Short abstract
Cardiac fibroblasts and their activated derivatives, myofibroblasts, play a critical role in wound healing after myocardial injury and often contribute to long-term pathological outcomes, such as excessive fibrosis. Thus, defining the microenvironmental factors that regulate the phenotype of cardiac fibroblasts and myofibroblasts could lead to new therapeutic strategies. Both chemical and biomechanical cues have previously been shown to induce myofibroblast differentiation in many organs and species. For example, transforming growth factor beta 1, a cytokine secreted by neutrophils, and rigid extracellular matrix environments have both been shown to promote differentiation. However, the relative contributions of transforming growth factor beta 1 and extracellular matrix rigidity, two hallmark cues in many pathological myocardial microenvironments, to the phenotype of human cardiac fibroblasts are unclear. We hypothesized that transforming growth factor beta 1 and rigid extracellular matrix environments would potentially have a synergistic effect on the differentiation of human cardiac fibroblasts to myofibroblasts. To test this, we seeded primary human adult cardiac fibroblasts onto coverslips coated with polydimethylsiloxane of various elastic moduli, introduced transforming growth factor beta 1, and longitudinally quantified cell phenotype by measuring expression of α-smooth muscle actin, the most robust indicator of myofibroblasts. Our data indicate that, although extracellular matrix rigidity influenced differentiation after one day of transforming growth factor beta 1 treatment, ultimately transforming growth factor beta 1 superseded extracellular matrix rigidity as the primary regulator of myofibroblast differentiation. We also measured expression of POSTN, FAP, and FSP1, proposed secondary indicators of fibroblast/myofibroblast phenotypes. Although these genes partially trended with α-smooth muscle actin expression, they were relatively inconsistent. Finally, we demonstrated that activated myofibroblasts incompletely revert to a fibroblast phenotype after they are re-plated onto new surfaces without transforming growth factor beta 1, suggesting differentiation is partially reversible. Our results provide new insights into how microenvironmental cues affect human cardiac fibroblast differentiation in the context of myocardial pathology, which is important for identifying effective therapeutic targets and dictating supporting cell phenotypes for engineered human cardiac disease models.
Impact statement
Heart disease is the leading cause of death worldwide. Many forms of heart disease are associated with fibrosis, which increases extracellular matrix (ECM) rigidity and compromises cardiac output. Fibrotic tissue is synthesized primarily by myofibroblasts differentiated from fibroblasts. Thus, defining the cues that regulate myofibroblast differentiation is important for understanding the mechanisms of fibrosis. However, previous studies have focused on non-human cardiac fibroblasts and have not tested combinations of chemical and mechanical cues. We tested the effects of TGF-β1, a cytokine secreted by immune cells after injury, and ECM rigidity on the differentiation of human cardiac fibroblasts to myofibroblasts. Our results indicate that differentiation is initially influenced by ECM rigidity, but is ultimately superseded by TGF-β1. This suggests that targeting TGF-β signaling pathways in cardiac fibroblasts may have therapeutic potential for attenuating fibrosis, even in rigid microenvironments. Additionally, our approach can be leveraged to engineer more precise multi-cellular human cardiac tissue models.
Keywords: Extracellular matrix, elastic modulus, transforming growth factor beta 1, α-smooth muscle actin
Introduction
Ventricular myocardium consists of cardiac myocytes embedded in an extracellular matrix (ECM) network synthesized and maintained primarily by cardiac fibroblasts. After many forms of injury, cardiac fibroblasts and their activated derivatives, myofibroblasts, play a critical role in the wound healing response. For example, after a myocardial infarction, cardiac myocytes downstream of an occluded coronary artery undergo injury that can progress to apoptosis. This initiates an inflammatory response and recruitment of macrophages and neutrophils.1,2 Neutrophils secrete various cytokines and growth factors, including transforming growth factor beta 1 (TGF-β1), which binds to TGF-β receptors and activates the TGF-β/Smad pathway in fibroblasts.3 Smad proteins translocate to the nucleus and upregulate transcription of ECM proteins and alpha smooth muscle actin (α-SMA), a critical component of contractile microfilaments is expressed in myofibroblasts.4,5 Consequently, myofibroblasts deposit more ECM molecules and are more contractile than quiescent fibroblasts, exhibiting a phenotype intermediate to fibroblasts and smooth muscle cells.6 After an infarction, activated myofibroblasts proliferate, contract, and deposit ECM molecules at the site of injury, ultimately forming stiff, fibrotic tissue7 and providing mechanical stability to compensate for any loss of myocytes.8 In other tissue types, such as skin, myofibroblasts typically undergo apoptosis after repairing minor injuries.9 However, myofibroblasts persist in the myocardium due to the nominal regeneration of cardiac myocytes. These lingering myofibroblasts can contribute to many pathological outcomes,10,11 including arrhythmias by interfering with cardiac myocyte cell–cell communication.12–15
Although cardiac myofibroblasts are known to contribute to many injury responses and pathological remodeling processes in the ventricle, the cues that induce the differentiation of human cardiac myofibroblasts from fibroblasts are still incompletely understood. In animal models, TGF-β1 has been shown to activate the differentiation of fibroblasts to myofibroblasts and contribute to cardiac hypertrophy and fibrosis.16,17 Similarly, exogenous TGF-β1 applied in vitro has been shown to induce myofibroblast differentiation in skin, lung, kidney, and cardiac fibroblasts.5,18–21 Mechanical stimuli have also been shown to activate the fibroblast to myofibroblast transition. For example, the in vitro differentiation of bronchial,22 valvular,23 and cardiac24 fibroblasts to myofibroblasts increases with increasing rigidity of the substrate, suggesting that fibrotic scar tissue, which is deposited by myofibroblasts themselves, could positively reinforce myofibroblast phenotypes. Other forms of mechanical stimulation, including cyclic stretch25 and perpendicularly-applied forces,26 have also been shown to induce differentiation of cardiac fibroblasts into myofibroblasts. Additionally, increased substrate rigidity has been shown to promote the TGF-β1-induced differentiation of bronchial27 and portal28 fibroblasts to myofibroblasts, suggesting that chemical and biomechanical cues can have combinatorial, or potentially synergistic, effects on fibroblast-myofibroblast phenotypes. However, the relative contributions of TGF-β1 and ECM rigidity to cardiac fibroblast-myofibroblast differentiation have not been established, which is important for delineating the microenvironmental cues that have the greatest impact and potential as therapeutic targets after myocardial injury. Additionally, most studies with cardiac fibroblasts to date have been limited to primary rodent fibroblasts, which may not translate to humans.
Our objective was to determine how increases in ECM rigidity and TGF-β1 exposure independently and jointly regulate human cardiac fibroblast-myofibroblast phenotype. We chose these two cues because they are both present in injured myocardium and have previously been shown to independently induce differentiation of fibroblasts to myofibroblasts, although primarily in non-human cell types.24,29 First, we cultured primary human adult cardiac fibroblasts on coverslips coated with polydimethylsiloxane (PDMS) of three distinct elastic moduli, treated cells with TGF-β1, and quantified α-SMA expression over time by immunostaining and quantitative real-time PCR (RT-PCR). Overall, our results indicate that α-SMA expression on the gene and protein level is more dominantly regulated by exogenous TGF-β1 compared to substrate rigidity. We also used RT-PCR to quantify expression of other proposed fibroblast/myofibroblast markers, such as periostin. However, the secondary markers that we evaluated were less robustly regulated by TGF-β1 compared to α-SMA. Due to the proposed role of cardiac fibroblasts/myofibroblasts in arrhythmogenesis, we also quantified the expression of GJA1, which encodes for connexin 43 (Cx43) protein. However, we did not identify any significant differences in GJA1 expression due to TGF-β1 or matrix rigidity. Finally, to determine if myofibroblast phenotype is reversible, we re-plated myofibroblasts activated by TGF-β1 onto new coverslips and maintained them without TGF-β1, which led to a partial reversal to the fibroblast phenotype. Collectively, these results provide new insights into how human cardiac fibroblast and myofibroblast phenotypes are differentially regulated by chemical and biomechanical cues present in pathological cardiac microenvironments. These data can contribute to the identification of new therapeutic targets for slowing or reversing the transition of fibroblasts to myofibroblasts, which could ultimately minimize fibrosis. Additionally, the approaches we developed to dictate human cardiac fibroblast and myofibroblast phenotypes can be leveraged for future in vitro studies, such as co-culturing with cardiac myocytes or engineering more precise multi-cellular human cardiac disease models.
