Abstract
The pantropical Picrodendraceae produce mostly spheroidal to slightly oblate, echinate pollen grains equipped with narrow circular to elliptic pori that can be hard to identify to family level in both extant and fossil material using light microscopy only. Fossil pollen of the family have been described from the Paleogene of America, Antarctica, Australia, New Zealand, and Europe, but until now none have been reported from Afro-India. Extant pollen described here include representatives from all recent Picrodendraceae genera naturally occurring in Africa and/or Madagascar and south India and selected closely related tropical American taxa. Our analyses, using combined light microscopy and scanning electron microscopy, show that pollen of the Afro-Indian genera encompass three morphological types: Type 1, comprising only Hyaenanche; Type 2, including Aristogeitonia, Mischodon, Oldfieldia and Voatamalo; Type 3, comprising the remaining two genera, Androstachys and Stachyandra. Based on the pollen morphology presented here it is evident that some previous light microscopic accounts of spherical and echinate fossil pollen affiliated with Arecaceae, Asteraceae, Malvaceae, and Myristicaceae from the African continent could belong to Picrodendraceae. The pollen morphology of Picrodendraceae, fossil pollen records, a dated intra-familial phylogeny, seed dispersal modes, and the regional Late Cretaceous to early Cenozoic paleogeography, together suggest the family originated in the Americas and dispersed from southern America across Antarctica and into Australasia. A second dispersal route is believed to have occurred from the Americas into continental Africa via the North Atlantic Land Bridge and Europe.
Keywords: Androstachys, autochory, Aristogeitonia, Hyaenanche, myrmecochory, Oldfieldia, Piranhea, Stachyandra, Tetracoccus, Voatamalo
Picrodendraceae are a small pantropical woody family of 25 genera and 96 species in the order Malpighiales (WCSP 2018; see Supporting Information). Prior to changes in the classification of orders and families of flowering plants as a result of increased morphological, anatomical, and especially molecular phylogenetic information, the genera constituted the three tribes of subfamily Oldfieldioideae in the Euphorbiaceae sensu lato (Webster 1994a, 1994b). The distribution of Picrodendraceae is disjunct across the subtropical and tropical Southern Hemisphere, with some limited representation in cooler temperate zones. Representatives of the family occur in the Americas (five genera), Africa (six genera; Table I), south India and Sri Lanka (one genus; Table I) and in Australasia (13 genera; see Supporting Information). The greatest diversity at both the genus and species levels occurs in Australia with ten genera (seven endemic) and approximately 40 spp (WCSP 2018). The largest genus is Austrobuxus with 22 spp, of which 15 are endemic to New Caledonia (WCSP 2018; see Supporting Information). The extensive vegetative diversity of Picrodendraceae is reflected in the widely different habitats and environments the family occupies (noted from herbarium sheets examined for this study): a range of altitudes from near sea level to over 1800 m; in flooded forests in the Amazon; deserts in the south-western United States and northern Mexico; dry deciduous forests, humid evergreen forests, woodlands, in sand and on rocky hills in Africa, Madagascar, and south India; dry fynbos and savanna in South Africa (Dyer 1975); drier rainforests and woodlands on sandy or serpentine soils in Australia; and rain forests in New Caledonia.
Table I.
Afro-Indian Picrodendraceae and their distribution.
| Genus | Species number | Taxon | Occurence | Geographic region | Pollen samples used for this study |
|---|---|---|---|---|---|
| Androstachys | 1 | A. johnsonii Prain | Mozambique to South Africa, Madagascar | Africa | van der Schyff, 978 (PRE) |
| Aristogeitonia | 7 | A. gabonica Breteler | Gabon | Africa | Sosef, 1794 (MO) |
| A. limoniifolia Prain | Angola | Africa | |||
| A. lophirifolia Radcl.-Sm. | North and west Madagascar | Madagascar | Capuron, 23.156-SF (MO) | ||
| A. magnistipula Radcl.-Sm. | Tanzania | Africa | |||
| A. monophylla Airy Shaw | Southeast Kenya, east Tanzania | Africa | Phillipson, 4954 (MO) | ||
| A. perrieri (Leandri) Radcl.-Sm. | North and west Madagascar | Madagascar | Capuron, 18.462-SF (MO) | ||
| A. uapacifolia Radcl.-Sm. | Madagascar (Toliara Province) | Madagascar | |||
| Hyaenanche | 1 | H. globosa (Gaert.) Lamb. & Vahl | Cape Province in South Africa | Africa | Hall 3912 (PRE); unknown s.n. (WAG) |
| Mischodon | 1 | M. zeylanicus Thwaites | Sri Lanka, South India, Andaman Is. | India | unknown s.n. (WAG) |
| Oldfieldia | 4 | O. africana Benth. & Hook.f. | West and west/central tropical Africa | Africa | Small 621 (K); Leeuwenberg 3780 (WAG) |
| O. dactylophylla (Welw. ex Oliv.) J.Léonard | Tanzania to south tropical Africa | Africa | Meuangulango 1279 (MO) | ||
| O. macrocarpa J.Léonard | Central and southeast DR Congo | Africa | |||
| O. somalensis (Chiov.) Milne-Redh. | Somalia to Mozambique | Africa | Bally 6880 (EA) | ||
| Stachyandra | 4 | S. imberbis (Air Shaw) Radcl.-Sm. | Northwest Madagascar | Madagascar | |
| S. merana (Airy Shaw) J.-F.Leroy ex Radcl.-Sm. | Northwest Madagascar | Madagascar | Capuron, 23.335-SF (MO) | ||
| S. rufibarbis (Airy Shaw) Radcl.-Sm. | Northeast Madagascar | Madagascar | |||
| S. viticifolia (Airy Shaw) Radcl.-Sm. | Northwest Madagascar | Madagascar | |||
| Voatamalo | 2 | V. capuronii Bosser | Northeast Madagascar | Madagascar | |
| V. eugenioides Capuron ex Bosser | East Madagascar | Madagascar | Capuron, 22.327-SF (MO) |
Note: Data extracted from WCSP (2018).
Picrodendraceae includes monoecious or dioecious subshrubs, shrubs, and small to large trees with simple or palmately-compound leaves; tiny, apetalous, mostly unisexual flowers; anthers of male flowers few or to as many as 55; ovaries 2–7-locular with biovulate locules; fruit capsular and dehiscent or drupaceous; seeds one or two per locule; and copious endosperm. The floral structure indicates a close relationship to the Phyllanthaceae (formerly fam. Euphorbiaceae s.l., subfam. Phyllanthoideae), which is supported in molecular phylogenetic analyses of the Euphorbiaceae (Wurdack et al. 2004; Wurdack & Davis 2009) and Malpighiales (Soltis et al. 2011; Xi et al. 2012). Morphologically, Picrodendraceae and Phyllanthaceae are united by unspecialised monopodial branching patterns, a lack of laticifers, and biovulate locules in the capsular fruit (Webster 1994a, 1994b; Merino Sutter et al. 2006). In an extensive phylogeny of the order Malpighiales, sequences of 82 plastid genes produced a fully supported tree in which Picrodendraceae and Phyllanthaceae were confirmed as sister families (Xi et al. 2012). The South American genus Podocalyx was retrieved as sister to the other Picrodendraceae, with Tetracoccus + Androstachys, sister to a clade of Petalostigma, Dissiliaria, and Micrantheum + Austrobuxus.
The unique morphology of Picrodendraceae pollen (± spherical, echinate, stephanoporate) and its systematic value was first pointed out by Erdtman (1952) and later by Punt (1962) and Köhler (1965). The pollen morphology of Afro-Indian Picrodendraceae has been studied to some extent (Table II). Among 20 species, eight have been studied using transmission electron microscopy (TEM) (Hayden et al. 1984; Levin & Simpson 1994; Simpson & Levin 1994), six have been studied using scanning electron microscopy (SEM) (Hayden et al. 1984; Levin & Simpson 1994; Lobreau-Callen & Cervera 1994; Simpson & Levin 1994), and four have been illustrated using light microscopy (LM) micrographs (Köhler 1965). These studies documented some variation in the pollen morphology among genera or at the species level of Afro-Indian Picrodendraceae that suggested fossil Picrodendraceae pollen grains could be associated with specific intrafamilial lineages.
Table II.
Previously illustrated pollen of Afro-Indian Picrodendraceae species.
| Taxon (Type) | Figured in | Micrograph Nr. | Micrograph type |
|---|---|---|---|
| Androstachys johnsonii | Simpson & Levin 1994 (Levin & Simpson 1994) |
Fig. 35 (Fig. 7) |
SEM, overview |
| Simpson & Levin 1994 (Levin & Simpson 1994) |
Fig. 36 (Fig. 8) |
SEM, close-up | |
| Simpson & Levin 1994 (Levin & Simpson 1994) |
Fig. 45 (Fig. 22) |
TEM, non-apertural wall | |
| Simpson & Levin 1994 | Fig. 46 | TEM, apertural region | |
| Köhler 1965 | Plate 9, fig. 9 | LM, overview | |
| Punt 1962 | Plate VII, fig. 1 | LM (drawing) | |
| Aristogeitonia limoniifolia | Punt 1962 | Plate VI, fig. 5 | LM (drawing) |
| Aristogeitonia monophylla | Simpson & Levin 1994 | Fig. 52 | TEM, non-apertural wall |
| Simpson & Levin 1994 | Fig. 53 | TEM, apertural region | |
| Aristogeitonia perrieri | Lobreau-Callen & Cervera 1994 | Plate 5, fig. G | SEM, overview |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. H | SEM, close-up | |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. I | SEM, break in wall | |
| Hyaenanche globosa | Simpson & Levin 1994 | Fig. 54 | TEM, non-apertural wall |
| Simpson & Levin 1994 | Fig. 55 | TEM, apertural region | |
| Punt 1987 | Fig. 7 | SEM, overview | |
| Köhler 1965 | Plate 8, fig. 18 | LM, overview | |
| Punt 1962 | Plate 6, fig. 6 | LM (drawing) | |
| Mischodon zeylanicus | Simpson & Levin 1994 | Fig. 51 | TEM, apertural region |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. A | SEM, overview | |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. B | SEM, close-up | |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. C | SEM, break in wall | |
| Köhler 1965 | Plate 8, fig. 17 | LM, overview | |
| Oldfieldia africana | Simpson & Levin 1994 (Levin & Simpson 1994) |
Fig. 44 (Fig. 18) |
TEM, non-apertural wall |
| Hayden et al. 1984 | Fig. 16 | SEM, overview | |
| Oldfieldia dactylophylla | Köhler 1965 | Plate 8, fig. 16 | LM, overview |
| Stachyandra merana | Simpson & Levin 1994 | Fig. 47 | TEM, non-apertural wall |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. D | SEM, overview | |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. E | SEM, close-up | |
| Lobreau-Callen & Cervera 1994 | Plate 5, fig. F | SEM, break in wall | |
| Stachyandra rufibarbis | Simpson & Levin 1994 | Fig. 48 | TEM, non-apertural wall |
| Simpson & Levin 1994 | Fig. 49 | TEM, apertural region | |
| Voatamalo eugenioides | Simpson & Levin 1994 | Fig. 50 | TEM, non-apertural wall |
The current disjunct tropical to subtropical distribution of Picrodendraceae implies that it already had a worldwide distribution in early Cenozoic times. This is supported by fossil pollen records from the Paleocene and/or Eocene of Australia (Martin 1974, 1978, 1982), New Zealand (Raine et al. 2011), Europe (Zetter & Hofmann 2008; Hofmann et al. 2011; Zetter et al. 2011) and North America (Tschudy & van Loenen 1970; Tschudy 1973; Graham et al. 2000). Interestingly, there is not a single fossil record of Picrodendraceae from Afro-India.
Here we describe and illustrate pollen from 12 of the 20 (12/20) extant taxa currently accepted in Afro-India (Table III), including: Androstachys (1/1), Aristogeitonia (4/7), Hyaenanche (1/1), Oldfieldia (3/4), Stachyandra (1/4), Voatamalo (1/2) and Mischodon (1/1). Pollen of Tetracoccus (1/4) and Piranhea (2/4), from the Americas, are also portrayed as they represent early branching taxa/lineages of the American-Afro-Indian clade. The extant African pollen grains are compared, grouped by morphological type, and their diagnostic features highlighted. Fossil Picrodendraceae pollen from the Miocene of Africa and their Eocene predecessors from Europe are also described. The morphological traits of extant taxa are here used to associate fossil Picrodendraceae pollen grains from Europe and Africa with extant genera and/or lineages. Further, the pollen morphology is discussed in relation to dispersal methods, the fossil record, paleogeography, and the current molecular phylogenetic framework, to unravel the paleophytogeographic history of this family in Afro-India. A dated phylogeny is presented supporting the hypothesis that Picrodendraceae originated in the Upper Cretaceous of the Americas from where they dispersed across the world during the Cenozoic.
Table III.
Herbarium material used for this study.
| Taxon | Collector | Coll. Nr. | Country | Herbarium |
|---|---|---|---|---|
| Androstachys johnsonii Prain | H.P.v.d. Schyff | 978 | South Africa | PRE |
| Aristogeitonia gabonica Breteler | M.S.M. Sosef | 1794 | Gabon | MO |
| Aristogeitonia lophirifolia Radcl.-Sm. | R. Capuron | 23.156-SF | Madagascar | MO |
| Aristogeitonia monophylla Airy Shaw | P.B. Phillipson | 4954 | Tanzania | MO |
| Aristogeitonia perrieri (Leandri) Radcl.-Sm. | R. Capuron | 18.462-SF | Madagascar | MO |
| Hyaenanche globosa (Gaert.) Lamb. & Vahl | H. Hall | 3912 | Namibia | PRE |
| Hyaenanche globosa (Gaert.) Lamb. & Vahl | Unknown | s.n. | South Africa | WAG |
| Mischodon zeylanicus Thwaites | Unknown | s.n. | India | WAG |
| Oldfieldia africana Benth. & Hook.f. | D. Small | 621 | Sierra Leone | K |
| Oldfieldia africana Benth. & Hook.f. | Leeuwenberg | 3780 | Ivory Coast | WAG |
| Oldfieldia dactylophylla (Welw. Ex Oliv.) J.Léonard | N.A. Meuangulango | 1279 | Tanzania | MO |
| Oldfieldia somalensis (Chiov.) Milne-Redh. | P.C. Bally | 6880 | Tanzania | EA |
| Piranhea longepedunculata Jabl. | Liesner & Gonzalez | 5859 | Venezuela | WAG |
| Piranhea trifoliata Baill. | Berg et al. | P19790 | Brazil | WAG |
| Stachyandra merana (Airy Shaw) J.-F.Leroy ex Radcl.-Sm. | R. Capuron | 23.335-SF | Madagascar | MO |
| Tetracoccus fasciculatus (S.Watson) Croizat | Langenberg | s.n. | USA | WAG |
| Voatamalo eugenioides Capuron ex Bosser | R. Capuron | 22.327-SF | Madagascar | MO |
Material and methods
Origin and preparation of samples
Extant flower material (see Table III) from the Missouri Botanical Garden (MO), the Royal Botanic Gardens, Kew (K), the South African National Biodiversity Institute (PRE), the National Museums of Kenya (EA), and Naturalis (WAG) was prepared according to the protocol outlined in Grímsson et al. (2017a, 2018b) and Halbritter et al. (2018).
The fossil Picrodendraceae pollen identified during this study occurred in five different sedimentary rock samples: (1) the Holzer Formation (early Eocene) at Krappfeld, Pemberg Quarry, west of Klein St Paul, Carinthia, Austria, (2) the Borkener coal measures (middle Eocene) of the Stolzenbach underground coal mine, near Kassel, Germany, (3) the Profen Formation (middle Eocene) of the Profen opencast mine, close to Leipzig, Germany, (4) the Mush Valley fossil beds (sample MU07; early Miocene) of the central Ethiopian Plateau, Ethiopia, and (5) the Elandsfontyn Formation (early Miocene), core sample #114755 from Saldanha Bay, South Africa. For details on the geographic positions, geology, palaeoecology, and previously reported fossil plants from these formations and localities see Table IV and references cited therein.
Table IV.
Information on sample sites.
| Krappfeld MT | Stolzenbach MT | Profen MT | Mush MT | Saldanha MT | |
|---|---|---|---|---|---|
| Location | Krappfeld area, Carinthia, Austria | Stolzenbach underground coalmine, Kassel, Germany | Profen opencast mine, close to Leipzig, Germany | Mush Valley, Debre Birhan Woreda, Ethiopia | Saldanha Bay drill core, South Africa |
| Latitude and longitude (c.) | 46°50′N, 14°31′E | 51°0′N, 9°17′E | 51°09′N, 12°11′E | 9°47′N, 39°39′E | 33°01′S, 17°59′E |
| Lithostratigraphy | Holzer Formation | Borkener coal measures | Profen Formation | Unnamed lacustrine sediments (sample MU07) | Elandsfontyn Formation (core sample #114755) |
| Epocha | Ypresian | Lutetian | Bartonian | Aquitanian | Aquitanian |
| Age (Ma)a | 56.0–47.8a | 47.8–41.2a | 41.2–38a | 22.63–21.73 (absolute dating) | 23.03–20.44a |
| Age according to | Litho- and biostratigraphy | Litho- and biostratigraphy | Litho- and biostratigraphy | Chrono- and lithostratigraphy | Litho- and biostratigraphy |
| For further info on the geological background, stratigraphy (S), paleoenvironment, paleoclimate, and plant fossils (P) | Hofmann & Zetter 2001 (S/P); Zetter & Hofmann 2001 (P); Hofmann et al. 2012, Hofmann et al. 2015a, 2015b (S/P); Hofmann 2018 (P) | Oschkinis & Gregor 1992 (P/S); Gregor 2005 (P); Hottenrott et al. 2010 (P); Gregor & Oschkinis 2013 (P); Manchester et al. 2015 (P); Grímsson et al. 2017b (P); Hofmann 2018 (P), Hofmann & Gregor 2018 (P) | Krutzsch & Lenk 1973 (P/S); Pälchen & Walter 2011 (S); Manchester et al. 2015 (P); Grímsson et al. 2017c (P) | Danehy 2010 (S/P); Pan et al. 2012 (S/P), 2014 (P), Bush et al. 2017(P), Tesfamichael et al. 2017 (S/P) | Roberts et al. 2017 (S/P); Coetzee 1981, 1983 (P); Coetzee & Muller 1984 (P), Coetzee & Praglowski 1984, 1988 (P); Grímsson et al. 2018c (P). |
aFollowing ICS (2017)
The sedimentary rock samples were processed and fossil pollen grains extracted according to the method explained in Grímsson et al. (2008). The fossil Picrodendraceae pollen grains were investigated both by LM and SEM using the single grain method as described by Zetter (1989) and Halbritter et al. (2018). SEM stubs with extant and fossil Picrodendraceae pollen produced under this study are stored in the collection of the Department of Paleontology, University of Vienna, Austria.
Tree inference
Sequences for five mitochondrial loci (ccmB, cob, nad1, nad6, mt-rps3), three nuclear loci (18S, EMB2765, PHYC), and four plastid loci (atpB, matK, ndhF, rbcL) from Picrodendraceae were downloaded from NCBI GenBank. Sequences for each locus were aligned using MAFFT v.7.273 (Katoh & Standley 2013) using the L-INS-I algorithm for all loci. Alignments were visually inspected for irregularities and truncated at the end for portions covering data of less than seven genera. The single locus alignments were then concatenated using MESQUITE (Maddison & Maddison 2011). Phylogenetic analyses were run using RaxML v.8.2.10 (Stamatakis 2014), which we used to obtain 1000 (fast) bootstrap replicates, and MrBayes v.3.2.6 (Ronquist et al. 2012), where we conducted two runs with one cold chain and three heated chains for 1 900 000 mcmc generations, with 25% of the generations discarded as burnin. Parameters of the mcmc runs were inspected using Tracer (Rambaut & Drummond 2003) to check for convergence. In both cases, data were partitioned by genome (mitochondrial, plastidial, and nuclear), and an unlinked GTR model with gamma-distributed rate variation was employed. RaxML bootstrap replicates and post-burnin samples from both runs were synthesised using consensus networks (Holland & Moulton 2003) in SplitsTree (Huson & Bryant 2006), using a 20% cutoff.
To obtain a timescale for Picrodendraceae, we conducted a molecular dating analysis with the fossilised birth–death prior (Heath et al. 2014) as implemented in MrBayes v.3.2.6. Fossil pollen taxa compiled for this study were coded as tips, with a uniform prior on their age reflecting uncertainty in the dates. Fossils were constrained in clades with their most probable extant relatives based on informal assessment of their micromorphology. For the molecular data, we employed a relaxed IGR clock, with the same substitution model implemented for the non-clock analysis (see earlier). We set the sampling probability of the extant taxa as 0.26 (based on species count of extant Picrodendraceae), and we set the sample strategy as diversified. We ran two independent runs with four chains (one cold, four heated) for 50 000 000 generations. A consensus tree was generated using all compatible splits.
Systematic palynology
The pollen terminology follows Punt et al. (2007; LM) and Halbritter et al. (2018; SEM). The classification and author names of extant taxa follow WCSP (2018). Genera and species are arranged in alphabetical order with the pollen of each taxon described individually, followed by descriptions of the fossils at the end. Pollen grains of all the extant American-Afro-Indian Picrodendraceae species studied here are compared in Table V. Three morphological types are recognised, referred to here as PT 1–PT 3. Fossil Picrodendraceae pollen grains are compared in Table VI. For practical reasons all the fossil pollen grains are classified as morphotypes (MT) named after the locality where they were found.
Table V.
