Abstract
Background: Compensatory movement, such as flexing the trunk during reaching, may negatively affect motor improvement during task-based practice for persons with stroke. Shaping, or incrementally decreasing, the amount of compensation used during rehabilitation may be a viable strategy with methods using virtual reality.
Methods: A virtual reality tool was designed to (1) monitor upper extremity movement kinematics with an off-the-shelf motion sensor (Microsoft Kinect V2), (2) convert movements into control of widely available computer games, and (3) provide real-time feedback to shape trunk compensation. This system was tested for feasibility by a small cohort of participants with chronic stroke (n = 5) during a 1-h session involving 40 min of virtual reality interaction. Outcomes related to repetitions, compensation, movement kinematics, usability, motivation, and sense of presence were collected.
Results: Participants achieved a very high dose of reaching repetitions (461 ± 184), with an average of 81% being successful and 19% involving compensatory trunk flexion. Participants rated the system as highly usable, motivating, engaging, and safe.
Conclusions: VRShape is feasible to use as a tool for increasing repetition rates, measuring and shaping compensation, and enhancing motivation for upper extremity therapy. Future research should focus on software improvements and investigation of efficacy during a virtual reality-based motor intervention.
Keywords: Virtual reality, stroke rehabilitation, occupational therapy, neurorehabilitation, motion/posture analysis, motion analysis systems
Background
Hemiparesis is the most common motor deficit resulting from stroke, with up to two-thirds of stroke survivors living with its associated chronic impairments and reduced quality of life.1,2 Resulting long-term impairments to strength, motor control, and range of motion (ROM) in the upper extremity (UE) can make it difficult for many stroke survivors to adequately perform important activities or participate in the flow of daily life.3
Rehabilitation strategies for persons with hemiparetic stroke rely on principles of neuroplasticity that state thousands of specific, intense, task-oriented movement repetitions must be performed to drive reorganization of cortical motor function from injured to healthy areas of the brain.4 Many physical and occupational therapy interventions capitalize on this tenet of motor learning to target improvements in post-stroke motor function. For example, constraint-induced movement therapy (CIMT) has been shown to improve motor function as a result of restraining the unimpaired arm and forcing large doses of repetitive practice with the paretic arm.5 However, such intense protocols are often impossible in typical rehabilitation settings due to time constraints, funding limitations, and client noncompliance.6 This is reflected in recent research that suggests typical outpatient therapy sessions achieve very few movement repetitions relative to the dose required for salient motor learning.7
Furthermore, few current interventions acknowledge that two competing mechanisms of functional motor improvement may be occurring simultaneously: motor recovery and compensation. True motor recovery refers to the reacquisition of pre-stroke movement patterns and motor skills, while compensation refers to the substitution of novel movement patterns or skills to complete tasks.8 Common compensatory strategies involve excessive flexion and rotation at the trunk to move the hand into position during reaching.9,10 The extreme case of maladaptive compensation is “learned non-use”, defined when a person learns to solely perform tasks with the unimpaired arm.5 While motor recovery is the ideal goal of most post-stroke treatments, in fact, compensatory strategies are often prescribed in lieu of normal motor functioning. It is hypothesized that frequent use of such compensations, or “learned bad-use”, may lead to long-term chronic pain in overused joints and suboptimal motor recovery in the impaired arm due to limited repetitive practice.8,11,12 While a paucity of evidence exists, many interventions, including CIMT, are suspected of inadvertently teaching compensatory strategies instead of promoting true motor recovery in persons with stroke.13
Virtual reality (VR) has emerged as a prominent tool for addressing some shortcomings in current motor rehabilitation strategies. VR is defined as a human–computer interface that allows a user to interact with a virtual environment (VE) through physical movement.14 Contemporary research has shown that VR-based therapy can elicit very large doses of movement repetitions and similar, sometimes superior, improvements in UE motor impairment, function, and activity performance relative to no therapy or conventional therapy.15–17 The most recent Cochrane review included 72 randomized controlled trials involving over 2000 participants with stroke and concluded that VR use can significantly improve UE motor function and performance of activities of daily living (ADLs) in persons with chronic stroke.16 VR has also been found efficacious for improving mobility, gait, and balance for persons with stroke.18,19 The primary advantages of VR are related to objective measurement, immediate feedback, and high user motivation that may improve aspects of motor learning and subsequent true motor recovery when combined with principles of neuroplasticity.20 Importantly, VR systems may also be able to capitalize on advancements in motion capture technology to measure compensation during repetitive practice with the impaired arm in the hopes of further enhancing motor outcomes for persons with stroke.
We have previously successfully applied a VR-based motor therapy strategy that incorporates the Microsoft Kinect sensor (Microsoft Corp., Redmond, WA), customizable software, and freely available computer games to different populations with motor deficits including chronic stroke, cerebral palsy (CP), and Rett syndrome. In a small observational study of persons with stroke, we found that the first generation Kinect (V1) and the Flexible and Articulated Skeleton Toolkit (FAAST) can be used to create intense and motivating motor therapy.21,22 In multiple case studies involving persons with stroke, we found that this strategy was feasible and capable of improving functional and occupational performance through use in a clinical setting and as a home exercise program.23 In a case series of children with CP, we found that this same strategy could be transitioned from in-laboratory to in-home therapy over the course of 12 weeks, was highly customizable and usable with as many as 26 different computer games, and was capable of facilitating improvement in some aspects of UE movement kinematics and function.24 In a case study of a single person with Rett syndrome, we found that this strategy could improve the performance of self-care activities and decrease stereotypical hand movements by facilitating an increase in the number of targeted reaches performed during therapy.25
None of our previous work has investigated the use of compensation during repetitive practice of UE tasks, and yet the excessive use of compensation may affect the amount of motor recovery that can be achieved.8,11,12 A few studies have addressed compensation during task-based training and CIMT through the use of physical restraints or static feedback.26–28 Existing robotic or VR-based interventions have provided auditory, visual, or tactile feedback based on variable compensation thresholds.29,30 Shaping, or the incremental adjustment of task difficulty, is used often in occupational therapy and interventions such as CIMT,5 may be a more useful technique for reducing compensation, and can be integrated into existing VR methods. The purposes of this study were to design and assess the feasibility of a VR tool capable of measuring and shaping compensatory movements during repetitive UE practice for persons with chronic stroke.
Methods
System design
We developed a VR tool called VRShape that (1) monitors movement kinematics in real time using an affordable, off-the-shelf motion sensor; (2) recognizes a variety of customizable, targeted UE movements; (3) converts these UE movements into control of nearly any freely available computer game; (4) records and reports clinically-relevant performance metrics; and (5) provides feedback about compensatory trunk movements in real time. Each of these elements combine to create a VR-based therapy that is client-centered, motivating, and designed to encourage clients to perform large doses of high-quality movement repetitions with incrementally decreased compensation (Figure 1).
