Abstract
Data released by the U.S. Centers for Disease Control and Prevention (CDC) on March 5, 2019 showed that Staph aureus infections are a major problem in the United States, with 119,000 infections and almost 20,000 deaths in 2017. Rates of decline for hospital-onset MRSA have slowed since 2012 and the United States is not on track for meeting the 2015 U.S. Dept. of Health and Human Services’ goal of a 50% reduction by 2020. There is a need for improved standards for control of dangerous pathogens. Currently, the World Health Organization’s recommendation of preoperatively screening patients for Staph aureus has not become a standard of care in the United States.
The U.S. Veterans Health Administration also released data which found a much larger decrease in hospital-onset MRSA infections as opposed to hospital-onset MSSA using various infectious disease bundles that all included universal MRSA surveillance and isolation for MRSA carriers. These results mirror the results obtained by the United Kingdom’s National Health Service. These findings support the contention that the marked decline in hospital-onset MRSA infections observed in these studies is due to interventions which are specifically targeted towards MRSA.
A case is made that concerns with the integrity of healthcare policy research, along with industrial conflicts-of-interest have inhibited effective formulation of infectious disease policy in the United States. Because MRSA has become endemic in the general U.S. population (approximately 2%), the author advocates that universal facility-wide screening of MRSA on admission be included in infection prevention bundles used at U.S. hospital.
Keywords: MRSA, MSSA, CDC, Surveillance, Isolation, Veterans health administration, VA, Standards, SIR, Risk adjustment
Background
On March 4th 2019, the Centers for Disease Control and Prevention (CDC) released a Vital Signs report regarding efforts in the United States to control Staph aureus and MRSA. The results were not good. In 2017, there were 119,000 Staph aureus infections and almost 20,000 deaths [1]. The reduction in MRSA bloodstream infections stalled between 2012 and 2016. Risk-adjusted data using the CDC’s Standardized Infection Ratio (SIR) reported an 8% reduction in 2017 compared to 2016 [2]. However, there is still wide variation in performance [3]. The United States is nowhere near on track to meet the 2015 U.S. Dept of Health and Human Services goal of a 50% reduction by 2020 [4]. It is the purpose of this commentary to review the formulation and history of MRSA control policy in the United States with emphasis on the role of carrier surveillance, along with policy implications of the newly released CDC data and Veterans Health Administration MRSA infection control report..
Implications of differing control rates for MRSA and MSSA
In the Vital Signs report, [1] the CDC did not make many new recommendations. However, they did highlight the large decrease in MRSA which was achieved by the U.S. Veterans Health Administration. The report observed a 66% decrease in hospital-onset MRSA, but only a 19% decrease in MSSA [5]. The emphasis was that improvement can take place, but what was not said was even more important.
The relatively small decrease observed in MSSA strongly supports the contention that strategies specifically directed at MRSA, as opposed to global strategies were responsible for the large fall in MRSA infections. In Veterans Health Administration, this comprised universal surveillance of all admitted patients and isolation of carriers. A similar observations was also found buried in the Electronic Medical Record data in the Vital Signs Report [1]. Hospital-onset MRSA bloodstream infections showed a significant decrease but hospital-onset rates of MSSA were unchanged.
All of this was foretold by a 2017 ARIC article which reported that rates of hospital-onset MRSA bloodstream infections were not falling in the United States and suggested that a reexamination of the Veterans Health Administration MRSA infection data might hold lessons on how to reduce MRSA infection rates [6].
We have identified a total of four reports which have observed a marked decrease in hospital-onset MRSA infections and little or no decease hospital-onset MSSA infections.
United States - Veterans Health Administration [5].
Seville, Spain - Jesus Rodriguez-Bano, et al [9].
United States - CDC Electronic Medical Record Data from 447 hospitals [1].
In these four reports one may conclude that strategies designed specifically for MRSA must have been the driver in the observed reduction in MRSA infections. Infectious disease prevention strategies used in the first three reports included surveillance on admission for MRSA carriers with implementation of isolation and/or decolonization. Infectious disease prevention protocols were not described in the fourth report. In the United States private sector, Rodriguez-Bano, et al., the VA and NHS also implemented protocols to promote hand hygiene. However, if hand hygiene was the driver in hospital-onset MRSA reduction, we should also have observed a reduction in hospital-onset MSSA.
How did the United States largely abandon surveillance
The main interventions in the United States to stop the MRSA epidemic have not centered on surveillance and isolation / decolonization. Two papers, Harbarth, et al., published in JAMA [10] and Huskins, et al., published in NEJM [11] have been used by policymakers to not recommend the expanded use of surveillance and isolation [12–16]. In addition, there has been a demand for rigorous studies after a review by Cooper, et al., found that most surveillance studies were not well controlled. [17, 18] This philosophy continued despite Harbarth, et al., and Huskins, et al. being criticized for major design flaws [19, 20] and a major controlled study demonstrating the efficacy of universal facility-wide surveillance in decreasing MRSA infections [21].
