Short abstract
Intervertebral disc degeneration is a complex disease involving genetic and environmental factors and multiple cellular processes. The role and expression of the lncRNA NEAT1 were assessed in intervertebral disc degeneration. NEAT1 expression was assessed in degenerative and control nucleus pulposus using RT-PCR. Western blotting and RT-PCR were also used to investigate p53 and p21 levels in nucleus pulposus tissues. NEAT1 function in degenerative nucleus pulposus cells was assessed with gain- and loss-of-function experiments. ERK/MAPK signaling was also examined. NEAT1, p53, and p21 were dramatically upregulated in intervertebral disc degeneration. Furthermore, catabolic MMP13 and ADAMTS5 were dysregulated and collagen II and aggrecan were downregulated after NEAT1 overexpression. This effect was reversed by transfection with si-NEAT1 in degenerative nucleus pulposus cells. In addition, NEAT1 was found to affect the activation of the ERK/MAPK pathway. The NEAT1-induced ECM degradation may involve ERK1/2/MAPK signaling. LncRNA NEAT1 may represent a novel molecular target for intervertebral disc degeneration treatment by preventing nucleus pulposus ECM degradation.
Impact statement
For the first time, our study demonstrates that lncRNA NEAT1 plays a role in the occurrence and development of IDD by participating in extracellular matrix remodeling. This lncRNA regulates catabolic MMP13 and ADAMTS5 and anabolic collagen II and aggrecan by affecting the ERK/MAPK signaling pathway in degenerative human nucleus pulposus (NP) cells. Our research provides a scientific basis for targeting of NEAT1 for the IDD.
Keywords: NEAT1, degeneration, intervertebral disc, nucleus pulposus cells, ERK/MARK
Introduction
Intervertebral disc degeneration (IDD) is deeply involved in low back pain, which has deleterious health and social consequences,1 with a cost of more than $100 billion per year in America.2 The potential etiological factors for IDD include abnormal process of cell degradation associated with genetics and age.3 The exact pathogenesis of IDD remains poorly understood.
Emerging evidence has indicated that lncRNAs is involved in pathogenesis of IDD by regulating human nucleus pulposus (NP) cells.4,5 LncRNAs are defined as RNAs at least 200 nucleotides (nt) long that are independently transcribed; they resemble mRNAs at the molecular level, but do not have recognizable potential for encoding functional proteins. Genomic studies based on EST and cDNA sequencing, tiling microarrays, and RNA sequencing have identified thousands of lncRNAs in diverse animal and plant genomes. However, their involvement in human disease is only beginning to be understood.
NEAT1 (nuclear enriched abundant transcript 1) is a lncRNA essential for the integrity of the nuclear paraspeckle substructure.6 Although NEAT1 expression is dysregulated in many human malignancies, including downregulation in gastric, prostate, esophageal, and lung cancer and acute promyelocytic leukemia,7–11 a previous lncRNA–mRNA microarray study showed that anomalous NEAT1 expression was higher in IDD than in normal discs.12
In this study, we verified that NEAT1expression increases with the progression of degeneration. Our findings indicate that NEAT1 is deeply associated with the generation of IDD by participating in NP ECM degradation. Furthermore, we confirmed that this effect involved the ERK/MAPK signaling pathway.
Materials and methods
Patients and samples
The study protocol was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from all participants. Human lumbar NP specimens used as normal controls were collected from six patients (n = 3 women and 3 men; mean age, 22 years; range, 18–32 years) with spinal fracture who underwent fusion surgery. Degenerative human lumbar NP specimens were collected from 10 patients (n = 4 women and 6 men; mean age, 38.4 years; range, 27–57 years) with IDD who underwent disc excision and spinal fusion surgery. The degree of IDD was assessed according to the modified Pfirrmann grading system using preoperative MRI scans.13
Human NP cells
Ten degenerative NP tissue samples were collected from IDD. Human degenerative NP cells were isolated and cultured as described previously.14 Cells were passaged two to three times for use in the following experiments.