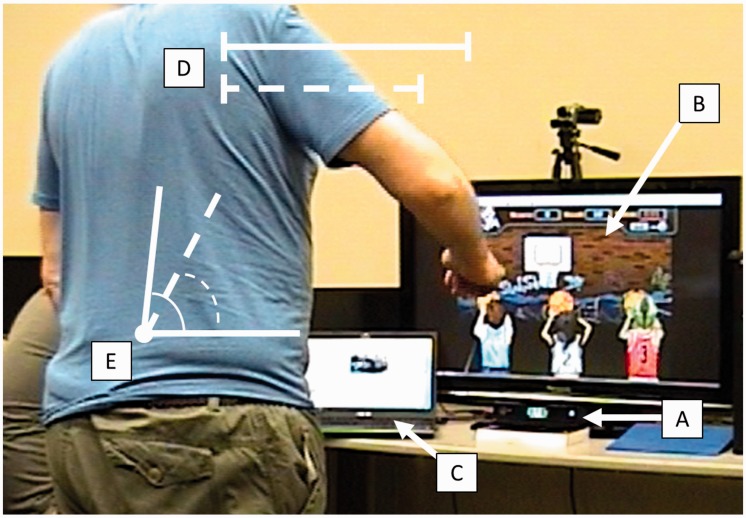
Figure 1.
Example of experimental setup using VRShape involving (A) the Microsoft Kinect V2, (B) a client-chosen computer game (Hoops Mania), and (C) host computer providing feedback. A simple representation of reaching ROM (solid) and calibrated reaching threshold (dotted) is shown (D). A simple representation of trunk flexion (solid) and calibrated trunk flexion threshold (dotted) is also shown (E).
VRShape uses the second generation (V2) of the Microsoft Kinect sensor to identify bodily movement. The Kinect V2 combines a typical camera and an infrared depth sensor to capture the movement of up to 25 joints and body segments in real time without the use of wearable trackers. The sensor connects to a host computer via a USB 3.0 connection and driver software, namely the Kinect Software Development Kit (SDK) (v2.0). Several studies have established the Kinect V2 to be adequately valid and reliable for measuring UE and postural movements relative to more expensive and accurate motion capture systems.31,32 In a previous investigation, we found both the Kinect V1 and V2 to have good validity and reliability for measuring arm displacements and shoulder angles relative to an 8-camera video motion capture system (VMC), but found the Kinect V2 to be closer in magnitude to VMC for the majority of kinematic variables.33
VRShape was developed primarily within the MATLAB programming environment utilizing an interface for the Kinect V2 (r2016a, Mathworks Inc., Natick, MA). Relevant information is defined and passed through a series of graphical–user interfaces (GUIs) including a login page, a main dashboard, a calibration screen, and several performance reports to make up the general workflow of the software (Figure 2). These GUIs are the main interface for the person controlling an intervention session, whether it be a researcher, a therapist, or the user him- or herself. Each user can define a personalized login name, under which all subsequent setup and performance data will be saved. The user’s experience can be customized according to the movement that is targeted for practice, the ROM threshold for the movement, and the desired computer game. Performance metrics related to time played, number of repetitions achieved, movement used, game used, and ROM achieved can be displayed in numerical or graphical form in a series of optional GUIs. These data can also be shown longitudinally to track progress over the course of several sessions.
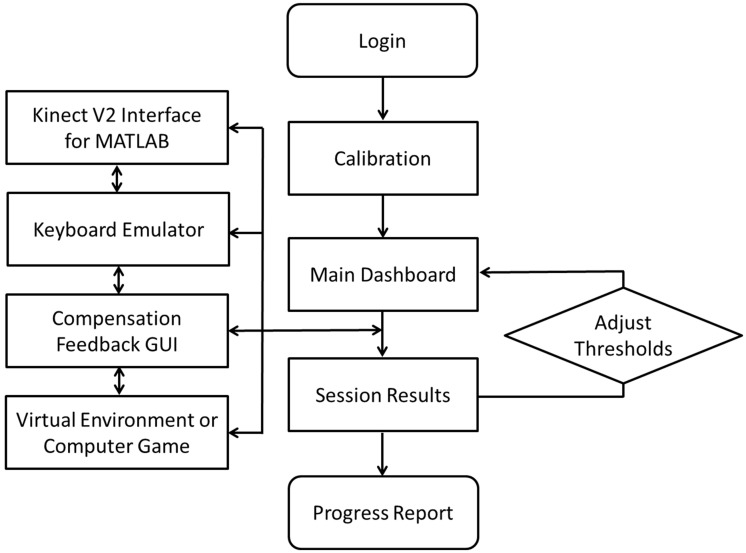
Figure 2.
Flow diagram of VRShape processes and interfaces. Once logged in, the system is calibrated for reaching and compensation thresholds. The main dashboard allows setting of all parameters and display of the Kinect V2 data feed. Once a session is started, parallel processes use Kinect data to control a VE through a keyboard emulator. Compensation feedback is provided in a separate GUI. Results are provided and thresholds can be adjusted before transitioning to another VE. At the end of a session, a progress report is presented and saved.
VRShape has the built-in capability to recognize a variety of therapy-relevant UE movements that are common targets of repetitive training including forward, side, and vertical reaching; shoulder flexion, abduction, and internal rotation; elbow flexion; and wrist flexion and deviation. These movements are commonly affected by damage to the corticospinal tract due to stoke and resulting hemiparesis.34 The software also has the capability to recognize trunk flexion, lateral flexion, and axial rotation simultaneously during the performance of UE movements. Post-stroke deficits during functional reaching are well researched and often defined by decreased endpoint precision, increased time (slower reaches), fractionation of movement, and reduced ROM.9,10,35 Research in post-stroke reaching also provides the most robust evidence for the nature of compensatory movements post-stroke, identifying trunk movements as the most common.9,10,35 Our previous investigations have identified that the Kinect V2 is highly reliable and valid for measuring reaching.33 For these reasons, the remainder of this investigation focuses on repetitive reaching and the associated trunk flexion compensations.