Material and methods
Fabrication of tunable PDMS substrates
Three types of PDMS with distinct elastic moduli were prepared using Sylgard 184 silicone elastomer and Sylgard 527 silicone dielectric gel (Dow Corning, Midland, MI, USA). Pure Sylgard 184, referred to as high, was prepared by mixing the base component with the curing agent at a 10:1 mass ratio. Pure Sylgard 527, referred to as low, was prepared by mixing components A and B at a 1:1 mass ratio. A 1:20 mass ratio of Sylgard 184 and Sylgard 527, referred to as moderate, was also prepared, similar to previous studies.30–32 Each substrate was mixed and degassed using a planetary centrifugal mixer (AR-100, Thinky, Japan) and spin-coated onto 25 mm diameter glass coverslips (Electron Microscopy Sciences, Hatfield, PA, USA) using a G3P-8 spincoater (Specialty Coating Systems, Indianapolis, IN, USA), as described previously.33 PDMS-coated coverslips were treated with ultraviolet ozone cleaner (Jelight Company Inc., Irvine, CA, USA) for 8 min, uniformly coated with 150 μL of human fibronectin (50 μg/mL, Corning, Corning, NY, USA) or rat tail collagen type I (200 μg/mL, Corning) for 2 min, rinsed with PBS, and maintained at room temperature until seeded with cells on the same day.
Cell culture
Primary human adult cardiac fibroblasts (Lot 3131, Cell Applications Inc., San Diego, CA, USA) were thawed into a 75 cm2 cell culture flask with fibroblast growth medium, consisting of low glucose DMEM (1 g/L glucose) supplemented with 10% v/v fetal bovine serum (FBS) (Gibco, Waltham, MA, USA) and 1% v/v penicillin-streptomycin solution (10,000 U/mL penicillin, 10,000 μg/mL streptomycin). Cells were passaged at 90% confluence into 175 cm2 cell culture flasks by incubating the cells with trypsin-EDTA for 4 min at room temperature, immediately adding trypsin neutralizing solution, centrifuging the cell solution at 300g for 5 min, and re-suspending the cell pellet in fibroblast growth medium. These cells were subsequently seeded onto PDMS coverslips in six-well plates at a density of 36,500 cells/cm2 and serum-arrested with 0.1% or 0.5% serum the following day. Cells used for experiments were between passages four and eight. After an additional 48 h, TGF-β1 (2 or 10 ng/mL, R&D Systems, Minneapolis, MN, USA) was added to select wells. Media was replenished every 48 h, including the re-application of TGF-β1 for treated wells.
For re-plating experiments, TGF-β1-treated and untreated fibroblasts maintained on high PDMS-coated coverslips for six days were dissociated with trypsin-EDTA for 2 min. Constructs were placed in the incubator for 1 min during dissociation to enhance cell detachment. The trypsin solution was immediately neutralized with trypsin neutralizing solution and the cell solution was centrifuged at 300g for 5 min at 4°C. The supernatant was aspirated and 2 mL of growth media was added to re-suspend the cell pellet. The cells were seeded onto new PDMS-coated coverslips of high stiffness and serum-arrested with 0.1% serum the following day.
Immunostaining
Cells were fixed with 4% paraformaldehyde for 10 min and subsequently permeabilized with 0.2% Triton X-100 solution for 10 min. Fixed cells were incubated with a monoclonal mouse anti-α-SMA (1:200, Thermo Fisher Scientific, Waltham, MA, USA) primary antibody overnight at 4°C. After PBS rinses, cells were incubated with DAPI (1:200, Life Technologies, Waltham, MA, USA), Alexa Fluor 488 Phalloidin (1:200, Life Technologies), and Alexa Fluor 546 goat anti-mouse secondary antibody (1:200, Life Technologies) for 90 min at room temperature. Coverslips were mounted onto glass slides with a drop of ProLong Gold Anti-Fade Mountant (Life Technologies), and sealed with nail polish.
Microscopy and image analysis
High-resolution fluorescent images were captured using a 60× oil objective on a Nikon C2 point-scanning confocal microscope. Lower resolution fluorescent images at nine locations dispersed across the coverslips were captured using a 20× air objective on a Nikon Eclipse Ti-S inverted fluorescent microscope and an Andor Zyla scientific CMOS camera. A 2 × 2 tile scan with 15% overlap was utilized to increase sampling area (total field of view: 1.56 mm × 1.32 mm).
To analyze tile scan images, a custom macro was written in ImageJ to automatically count the total number of nuclei per field of view based on DAPI fluorescence. A Gaussian Blur (value of 4.0) was first applied to blur the image and minimize variances in pixel color exposure. Next, manual thresholding of the image was used to generate a black background with white ellipses corresponding to nuclei. The Watershed function was applied to delineate and separate overlapping nuclei. The Analyze Particles function was used to set the size range of the particles from 20 μm2 to infinity to ensure only nuclei were counted and small background noise was omitted. Manual thresholding of actin and α-SMA images was used to quantify the total actin-positive pixels and the total α-SMA-positive pixels, respectively. These values were divided by the total number of pixels in the image to determine actin and α-SMA coverage, respectively.
Gene expression analysis
mRNA transcripts were isolated and collected using the Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA, USA). mRNA concentrations and 260/280 ratios were measured with a Nanodrop (Thermo Fisher Scientific). As needed, a SpeedVac Concentrator (Thermo Fisher Scientific) was used to increase mRNA concentration. Only mRNA concentrations above 90 ng/μL and 260/280 ratios above 2.0 were used for cDNA synthesis. cDNA was then synthesized via reverse transcription using the iScript Reverse Transcription Supermix for RT-qPCR kit (Bio-Rad) and was stored at −80°C. qPCR was performed by mixing SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), cDNA, and the various primers (Bio-Rad) listed in Supplemental Table S1 into a 384-well PCR plate, according to the instructions of the manufacturer. The plate was inserted into the CFX384 Touch Real-Time PCR Detection System (Bio-Rad) to obtain cycle threshold (Ct) data sets for each condition. Gene expression was normalized relative to the housekeeping gene GAPDH. Average expression values were computed using the standard comparative Ct method, as described previously.34
Statistics
All data sets were first validated for normality using the Kolmogorov–Smirnov test in MATLAB (MathWorks, Natick, MA, USA). The data were then analyzed using one-way and/or two-way ANOVA followed by Tukey’s test for multiple comparisons in MATLAB, with α set to 0.05. Data for each condition were gathered from at least four independent experiments and multiple regions of interest per sample were collected and averaged for image analysis.