Pollen morphology of extant Picrodendraceae.
| Androstachys johnsonii | Aristogeitonia gabonica | Aristogeitonia lophirifolia | Aristogeitonia monophylla | Aristogeitonia perrieri | Hyaenanche globosa | Mischodon zeylanicus | |
|---|---|---|---|---|---|---|---|
| P/E ratio | Spheroidal to oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate |
| Outline in polar and equatorial view | Elliptic to circular to slightly angular | Circular | Circular | Circular | Circular | Circular | Circular |
| Equatorial diameter including echini (LM) | 34–38 | 27–31 | 25–29 | 32–36 | 27–29 | 32–39 | 32–38 |
| Equatorial diameter including echini (SEM) | 33–36 | 26–28 | 25–28 | 32–34 | 25–29 | 30–38 | 32–37 |
| Equatorial diameter excluding echini (LM) | N/A | 21–25 | 20–24 | 25–30 | 22–25 | 29–35 | 27–33 |
| Equatorial diameter excluding echini (SEM) | 32–34 | 21–23 | 21–23 | 25–27 | 21–25 | 28–34 | 27–32 |
| Polar axis including echini (LM) | 30–33 | 25–28 | 25–28 | 32–35 | 26–28 | 30–35 | 30–34 |
| Polar axis including echini (SEM) | 29–32 | 23–25 | 23–25 | 31–33 | 24–27 | 29–34 | 31–34 |
| Polar axis excluding echini (LM) | N/A | 20–23 | 21–23 | 26–28 | 22–24 | 29–33 | 26–30 |
| Polar axis excluding echini (SEM) | 28–31 | 19–21 | 19–21 | 25–27 | 20–23 | 26–32 | 26–28 |
| Aperture type and number | Stephano(5-7)porate | Stephano(5-6)porate | Stephano(6-7)porate | Stephano(7)porate | Stephano(6-7)porate | Stephano(6-7)porate | Stephano(5-7)porate |
| Aperture position | Pori often at irregular intervals, one or two pori can be positioned outside of the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator |
| Aperture diameter (SEM) | 2.5–4.5 | 2.5–3.5 | 2.5–4.0 | 3.5–5.0 | 2.5–3.5 | 3.0–5.5 | 3.5–4.5 |
| Exine thickness (LM) | 1.2–1.3 | 1.2–1.5 | 1.1–1.2 | 1.6–1.7 | 1.1–1.5 | 1.4–1.6 | 1.3–1.8 |
| Pollen wall (SEM) | Tectate | Tectate | Tectate | Tectate | Tectate | Tectate | Tectate |
| Sculpture (LM) | Echinate | Echinate | Echinate | Echinate | Echinate | Echinate | Echinate |
| Sculpture (SEM) | Microechinate; granulate and perforate in areas between echini | Echinate; nanogemmate to nanorugulate in areas between echini | Echinate; nanogemmate to granulate in areas between echini | Echinate; nanogemmate to nanorugulate in areas between echini | Echinate; nanogemmate to granulate in areas between echini | Echinate; fossulate, perforate and nanogemmate in areas between echini | Echinate; nanogemmate to granulate in areas between echini |
| Number of echini in central polar area (SEM) | 30–45 per 100 µm2 | 5–11 per 100 µm2 | 9–13 per 100 µm2 | 3–7 per 100 µm2 | 9–12 per 100 µm2 | 15–30 per 100 µm2 | 3–7 per 100 µm2 |
| Echini height (SEM) | 0.7–1.3 | 3.0–4.5 | 2.0–3.5 | 3.0–4.5 | 2.0–3.0 | 1.0–2.0 | 3.0–4.5 |
| Aperture membrane (SEM) | Nanoechinate, nanogemmate and granulate | Nanogemmate to nanorugulate | Nanogemmate to granulate | Nanogemmate to nanorugulate | Nanogemmate to granulate | Nanogemmate | Nanogemmate to granulate |
| Pollen Type | 3 | 2 | 2 | 2 | 2 | 1 | 2 |
| Oldfieldia africana | Oldfieldia dactylophylla | Oldfieldia somalensis | Piranhea longepedunculata | Piranhea trifoliata | Stachyandra merana | Tetracoccus fasciculatus | Voatamalo eugenioides |
|---|---|---|---|---|---|---|---|
| Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to oblate | Spheroidal to oblate | Spheroidal to slightly oblate |
| Circular | Circular | Circular | Circular | Circular | Elliptic to circular to slightly angular | Circular | Circular |
| 30–34 | 32–38 | 30–37 | 27–30 | 25–31 | 32–40 | 31–35 | 35–38 |
| 26–36 | 28–34 | 28–35 | 25–29 | 24–29 | 33–38 | 30–33 | 28–37 |
| 25–29 | 27–34 | 25–30 | 24–27 | 24–29 | N/A | 30–33 | 28–33 |
| 22–29 | 22–27 | 22–28 | 22–24 | 21–26 | 31–36 | 28–30 | 22–31 |
| 28–30 | 31–35 | 28–32 | 25–28 | 24–28 | 33–35 | 29–31 | 32–35 |
| 26–28 | 27–32 | 29–31 | 21–24 | 23–26 | 33–35 | 27–28 | 28–31 |
| 23–25 | 25–28 | 23–27 | 22–25 | 22–25 | N/A | 27–30 | 27–32 |
| 22–24 | 21–25 | 23–25 | 20–22 | 21–23 | 32–34 | 25–26 | 23–26 |
| Stephano(6-8)porate | Stephano(7-8)porate | Stephano(6-8)porate | Stephano(6-7)porate | Stephano(6-7)porate | Stephano(4-6)porate | Stephano(6)porate | Stephano(6-7)porate |
| Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori often at irregular intervals, one or two pori can be positioned outside of the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator |
| 3.0–4.0 | 3.0–3.5 | 3.0–4.5 | 3.6–4.4 | 3.3–4.2 | 2.5–4.5 | 3.0–4.4 | 2.5–4.0 |
| 1.6–1.9 | 1.5–1.8 | 1.5–1.7 | 1.7–2.0 | 1.7–2.0 | 1.1–1.3 | 2.0–2.5 | 1.3–1.6 |
| Tectate | Tectate | Tectate | Tectate | Tectate | Tectate | Tectate | Tectate |
| Echinate | Echinate | Echinate | Echinate | Echinate | Echinate | Echinate | Echinate |
| Echinate; nanogemmate to nanorugulate in areas between echini | Echinate; nanogemmate to nanorugulate to granulate in areas between echini | Echinate; nanogemmate to nanorugulate in areas between echini | Echinate; fossulate, perforate and nanogemmate in areas between echini | Echinate; fossulate, perforate and nanogemmate in areas between echini | Microechinate; granulate and perforate in areas between echini | Echinate; nanogemmate to granulate, perforate in areas between echini | Echinate; nanogemmate to nanorugulate in areas between echini |
| 8–12 per 100 µm2 | 6–8 per 100 µm2 | 6–9 per 100 µm2 | 12–16 per 100 µm2 | 13–20 per 100 µm2 | 30–45 per 100 µm2 | 20–25 per 100 µm2 | 6–11 per 100 µm2 |
| 2.0–4.0 | 3.0–4.0 | 2.5–3.5 | 1.6–2.6 | 1.6–2.7 | 0.7–1.2 | 1.1–2.2 | 2.5–4.0 |
| Nanogemmate to nanorugulate | Nanogemmate to nanorugulate to granulate | Nanogemmate to nanorugulate | Nanogemmate | Nanogemmate | Nanoechinate, nanogemmate and granulate | Nanogemmate to granulate | Nanogemmate to nanorugulate |
| 2 | 2 | 2 | N/A | N/A | 3 | N/A | 2 |
Note: All measurements include only those from this study and are given in µm. Most diagnostic features appear in bold font. N/A = not applicable.
Table VI.
Pollen morphology of fossil European and African Picrodendraceae.
| Krappfeld MT | Stolzenbach MT | Profen MT | Mush MT | Saldanha MT | |
|---|---|---|---|---|---|
| P/E ratio | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate | Spheroidal to slightly oblate |
| Outline in polar and equatorial view | Circular | Circular | Circular | Circular | Circular |
| Equatorial diameter including echini (LM) | 23–25 | 24–32 | 28–30 | 40–43 | 28–38 |
| Equatorial diameter including echini (SEM) | 21–23 | 22–30 | 26–30 | 36–39 | 26–35 |
| Equatorial diameter excluding echini (LM) | 20–22 | 22–30 | 26–27 | 32–35 | 25–35 |
| Equatorial diameter excluding echini (SEM) | 16–18 | 20–28 | 23–27 | 31–32 | 23–32 |
| Polar axis including echini (LM) | 19–21 | 22–30 | N/O | 34–40 | 27–30 |
| Polar axis including echini (SEM) | N/O | N/O | N/O | 33–35 | 25–27 |
| Polar axis excluding echini (LM) | 15–17 | 20–28 | N/O | 26–33 | 25–28 |
| Polar axis excluding echini (SEM) | N/O | N/O | N/O | 29–30 | 22–24 |
| Aperture type and number | Stephano(7)porate | Stephano(7)porate | Stephano(7)porate | Stephano(5-6)porate | Stephano(7)porate |
| Aperture position | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator | Pori at regular intervals, positioned at the equator |
| Aperture diameter (SEM) | 3.5–4.0 | 2.1–2.6 | 1.0–2.3 | 2.3–3.1 | 3.0–4.5 |
| Exine thickness (LM) | 1.3–1.5 | 1.2–1.4 | 1.2–1.3 | 2.0–2.2 | 1.2–1.4 |
| Pollen wall (SEM) | Tectate | Tectate | Tectate | Tectate | Tectate |
| Sculpture (LM) | Echinate | Echinate | Echinate | Echinate | Echinate |
| Sculpture (SEM) | Echinate; fossulate, perforate and nanogemmate in areas between echini | Echinate; fossulate, perforate and nanogemmate in areas between echini | Echinate; fossulate, perforate and nanogemmate in areas between echini | Echinate; nanogemmate to naorugulate in areas between echini | Echinate; fossulate, perforate and nanogemmate in areas between echini |
| Number of echini in central polar area (SEM) | 20–25 per 100 µm2 | 19–30 per 100 µm2 | 17–25 per 100 µm2 | 4–8 per 100 µm2 | 15–30 per 100 µm2 |
| Echini height (SEM) | 2.3–2.6 | 0.9–2.7 | 1.7–3.1 | 4.1–5.2 | 1.0–2.5 |
| Aperture membrane (SEM) | Nanogemmate | Nanogemmate | Nanogemmate | Nanogemmate to naorugulate | Nanogemmate |
| Affiliation | Pollen close to Piranhea | Pollen close to Piranhea | Pollen close to Piranhea | Pollen of the Aristogeitonia/Mischodon/Oldfieldia/Voatamalo clade | PT 1: aff. Hyaenanche |
Note: All measurements are given in µm. N/O = not observed.
Note on the SEM based pollen morphology. — Fossil European Picrodendraceae pollen grains are characterised by finely striate sculpture on the echini and around the basal area of the echini (e.g. Zetter & Hofmann 2008; Zetter et al. 2011). This feature is hard to see in extant material, but occurs in some of the SEM-studied genera/taxa (see descriptions of Hyaenanche, Aristogeitonia and Oldfieldia). It is suggestive that all American and Afro-Indian taxa have such striae, but the pollen surface can be covered by a thin sporopollenin layer obscuring this feature. This sporopollenin layer, which appears granulate in SEM, is not easily removed during preparation, but is often eroded during fossilisation revealing the distinctive striate sculpture.
Pollen descriptions of extant taxa
Family Picrodendraceae
Genus Androstachys
Species Androstachys johnsonii Prain (van der Schyff, 978 [PRE])
Figure 1.
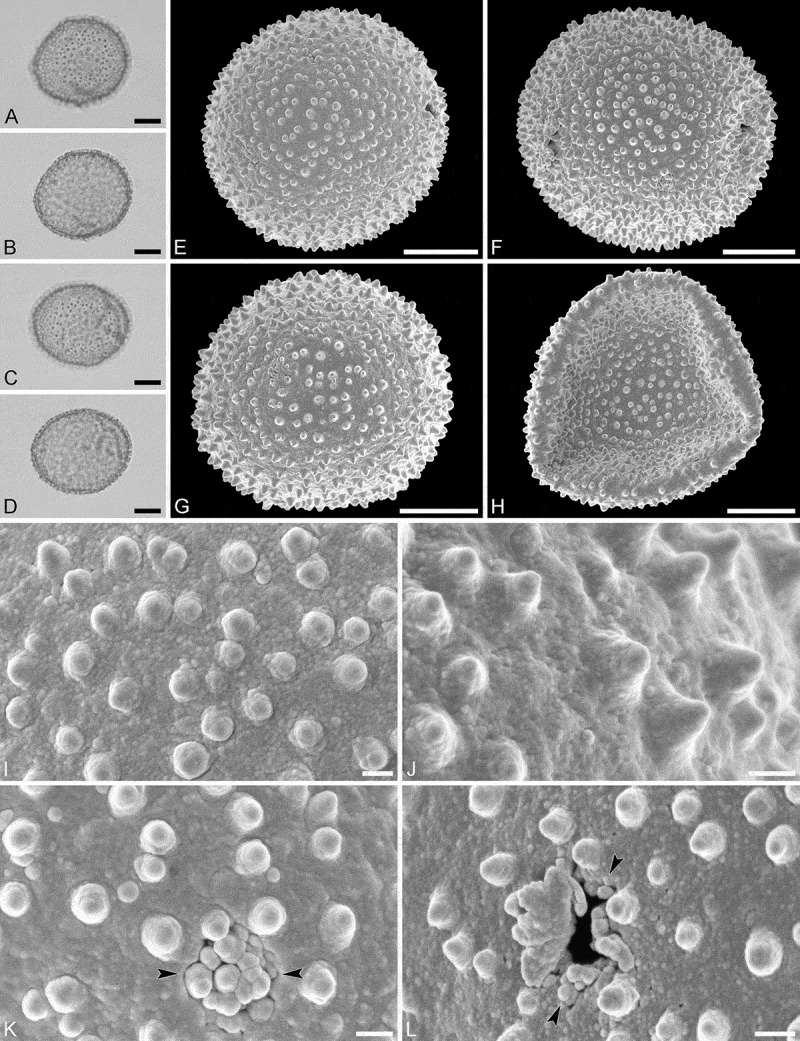
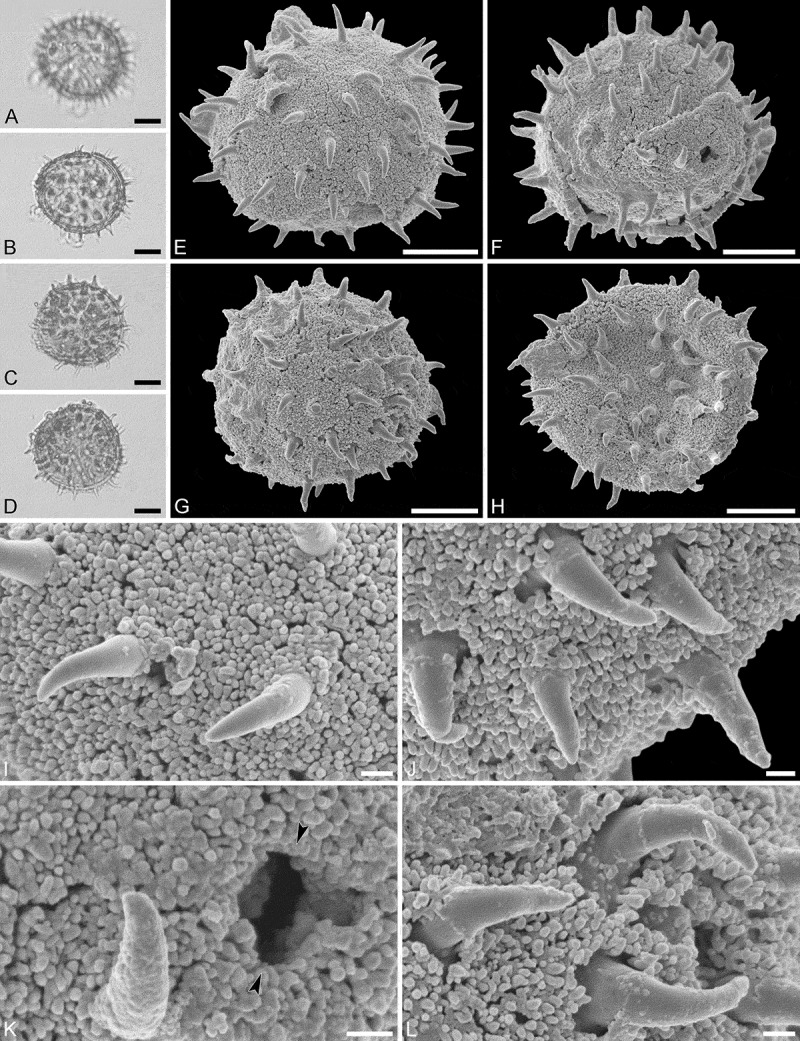
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Androstachys johnsonii (from South Africa, coll. van der Schyff, 978 [PRE]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Oblique polar view. F. Oblique equatorial view. G. Polar view, note displaced porus. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, polar/equatorial (P/E) ratio spheroidal to oblate, outline elliptic to circular to slightly angular in polar and equatorial view; equatorial diameter including echini 34–38 µm in LM, 33–36 µm in SEM, equatorial diameter excluding echini 32–34 µm in SEM, polar axis including echini 30–33 µm in LM, 29–32 µm in SEM, polar axis excluding echini 28–31 µm in SEM; stephano(5–7)porate, pori often at irregular intervals, one or two pori sometimes outside of the equator, pori elliptic, 2.5–4.5 µm in diameter (SEM); exine 1.2–1.3 µm thick; pollen wall tectate; sculpture echinate in LM, microechinate in SEM, granulate and perforate in areas between echini (SEM); 30–45 echini per 100 µm2 in central polar area, echini at irregular intervals, echini 0.7–1.3 µm in height; aperture membrane nanoechinate, nanogemmate and granulate (SEM).
Remarks
The irregular interval and displacement of pori outside of the equator gives the pollen a slightly angular outline in polar and equatorial view. Pollen of this taxon were figured by Köhler (1965, plate 9, figure 9 [LM]) and Simpson and Levin (1994, figures 35, 36 [SEM]). The SEM close-up figured in Simpson and Levin (1994) shows echini that look similar in size and outline to what is observed in the material studied herein, but lacks the resolution needed to identify any sculpture elements in the areas between the echini. Micrographs showing the ultrastructure of Androstachys pollen, from both non-apertural and apertural regions, have also been provided by Simpson and Levin (1994, figures 45, 46 [TEM]).
Genus Aristogeitonia
Species Aristogeitonia gabonica Breteler (Sosef, 1794 [MO])
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 27–31 µm in LM, 26–28 µm in SEM, equatorial diameter excluding echini 21–25 µm in LM, 21–23 µm in SEM, polar axis including echini 25–28 µm in LM, 23–25 µm in SEM, polar axis excluding echini 20–23 µm in LM, 19–21 µm in SEM; stephano(5–6)porate; pori at regular intervals, positioned at the equator, elliptic, 2.5–3.5 µm in diameter (SEM); exine 1.2–1.5 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate in areas between echini (SEM); 5–11 echini per 100 µm2 in central polar area; echini at irregular intervals, 3.0–4.5 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to nanorugulate (SEM).
Species Aristogeitonia lophirifolia Radcl.-Sm. (Capuron, 23.156-SF [MO])
Figure 3.
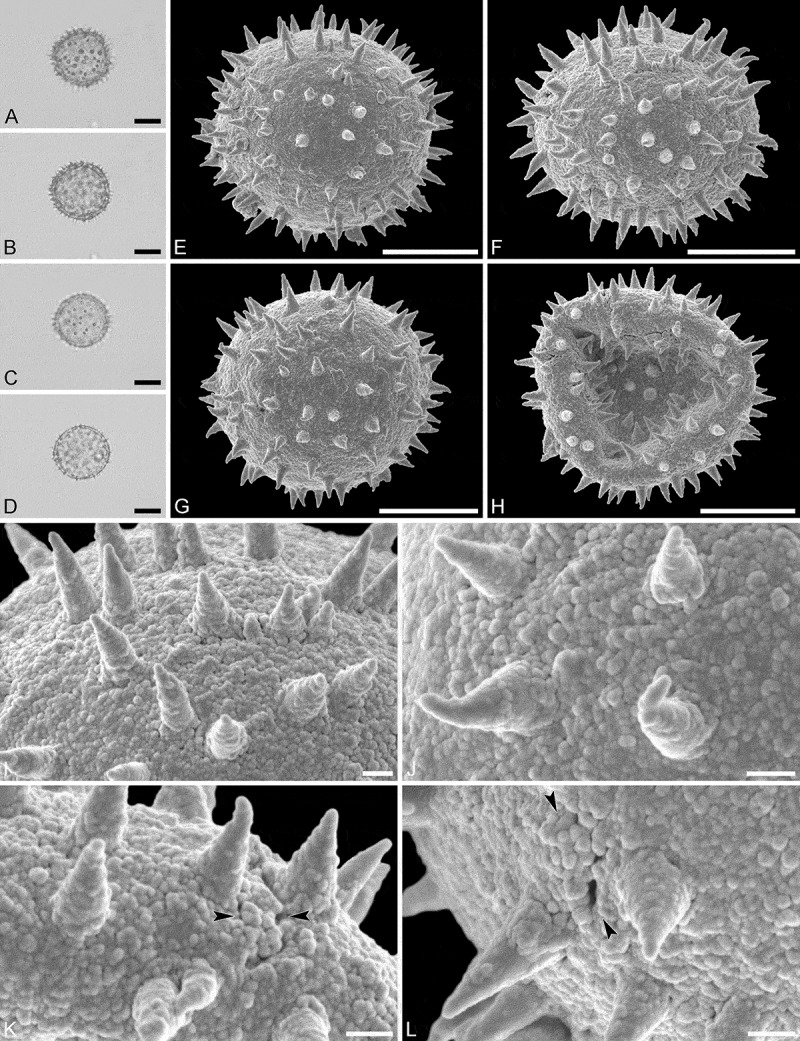
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Aristogeitonia lophirifolia (from Madagascar, coll. Capuron, 23.156-SF [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Oblique polar view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal, outline circular in polar and equatorial view; equatorial diameter including echini 25–29 µm in LM, 25–28 µm in SEM, equatorial diameter excluding echini 20–24 µm in LM, 21–23 µm in SEM, polar axis including echini 25–28 µm in LM, 23–25 µm in SEM, polar axis excluding echini 21–23 µm in LM, 19–21 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 2.5–4.0 µm in diameter (SEM); exine 1.1–1.2 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to granulate in areas between echini (SEM); 9–13 echini per 100 µm2 in central polar area; echini at irregular intervals, 2.0–3.5 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to granulate (SEM).
Species Aristogeitonia monophylla Airy Shaw (Phillipson, 4954 [MO])
Figure 4.