To interact with the system, the user either stands or sits in a chair facing the Kinect sensor at a distance of approximately 2.0 m with the sensor situated at a height of approximately 1.2 m above the ground (Figure 1). Because of the relatively long distance from user to sensor, a large television screen is used to display the computer game. The software recognizes a movement repetition as completed when it surpasses a defined threshold, most commonly the user’s targeted ROM in terms of linear or angular displacement (Figure 3). For reaching movements, this threshold is defined as a minimum Euclidean distance of the hand relative to the initial position of the shoulder, which can be defined by an automatic calibration algorithm (maximum ROM during a preliminary set of reaches) or manually by the supervising researcher or therapist. A keyboard emulator activates when this threshold is met, allowing for the control of a computer game (Figure 4). The keyboard emulator can be programmed with any key press or mouse movement required for a specific application. For example, VRShape can be calibrated to press the spacebar when the user reaches in the sagittal plane with his or her right arm by a distance of 40 cm in order to activate a virtual action requiring the spacebar in a specific computer game. This algorithm is similar to strategies that have been used in other research works performed within our laboratory for persons with a variety of motor impairments.21–25
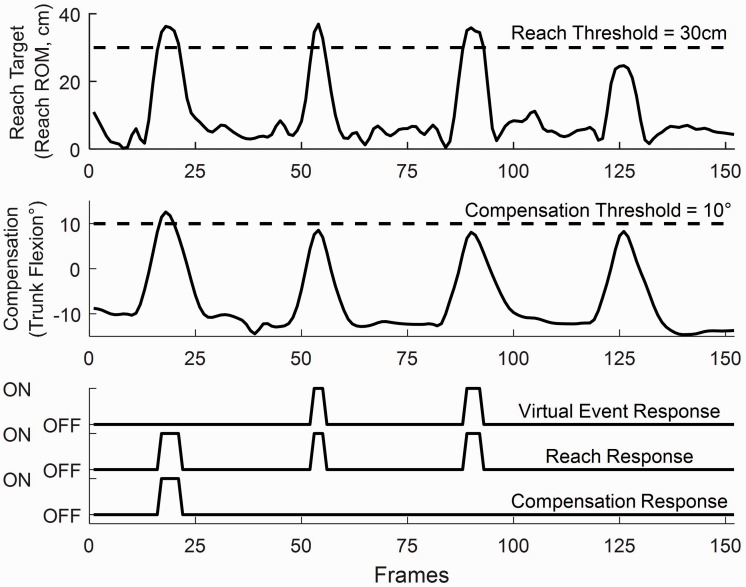
Figure 3.
Examples of signals for reaching movement (top axes), compensatory movement (middle axes), and the response of the VRShape software (bottom axes). During the first attempt, the participant exceeded the compensation threshold (middle). Because virtual events only occur when reaching without compensation, there was no output for the first attempt. The subsequent two attempts resulted in a virtual event, while the last did not because the participant failed to reach far past the threshold.
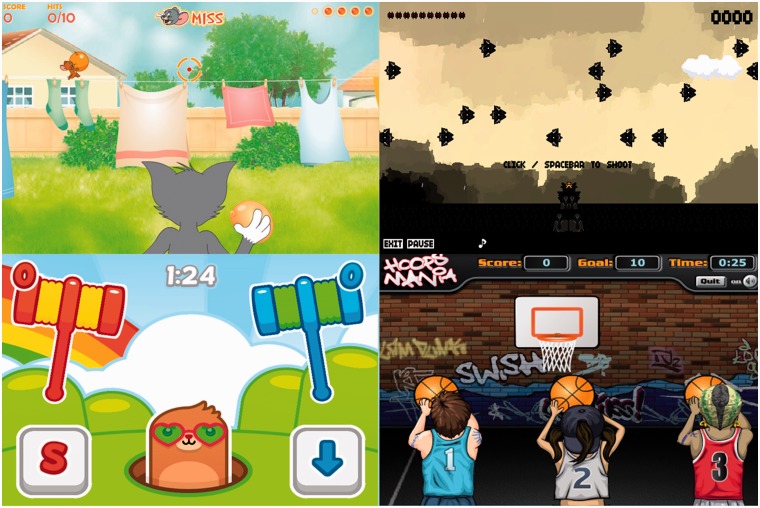
Figure 4.
Examples of the four most common VEs used during this investigation. Tom and Jerry (top left) requires the client to reach to trigger Tom to throw a water balloon at Jerry. Ten Bullets (top right) requires the client to reach to shoot at spaceships across the sky. Mole Hammers (bottom left) requires the client to reach to slam a hammer on a mole’s head. Hoops Mania (bottom right) requires the client to reach to shoot at a moving basketball hoop. Written consent was obtained from game publishers for the reproduction and publication of these screenshots.
The most novel design feature of VRShape is its ability to “shape” compensatory trunk movements. Shaping is a technique founded in behavioral science and utilized as a key component of CIMT to incrementally match the difficulty of tasks to the abilities and characteristics of the client.5 Our software has the ability to shape compensations by incrementally decreasing the allowable amount of trunk flexion over the course of an intervention based on the client’s movements. An automatic calibration algorithm for defining a compensation threshold can be employed before a session. This algorithm measures the average trunk flexion used during the performance of multiple unconstrained UE movements over the course of a small timespan, and uses 90% of this average as the trigger for providing feedback during subsequent therapy. This threshold can be manually adjusted at the discretion of the researcher in order to provide the best experience for the user and avoid frustration, but the value of 90% is intended to keep the user at an adequate level of challenge similar to existing rules for task grading in CIMT and task-oriented training.36–38 The reaching ROM threshold can also be established in this way: the average local extrema for shoulder and hand position can be used to automatically calculate reach distance, or it can be manually set by client or therapist. For the purposes of this study, the compensation and reaching thresholds were calibrated at the beginning and held constant at 90% and 100%, respectively, throughout each session. When implemented within an intervention in the future, these thresholds will be recalibrated at the beginning of each subsequent session.
Feedback about compensatory movement is provided in three different ways. Once a compensation threshold is defined, feedback is provided during gameplay by means of audio, visual, or virtual event suppression. Audio feedback can be provided in the form of a loud alarm that triggers when the user moves his or her trunk past the threshold. Visual feedback can be provided in the form of a graphical movement trace with a prominent line representing the maximum allowable trunk movement. When the compensation threshold is exceeded, a large stoplight image can be displayed over this movement graph. Finally, virtual event suppression cancels the outcome of a completed movement repetition if the user has compensated; in the above example, this would mean that the spacebar would not be pressed if the user flexed their trunk too far even while reaching beyond the 40 cm threshold. The combination of a customizable reaching trigger for interacting with a VE and a customizable compensation threshold beyond which multimodal feedback is provided are theory-based design features intended to encourage simultaneous increases in ROM and decreases in compensation utilization.
Feasibility testing
Five participants with chronic stroke (4 males, 1 female; mean age 63.2 years) were recruited for this study from the greater St. Louis area. Participants were eligible for inclusion if they (1) were aged 40–80 years, (2) experienced an ischemic stroke greater than six months prior, (3) exhibited persistent hemiparesis as noted by a score of 1–3 on the motor arm subscale of the National Institutes of Health Stroke Scale39 (NIHSS), (4) displayed some voluntary activity in proximal or distal UE joints when asked to reach for an item in their immediate space, and (5) utilized noticeable trunk compensation (>20°) when performing reaching movements with the impaired arm. Participants were excluded if they had any medical conditions that would impair their ability to play computer games, such as significant comprehension difficulties, attentional disorders, or visual field deficits (Table 1). All participants provided written consent and the Institutional Review Board (IRB) of the Washington University School of Medicine approved all study activities.