Results
Effects of ECM ligand, serum concentration, and TGF-β1 concentration on human cardiac fibroblast adhesion and differentiation
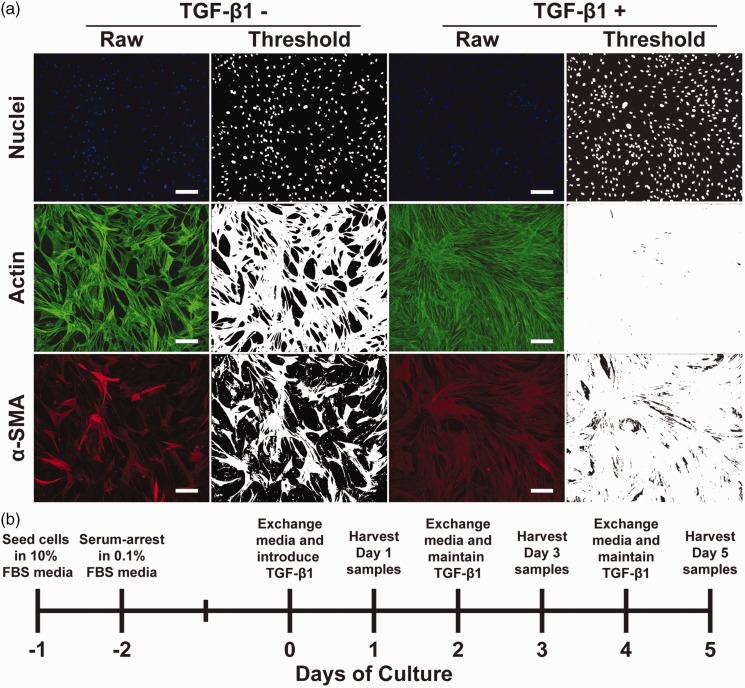
To determine the independent and combined effects of substrate rigidity and TGF-β1 on the phenotype of primary human cardiac fibroblasts, we first established our experimental parameters in a series of pilot experiments. To determine the optimal ECM ligand for long-term cell adhesion, we coated high PDMS coverslips with 50 μg/mL of human fibronectin or 200 μg/mL rat tail collagen type I. We seeded substrates with primary human adult cardiac fibroblasts and, after one day, reduced FBS levels to 0.1% or 0.5%. We tested these two levels of FBS because high-serum can cause uncontrolled myofibroblast differentiation due to the presence of many confounding molecules in FBS, but low-serum can compromise cell adhesion. Next, we treated cells with 10 ng/mL TGF-β1 (a relatively high dose) and stained cells for nuclei, actin, and α-SMA after five days. To characterize cell phenotypes, we quantitatively analyzed our immunostained images (Figure 1(a)). To access cell density, we applied a threshold to images of nuclei and quantified nuclei per mm2. Because myofibroblasts are morphologically flatter and more elongated compared to small, spindle-like fibroblasts,35,36 we also quantified actin coverage as proxy for cell size. As shown in Supplementary Figure S1(a) and (b), cell density and actin coverage were similar in both FBS concentrations, but were much lower on collagen I compared to fibronectin. Next, we quantified α-SMA/actin coverage because α-SMA is only expressed by myofibroblasts and therefore α-SMA/actin coverage reflects the percentage of myofibroblasts. α-SMA/actin coverage was high in all conditions, as expected due to TGF-β1 treatment. Additionally, α-SMA/actin coverage was independent of ECM ligand and serum concentration (Supplementary Figure S1(c)), suggesting that myofibroblast differentiation was unaffected by either variable. Thus, based on these pilot experiments, we selected fibronectin as our ECM ligand and 0.1% serum to maximize cell adhesion and minimize proliferation and any other confounding effects of serum.
Figure 1.
Image processing techniques and experimental timeline. (a) Representative raw and thresholded images of nuclei, actin, and α-SMA in untreated and TGF-β1-treated human cardiac fibroblasts (scale bars: 200 μm). (b) Experimental timeline for culturing cardiac fibroblasts with reduced serum and TGF-β1 treatment. (A color version of this figure is available in the online journal.)
Previous studies have utilized TGF-β1 concentrations ranging from 2 to 10 ng/mL and maintained fibroblasts and myofibroblasts anywhere from two to five days.19,20,37–39 Thus, we next characterized the effects of 2 and 10 ng/mL TGF-β1 over five days in culture to establish the optimal dosing and time course of TGF-β1 activation. As shown in Supplementary Figure S2(a), cell density was relatively similar between control and 2 and 10 ng/mL of TGF-β1, with a slight decrease over time in culture in all conditions. However, actin (Supplementary Figure S2(b)) and α-SMA/actin (Supplementary Figure S2(c)) coverage in both TGF-β1 conditions were similar and higher than the control condition, suggesting that 2 and 10 ng/mL TGF-β1 have a similar effect on myofibroblast differentiation. Additionally, nearly all cells were differentiated after three and five days of treatment. Given these results, we selected 2 ng/mL TGF-β1 and five days of treatment as the upper limit for remaining experiments.
Regulation of cardiac myofibroblast differentiation by TGF-β1 and ECM rigidity
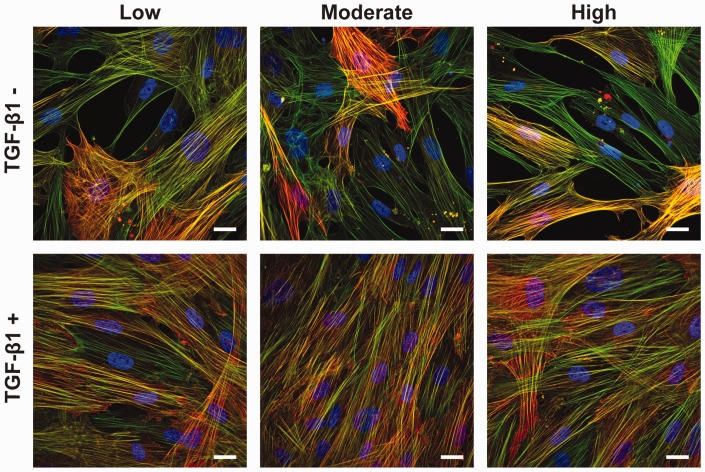
To test the impact of ECM rigidity and TGF-β1 on the differentiation of human cardiac fibroblasts to myofibroblasts, we next fabricated three types of PDMS-coated coverslips with elastic moduli of 1.61 kPa, 27.4 kPa, and 2.68 MPa,32 referred to as low, moderate, and high. These substrates were selected because they roughly mimic the rigidity and/or mechanical load experienced by fibroblasts in a developing heart, a healthy heart, and a fibrotic and/or pressure overload heart.7,40 Based on our optimization experiments, we coated these coverslips with human fibronectin, seeded them with primary human cardiac fibroblasts, and reduced serum to 0.1% after one day. After an additional two days in culture, we introduced 2 ng/mL of TGF-β1 to select coverslips and immunostained both untreated and treated cells after an additional one, three, and five days in culture (days 1, 3, and 5), as shown in Figure 1(b). Next, we captured high resolution images to characterize α-SMA expression and localization. As shown in Figure 2, we observed actin fibers in all cells in all conditions. In select cells, we also observed α-SMA signal co-localized with actin fibers, which is a hallmark of myofibroblasts. We also observed that most cells treated with TGF-β1 were positive for α-SMA on low, moderate, and high PDMS substrates. However, without TGF-β1 treatment, fewer than half the cells were positive for α-SMA on all PDMS substrates. Additionally, cells treated with TGF-β1 qualitatively occupied more surface area compared to untreated cells. Thus, TGF-β1 appeared to have a more substantial effect on myofibroblast differentiation compared to ECM rigidity.
Figure 2.
Cell morphology and α-SMA expression due to ECM rigidity and TGF-β1 treatment. Representative images of human cardiac fibroblasts cultured for five days on low, moderate, and high PDMS-coated coverslips with or without 2 ng/mL TGF-β1 (blue: nuclei, green: actin, red: α-SMA, scale bars: 25 μm).