Research integrity issues with underlying health policy research
In the United States, hand hygiene has been one of the cornerstones of infectious disease policy. However, its effectiveness as a primary intervention appears to have been overstated, as illustrated by at least one article [22]. In this instance, industrial conflicts of interest appear to exist at both the research and editorial level [23]. Hand hygiene is an important part of an infectious disease control bundle, but as supported by the results in the above four research reports, targeted pathogen specific interventions are needed to markedly decrease infection rates.
Universal use of chlorohexidine has also been advocated and has distracted some institutions in their efforts to control MRSA. The basic research behind widening the applications of chlorhexidine has been riddled with research integrity problems and industrial conflicts of interest. Numerous research papers have performed two-to-one comparisons, comparing the efficacy of chlorhexidine plus alcohol to alcohol alone [24]. Industrial conflict of interest was brought to a head by the Charles Denham scandal which involved the National Quality Forum, a non-profit organization in the United States that advises CMS on quality measurements and patient safety indicators [25].
A major report on the efficacy of universal daily chlorhexidine bathing in the ICU [26] had debatable conclusions, showing a non-significant decrease in MRSA bloodstream infections with the most significant effect on commensal bacteria and yeast. This report also appeared to have issues of research integrity and spinning [20] and was the subject of a Reuters Investigative report which found apparent industrial conflicts of interest. [27] On a facility-wide basis, universal chlorhexidine bathing was found not be effective in the prevention of MRSA [28, 29]. However, certain high-risk patients with medical devices may benefit from this intervention [28].
Interventions which currently are receiving emphasis in the United States
Despite the mounting evidence for the need for pathogen-specific targeted interventions, many institutions in the United States continued to exclusively implement non-specific global interventions. These have included:
Hand hygiene. Hand hygiene is extremely important. It is the “plastic straw” of infection control. But by itself it is of questionable value. In the context of MDROs, hand hygiene should be viewed as a backup measure, since these organisms should not be on a healthcare workers’ hands in the first place. And if they are, there is a problem with containment and control.
Focusing on the farm and not healthcare. The justification for this argument is that even though rates of usage are dropping, almost 11 million kg of antibiotics were consumed by U.S. farm animals in 2017 [30]. This argument has been used in an attempt to bolster risk adjustment for hospital-onset MRSA infections using community levels of MRSA infections. However, recent epidemiological research from the European Union has brought the concept of agriculture antibiotic usage as a major driver of human infections into question [31]. And one must ask, if MRSA is so prevalent in the community, regardless of the source, why are institutions not screening all patients upon admission?
Antibiotic stewardship. This is extremely important, but there is no guarantee this will reverse the current epidemic or stop new resistant organisms from developing. Even if usage is cut by 50%, there will still be billions of bacteria exposed to antibiotics and resistance may still develop, but hopefully at a slower rate. In addition, antibiotic stewardship’s efficacy in stopping an epidemic caused by endemic pathogens may differ from its efficacy in preventing future epidemics from emerging drug-resistant pathogens.
Universal daily bathing with chlorhexidine. Because of the history of research integrity problems surrounding this product, [16, 24] along with the recent well-controlled study showing its lack of effectiveness in preventing MRSA infections on a facility-wide basis (with the exception of patients with medical devices), [28] we agree with Olivier Mimoz and Jérémy Guenezan who stated in a recent Lancet commentary that “Chlorhexidine use should consequently be limited to situations presenting a clear patient benefit” [29].
Common excuses for the high rates of hospital-onset MRSA in the United States
There are a number of common excuses which are used to justify the high rates of hospital-onset MRSA and the inertia in implementing protocols to lower infections.
Citing the epidemic of illicit opioid injection injecting opioids for the increased in community MRSA and for the increase risk of obtaining a hospital acquired infection, has become another common excuse to justify inaction. There are calls by the industry to increase risk adjustment because of the increase risk of infections caused by the opioid epidemic. However, the proportion of total MRSA infections represented by patients who inject opioids only increased by 5% (4% in 2011 to 9% in 2016) [32]. Thus, one could argue that instead of risk adjusting the effects of the opioid epidemic away, United States’ facilities should place more emphasis on screening and isolation / decolonization.