Cell transfection
NEAT1 has two isoforms, a smaller 3.7 kb (NEAT1–1) and a larger 23 kb isoform (NEAT1–2). A specific vector targeting NEAT1 (pcDNA3.1-NEAT1) and an empty vector (used as a negative control) were purchased from Invitrogen. Human degenerative NP cells were then transfected with the control vector or pcDNA3.1-NEAT1using Lipofectamine, 2000 (Invitrogen). In addition, short interfering (si)RNA against NEAT1 (si-NEAT1) and si-NC (control group) were purchased from RiboBio (Guangzhou, China), and transfected into NP cells by manufacturer’s instructions. The NEAT1 siRNA primer sequences were: siRNA-1:GUGAGAAGUUGCUUA GAAAUU, siRNA-2:UGGUAAUGGUGGAGGAAGAUU,siRNA-3: GGAGGAGUCAGGAGGAAUAUU.
Quantitative real-time PCR
We used TRIzol reagent to extract RNA from NP tissues and cells. Total RNA was used to synthesize cDNA with a cDNA Synthesis Kit (TaKaRa Biotechnology, Otsu, Japan). Real-time PCR was performed and products were analyzed, respectively, with SYBR Green kit Master Mix (Applied Biosystems) and the ABI, 7500 Sequencing Detection System. The primer sequences: NEAT1forward: ATGCCACAACGCAGATTGAT, reverse: CGAGAAACGC ACAAGAAGG. Aggrecan forward: ACTCTGGGTTTTC GTGACTCT, reverse: ACACTCAGCGAGTTGTCATGG. MMP13 forward: ACTGAGAGGCTCCGAGAAATG, reverse: GAACCCCGCATCTTGGCTT. ADAMTS4 forward: ACCCAAGCATCCGCAATC, reverse: TGCCCAC ATCAGCCATAC. Homo β-actin forward: AGCGAGC ATCCCCCAAAGTT, reverse: GGGCACGAAGGCTCATC ATT.
Western blotting
Western blotting was performed as described previously with antibodies specific for type II collagen, aggrecan, MMP-13, ADAMTS-4, p53, p21,phospho-ERK1/2 (p-ERK), ERK1/2, and β-actin (all from Abcam, Cambridge, MA, USA).
Statistical analysis
Data are expressed as the mean ± SD. Data are analyzed with Student’s t-test or by analysis of variance using SPSS v.18.0 software (SPSS Inc., Chicago, IL, USA). The statistical significance is defined as P< 0.05.
Results
NEAT1 is upregulated in degenerative human NP tissues
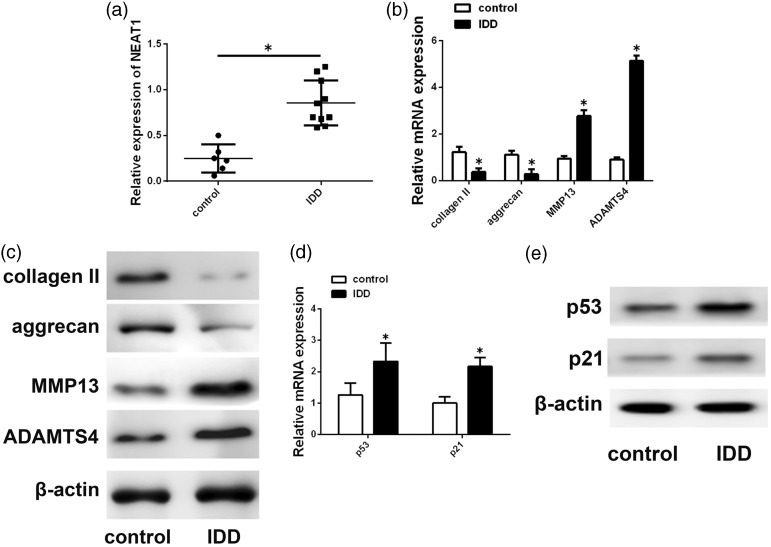
To assess whether NEAT1expression is altered in IDD, NEAT1 expression was measured in 6 control NP tissues and 10 degenerative tissues. As shown in Figure 1(a), NEAT1 expression was significantly upregulated in degenerative NP tissues compared with that in controls. Moreover, differential expression of several ECM-related molecules was detected. ADAMTS-4 and MMP-13 levels were increased and aggrecan and type II collagen levels are decreased in IDD (Figure 1(b) and (c)), which is in agreement with findings of previous studies. Because previous experiment showed that NEAT1 is a direct transcriptional target of p53,15,16 we assessed the expression of p53 and its target gene p21.p53 and p21 levels were increased in degenerative tissues (Figure 1(d) and (e)).
Figure 1.