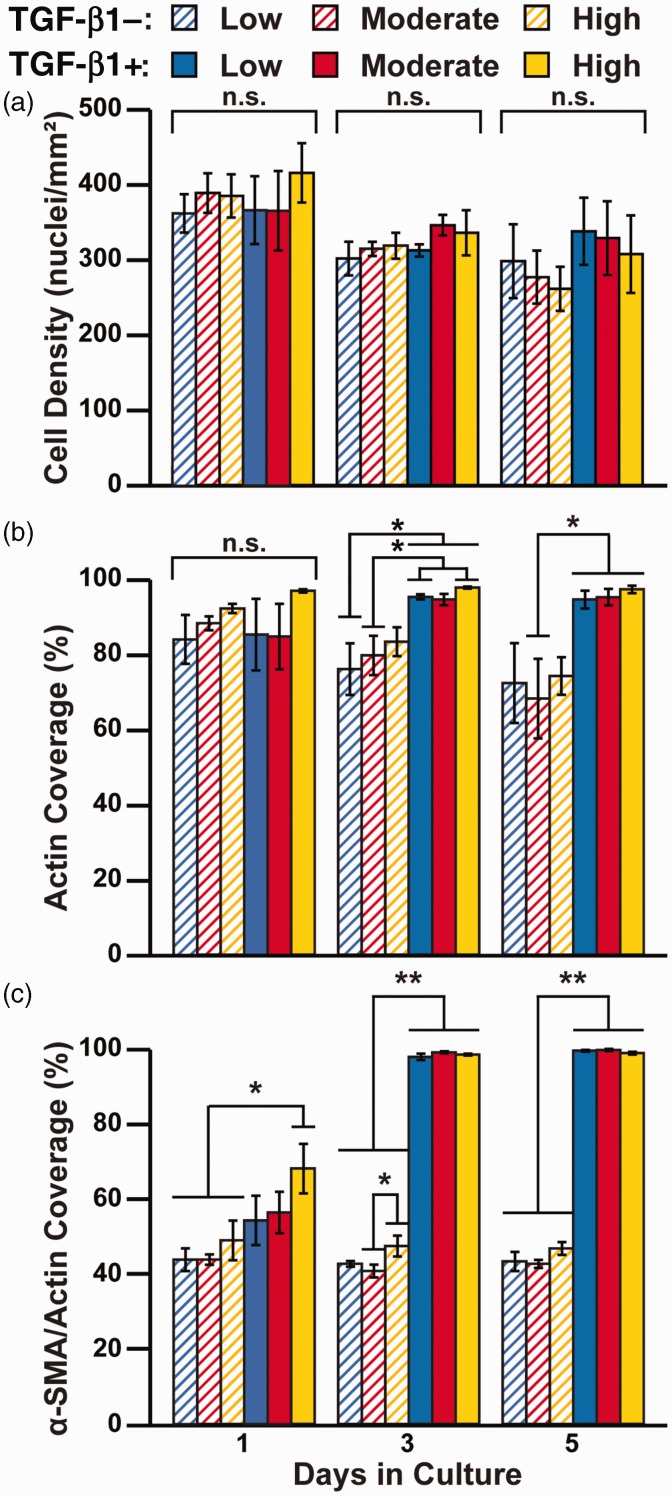
To quantify differences in phenotype, we next captured multiple lower resolution tile scans to increase our sampling area and quantified nuclei density, actin coverage, and α-SMA/actin coverage, as described above (Figure 1(a)). Within each time point, we first compared cell density across all conditions using one-way ANOVA and multiple comparisons and found no significant differences (Figure 3(a)). However, we did observe a general downward trend in cell density with time, which is likely because the low-serum media arrested proliferation. We also conducted a two-way ANOVA to quantify the independent effects of substrate rigidity and TGF-β1 treatment on nuclei density (Table 1) and similarly found no significant differences at any timepoint. These results suggest that neither substrate rigidity nor TGF-β1 treatment had a significant impact on cell adhesion, proliferation, and/or apoptosis.
Figure 3.
Quantification of cell morphology and α-SMA expression due to ECM rigidity and TGF-β1 treatment. Human cardiac fibroblasts were cultured on low, moderate, and high PDMS-coated coated coverslips with or without 2 ng/mL TGF-β1 for one, three, and five days. Cells were immunostained and cell density (a), actin coverage (b), and α-SMA/actin coverage (c) were quantified from immunostained images (n = 4, bars indicate mean ± standard error of the mean, *P < 0.05 and **P < 0.01 according to one-way ANOVA and Tukey’s test for multiple comparisons. For detailed statistical analysis, see Supplementary Tables S2 to S4). (A color version of this figure is available in the online journal.)
Table 1.
Two-way ANOVA P-values.
| Day | ECMRigidity | TGF-β1 | Interaction | |
|---|---|---|---|---|
| Cell density | 1 | 0.5316 | 0.8926 | 0.7083 |
| 3 | 0.3082 | 0.1459 | 0.8041 | |
| 5 | 0.6812 | 0.1569 | 0.9875 | |
| Actin coverage | 1 | 0.1268 | 0.8791 | 0.7008 |
| 3 | 0.3607 | <0.0001* | 0.7373 | |
| 5 | 0.7807 | <0.0001* | 0.9068 | |
| α-SMA/actin coverage | 1 | 0.0655 | <0.0001* | 0.5635 |
| 3 | 0.0474* | <0.0001* | 0.0283* | |
| 5 | 0.3342 | <0.0001* | 0.1021 | |
| ACTA2 relative expression | 1 | 0.3255 | <0.0001* | 0.4763 |
| 3 | 0.6160 | <0.0001* | 0.7983 | |
| POSTN relative expression | 1 | 0.8103 | <0.0001* | 0.2997 |
| 3 | 0.6836 | 0.1872 | 0.4287 | |
| FAP relative expression | 1 | 0.8928 | 0.0032* | 0.3781 |
| 3 | 0.4506 | 0.0016* | 0.0670 | |
| FSP1 relative expression | 1 | 0.5935 | <0.0001* | 0.1548 |
| 3 | 0.5417 | 0.0172* | 0.5088 | |
| GJA1 relative expression | 1 | 0.8300 | 0.8398 | 0.2188 |
| 3 | 0.0953 | 0.9058 | 0.0609 |
Note: Data for all conditions were normally distributed, as determined by the Kolmogorov–Smirnov test (n = 4, *P < 0.05).
ECM: extracellular matrix.
We next quantified actin coverage to characterize cell size. Based on one-way ANOVA and multiple comparison analysis, we observed no significant differences after one day of TGF-β1 treatment (Figure 3(b)). On days 3 and 5, we observed several instances of statistically higher actin coverage in TGF-β1-treated cells compared to untreated cells, as shown in Figure 3(b), but no differences due to rigidity. Our two-way ANOVA analysis similarly demonstrated that actin coverage was regulated by TGF-β1, but not ECM rigidity, on days 3 and 5. Higher actin coverage combined with a relatively similar nuclei count implies that cells treated with TGF-β1 had a larger surface area than those without TGF-β1, which is consistent with a myofibroblast phenotype in TGF-β1-treated cells.
We next compared α-SMA/actin coverage within each timepoint because α-SMA is the most widely used marker for cardiac myofibroblasts. On Day 1, our one-way ANOVA and multiple comparison tests revealed that TGF-β1-treated cells on high PDMS had significantly greater α-SMA/actin coverage compared to all untreated cells (Figure 3(c)), suggesting that high substrate rigidity promoted TGF-β1-mediated differentiation to myofibroblasts. Additionally, on day 3, cells on high PDMS without TGF-β1 had higher α-SMA/actin coverage compared to cells on moderate PDMS without TGF-β1, potentially because high substrate rigidity promoted myofibroblast differentiation, even without TGF-β1. However, on days 3 and 5, TGF-β1 was the dominant regulator of fibroblast differentiation, as all treated cells had significantly higher α-SMA/actin coverage than all untreated cells, independent of substrate rigidity. Our two-way ANOVA analysis (Table 1) identified that α-SMA/actin coverage was regulated by TGF-β1 on days 1, 3, and 5. However, substrate rigidity regulated α-SMA/actin coverage on day 3, suggesting that this parameter also has an impact, although less prominent than TGF-β1. Collectively, these data suggest that the differentiation of fibroblasts to myofibroblasts is regulated predominantly by TGF-β1 compared to ECM rigidity, but that ECM rigidity potentially accelerates the differentiation process.