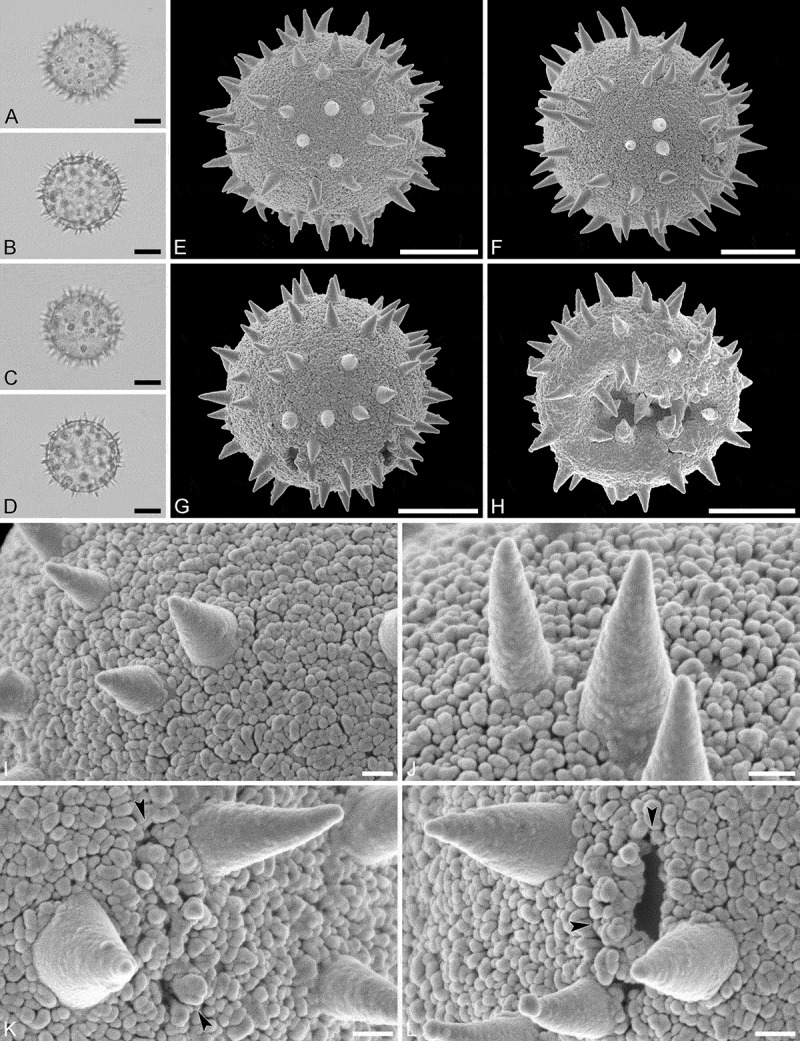
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Aristogeitonia monophylla (from Tanzania, coll. Phillipson, 4954 [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Oblique polar view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 32–36 µm in LM, 32–34 µm in SEM, equatorial diameter excluding echini 25–30 µm in LM, 25–27 µm in SEM, polar axis including echini 32–35 µm in LM, 31–33 µm in SEM, polar axis excluding echini 26–28 µm in LM, 25–27 µm in SEM; stephano(7)porate; pori at regular intervals, positioned at the equator, elliptic, 3.5–5.0 µm in diameter (SEM); exine 1.6–1.7 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate in areas between echini (SEM); 3–7 echini per 100 µm2 in central polar area; echini at irregular intervals, 3.0–4.5 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to nanorugulate (SEM).
Remarks
Micrographs showing the ultrastructure of Aristogeitonia monophylla pollen are provided by Simpson and Levin (1994, figures 52, 53 [TEM]).
Species Aristogeitonia perrieri (Leandri) Radcl.-Sm. (Capuron, 18.462-SF [MO])
Figure 5.
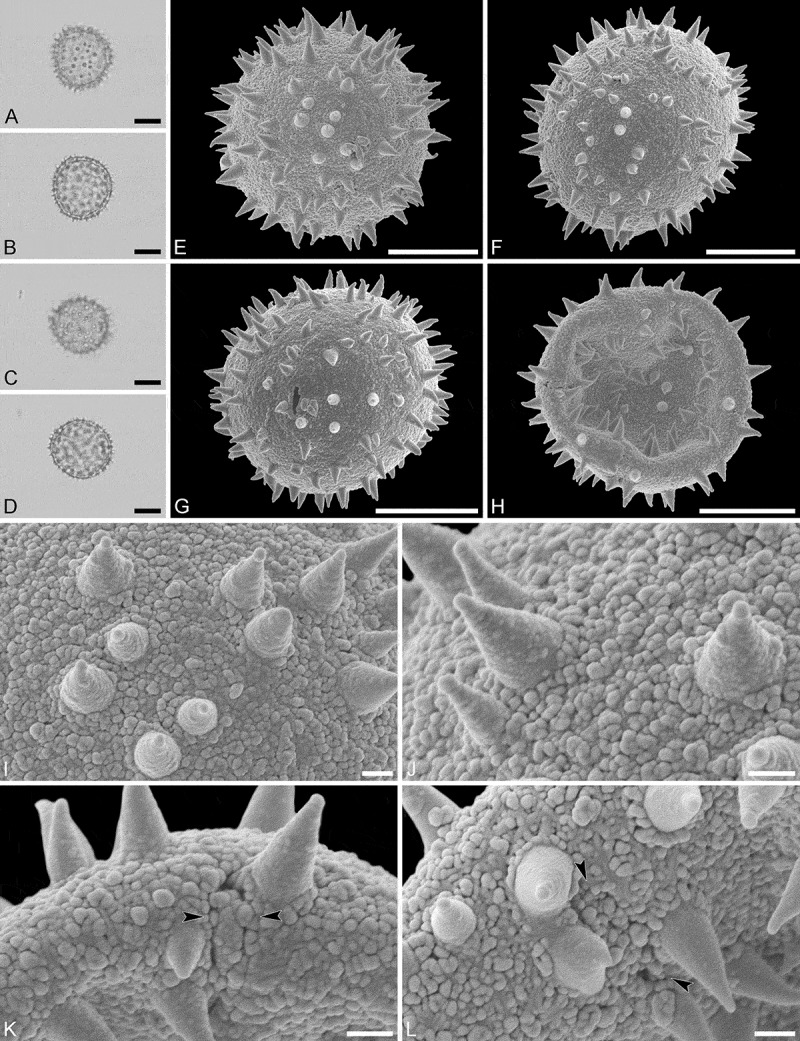
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Aristogeitonia perrieri (from Madagascar, coll. Capuron, 18.462-SF [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 27–29 µm in LM, 25–29 µm in SEM, equatorial diameter excluding echini 22–25 µm in LM, 21–25 µm in SEM, polar axis including echini 26–28 µm in LM, 24–27 µm in SEM, polar axis excluding echini 22–24 µm in LM, 20–23 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 2.5–3.5 µm in diameter (SEM); exine 1.1–1.5 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to granulate in areas between echini (SEM); 9–12 echini per 100 µm2 in central polar area; echini at irregular intervals, 2.0–3.0 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to granulate (SEM).
Remarks
The SEM sculpture of the material presented here corresponds to features detectable in micrographs by Lobreau-Callen and Cervera (1994, plate V, figures G–I) from the same taxon.
Genus Hyaenanche
Species Hyaenanche globosa (Gaert.) Lamb. et Vahl (Hall, 3912 [PRE]; unknown, s.n. [WAG])
Figure 6.
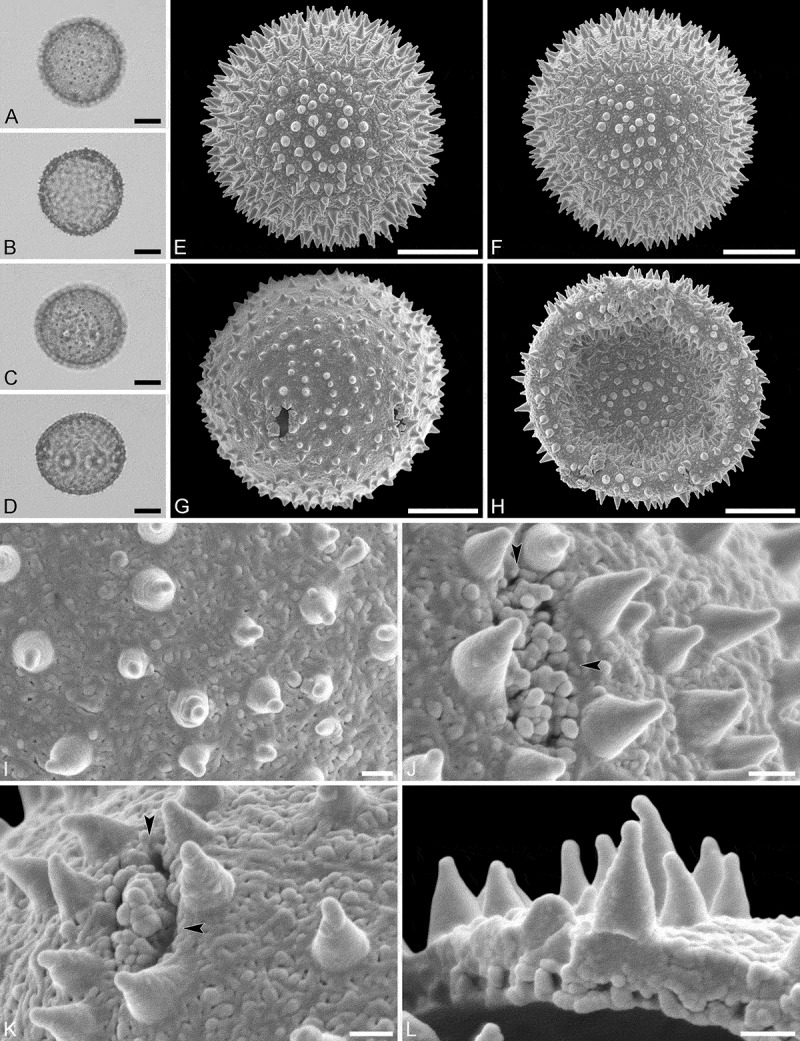
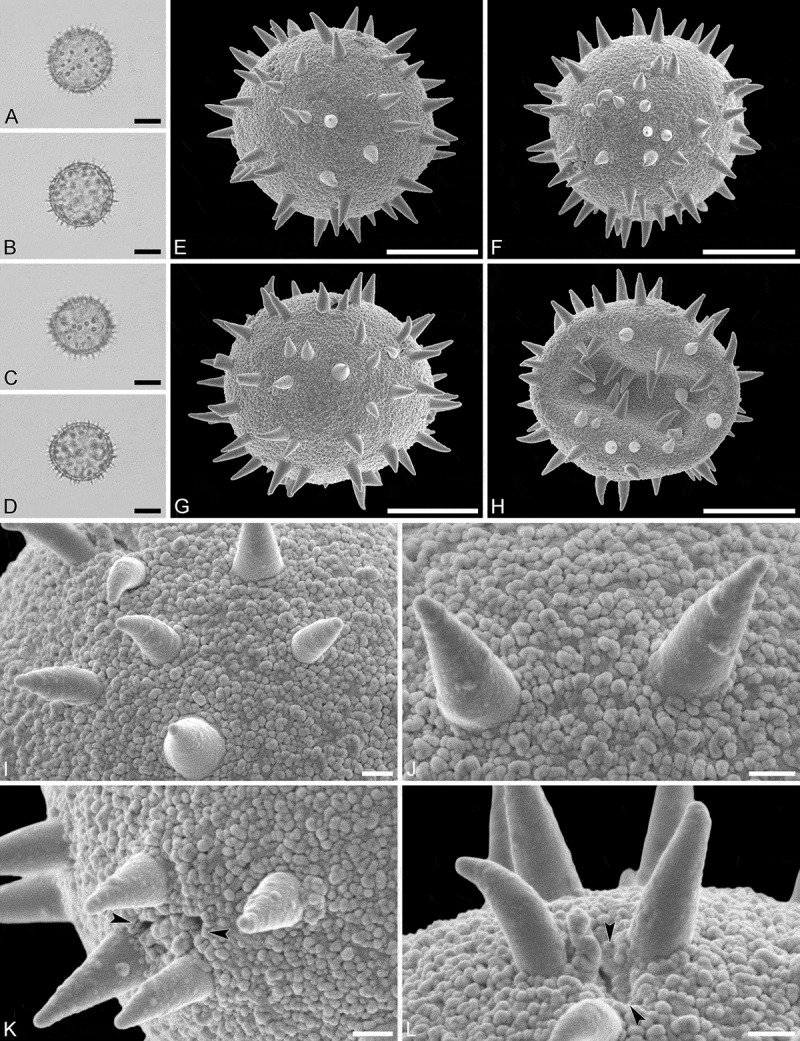
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Hyaenanche globosa (from South Africa, coll. unknown, s.n. [WAG: E, F, H, J, L]; from Namibia, coll. Hall, 3912 [PRE: A–D, G, I, K]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of aperture, showing membrane (arrows). K. Close-up of aperture, showing membrane (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 32–39 µm in LM, 30–38 µm in SEM, equatorial diameter excluding echini 29–35 µm in LM, 28–34 µm in SEM, polar axis including echini 30–35 µm in LM, 29–34 µm in SEM, polar axis excluding echini 29–33 µm in LM, 26–32 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 3.0–5.5 µm in diameter (SEM); exine 1.4–1.6 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 15–30 echini per 100 µm2 in central polar area; echini at irregular intervals, 1.0–2.0 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate (SEM).
Remarks
Köhler (1965, plate 8, figure 18, as Toxicodendron globosum) provides an LM micrograph and Punt (1987, figure 7, as Hyaenanche capensis) an SEM overview showing Hyaenanche globosa pollen that look similar to the material presented here. Also, micrographs displaying the ultrastructure of this taxon are provided by Simpson and Levin (1994, figures 54, 55 [TEM]).
Genus Mischodon
Species Mischodon zeylanicus Thwaites (unknown, s.n. [WAG])
Figure 7.
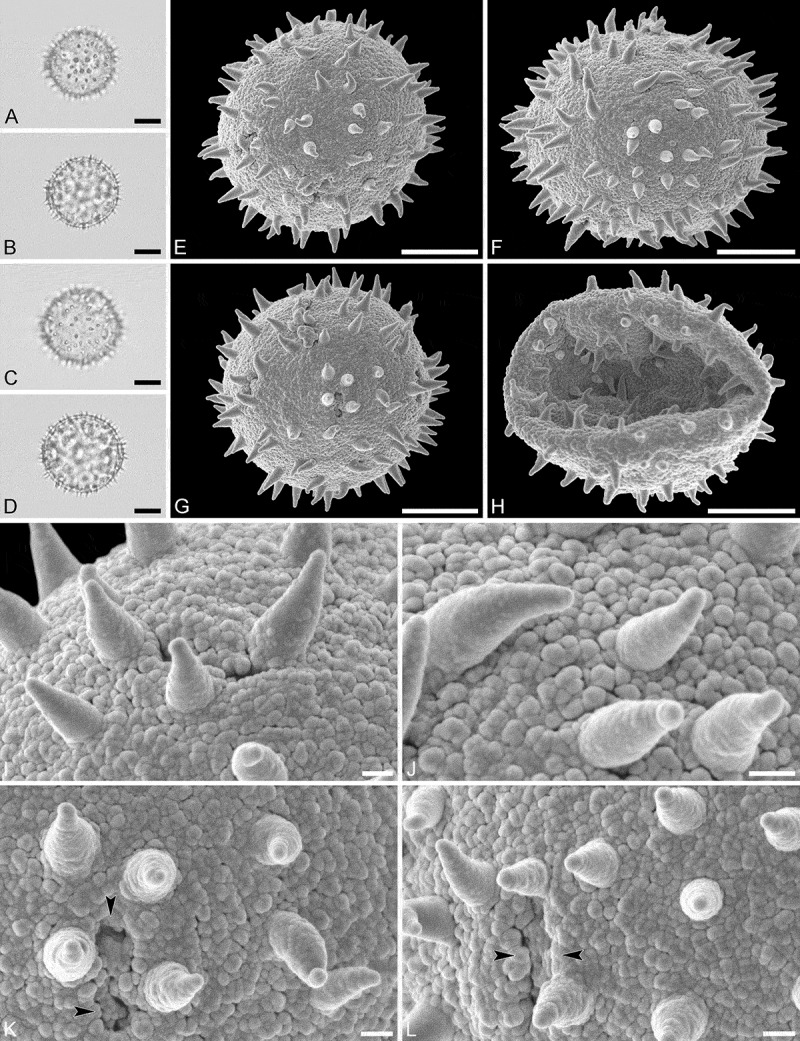
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Mischodon zeylanicus (from India, coll. unknown, s.n. [WAG]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross section. E. Oblique polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 32–38 µm in LM, 32–37 µm in SEM, equatorial diameter excluding echini 27–33 µm in LM, 27–32 µm in SEM, polar axis including echini 30–34 µm in LM, 31–34 µm in SEM, polar axis excluding echini 26–30 µm in LM, 26–28 µm in SEM; stephano(5–7)porate; at regular intervals, positioned at the equator, elliptic, 3.5–4.5 µm in diameter (SEM); exine 1.3–1.8 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to granulate in areas between echini (SEM); 5–10 echini per 100 µm2 in central polar area; echini at irregular intervals, 3.0–4.5 µm in height; aperture membrane nanogemmate to granulate (SEM).
Remarks
Mischodon zeylanicus pollen has been studied previously using LM, SEM and TEM. Köhler (1965, plate 8, figure 17) provides an LM overview that corresponds well to the present observations. SEM micrographs are provided by Lobreau-Callen and Cervera (1994, plate V, figures A–C), showing a single grain in overview, a close-up of aperture regions, as well the wall structure via a break in the pollen wall. The pollen ultrastructure of this taxon is figured in Simpson and Levin (1994, figure 51 [TEM]). The SEM micrographs by Lobreau-Callen and Cervera (1994) illustrate sculpture elements identical to those observed in the pollen material presented here.
Genus Oldfieldia
Species Oldfieldia africana Benth. et Hook.f. (Small, 621 [K]; Leeuwenberg, 3780 [WAG])
Figure 8.
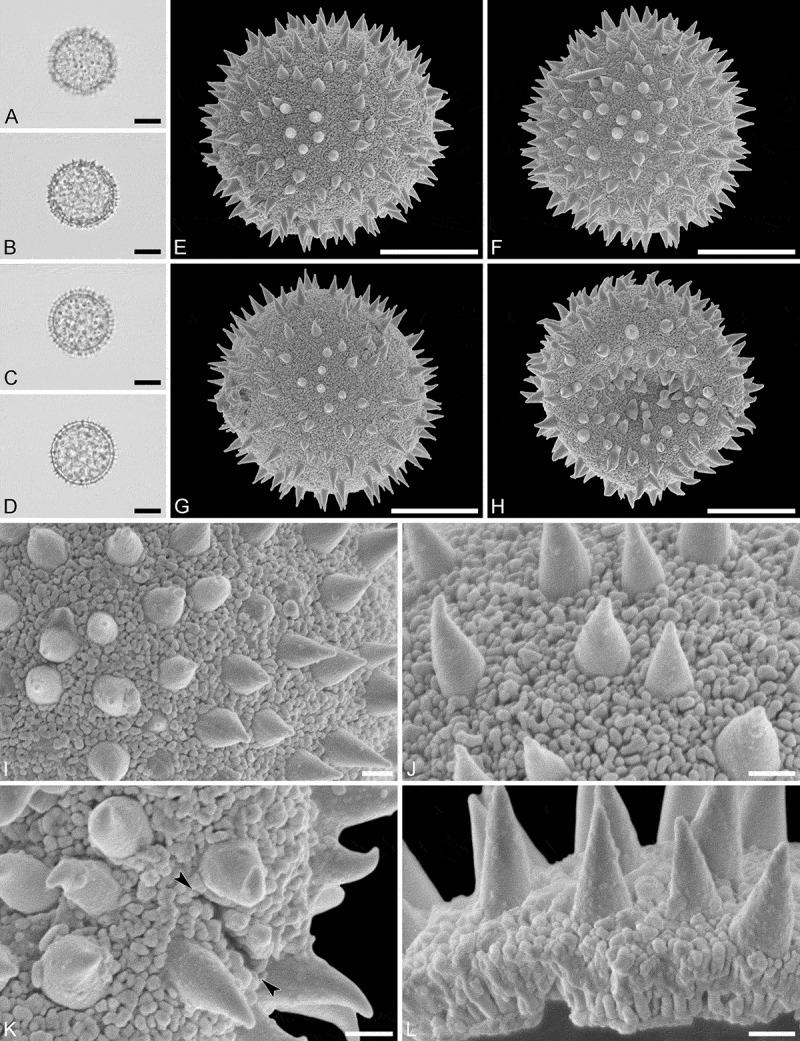
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Oldfieldia africana (from Ivory Coast, coll. Leeuwenberg, 3780 [WAG]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Polar view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 30–34 µm in LM, 26–36 µm in SEM, equatorial diameter excluding echini 25–29 µm in LM, 22–29 µm in SEM, polar axis including echini 28–30 µm in LM, 26–28 µm in SEM, polar axis excluding echini 23–25 µm in LM, 22–24 µm in SEM; stephano(6–8)porate; pori at regular intervals, positioned at the equator, elliptic, 3.0–4.0 µm in diameter (SEM); exine 1.6–1.9 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate in areas between echini (SEM); 8–12 echini per 100 µm2 in central polar area; echini at irregular intervals, 2.0–4.0 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to nanorugulate (SEM).
Remarks
The single SEM overview of an Oldfieldia africana pollen grain by Hayden et al. (1984, figure 16) does not allow for detailed comparison with the material presented here. A micrograph showing the ultrastructure of Oldfieldia africana pollen is provided by Simpson and Levin (1994, figure 44 [TEM]).
Species Oldfieldia dactylophylla (Welw. ex Oliv.) J.Léonard (Meuangulango, 1279 [MO])
Figure 9.
