North Texas Health Care System, Medical Service, Dallas VA Medical Center, 4500 South Lancaster Road, Dallas, TX 75216, USA.
Per Soelberg Soerensen, Danish MS Center, Department of Neurology, University of Copenhagen, Rigshospitalet, Copenhagen, Denmark.
Thomas Leist, Division of Clinical Neuroimmunology, Jefferson University, Comprehensive MS Center, Philadelphia, PA, USA.
Gavin Giovannoni, Queen Mary University of London, Blizard Institute, Barts and The London School of Medicine and Dentistry, London, UK.
Yann Hyvert, EMD Serono, Inc, Billerica, MA, USA.
Doris Damian, EMD Serono, Inc, Billerica, MA, USA.
Fernando Dangond, EMD Serono, Inc, Billerica, MA, USA.
Ursula Boschert, EMD Serono, Inc, Billerica, MA, USA.
References
- 1. Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron 2018; 97: 742–768. [DOI] [PubMed] [Google Scholar]
- 2. Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol 2012; 11: 157–169. [DOI] [PubMed] [Google Scholar]
- 3. Bates D. Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials. Neurology 2011; 76: S14–S25. [DOI] [PubMed] [Google Scholar]
- 4. Kappos L, Kuhle J, Multanen J, et al. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry 2015; 86: 1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Constantinescu CS, Gran B. The essential role of T cells in multiple sclerosis: a reappraisal. Biomed J 2014; 37: 34–40. [DOI] [PubMed] [Google Scholar]
- 6. Posova H, Horakova D, Capek V, et al. Peripheral blood lymphocytes immunophenotyping predicts disease activity in clinically isolated syndrome patients. BMC Neurol 2017; 17: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics 2017; 14: 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiendl H. Cladribine - an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol 2017; 13: 573–574. [DOI] [PubMed] [Google Scholar]
- 9. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 10. De Stefano N, Giorgio A, Battaglini M, et al. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler 2018; 24: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comi G, Cook SD, Giovannoni G, et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol 2013; 260: 1136–1146. [DOI] [PubMed] [Google Scholar]
- 12. Comi G, Cook S, Rammohan K, et al. Long-term effects of cladribine tablets on MRI activity outcomes in patients with relapsing-remitting multiple sclerosis: the CLARITY Extension study. 2018; 11: 1756285617753365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giovannoni G, Soelberg-Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing–remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018; 24: 1594–1604. [DOI] [PubMed] [Google Scholar]
- 14. Leist TP, Comi G, Cree BA, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol 2014; 13: 257–267. [DOI] [PubMed] [Google Scholar]
- 15. Freedman MS, Leist TP, Comi G, et al. The efficacy of cladribine tablets in CIS patients retrospectively assigned the diagnosis of MS using modern criteria: results from the ORACLE-MS study. Mult Scler J Exp Transl Clin 2017; 3: 2055217317732802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm 2017; 4: e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol 2018; 265: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merck Serono Europe Limited. MAVENCLAD 10 mg tablets; summary of product characteristics, https://www.medicines.org.uk/emc/product/8435/smpc (2017, accessed May 2019).
- 19. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 20. Cook S, Vermersch P, Comi G, et al. Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult Scler 2011; 17: 578–593. [DOI] [PubMed] [Google Scholar]
- 21. Stuve O, Soelberg-Sorenson P, Giovannoni G, et al. Cladribine tablets produce selective and discontinuous reduction of B and T lymphocytes and natural killer cells in patients with early and relapsing multiple sclerosis (ORACLE-MS, CLARITY and CLARITY Extension). Mult Scler J 2017; 23: 335–336. [Google Scholar]
- 22. Giunti D, Borsellino G, Benelli R, et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol 2003; 73: 584–590. [DOI] [PubMed] [Google Scholar]
- 23. Kivisakk P, Mahad DJ, Callahan MK, et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol 2004; 55: 627–638. [DOI] [PubMed] [Google Scholar]
- 24. Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol 2016; 16: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30: 636–645. [DOI] [PubMed] [Google Scholar]
- 26. Rakebrandt N, Littringer K, Joller N. Regulatory T cells: balancing protection versus pathology. Swiss Med Wkly 2016; 146: w14343. [DOI] [PubMed] [Google Scholar]
- 27. Sambucci M, Gargano F, De Rosa V, et al. FoxP3 isoforms and PD-1 expression by T regulatory cells in multiple sclerosis. Sci Rep 2018; 8: 3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soelberg-Sorensen P, Dangond F, Hicking C, et al. Long-term lymphocyte counts in patients with relapsing-remitting multiple sclerosis (RRMS) treated with cladribine tablets 3.5 mg/kg: total lymphocytes, B and T cell subsets. Mult Scler J 2017; 23: 310. [Google Scholar]
- 29. Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol 2017; 74: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willis M, Robertson NP. Drug safety evaluation of alemtuzumab for multiple sclerosis. Expert Opin Drug Saf 2014; 13: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 31. Zwang NA, Turka LA. Homeostatic expansion as a barrier to lymphocyte depletion strategies. Curr Opin Organ Transplant 2014; 19: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones JL, Thompson SAJ, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. PNAS 2013; 110: 20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker D, Marta M, Pryce G, et al. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017; 16: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 209–220. [DOI] [PubMed] [Google Scholar]
- 35. Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010; 67: 452–461. [DOI] [PubMed] [Google Scholar]
- 36. Cross AH, Stark JL, Lauber J, et al. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 2006; 180: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jelcic I, Al Nimer F, Wang J, et al. Memory B cells activate brain-homing, autoreactive CD4(+) T cells in multiple sclerosis. Cell 2018; 175: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Claes N, Dhaeze T, Fraussen J, et al. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS One 2014; 9: e111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dooley J, Pauwels I, Franckaert D, et al. Immunologic profiles of multiple sclerosis treatments reveal shared early B cell alterations. Neurol Neuroimmunol Neuroinflamm 2016; 3: e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kappos L, Hartung HP, Freedman MS, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol 2014; 13: 353–363. [DOI] [PubMed] [Google Scholar]
- 41. Cook S, Comi G, Giovannoni G, et al. Rates of lymphopenia year-by-year in patients with relapsing multiple sclerosis treated and retreated with cladribine tablets 3.5mg/kg. Mult Scler J 2017; 23: 317. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials for Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers by Olaf Stuve, Per Soelberg Soerensen, Thomas Leist, Gavin Giovannoni, Yann Hyvert, Doris Damian, Fernando Dangond and Ursula Boschert in Therapeutic Advances in Neurological Disorders