Expression of fibroblast- and myofibroblast-associated genes due to TGF-β1 and ECM rigidity
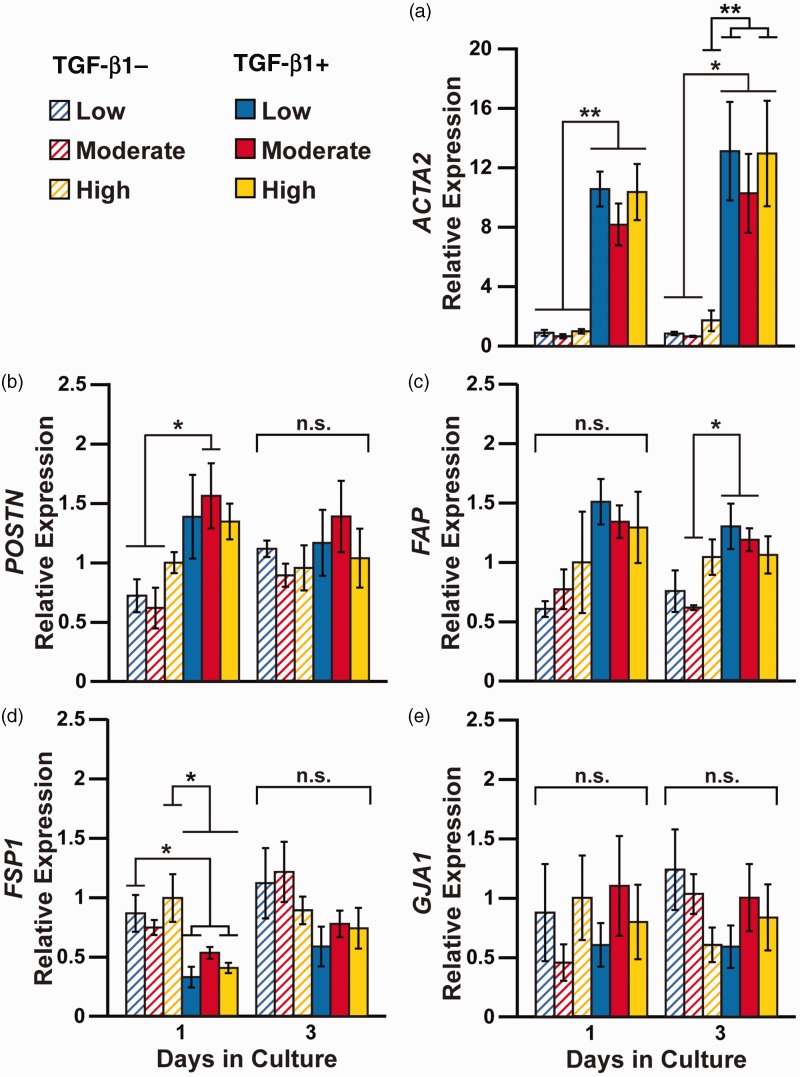
To determine if ECM rigidity and/or TGF-β1 impact transcription, we first performed RT-PCR on days 1 and 3 to quantify expression of the ACTA2 gene, which encodes for α-SMA. Our one-way ANOVA and multiple comparison analysis indicated that ACTA2 was significantly upregulated in nearly all TGF-β1-treated cells on both day 1 and day 3 compared to untreated cells, independent of substrate rigidity (Figure 4(a)). This result was corroborated with our two-way ANOVA test (Table 1), which indicated that ACTA2 expression was regulated by TGF-β1, but not substrate rigidity, on days 1 and 3. Compared to our RT-PCR data, our immunostaining results did not show stark distinctions due to TGF-β1 treatment as early as day 1, but this could be due to the delay between transcription and translation. Also, unlike our immunostaining results, we did not detect any differences in ACTA2 expression due to rigidity. However, the differences we observed with immunostaining were relatively subtle and could be attributed to differences in assay sensitivity. Thus, our RT-PCR data for ACTA2 are mostly consistent with our immunostaining results for α-SMA, as both datasets suggest that TGF-β1 dominates over ECM rigidity in differentiating human cardiac fibroblasts to myofibroblasts.
Figure 4.
Relative changes in gene expression due to ECM rigidity and TGF-β1 treatment. Human cardiac fibroblasts were cultured on low, moderate, and high PDMS-coated coated coverslips with or without 2 ng/mL TGF-β1 for one, three, and five days. RT-PCR was used to quantify the relative expression of (a) ACTA2, (b) POSTN, (c) FAP, (d) FSP1, and (e) GJA1. All data were normalized to high PDMS control group on Day 1. (n = 4, bars indicate mean ±standard error of the mean, *P < 0.05 and **P < 0.01 according to one-way ANOVA and Tukey’s test for multiple comparisons. For detailed statistical analysis, see Supplementary Tables S5 to S9). (A color version of this figure is available in the online journal.)
We also used RT-PCR to quantify expression of POSTN and FAP because these genes have been proposed in the literature as markers of myofibroblasts. POSTN encodes for periostin, an osteogenic protein commonly expressed in mesenchymal cells that interacts with structural components of the ECM and is associated with fibrosis. Studies have shown that periostin is expressed by myofibroblasts after myocardial infarction41 and in dermal wounds.42 On day 1, we observed increases in POSTN expression in TGF-β1-treated cells on moderate PDMS compared to untreated cells on soft and moderate PDMS by one-way ANOVA and multiple comparisons (Figure 4(b)). Our two-way ANOVA analysis revealed that POSTN expression is regulated by TGF-β1 on day 1, but not substrate rigidity (Table 1). On day 3, we observed no differences in POSTN expression by one-way or two-way ANOVA, suggesting that POSTN sensitivity to TGF-β1 decreases over exposure time. FAP encodes for fibroblast activation protein (FAP), which has been associated with proliferation and migration of activated stromal fibroblasts in human carcinomas.43 Myofibroblasts were also shown to express FAP after myocardial infarction in vivo and in response to TGF-β1 in vitro.44 In our study, the only statistical differences we observed through one-way ANOVA and multiple comparisons were an increase in FAP expression in TGF-β1-treated cells on low and moderate PDMS compared to untreated cells on moderate PDMS on day 3 (Figure 4(c)). However, our two-way ANOVA analysis indicated that FAP expression is regulated by TGF-β1 on day 1 and day 3. Thus, in several instances, both POSTN and FAP expression was higher in TGF-β1-treated cells compared to untreated cells, although results were not as consistent as ACTA2.
We next measured FSP1 expression using RT-PCR. FSP1 encodes for fibroblast specific protein-1 (FSP1), which promotes fibrosis and is shown to be upregulated in rat cardiac fibroblasts after myocardial infarction.45 However, FSP1 is also known to be expressed by many non-fibroblast cell types after an infarction, including endothelial cells and hematopoietic cells.46 Thus, it is unclear if FSP1 is a robust marker for identifying fibroblasts versus myofibroblasts. Our one-way ANOVA test revealed several decreases in FSP1 expression in TGF-β1-treated cells compared to untreated cells (Figure 4(d)), but no differences on day 3. Our two-way ANOVA test indicated that FSP1 expression is regulated by TGF-β1 on both day 1 and day 3 (Table 1). These results suggest that FSP1 expression is reduced due to TGF-β1 treatment in primary human cardiac fibroblasts and mostly unaffected by matrix rigidity.
GJA1 encodes for Cx43, the major gap junction protein expressed by cardiac myocytes in ventricular myocardium to facilitate cell-to-cell action potential propagation.47 The ability of fibroblasts and/or myofibroblasts to express Cx43 and form functional gap junction channels with cardiac myocytes has been widely debated.12–15 Thus, we used RT-PCR to investigate if GJA1 expression is impacted by TGF-β1 and/or matrix rigidity in human cardiac fibroblasts. Both our one-way (Figure 4(e)) and two-way ANOVA (Table 1) analysis indicated no significant differences in GJA1 expression between untreated and TGF-β1-treated cells at day 1 or 3, suggesting that fibroblasts and myofibroblasts in monoculture express similar levels of GJA1.