-
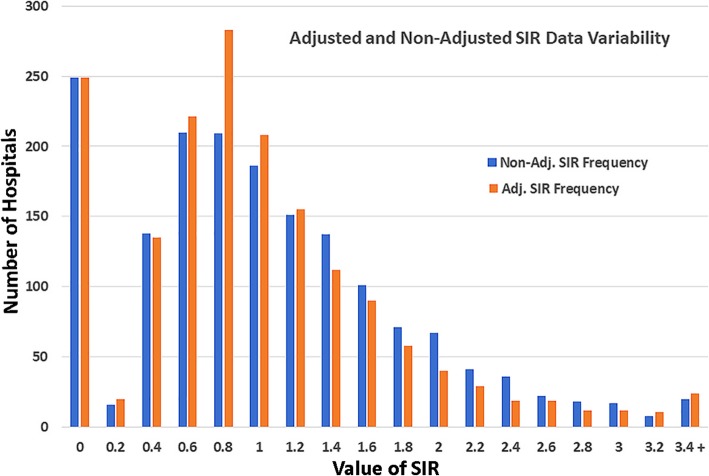
Citing the high rate of MRSA in the community. Similar to the opioid epidemic, high rates of MRSA in the community have increased calls for risk adjustment of hospital acquired infection rates. Currently, risk adjustment can decrease the reported Standardized Infection Ratio (SIR) or adjusted infection rate of a facility by over 50% (see Table 1). Instead of improving reported performance by mathematically lowering infection rates with the SIR, a better strategy would be to implement pathogen specific interventions, such as surveillance and isolation, to actually decrease infections which are associated with community environmental pressure. This type of risk adjustment also causes an aberration of increasing the SIR in some “low risk” facilities. The overall effect is to decrease interfacility variability (see Fig. 1) and disincentivizes support for infection control. Thus, the concern is that risk adjustment is not adjusting only for patient risk but for facility underperformance.
Similarly, changing the grace period for the diagnosis of healthcare associated hospital acquired MRSA bloodstream infections in the United States from 2 to 3 days lowers the number of infections reported. The smaller numbers make interfacility differences less likely to reach statistical significance, resulting in more facilities being designated as “No Different Than National Benchmark”.
Citing the staff’s hand washing compliance is also used to justify inaction. The reason given is that if we cannot get the staff to reliably wash their hands, then everything else is unlikely to work. There is no significant evidence that destroying the microbiome on a healthcare workers hands 100 times a day is a prerequisite to infection control. Few if any centers have been able to reliably accomplish this. However, the above four reports showing a marked decrease in hospital-onset MRSA as compared to hospital-onset MSSA present a strong combined argument that targeted infection control can be effective, even if hand hygiene compliance is not 100%.
Believing that decolonization cannot impact the health of a patient or healthcare worker who is colonized with MRSA. However, decolonization can be effective for both healthcare staff and patients. Albrich, et al., reported that 88% of 510 healthcare workers were successfully decolonized for MRSA [33]. Huang SS, et al., in a recent NEJM article, reported a well-controlled large prospective study (Project CLEAR) which found that outpatient decolonization of patients after hospitalizations can significantly decrease infections. [34] The World Health Organization recommends that all presurgical patients undergo surveillance for Staph aureus, along with decolonization. [35] However, in the United States, this has yet to become a standard of care, not even for the most dangerous form of Staph aureus, MRSA.
Table 1.
MRSA Bloodstream Infections in US Hospitals Having Major Risk Adjustment. Data Acquisition Dates 1/4/2017 to 31/3/2018
| Hospital Name | City | State | Patient Days | Risk Adj. Perdicted Cases | Non-Risk Adj. Perdicted Cases* | Observed Cases | Risk Adj. SIR | Non-Risk Adj SIR* | Percent difference** |
|---|---|---|---|---|---|---|---|---|---|
| HOSPITAL FOR SPECIAL SURGERY | NEW YORK | NY | 50,032 | 1.148 | 2.606 | 1 | 0.871 | 0.384 | 127.01% |
| HIALEAH HOSPITAL | HIALEAH | FL | 54,002 | 1.348 | 2.813 | 6 | 4.451 | 2.133 | 108.69% |
| MAGEE WOMENS HOSP. OF UPMC HEALTH SYSTEM | PITTSBURGH | PA | 94,175 | 2.461 | 4.906 | 3 | 1.219 | 0.612 | 99.35% |
| WOMEN & INFANTS HOSPITAL OF RHODE ISLAND | PROVIDENCE | RI | 77,556 | 2.082 | 4.040 | 2 | 0.961 | 0.495 | 94.13% |
| WOMANS HOSPITAL OF TEXAS,THE | HOUSTON | TX | 110,317 | 2.984 | 5.747 | 2 | 0.670 | 0.348 | 92.52% |
| MEDSTAR GEORGETOWN UNIVERSITY HOSPITAL | WASHINGTON | DC | 121,973 | 13.276 | 6.354 | 10 | 0.753 | 1.574 | −52.15% |
| PENN PRESBYTERIAN MEDICAL CENTER | PHILADELPHIA | PA | 90,041 | 9.801 | 4.691 | 6 | 0.612 | 1.279 | −52.16% |
| UNIVERSITY HEALTH SYSTEM | SAN ANTONIO | TX | 180,652 | 19.662 | 9.411 | 12 | 0.610 | 1.275 | −52.16% |
| UNIVERSITY OF IOWA HOSPITAL & CLINICS | IOWA CITY | IA | 213,095 | 23.193 | 11.101 | 11 | 0.474 | 0.991 | −52.16% |
| INDIANA UNIV. HEALTH BALL MEMORIAL HOSPITAL | MUNCIE | IN | 88,914 | 9.678 | 4.632 | 4 | 0.413 | 0.864 | −52.18% |
A smaller SIR denotes better performance
*Estimated using total number of U.S. MRSA Bloodstream Infections and total number of U.S. Hospital Patient Days
**Negative values increases performance with a smaller SIR, postive values decreases performance with a larger SIR
Non-Risk Adjusted SIR was calculated using a ratio of the hospital’s observed cases / the hospital’s Non-Risk Adjusted Perdicted Cases
Non-Risk Adjusted Perdicted Cases was calucated by multiplying the Non-Risk Adjusted National Infection Rate by the number of facility Patient Days
Non-Risk Adjusted National Infection Rate equals the the sum of the national total Observed Cases divided by the national total number of Patient Days
N equaled 3917 U.S. facilities
Fig. 1.