NEAT1expression in human NP tissue. (a) NEAT1 exhibited higher expression in degenerative NP tissues. (b, c) MRNA and protein levels of ADAMTS4, aggrecan, type II collagen andMMP13 were analyzed by Western blotting and qRT-PCR. (d, e) p53 and p21 expression level were analyzed. Data represent the mean ± SD. *P < 0.05 vs. control group.
Silencing of NEAT1 inhibits ECM degradation of degenerative NP cells
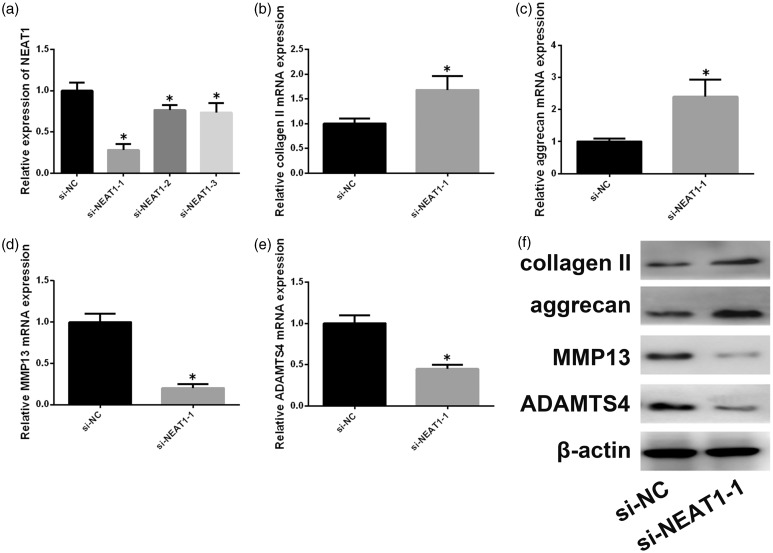
We next studied the effect of silencing NEAT1 on NP ECM metabolism. Three siRNAs were designed to silence NEAT1 (Figure 2(a)); si-NEAT1–1 showed the best silencing effect and was used for the subsequent experiments. Aggrecan and type II collagen expression was increased in the NEAT1-silenced group compared to the control (Figure 2(b), (c), and (f)). MMPs and ADAMTSs are involved in NP ECM degradation. We thus assessed ADAMTS-4and MMP-13 levels in degenerative NP cells. NEAT1 inhibition decreased the expression of ADAMTS-4 andMMP-13 relative to that in the control (Figure 2(d) to (f)).
Figure 2.
NEAT1 inhibition affects ECM degradation in degenerative NP cells. (a) Effects of three different siRNAs against NEAT1were assessed by qRT-PCR. (b–e) mRNA expression levels of ADAMTS-4, aggrecan, MMP-13, and type II collagen were determined after silencing of NEAT1 by siRNA. (f) Effect of silencing ofNEAT1 onADAMTS-4, aggrecan, MMP-13, and type II collagen protein expression. Data represent the mean ± SD. *P < 0.05 vs. control group, n = 3.
Overexpression of NEAT1 promotes ECM degradation in degenerative NP cells
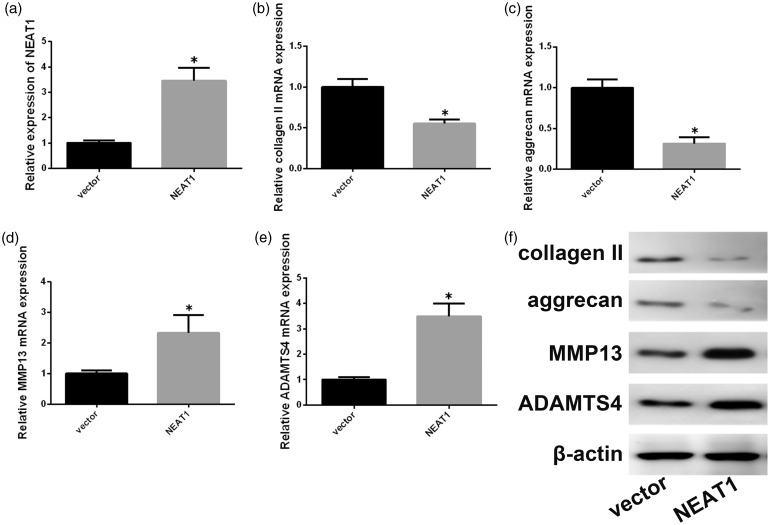
We also investigated the effect of NEAT1 overexpression on NP ECM degradation. As shown in Figure 3(a), NEAT1 overexpression vector was used to overexpress NEAT1, which resulted in increases in ADAMTS-4 and MMP-13 and decreases in aggrecan, type II collagen at the mRNA, protein levels (Figure 3(b) to (f)). These findings show that NEAT1 is related to NP ECM degradation in IDD.