Table 1.
Participant characteristics. Basic demographic data are shown along with the NIHSS arm/motor subscale, data about computer knowledge and usage, and data about VR knowledge and usage obtained from the ITC-SOPI.
| Participants |
|||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |
| Age | 62 | 63 | 44 | 69 | 78 |
| Gender | M | M | M | F | M |
| Years post-stroke | 2 | 1 | 4 | 6 | 1 |
| Affected side | R | R | R | L | L |
| NIHSS arm/motor | 1 | 1 | 1 | 2 | 1 |
| Computer experience | None | Expert | Intermediate | None | Expert |
| Computer game use | Never | Often | Every day | Never | Every day |
| VR knowledge | None | Intermediate | None | None | None |
| VR usage | No | Yes | No | No | No |
M: male; F: female; R: right; L: left; Afr. Amer.: African American; NIHSS: National Institutes of Health Stroke Scale; ITC-SOPI: Independent Television Commission Sense of Presence Inventory.
Each participant took part in a one-hour session during which VRShape was used to control four separate computer games by means of reaching movements calibrated to each participant’s abilities and ROM. Movements were performed in the scaption plane (45° between sagittal and frontal) to optimize tracking accuracy with the Kinect and translation to everyday functional movements. Two of these games were used consistently across individuals and two were chosen by each individual from a previously defined list of games known be compatible with the VRShape software. Each game was played for approximately 10 minutes and 2-3 minutes were allowed for calibration and transitions between games. The 2–3 min calibration period involved approximately 20-30 warmup repetitions. Outcomes measures were collected following the session. During calibration, the participants were asked to reach as far as possible in the scaption plane while VRShape recorded their average trunk flexion and reach distance; for the remainder of the session, trunk flexion was limited to 90% and reach distance was set to 100% of these values. In this way, the participants were asked to use less trunk flexion and more shoulder flexion, shoulder abduction, and elbow extension to transport their hand the same or greater distance as used in the unconstrained calibration period. The primary outcome measures for this feasibility study were related to usability, motivation for use, and sense of presence during use of the VRShape software.
The System Usability Scale (SUS) is a 10-item questionnaire that uses a five-point Likert scale to assess usability, which may be defined as a technological system’s appropriateness for its designated task within its intended context.40 The SUS has been used in numerous publications, demonstrating excellent reliability and validity and taking less than 10 min to administer.41 A sum score on a scale from 0 to 100, also representing percentile rank, is tabulated after the evaluation of each individual item. A sum score of 68 is considered “average”,42 and an adjective rating scale has been validated to describe a system as “worst imaginable” (0–20.3), “awful” (20.3–35.7), “poor” (35.7–50.9), “OK” (50.9–71.4), “good” (71.4–85.5), “excellent” (85.5–90.9), and “best imaginable” (90.9–100).43
The Intrinsic Motivation Inventory (IMI) is a multidimensional assessment that has demonstrated good psychometrics for measuring subjective experience of an activity related to interest/enjoyment, perceived competence, effort/importance, and pressure/tension.44 Specifically, the interest/enjoyment subscale takes less than 10 min to administer and consists of seven items measured on a seven-point Likert scale that can be summed for a total score from 0 to 49. This subscale has previously been used within our laboratory.21,23,24 While classification ranges have not been established for the IMI, an approximate average item score of six (“mostly agree”) and a total subscale score of 42 is generally considered to be highly motivating in existing literature that has used the interest/enjoyment subscale for assessing VR-based rehabilitation.45,46
The Independent Television Commission-Sense of Presence Inventory (ITC-SOPI) measures a user’s sense of presence, or the overall subjective sensation of “being there”, across domains related to spatial presence, engagement, ecological validity, and negative effects.47 It consists of 44 items measured on a five-point Likert scale; these ordinal responses are subsequently averaged within each of the four domains to produce summary scores. The validity of the ITC-SOPI has been well established and score ranges exist for multiple media formats including television, computer games, and VR.47,48 There are three parts to the ITC-SOPI: background information, Part A, and Part B. The background information section asks questions about prior knowledge and experience with computers, television, and VR (selected questions, Table 1). Part A includes 10 questions on engagement and negative experiences, and Part B contains the remaining 34 questions from all four domains. The entire assessment takes about 15 min to administer.
VRShape automatically collected performance metrics at an approximate rate of 30 Hz during each session. The most important of these metrics were the number of repetitions and ROM achieved with the UE during reaching. The software also collected XYZ position data for all body landmarks viewed by the Kinect during interaction with the system. These data were filtered (6th order, 6 Hz Butterworth) and post-processed in MATLAB to calculate kinematic variables including reaching ROM, sagittal and frontal planar reach distance, shoulder flexion and abduction, trunk flexion and lateral flexion, and elbow flexion. In the event of occluded or mislabeled joint data from the Kinect identified by abnormal and abrupt changes in signal, spline interpolation was used to estimate relevant kinematic variables. A peak detection algorithm was applied to find the average maximum value for each of these kinematic variables over the course of several minutes of therapy and thousands of collected frames. In the event of missing data, which intermittently occurred due to technical issues and the novelty of using the system with stroke participants, metrics were extrapolated to the length of the session based on repetition and compensation rates.
Finally, at the conclusion of the session, a qualitative interview was performed to identify facilitators/barriers, likes/dislikes, and suggestions for improvement for the VRShape software. Simple questions including “What would you change about the system?” and “What would encourage or stop you from using the system regularly?” were used to facilitate discussion, similar to custom usability questionnaires utilized in prior research.49
To assess the effect of prior knowledge or experience with computers or VR, selected items from the ITC-SOPI background assessment were converted to ordinal scales and compared to SUS sum scores, IMI interest/enjoyment scores, and ITC-SOPI subdomains. Spearman’s rank-order correlations (ρ) were used due to the small sample size and ordinal type data.
Results
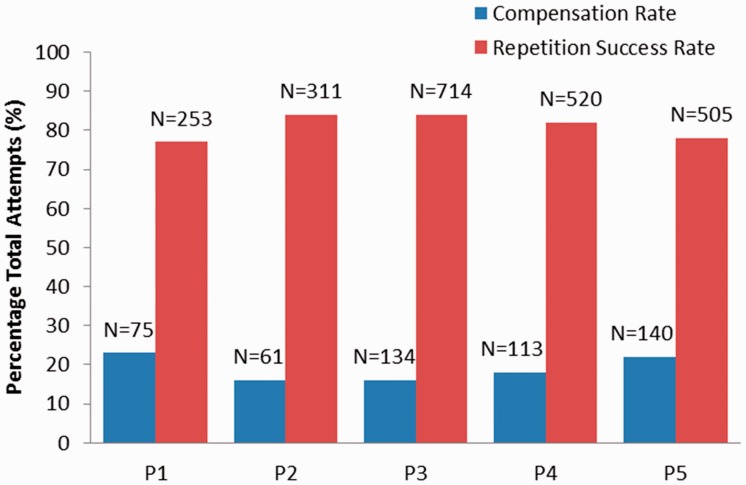
The participants performed an average of 461 (SD = 184) repetitions in only 40 min of gameplay with VRShape (Table 2). Seven different games were successfully played with VRShape and the average number of completed repetitions varied by game (range = 21–239). To achieve this number of successful repetitions, the participants exceeded their individualized compensation thresholds (used too much trunk flexion) and triggered feedback an average of 105 (SD = 35) times per session. The result is that the participants only performed “bad” repetitions involving compensation at a rate between 16% and 22% and achieved success at a rate between 77% and 84% (Figure 5).