Risk Adjustment Data Variability in the Standardized Infection Ratio. Acquisition Dates 1/4/2017 to 31/3/2018, 1697 hospitals analyzed
Need for transparency and more comprehensive reporting
The healthcare industry in the United States is not making the hard decisions and allocation of resources needed to reverse this epidemic. The industry has largely avoided the issue of identification and reporting of colonized patients and healthcare workers, along with the risk these colonized pathogens pose to their health, and the health of their families and patients.
In addition, the United States has changed metric definitions [36] and uses stringent risk adjustments for reporting MRSA along with other hospital acquired infections. This has the potential of severely mitigating the actual numbers of patients. In the historical review “The Pandemic Century” [37], it is apparent this strategy has been used in many past pandemics, with governments trying to avoid accountability. For example: During the 2014 Ebola epidemic, the government of Guiana insisted that only laboratory confirmed cases be counted. In a country with sparse medical resources this created an underestimation of cases and the appearance that the epidemic was being brought under control, greatly delaying the international response and setting the stage for the carnage that followed. And, in the 2015 Zika epidemic in Brazil, the definition of microcephaly was changed from a head circumference of 33 cm to 32 cm which reduced the number of cases.
When risk adjustment is performed, it should be based on high performance facilities which have optimally implemented preventative strategies. However, when this is done, even with high risk populations as serviced by the Veterans Administration, MRSA infection rates have been observed to fall to an extremely low level [38] questioning the need for adjustment. The same is true for Central Line Bloodstream Infections (CLABSIs) [39].
Conclusion
In the face of an emerging epidemic, medicine must not stagnate by waiting for the performance of randomized controlled trials(RCT). This is what appears to have happened in the United States and in the review by Cooper, et al. [18] was used as justification [17]. However, epidemics evolve faster than randomized controlled trials can be planned, approved and conducted. Conditions change. In the United States, the epidemic of MRSA has progressed becoming endemic in its population. Not since the Spanish Flu Outbreak of 1918 has an epidemic of this magnitude existed in the United States and similar tactics may need to be adopted.
It is known how to control the spread of MRSA in acute care facilities. The use of facility-wide admission screening is a strategy reminiscent of reverse isolation used in the Spanish flu epidemic, where a barrier is placed between the community and the facility to protect the most vulnerable of individuals, patients in hospitals. The VA has had great success with this strategy, it now needs systemwide implementation in the United States. Additional measures which should be enacted include instructing visitors regarding good hygiene and the prohibition of young children from visiting a facility, both from the standpoint of safety of the child and patients. The use of daily Chlohexidine bathing is associated with concerns of fostering resistance [40, 41] and on a facility wide basis it has not been shown to be effective in preventing MRSA infections, except in patients with medical devices [28].
Control of MRSA in the community is another issue. MRSA is endemic in the United States, with the CDC estimating a 2% rate of carriage in our general population. It is known that this carriage carries a greater risk of infections, [34, 42] many of which can be prevented by decolonization [34]. Households have been shown to be an important reservoir for MRSA, where it may persist for 2 to 8 years and decolonization of household members may be an important component of an MRSA control strategy [43]. Additional research is needed on how to best decolonize citizens, the best ways to clean the environment and how to prevent reconversion.
The cost will be high. For example, Project CLEAR took approximately 8 years from commencement to publication [44] at a cost of almost 10,000,000 USD from AHRQ funding alone [45]. The United States Government has limited resources to fund these large studies, especially when strapped with funding the most expensive healthcare system in the world [46] where the average CEO‘s salary at 22 major non-profit hospitals is 3.1 million dollars per year [47]. Obviously, we cannot wait for RCT design and completion before taking decisive action to confront epidemics of emerging and everchanging drug resistant bacteria.
The United States needs to regain its leadership role in the World in confronting these pathogens, which pose both a national and international risk and not fall into the trap of avoiding setting standards by using the excuse of “One size does not fit all”. The lack of firm standards has also led to an almost lackadaisical attitude in the control of dangerous pathogens with some U.S. facilities viewing MRSA carriage as “no big deal” [48] and not even placing patients who have MRSA infections or colonizations in full contact precautions .