Reversibility of myofibroblast phenotype
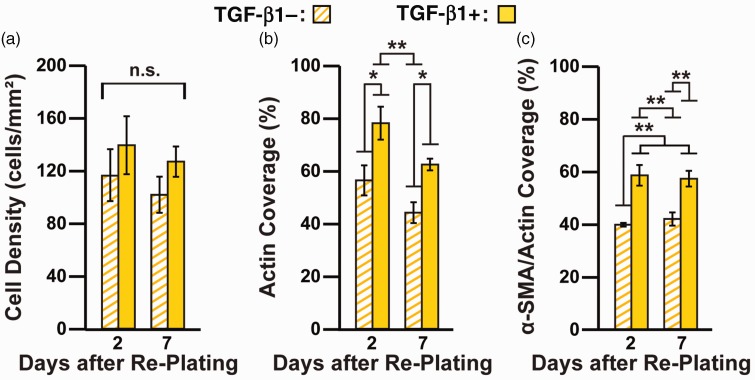
Myofibroblasts are thought to de-differentiate into quiescent fibroblasts after differentiation stimuli are removed.23,48 However, the extent of reversibility has not been thoroughly investigated, especially in human cardiac myofibroblasts. To establish if human cardiac myofibroblasts can de-differentiate, we first cultured fibroblasts for three days in the absence and presence of TGF-β1 on high PDMS to generate fibroblasts and myofibroblasts, respectively. Because ECM rigidity had a minimal impact on phenotype, we excluded this as a variable for these experiments. Next, we trypsinized and re-plated the cells onto high PDMS-coated coverslips and maintained them in the absence of TGF-β1. After an additional two and seven days, we immunostained re-plated cells for nuclei, actin, and α-SMA and characterized their phenotype using the image processing tools described above. Cell density was similar in all conditions and did not significantly change over the seven days (Figure 5(a)). However, actin coverage was significantly higher in cells pre-treated with TGF-β1 compared to untreated cells, although the difference became smaller over time (Figure 5(b)). α-SMA/actin coverage was significantly higher in cells pre-treated with TGF-β1 compared to untreated cells on day 2 and day 7 (Figure 5(c)), although the differences were not as substantial compared to our earlier experiments with sustained TGF-β1 treatment (Figure 3). These data indicate that myofibroblasts partially maintain their phenotype for at least a week after re-plating and TGF-β1 withdrawal.
Figure 5.
Quantification of cell morphology and α-SMA expression after re-plating untreated and TGF-β1-treated cells in the absence of TGF-β1. On day 3, untreated and TGF-β1-treated cells on high PDMS coverslips were trypsinized, re-plated onto high PDMS coverslips, and maintained without TGF-β1 for an additional two and seven days. Cells were immunostained and cell density (a), actin coverage (b), and α-SMA/actin coverage (c) were quantified from immunostained images (n = 4, bars indicate mean ± standard error of the mean, *P< 0.05 and **P< 0.01 according to one-way ANOVA and Tukey’s test for multiple comparisons. For detailed statistical analysis, see Supplementary Tables S10 to S12). (A color version of this figure is available in the online journal.)
Discussion
In the myocardium, many forms of injury and pathological remodeling are associated with increases in both ECM rigidity and TGF-β1 secretion by immune cells. However, the relative contribution of each of these microenvironmental cues in the differentiation of human cardiac fibroblasts to myofibroblasts has not been clearly established with existing in vivo and in vitro models. To address this, we cultured primary human cardiac fibroblasts on tunable PDMS substrates to control ECM rigidity and selectively treated cells with TGF-β1. Our results suggest that the rigidity of the microenvironment has some subtle effects on differentiation, but overall, TGF-β1 treatment dominates myofibroblast differentiation. These data suggest that the differentiation of cardiac fibroblasts to myofibroblasts is more sensitive to inflammatory responses after injury rather than increases in ECM rigidity secondary to fibrotic remodeling. We also quantified the expression of several genes previously associated with fibroblasts or myofibroblasts to establish their robustness as indicators of cell phenotype in vitro. Although some secondary markers trended with one phenotype, ACTA2 was the only gene consistently regulated by TGF-β1 treatment.
In our study, we first used cell density, actin coverage, and α-SMA/actin coverage as benchmarks to select an ECM ligand type, serum concentration, and TGF-β1 dose that maintained cell adhesion and viability and minimized any confounding effects due to these factors. Based on these data, we cultured cells on fibronectin instead of collagen I because cell adhesion was substantially higher on fibronectin compared to collagen I. However, α-SMA/actin coverage was similar on both substrates, which indicates that myofibroblast activation was unaffected by ECM ligand. In many fibrotic conditions, such as after a myocardial infarction, both collagen I and fibronectin are increased.1,49 Thus, both ligands represent aspects of the native cardiac microenvironment. We also found that cell density, actin coverage, and α-SMA/actin coverage were similar in 0.1% and 0.5% FBS and with 2 and 10 ng/mL TGF-β1, suggesting that our results are likely independent of FBS and TGF-β1 concentrations within these ranges.
To delineate the impact of ECM rigidity and TGF-β1, we uniformly coated fibronectin onto PDMS-coated coverslips with elastic moduli of 1.61 kPa, 27.4 kPa, and 2.68 MPa. These values roughly correspond to developing myocardium, healthy myocardium,40,50 and myocardium that is highly fibrotic and/or under high pressure overload,7,51 two conditions commonly observed after an infarction and other forms of injury. Although PDMS has highly synthetic properties, it is easier to controllably fabricate and handle for experimental measurements (especially microscopy) compared to many ECM-derived biomaterials. Thus, we could monitor fibroblast differentiation due to substrate rigidity and TGF-β1 in simple, yet relatively physiologically-relevant, constructs. We found that cell density was constant across all conditions at each time point, indicating that substrate rigidity and TGF-β1 had minimal impact on phenotypes such as cell adhesion or proliferation. In several instances, actin coverage increased only due to TGF-β1 treatment, suggesting that TGF-β1, but not ECM rigidity, induces an increase in cell size, characteristic of myofibroblasts. We also found that TGF-β1 treatment caused an increase in ACTA2 gene expression as early as day 1. This was followed by a substantial increase in α-SMA/actin coverage on days 3 and 5 in TGF-β1-treated cells. ECM rigidity also had some subtle effects on differentiation. For example, α-SMA/actin coverage was increased due to TGF-β1 treatment only on high PDMS on day 1, suggesting that fibroblasts have increased sensitivity to TGF-β1 on stiffer substrates and/or differentiate more rapidly on stiffer substrates. However, overall, TGF-β1 had a much stronger effect on phenotype compared to ECM rigidity. Our data are mostly in agreement with previous studies, which have also shown stark increases in α-SMA due to TGF-β1.17,38,52,53
Besides α-SMA, there are limited definitive markers that clearly distinguish cardiac fibroblasts from myofibroblasts.54 Here, we measured the expression of the genes for periostin (POSTN), FAP (FAP), and FSP1 (FSP1) because they have each been postulated to be differentially expressed by fibroblasts and myofibroblasts. Periostin is a secreted osteogenic protein that interacts with the ECM and promotes wound repair. Several studies have shown that cardiac myofibroblasts, but not fibroblasts, express periostin after injury in vivo.41,55,56 Similarly, periostin is up-regulated in dermal mouse myofibroblasts during cutaneous wound repair in vivo.42 In our experiments, we observed that POSTN expression was higher in TGF-β1-treated cells on day 1, but these differences were attenuated by day 3. This suggests that periostin is acutely up-regulated in cardiac fibroblast/myofibroblasts after TGF-β1 exposure, but maybe not for prolonged periods of time. Similar to periostin, FAP also promotes wound repair after an infarction. Elevated FAP expression has been observed in rat cardiac myofibroblasts after myocardial infarction in vivo and in human cardiac myofibroblasts after TGF-β1 treatment in vitro.44 We observed that FAP expression was increased due to TGF-β1 treatment on both days 1 and 3 of treatment. Thus, although POSTN and FAP were not as consistently or strikingly impacted by TGF-β1 treatment compared to α-SMA, they still trended with a myofibroblast phenotype, suggesting they are relevant secondary markers for human cardiac myofibroblasts.