Table 2.
Results for repetitions, compensation, and kinematic variables presented for each participant and as group mean (SD). Repetitions and compensations are calculated from performance relative to individualized reach and trunk flexion thresholds within VRShape. The average percentage magnitude achieved beyond (reach) or below (compensation) is also presented. Baseline trunk flexion angles are calculated from calibration data.
| Variable | P1 | P2 | P3 | P4 | P5 | Mean (SD) |
|---|---|---|---|---|---|---|
| Successful repetitions (%)a | 253 (77) | 311 (84) | 714 (84) | 520 (82) | 505 (78) | 461 (184) |
| Compensations (%)* | 75 (23) | 61 (16) | 134 (16) | 113 (18) | 140 (22) | 105 (35) |
| Percent reach target (%) | 108.7 | 186.0 | 110.5 | 121.6 | 106.6 | 126.7 (33.7) |
| Percent compensation limit (%) | 72.0 | 69.0 | 59.9 | 77.0 | 81.0 | 71.8 (8.1) |
| Reach ROM (cm) | 49.5 | 55.6 | 51.4 | 50.9 | 49.8 | 51.4 (2.5) |
| Sagittal reach distance (cm) | 32.6 | 46.5 | 43.1 | 38.1 | 42.2 | 40.5 (5.3) |
| Front reach distance (cm) | 12.7 | 18.7 | 14.2 | 9.5 | 11.3 | 13.3 (3.5) |
| Shoulder flexion (deg) | 13.5 | 12.2 | 11.4 | 15.7 | 18.3 | 14.2 (2.8) |
| Shoulder abduction (deg) | 39.1 | 29.5 | 40.0 | 24.3 | 31.9 | 33.0 (6.6) |
| Baseline trunk flexion (deg) | 10.9 | −6.6 | −9.7 | −4.8 | −18.5 | −5.7 (10.7) |
| Trunk flexion (deg) | 7.2 | −14.5 | −16.7 | −7.7 | −24.7 | −11.3 (12.0) |
| ΔTrunk flexion (deg) | 3.7 | 7.9 | 7.0 | 2.9 | 6.2 | 5.5 (2.2) |
| Trunk lateral flexion (deg) | 2.3 | 2.6 | 1.9 | 1.4 | 3.1 | 2.3 (0.7) |
| Elbow flexion (deg) | 96.5 | 86.2 | 82.7 | 91.7 | 74.6 | 86.3 (8.4) |
Parentheticals are the percentage of successful reps and reps with compensation relative to an estimate of the total number of repetitions attempted.
Figure 5.
Comparison of repetition success rate and compensation rate for each participant. The raw number of successful repetitions and the number of compensations are shown above each column.
In terms of movement kinematics, the participants achieved an average of approximately 126.7% (SD = 33.7) of the threshold required for reaching ROM while using only 71.8% (SD = 8.1) of the trunk flexion feedback threshold (Table 2). Planar reach distances, in general, show that the majority of participants were reaching more in the sagittal plane (M = 40.5 cm, SD = 5.3) than in the frontal plane (M = 13.3 cm, SD = 13.3) during the scaption reach. More shoulder abduction (M = 3.0°, SD = 6.6) than flexion (M = 14.2°, SD = 2.8) was utilized to transport the arm and achieve the required reach distance. Trunk flexion varied across individuals, but remained below feedback thresholds for the majority of repetition attempts (M = −11.3°, SD = 12.0). All participants decreased their trunk flexion during the session relative to baseline thresholds (M = 5.5, SD = 2.2). Lateral flexion was not heavily utilized during reaches (M = 2.3°, SD = 0.7). Finally, elbow flexion remained similar across individuals during reaching (M = 86.3°, SD = 8.4). For trunk flexion values at baseline and during the session (Table 2), it should be noted that average maximums are negative in four of the participants (P2–P5) due to (1) variance in landmark tracking of the Kinect in seated positions and (2) extended neutral postures of participants seated in wheelchairs and with impaired postural control. The system in fact reported trunk flexion values to a greater negative extent when these participants were not reaching and seated in neutral.
The participants found the system’s usability to be “OK” according to the average SUS sum score (M = 69.0, SD = 24.66) (Table 3). Individual usability ratings varied, with one participant reporting “best imaginable”, one participant reporting “excellent”, two participants reporting “OK”, and one participant reporting “poor.” The participant (P1) that rated the system as “poor” reported the system to be complex (question 2) and require extensive learning before use (question 10). The participants also found the system to be highly motivating according to the average IMI interest/enjoyment score (M = 43.2, SD = 7.66). Individual IMI scores also varied.
Table 3.
Results for each participant for SUS, IMI, and ITC-SOPI. Only the interest/enjoyment subscale of the IMI was utilized. ITC-SOPI scores are divided into each subscale.
| Variable | P1 | P2 | P3 | P4 | P5 | Mean (± SD) |
|---|---|---|---|---|---|---|
| SUS sum score | 42.5 | 90.0 | 55.0 | 57.5 | 100.0 | 69.0 ± 24.7 |
| IMI interest/enjoyment | 44.0 | 45.0 | 48.0 | 30.0 | 49.0 | 43.2 ± 7.7 |
| ITC-SOPI spatial presence | 1.4 | 1.5 | 3.2 | 2.1 | 4.1 | 2.4 ± 1.2 |
| ITC-SOPI engagement | 2.8 | 3.3 | 4.4 | 2.9 | 4.4 | 3.6 ± 0.8 |
| ITC-SOPI ecological validity | 1.0 | 1.2 | 5.0 | 1.8 | 4.0 | 2.6 ± 1.8 |
| ITC-SOPI negative effects | 1.7 | 1.8 | 1.0 | 1.8 | 1.0 | 1.5 ± 0.4 |
SUS: System Usability Scale; IMI: Intrinsic Motivation Inventory; ITC-SOPI: Independent Television Commission Sense of Presence Inventory.