The principal deputy director of the CDC was quoted by USA Today as stating the stalling in the reduction of MRSA infections in the United States might indicate that healthcare facilities are “wondering whether it’s worth their trouble” to take action against these dangerous pathogens [49]. First and foremost, the United States must regain control of its fractured and disparate healthcare system. Uniform action must now take place. Similar to the Plague outbreak in San Francisco in 1924, the United States government may be able to obtain limited statutory authority to mandate uniform reporting of highly dangerous pathogens and oversee containment and control by using the 2005 WHO International Health Regulations [50], which arguably could be applied to any State with an air or sea port of entry.
The United States has the knowledge and resources, but it has yet to prove it has the political and economic will to contain this epidemic.
Acknowledgements
Not applicable.
Abbreviations
- ARIC
Antimicrobial Resistance and Infection Control
- CDC
Centers for Disease Control and Prevention
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-sensitive Staphylococcus aureus
- NHS
National Health Service
- SIR
Standardized Infection Ratio
Author’s contributions
KTK authored this manuscript. The author read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data can be downloaded from Hospital Compare datasets. Data.Medicare.gov. Mar. 21, 2019. https://data.medicare.gov/data/hospital-compare Accessed on Mar. 23, 2019.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Kevin Kavanagh has received partial conference attendance and meeting support from the U.S. Dept. of Health and Human Services, National Quality Forum, National Patient Safety Foundation (NPSF), The Leapfrog Group, National Quality Forum, Consumer Union and the Anesthesia Patient Safety Foundation. He has served on the Centers for Medicare and Medicaid Services’ Technical Expert Panel for Hospital Acquired Conditions, and most recently on the Strategic Working Group for AHRQ for quality indicators, and AHRQ Health Care Effectiveness and Outcomes Research (HEOR) Study Section. He is an Associate Editor for the Journal of Patient Safety for which he receives an honorarium. He has a first degree relative, who is employed by a state university, and is involved with the development of cancer chemotherapeutic and diagnostic agents.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM; Emerging Infections Program MRSA author group, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo D. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214–219. 10.15585/mmwr.mm6809e1. https://www.cdc.gov/mmwr/volumes/68/wr/mm6809e1.htm Accessed on 24 May 2019. [DOI] [PMC free article] [PubMed]
- 2.National Data for Acute Care Hospitals, Year 2017. Centers for Disease Control and Prevention. 2019. https://gis.cdc.gov/grasp/PSA/HAIreport.html Accessed on 24 May 2019.
- 3.Hospital Compare datasets. Data.Medicare.gov. Mar. 21 2019. https://data.medicare.gov/data/hospital-compare Accessed on 24 May 2019.
- 4.Office of Disease Prevention and Health Promotion. National Targets and Metrics US Dept Health and Human Services 2019. https://health.gov/hcq/prevent-hai-measures.asp Accessed on 24 May 2019.
- 5.Jones M, Jernigan JA, Evans ME, Roselle GA, Hatfield KM, Samore MH. Vital signs: trends in Staphylococcus aureus infections in veterans affairs medical centers - United States, 2005-2017. MMWR Morb Mortal Wkly Rep 2019;68(9):220–224. 10.15585/mmwr.mm6809e2. https://www.cdc.gov/mmwr/volumes/68/wr/mm6809e2.htm. Accessed 24 May 2019. [DOI] [PMC free article] [PubMed]
- 6.Kavanagh KT, Abusalem S, Calderon LE. The incidence of MRSA infections in the United States: is a more comprehensive tracking system needed? Antimicrob Resist Infect Control 2017;6:34. 10.1186/s13756-017-0193-0. eCollection 2017. https://aricjournal.biomedcentral.com/articles/10.1186/s13756-017-0193-0. [DOI] [PMC free article] [PubMed]
- 7.Otter, J. The English MRSA miracle. Reflections on Infection Prevention and Control 3 2015. https://reflectionsipc.com/2015/03/03/the-english-mrsa-miracle/ Accessed on 19 Mar. 2019.