FSP1 encodes for FSP1, which has been shown to be expressed by rat cardiac fibroblasts and up-regulated in injured myocardium in vivo.45,57 In our study, we observed a decrease in FSP1 expression due to TGF-β1 treatment. The discrepancy in our data compared to previous in vivo studies could be due to the number of additional factors and cues in vivo. For example, FSP1 has been shown to be expressed strongly by non-fibroblasts, such as endothelial cells and hematopoietic cells, after myocardial injury,46 which could confound the in vivo results. Furthermore, our experiments were in primary human cardiac fibroblasts, whereas most previous studies with this gene and protein were in rodents. Thus, more studies are needed to further establish the role of FSP1 in human cardiac fibroblasts and myofibroblasts.
The ability of fibroblasts and/or myofibroblasts to couple to cardiac myocytes via Cx43 gap junction channels has been a relatively controversial topic. For example, in vivo and ex vivo studies have shown that fibroblasts/myofibroblasts and cardiac myocytes do not form gap junctions in healthy myocardium58 or after myocardial infarction.59 However, other studies have shown that fibroblasts/myofibroblasts express Cx4360 and conduct signals across scar tissue61 after an infarction. In vitro, fibroblasts62 and myofibroblasts15 have been shown to propagate action potentials from cardiac myocytes, with an increase in coupling after TGF-β1-treatment.63 Furthermore, silencing Cx43 in myofibroblasts has been shown to reduce arrhythmogenesis in co-culture models.64 Collectively, most studies suggest that Cx43 is up-regulated in myofibroblasts after myocardial injury, although this has been investigated primarily in rodent cells. Here, we tested if GJA1 expression, which encodes for Cx43 protein, is up-regulated due to matrix rigidity and/or TGF-β1 treatment in primary human cardiac fibroblasts. We found no significant difference in GJA1 expression due to these two variables, which suggests that expression of GJA1 is similar in human cardiac fibroblasts and myofibroblasts. However, one important consideration is that expression of GJA1 in fibroblasts/myofibroblasts could be influenced by the presence of cardiac myocytes, which are not present in our system. Additionally, our simplified in vitro system does not recapitulate all the diverse cues present in vivo, which could have a more substantial impact on GJA1 expression.
In many fibroblasts of non-cardiac origins, ECM rigidity and TGF-β1 have been shown to jointly promote differentiation to myofibroblasts. For example, freshly isolated rat portal fibroblasts,28 rat hepatic stellate cells,65 and rat bronchial fibroblasts27 have the highest expression of myofibroblast markers when treated with TGF-β1 and cultured on stiffer surfaces. In our study, we observed more subtle effects of ECM rigidity. For example, α-SMA/actin coverage was significantly higher in TGF-β1-treated cells at our earliest time point only on the most rigid substrate. Beyond this first timepoint, TGF-β1 activated myofibroblast differentiation equally on all substrates. However, the previous studies mentioned above used rat fibroblasts at very early passages, which was logistically impossible for our study because we used primary human cardiac fibroblasts, which are in extremely limited supply. Thus, we were forced to expand our cells in polystyrene flasks prior to experiments, which could have reduced their sensitivity to ECM rigidity. Additionally, myofibroblast differentiation is likely distinct in fibroblasts from different species and/or organs.
In general, myofibroblasts are thought to de-differentiate into quiescent fibroblasts when differentiation stimuli, such as TGF-β1 or mechanical stress, are removed. For example, α-SMA expression decreased in cultured synovial fibroblasts after removal of exogenous TGF-β1.35 However, in this study, expression levels were still higher than quiescent fibroblasts, suggestive of an incomplete reversal of phenotype. Similarly, valvular myofibroblasts cultured on stiff surfaces reduced α-SMA expression when matrix stiffness was decreased.23 Our data are consistent with these studies, as we also observed a partial reversibility in myofibroblast phenotype after we re-plated TGF-β1-treated cells onto new substrates and excluded TGF-β1 from the culture media. Our rationale for transferring our cells to a new platform was two-fold. First, the cells often detached from their substrates after approximately one week, which is insufficient time to treat cells with TGF-β1 to activate differentiation, withdraw TGF-β1, and monitor de-differentiation. Second, we are interested in controllably generating cardiac fibroblasts and myofibroblasts in culture and then re-plating them on new substrates for additional downstream experiments, such as co-culture with cardiac myocytes. However, our results suggest that TGF-β1 must be sustained to maintain high levels of differentiated myofibroblasts.
As mentioned above, one limitation of our study is that we did not incorporate cardiac myocytes that coexist with fibroblasts in the native myocardium and likely have an impact on fibroblast/myofibroblast phenotype. However, for this study, our goal was to minimize the number of variables and focus on how ECM rigidity and TGF-β1 specifically impact fibroblast differentiation. Future studies will focus on investigating interactions between human cardiac myocytes and fibroblasts/myofibroblasts. We also neglected to investigate the impact of other cytokines, such as tumor necrosis factors and interleukin proteins that are also secreted by neutrophils during inflammation and likely also affect human cardiac myofibroblast differentiation.66 These are also important topics for follow-up studies.
In summary, we investigated the independent and combined effects of ECM rigidity and TGF-β1 on the phenotype of primary human cardiac fibroblasts. Our findings demonstrate that differentiation to myofibroblasts is predominantly regulated by TGF-β1 treatment rather than ECM rigidity. This suggests that targeting the TGF-β/Smad signaling pathway could have therapeutic potential to minimize fibrosis after an infarction, even in rigid, fibrotic microenvironments. Additionally, fibroblasts and myofibroblasts are key players in many physiological and pathological process in the myocardium, but have been mostly excluded from in vitro studies. Here, we established parameters for robustly generating human cardiac myofibroblasts in vitro. This approach can be used for controlled in vitro studies to further investigate the coupling between myocytes, fibroblasts, and myofibroblasts, for example. By controlling the phenotype of fibroblasts and myofibroblasts, these cells can also be selectively incorporated into more physiologically relevant in vitro models of healthy and diseased myocardium “on a chip,” which have many applications for human disease modeling and drug screening.
Supplemental Material
Supplemental material for TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts by Nathan Cho, Shadi E Razipour and Megan L McCain in Experimental Biology and Medicine
Authors’ contributions
All authors participated in the analysis of the data and review of the manuscript; NC and SER conducted the experiments; NC and MLM designed the experiments, interpreted the data, and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with research, authorship, and/or publication of this article.
Funding
This work was sponsored by the University of Southern California (USC) Viterbi School of Engineering, USC Provost’s Fellowship, USC Women in Science and Engineering, and the American Heart Association [16SDG29950005].