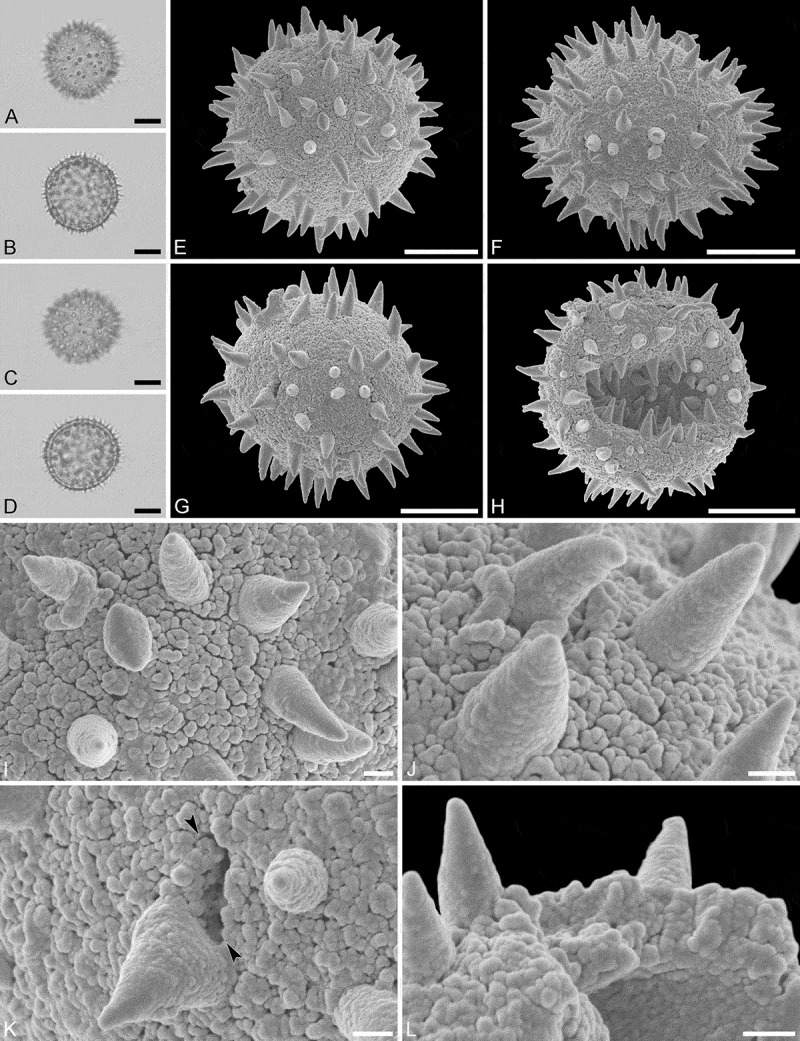
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Oldfieldia dactylophylla (from Tanzania, coll. Meuangulango, 1279 [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 32–38 µm in LM, 28–34 µm in SEM, equatorial diameter excluding echini 27–34 µm in LM, 22–27 µm in SEM, polar axis including echini 31–35 µm in LM, 27–32 µm in SEM, polar axis excluding echini 25–28 µm in LM, 21–25 µm in SEM; stephano(7–8)porate; pori at regular intervals, positioned at the equator, elliptic, 3.0–3.5 µm in diameter (SEM); exine 1.5–1.8 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate to granulate in areas between echini (SEM); 6–8 echini per 100 µm2 in central polar area; echini at irregular intervals, 3.0–4.0 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to nanorugulate to granulate (SEM).
Remarks
The LM micrograph of Oldfieldia dactylophylla pollen in Köhler (1965, plate 6, figure 16) corresponds to the observations in this study.
Species Oldfieldia somalensis (Chiov.) Milne-Redh. (Bally, 6880 [EA])
Figure 10.
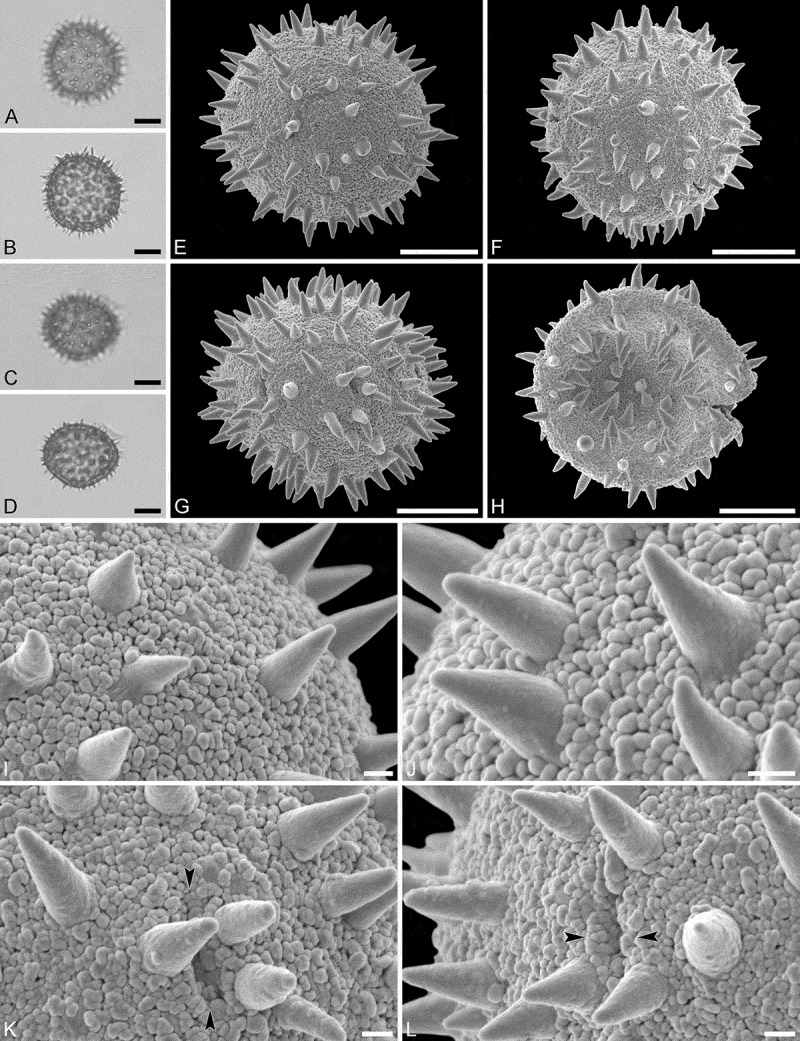
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Oldfieldia somalensis (from Tanzania, coll. Bally, 6880 [EA]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 30–37 µm in LM, 28–35 µm in SEM, equatorial diameter excluding echini 25–30 µm in LM, 22–28 µm in SEM, polar axis including echini 28–32 µm in LM, 29–31 µm in SEM, polar axis excluding echini 23–27 µm in LM, 23–25 µm in SEM; stephano(6–8)porate; pori at regular intervals, positioned at the equator, elliptic, 3.0–4.5 µm in diameter (SEM); exine 1.5–1.7 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate in areas between echini (SEM); 6–9 echini per 100 µm2 in central polar area; echini at irregular intervals, 2.5–3.5 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to nanorugulate (SEM).
Genus Piranhea Species Piranhea longepedunculata Jabl. (Liesner & Gonzalez, 5859 [WAG])
Figure 11.
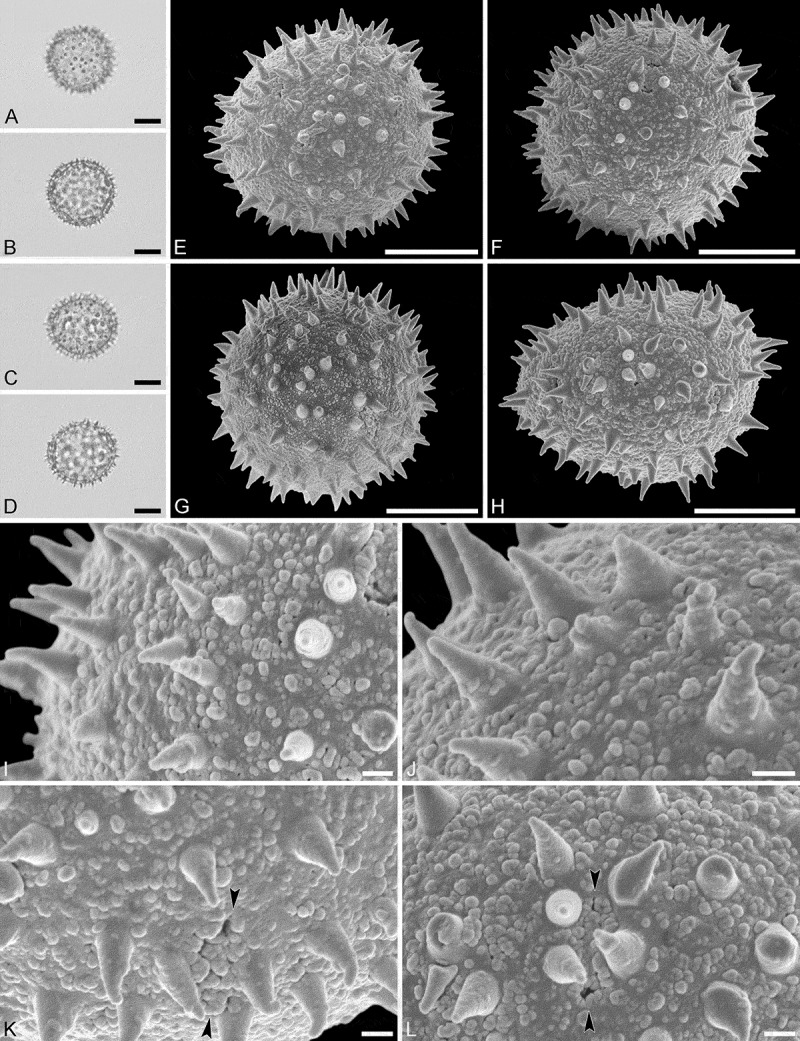
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Piranhea longepedunculata (from Venezuela, coll. Liesner & Gonzalez, 5859 [WAG]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Oblique polar view. F. Oblique polar view. G. Oblique equatorial view. H. Equatorial view. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 27–30 µm in LM, 25–29 µm in SEM, equatorial diameter excluding echini 24–27 µm in LM, 22–24 µm in SEM, polar axis including echini 25–28 µm in LM, 21–24 µm in SEM, polar axis excluding echini 22–25 µm in LM, 20–22 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 3.6–4.4 µm in diameter (SEM); exine 1.7–2.0 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 12–16 echini per 100 µm2 in central polar area; echini at irregular intervals, 1.6–2.6 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate (SEM).
Remarks
The only SEM overview of an Piranhea longepedunculata pollen grain by Hayden et al. (1984, figure 18) corresponds to the material presented here.
Species Piranhea trifoliata Baill. (Berg et al., P19790 [WAG])
Figure 12.
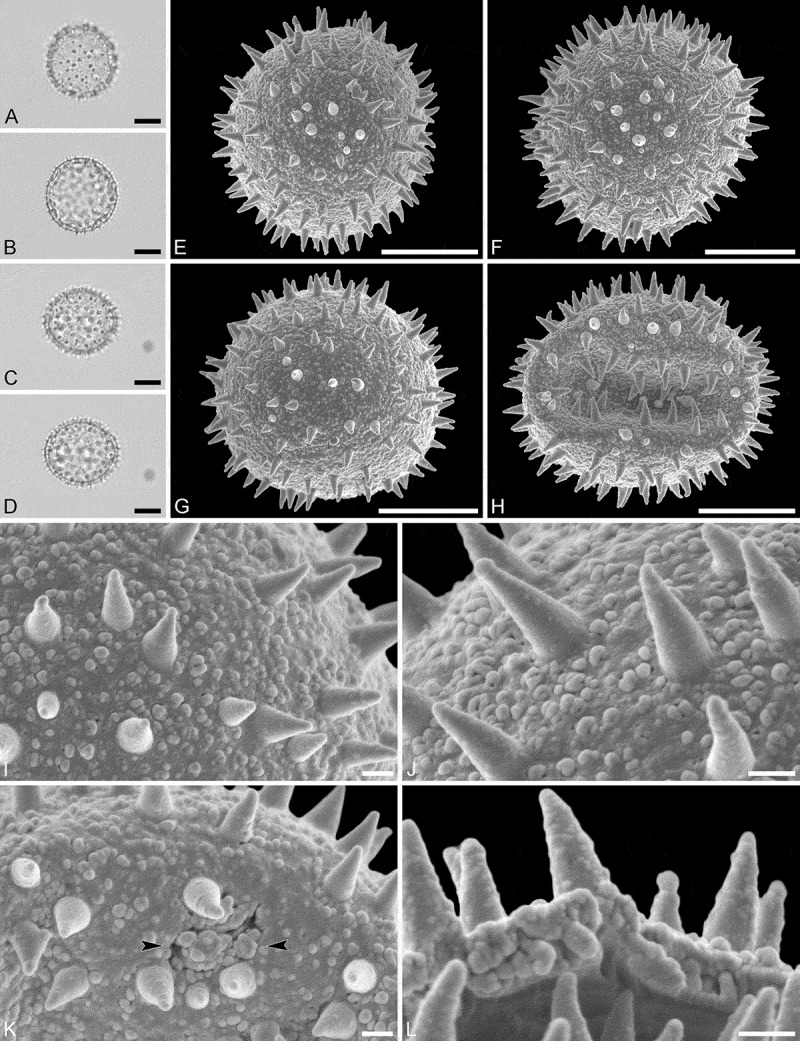
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Piranhea trifoliata (from Brazil, coll. Berg et al., P19790 [WAG]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Polar view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 25–31 µm in LM, 24–29 µm in SEM, equatorial diameter excluding echini 24–29 µm in LM, 21–26 µm in SEM, polar axis including echini 24–28 µm in LM, 23–26 µm in SEM, polar axis excluding echini 22–25 µm in LM, 21–23 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 3.3–4.2 µm in diameter (SEM); exine 1.7–2.0 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 13–20 echini per 100 µm2 in central polar area; echini at irregular intervals, 1.6–2.7 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate (SEM).
Remarks
The pollen ultrastructure (non-apertural wall) of this taxon is figured by Simpson and Levin (1994, figure 41 [TEM]).
Genus Stachyandra
Species Stachyandra merana (Airy Shaw) J.-F.Leroy ex Radcl.-Sm. (Capuron, 23.335-SF [MO])
Figure 13.
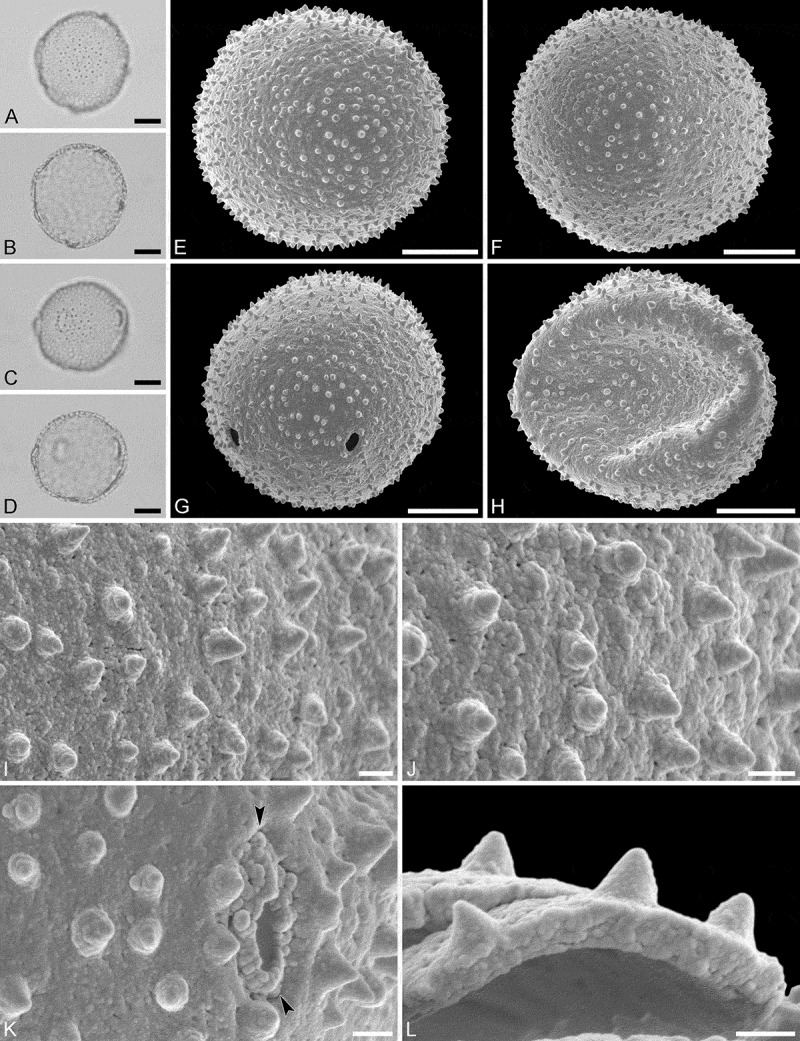
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Stachyandra merana (from Madagascar, coll. Capuron, 23.335-SF [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to oblate, outline elliptic to circular to slightly angular in polar and equatorial view; equatorial diameter including echini 32–40 µm in LM, 33–38 µm in SEM, equatorial diameter excluding echini 31–36 µm in SEM, polar axis including echini 33–35 µm in LM, 33–35 µm in SEM, polar axis excluding echini 32–34 µm in SEM; stephano(4–6)porate; pori often at irregular intervals, one or two can be positioned outside of the equator, pori elliptic, 2.5–4.5 µm in diameter (SEM); exine 1.1–1.3 µm thick; pollen wall tectate; sculpture echinate in LM, microechinate in SEM, granulate and perforate in areas between echini (SEM); 30–45 echini per 100 µm2 in central polar area; echini at irregular intervals, 0.7–1.2 µm in height; aperture membrane nanoechinate, nanogemmate and granulate (SEM).
Remarks
The SEM overview of a Stachyandra merana pollen grain in Lobreau-Callen and Cervera (1994, plate V, figure D, as Androstachys murana) looks very similar to those presented here. The close-ups provided (Lobreau-Callen & Cervera 1994, plate V, figures E, F) also suggest that the echini can be much sharper, and the perforation in the areas between the echini can be bigger and more conspicuous than observed in the present material. The ultrastructure of Stachyandra merana pollen is figured by Simpson and Levin (1994, figure 47 [TEM]).
Genus Tetracoccus
Species Tetracoccus fasciculatus (S.Watson) Croizat (Langenberg, s.n. [WAG])
Figure 14.
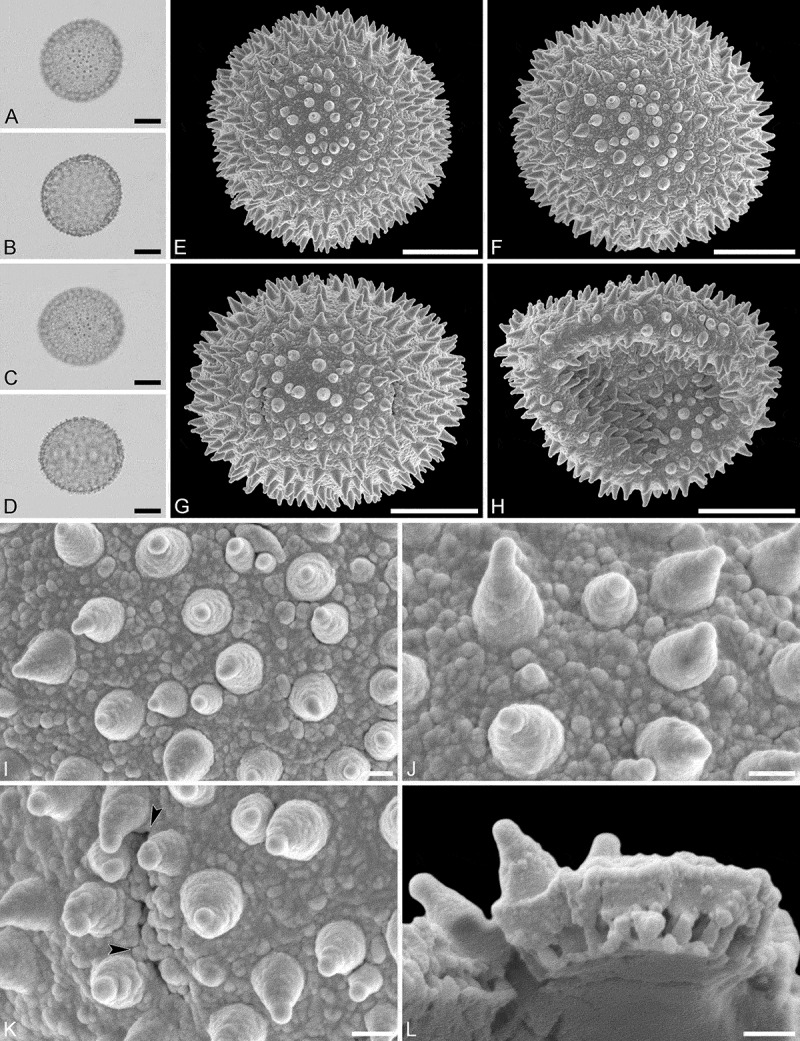
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Tetracoccus fasciculatus (from USA, coll. Langenberg, s.n. [WAG]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 31–35 µm in LM, 30–33 µm in SEM, equatorial diameter excluding echini 30–33 µm in LM, 28–30 µm in SEM, polar axis including echini 29–31 µm in LM, 27–28 µm in SEM, polar axis excluding echini 27–30 µm in LM, 25–26 µm in SEM; stephano(6)porate; pori at regular intervals, positioned at the equator, elliptic, 3.0–4.4 µm in diameter (SEM); exine 2.0–2.5 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to granulate, perforate in areas between echini (SEM); 20–25 echini per 100 µm2 in central polar area; echini at irregular intervals, 1.1–2.2 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to granulate (SEM).
Genus Voatamalo
Species Voatamalo eugenioides Capuron ex Bosser (Capuron, 22.327-SF [MO])
Figure 15.