- 8.Dancer. S. Response to: evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ 2012;344:e3005. https://www.bmj.com/content/344/bmj.e3005/rapid-responses. Accessed on 19 Mar. 2019. [DOI] [PMC free article] [PubMed]
- 9.Rodríguez-Baño J, García L, Ramírez E, Lupión C, Muniain MA, Velasco C, Gálvez J, del Toro MD, Millán AB, López-Cerero L, Pascual A. Long-term control of endemic hospital-wide methicillin-resistant Staphylococcus aureus (MRSA): the impact of targeted active surveillance for MRSA in patients and healthcare workers. Infect Control Hosp Epidemiol 2010;31(8):786–795. 10.1086/654003. https://www.ncbi.nlm.nih.gov/pubmed/20524852 Accessed on 22 May 2019. [DOI] [PubMed]
- 10.Harbarth S, Fankhauser C, Schrenzel J, Christenson J, Gervaz P, Bandiera-Clerc C, Renzi G, Vernaz N, Sax H, Pittet D. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA. 2008;299(10):1149–57. 10.1001/jama.299.10.1149. https://jamanetwork.com/journals/jama/fullarticle/181604. Accessed on 22 May 2019. [DOI] [PubMed]
- 11.Huskins WC, Huckabee CM, O'Grady NP, Murray P, Kopetskie H, Zimmer L, Walker ME, Sinkowitz-Cochran RL, Jernigan JA, Samore M, Wallace D, Goldmann DA; STAR*ICU Trial Investigators. Intervention to reduce transmission of resistant Bacteria in intensive care. N Engl J Med 2011;364(15):1407–1418. 10.1056/NEJMoa1000373. https://www.nejm.org/doi/full/10.1056/nejmoa1000373 Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 12.House of Representatives. Healthcare-Associated Infections: A preventable epidemic. Hearing before the committee on oversight and government reform house of representatives. 2008. https://www.govinfo.gov/content/pkg/CHRG-110hhrg47541/html/CHRG-110hhrg47541.htm. Accessed 22 May 2019.
- 13.Platt R. Time for a culture change? N Engl J Med 2011;364(15):1464–1465. 10.1056/NEJMe1014292 PMID: 21488769 https://www.nejm.org/doi/full/10.1056/NEJMe1014292. Accessed on 22 May 2019. [DOI] [PubMed]
- 14.Jernigan JA. Preventing MRSA in healthcare- is there a silver bullet? (part 1 of 3) centers for diseases control and prevention. 2011. HW USA article archive.http://www.healthwatchusa.org/downloads/20110413-Jernigan-CDC-2011-p1542.pdf. Accessed 22 May 2019.
- 15.Screening for methicillin-resistant Staphylococcus aureus (MRSA). Comparative effectiveness review. Number 102. Agency for Healthcare Research and Quality. http://www.effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productID=1550&ECem=130620. Accessed on 22 May 2019.
- 16.Kavanagh KT, Saman DM, Yu Y. A perspective on how the United States fell behind northern Europe in the battle against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2013;57(12):5789–5791. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3837914/. Accessed 22 May 2019. [DOI] [PMC free article] [PubMed]
- 17.Jane D. Siegel, MD; Emily Rhinehart, RN MPH CIC; Marguerite Jackson, PhD; Linda Chiarello, RN MS; the Healthcare Infection Control Practices Advisory Committee. Management of Multidrug-Resistant Organisms In Healthcare Settings, 2006. Centers for Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/pdf/guidelines/mdro-guidelines.pdf. Accessed on 22 May 2019.
- 18.Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, Duckworth GJ, Lai R, Ebrahim S. Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling. Health Technol Assess 2003;7(39):1–194. https://www.journalslibrary.nihr.ac.uk/hta/hta7390#/abstract. Accessed on 22 May 2019. [DOI] [PubMed]
- 19.Kavanagh K, Abusalem S, Saman DM. A perspective on the evidence regarding methicillin-resistant Staphylococcus aureus surveillance. J Patient Saf 2012;8(3):140–143. 10.1097/PTS.0b013e3182627b89. PMID 22874134 https://journals.lww.com/journalpatientsafety/fulltext/2012/09000/A_Perspective_on_the_Evidence_Regarding.6.aspx. Accessed on 22 May 2019. [DOI] [PubMed]
- 20.Kavanagh KT, Tower SS, Saman DMA. Perspective on the principles of integrity in infectious disease research. J Patient Saf. 2016;12(2):57–62. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4915748/. Accessed 22 May 2019. [DOI] [PMC free article] [PubMed]
- 21.Robicsek A, Beaumont JL, Paule SM, Hacek DM, Thomson RB Jr, Kaul KL, King P, Peterson LR. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148(6):409–418. PMID: 18347349http://www.ncbi.nlm.nih.gov/pubmed/18347349. Accessed on 22 May 2019. [DOI] [PubMed]
- 22.Kelly, J.W., Blackhurst, D., McAtee, W., and Steed, C. Electronic hand hygiene monitoring as a tool for reducing health care-associated methicillin-resistant Staphylococcus aureus infection. Am J Infect Control 2016; 44: 956–995. https://www.ajicjournal.org/article/S0196-6553(16)30340-6/fulltext Accessed on 22 May 2019. [DOI] [PubMed]
- 23.Kavanagh KT. Comment on electronic hand hygiene monitoring as a tool for reducing health care-associated methicillin-resistant Staphylococcus aureus infection. PubPeer. https://pubpeer.com/publications/B1ECD44FC56A8536E5E0355D0396CF. Accessed on 19 Mar. 2019. [DOI] [PubMed]
- 24.Maiwald M, Chan ES. Pitfalls in evidence assessment: the case of chlorhexidine and alcohol in skin antisepsis. J Antimicrob Chemother 2014;69(8):2017–2021. 10.1093/jac/dku121. https://academic.oup.com/jac/article/69/8/2017/2911297. Accessed on 22 May 2019. [DOI] [PubMed]
- 25.Wu AW, Kavanagh KT, Pronovost PJ, Bates DW. Conflict of interest, Dr Charles Denham and the journal of patient safety. J Patient Saf. 2014;10(4):181–185. 10.1097/PTS.0000000000000144. http://journals.lww.com/journalpatientsafety/Fulltext/2014/12000/Conflict_of_Interest,_Dr_Charles_Denham_and_the.1.aspx Accessed on 22 May 2019. [DOI] [PubMed]
- 26.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford F, Hayden MK, Jernigan JA, Weinstein RA, Fraser VJ, Haffenreffer K, Cui E, Kaganov RE, Lolans K, Perlin JB, Platt R; the CDC prevention epicenters program; the AHRQ DECIDE network and healthcare-associated infections program. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013; PMID:23718152 http://www.ncbi.nlm.nih.gov/pubmed/23718152. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 27.Nelson DJ, McNeill R. Money from infection-control industry muddies research into beating back superbugs Reuters news service.2017. http://www.reuters.com/investigates/special-report/usa-superbugs-research/ Accessed on 22 May 2019.