Figure 3.
NEAT1 affects NP ECM metabolism. (a) Expression of NEAT1 in degenerative NP cells transfected with pcDNA3.1-NEAT1 (NEAT1) or the control plasmid was determined with qRT-PCR. (b–e) mRNA expression levels of ADAMTS-4, aggrecan, MMP-13, and type II collagen were determined after NEAT1 overexpression. (f) Effects of NEAT1 overexpression on ADAMTS-4, aggrecan, MMP-13, and type II collagen protein expression. Data represent the mean ± SD. *P < 0.05 vs. control group, n = 3.
NEAT1 affects MAPK pathway activation
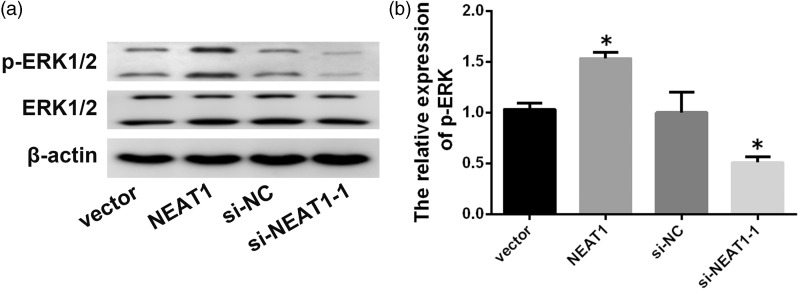
To obtain a preliminary understanding of the exact molecular mechanism by which NEAT1 promotes NP ECM degradation, Western blotting was performed to assess the effects of NEAT1 down- and up-regulation on the ERK/MAPK pathway, which is often aberrantly activated in IDD and contributes to NP ECM degradation. Western blotting analysis showed that downregulation of NEAT1 significantly reduced the levels of phosphorylated ERK1/2, whereas upregulation of NEAT1 promoted the phosphorylation (Figure 4(a) and (b)). These results show that the ERK/MAPK pathway activation by NEAT1 overexpression may contribute to degenerative NP ECM degradation.
Figure 4.
NEAT1 activates ERK/MAPK in NP cells. (a, b) Protein levels of p-ERK and total protein levels of ERK in degenerative NP cells transfected using empty vector, pcDNA3.1-NEAT1, si-NC, or si-NEAT1–1 for 48 h. Data represent the mean ± SD. *P < 0.05 vs. control group.
Discussion
IDD is characterized by abnormal NP apoptosis, the increased expression of various inflammatory factors, and disordered ECM metabolism in NP cells.17–19 The molecular mechanisms of IDD remain unclear, although various factors are associated with IDD etiology. Accumulating evidence has shown that non-coding RNAs, including miRNAs and lncRNAs, play a vital role in IDD. One experiment revealed that the lncRNA RP11–296A18.3 promotes HIF1A expression by sequesteringmiR-138, thus promoting human NP cell proliferation and ECM synthesis.4 Another recent study showed that silencing ofTUG1 may not only protect human NP cells from TNF-α-induced apoptosis and senescence but may also promote cell proliferation by blocking the Wnt/β-catenin pathway.20 In this regard, the data presented in this study are consistent with previous studies indicating that the lncRNA NEAT1 may be a causative factor in the development of IDD. Increased NEAT1 expression decreased aggrecan and collagen levels and increased ADAMTS4 and MMP-13 levels.