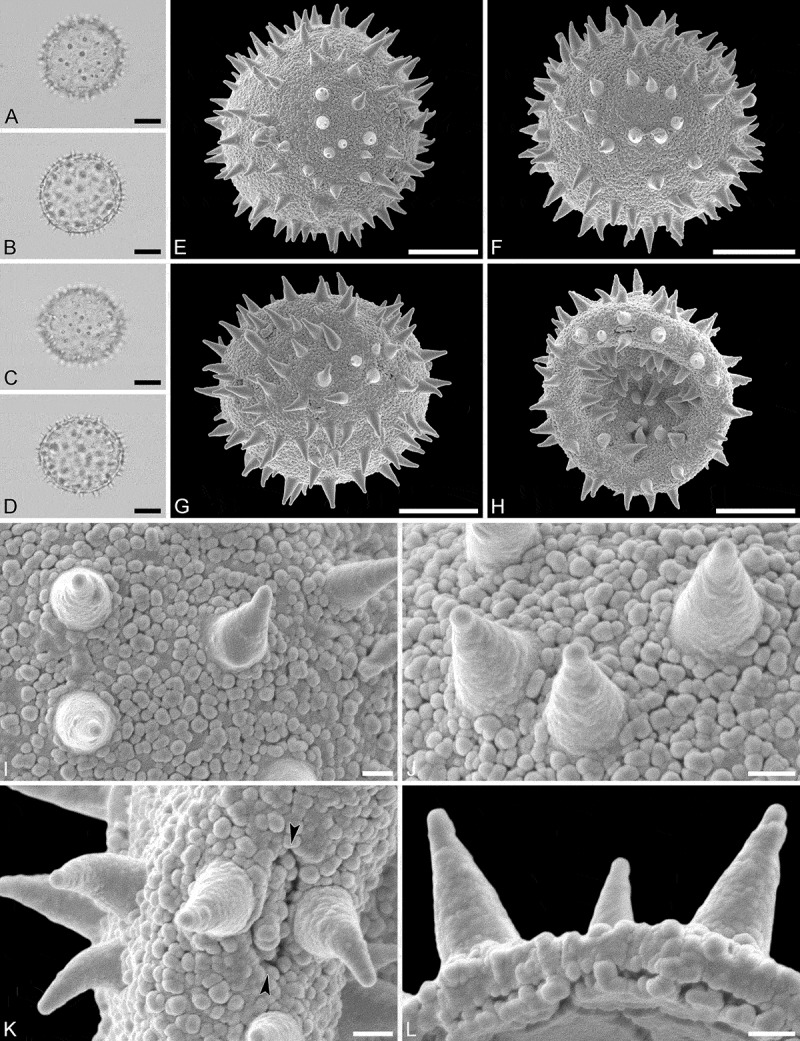
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Voatamalo eugenioides (from Madagascar, coll. Capuron, 22.327-SF [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Equatorial view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up showing section through pollen wall. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 35–38 µm in LM, 28–37 µm in SEM, equatorial diameter excluding echini 28–33 µm in LM, 22–31 µm in SEM, polar axis including echini 32–35 µm in LM, 28–31 µm in SEM, polar axis excluding echini 27–32 µm in LM, 23–26 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 2.5–4.0 µm in diameter (SEM); exine 1.3–1.6 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate in areas between echini (SEM); 6–11 echini per 100 µm2 in central polar area; echini at irregular intervals, 2.5–4.0 µm in height; aperture membrane nanogemmate to nanorugulate (SEM).
Remarks
The ultrastructure of Voatamalo eugenioides pollen is illustrated by Simpson and Levin (1994, figure 50 [TEM]).
Pollen descriptions of fossil taxa
Krappfeld MT, pollen close to Piranhea
Figure 16.
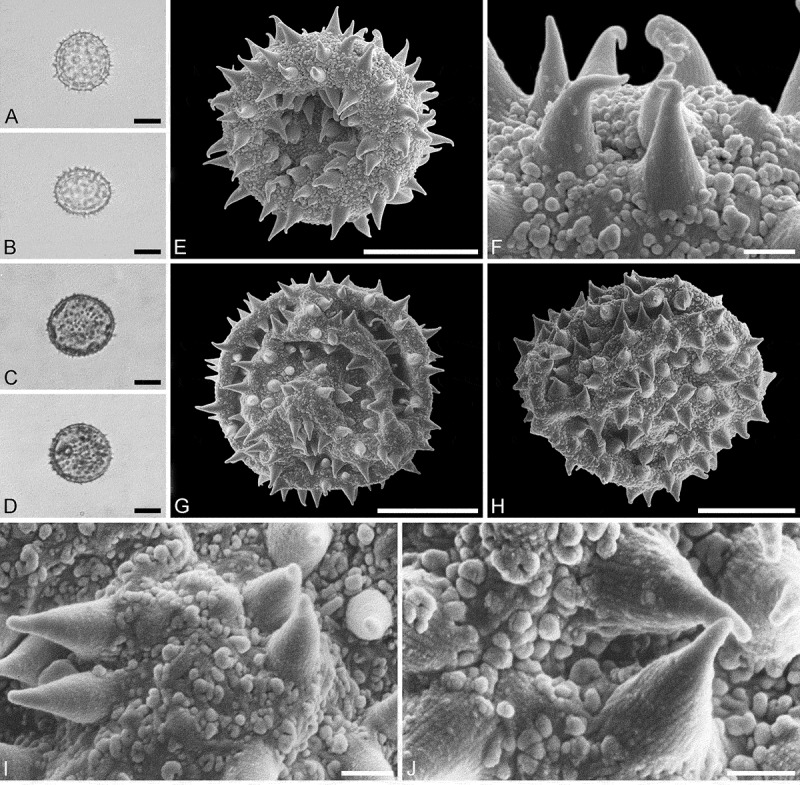
Light microscopy (A–D) and scanning electron microscopy (E–J) micrographs of the Krappfeld MT (same grain: A, B, E, F) and Stolzenbach MT (same grain: C, D, G, I; same grain: H, J). A. Polar view. B. Equatorial view. C. Polar view. D. Equatorial view. E. Polar view, infolded grain. F. Close-up of interapertural area. G. Polar view, infolded grain. H. Oblique polar view, infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. Scale bars – 10 µm (A–E, G, H), 1 µm (F, I, J).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 23–25 µm in LM, 21–23 µm in SEM, equatorial diameter excluding echini 22–23 µm in LM, 20–22 µm in SEM, polar axis including echini 19–21 µm in LM, not observed in SEM, polar axis excluding echini 15–17 µm in LM, not observed in SEM; stephano(7)porate; pori at regular intervals, positioned at the equator, elliptic, 3.5–4.0 µm in diameter (SEM); exine 1.3–1.5 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 20–25 echini per 100 µm2 in central polar area; echini at irregular intervals, 2.3–2.6 µm in height, striate at base; aperture membrane nanogemmate (SEM).
Locality
Krappfeld, Pemberg Quarry, west of Klein St Paul, Carinthia, Austria, early Eocene (Table IV).
Remarks
This is an extremely rare element in the Krappfeld palynoflora. The measurements presented earlier are based on a single specimen (versus 20 in extant material) and most likely do not convey the complete natural size ranges of this MT. The Krappfeld MT is not identical to pollen from any extant African Picrodendraceae, but shares most features with the African Hyaenanche and especially the American Piranhea. The Krappfeld MT pollen in size is closer to that of Piranhea than to the larger Hyaenanche pollen, and it also has numerous prominent nanogemmae that seem to encircle perforations as in Piranhea. In fact, the main difference discriminating the Krappfeld MT pollen grain from those of Piranhea is the shape and outline of echini and especially their prominently striate surface. The pollen morphology suggests that the Krappfeld MT represents an extinct early diverging taxon of the American-Afro-Indian clade, positioned close to the Piranhea lineage, and is ancestral form leading to the Afro-Indian clade (including Hyaenanche). The fossil Picrodendraceae pollen from the Eocene of Austria (Krappfeld MT) and Germany (Stolzenbach MT and Profen MT) are very similar. They clearly represent a closely related stock and might even originate from the same biological taxon.
Stolzenbach MT, pollen close to Piranhea
(Figures 16, 17, Table VI)
Figure 17.
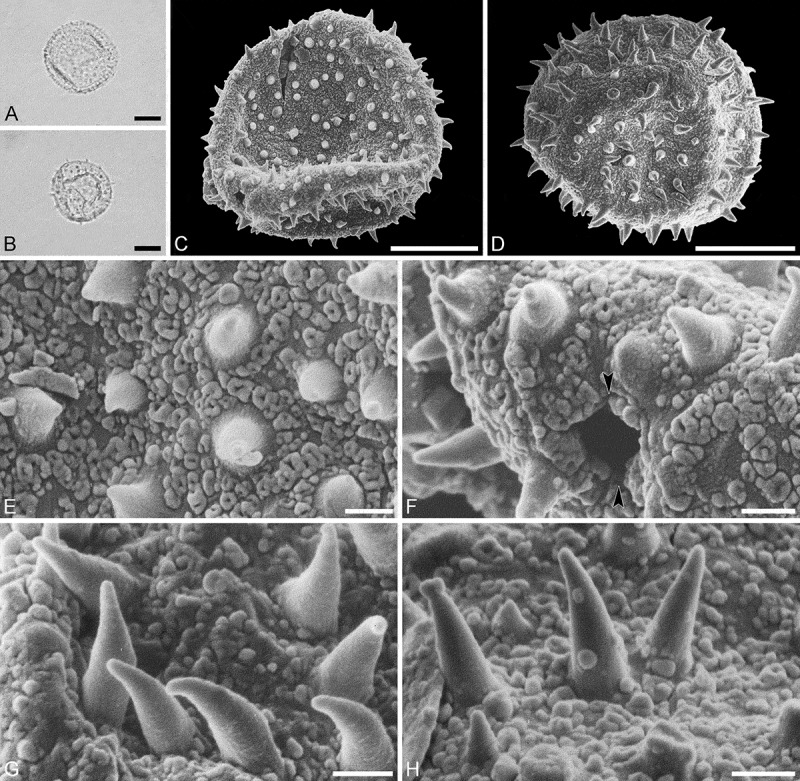
Light microscopy (A, B) and scanning electron microscopy (C–H) micrographs of the Stolzenbach MT (same grain: A, C, E–G; same grain: B, D, H). A. Polar view. B. Polar view. C. Polar view, infolded grain. D. Polar view. E. Close-up of interapertural area. F. Close-up of aperture (arrows). G. Close-up of interapertural area. H. Close-up of interapertural area. Scale bars – 10 µm (A–D), 1 µm (E–H).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 24–32 µm in LM, 22–30 µm in SEM, equatorial diameter excluding echini 22–30 µm in LM, 20–28 µm in SEM, polar axis including echini 22–30 µm in LM, not observed in SEM, polar axis excluding echini 20–28 µm in LM, not observed in SEM; stephano(7)porate; pori at regular intervals, positioned at the equator, elliptic, 2.1–2.6 µm in diameter (SEM); exine 1.2–1.4 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 19–30 echini per 100 µm2 in central polar area; echini at irregular intervals, 0.9–2.7 µm in height, striate at base; aperture membrane nanogemmate (SEM).
Locality
Stolzenbach underground coalmine, Kassel, Germany, middle Eocene (Table IV).
Remarks
This is a rare element in the Stolzenbach palynoflora. The measurements presented earlier are based on four specimens (versus 20 in extant material) and most likely do not convey the complete natural size ranges of this MT. For further notes see ‘Remarks’ of the Krappfeld MT.
Profen MT, pollen close to Piranhea
Figure 18.
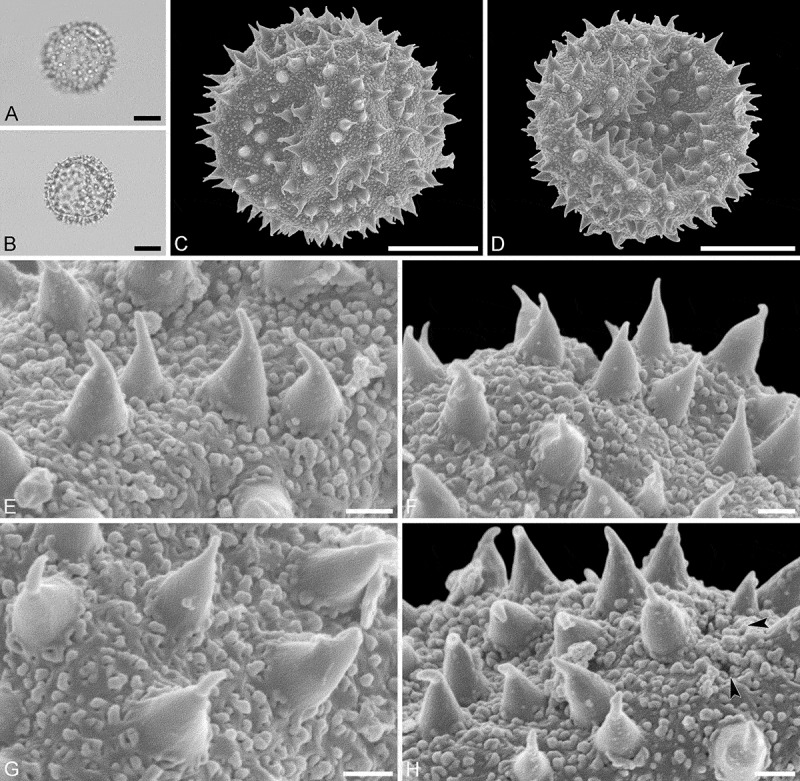
Light microscopy (A, B) and scanning electron microscopy (C–H) micrographs of the Profen MT (same grain: A–H). A. Polar view, high focus. B. Polar view, optical cross-section. C. Polar view. D. Polar view, opposite site, grain infolded. E. Close-up of interapertural area. F. Close-up of interapertural area. G. Close-up of interapertural area. H. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–D), 1 µm (E–H).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 28–30 µm in LM, 26–30 µm in SEM, equatorial diameter excluding echini 26–27 µm in LM, 23–27 µm in SEM, polar axis including echini not observed in LM or SEM, polar axis excluding echini not observed in LM or SEM; stephano(7)porate; pori at regular intervals, positioned at the equator, elliptic, 1.0–1.5 µm in diameter (SEM); exine 1.2–1.3 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 17–25 echini per 100 µm2 in central polar area; echini at irregular intervals, 1.7–3.1 µm in height, striate at base; aperture membrane nanogemmate (SEM).
Locality
Profen, Leipzig, central Germany, middle Eocene (Table IV).
Remarks
This is an extremely rare element in the Profen palynoflora. The measurements presented earlier are based on a single specimen (versus 20 in extant material) and most likely do not convey the complete natural size ranges of this MT. For further notes see ‘Remarks’ of the Krappfeld MT.
Mush MT, pollen of the Aristogeitonia/Mischodon/Oldfieldia/Voatamalo clade
(Figures 19, 20, Table VI)
Figure 20.
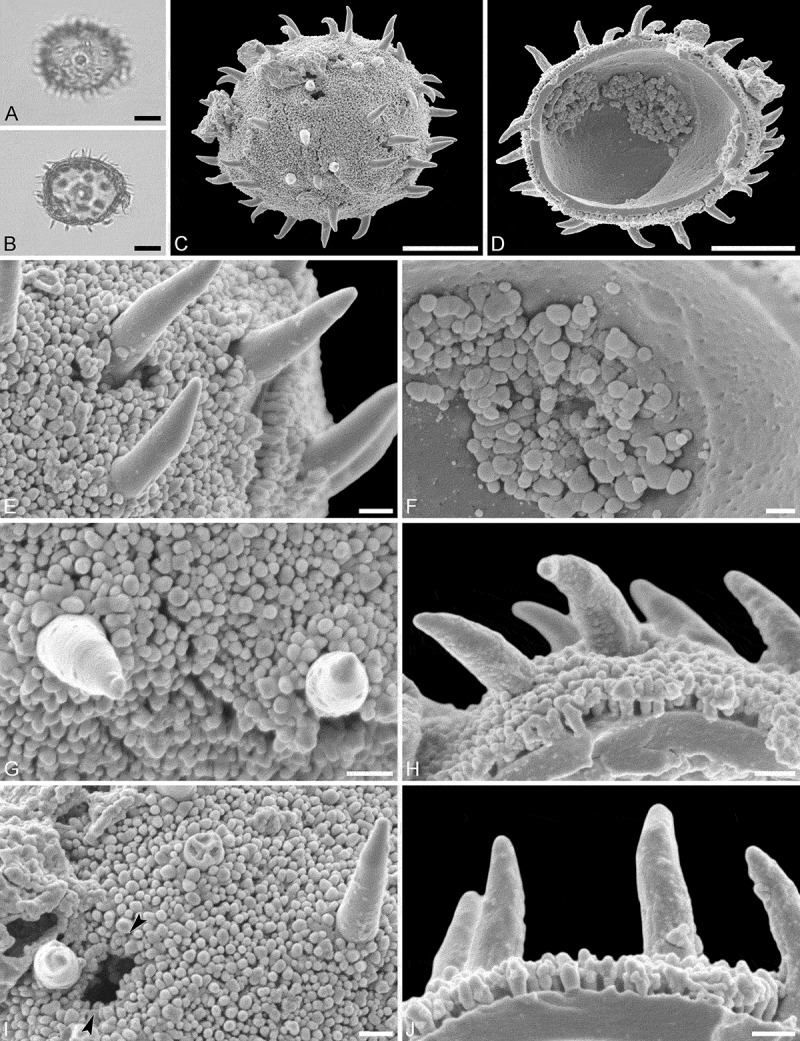
Light microscopy (A, B) and scanning electron microscopy (C–J) micrographs of the Mush MT (same grain: A–J). A. Equatorial view, high focus. B. Equatorial view, optical cross-section. C. Oblique equatorial view. D. Grain broken, showing the inner side of the pollen. E. Close-up of interapertural area. F. Close-up showing thickening around aperture on the inner side of pollen wall. G. Close-up of interapertural area. H. Close-up showing section through pollen wall. I. Close-up of aperture (arrows). J. Close-up showing section through pollen wall. Scale bars – 10 µm (A–D), 1 µm (E–J).
Description
Pollen monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular in polar and equatorial view; equatorial diameter including echini 40–43 µm in LM, 36–39 µm in SEM, equatorial diameter excluding echini 32–35 µm in LM, 31–32 µm in SEM, polar axis including echini 34–40 µm in LM, 33–35 µm in SEM, polar axis excluding echini 26–33 µm in LM, 29–30 µm in SEM; stephano(5–7)porate; pori at regular intervals, positioned at the equator, elliptic, 2.3–3.1 µm in diameter (SEM); exine 2.0–2.2 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, nanogemmate to nanorugulate in areas between echini (SEM); 4–8 echini per 100 µm2 in central polar area; echini at irregular intervals, 4.1–5.2 µm in height, can be faintly striate especially at base; aperture membrane nanogemmate to nanorugulate (SEM).
Locality
Mush Valley, Debre Birhan Woreda, Ethiopia, early Miocene (Table IV).
Remarks
This is not a rare element in the Mush palynoflora and can be found in various stages, in both perfect preservation or compressed and/or broken. The combined features observed in LM and SEM (± spherical, stephanoporate, elliptic pori, echinate sculpture) clearly place these fossil pollen grains in Picrodendraceae. The fossil Mush MT differs considerably from that of Hyaenanche globosa (PT 1). The fossil pollen grain is larger, it has much fewer echini per 100 µm2 in the central polar area (4–8 versus 15–30) and the echini are also higher (4.1–5.2 versus 1.0–2.0 µm). The sculpture between the echini is nanogemmate to nanorugulate in the Mush MT, but fossulate, perforate, and nanogemmate in Hyaenanche. The Mush MT also differs in outline from pollen of Androstachys (PT 3) and Stachyandra (PT 3) (circular versus elliptic to slightly angular) and in aperture position (regular intervals and at the equator versus irregular intervals and displaced). The echini are also much higher in the Mush MT (4.1–5.2 versus 0.7–1.3 µm) and of different shape and wider apart (4–8 per 100 µm2 versus 35–45 per 100 µm2) than in both Androstachys and Stachyandra. Also, the SEM sculpture is nanogemmate to nanorugulate in the Mush MT, but clearly granulate and perforate in Androstachys and Stachyandra (Tables V, VI). The Mush MT shares many features with extant pollen of PT 2 (Aristogeitonia, Mischodon, Oldfieldia and Voatamalo). The pollen body of the Mush MT is very large, between 32 and 35 µm in diameter (excluding echini); similar sized pollen is observed in extant Aristogeitonia monophylla, Mischodon, Oldfieldia, and Voatamalo eugenioides (Table V). All these extant genera also display few (less than 15 per 100 µm2) widely spaced echini in the central polar area, that are relatively high (up to 4.5 µm), a feature also characteristic for the Mush MT. The SEM sculpture observed between the echini in the Mush MT is also very similar to that of PT 2 pollen. There is one prominent difference separating the fossil Mush MT from the extant PT 2 pollen and that is the massive thickness of the solid nexine in the Mush MT (see SEM break in Figure 20D, H, J). The pollen morphology suggests that the Mush MT represents a diverging taxon of the Afro-Indian clade, demonstrating an ancestral or extinct form of the Aristogeitonia-Mischodon-Oldfieldia-Voatamalo clade.
Saldanha MT, aff. Hyaenanche
(Figures 21, 22, Table VI)
Figure 21.
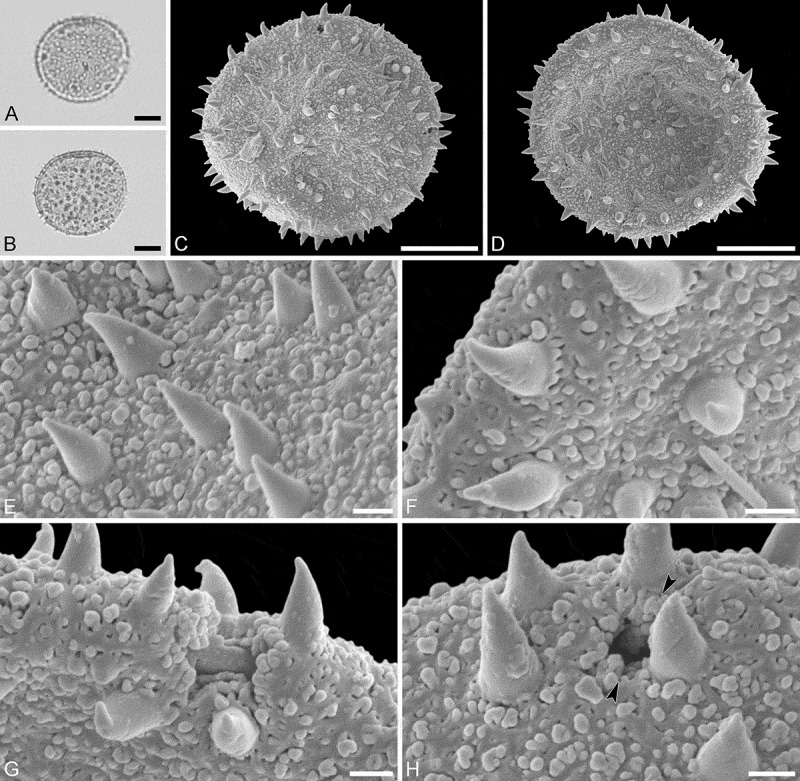
Light microscopy (A, B) and scanning electron microscopy (C–H) micrographs of the Saldanha MT (same grain: A–H). A. Polar view, high focus. B. Polar view, optical cross-section. C. Polar view. D. Polar view, opposite site, grain infolded. E. Close-up of interapertural area. F. Close-up of interapertural area. G. Close-up of aperture, showing part of membrane (arrows). L. Close-up of aperture, showing part of membrane (arrows). Scale bars – 10 µm (A–D), 1 µm (E–H).