- 28.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Heim L, Gombosev A, Avery TR, Haffenreffer K, Shimelman L, Hayden MK, Weinstein RA, Spencer-Smith C, Kaganov RE, Murphy MV, Forehand T, Lankiewicz J, Coady MH, Portillo L, Sarup-Patel J, Jernigan JA, Perlin JB, Platt R; ABATE Infection trial team. Chlorhexidine versus routine bathing to prevent multidrug-resistant organisms and all-cause bloodstream infections in general medical and surgical units (ABATE Infection trial): a cluster-randomised trial. Lancet. 4 Mar. 2019. pii: S0140–6736(18)32593–5. 10.1016/S0140-6736(18)32593-5. [Epub ahead of print] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32593-5/fulltext. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 29.Mimoz O, Guenezan J. No benefit of chlorhexidine bathing in non-critical care units. Lancet. 4 Mar. 2019. pii: S0140-6736(18)33130-1. 10.1016/S0140-6736(18)33130-1. [Epub ahead of print] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)33130-1/ppt. Accessed on 22 May 2019. [DOI] [PubMed]
- 30.2017 summary report on antimicrobials sold or distributed for use in food-producing animals. U.S. Food & drug administration. 2018. https://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM628538.pdf. Accessed on 22 May 2019.
- 31.Fisher MM, Bild M. Hospital use of antibiotics as the main driver of infections with antibioticresistant bacteria - a reanalysis of recent data from the European Union. bioRxiv preprint; 2019. https://www.biorxiv.org/content/10.1101/553537v1. Accessed 22 May 2019.
- 32.Jackson KA, Bohm MK, Brooks JT, Asher A, Nadle J, Bamberg WM, Petit S, Ray SM, Harrison LH, Lynfield R, Dumyati G, Schaffner W, Townes JM, See I. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs — six sites, 2005–2016. MMWR Morb Mortal Wkly Rep. 2018;67(22):625–8. 10.15585/mmwr.mm6722a2. Accessed 22 May 2019. [DOI] [PMC free article] [PubMed]
- 33.Albrich,W.C.,and Harbarth,S.(2008).Health-careworkers: source, vector, or victim of MRSA? Lancet Infect Dis 8,289–301. 10.1016/S1473-3099(08)70097-5http://www.thelancet.com/journals/laninf/article/PIIS1473309908700975/abstract. Accessed on 26 May 2019. [DOI] [PubMed]
- 34.Huang SS, Singh R, McKinnell JA, Park S, Gombosev A, Eells SJ, Gillen DL, Kim D, Rashid S, Macias-Gil R, Bolaris MA, Tjoa T, Cao C, Hong SS, Lequieu J, Cui E, Chang J, He J, Evans K, Peterson E, Simpson G, Robinson P, Choi C, Bailey CC Jr, Leo JD, Amin A, Goldmann D, Jernigan JA, Platt R, Septimus E, Weinstein RA, Hayden MK, Miller LG; project CLEAR Trial. Decolonization to reduce Postdischarge infection risk among MRSA carriers. N Engl J Med 2019;380(7):638–650. 10.1056/NEJMoa1716771. https://www.nejm.org/doi/full/10.1056/NEJMoa1716771. Accessed 22 May 2019. [DOI] [PMC free article] [PubMed]
- 35.Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, Abbas M, Atema JJ, Gans S, van Rijen M, Boermeester MA, Egger M, Kluytmans J, Pittet D, Solomkin JS, Guidelines Development WHO. Group. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e276–87. https://www.thelancet.com/journals/laninf/article/PIIS1473.30991630398.X/fulltext. Accessed 22 May 2019. [DOI] [PubMed]
- 36.Kavanagh KT. Collective ignorance and government timidity are public health threats. Los Angeles Times 16 2019. https://www.latimes.com/opinion/op-ed/la-oe-kavanagh-epidemics-health-public-policy-20190516-story.html.