NEAT1 exerts a regulatory function in proliferation and differentiation, and it is upregulated and plays an oncogenic role in most types of solid tumor. In contrast, NEAT1 is downregulated in acute promyelocytic leukemia, where it promotes leukocyte differentiation.21 In osteoarthritis (OA) tissues, NEAT1 is upregulated and represses synoviocyte proliferation. After NEAT1 knockdown, MMP13, IL-6, and IL-8 expression is reduced.22 In our study, the expression of NEAT1 in NP tissues from IDD patients was higher than that in controls. Further studies showed levels of p53 and p21, a canonical p53 target gene, were higher than those in controls. These findings suggest that NEAT1 might be upregulated in a p53-dependent manner. This is consistent with published reports that NEAT1 expression was induced by p53 activation.15,16
Accumulating studies have highlighted the role of NP cell degeneration and senescence in IDD.23 Anabolic and catabolic processes control the homeostasis between ECM synthesis and degradation.24 A recent study showed that epoxy eicosanoids prevent intervertebral disc ECM degeneration, perhaps by inhibiting the matrix remodeling response of NP cells to TNF-α.14 Therefore, we investigated the function of NEAT1 in ECM degradation in degenerative NP cells, NEAT1 was shown to participate in ECM degradation. The levels of catabolic MMP-13 and ADAMTS-5 and anabolic collagen II and aggrecan were affected by NEAT1expression, further indicating that high expression of NEAT1 may have significant impacts on the occurrence and development of IDD. NEAT1 has thus been demonstrated to participate in ECM remodeling.
Multiple studies have shown an important role of the ERK/MAPK pathway in IDD. Beta1 integrin was shown to decrease apoptosis in annulus fibrosus (AF) cells via the ERK1/2 MAPK pathway.25 Tensile stress promotes MAPK activation in AF cells, which is associated with proinflammatory gene expression and drives an inflammatory reaction.26 The tolerance of NP cells to hyperosmotic milieu is associated with ERK/MAPK pathway activation.27 Loss-of-function studies have confirmed that ERK/MAPK signaling contributes to the cytokine-dependent induction promoter activity of MMP3, which is important in IDD.28 Inhibition of this pathway reduces or abrogates IL-1β-mediated catabolic effects in disc degeneration. Furthermore, a previous study indicated that ERK/MAPK signaling increases with the progression of IDD,29 which is in line with our findings. In addition, the level of phosphorylated ERK1/2 was regulated byNEAT1 in degenerative NP cells from IDD patients, further indicating that high expression of NEAT1 may have significant impacts on the occurrence and development of IDD by specifically activating the ERK/MAPK signaling pathway.
In conclusion, we demonstrated the effects of NEAT1 on IDD. The data from the current study provide new evidence showing that NEAT1 may activate ERK/MARK signaling to exacerbate the imbalance in ECM metabolism in degenerative human NP cells. This is important in the treatment of IDD, which induces the progression of LBP. Further studies are needed to demonstrate the effect of NEAT1 expression in normal NP cells to promote the clinical treatment of IDD.
Authors’ contributions
FL designed the study. FL and ZR wrote the manuscript. ZR, HM and JL conducted the experiments. HYL and HRJ analyzed the data. FL reviewed and revised the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81472133) and Natural Science Foundation of Hubei Province (No.2014CFA058).
References
- 1.Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012; 37:E668–77 [DOI] [PubMed] [Google Scholar]
- 2.Cheung KM, Karppinen J, Chan D, Ho DW, Song YQ, Sham P, Cheah KS, Leong JC, Luk KD. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009; 34:934–40 [DOI] [PubMed] [Google Scholar]
- 3.Mern DS, Beierfuss A, Thome C, Hegewald AA. Enhancing human nucleus pulposus cells for biological treatment approaches of degenerative intervertebral disc diseases: a systematic review. J Tissue Eng Regen Med 2014; 8:925–36 [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Lv G, Li J, Wang B, Zhang Q, Lu C. LncRNA-RP11-296A18.3/miR-138/HIF1A pathway regulates the proliferation ECM synthesis of human nucleus pulposus cells (HNPCs). J Cell Biochem 2017; 118:4862–71 [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Song Y, Liu W, Wu X, Zhang Y, Li S, Kang L, Tu J, Zhao K, Hua W, Yang C. The noncoding RNA linc-ADAMTS5 cooperates with RREB1 to protect from intervertebral disc degeneration through inhibiting ADAMTS5 expression. Clin Sci 2017; 131:965–79 [DOI] [PubMed] [Google Scholar]