References
- 1.Ma Y, de Castro Brás LE, Toba H, Iyer RP, Hall ME, Winniford MD, Lange RA, Tyagi SC, Lindsey ML. Myofibroblasts and the extracellular matrix network in post-myocardial infarction cardiac remodeling. Pflugers Arch 2014; 466:1113–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 2013; 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004; 18:816–27 [DOI] [PubMed] [Google Scholar]
- 4.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 2010; 106:1675–80 [DOI] [PubMed] [Google Scholar]
- 5.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993; 122:103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt-Gräff A, Desmoulière A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch 1994; 425:3–24 [DOI] [PubMed] [Google Scholar]
- 7.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 2006; 290:H2196–203 [DOI] [PubMed] [Google Scholar]
- 8.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res 2006; 69:604–13 [DOI] [PubMed] [Google Scholar]
- 9.Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995; 146:56–66 [PMC free article] [PubMed] [Google Scholar]
- 10.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 2013; 10:15–26 [DOI] [PubMed] [Google Scholar]
- 11.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 2010; 7:30–7 [DOI] [PubMed] [Google Scholar]
- 12.McDowell KS, Arevalo HJ, Maleckar MM, Trayanova NA. Susceptibility to arrhythmia in the infarcted heart depends on myofibroblast density. Biophys J 2011; 101:1307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res 2010; 107:1011–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson SA, Blazeski A, Copeland CR, Cohen DM, Chen CS, Reich DM, Tung L. Acute slowing of cardiac conduction in response to myofibroblast coupling to cardiomyocytes through N-cadherin. J Mol Cell Cardiol 2014; 68:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 2007; 101:755–8 [DOI] [PubMed] [Google Scholar]
- 16.Schultz JlJ, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 2002; 109:787–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017; 127:3770–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 2007; 282:23337–47 [DOI] [PubMed] [Google Scholar]
- 19.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts. Matrix Biol 2015; 42:74–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding J, Kwan P, Ma Z, Iwashina T, Wang J, Shankowsky HA, Tredget EE. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns 2016; 42:1277–86 [DOI] [PubMed] [Google Scholar]
- 21.Poosti F, Bansal R, Yazdani S, Prakash J, Post E, Klok P, van den Born J, de Borst MH, van Goor H, Poelstra K, Hillebrands JL. Selective delivery of IFN-γ to renal interstitial myofibroblasts: a novel strategy for the treatment of renal fibrosis. FASEB J 2015; 29:1029–42 [DOI] [PubMed] [Google Scholar]
- 22.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol 2012; 4:410–21 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 2012; 7:e39969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Li X, Zhao S, Zeng Y, Zhao L, Ding H, Sun W, Du Y. Microengineered in vitro model of cardiac fibrosis through modulating myofibroblast mechanotransduction. Biofabrication 2014; 6:045009. [DOI] [PubMed] [Google Scholar]
- 25.Balestrini JL, Billiar KL. Magnitude and duration of stretch modulate fibroblast remodeling. J Biomech Eng 2009; 131:051005. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Seth A, McCulloch CA. Force regulates smooth muscle actin in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 2000; 279:H2776–85 [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Dong Y, Duan Y, Jiang X, Chen C, Deng L. Substrate stiffness influences TGF-β1-induced differentiation of bronchial fibroblasts into myofibroblasts in airway remodeling. Mol Med Rep 2013; 7:419–24 [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 2007; 46:1246–56 [DOI] [PubMed] [Google Scholar]
- 29.Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol 2003; 25:79–86 [DOI] [PubMed] [Google Scholar]
- 30.Palchesko RN, Zhang L, Sun Y, Feinberg AW. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PloS One 2012; 7:e51499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettadapur A, Suh GC, Geisse NA, Wang ER, Hua C, Huber HA, Viscio AA, Kim JY, Strickland JB, McCain ML. Prolonged culture of aligned skeletal myotubes on micromolded gelatin hydrogels. Sci Rep 2016; 6:28855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyra-Leite DM, Andres AM, Petersen AP, Ariyasinghe NR, Cho N, Lee JA, Gottlieb RA, McCain ML. Mitochondrial function in engineered cardiac tissues is regulated by extracellular matrix elasticity and tissue alignment. Am J Physiol Heart Circ Physiol 2017; 313:H757–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton 2008; 65:641–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101–8 [DOI] [PubMed] [Google Scholar]
- 35.Mattey DL, Dawes PT, Nixon NB, Slater H. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis 1997; 56:426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chitturi RT, Balasubramaniam AM, Parameswar RA, Kesavan G, Haris KT, Mohideen K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J Int Oral Health 2015; 7:75–80 [PMC free article] [PubMed] [Google Scholar]
- 37.Kural MH, Billiar KL. Myofibroblast persistence with real-time changes in boundary stiffness. Acta Biomater 2016; 32:223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard M, Dieudé M, Yang B, Hamelin K, Underwood K, Hébert MJ. Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF. Autophagy 2014; 10:2193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 2003; 278:12384–9 [DOI] [PubMed] [Google Scholar]
- 40.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. Cell Sci 2008; 121:3794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, J Lin SC, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016; 7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci 2012; 125:121–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Yang WW, Wen QT, Xu L, Chen M. TGF-beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected]. Exp Mol Pathol 2009; 87:189–94 [DOI] [PubMed] [Google Scholar]
- 44.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol 2015; 87:194–203 [DOI] [PubMed] [Google Scholar]
- 45.Tamaki Y, Iwanaga Y, Niizuma S, Kawashima T, Kato T, Inuzuka Y, Horie T, Morooka H, Takase T, Akahashi Y, Kobuke K, Ono K, Shioi T, Sheikh SP, Ambartsumian N, Lukanidin E, Koshimizu TA, Miyazaki S, Kimura T. Metastasis-associated protein, S100A4 mediates cardiac fibrosis potentially through the modulation of p53 in cardiac fibroblasts. J Mol Cell Cardiol 2013; 57:72–81 [DOI] [PubMed] [Google Scholar]
- 46.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol 2013; 305:H1363–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanter HL, Saffitz JE, Beyer EC. Cardiac myocytes express multiple gap junction proteins. Circ Res 1992; 70:438–44 [DOI] [PubMed] [Google Scholar]
- 48.Driesen RB, Nagaraju CK, Abi-Char J, Coenen T, Lijnen PJ, Fagard RH, Sipido KR, Petrov VV. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res. 2014; 101:411–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: opportunities for new therapeutic intervention. Adv Wound Care 2013; 2:122–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and Myosin expression to optimize beating. Curr Biol 2013; 23:2434–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doering CW, Jalil JE, Janicki JS, Pick R, Aghili S, Abrahams C, et al. Collagen network remodelling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res 1988; 22:686–95 [DOI] [PubMed] [Google Scholar]
- 52.Asazuma-Nakamura Y, Dai P, Harada Y, Jiang Y, Hamaoka K, Takamatsu T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res 2009; 315:1190–9 [DOI] [PubMed] [Google Scholar]
- 53.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A 2005; 102:437–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lajiness JD, Conway SJ. Origin, development, and differentiation of cardiac fibroblasts. J Mol Cell Cardiol 2014; 70:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res 2009; 105:934–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 2010; 120:254–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider M, Kostin S, Strøm CC, Aplin M, Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E, Lerche Hansen J, Sheikh SP. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res 2007; 75:40–50 [DOI] [PubMed] [Google Scholar]
- 58.Hucker WJ, McCain ML, Laughner JI, Iaizzo PA, Efimov IR. Connexin 43 expression delineates two discrete pathways in the human atrioventricular junction. Anat Rec 2008; 291:204–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baum JR, Long B, Cabo C, Duffy HS. Myofibroblasts cause heterogeneous Cx43 reduction and are unlikely to be coupled to myocytes in the healing canine infarct. Am J Physiol Heart Circ Physiol 2012; 302:H790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol 2010; 19:e233–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahoney VM, Mezzano V, Mirams GR, Maass K, Li Z, Cerrone M, Vasquez C, Bapat A, Delmar M, Morley GE. Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Sci Rep 2016; 6:26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res 2003; 93:421–8 [DOI] [PubMed] [Google Scholar]
- 63.Salvarani N, Maguy A, De Simone SA, Miragoli M, Jousset F, Rohr STGF. Β1 (Transforming growth factor-β1) plays a pivotal role in cardiac myofibroblast arrhythmogenicity. Circ Arrhythm Electrophysiol 2017; 10:e004567. [DOI] [PubMed] [Google Scholar]
- 64.Askar SF, Bingen BO, Swildens J, Ypey DL, van der Laarse A, Atsma DE, Zeppenfeld K, Schalij MJ, de Vries AA, Pijnappels DA. Connexin43 silencing in myofibroblasts prevents arrhythmias in myocardial cultures: role of maximal diastolic potential. Cardiovasc Res 2012; 93:434–44 [DOI] [PubMed] [Google Scholar]
- 65.Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 2011; 301:G110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 2004; 94:1543–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts by Nathan Cho, Shadi E Razipour and Megan L McCain in Experimental Biology and Medicine