Figure 22.
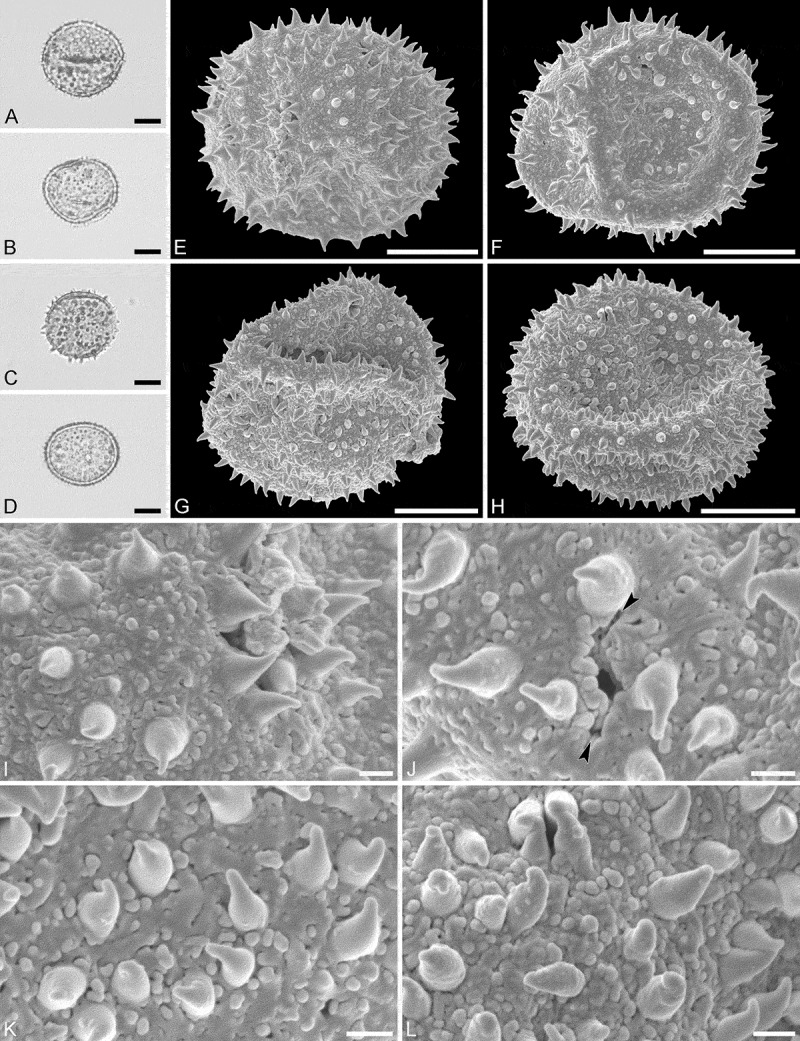
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of the Saldanha MT (same grain: A, E, I; same grain B, F, J; same grain C, G, K; same grain: D, H, L). A. Polar view. B. Oblique polar view. C. Oblique equatorial view. D. Equatorial view. E. Oblique equatorial view. F. Oblique equatorial view, infolded grain. G. Oblique polar view, folded grain. H. Polar view, folded grain. I. Close-up of interapertural area. J. Close-up of aperture, showing part of membrane (arrows). K. Close-up of interapertural area. L. Close-up of interapertural area. Scale bars – 10 µm (A–H), 1 µm (I–L).
Description
Pollen, monad, isopolar, P/E ratio spheroidal to slightly oblate, outline circular to elliptic in polar and equatorial view; equatorial diameter including echini 28–38 µm in LM, 26–35 µm in SEM, equatorial diameter excluding echini 25–35 µm in LM, 23–32 µm in SEM, polar axis including echini 27–30 µm in LM, 25–27 µm in SEM, polar axis excluding echini 25–28 µm in LM, 22–24 µm in SEM; stephano(6–7)porate; pori at regular intervals, positioned at the equator, elliptic, 3.0–4.5 µm in diameter (SEM); exine 1.2–1.4 µm thick; pollen wall tectate; sculpture echinate in LM and SEM, fossulate, perforate and nanogemmate in areas between echini (SEM); 15–30 echini per 100 µm2 in central polar area; echini at irregular intervals, 1.0–2.5 µm in height; aperture membrane nanogemmate (SEM).
Locality
Saldanha Bay drill core, South Africa, early Miocene (Table IV).
Remarks
Most of the fossil pollen grains are infolded or compressed (flattened). The range of the polar axis is based on only two measurements (versus 20 in extant material) and most likely does not convey the complete natural length between the poles in this MT.
Based on the pollen morphology of extant Afro-Indian Picrodendraceae genera it is clear that the pollen should be assigned to genus Hyaenanche (PT 1). The fossil Saldanha MT pollen grains differ in outline from those of Androstachys and Stachyandra (PT 3; elliptic to slightly angular versus circular) and in aperture position (irregular intervals and displaced versus regular intervals and at the equator; Tables V, VI). The echini are also shorter (0.7–1.3 versus 1.0–2.5 µm), of different shape and more densely packed (35–45 per 100 µm2 versus 15–30 per 100 µm2) in both Androstachys and Stachyandra than in the fossil pollen. The SEM sculpture in areas between echini is granulate and perforate in Androstachys and Stachyandra, but clearly fossulate, perforate and nanogemmate in the fossil pollen (Tables V, VI). The fossil Saldanha MT pollen grains differ also from those of Aristogeitonia, Mischodon, Oldfieldia and Voatamalo (PT 2). The four genera produce pollen grains that have fewer than 15 echini per 100 µm2 in the central polar area, whereas the fossil pollen grains have 15–30 echini per 100 µm2 at the central pole. Most of these extant taxa also produce pollen grains equipped with higher echini, 2.0–4.5 µm in height; the echini of the fossil pollen are usually between 1.0 and 2.0 µm and sometimes reach 2.5 µm. The SEM sculpture in areas between the echini is nanogemmate to nanorugulate to granulate in Aristogeitonia, Mischodon, Oldfieldia and Voatamalo, but fossulate, perforate and nanogemmate in extant Hyaenanche (Table V). The fossil Saldanha MT pollen grains are extremely similar to those from the extant species, Hyaenanche globosa. The outline and size of the pollen are similar, the position, number, size and outline of the pori are also the same, and they show the same sculpture in SEM. The echini are of similar size, shape and number in the central polar area, the sculpture in areas between echini compares closely, and the preserved parts of the aperture membrane observed in the fossil pollen suggest that it is the same as in the extant species (Tables V, VI).
Discussion
Taxonomic value of Afro-Indian Picrodendraceae pollen
Extant Afro-Indian Picrodendraceae pollen can be divided into three morphological types, PT 1–PT 3. The pollen of Hyaenanche (PT 1) is clearly unique among the Afro-Indian taxa. The sculpture is echinate, but fossulate, perforate and nanogemmate in areas between echini, and there are 15–30 echini per 100 µm2 in the central polar area (Figure 6, Table V). The echini are of intermediate size (1.0–2.0 µm high) and the aperture membranes are nanogemmate.
Many of the Afro-Indian genera produce PT 2. This includes, Aristogeitonia (Figures 2–5), Mischodon (Figure 7), Oldfieldia (Figures 8–10), and Voatamalo (Figure 15). PT 2 is mostly spheroidal (P/E ratio) and circular in outline (polar and equatorial). The pori are placed at regular intervals around the equator. The sculpture is echinate, with tall (2.0–4.5 µm) but few (3–13 per 100 µm2) echini, and nanogemmate to granulate in areas between echini and on the aperture membrane. There are some subtle differences observed among species in the size of the PT 2 pollen grains and their sculpture elements, but there is a complete overlap among the genera (Table V). Taxa from the same geographic region (e.g. Voatamalo versus Aristogeitonia on Madagascar; Oldfieldia versus Aristogeitonia in eastern Africa; Figure 23) are impossible to distinguish on the basis of their dispersed pollen.
Figure 19.
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of the Mush MT (same grain: A, B, E, F, I, K; same grain: C, D, G, H, J, L). A. Equatorial view, high focus. B. Equatorial view, optical cross-section. C. Oblique equatorial view. D. Oblique equatorial view. E. Oblique equatorial view. F. Oblique equatorial view, opposite site, grain broken. G. Polar view. H. Polar view, opposite site, grain infolded. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture (arrows). L. Close-up of interapertural area. Scale bars – 10 µm (A–H), 1 µm (I–L).
Figure 2.
Light microscopy (A–D) and scanning electron microscopy (E–L) micrographs of Aristogeitonia gabonica (from Gabon, coll. Sosef, 1794 [MO]). A. Polar view, high focus. B. Polar view, optical cross-section. C. Equatorial view, high focus. D. Equatorial view, optical cross-section. E. Polar view. F. Polar view. G. Polar view. H. Infolded grain. I. Close-up of interapertural area. J. Close-up of interapertural area. K. Close-up of aperture, showing membrane (arrows). L. Close-up of aperture, showing membrane (arrows). Scale bars – 10 µm (A–H), 1 µm (I–L).
Figure 23.
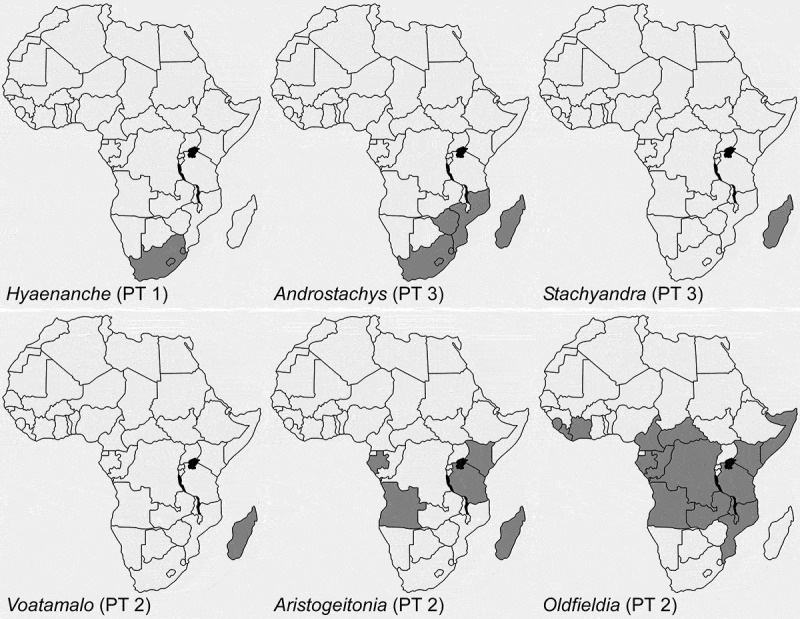
Maps showing the African countries (dark grey) where Picrodendraceae have been reported as part of the modern flora. The pollen types (PT 1–PT 3) are noted.
PT 3 occurs in two genera, Androstachys (Figure 1) and Stachyandra (Figure 13). This pollen is characterised by irregularly placed pori, microechinate sculpture (echini 0.7–1.3 µm high and 30–45 per 100 µm2) and nanoechinate aperture membranes (Table V). The pollen grains of the two taxa studied, Androstachy johnsonii and Stachyandra merana, are so similar that there is no way to distinguish them using LM and/or SEM. The two genera are debatably congeneric (Radcliffe-Smith 2001; Webster 2014).
Origin, divergence, and dispersal of Afro-Indian Picrodendraceae
Previous molecular studies using fossil age constraints suggested that crown Malpighiales began to radiate in the late Early Cretaceous (middle Aptian to middle Albian), at 119.4–110.7 Ma (Davis et al. 2005) or 113.1–106.1 Ma (Xi et al. 2012) (but see also Wikström et al. 2001; Magallón & Castillo 2009; Bell et al. 2010). So far, estimates for the age of the Phyllanthaceae/Picrodendraceae clade fall in the latest Early Cretaceous (Albian), at 114–105.8 Ma (Davis et al. 2005) or in the early Late Cretaceous (Cenomanian to Coniacian), at 101–86.5 Ma (Xi et al. 2012). Estimates for the age of crown-group Picrodendraceae suggest a middle Late Cretaceous (Turonian to Campanian) origin at 92.9–72 Ma (Xi et al. 2012).
Since all the Afro-Indian Picrodendraceae form a monophyletic clade (Figures 24, 25; Wurdack 2008; Wurdack & Davis 2009), it is clear they descended from a single common ancestor that at some point dispersed into Africa. The molecular phylograms of Wurdack and Davis (2009), and Xi et al. (2012), with seven genera, place the South American Podocalyx as sister to the other six genera of the Picrodendraceae that were sampled, which form a grade from Tetracoccus to Austrobuxus. The bipartition network presented here (Figure 24) suggests two major clades, one including all American and African taxa, and the other including all Australasian taxa, with a potential divergence (or origin) point to be placed somewhere close to Podocalyx.
Figure 24.
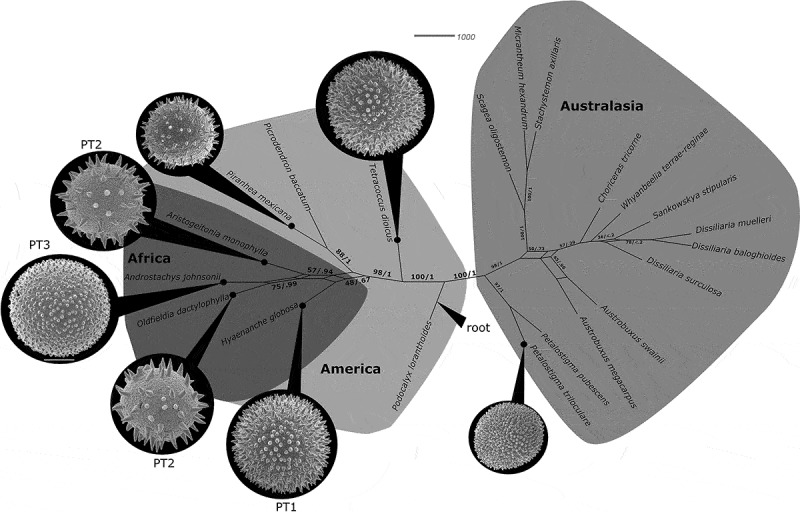
Consensus network based on ML bootstrap replicate summarised with a 20% cutoff. Support values for the split are shown as ML bootstrap/BI posterior probability. Pollen types of different taxa are illustrated at the same scale (scale bar = 10 µm). Geography of the different clades are indicated by different background colour.
Figure 25.
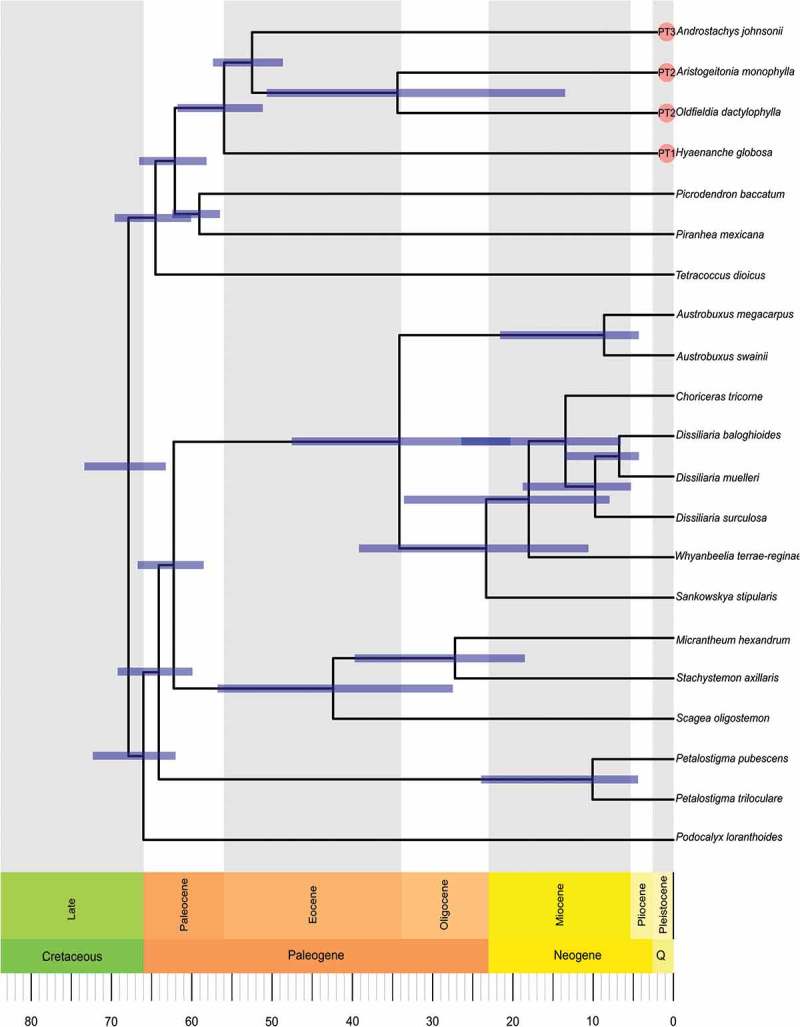
Maximum Clade Credibility consensus timetree of Picrodendraceae obtained using the fossilised birth–death prior. All fossils (see Table VII) have been trimmed off after the construction of the consensus tree. Pollen types for the Afro-Indian taxa are indicated. Bars indicate 95% highest posterier density (HPD) intervals.
It is interesting that PT 1, found in the African Hyaenanche, is extremely similar to pollen occurring in the early branching Tetracoccus (second branching American lineage; Figure 24) and in Piranhea, that is part of the sister clade to the Afro-Indian taxa. PT 1 is clearly a basal or primitive (plesiomorphic) pollen occurring in early diverging/branching Picrodendraceae. This suggests that PT 1 is the ‘original’ pollen type of the lineage that dispersed into Africa from the Americas; therefore PT 1 is ancestral to PT 2, a pollen type occurring in most of the extant Afro-Indian taxa (Aristogeitonia, Mischodon, Oldfieldia and Voatamalo) and showing the widest extant distribution (Figure 23). Lineages with PT 2 subsequently led to the evolution of PT 3, occurring in both Androstachys and Stachyandra.
Picrodendraceae bear mostly explosive dehiscent schizocarp (capsular) fruits (Androstachys, Aristogeitonia, Austrobuxus, Choriceras, Dissiliaria, Hyaenanche, Kairothamnus, Longetia, Micrantheum, Mischodon, Neoroepera, Paradiodendron, Piranhea, Podocalyx, Pseudanthus, Sankowskya, Scagea, Stachyandra, Stachystemon, Tetracoccus, Voatamalo, Whyanbeelia) resulting in very restricted autochorous dispersal (up to a few metres), believed to be primitive in the family (Webster 1994a, 2014). About half of the genera produce carunculate seeds (Androstachys, Austrobuxus, Hyaenanche, Longetia, Micrantheum, Neoroepera, Oldfieldia, Petalostigma, Pseudanthus, Sankowskya, Scagea, Stachyandra, Stachystemon, Tetracoccus, Voatamalo, Whyanbeelia) that are dispersed by ants (myrmecochory; Webster 1994a, 2014). Only three genera have fleshy or drupaceous seeds/fruits (Oldfieldia, Petalostigma, Picrodendron) that are primarily dispersed by birds and/or mammals (endozoochory/ornithochory), a trait believed to be derived in the family (Webster 1994a, 2014). The African Oldfieldia are dispersed by mammals, mostly primates (Koné et al. 2008), the Australian Petalostigma by emus (Clifford & Monteith 1989), and the West Indies Picrodendron by birds and iguanas (Alberts 1999; Hines 2016). According to Webster (1994a, 2014) the basal Phyllanthaceae, Picrodendraceae and Euphorbiaceae typically bear capsular fruits with dry seed-coats excluding long distance dispersal across wide ocean barriers. The predominant autochorous and myrmecochorous dispersal of basal Picrodendraceae and their current distribution suggests they could have dispersed across the Southern Hemisphere prior to later stages of the Gondwana breakup when ocean barriers became too wide.
The Gondwana landmass started to fragment during the Early to Middle Jurassic, at 180–160 Ma (summarised by: e.g. McLoughlin 2001; Chatterjee et al. 2013; Torsvik & Cocks 2013). In the Early Cretaceous, at ~130 Ma (Barremian), Gondwana started to separate into two approximately equal halves, (1) West Gondwana composed of South America and Africa, and (2) East Gondwana composed of Madagascar, India, Antarctica and Australia. Contemporaneously, Madagascar/India started to drift apart from Antarctica/Australia. This scenario continued until c. 90 Ma, when India started to break away from Madagascar, and India then continued its northward journey towards Asia. From that time on Madagascar became part of the African plate (Chatterjee et al. 2013). Also, eastern South America and west/central Africa were closely aligned until 90–80 Ma (Gaina et al. 2013), with potential pathways for plant dispersal between the two continents. Furthermore, tectonic models of East Gondwana (Gibbons et al. 2013) suggest that Africa, Madagascar and India were close until 80–70 Ma, allowing for plant dispersal among these landmasses until at least the latest Upper Cretaceous (Campanian/Maastrichtian). Additionally, paleogeographic reconstructions and terrestrial fossil records suggest that southern South America and West Antarctica were connected by a land bridge (Weddellian Isthmus) believed to have been functional until the late Paleocene (Reguero et al. 2014, terrestrial vertebrates) or the middle Eocene (Ghiglione et al. 2013, terrestrial vertebrates), or even the early Oligocene (Graham 2018b, plants). Based on the paleogeography and taking into account dispersal mechanisms of the family it is clear that if Picrodendraceae dispersed across a southern route into Africa they must have done so prior to the end Cretaceous. Also, since Mischodon has an advanced pollen type (PT 2) and is nested within the African clade (Wurdack 2008) that scenario suggests they must have dispersed from Africa/Madagascar into India in the latest Cretaceous (c. 80–70 Ma; Gibbson et al. 2013) or the early Cenozoic. But that does not fit with the fossil record of the family.