- 6.Naganuma T, Hirose T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol 2013; 10:456–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Liu L, Yan F, Wei W, Deng J, Sun J. Enhanced expression of long non-coding RNA NEAT1 is associated with the progression of gastric adenocarcinomas. World J Surg Onc 2016; 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M, MacDonald TY, Fontugne J, Erho N, Vergara IA, Ghadessi M, Davicioni E, Jenkins RB, Palanisamy N, Chen Z, Nakagawa S, Hirose T, Bander NH, Beltran H, Fox AH, Elemento O, Rubin MA. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 2014; 5:5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Kong J, Ma Z, Gao S, Feng X. Up regulation of the long non-coding RNA NEAT1 promotes esophageal squamous cell carcinoma cell progression and correlates with poor prognosis. Am J Cancer Res 2015; 5:2808–15 [PMC free article] [PubMed] [Google Scholar]
- 10.Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer 2017; 16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L, Chen S, Li Y. Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 2014; 14:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan PH, Liu ZH, Pei YJ, Wu ZG, Yu Y, Yang YF, Liu X, Che L, Ma CJ, Xie YK, Hu QJ, Wan ZY, Wang HQ. Landscape of RNAs in human lumbar disc degeneration. Oncotarget 2016; 7:63166–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001; 26:1873–8 [DOI] [PubMed] [Google Scholar]
- 14.Li J, Guan H, Liu H, Zhao L, Li L, Zhang Y, Tan P, Mi B, Li F. Epoxyeicosanoids prevent intervertebral disc degeneration in vitro and in vivo. Oncotarget 2017; 8:3781–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adriaens C, Standaert L, Barra J, Latil M, Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W, Leucci E, Lapouge G, Beck B, van den Oord J, Nakagawa S, Hirose T, Sablina AA, Lambrechts D, Aerts S, Blanpain C, Marine JC. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med 2016; 22:861. [DOI] [PubMed] [Google Scholar]
- 16.Idogawa M, Ohashi T, Sasaki Y, Nakase H, Tokino T. Long non-coding RNA NEAT1 is a transcriptional target of p53 and modulates p53-induced transactivation and tumor-suppressor function. Int J Cancer 2017; 140:2785–91 [DOI] [PubMed] [Google Scholar]
- 17.Fontana G, See E, Pandit A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv Drug Deliv Rev 2015; 84:146–58 [DOI] [PubMed] [Google Scholar]
- 18.Cai XY, Xia Y, Yang SH, Liu XZ, Shao ZW, Liu YL, Yang W, Xiong LM. Ropivacaine- and bupivacaine-induced death of rabbit annulus fibrosus cells in vitro: involvement of the mitochondrial apoptotic pathway. Osteoarthr Cartil 2015; 23:1763–75 [DOI] [PubMed] [Google Scholar]
- 19.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014; 10:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Jia YS, Liu GZ, Sun Q, Zhang F, Ma S, Wang YJ. Role of LncRNA TUG1 in intervertebral disc degeneration and nucleus pulposus cells via regulating Wnt/beta-catenin signaling pathway. Biochem Biophys Res Commun 2017; 49:668–74 [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: a novel cancer-related long non-coding RNA. Cell Prolif 2017; 50:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Wang W, Zhang F, Deng Y, Long Z. NEAT1/miR-181c regulates osteopontin (OPN)-mediated synoviocyte proliferation in osteoarthritis. J Cell Biochem 2017; 118:3775–84 [DOI] [PubMed] [Google Scholar]
- 23.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 2002; 27:2631–44 [DOI] [PubMed] [Google Scholar]
- 24.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 2004; 29:2691–9 [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Ding W, Sun W, Sun XJ, Xie YZ, Zhao CQ, Zhao J. Beta1 integrin inhibits apoptosis induced by cyclic stretch in annulus fibrosus cells via ERK1/2 MAPK pathway. Apoptosis 2016; 21:13–24 [DOI] [PubMed] [Google Scholar]
- 26.Pratsinis H, Papadopoulou A, Neidlinger-Wilke C, Brayda-Bruno M, Wilke HJ, Kletsas D. Cyclic tensile stress of human annulus fibrosus cells induces MAPK activation: involvement in proinflammatory gene expression. Osteoarthr Cartil 2016; 24:679–87 [DOI] [PubMed] [Google Scholar]
- 27.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res 2007; 22:965–74 [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Wang H, Yang H, Li J, Cai Q, Shapiro IM, Risbud MV. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: implications in inflammatory disc disease. Am J Pathol 2014; 184:2560–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels J, Binch AA, Le Maitre CL. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J Orthop Res 2017; 35:74–85 [DOI] [PubMed] [Google Scholar]