ITC-SOPI scores are separated into each subscale: spatial presence, engagement, ecological validity, and negative effects. Since the Likert scale score does not carry inherent meaning, similar validated media forms evaluated by the creators of the ITC-SOPI are included for reference.47 The participants experienced a modest sense of presence according to the average spatial presence subscale score (M = 2.44, SD = 1.19), representing an experience similar to that of viewing a movie at the cinema. The participants were highly engaged during their experience with the system, exemplified by the relatively high average score on the engagement subscale (M = 3.57, SD = 0.77), most similar to the experience of playing a commercially-developed computer game. Ecological validity of the system was found to be modest, exhibited by the modest ecological validity subscale score (M = 2.60, SD = 1.79). This was most similar to the experience of viewing a movie in a group setting on a low definition television. Finally, the participants experienced very few negative effects, exemplified by the low average negative effects subscale score (M = 1.47, SD = 0.43). This score was lower than any media format validated with the ITC-SOPI.
Significant correlations were found between computer game use and IMI ratings (ρ = 0.95, p = 0.04) and ITC-SOPI engagement ratings (ρ = 0.97, p < 0.01). This signifies that the participants that used computer games more often were also more motivated and more engaged during use of VRShape. No other significant correlations were found, but in general, prior computer knowledge or use showed greater association with outcomes than did VR knowledge or use.
Four out of five participants noted that he or she would need more time with the system to adequately comment on its positive and negative attributes. Each participant expressed excitement to continue use of VRShape in the context of an intervention. Three out of five participants, in one form or another, expressed interest for games beyond those that they used in their session, including genres like card games, board games, hunting games, and sports games. One participant (P5) stated that he felt like the system was making him do more than he was accustomed to from his previous rehabilitation experience: “I can feel it in my arm, it’s making me move more than regular therapy”. Small technical issues were noted during sessions, including some difficulty viewing or hearing feedback related to trunk compensation, issues navigating game menus while motion capture was active, and delays while switching games or adjusting movement thresholds.
Discussion
The purpose of this study was to develop and test a VR tool designed to elicit high doses of UE movement repetitions while measuring and shaping compensation for persons with stroke. The resulting software, VRShape, demonstrated the ability to engage participants in an intense quantity of reaching movements while shaping success and providing feedback based on excessive trunk flexion. The participants found the software to be usable, motivating, and safe.
Several technical limitations were identified as a result of this initial testing with VRShape. First, the workflow of GUIs and background data was not optimal and therefore sometimes caused brief time delays during sessions. The process for transitioning between computer games, adjusting movements, and displaying post-session data often took slightly longer than expected. Data flow and GUI layouts should be streamlined to make therapy sessions more efficient and decrease the likelihood of client disengagement. Second, a more expansive library of computer games must be identified; several participants gave suggestions for interesting game genres that were not yet a part of the VRShape software. These games may also include the use of a wireless mouse for gameplay and menu navigation. Along with that, it is clear that repetition rates may be bounded by the type of game being played, exemplified by the large range of repetitions. For example, a whack-a-mole game, Mole Hammers, elicited about double the repetitions (N = 242) of a basketball game, Hoops Mania (N = 124). Third, the presentation of feedback may not be adequate for some participants due to (1) distance from the display screens, (2) the use of a second display for visual feedback, and (3) competing audio between feedback mechanisms and computer games. To mitigate issues with visual feedback, large screens or projectors (48”+) should be incorporated for side-by-side displays and the distance to the sensor should be reduced to the lowest possible while maintaining Kinect V2 tracking fidelity. Visual feedback might also be displayed within the same window as the computer game to avoid issues with divided attention. Methods to change the type, volume, and duration of auditory feedback would enhance salience and distinguish from in-game audio effects. Finally, motion tracking with the Kinect V2 was not completely reliable and intermittently resulted in occluded or mislabeled joints, difficulties with computer game interaction, and errors in feedback. Tracking issues and the associated inaccurate feedback could negatively affect rehabilitation outcomes and create frustration for clients; however, it should be noted that usability was rated near average, no participants specifically reported such technical issues, and these occurrences were relatively sparse within the collected kinematic data. Future work should seek to assess and reduce these motion capture issues and incorporate new-and-improved sensor technology as it becomes available.
An average of 81% of repetitions was performed successfully by the participants and the remaining 19% were performed with excessive trunk compensation (Table 2). Compensation thresholds were rounded to 90% of the trunk flexion used in an initial calibration period at the beginning of the session, and VRShape was designed to increase task difficulty (restrain compensation) in these 10% increments at the beginning of each session over the course of an intervention. The process of shaping is generally defined by rules for increasing or decreasing task difficulty related to rate of success. In CIMT, a general rule is to make the task slightly more difficult if a participant achieves five successful repetitions in a row38 or if performance has plateaued for as many as 10 consecutive trials.37 In task-based training protocols, it is recommended that task difficulty be graded up if a participant successfully completes more than 100 repetitions in 15 min, and graded down if a participant fails more than 50 repetitions in 15 min.36 The average repetition rate with VRShape translates to approximately 170 successful and 40 “bad” repetitions using compensation per 15 min. Furthermore, the participants were able to achieve well over their reaching thresholds (127%) while using less than their compensation threshold (72%), signifying that most repetitions were highly successful. While the participants did not express frustration or boredom in this single session, it may indeed be a limitation that the prescribed activity was not adequately challenging to affect within-session or long-term motor improvements. Similar research using VR and robotics shows single-session changes in trunk flexion given compensation limitations up to 50% of initially calibrated values.30 Following warm-up and with increasing familiarity with the system and computer games, the performance may significantly improve within just 40 min of active use. More stringent compensation limitations (<90% of calibration), tougher reaching targets (>100% of calibration), and algorithms to change these thresholds dynamically within a session may create a more challenging experience and enhance ultimate rehabilitation outcomes.
Contemporary motor learning research suggests that thousands of repetitions are required to retrain the brain in order to acquire a new motor skill or make up for injured neural areas resulting from a stroke.4 Traditional physical and occupational therapy may only involve 30–40 repetitions in a single session.8 In a study translating research in animal dosing to persons with stroke, Birkenmeier et al.36 had participants perform an average of 322 repetitions per 1-h session, resulting in improvements in motor function, activity performance, and participation. In a recent large-scaled dosing study, Lang et al.50 included an individualized maximum group that was able to achieve an average of 10,808 repetitions over an average of 36 sessions, resulting in modest improvements in motor function. In our study, the participants were able to achieve an average of 461 repetitions in 40 minutes of using VRShape, exceeding repetition rates documented for these traditionally administered task-based training procedures. This repetition rate is similar to that seen in other VR systems (300–600 per session) and represents an advantage of VR-based motor therapy.21,23–25 Extrapolated over the course of an intervention, it would require only approximately 22 sessions to achieve the colloquial 10,000 repetition threshold achieved in recent dosing studies and required for retained motor learning.