Fossil records of the Phyllanthaceae/Picrodendraceae clade can be traced back to the Upper Cretaceous. Webster (2014) summarised the macrofossil record of Euphorbiaceae s.l. citing fossil woods, fruits and inflorescences. However, neither Webster nor previous authors affiliate any macrofossil directly to the Picrodendraceae (or Oldfieldioideae of the Euphorbiaceae s.l.). Long before, Metcalfe and Chalk (1950) presented a clear overview of the wood anatomy of Euphorbiaceae s.l., in which they recognised three groups within the so-called ‘Phyllanthoideae’ (= today’s Phyllanthaceae and Picrodendraceae): Group A (Aporosa or Aporusa Type), Group B (Glochidion Type) and ‘Other genera of the Phyllanthoideae’, which mainly represent Picrodendraceae (Hayden 1994). Many fossil wood genera (Paraphyllanthoxylon, Phyllanthinium, Glochidioxylon, Bridelioxylon [syn. Bischofioxylon], Securinegoxylon and Bischofinium) have been proposed to accommodate the ‘Phyllanthoideae’ sensu Metcalfe and Chalk (1950; see also Mädel 1962; Prakash et al. 1986). Of these, only Paraphyllanthoxylon (taxa of species group B sensu Herendeen 1991) with numerous uniseriate rays and longer marginal rows, or more pronounced ray heterogeneity, can be labelled as ‘phyllanthoid’. Paraphyllanthoxylon is a very broadly defined fossil genus with numerous species of various ages (Upper Cretaceous to late Cainozoic) described from many parts of the world (for a summary see Gryc et al. 2009; Nunes et al. 2018). The oldest fossil wood record of Phyllanthaceae is represented by the type species of Paraphyllanthoxylon, Paraphyllanthoxylon arizonense Bailey, from the Cenomanian of Arizona (Bailey 1924).
The worldwide Cretaceous ‘Phyllanthoideae’ wood records are accompanied by Phyllanthaceae fruit/seeds from the late Maastrichtian of India (Kapgate et al. 2017). The earliest macrofossils showing morphology of modern Picrodendraceae (Table VII) are inflorescences and flowers from the late Paleocene of Tennessee (as Protoarecoidea buchananensis; Feldman 1990). These flowers also contain in situ pollen that represent the earliest known pollen records of this family, along with dispersed pollen described from the late Paleocene of southeast Australia (Harris 1965; Stover & Partridge 1973). Following the Paleocene, fossil Picrodendraceae pollen (Table VII) are known from the Eocene of Europe (this study; Zetter & Hofmann 2008; Hofmann et al. 2011; Zetter et al. 2011), the Americas (Tschudy & van Loenen 1970; Tschudy 1973; Graham et al. 2000), Australasia (Stover & Partridge 1973) and Antarctica (Truswell & Macphail 2009).
Table VII.
Fossil record of Picrodendraceae.
| Taxon | Organ(s) | Affinity | Locality | Geography | Continent | Period/epoch/stage | Age (ICS 2017) | Range | References |
|---|---|---|---|---|---|---|---|---|---|
| Protoarecoidea buchananensis | Inflorescence, flowers, pollen (LM, SEM, TEM) | Tetracoccus, Piranhea, Picrodendron | Buchanan, Tennessee | USA | North America | Late Paleocene (Thanetian) | 59.2–56.0 | Feldman 1990 | |
| Echiperiporites | Pollen (LM) | Picrodendron | Mississippi embayment area | USA | North America | Early Eocene (Ypresian) | 59.2–56.0 | Tschudy & van Loenen 1970; Tschudy 1973 | |
| Malvacipollis tschudyi | Pollen (LM) | Picrodendron | Saramaguacán Formation | Cuba | North America | Middle Eocene (Lutetian-Bartonian) | 47.8–37.8 | Graham et al. 2000 | |
| Aristogeitonia type | Pollen (LM, SEM) | Piranhea, Hyaenanche | St Pankraz, Salzburg | Austria | Europe | Early Eocene (Ypresian) | 56–47.8 | Hofmann et al. 2011 | |
| Krappfeld MT | Pollen (LM, SEM) | Piranhea, Hyaenanche | Krappfeld | Austria | Europe | Early Eocene (Ypresian) | 56–47.8 | This study | |
| Stolzenbach MT | Pollen (LM, SEM) | Piranhea, Hyaenanche | Stolzenbach | Germany | Europe | Middle Eocene (Lutetian) | 47.8–41.2 | This study | |
| Multiporopollenites; Nothofagidites sp. aff. N. echinata | Pollen (LM) | Piranhea, Hyaenanche | Helmstedt, Wulfersdorfer Flözgruppe | Germany | Europe | Middle Eocene (Lutetian) | 47.8–41.2 | Lenz 2000, 2005 | |
| Profen MT | Pollen (LM, SEM) | Piranhea, Hyaenanche | Profen | Germany | Europe | Middle Eocene (Bartonian) | 41.2–37.8 | This study | |
| Saldanha MT | Pollen (LM, SEM) | Hyaenanche | Saldanha Bay | South Africa | Africa | Early Miocene (Aquitanian-Burdigalian) | 23.03–15.97 | This study | |
| Mush MT | Pollen (LM, SEM) | Aristogeitonia, Mischodon, Oldfieldia, Voatamalo | Mush Valley | Ethiopia | Africa | Early Miocene (Aquitanian) | 22.63–21.73 (absolute dating) | This study | |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | Gippsland Basin | SE Australia | Australasia | Late Paleocene (Thanetian) | 59.2–56.0 | Late Paleocene through early Eocene | Stover & Partridge 1973 |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | Princetown area, Victoria | SE Australia | Australasia | Late Paleocene (Thanetian) | 59.2–56.0 | Harris 1965 | |
| Malvacipollis subtilis | Pollen (LM) | Austrobuxus, Dissiliaria | Gippsland Basin | SE Australia | Australasia | Early Eocene (Ypresian) | 56–47.8 | Early Eocene through late Miocene | Stover & Partridge 1973 |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | New South Wales | E Australia | Australasia | Early Eocene (Ypresian) | 56–47.8 | Early Eocene through Pliocene | Martin 1974 |
| Plagianthus | Pollen (LM) | Austrobuxus, Dissiliaria | Dannevirke Series | New Zealand | Australasia | Early Eocene (Ypresian) | 56–47.8 | Early Eocene through Holocene | Couper 1960 |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | One Tree Hill, St Vincent Basin | S Australia | Australasia | Middle Eocene (Lutetian-Bartonian) | 47.8–37.8 | Alley & Broadbridge 1992 | |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | Murray Basin | SE Australia | Australasia | Middle Eocene (Bartonian) | 41.2–37.8 | Middle Eocene through late Eocene | Macphail 1999 |
| Malvacipollis subtilis | Pollen (LM) | Austrobuxus, Dissiliaria | Murray Basin | SE Australia | Australasia | Middle Eocene (Bartonian) | 41.2–37.8 | Middle Eocene through Pliocene | Macphail 1999 |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | Werillup Formation | W Australia | Australasia | Middle Eocene (Bartonian) | 41.2–37.8 | Late middle Eocene through early late Eocene | Hos 1975; Stover & Partridge 1982 |
| Malvacipollis diversus | Pollen (LM) | Austrobuxus, Dissiliaria | Prydz Bay | E Antarctica | Antarctica | Late Eocene (Priabonian) | 37.8–33.9 | Truswell & Macphail 2009 | |
| Malvacipollis subtilis | Pollen (LM) | Austrobuxus, Dissiliaria | Prydz Bay | E Antarctica | Antarctica | Late Eocene (Priabonian) | 37.8–33.9 | Truswell & Macphail 2009 | |
| Malvacipollis (Micrantheum) spinyspora | Pollen (LM) | Micrantheum, Neoroepera | Manuherikia Group, Central Otago | New Zealand | Australasia | Early Miocene (Aquitanian-Burdigalian) | 23.03–15.97 | Early Miocene through Pliocene | Mildenhall 1989 |
| Malvacipollis (Micrantheum) spinyspora | Pollen (LM) | Micrantheum, Neoroepera | Murray Basin | SE Australia | Australasia | Late Miocene (Tortonian-Messinian) | 11.63–5.33 | Late Miocene through Pliocene | Knight & Martin 1989; Mcphail & Truswell 1993 |
| Malvacipollis (Micrantheum) spinyspora | Pollen (LM) | Micrantheum, Neoroepera | New South Wales | E Australia | Australasia | Pliocene (Zanclean-Piacenzian) | 5.333–2.58 | Pliocene through Pleistocene | Martin 1974 |
| Helciporites astrus | Pollen (LM) | Longetia | Gippsland Basin | SE Australia | Australasia | Early Eocene (Ypresian) | 56.0–47.8 | Early Eocene through late Eocene | Stover & Partridge 1973 |
| Polyorificites oblatus | Pollen (LM) | Longetia | New South Wales | E Australia | Australasia | Late Eocene (Priabonian) | 37.8–33.9 | Late Eocene through Miocene | Martin 1974 |
The fossil record supports an American origin of Picrodendraceae (Table VII). The fossils at hand suggest that the family was well established in the Americas during the late Paleocene. The fossil record also supports a southern dispersal route, from South America via Antarctica into Australasia, during the late Paleocene, a migration route believed to have been open for plant dispersal during most of the Paleogene (Ghiglione et al. 2013; Reguero et al. 2014; Graham 2018a, 2018b). There are, however, no fossil records from Africa, Madagascar or India from the late Cretaceous or Paleocene to demonstrate the presence of Picrodendraceae in this part of the world during that time. Thus, the European Eocene Krappfeld, Stolzenbach and the Profen fossil pollen MTs become important as the only known representatives of an extinct early diverging lineage of the American-Afro-Indian clade, positioned close to Piranhea. The current Picrodendraceae fossil records indicate the family dispersed into Africa from Europe via a second dispersal route, namely from the Americas via the North Atlantic Land bridge during periods of elevated temperatures in the Paleocene and/or Eocene. The North Atlantic Land Bridge is known as a gateway for numerous thermophilic plants, even some of today’s tropical to subtropical plant groups, that were able to disperse freely between the Americas (Greenland) and Europe until the late Eocene (e.g. Tiffney 1985, 2000; Grímsson et al. 2018a). More cold tolerant plants were able to follow this route until the middle to late Miocene (Denk et al. 2010, 2011; Graham 2018a, 2018b). Such a ‘northern route’ has recently been proposed for another predominantly southern hemispheric group, the Loranthaceae, believed to have conquered Africa from Asia during the Eocene (Grímsson et al. 2017b, 2018c). During the Eocene, Europe was influenced by hot and humid climate (Zachos et al. 2001; Mosbrugger et al. 2005), comparable to that of present day tropics to subtropics. In Europe, this paleoclimate sustained a special thermophilic flora, so-called Paratropical Rainforest (e.g. Mai 1995), that disappeared at the end of the Eocene. With tropical and subtropical climate equivalents and corresponding vegetation reaching from the equator towards mid latitudes and even high latitudes, temperatures would not have been a barrier to a southward dispersal of Picrodendraceae from Europe into Africa during the Eocene. The African fossil records show that Picrodendraceae had diverged into at least two different lineages prior to the early Miocene. This is evident by the early Miocene Saldanha MT pollen, that is clearly of the extant PT 1 lineage, and the early Miocene Mush MT, that represents an extinct ancestral taxon of the extant PT 2 lineage. Studies by some of the present authors on late Oligocene to early Miocene palynofloras from Ethiopia and South Africa have yielded no additional fossil pollen that truly represent the extant PT 2 pollen. The only pollen discovered so far are the primitive basal PT 1 from Saldanha Bay (South Africa) and the extinct ancestral type to the PT 2 lineage from Mush (Ethiopia). Therefore, the current fossil data suggests that both the extant PT 2 and PT 3 pollen had not evolved during the latest Oligocene or the earliest Miocene, but are post earliest Miocene taxa. Still, this could easily change in the future as more African palynofloras are studied and screened for Picrodendraceae pollen.
The respective ages of the different Picrodendraceae lineages in the dated phylogeny (Figure 25) support a primary dispersal from South America to Australasia via Antarctica. The Australasian clade supposedly originated between the Late Cretaceous and the Paleocene (69.2–59.9 Ma). The Afro-Indian clade apparently split from the Piranhea-Picrodendron clade between the latest Cretaceous and the Paleocene (66.8–58.1 Ma; Figure 25). The age of the Afro-Indian crown group spans between the Paleocene and the Eocene (61.8–51.1 Ma). These relatively young ages are in agreement with the fossil records and suggestive of a boreotropical distribution with dispersal across the North Atlantic Land Bridge. However, since the earliest fossil records representing the Afro-Indian clade are mostly from European localities, that could bias the inferred splits towards younger ages by missing potential diversity in Afro-India. Similar biases have been reported for other groups of southern origin with mostly European records, like Solanaceae (Wilf et al. 2017).
If results are accepted from the analysis of the current African fossil records and the dated phylogeny (Figure 25) indicating PT 2 pollen (occurring in present day Aristogeitonia, Mischodon, Oldfieldia, Voatamalo) did not evolve until the early to middle Miocene, then dispersal of that lineage from continental Africa to Madagascar and other islands on the eastern side of Africa, and especially to India must have taken place via island hopping and/or long-distance dispersal. It is speculative, but possible that birds transported seeds from the continent, or that seeds along with ants (their primary disperser) and/or even mammals or reptiles, were transported eastwards on floating debris, by one or more successive dispersal events.
Alternatively, the lack of fossil Picrodendraceae pollen from the Eocene to Oligocene of continental Africa may not be because these plants did not thrive in these parts of the world during that time, but more due to the fact that not enough samples have been studied or that they have simply been overlooked or misidentified due to their superficial similarities to e.g. Asteraceae, Malvaceae and Arecaceae. The fossil pollen record to date (Table VII) has been compiled by the Vienna working group, routinely using SEM, and the Australasian palynological community. Palynologists working in Africa may not yet be fully aware of the presence of this pollen group or of its affiliation. This is supported by the current identification of the Miocene Mush MT pollen in the Mush Valley palynoflora (Ethiopia), where this pollen type was most likely previously referred to Myristicaceae (Danehy 2010), and the Saldanha MT (Hyaenanche lineage) pollen in the Saldanha Bay palynoflora (South Africa), a well-studied Miocene flora, where this type of pollen was previously referred to Baumannipollis with affiliation to Malvaceae (Roberts et al. 2017).
Conclusion and outlook
Picrodendraceae produce unique pollen grains that are easily identified using the combination of LM and SEM. The extant Afro-Indian pollen can be grouped into three morphological types (geographical lineages). The PT 1 occurring in Hyaenanche is clearly the basal most surviving Afro-Indian pollen type that gave off subsequent pollen types (PT 2 and PT 3).
Combining evidence from the pollen morphology of Picrodendraceae along with their fossil records, the current phylogenetic framework, a new dated intra-familial phylogeny, and relevant paleogeographic scenarios from the Late Cretaceous to early Cenozoic, we conclude that: (1) Picrodendraceae originated in the Americas, (2) the family dispersed from Southern America across Antarctica and into Australasia during the late Paleocene, (3) a second migration (of a different lineage) took place from the Americas across the North Atlantic Land Bridge and into Europe during the Paleocene and/or Eocene, (4) the Picrodendraceae reached their maximum north–south distribution prior to the end of the Eocene, (5) the family dispersed into Africa from Europe during the Eocene, (6) and dispersed from continental Africa to islands east of the continent and to India in the Neogene via island hopping or long distance dispersal.
Still, to fully understand the early divergence and evolution of Afro-Indian Picrodendraceae, palynological studies need to focus on Late Cretaceous to early Cenozoic floras from the Americas and Afro-India using the morphological data presented here. This will enable scientists to correctly affiliate pollen of this group to particular lineages and to fill some of the gaps in the family’s early history. Also, the expansion of the molecular phylogeny is needed to more completely understand the historical evolutionary relationship of all the Afro-Indian, American and Australasian taxa of this family.
Supplementary Material
Funding Statement
This work was supported by the Austrian Science Fund (P29501-B25); the National Science Foundation (EAR-1053549).
Acknowledgements
This study was funded by the Austrian Science Fund (FWF) with a grant to FG, project number P29501-B25. FHN was funded by a post-doctoral fellowship at the UKZN/UFS and LS by the National Research Foundation, South Africa. MC thanks H. Peter Linder for his support. Funding for research in Ethiopia came from the National Science Foundation (NSF) grant EAR-1053549 and the National Geographic Society Committee for Research and Exploration
Disclosure statement
No potential conflict of interest was reported by the authors.
Supporting information
Supporting Information for this article can be accessed here.