- 37.Honigsbaum M. The pandemic century. W.W. Norton & Company. New York New York. 2019. https://www.kirkusreviews.com/book-reviews/mark-honigsbaum/the-pandemic-century/.
- 38.Jones M, Jernigan JA, Evans ME, Roselle GA, Hatfield KM, Samore MH. Vital signs: trends in Staphylococcus aureus infections in veterans affairs medical centers - United States, 2005-2017. MMWR Morb Mortal Wkly Rep 2019;68(9):220–224. 10.15585/mmwr.mm6809e2. https://www.cdc.gov/mmwr/volumes/68/wr/mm6809e2.htm. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 39.Saman DM, Kavanagh KT. Assessing the necessity of the standardized infection ratio for reporting central line associated bloodstream infections. Plos one. 4 Nov 2013. PMID 24223966. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0079554. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 40.Naparstek L, Carmeli Y, Chmelnitsky I, Banin E, Navon-Venezia S. Reduced susceptibility to chlorhexidine among extremely-drug-resistant strains of Klebsiella pneumoniae. J Hosp Infect 2012;81(1):15–19. https://www.ncbi.nlm.nih.gov/pubmed/22463977. Accessed 22 May 2019. [DOI] [PubMed]
- 41.Copin R, Sause WE, Fulmer Y, Balasubramanian D, Dyzenhaus S, Ahmed JM, Kumar K, Lees J, Stachel A, Fisher JC, Drlica K, Phillips M, Weiser JN, Planet PJ, Uhlemann AC, Altman DR, Sebra R, van Bakel H, Lighter J, Torres VJ, Shopsin B. Sequential evolution of virulence and resistance during clonal spread of community-acquired methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2019;116(5):1745–1754. 10.1073/pnas.1814265116. https://www.ncbi.nlm.nih.gov/pubmed/30635416/. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 42.Cadena J, Thinwa J, Walter EA, Frei CR. Risk factors for the development of active methicillin-resistant Staphylococcus aureus (MRSA) infection in patients colonized with MRSA at hospital admission. Am J Infect Control 2016;44(12):1617–1621. doi: 10.1016/j.ajic.2016.05.009. Epub 29 Jun 2016. https://www.ajicjournal.org/article/S0196-6553(16)30483-7/fulltext Accessed on 22 May 2019. [DOI] [PubMed]
- 43.Alam MT, Read TD, Petit RA 3rd, Boyle-Vavra S, Miller LG, Eells SJ, Daum RS, David MZ. Transmission and microevolution of USA300 MRSA in U.S. households: evidence from whole-genome sequencing. MBio. 2015;6(2):e00054. doi: 10.1128/mBio.00054-15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4453535/. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 44.Clinical Trails.gov Identifier: NCT01209234 https://clinicaltrials.gov/ct2/show/NCT01209234. Accessed on 22 May 2019.
- 45.Federal RePORTER Project Number R01HS019388 https://federalreporter.nih.gov/projects/switchQueryForm?mode=Advanced. Accessed on 22 May 2019.
- 46.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039. 10.1001/jama.2018.1150. https://jamanetwork.com/journals/jama/article-abstract/2674671. Accessed on 22 May 2019. [DOI] [PubMed]
- 47.Du JY, Rascoe AS, Marcus RE. The growing executive-physician wage gap in major US nonprofit hospitals and burden of nonclinical workers on the US healthcare system. Clin Orthop Relat Res 2018;476(10):1910–1919. 10.1097/CORR.0000000000000394. https://www.ncbi.nlm.nih.gov/pubmed/30001293. Accessed on 22 May 2019. [DOI] [PMC free article] [PubMed]
- 48.McCarthy M. What superbug hunters know that we Don’t. The New York times. 2019. https://www.nytimes.com/2019/05/20/opinion/hospitals-antibiotic-resistant-bacteria-superbugs.html. Accessed on 22 May 2019.
- 49.O’Donnell, J. Hospitals know how to reduce or stop staph infections. So why are thousands still dying? 2019. https://www.usatoday.com/story/news/health/2019/03/05/mrsa-staph-infection-hospital-deadly-centers-disease-control-medicare-medicaid/3032939002/. Accessed 22 May 2019.
- 50.WHO International Health Regulations (2005). https://www.who.int/ihr/9789241596664/en/. Accessed on 22 May 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be downloaded from Hospital Compare datasets. Data.Medicare.gov. Mar. 21, 2019. https://data.medicare.gov/data/hospital-compare Accessed on Mar. 23, 2019.