The usability of the system was found to be “OK” and motivation for using the system was found to be high, but these values varied drastically across individuals. A mean SUS score of 69.0 is very near the global average, and is similar to other preliminary investigations of VR-based therapy systems in the literature.41 In a study involving a similar customizable VR-based system for in-home therapy, Proffitt et al.51 found scores in the same usability range. An average IMI of 43.2 is considered highly motivating and is also similar to existing VR-based research. In a study involving a more immersive VR-based system utilizing a mechanical device and custom-built computer games, Sampson et al.46 found a similar interest/enjoyment rating (M = 43.4) in a small sample of stroke participants. Wide variations in the ability and motivation to use technology may be mitigated by previous knowledge and experience with computers and VR (Table 1).
The subjective experience of spatial presence and ecological validity, both aspects of the overall sense of presence in a VE, was found to be modest following use of VRShape. The sense of presence is theoretically and empirically related to the provision of an extensive, surrounding display that vividly engages multiple senses to make the user feel included in the virtual experience.52 Recent research suggests that VR systems may be divided into two different categories: “immersive” and “non-immersive”.15 VRShape may be considered to be a nonimmersive system, because it does not include technology such as large screen projection, head-mounted displays, haptic feedback systems, or complex, custom-designed VEs that are required for immersive systems. It is therefore fitting that the participants scored their sense of spatial presence and ecological validity within VRShape similarly to other validated, nonimmersive systems, namely viewing a movie in a group setting either at the cinema or on a television.47
Correlation results show that the participants’ experience of VRShape may be affected by literacy and current usage of computers and VR. For example, the participant (P1) that rated usability as “poor” according to the SUS reported no prior knowledge and never interacted with computers or VR in his daily life (Table 1). Persons with stroke often have difficulties with or give up everyday technology use, including videogames, which is associated with limitations in ADL performance, reduced quality of life, and barriers to participation.53 Due to the small size and large variation within our sample, it is difficult to make definitive conclusions, but future work should consider those that are most appropriate for VRShape based on motivation and value for technology use and technology-related rehabilitation goals.
There are a number of modern VR tools designed to provide a fun, engaging medium for performing UE motor therapy.16,17 To our knowledge, there are very few existing tools designed specifically to shape the amount of trunk compensation utilized over time through the provision of feedback and user-specific shaping algorithms.54 The main ingredients of a theory-driven intervention targeting salient motor learning and associated neuroplastic change are (1) the repetitive practice of meaningful tasks, (2) progressive task difficulty matched to the person’s abilities, (3) involvement of problem-solving mechanisms, (4) engagement and motivation to improve, (5) feedback about performance and results of practice.4,20 In this study, we have demonstrated that VRShape is designed with each of these areas in mind and is capable of providing intense, motivating, challenging motor therapy for persons with stroke. These advantages, combined with its low-cost, ease-of-use, and focus on objective compensation measurement, provide tremendous potential for use as a tool for both clinical application and rehabilitation science research.
Conclusion
The present study described the development of a novel VR tool, namely VRShape, and its initial feasibility testing with a small cohort of persons with hemiparetic stroke. VRShape proved to be a capable tool for eliciting high doses of UE repetitions while providing feedback about trunk compensation during a VR-based motor therapy session. The system was found to be usable, highly motivating, and safe while providing a modest sense of virtual presence. Areas requiring improvement were identified and will be addressed, and future research is needed to establish the long-term feasibility and preliminary efficacy of VRShape for use as the basis of VR-based motor rehabilitation.
Acknowledgements
The authors would like to thank the study participants and members of the Human Performance Laboratory for offering their time and effort.
Contributorship
MF conceived this research, executed all aspects of the study protocol, and wrote the manuscript. JR served as research mentor, oversaw the study design and execution, and reviewed and approved the manuscript text.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Both MF and JE have financial interests in Accelerated Rehabilitation Technologies (ART) and may benefit if the company is successful in licensing and marketing software related to VRShape.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to thank the Program in Occupational Therapy at Washington University in St. Louis for supporting this research.
Guarantor
MF
References
- 1.Nichols-Larsen DS, Clark PC, Zeringue A, et al. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 2005; 36: 1480–1484. [DOI] [PubMed] [Google Scholar]
- 2.Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003; 34: 2181–2186. [DOI] [PubMed] [Google Scholar]
- 3.Lai SM, Studenski S, Duncan PW, et al. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002; 33: 1840–1844. [DOI] [PubMed] [Google Scholar]
- 4.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 2008; 51: S225–239. [DOI] [PubMed] [Google Scholar]
- 5.Taub E, Crago JE, Burgio LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav 1994; 61: 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jutai JW, Teasell RW. The necessity and limitations of evidence-based practice in stroke rehabilitation. Top Stroke Rehabil 2003; 10: 71–78. [DOI] [PubMed] [Google Scholar]
- 7.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther 2007; 31: 3–10. [DOI] [PubMed] [Google Scholar]
- 8.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 2009; 23: 313–319. [DOI] [PubMed] [Google Scholar]
- 9.Cirstea M, Levin MF. Compensatory strategies for reaching in stroke. Brain 2000; 123: 940–953. [DOI] [PubMed] [Google Scholar]
- 10.Levin MF, Michaelsen SM, Cirstea CM, et al. Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Exp Brain Res 2002; 143: 171–180. [DOI] [PubMed] [Google Scholar]
- 11.Alaverdashvili M, Foroud A, Lim DH, et al. “Learned baduse” limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: a new analysis of the effect of gestures on success. Behav Brain Res 2008; 188: 281–290. [DOI] [PubMed] [Google Scholar]
- 12.Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci 2010; 124: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitago T, Liang J, Huang VS, et al. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair 2013; 27: 99–109. [DOI] [PubMed] [Google Scholar]
- 14.Holden MK. Virtual environments for motor rehabilitation: review. Cyberpsychol Behav 2005; 8: 187–211. [DOI] [PubMed] [Google Scholar]