References
- Alberts A.1999. West Indian Iguanas: Status survey and conservation action plan. Gland, Switzerland, Cambridge, UK: IUCN. [Google Scholar]
- Alley NF, Broadbridge LM. 1992. Middle Eocene palynofloras from the One Tree Hill area, St Vincent Basin, South Australia. Alcheringa 16: 241–267. doi: 10.1080/03115519208619121. [DOI] [Google Scholar]
- Bailey IW. 1924. The problem of identifying the wood of Cretaceous and later dicotyledons: Paraphyllanthoxylon arizonense. Annals of Botany 38: 439–451. doi: 10.1093/oxfordjournals.aob.a089910. [DOI] [Google Scholar]
- Bell CD, Soltis DE, Soltis PS. 2010. The age and diversification of the angiosperms re-revisited. American Journal of Botany 97: 1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- Bush RT, Wallace J, Currano ED, Jacobs BF, McInerney FA, Dunn RE, Tabor NJ. 2017. Cell anatomy and leaf δ13C as proxies for shading and canopy structure in a Miocene forest from Ethiopia. Palaeogeography, Palaeoclimatology, Palaeoecology 485: 593–604. doi: 10.1016/j.palaeo.2017.07.015. [DOI] [Google Scholar]
- Chatterjee S, Goswami A, Scotese CR. 2013. The longest voyage: Tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Research 23: 238–267. doi: 10.1016/j.gr.2012.07.001. [DOI] [Google Scholar]
- Clifford HT, Monteith GB. 1989. A three phase seed dispersal mechanism in Australian quinine bush (Petalostigma pubescens Domin). Biotropica 21: 284–286. doi: 10.2307/2388659. [DOI] [Google Scholar]
- Coetzee JA. 1981. A palynological record of very primitive angiosperms in Tertiary deposits of the south-western Cape Province, South Africa. South African Journal of Science 77: 341–343. [Google Scholar]
- Coetzee JA. 1983. Intimation on the Tertiary vegetation of southern Africa. Bothalia 14: 345–354. doi: 10.4102/abc.v14i3/4.1179. [DOI] [Google Scholar]
- Coetzee JA, Muller J. 1984. The phytogeographic significance of some extinct Gondwana pollen types from the Tertiary of the southwestern Cape (South Africa). Annals of the Missouri Botanical Garden 71: 1088–1099. doi: 10.2307/2399246. [DOI] [Google Scholar]
- Coetzee JA, Praglowski J. 1984. Pollen evidence for the occurrence of Casuarina and Myrica in the Tertiary of South Africa. Grana 23: 23–41. doi: 10.1080/00173138409428875. [DOI] [Google Scholar]
- Coetzee JA, Praglowski J. 1988. Winteraceae pollen from the Miocene of the southwestern Cape (South Africa). Grana 27: 27–37. doi: 10.1080/00173138809427730. [DOI] [Google Scholar]
- Couper RA. 1960. New Zealand Mesozoic and Cainozoic plant microfossils. New Zealand Geological Survey Palaeontological Bulletin 32: 1–88. [Google Scholar]
- Danehy DR.2010. Terrestrial vegetation reconstructions spanning the Paleogene – Neogene boundary in the Ethiopian highlands. MS Thesis, Southern Methodist University, Dallas, TX, USA. [Google Scholar]
- Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ. 2005. Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. The American Naturalist 165: E36–E65. doi: 10.1086/428296. [DOI] [PubMed] [Google Scholar]
- Denk T, Grímsson F, Zetter R. 2010. Episodic migration of oaks to Iceland: Evidence for a North Atlantic “land bridge” in the latest Miocene. American Journal of Botany 97: 276–287. doi: 10.3732/ajb.0900195. [DOI] [PubMed] [Google Scholar]
- Denk T, Grímsson F, Zetter R, Símonarson LA. 2011. Late Cainozoic floras of Iceland: 15 million years of vegetation and climate history in the northern North Atlantic. Heidelberg, Germany: Springer. [Google Scholar]
- Dyer RA. 1975. Flora of Southern Africa. The Genera of Southern African Flowering Plants Volume 1 Dicotyledones. Pretoria, South Africa: Department of Agricultural Technical Services. [Google Scholar]
- Erdtman G. 1952. Pollen morphology and plant taxonomy – Angiosperms. Stockholm, Sweden: Almqvist & Wiksell. [Google Scholar]
- Feldman GD.1990. Evidence of early insect pollination in the Arecaceae: A palynological investigation and a new palm flower from the Paleocene/Eocene of the Mississippi embayment. PhD Thesis, University of Connecticut, Storrs, CT. doi: 10.1099/00221287-136-2-327 [DOI] [Google Scholar]
- Gaina C, Torsvik TH, van Hinsbergen DJJ, Medvedev S, Werner SC, Labails C. 2013. The African Plate: A history of oceanic crust accretion and subduction since the Jurassic. Tectonophysics 604: 4–25. doi: 10.1016/j.tecto.2013.05.037. [DOI] [Google Scholar]
- Ghiglione MC, Navarrete-Rodríguez AT, González-Guillot M, Bujalesky G. 2013. The opening of the Magellan Strait and its geodynamic implications. Terra Nova 25: 13–20. doi: 10.1111/ter.2013.25.issue-1. [DOI] [Google Scholar]
- Gibbons AD, Whittaker JM, Müller RD. 2013. The breakup of East Gondwana: Assimilating constraints from Cretaceous ocean basins around India into a best-fit tectonic model. Journal of Geophysical Research Solid Earth 118: 808–822. doi: 10.1002/jgrb.50079. [DOI] [Google Scholar]
- Graham A. 2018a. Land Bridges, ancient environments, plant migrations, and New World connections. Chicago, IL: University of Chicago Press. [Google Scholar]
- Graham A. 2018b. The role of land bridges, ancient environments, and migrations in the assembly of the North American flora. Journal of Systematics and Evolution 46: 405–429. doi: 10.1111/jse.12302 [DOI] [Google Scholar]
- Graham A, Cozadd D, Areces-Mallea A, Frederiksen NO. 2000. Studies in Neotropical paleobotany. XIV. A palynoflora from the Middle Eocene Saramaguacán Formation of Cuba. American Journal of Botany 87: 1526–1539. doi: 10.2307/2656879. [DOI] [PubMed] [Google Scholar]
- Gregor H-J. 2005. Pflanzen und Tiere aus den eozänen Braunkohlen des Untertagebaues Stolzenbach bei Kassel. Philippia 12: 147–181. [Google Scholar]
- Gregor H-J, Oschkinis V. 2013. Die eozänen Braunkohleschichten aus dem Untertagebau Stolzenbach bei Kassel (PreußenElektra, Niederhessen) XI. Die tierischen Reste - Insekten. Documenta Naturae 193: 51–56. [Google Scholar]
- Grímsson F, Denk T, Zetter R. 2008. Pollen, fruits, and leaves of Tetracentron (Trochodendraceae) from the Cainozoic of Iceland and western North America and their palaeobiogeographic implications. Grana 47: 1–14. doi: 10.1080/00173130701873081. [DOI] [Google Scholar]
- Grímsson F, Grimm GW, Potts AJ, Zetter R, Renner SS. 2018a. A Winteraceae pollen tetrad from the early Paleocene of western Greenland, and the fossil record of Winteraceae in Laurasia and Gondwana. Journal of Biogeography 45: 567–581. doi: 10.1111/jbi.2018.45.issue-3. [DOI] [Google Scholar]
- Grímsson F, Grimm GW, Zetter R. 2017a. Tiny pollen grains: First evidence of Saururaceae from the Late Cretaceous of western North America. PeerJ 5: e3434. doi: 10.7717/peerj.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grímsson F, Grimm GW, Zetter R. 2018b. Evolution of pollen morphology in Loranthaceae. Grana 57: 16–116. doi: 10.1080/00173134.2016.1261939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grímsson F, Kapli P, Hofmann C-C, Zetter R, Grimm GW. 2017b. Eocene Loranthaceae pollen pushes back divergence ages for major splits in the family. PeerJ 5: e3373. doi: 10.7717/peerj.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grímsson F, Xafis A, Neumann FH, Scott L, Bamford MK, Zetter R. 2018c. The first Loranthaceae fossils from Africa. Grana 57: 249–259. doi: 10.1080/00173134.2018.1430167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryc V, Vavrčík H, Sakala J. 2009. Cenomanian angiosperm wood from the Bohemian Cretaceous Basin, Czech Republic. IAWA Journal 30: 319–329. doi: 10.1163/22941932-90000221. [DOI] [Google Scholar]
- Halbritter H, Ulrich S, Grímsson F, Weber M, Zetter R, Hesse M, Buchner R, Svojtka M, Frosch-Radivo A. 2018. Illustrated pollen terminology (Second edition). Vienna, Austria: Springer. [Google Scholar]
- Harris WK. 1965. Basal Tertiary microfloras from the Princetown area, Victoria, Australia. Palaeontographica Abteilung B 115: 75–106. [Google Scholar]
- Hayden WJ. 1994. Systematic anatomy of Euphorbiaceae subfamily Oldfieldioidae. I. Overview. Annals of the Missouri Botanical Garden 81: 180–202. doi: 10.2307/2992093. [DOI] [Google Scholar]
- Hayden WJ, Gillis WT, Stone DE, Broome CR, Webster GL. 1984. Systematics and palynology of Picrodendron: Further evidence for relationship with the Oldfieldioideae (Euphorbiaceae). Journal of the Arnold Arboretum 65: 105–127. [Google Scholar]
- Heath TA, Huelsenbeck JP, Stadler T. 2014. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proceedings of the National Academy of Sciences of the United States of America 111: E2957–E2966. doi: 10.1073/pnas.1319091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen PS. 1991. Lauraceous wood from the mid-Cretaceous of eastern North America: Paraphyllanthoxylon marylandense sp. nov. Review of Palaeobotany and Palynology 69: 277–290. doi: 10.1016/0034-6667(91)90032-X. [DOI] [Google Scholar]
- Hines KN. 2016. Food habits of Northern Bahamian rock iguanas (Cyclura cychlura) in the Exuma Islands, with a dietary review of rock iguanas (genus Cyclura). Herpetological Conservation and Biology 11: 121–138. [Google Scholar]
- Hofmann C-C. 2018. Light and scanning electron microscopic investigations of pollen of Ericales (Ericaceae, Sapotaceae, Ebenaceae, Styracaceae and Theaceae) from five lower and mid-Eocene localities. Botanical Journal of the Linnean Society 187: 550–578. doi: 10.1093/botlinnean/boy035. [DOI] [Google Scholar]
- Hofmann C-C, Egger H, King C. 2015a. LM and SEM investigations of pollen from PETM and EECO localities of Austria and Great Britain: New findings of Atherospermataceae, Annonaceae, Araceae and Arecaceae from the Lower Eocene. Plant Systematics and Evolution 301: 773–793. doi: 10.1007/s00606-014-1116-7. [DOI] [Google Scholar]
- Hofmann C-C, Egger H, King C. 2015b. SEM investigation of pollen from the lower Eocene (Carinthia and Salzburg in Austria and Brixton, London area, in England): New findings of Vitaceae, Euphorbiaceae, Phyllanthaceae, Fabaceae, Anacardiaceae, Araliaceae and Apiaceae. Plant Systematics and Evolution 301: 2291–2312. doi: 10.1007/s00606-015-1229-7. [DOI] [Google Scholar]
- Hofmann C-C, Gregor H-J. 2018. Scanning electron microscope and light microscope investigation of pollen from an atypical mid-Eocene coal facies in Stolzenbach mine (PreußenElektra) near Borjen (Kassel, Lower Hesse, Germany). Review of Palaeobotany and Palynology 252: 41–63. doi: 10.1016/j.revpalbo.2018.02.004. [DOI] [Google Scholar]
- Hofmann C-C, Pancost R, Ottner F, Egger H, Taylor K, Mohamed O, Zetter R. 2012. Palynology, biomarker assemblages and clay mineralogy of the Early Eocene Climate Optimum (EECO) in the transgressive Krappfeld succession (Eastern Alps, Austria). Austrian Journal of Earth Sciences 105: 224–239. [Google Scholar]
- Hofmann C-C, Zetter R. 2001. Palynological investigation of the Krappfeld area, Paleocene/ Eocene,Carinthia (Austria). Palaeontographica Abteilung B 259: 47–67. [Google Scholar]
- Hofmann C-C, Zetter R, Jin J. 2011. Pollen grains of Picrodendraceae, Phyllanthaceae, Euphorbiaceae (former Euphorbiaceae) from Paleogene strata of Central Europe and South China. Berichte der Geologischen Bundesanstalt 85: 90. [Google Scholar]
- Holland B, Moulton V. 2003. Consensus networks: A method for visualising incompatibilities in collections of trees In: Benson G, Page RDM, eds. Algorithms in bioinformatics, 165–176. Berlin, Germany: Springer. [Google Scholar]
- Hos D. 1975. Preliminary investigation of the palynology of the upper eocene werillup formation, Western Australia. Journal of the Royal Society of Western Australia 58: 1–14. [Google Scholar]
- Hottenrott M, Gregor H-J, Oschkinis V. 2010. Die eozänen Braunkohleschichten aus dem Untertagebau Stolzenbach bei Kassel (PreußenElektra, Niederhessen) VII. Die Mikroflora. Documenta Naturae 181: 29–43. [Google Scholar]
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biolology and Evolution 23: 254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- ICS 2017. International Chronostratigraphic Chart v2017/02. http://www.stratigraphy.org/ICSchart/ChronostratChart2017-02.pdf
- Kapgate D, Manchester SR, Stuppy W. 2017. Oldest fruit of Phyllanthaceae from the Deccan Intertrappean Beds of Singpur, Madhya Pradesh, India. Acta Palaeobotanica 57: 33–38. doi: 10.1515/acpa-2017-0004. [DOI] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MJ, Martin HA. 1989. Origin of groundwater salinity near Tesco, northwest Victoria. Journal of Australian Geology & Geophysics 11: 285–289. [Google Scholar]
- Köhler E. 1965. Die Pollenmorphologie der biovulaten Euphorbiaceae und ihre Bedeutung für die Taxonomie. Grana 6: 26–120. [Google Scholar]
- Koné I, Lambert JE, Refisch J, Bakayoko A. 2008. Primate seed dispersal and its potential role in maintaining useful tree species in the Taï region, Côte-d’Ivoire: Implications for the conservation of forest fragments. Tropical Conservation Science 1: 293–306. doi: 10.1177/194008290800100309. [DOI] [Google Scholar]
- Krutzsch W, Lenk G. 1973. Sporenpaläontologische Untersuchungen im Alttertiär des Weißelster-Beckens. I. Die stratigraphisch wichtigen Pollen- und Sporenformen aus dem Profil des Tagebaus Profen. Abhandlungen des Zentralen Geologischen Instituts 18: 59–76. [Google Scholar]
- Lenz OK.2000. Paläoökologie eines Küstenmoores aus dem Eozän Mitteleuropas am Beispiel der Wulfersdorfer Flöze und deren Begleitschichten (Helmstedt Oberflözgruppe, Tagebau Helmstedt). PhD Thesis, University of Göttingen, Germany. [Google Scholar]
- Lenz OK. 2005. Palynologie und Paläoökologie eines Küstenmoores aus dem Mittleren Eozän Mitteleuropas – Die Wulfersdorfer Flözgruppe aus dem Tagebau Helmstedt, Niedersachsen. Palaeontographica Abteilung B 271: 1–157. doi: 10.1127/palb/271/2005/1. [DOI] [Google Scholar]
- Levin GA, Simpson MG. 1994. Phylogenetic implications of pollen ultrastructure in the Oldfieldioideae (Euphorbiaceae). Annals of the Missouri Botanic Garden 81: 203–238. doi: 10.2307/2992094. [DOI] [Google Scholar]
- Lobreau-Callen D, Cervera MS. 1994. Pollen ultrastructure of Hymenocardia Wallich ex Lindley and comparison with other Euphorbiaceae. Review of Palaeobotany and Palynology 81: 257–278. doi: 10.1016/0034-6667(94)90111-2. [DOI] [Google Scholar]
- Macphail MK. 1999. Palynostratigraphy of the Murray Basin, inland Southeastern Australia. Palynology 23: 197–240. doi: 10.1080/01916122.1999.9989528. [DOI] [Google Scholar]
- Macphail MK, Truswell EM. 1993. Palynostratigraphy of the Bookpurnong Beds and related Late Miocene-Early Pliocene facies in the central west Murray Basin, part 2: Spores and pollens. Journal of Australian Geology & Geophysics 14: 383–409. [Google Scholar]
- Maddison WP, Maddison DR. 2011. Mesquite: A Modular System for Evolutionary Analysis. Version 2.75, http://mesquiteproject.org; accessed 20 March 2013.
- Mädel E. 1962. Die fossilen Euphorbiaceen-Hölzer mit besonderer Berücksichtigung neuer Funde aus der Oberkreide Süd-Afrikas. Senckenbergiana Lethaea 43: 283–321. [Google Scholar]
- Magallón S, Castillo A. 2009. Angiosperm diversification through time. American Journal of Botany 96: 349–365. doi: 10.3732/ajb.0800060. [DOI] [PubMed] [Google Scholar]
- Mai DH. 1995. Tertiäre Vegetationsgeschichte Europas. Jena, Germany: Gustav Fischer. [Google Scholar]
- Manchester SR, Grímsson F, Zetter R. 2015. Assessing the fossil record of asterids in the context of our current phylogenetic framework. Annals of the Missouri Botanical Garden 100: 329–363. doi: 10.3417/2014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HA. 1974. The identification of some Tertiary pollen belonging to the family Euphorbiaceae. Australian Journal of Botany 22: 271–279. doi: 10.1071/BT9740271. [DOI] [Google Scholar]
- Martin HA. 1978. Evolution of the Australian flora and vegetation through the Tertiary: Evidence from pollen. Alcheringa 2: 181–201. doi: 10.1080/03115517808527776. [DOI] [Google Scholar]
- Martin HA. 1982. Changing Cenozoic barriers and the Australian paleobotanical record. Annals of the Missouri Botanical Garden 69: 625–667. doi: 10.2307/2399086. [DOI] [Google Scholar]
- McLoughlin S. 2001. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany 49: 271–300. doi: 10.1071/BT00023. [DOI] [Google Scholar]
- Merino Sutter D, Forster PI, Endress PK. 2006. Female flowers and systematic position of Picrodendraceae (Euphorbiaceae s.l., Malpighiales). Plant Systematics and Evolution 261: 187–215. doi: 10.1007/s00606-006-0414-0. [DOI] [Google Scholar]
- Metcalfe CR, Chalk L. 1950. Anatomy of the Dicotyledons. Oxford: Clarendon Press. [Google Scholar]
- Mildenhall DC. 1989. Summary of the age and paleoecology of the Miocene Manuherikia Group, Central Otago, New Zealand. Journal of the Royal Society of New Zealand 19: 19–29. doi: 10.1080/03036758.1989.10426452. [DOI] [Google Scholar]
- Mosbrugger V, Utescher T, Dilcher DL. 2005. Cenozoic continental climate evolution of Central Europe. Proceedings of the National Academy of Sciences of the United States of America 102: 14964–14969. doi: 10.1073/pnas.0505267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes CI, Pujana RR, Escapa IH, Gandolfo MA, Cúneo NR. 2018. A new species of Carlquistoxylon from the Early Cretaceous of Patagonia (Chubut province, Argentina): the oldest record of angiosperm wood from South America. IAWA Journal 39: 406–426. doi: 10.1163/22941932-20170206. [DOI] [Google Scholar]
- Oschkinis V, Gregor H-J. 1992. Paläontologische Funde aus der eozänen Braunkohle des Untertagebaus Stolzenbach (PreußenElektra) in Niederhessen – I. Die Flora. Documenta Naturae 72: 1–31. [Google Scholar]
- Pälchen W, Walter H. 2011. Geologie von Sachsen I. Geologischer Bau und Entwicklungsgeschichte. 2. Auflage. Stuttgart, Germany: E. Schweizerbart’sche Verlagsbuchhandlung. [Google Scholar]
- Pan AD, Currano ED, Jacobs BF, Feseha M, Tabor N, Herendeen PS. 2012. Fossil Newtonia (Fabaceae) seeds from the early Miocene (22-21 Ma) Mush Valley in Ethiopia. International Journal of Plant Sciences 173: 290–296. doi: 10.1086/663967. [DOI] [Google Scholar]
- Pan AD, Jacobs BF, Currano ED. 2014. Dioscoreaceae fossils from the Late Oligocene and early Miocene of Ethiopia. Botanical Journal of the Linnean Society 175: 17–28. doi: 10.1111/boj.12150 [DOI] [Google Scholar]
- Prakash U, Bande MB, Lalitha V. 1986. The genus Phyllanthus from the Tertiary of India with critical remarks on the nomenclature of fossil woods of Euphorbiaceae. Palaeobotanist 35: 106–114. [Google Scholar]
- Punt W. 1962. Pollen morphology of the Euphorbiaceae with special reference to taxonomy. Wentia 7: 1–116. doi: 10.1111/plb.1962.7.issue-S1. [DOI] [Google Scholar]
- Punt W. 1987. A survey of pollen morphology in Euphorbiaceae with special reference to Phyllanthus. Botanical Journal of the Linnean Society 94: 127–142. doi: 10.1111/j.1095-8339.1987.tb01042.x. [DOI] [Google Scholar]
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1–81. doi: 10.1016/j.revpalbo.2006.06.008. [DOI] [Google Scholar]
- Radcliffe-Smith A. 2001. Genera euphorbiacearum. Kew: Royal Botanic Gardens. [Google Scholar]
- Raine JI, Mildenhall DC, Kennedy EM. 2011. New Zealand fossil spores and pollen: An illustrated catalogue. 4th edition. GNS Science miscellaneous series no. 4, http://data.gns.cri.nz/sporepollen/index.htm
- Rambaut A, Drummond A. 2003. Tracer: A program for analysing results from Bayesian MCMC programs such as BEAST & MrBayes. Edinburgh, UK: University of Edinburgh. [Google Scholar]
- Reguero MA, Gelfo JN, López GM, Bond M, Abello A, Santillama SN, Marenssi SA. 2014. Final Gondwana breakup: The Paleogene South American native ungulates and the demise of the South American-Antarctica land connection. Global and Planetary Change 123B: 400–413. doi: 10.1016/j.gloplacha.2014.07.016. [DOI] [Google Scholar]
- Roberts DL, Neumann FH, Cawthra HC, Carr AS, Scott L, Durugbo EU, Humphries MS, Cowling RM, Bamford MK, Musekiwa C, MacHutchon M. 2017. Palaeoenvironments during a terminal Oligocene or early Miocene transgression in a fluvial system at the southwestern tip of Africa. Global and Planetary Change 150: 1–23. doi: 10.1016/j.gloplacha.2017.01.007. [DOI] [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MG, Levin GA. 1994. Pollen ultrastructure of the biovulate Euphorbiaceae. International Journal of Plant Sciences 155: 313–341. doi: 10.1086/297170. [DOI] [Google Scholar]
- Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Latvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu YL, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS. 2011. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98: 704–730. doi: 10.3732/ajb.1000251. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover LE, Partridge AD. 1973. Tertiary and Late Cretaceous spores and pollen from the Gippsland Basin, southeastern Australia. Proceedings of the Royal Society of Victoria 85: 237–286. [Google Scholar]
- Stover LE, Partridge AD. 1982. Eocene spore-pollen from the Werillup Formation, Western Australia. Palynology 6: 69–96. doi: 10.1080/01916122.1982.9989235. [DOI] [Google Scholar]
- Tesfamichael T, Jacobs BF, Tabor N, Michel L, Currano E, Feseha M, Barclay R, Kappelmann J, Schmitz M. 2017. Settling the issue of “decoupling” between atmospheric carbon dioxide and global temperature: [CO2]atm reconstructions across the warming Paleogene-Neogene divide. Geology 45: 999–1002. doi: 10.1130/G39048.1. [DOI] [Google Scholar]
- Tiffney BH. 1985. The Eocene North Atlantic Land Bridge: Its importance in Tertiary and modern phytogeography of the Northern Hemisphere. Journal of the Arnold Arboretum 66: 243–273. doi: 10.5962/bhl.part.13183. [DOI] [Google Scholar]
- Tiffney BH. 2000. Geographical and climatic influences on the Cretaceous and Tertiary history of Euramerican floristic similarity. Acta Universitatis Carolinae - Geologica 44: 5–16. [Google Scholar]
- Torsvik TH, Cocks RM. 2013. Gondwana from top to base in space and time. Gondwana Research 24: 999–1030. doi: 10.1016/j.gr.2013.06.012. [DOI] [Google Scholar]
- Truswell EM, Macphail MK. 2009. Polar forests on the edge of extinction: What does the fossil spore and pollen evidence from East Antarctica say?. Australian Systematic Botany 22: 57–106. doi: 10.1071/SB08046. [DOI] [Google Scholar]
- Tschudy RH. 1973. Stratigraphic distribution of significant Eocene palynomorphs of the Mississippi Embayment. U.S. Geological Survey Professional Paper 743-B: B1–B24. [Google Scholar]
- Tschudy RH, van Loenen SD. 1970. Illustrations of plant microfossils from the Yazoo Clay (Jackson Group, Upper Eocene) Mississippi. Geological Survey Professional Paper 643-E: E1–E3. [Google Scholar]
- WCSP 2018. World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. http://wcsp.science.kew.org/; accessed6February2018.
- Webster GL. 1994a. Classification of the Euphorbiaceae. Annals of the Missouri Botanic Garden 81: 3–32. doi: 10.2307/2399908. [DOI] [Google Scholar]
- Webster GL. 1994b. Synopsis of the genera and suprageneric taxa of Euphorbiaceae. Annals of the Missouri Botanic Garden 81: 33–144. doi: 10.2307/2399909. [DOI] [Google Scholar]
- Webster GL. 2014. Euphorbiaceae In: Kubitzki K, ed. The families and genera of vascular plants 11, 51–216. Heidelberg, Germany: Springer. [Google Scholar]
- Wikström N, Savolainen V, Chase MW. 2001. Evolution of the angiosperms: Calibrating the family tree. Proceedings of the Royal Society B 268: 2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilf P, Carvalho MR, Gandolfo MA, Cúneo NR. 2017. Eocene lantern fruits from Gondwanan Patagonia and the early origins of Solanaceae. Science 355: 71–75. doi: 10.1126/science.aag2737. [DOI] [PubMed] [Google Scholar]
- Wurdack KJ.2008. Evolution and biogeography of Picrodendraceae: saga of the spiny-pollen spurges. Botany 2008, British Columbia, Vancouver BC, Canada, 26–30 July 2008. [Google Scholar]
- Wurdack KJ, Davis CC. 2009. Malpighiales phylogenetics: Gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. American Journal of Botany 96: 1551–1570. doi: 10.3732/ajb.0800207. [DOI] [PubMed] [Google Scholar]
- Wurdack KJ, Hoffmann P, Samuel R, de Bruijn A, van der Bank M, Chase MW. 2004. Molecular phylogenetic analysis of Phyllanthaceae (Phyllanthoideae pro parte, Euphorbiaceae sensu lato) using plastid rbcL sequences. American Journal of Botany 91: 1882–1900. doi: 10.3732/ajb.91.11.1882. [DOI] [PubMed] [Google Scholar]
- Xi Z, Ruhfel BR, Schaefer H, Amorim AM, Sugumaran M, Wurdack KJ, Endress PK, Matthews ML, Stevens PF, Mathews S, Davis CC. 2012. Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proceedings of the National Academy of Sciences of the United States of America 109: 17519–17524. doi: 10.1073/pnas.1205818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos JC, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693. doi: 10.1126/science.292.5522.1627b. [DOI] [PubMed] [Google Scholar]
- Zetter R. 1989. Methodik und Bedeutung einer routinemäßig kombinierten lichtmikroskopischen und rasterelektonenmikroskopischen Untersuchung fossiler Mikrofloren. Courier Forschungsinstitut Senckenberg 109: 41–50. [Google Scholar]
- Zetter R, Hofmann C-C. 2001. New aspects of the palynoflora of the lowermost Eocene (Krappfeld area, Carinthia). Österreichische Akademie der Wissenschaften. Schriftenreihe der Erdwissenschaftlichen Kommissionen 14: 473–507. [Google Scholar]
- Zetter R, Hofmann C-C. 2008. Occurrences of Aristogeitonia-type pollen (Euphorbiaceae) in microfloras from Central Europe during the Eocene Thermal Maximum. Terra Nostra 2008/2: 317. [Google Scholar]
- Zetter R, Hofmann C-C, Grímsson F. 2011. Kurzzeitiges Auftreten tropischer Florenelemente im Paläogen von Mittel- und Westeuropa. Beiträge zur Paläontologie 32: 43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