- 15.Henderson A, Korner-Bitensky N, Levin M. Virtual reality in stroke rehabilitation: a systematic review of its effectiveness for upper limb motor recovery. Top Stroke Rehabil 2007; 14: 52–61. [DOI] [PubMed] [Google Scholar]
- 16.Laver KE, Lange B, George S, et al. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2017; 11: CD008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohse KR, Hilderman CG, Cheung KL, et al. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS One 2014; 9: e93318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbetta D, Imeri F, Gatti R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: a systematic review. J Physiother 2015; 61: 117–124. [DOI] [PubMed] [Google Scholar]
- 19.Darekar A, McFadyen BJ, Lamontagne A, et al. Efficacy of virtual reality-based intervention on balance and mobility disorders post-stroke: a scoping review. J Neuroeng Rehabil 2015; 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin MF, Weiss PL, Keshner EA. Emergence of virtual reality as a tool for upper limb rehabilitation: incorporation of motor control and motor learning principles. Phys Ther 2015; 95: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauterbach SA, Foreman MH, Engsberg JR. Computer games as therapy for persons with stroke. Games Health J 2013; 2: 24–28. [DOI] [PubMed] [Google Scholar]
- 22.Suma EA, Krum DM, Lange B, et al. Adapting user interfaces for gestural interaction with the flexible action and articulated skeleton toolkit. Comput Graphics 2013; 37: 193–201. [Google Scholar]
- 23.Behar C, Lustick M, Foreman MH, et al. Personalized virtual reality for upper extremity rehabilitation: moving from the clinic to a home exercise program. J Intellect Disabil Diagn Treat 2016; 4: 160–169. [Google Scholar]
- 24.Sevick M, Eklund E, Mensch A, et al. Using free internet videogames in upper extremity motor training for children with cerebral palsy. Behav Sci 2016; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mraz K, Eisenberg G, Diener P, et al. The effects of virtual reality on the upper extremity skills of girls with Rett syndrome: a single case study. J Intellect Disabil Diagn Treat 2016; 4: 152–159. [Google Scholar]
- 26.Michaelsen SM, Dannenbaum R, Levin MF. Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke 2006; 37: 186–192. [DOI] [PubMed] [Google Scholar]
- 27.Thielman G, Kaminski T, Gentile AM. Rehabilitation of reaching after stroke: comparing 2 training protocols utilizing trunk restraint. Neurorehabil Neural Repair 2008; 22: 697–705. [DOI] [PubMed] [Google Scholar]
- 28.Woodbury ML, Howland DR, McGuirk TE, et al. Effects of trunk restraint combined with intensive task practice on poststroke upper extremity reach and function: a pilot study. Neurorehabil Neural Repair 2009; 23: 78–91. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian SK, Lourenco CB, Chilingaryan G, et al. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabil Neural Repair 2013; 27: 13–23. [DOI] [PubMed] [Google Scholar]
- 30.Valdes BA, Van der Loos HFM. Biofeedback vs. game scores for reducing trunk compensation after stroke: a randomized crossover trial. Top Stroke Rehabil 2018; 25: 96–113. [DOI] [PubMed] [Google Scholar]
- 31.Clark RA, Pua Y-H, Oliveira CC, et al. Reliability and concurrent validity of the Microsoft Xbox One Kinect for assessment of standing balance and postural control. Gait Posture 2015; 42: 210–213. [DOI] [PubMed] [Google Scholar]
- 32.Kuster RP, Heinlein B, Bauer CM, et al. Accuracy of KinectOne to quantify kinematics of the upper body. Gait Posture 2016; 47: 80–85. [DOI] [PubMed] [Google Scholar]
- 33.Reither LR, Foreman MH, Migotsky N, et al. Upper extremity movement reliability and validity of the Kinect version 2. Disabil Rehabil Assist Technol 2017; 13: 54–59. [DOI] [PubMed] [Google Scholar]
- 34.Lang CE, Bland MD, Bailey RR, et al. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther 2013; 26: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roby-Brami A, Feydy A, Combeaud M, et al. Motor compensation and recovery for reaching in stroke patients. Acta Neurol Scand 2003; 107: 369–381. [DOI] [PubMed] [Google Scholar]
- 36.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair 2010; 24: 620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taub E, Uswatte G, King DK, et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke 2006; 37: 1045–1049. [DOI] [PubMed] [Google Scholar]
- 38.Uswatte G, Taub E, Morris D, et al. Contribution of the shaping and restraint components of Constraint-Induced Movement therapy to treatment outcome. NeuroRehabilitation 2006; 21: 147–156. [PubMed] [Google Scholar]
- 39.Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke 1997; 28: 307–310. [DOI] [PubMed] [Google Scholar]
- 40.Brooke J. SUS-A quick and dirty usability scale. Usab Eval Ind 1996; 189: 4–7. [Google Scholar]
- 41.Brooke J. SUS: a retrospective. J Usab Stud 2013; 8: 29–40. [Google Scholar]
- 42.Sauro J. A practical guide to the system usability scale: background, benchmarks & best practices. Denver, CO: Measuring Usability LLC, 2011.
- 43.Bangor A, Kortum P, Miller J. Determining what individual SUS scores mean: adding an adjective rating scale. J Usab Stud 2009; 4: 114–123. [Google Scholar]
- 44.McAuley E, Duncan T, Tammen VV. Psychometric properties of the Intrinsic Motivation Inventory in a competitive sport setting: a confirmatory factor analysis. Res Q Exerc Sport 1989; 60: 48–58. [DOI] [PubMed] [Google Scholar]
- 45.Colombo R, Pisano F, Mazzone A, et al. Design strategies to improve patient motivation during robot-aided rehabilitation. J Neuroeng Rehabil 2007; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sampson M, Shau YW, King MJ. Bilateral upper limb trainer with virtual reality for post-stroke rehabilitation: case series report. Disabil Rehabil Assist Technol 2012; 7: 55–62. [DOI] [PubMed] [Google Scholar]
- 47.Lessiter J, Freeman J, Keogh E, et al. A cross-media presence questionnaire: the ITC-sense of presence inventory. Presence: Teleoper Virtual Environ 2001; 10: 282–297. [Google Scholar]
- 48.Schuemie MJ, Van Der Straaten P, Krijn M, et al. Research on presence in virtual reality: a survey. CyberPsychol Behav 2001; 4: 183–201. [DOI] [PubMed] [Google Scholar]
- 49.Cameirao MS, Badia SB, Duarte E, et al. The combined impact of virtual reality neurorehabilitation and its interfaces on upper extremity functional recovery in patients with chronic stroke. Stroke 2012; 43: 2720–2728. [DOI] [PubMed] [Google Scholar]
- 50.Lang CE, Strube MJ, Bland MD, et al. Dose response of task-specific upper limb training in people at least 6 months poststroke: a phase II, single-blind, randomized, controlled trial. Ann Neurol 2016; 80: 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proffitt R, Lange B. Feasibility of a customized, in-home, game-based stroke exercise program using the Microsoft Kinect(R) sensor. Int J Telerehabil 2015; 7: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slater M, Wilbur S. A framework for immersive virtual environments (FIVE): speculations on the role of presence in virtual environments. Presence: Teleoper Virtual Environ 1997; 6: 603–616. [Google Scholar]
- 53.Fallahpour M, Kottorp A, Nygard L, et al. Participation after acquired brain injury: associations with everyday technology and activities in daily life. Scand J Occup Ther 2015; 22: 366–376. [DOI] [PubMed] [Google Scholar]
- 54.Alankus G and Kelleher C. Reducing compensatory motions in video games for stroke rehabilitation. In: Proceedings of the SIGCHI conference on human factors in computing systems, Austin, TX, USA, 5–10 May 2012, pp. 2049–2058. ACM.